Abstract
Abstract
The investigational NEDD8‐activating enzyme inhibitor pevonedistat is being evaluated in combination with azacitidine versus single‐agent azacitidine in patients with higher‐risk myelodysplastic syndrome (higher‐risk MDS), higher‐risk chronic myelomonocytic leukemia (higher‐risk CMML), or low‐blast acute myeloid leukemia (AML) in a Phase 3 trial PANTHER. To support Asia‐inclusive global development, we applied multiregional clinical trial (MRCT) principles of the International Conference on Harmonisation E17 guidelines by evaluating similarity in drug‐related and disease‐related intrinsic and extrinsic factors. A PubMed literature review (January 2000–November 2019) supported similarity in epidemiology of higher‐risk MDS, AML, and CMML in Western and East Asian populations. Furthermore, the treatment of MDS/AML was similar in both East Asian and Western regions, with the same dose of azacitidine being the standard of care. Median overall survival in MDS following azacitidine treatment was generally comparable across regions, and the types and frequencies of molecular alterations in AML and MDS were comparable. Dose‐escalation studies established the same maximum tolerated dose of pevonedistat in combination with azacitidine in Western and East Asian populations. Pevonedistat clearance was similar across races. Taken together, conservation of drug‐related and disease‐related intrinsic and extrinsic factors supported design of an Asia‐inclusive Phase 3 trial and a pooled East Asian region. A sample size of ~ 30 East Asian patients (of ~ 450 randomized) was estimated as needed to demonstrate consistency in efficacy relative to the global population. This analysis is presented as an exemplar to illustrate application of clinical pharmacology and translational science principles in designing Asia‐inclusive MRCTs.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Azacitidine is the standard of care for myelodysplastic syndromes/low‐blast acute myeloid leukemia (AML) across Western and East Asian patients. The first‐in‐class small‐molecule inhibitor of NEDD8‐activating enzyme, pevonedistat, has been investigated as a single agent in multiple studies of hematologic and nonhematologic malignancies and in combination with azacitidine in elderly patients with untreated AML.
WHAT QUESTION DID THIS STUDY ADDRESS?
By applying clinical pharmacology and translational science and International Conference on Harmonisation E17 principles, this study designed an East Asian‐inclusive global pivotal Phase 3 trial of pevonedistat, taking into consideration drug‐related and disease‐related intrinsic and extrinsic factors.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
These analyses provide scientific rationale for Asia‐inclusive globalization of the pivotal, Phase 3 PANTHER trial and for pooling clinical data across the East Asian region for assessing consistency in efficacy.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
We developed a framework to facilitate efficient global clinical development of investigational therapies for rare cancers and orphan diseases in Asia‐inclusive multiregional clinical trials.
INTRODUCTION
Multiregional clinical trials (MRCTs) help decrease the lag in drug development and approval that often occurs in Asian countries compared with those in Europe and North America. 1 , 2 For example, delays due to country‐specific requirements for submission of local patient data can be mitigated if the MRCT is designed to enroll local patients. 1 The International Conference on Harmonisation of Technical Requirement for Registration of Pharmaceuticals for Human Use (ICH) E5 guidelines provide a framework for evaluating the impact of ethnic factors on the efficacy and safety of a particular study drug dose and regimen. 3 They also provide guidance on development strategies that assess the effect of ethnic factors while minimizing the duplication of clinical studies and expediting patient access to drugs. 3
Global MRCTs are on the rise, notably in oncology and rare disease/orphan drug development. 2 In 2017, the ICH issued E17 guidelines, which describe general principles for the planning and design of MRCTs with the aim of generating data that are applicable for global regulatory submissions. 4 The key principles of the E17 guidelines are to: conduct well‐designed MRCTs to increase drug development efficiency and support regulatory decision making across regions, understand relevant intrinsic and extrinsic factor effects early in MRCT design, allocate sample size by region to verify consistency in treatment effect while allowing feasibility in recruitment and timely trial conduct, pool prespecified regions based upon similarities, use a single primary analysis supported by structured exploration of consistency, ensure high‐quality trial design and conduct, and encourage efficient communication between sponsors and regulatory authorities during MRCT design. 4 Notably, ICH E17 principles can enable efficient design of MRCTs with regional sample size allocation that is based on scientific justification rather than traditional designs that may be driven by local (i.e., country‐level) regulatory considerations.
A key aspect of both the E5 and E17 guidelines is evaluating intrinsic and extrinsic factors as sources of variability in drug response. 3 , 4 This manuscript describes the application of ICH E5 and E17 principles to enable an Asia‐inclusive global development strategy for an investigational anticancer agent under evaluation for the treatment of rare hematologic malignancies.
Pevonedistat is the first small‐molecule inhibitor of the NEDD8‐activating enzyme (NAE). 5 NAE facilitates the activation of Cullin‐RING E3 ubiquitin ligases (CRLs) via binding of the small ubiquitin‐like protein NEDD8 (neural precursor cell expressed, developmentally downregulated 8). 5 , 6 Pevonedistat forms an adduct with NEDD8, preventing conjugation of NEDD8 to CRLs and ultimately leading to CRL substrate accumulation and cell death. 6 , 7 Pevonedistat has been investigated as a single agent in multiple studies of hematologic and nonhematologic malignancies and in combination with azacitidine in elderly patients with untreated acute myeloid leukemia (AML). 8 , 9 , 10 , 11 , 12 Based on the promising clinical data of pevonedistat in combination with azacitidine, a multicenter, global, randomized, controlled, open‐label, Phase 3 study (PANTHER, NCT03268954) is currently ongoing to investigate the efficacy and safety of pevonedistat in combination with azacitidine versus single‐agent azacitidine in patients with higher‐risk myelodysplastic syndrome (higher‐risk MDS), higher‐risk chronic myelomonocytic leukemia (higher‐risk CMML), or low‐blast AML (Figure S1). 13
To enable timely enrollment of East Asian patients into the PANTHER trial and to support the evaluation of pevonedistat plus azacitidine in both Western and East Asian patients with these rare diseases, we applied MRCT principles of the ICH E17 guidelines informed by ICH E5 to design an Asia‐inclusive study. The design of this MRCT was supported by assessment of drug‐related and disease‐related intrinsic and extrinsic factors, enabling successful reviews during the Clinical Trial Notification/Application (CTN/CTA) process by the Pharmaceuticals and Medical Devices Agency (PMDA, Japan), the Ministry of Food and Drug Safety (MFDS, South Korea), and the National Medical Products Administration (NMPA, China). Furthermore, a pooled East Asian region could be rationalized based on scientific considerations of commonality of intrinsic and extrinsic factors and statistical evaluations to define the number of East Asian patients required to evaluate consistency with the overall data.
METHODS
Literature review on AML and MDS epidemiology, mutational landscape, efficacy, and molecular pathology
We performed a targeted literature review (PubMed; January 2000–November 2019) to identify publications reporting clinical studies of azacitidine in higher‐risk MDS, AML, and CMML conducted in Western (Unites States/European Union) and East Asian regions, and epidemiological studies of incidence and characteristics of higher‐risk MDS, AML, and CMML by region. A comprehensive PubMed literature search was performed from September 2011 to March 2018 to compare the abundance of major mutated genes and cytogenetic abnormality profiles in MDS and AML between patients from Western and East Asian regions. The search terms for azacitidine efficacy were: azacitidine, myelodysplastic, efficacy, Phase 3, Phase 1/2, Korean, Japanese, and Chinese. The search terms for mutation and cytogenetic abnormalities were: myelodysplastic syndrome, acute myeloid leukemia, prognostic, molecular analysis, mutations, next generation sequencing, genetic abnormalities, cytogenetic analysis/abnormalities, karyotype, molecular pathology, and hypomethylation.
Population pharmacokinetic analyses
Pevonedistat plasma concentration–time data were collected from adult patients with advanced hematologic or nonhematologic malignancies (AML, MDS, multiple myeloma [MM], lymphoma, melanoma, or various solid tumors) who had participated in one of eight clinical studies (Table S1) in Western and East Asian regions. 8 , 9 , 10 , 11 , 12 , 14 Patients received single‐agent pevonedistat via a 1‐h i.v. infusion at dose levels of 25–278 mg/m2, across 6 different dosing schedules in 21‐day cycles. In the combination study, patients received pevonedistat 10, 20, or 30 mg/m2 via a 1‐h i.v. infusion in combination with azacitidine (i.v./s.c.) 75 mg/m2 in 28‐day cycles. All procedures performed in studies involving human participants were in accordance with the ICH Good Clinical Practice guidelines and appropriate regulatory requirements. All patients provided written informed consent.
Methods for bioanalysis of pevonedistat plasma concentrations have been previously described. 15 Pevonedistat pharmacokinetic (PK) data were analyzed using nonlinear mixed‐effects modeling (NONMEM version 7.3; ICON Development Solutions) as described previously. 15 Post hoc estimates of pevonedistat clearance in patients according to race (White, Black, and East Asian) were generated and compared.
Statistical considerations
At the time of calculating the sample size of the East Asian population in the PANTHER study, there were two primary end points: (1) overall response rate (ORR) by cycle 6 and (2) event‐free survival (EFS); overall survival (OS) was the key secondary end point. The protocol was subsequently amended to have one primary end point of EFS and one key secondary end point of OS. The appropriateness of the number of East Asian patients to be enrolled was assessed by calculating the probability of achieving consistent results between the overall population and the East Asian population for these end points. The consistency of the results was defined as follows: hazard ratio (pevonedistat in combination with azacitidine/single‐agent azacitidine) is smaller than one in the East Asian population for both EFS and OS, when the overall results are positive. Two treatment effect scenarios (A: median EFS of 22.2 m vs. 13 m, B: median EFS of 19.6 m vs. 13 m) for EFS that were used to determine minimum and maximum planned event size with adaptive EFS event size re‐estimation. Accordingly, these two scenarios (A and B) were used to calculate the consistency probabilities. The consistency probabilities were calculated by clinical trial simulations with 10,000 iterations per scenario. The simulation data for EFS and OS were randomly generated from an exponential distribution under the assumption that there is no difference in the efficacy of pevonedistat between the East Asian population and the overall population in the study, taking into consideration the enrollment projection and the timing of the analyses. Simulations were conducted using SAS version 9.2.
RESULTS
Epidemiology and standards of care
Incidences of MDS and AML by region are shown in Table 1. According to the National Cancer Institute in the United States, the incidence of MDS is estimated to be 4.5 per 100,000 people per year in the overall population, 0.1 per 100,000 in the subpopulation aged less than 40 years, 26.9 per 100,000 in the subpopulation aged 70–79 years, and 55.4 per 100,000 in the subpopulation aged greater than or equal to 80 years, showing increased incidences in higher age groups. 16 In the European Union, the incidence is estimated to be 4.15 per 100,000 people per year in the overall population, and 40–50 per 100,000 in the subpopulation aged greater than or equal to 70 years. 17 Age‐adjusted incidences of MDS in 2008 in Japan, standardized by the world standard population, were 1.6 cases per 100,000 men and 0.8 cases per 100,000 women. 18 In South Korea, age‐standardized incidence rates of MDS in 2008 were 1.3 cases per 100,000 men and 0.8 cases per 100,000 women. 19 In mainland China, no official large‐scale MDS incidence data are available. MDS incidence (based on World Health Organization [WHO] criteria) from 2004 to 2007 in Shanghai was 1.48 per 100,000 men and 1.54 per 100,000 women. These data were based on a survey that randomly selected 6 of 18 districts in Shanghai reflecting the incidence in an urban population in mainland China. 20 Azacitidine 75 mg/m2 (i.v. or s.c.) administered for 7 days in 28‐day cycles is approved in the United States, the European Union, Japan, South Korea, and China as first‐line treatment for higher‐risk MDS and CMML based on regional prescribing information. Azacitidine is also indicated for the treatment of AML with 20–30% blasts and multilineage dysplasia, according to WHO classification (United States and European Union) and AML with greater than 30% marrow blasts according to the WHO classification (European Union only). 21 , 22 Azacitidine is the standard treatment in these patient populations across the different countries.
Table 1.
Incidence of MDS and AML by region
| Region | MDS incidence | AML incidence |
|---|---|---|
| United States 16 | 4.5/100,000 people per year | 3.2/100,000 people per year |
| European Union 17 , 62 | 4.15/100,000 people per year | 3.7/100,000 people per year |
| Japan 18 , 63 | 1.6 cases per 100,000 men and 0.8 cases per 100,000 women | 1.9 per 100,000 people per year |
| South Korea 19 , 64 | 1.3 cases per 100,000 men and 0.8 cases per 100,000 women | 2.5 crude incidence rate per 100,000 |
| China 20 , 65 | 1.48 per 100,000 men and 1.54 per 100,000 women | 1.35 per 100,000 a |
Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplastic syndrome.
Nanjing during the period 2003–2007.
Treatment response and outcomes
A literature search identified a limited number of clinical studies (5 in total) investigating azacitidine in patients with MDS or low bone marrow blast count AML from East Asian or Western countries. A Phase 3 multicenter, randomized, open‐label trial (NCT00071799) conducted in 15 Western countries between February 2004 and August 2006 compared the efficacy of azacitidine 75 mg/m2 for 7 days in 28‐day cycles with that of conventional care regimens in the treatment of higher‐risk MDS. 23 Median OS for azacitidine was 24.5 months (interquartile range 9.9 was not reached). Median time to AML transformation was 17.8 months (95% confidence interval [CI] 13.6–23.6 months). Approximately one third of patients in this study were classified as AML with low bone marrow blast count and the median OS in this subset of patients was 19.1 months. 24 The ORR across all patients was 29%, with 17% complete remission (CR), 12% partial remission (PR), and 42% stable disease (SD). 23 Similar efficacy was observed in another randomized study conducted in Western countries investigating azacitidine 75 mg/m2 for 7 days every 28 days versus supportive care in patients with MDS. 25 The ORR was 60% in patients receiving azacitidine including 7% CR, 16% PR, and 37% hematologic improvement 25 ; median time to leukemic transformation or death was 21 months. 25 In a Phase 1/2 study of 53 Japanese patients with MDS who received azacitidine 75 mg/m2 s.c. or i.v. once daily for 7 consecutive days in a 28‐day cycle, hematologic improvement and hematologic response rates were 55% (28/51) and 28% (15/53; CR 15%, PR 0%, and marrow CR [mCR] 13%), respectively. 26 In a retrospective study of patients with MDS diagnosed and treated with azacitidine in the Korea MDS registry between 2004 and 2011, responses were observed in 46% (93/203) of patients (CR 11%, PR 6%, mCR 11%, and hematologic improvement 17%); median OS was 23.2 months. 27 A Phase 2 study conducted in Chinese patients with higher‐risk MDS reported a disease control rate of 96%, mainly driven by SD (CR 1% + PR 0% + SD 94%), and a hematologic improvement rate of 53% with s.c. azacitidine. The median OS was 22.0 months (95% CI 15.1–not reached) in Chinese patients, comparable with data from Western patients. 28 Based on the studies identified, median OS following azacitidine treatment is generally comparable among patients with MDS from East Asian and Western regions.
Mutational landscape and cytogenetic abnormalities
Genetic and cytogenetic alterations that are associated with the pathology and prognosis of AML, MDS, and CMML and which may be predictive of response to selected treatment options have been previously described. 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 It is possible that factors, such as geographic location, environment, lifestyle, genetic background, and others, may influence the mutational and cytogenetic landscape in patients from distinct ethnicities and may affect their sensitivity to specific drugs. In this study, we performed a comprehensive PubMed literature search to retrospectively compare the landscape and abundance of major mutated genes and the frequency of major cytogenetic abnormalities in MDS and AML between patients from Western and East Asian regions. The analysis showed that the mutational landscape and cytogenetic abnormalities of AML and MDS are similar among US, European, Japanese, and Korean populations (Figures 1, 2, Table S2). 29 , 43 Some differences in percentage mutation frequencies were noted in AML in DNMT3A (26–31, 16.2–23.1, and 7.5–14.9), IDH2 (14–20, 6.1–9, and 9.7), TP53 (8–9, 3.6–8, and 2.2), KRAS (6–12, 2–5.6, and 0–5.7), and ASXL1 (11, 2–2.5, and 8.6), and in MDS in SRSF2 (10–20, 5.2, and 0.9) and STAG2 (5–10, 2.9, and 0.9) between US plus European, and Japanese and Korean populations, respectively. These differences should be considered when correlating genetic and cytogenetic alteration with response and other end points of interest. However, in general, the types and frequencies of molecular alterations in AML and MDS are comparable across Western and East Asian populations, supporting the general conservation of disease‐related intrinsic factors.
FIGURE 1.
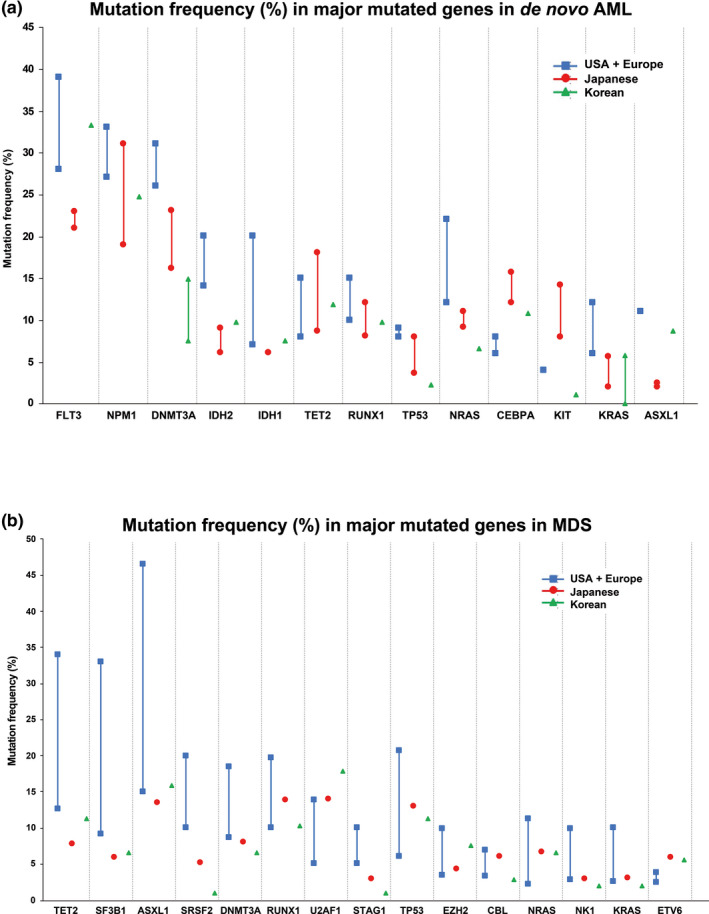
Comparative analysis of percentage frequency of major mutated genes in (a) de novo AML and (b) MDS across Western and East Asian populations. 29 , 50 , 66 , 67 , 68 , 69 , 70 AML, acute myeloid leukemia; MDS, myelodysplastic syndrome
FIGURE 2.
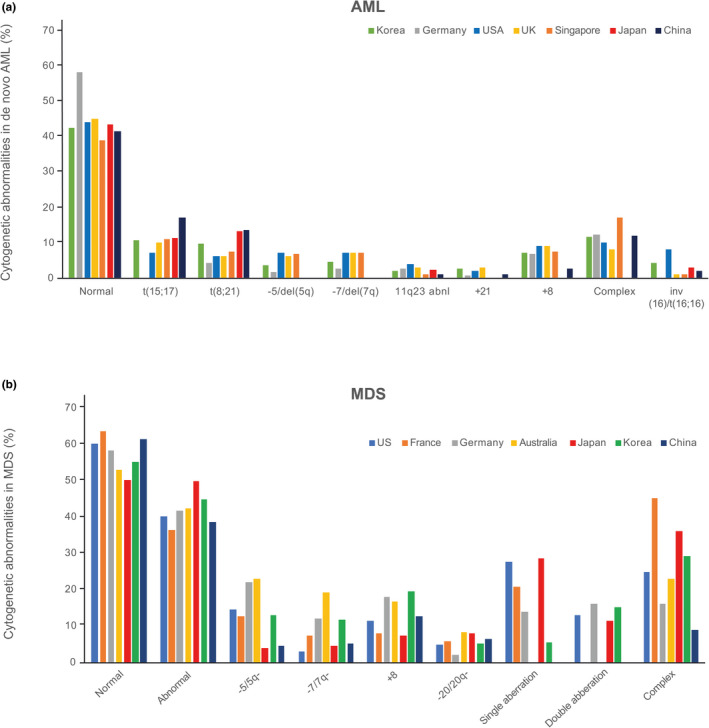
Cytogenetic abnormalities in de novo AML and MDS. 41 , 42 , 43 AML, acute myeloid leukemia; MDS, myelodysplastic syndrome
Pevonedistat dose, safety, and pharmacokinetics
Across Western (NCT01814826; conducted in the United States) and East Asian (NCT02782468; conducted in Japan, South Korea, and Taiwan) populations, Phase 1 dose escalation studies determined the same maximum tolerated dose (MTD) of pevonedistat (20 mg/m2 on days 1, 3, and 5 in 28‐day cycles) in combination with azacitidine (75 mg/m2 for 7 days in 28‐day cycles). 12 , 14 Population PK analyses were conducted to assess clinically important covariates and compare pevonedistat exposures between East Asian and Western patients. The analysis included data from 2 studies of pevonedistat in Western (NCT02610777) and East Asian (NCT02782468) patient populations in addition to 335 patients across 10 studies in a recently completed population PK analysis. 15 A total of 416 adult patients (receiving pevonedistat 15–278 mg/m2) contributed 4689 observations. Data were adequately described by a two‐compartment PK model. 15 Population PK analysis supported dose‐linear pevonedistat PK with body surface area (BSA) identified as a clinically important covariate on clearance, supporting BSA‐based dosing (Figure 3). After individualizing pevonedistat dose based on BSA, systemic pevonedistat clearance was similar when analyzed according to country/race (Figure 4).
FIGURE 3.
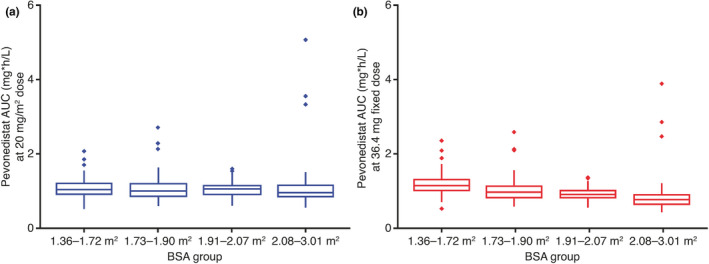
Comparison of simulated distribution of pevonedistat plasma exposures between (a) BSA‐adjusted dosing and (b) fixed dosing. AUC, area under the concentration‐time curve; BSA, body surface area
FIGURE 4.
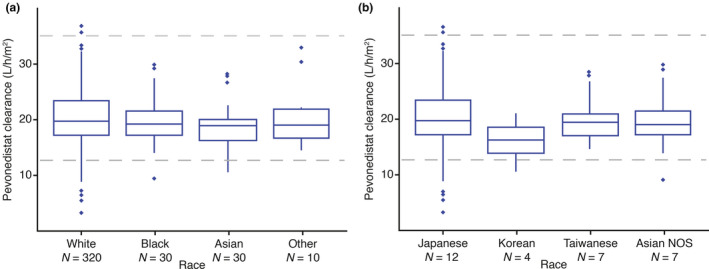
Pevonedistat BSA‐normalized clearance (a) by race and (b) in Asian patients by country/race. The horizontal lines comprising the box are the 25th, 50th (median), and 75th percentiles. The whisker ends denote 1.5 times the difference between the 25th and 75th percentiles, and the symbols beyond the whiskers are the outliers. The horizontal dashed lines represent 5th and 95th percentiles of clearance in the overall global analysis population. BSA, body surface area; NOS, not otherwise specified
Sample size of East Asian population in the PANTHER trial
Assuming that the treatment effects in Japanese/East Asian patients are similar to those in the overall global population, with a total of 30 East Asian patients, the estimated probabilities of achieving consistent efficacy outcomes are ~ 80% (measured by EFS and OS) between East Asian patients and the overall ~ 450 patients in the global, pivotal, Phase 3 PANTHER trial (Table 2, Figure S2).
Table 2.
Probability of consistency (East Asian vs. overall trial population)
| Parameter |
EFS Scenario A |
EFS Scenario B |
OS | OS |
|---|---|---|---|---|
| Analysis timepoint | Month 35–50 (IA3) a | Month 35–50 (IA3) a | Month 35–50 (IA3) a | Month 63 (FA) |
| Treatment effect (median) b |
Pevonedistat‐azacitidine arm: 22.2 months Azacitidine arm: 13 months |
Pevonedistat‐azacitidine arm: 19.6 months Azacitidine arm: 13 months |
Pevonedistat‐azacitidine arm: 36.95 months Azacitidine arm: 24.5 months |
Pevonedistat‐azacitidine arm: 36.95 months Azacitidine arm: 24.5 months |
| Number of overall patients randomized | ~450 | ~450 | ~450 | ~450 |
| Number of East Asian patients randomized | 30 | 30 | 30 | 30 |
| Expected number of events in East Asian patients | 17–21 | 18–22 | 13–17 | 19 |
| Consistency probability | 85.9–88.0% | 80.7–82.8% | 76.5–79.3% | 81.3% |
Abbreviations: EFS, event‐free survival; FA, final analysis; IA2, second interim analysis; IA3, third interim analysis; OS, overall survival.
The minimum and maximum EFS event size required for the IA3 in the overall population were specified as 147 and 249, respectively, in the study. The exact number of EFS events at IA3 depends on the IA2 results, where an event‐size re‐estimation is performed. It was projected at the start of the study that it would take 35 and 50 months to collect 147 and 249 EFS events, respectively. Expected number of events in East Asian patients and consistency probabilities were calculated and presented in intervals assuming IA3 is conducted sometime between 35 and 50 months after first patient is randomized.
There are two treatment effect scenarios (A and B) for EFS that were used to determine minimum and maximum planned event size. EFS and OS are assumed to be exponentially distributed.
DISCUSSION
Opportunities to increase efficiency in global drug development are increasing with the availability of the ICH E17 guideline that provides a scientific framework for MRCT design and analysis. 4 Herein, we have demonstrated the application of principles of clinical pharmacology and translational science in enabling the design of an Asia‐inclusive global pivotal Phase 3 trial in rare hematological malignancies for an investigational NAE inhibitor. This was accomplished through formulation of a pooled East Asian region rationalized by assessment of similarity in drug‐related and disease‐related intrinsic and extrinsic factors.
The Phase 3 PANTHER trial was designed to investigate the efficacy and safety of pevonedistat plus azacitidine versus single‐agent azacitidine in patients with higher‐risk MDS/CMML and low‐blast AML. We evaluated the regional characteristics of epidemiology, molecular pathology, standard‐of‐care treatment, and associated efficacy in higher‐risk MDS, CMML, and low‐blast AML to assess similarities in disease‐related intrinsic and extrinsic factors across Japanese, Korean, Chinese, and Western patient populations. Similarity in drug‐related intrinsic/extrinsic factors was confirmed based on the safety/tolerability and PKs of pevonedistat across these populations that supported a common global dose. Accordingly, we formulated a pooled East Asian region and determined, based on statistical considerations, that an associated sample size of ~ 30 of 450 randomized patients would be needed from this region to evaluate consistency in efficacy relative to the global population.
Epidemiology of the diseases of interest was inferred to be broadly comparable based on published age‐adjusted incidences of MDS across East Asian and Western regions. Consistent with current United States and European Union medical practice guidelines, azacitidine was identified as the standard of care for the treatment of MDS/AML in Japan, South Korea, and China, supporting inclusion of these countries in the common protocol evaluating pevonedistat in addition to azacitidine backbone therapy. Importantly, azacitidine dosage was conserved across these regions. Heterogeneity in efficacy evaluation of azacitidine, notably in selection of response rate end points, was apparent across the reported results of clinical trials. 23 , 26 , 28 , 44 Taken together with the heterogeneity in MDS, direct comparison of response rates to azacitidine across these clinical trials was not straightforward. Nevertheless, clinical survival outcomes were consistent across East Asian countries and the United States/Europe, supporting the inference of lack of clinically meaningful differences in azacitidine treatment outcome.
AML and MDS are heterogeneous diseases. Along with age and performance status, mutational profile and cytogenetic abnormalities are key clinical predictors of survival for an individual patient. Currently, cytogenetic status is incorporated into risk categorization schemes to guide treatment in both MDS and AML. The European LeukemiaNet integrates cytogenetics and the mutational status of six genes (FLT3 internal tandem duplication, CEBP, NPM1, RUNX1, ASXL1, and TP53) to classify patients with AML into three prognostic risk groups. 45 However, additional mutations in genes, such as ASXL1 and TET2, have more recently been shown to have prognostic value 46 , 47 and several novel risk‐categorization schemas have been proposed that include mutational status of these and other genes. 48 The Revised International Prognostic Scoring System for MDS incorporates cytogenetics as a way to determine the risk of transformation to AML. 49 Additionally, there are several recurrent somatic mutations, which are drivers of MDS pathogenesis and can be associated with clinical phenotype (e.g., RNA splicing factor mutations in ring‐sideroblasts) 50 , 51 or response to treatment. For example, mutations in TET2 have been shown to be associated with sensitivity to azacitidine, whereas patients with wild‐type TET2 have been shown to be resistant. 29 , 30 , 52 , 53 In MDS, patients with TP53 mutations initially respond well to hypomethylating agents, however, their duration of response is significantly shorter than in TP53 wild‐type patients. 35 In addition, TP53 mutation status has been shown to be the most significant predictor of mortality after hematopoietic stem cell transplantation. 29 In AML, TP53 alterations are the most important prognostic factor in complex karyotype‐AML, outweighing all other variables. 54
In this study, we explored whether the mutational and cytogenetic landscapes in MDS and AML are similar among Western and East Asian populations and whether it would be appropriate to include East Asian patients in the PANTHER trial. AML and MDS mutational landscape and cytogenetic abnormalities were found to be similar among US, European, Japanese, and Korean populations; however, some differences were noted in the frequencies of a few mutations. Compared with Japanese and Korean patients, higher percentage mutation frequencies were found in US and European patients in DNMT3A, IDH2, TP53, KRAS, and ASXL1 in AML, and in SRSF2 and STAG2 in MDS. Given the prognostic value of these genes, their correlation with response and other end points of interest should be carefully addressed while taking into consideration different ethnicities. Nevertheless, the overall concordance in molecular pathologies across East Asian and Western patient populations suggest that disease‐related intrinsic factors are generally preserved. This is further supported by the similarities in the survival outcomes observed in patients with MDS treated with azacitidine across regions. Collectively, these cytogenetic and mutational data support the inclusion of East Asian patients in the PANTHER trial.
Regional differences in disease severity, disease progression kinetics, and clinical outcomes, when present, can complicate the design of MRCTs. One such example is the setting of relapsed/refractory MM (RRMM). During global development of the oral proteasome inhibitor ixazomib, clinically and statistically significant progression‐free survival (PFS) benefit was observed in randomized evaluations of ixazomib plus lenalidomide‐dexamethasone versus placebo plus lenalidomide‐dexamethasone in both Chinese and global (primarily Western) populations. However, the absolute PFS achieved with ixazomib‐lenalidomide‐dexamethasone treatment in a China‐continuation study in patients with RRMM was markedly shorter than in a global Phase 3 study due to the later stage of diagnosis of Chinese patients, more advanced or refractory disease, and differences in prior treatments, which resulted in poorer outcomes relative to Western patients. 55 , 56 The global clinical development plan for ixazomib integrated China as a continuation study, allowing robust assessment of the efficacy of ixazomib across both the global and Chinese populations without confounding effects, such as the imbalance of clinical outcomes and prognostic factors in the disease of interest. Nevertheless, this example illustrates the importance of assessing disease‐related intrinsic and extrinsic factors when designing MRCTs across global patient populations.
A key aspect of designing MRCTs is to identify early whether ethnicity has any impact on PKs/pharmacodynamics, which might result in regional changes to dose selection. Pevonedistat undergoes oxidative metabolism primarily by CYP3A enzymes (Takeda data on file), although its PKs are not sensitive to CYP3A inhibition or induction based on results of clinical drug‐drug interaction studies. 57 , 58 A mass balance study has been recently completed and subsequent metabolite profiling analyses will shed further light on the clearance mechanisms of pevonedistat in humans. 59 Data from our population PK analysis demonstrated that systemic pevonedistat exposures were similar between East Asian and Western populations and across the major East Asian races after individualizing the pevonedistat dose based on BSA, a key covariate. We clearly demonstrated that fixed dosing resulted in a lower exposure among patients with a larger BSA. This would be expected to translate into regional differences if fixed dosing is used, as populations with smaller average BSA (e.g., East Asians) would be expected to be at risk for higher drug exposures. Our findings of similar exposures across the East Asian and Western populations following BSA‐based dosing of pevonedistat are reflected in a common MTD identified in the Phase 1/1b study of East Asian patients with AML or MDS. 14 The safety profiles of pevonedistat, alone or administrated with azacitidine, were also comparable in East Asian and Western patients in these studies, with the most common grade greater than or equal to 3 adverse events reported in both regions, including anemia, febrile neutropenia, and pneumonia. 11 , 14 Our population PK analysis identified BSA as an important source of PK variability for pevonedistat and illustrates how BSA‐based dosing achieved comparable drug exposures across East Asian and Western populations. This contrasts with drugs that demonstrate regional or race‐related differences in PKs. An example of this is the investigational Aurora A kinase inhibitor alisertib, for which studies have demonstrated a lower MTD in Asian versus Western patients that could be explained by PK differences (i.e., higher dose‐normalized systemic exposures of alisertib in East Asian populations). 60 For an investigational agent like alisertib, an exposure‐matched dosing strategy with a lower regional dose for Asian populations can be considered to preserve benefit/risk due to a clinically relevant difference in PKs between Asian and Western patient populations. 61 A lack of ethnic sensitivity in the clinical pharmacology of pevonedistat was concluded based on the totality of safety and PK data, and the same BSA‐based dose and regimen of pevonedistat in combination with azacitidine can be used across regions in the Phase 3 PANTHER trial. Furthermore, based on the lack of regional differences in disease biology and azacitidine efficacy, as long as the pooled East Asian population is adequately represented in the global population, the totality of the data in the group can be used to substantiate each region, and outcomes can be assessed in combined patient populations.
Taken together, based on the totality of data, intrinsic and extrinsic factors relevant to disease (higher‐risk MDS, CMML, and low‐blast AML) and drug (pevonedistat) were inferred to be similar across the planned countries of enrollment in the pooled East Asian region (Japan, South Korea, and China) and between East Asian and Western regions (Figure 5). Based on these considerations, clinical efficacy and safety data from the PANTHER trial should therefore be applicable to inform benefit‐risk assessment across Western and East Asian populations. Importantly, pooled data across patients enrolled in East Asia in this global MRCT will enable assessment of consistency in the benefit‐risk profile of pevonedistat in these patients versus the global data based on ICH E17 principles.
FIGURE 5.
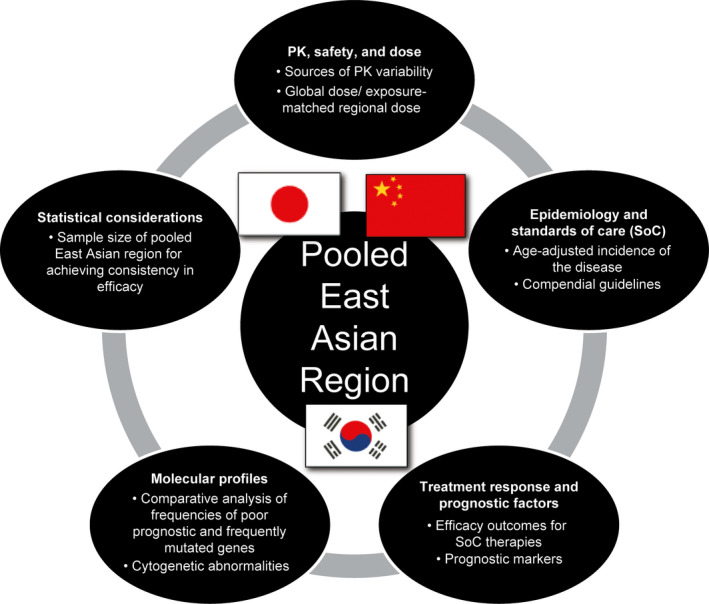
Framework for defining a pooled East Asian region using ICH E17 principles based on conservation of drug‐related and disease‐related intrinsic and extrinsic factors. ICH E17, International Conference on Harmonisation E17 guidelines; PK, pharmacokinetic
This analysis is intended to be an exemplar to illustrate the importance of quantitative pharmacological and translational scientific considerations in designing Asia‐inclusive MRCTs. During the course of this effort within our organization, it was evident that cross‐functional and cross‐regional partnerships were central to enabling the design of this MRCT and the synthesis of associated scientific rationale for discussion with Health Authorities worldwide. Viewed from a broader perspective, the framework described here (Figure 5) is applicable to the efficient global clinical development of investigational therapies for rare cancers and orphan diseases in Asia‐inclusive MRCTs based on systematic assessments of drug‐related and disease‐related intrinsic and extrinsic factors applying ICH E17 principles.
CONFLICT OF INTEREST
X.Z., S.F., E.K., F.S., Y.Yu., D.V.F., and S.B. are employees of Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. S.K., Y.Ya., K.H., K.N., and Y.D. are employees of Takeda Pharmaceutical Company Limited. Z.H. is an employee of Alnylam Pharmaceuticals Inc. K.V. is a former employee of Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, and is currently an employee of EMD Serono, Inc.
AUTHOR CONTRIBUTIONS
All authors wrote the manuscript. X.Z., S.F., E.K., Z.H., D.V.F., and K.V. designed the research. All authors performed the research. X.Z., S.F., D.V.F., and K.V. analyzed the data.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge writing support from Helen Wilkinson, PhD (FireKite, an Ashfield company, part of UDG Healthcare plc), which was funded by Millennium Pharmaceuticals, Inc., and complied with Good Publication Practice 3 ethical guidelines (Battisti et al. Ann Intern Med 2015; 163:461–4), and editorial support from Janice Y. Ahn, PhD, and Marcel Kuttab, PharmD, CMPP (Millennium Pharmaceuticals, Inc.).
Funding information
Writing support was funded by Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Contributor Information
Xiaofei Zhou, Email: xiaofei.zhou@takeda.com.
Karthik Venkatakrishnan, Email: venkatakrishnankarthik@gmail.com.
REFERENCES
- 1. Venkatakrishnan K, Burgess C, Gupta N, et al. Toward optimum benefit‐risk and reduced access lag for cancer drugs in Asia: a global development framework guided by clinical pharmacology principles. Clin Transl Sci. 2016;9:9‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asano K, Uyama Y, Tohkin M. Factors affecting drug‐development strategies in Asian global clinical trials for drug approval in Japan. Clin Transl Sci. 2018;11:182‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ICH E5 . Ethnic factors in the acceptability of foreign clinical data E5(R1), 1998. https://database.ich.org/sites/default/files/E5_R1__Guideline.pdf. Accessed November 12, 2020.
- 4. ICH E17 . General principles for planning and design of multi‐regional clinical trials E17, 2017. https://database.ich.org/sites/default/files/E17EWG_Step4_2017_1116.pdf. Accessed November 12, 2020.
- 5. Soucy TA, Smith PG, Rolfe M. Targeting NEDD8‐activated cullin‐RING ligases for the treatment of cancer. Clin Cancer Res. 2009;15:3912‐3916. [DOI] [PubMed] [Google Scholar]
- 6. Brownell JE, Sintchak MD, Gavin JM, et al. Substrate‐assisted inhibition of ubiquitin‐like protein‐activating enzymes: the NEDD8 E1 inhibitor MLN4924 forms a NEDD8‐AMP mimetic in situ. Mol Cell. 2010;37:102‐111. [DOI] [PubMed] [Google Scholar]
- 7. Swords RT, Kelly KR, Smith PG, et al. Inhibition of NEDD8‐activating enzyme: a novel approach for the treatment of acute myeloid leukemia. Blood. 2010;115:3796‐3800. [DOI] [PubMed] [Google Scholar]
- 8. Bhatia S, Pavlick AC, Boasberg P, et al. A phase I study of the investigational NEDD8‐activating enzyme inhibitor pevonedistat (TAK‐924/MLN4924) in patients with metastatic melanoma. Invest New Drugs. 2016;34:439‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarantopoulos J, Shapiro GI, Cohen RB, et al. Phase I study of the investigational NEDD8‐activating enzyme inhibitor pevonedistat (TAK‐924/MLN4924) in patients with advanced solid tumors. Clin Cancer Res. 2016;22:847‐857. [DOI] [PubMed] [Google Scholar]
- 10. Shah JJ, Jakubowiak AJ, O'Connor OA, et al. Phase I study of the novel investigational NEDD8‐activating enzyme inhibitor pevonedistat (MLN4924) in patients with relapsed/refractory multiple myeloma or lymphoma. Clin Cancer Res. 2016;22:34‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swords RT, Coutre S, Maris MB, et al. Pevonedistat, a first‐in‐class NEDD8‐activating enzyme inhibitor, combined with azacitidine in patients with AML. Blood. 2018;131:1415‐1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swords RT, Erba HP, DeAngelo DJ, et al. Pevonedistat (MLN4924), a first‐in‐class NEDD8‐activating enzyme inhibitor, in patients with acute myeloid leukaemia and myelodysplastic syndromes: a phase 1 study. Br J Haematol. 2015;169:534‐543. [DOI] [PubMed] [Google Scholar]
- 13. Sekeres MA, Fram RJ, Hua Z, Ades L. Phase 3 study of first line pevonedistat (PEV) + azacitidine (AZA) versus single‐agent AZA in patients with higher‐risk myelodysplastic syndromes (HR MDS), chronic myelomonocytic leukemia (CMML) or low‐blast acute myelogenous leukemia (AML). J Clin Oncol. 2018;36(15_suppl):TPS7077. [Google Scholar]
- 14. Wu S‐J, Cheong J‐W, Onishi Y, et al. Phase 1/1b study of pevonedistat as a single agent or combined with azacitidine in East Asian patients with acute myeloid leukemia or myelodysplastic syndrome. Poster presented at: 23rd Annual Congress of the European Hematology Association (EHA). June 14–17, 2018; Stockholm, Sweden. Poster S997, https://library.ehaweb.org/eha/2018/stockholm/215321/shang‐ju.wu.phase.1.1b.study.of.pevonedistat.%28pev%29.as.a.single.agent.or.html. Accessed November 13, 2020.
- 15. Faessel HM, Mould DR, Zhou X, et al. Population pharmacokinetics of pevonedistat alone or in combination with standard of care in patients with solid tumours or haematological malignancies. Br J Clin Pharmacol. 2019;85:2568‐2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howlader NE, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975‐2016, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019.
- 17. Neukirchen J, Schoonen WM, Strupp C, et al. Incidence and prevalence of myelodysplastic syndromes: data from the Dusseldorf MDS‐registry. Leuk Res. 2011;35:1591‐1596. [DOI] [PubMed] [Google Scholar]
- 18. Chihara D, Ito H, Katanoda K, et al. Incidence of myelodysplastic syndrome in Japan. J Epidemiol. 2014;24:469‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park HJ, Park E‐H, Jung K‐W, et al. Statistics of hematologic malignancies in Korea: incidence, prevalence and survival rates from 1999 to 2008. Korean J Hematol. 2012;47:28‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang W, Wang H, Wang XQ, Lin GW. First report of incidence of adult myelodysplastic syndrome in China. Ann Hematol. 2012;91:1321‐1322. [DOI] [PubMed] [Google Scholar]
- 21. Vidaza® (Azacitidine for Injection) [package insert] for Subcutaneous or Intravenous Use Prescribing Information. Summit, NJ: Celgene Corporation; 2004. [Google Scholar]
- 22. Vidaza® Summary of Product Characteristics [package insert]. Uxbridge, UK: Celgene Europe Ltd; 2008. [Google Scholar]
- 23. Fenaux P, Mufti GJ, Hellstrom‐Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher‐risk myelodysplastic syndromes: a randomised, open‐label, phase III study. Lancet Oncol. 2009;10:223‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fenaux P, Mufti GJ, Hellstrom‐Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562‐569. [DOI] [PubMed] [Google Scholar]
- 25. Silverman LR, Demakos EP, Petersono BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429‐2440. [DOI] [PubMed] [Google Scholar]
- 26. Uchida T, Ogawa Y, Kobayashi Y, et al. Phase I and II study of azacitidine in Japanese patients with myelodysplastic syndromes. Cancer Sci. 2011;102:1680‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee YG, Kim I, Yoon S‐S, et al. Comparative analysis between azacitidine and decitabine for the treatment of myelodysplastic syndromes. Br J Haematol. 2013;161:339‐347. [DOI] [PubMed] [Google Scholar]
- 28. Du X, Lai Y‐Y, Xiao Z, et al. Efficacy, safety and pharmacokinetics of subcutaneous azacitidine in Chinese patients with higher risk myelodysplastic syndromes: results from a multicenter, single‐arm, open‐label phase 2 study. Asia Pac J Clin Oncol. 2018;14:270‐278. [DOI] [PubMed] [Google Scholar]
- 29. Bejar R, Lord A, Stevenson K, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood. 2014;124:2705‐2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Itzykson R, Kosmider O, Cluzeau T, et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia. 2011;25:1147‐1152. [DOI] [PubMed] [Google Scholar]
- 31. Kihara R, Nagata Y, Kiyoi H, et al. Comprehensive analysis of genetic alterations and their prognostic impacts in adult acute myeloid leukemia patients. Leukemia. 2014;28:1586‐1595. [DOI] [PubMed] [Google Scholar]
- 32. Metzeler KH, Herold T, Rothenberg‐Thurley M, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood. 2016;128:686‐698. [DOI] [PubMed] [Google Scholar]
- 33. Ryotokuji T, Yamaguchi H, Ueki T, et al. Clinical characteristics and prognosis of acute myeloid leukemia associated with DNA‐methylation regulatory gene mutations. Haematologica. 2016;101:1074‐1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki T, Yamaguchi H, Ueki T, et al. Clinical characteristics and prognostic implications of NPM1 mutations in acute myeloid leukemia. Blood. 2005;106:2854‐2861. [DOI] [PubMed] [Google Scholar]
- 35. Takahashi K, Patel K, Bueso‐Ramos C, et al. Clinical implications of TP53 mutations in myelodysplastic syndromes treated with hypomethylating agents. Oncotarget. 2016;7:14172‐14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Traina F, Visconte V, Elson P, et al. Impact of molecular mutations on treatment response to DNMT inhibitors in myelodysplasia and related neoplasms. Leukemia. 2014;28:78‐87. [DOI] [PubMed] [Google Scholar]
- 37. Cancer Genome Atlas Research Network , Ley TJ, Miller C, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059‐2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haferlach T, Nagata Y, Grossmann V, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28:241‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harada H, Harada Y. Recent advances in myelodysplastic syndromes: molecular pathogenesis and its implications for targeted therapies. Cancer Sci. 2015;106:329‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wakita S, Yamaguchi H, Ueki T, et al. Complex molecular genetic abnormalities involving three or more genetic mutations are important prognostic factors for acute myeloid leukemia. Leukemia. 2016;30:545‐554. [DOI] [PubMed] [Google Scholar]
- 41. Byun JM, Kim YJ, Yoon H‐J, et al. Cytogenetic profiles of 2806 patients with acute myeloid leukemia‐a retrospective multicenter nationwide study. Ann Hematol. 2016;95:1223‐1232. [DOI] [PubMed] [Google Scholar]
- 42. Chen B, Zhao W‐L, Jin J, et al. Clinical and cytogenetic features of 508 Chinese patients with myelodysplastic syndrome and comparison with those in Western countries. Leukemia. 2005;19:767‐775. [DOI] [PubMed] [Google Scholar]
- 43. Li X, Li X, Xie W, et al. Comprehensive profile of cytogenetics in 2308 Chinese children and adults with de novo acute myeloid leukemia. Blood Cells Mol Dis. 2012;49:107‐113. [DOI] [PubMed] [Google Scholar]
- 44. Pharmaceuticals and Medical Devices Agency . PMDA List of approved drugs: FY 2010. https://www.pmda.go.jp/files/000232774.pdf. Accessed November 12, 2020.
- 45. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Metzeler KH, Becker H, Maharry K, et al. ASXL1 mutations identify a high‐risk subgroup of older patients with primary cytogenetically normal AML within the ELN favorable genetic category. Blood. 2011;118:6920‐6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Metzeler KH, Maharry K, Radmacher MD, et al. TET2 mutations improve the new European LeukemiaNet risk classification of acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2011;29:1373‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454‐2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bejar R, Stevenson K, Abdel‐Wahab O, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496‐2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Papaemmanuil E, Cazzola M, Boultwood J, et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N Engl J Med. 2011;365:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Visconte V, Selleri C, Maciejewski JP, Tiu RV. Molecular pathogenesis of myelodysplastic syndromes. Transl Med UniSa. 2014;8:19‐30. [PMC free article] [PubMed] [Google Scholar]
- 53. Ko M, Huant Y, Jankowska AM, et al. Impaired hydroxylation of 5‐methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468:839‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rücker FG, Schlenk RF, Bullinger L, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119:2114‐2121. [DOI] [PubMed] [Google Scholar]
- 55. Hou J, Jin J, Xu Y, et al. Randomized, double‐blind, placebo‐controlled phase III study of ixazomib plus lenalidomide‐dexamethasone in patients with relapsed/refractory multiple myeloma: China continuation study. J Hematol Oncol. 2017;10:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moreau P, Masszi T, Grzasko N, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621‐1634. [DOI] [PubMed] [Google Scholar]
- 57. Faessel H, Nemunaitis J, Bauer TM, et al. Effect of CYP3A inhibitors on the pharmacokinetics of pevonedistat in patients with advanced solid tumours. Br J Clin Pharmacol. 2019;85:1464‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhou X, Vaishampayan U, Mahalingam D, et al. A phase 1 study to evaluate the effects of rifampin, a strong CYP3A inducer, on pharmacokinetics of pevonedistat in patients with advanced malignancies. EHA Library. Abstract PB1983, https://library.ehaweb.org/eha/2020/eha25th/297899/xiaofei.zhou.a.phase.1.study.to.evaluate.the.effects.of.rifampin.a.strong.html?f=menu%3D6%2Abrowseby%3D8%2Asortby%3D2%2Amedia%3D3%2Ace_id%3D1766%2Aot_id%3D23227%2Amarker%3D758. Accessed November 13, 2020.
- 59. Zhou X, Sedarati F, Faller DV, et al. Phase I study assessing the mass balance, pharmacokinetics, and excretion of [(14)C]‐pevonedistat, a NEDD8‐activating enzyme inhibitor in patients with advanced solid tumors [published online ahead of print October 22]. Invest New Drugs. 2020. https://doi.org/10.1007/s10637‐020‐01017‐x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Venkatakrishnan K, Kim TM, Lin C‐C, et al. Phase 1 study of the investigational Aurora A kinase inhibitor alisertib (MLN8237) in East Asian cancer patients: pharmacokinetics and recommended phase 2 dose. Invest New Drugs. 2015;33:942‐953. [DOI] [PubMed] [Google Scholar]
- 61. Zhou X, Mould DR, Takubo T, et al. Global population pharmacokinetics of the investigational Aurora A kinase inhibitor alisertib in cancer patients: rationale for lower dosage in Asia. Br J Clin Pharmacol. 2018;84:35‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Visser O, Trama A, Maynadié M, et al. Incidence, survival and prevalence of myeloid malignancies in Europe. Eur J Cancer. 2012;48:3257‐3266. [DOI] [PubMed] [Google Scholar]
- 63. Chihara D, Ito H, Matsuda T, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164:536‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Park EH, Lee H, Won YJ, et al. Nationwide statistical analysis of myeloid malignancies in Korea: incidence and survival rate from 1999 to 2012. Blood Res. 2015;50:204‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen BA, Huang Z‐H, Zhang X‐P, et al. An epidemiological investigation of leukemia incidence between 2003 and 2007 in Nanjing, China. J Hematol Oncol. 2010;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jung SH, Kim YJ, Yim SH, et al. Somatic mutations predict outcomes of hypomethylating therapy in patients with myelodysplastic syndrome. Oncotarget. 2016;7:55264‐55275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Park MJ, Park SH, Park PW, et al. Frequency of KRAS mutations in adult Korean patients with acute myeloid leukemia. Int J Hematol. 2013;98:549‐557. [DOI] [PubMed] [Google Scholar]
- 68. Park SH, Choi J‐C, Kim SY, et al. Incidence and prognostic impact of DNMT3A mutations in Korean normal karyotype acute myeloid leukemia patients. Biomed Res Int. 2015;2015:723682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shin SY, Lee S‐T, Kim H‐J, et al. Mutation profiling of 19 candidate genes in acute myeloid leukemia suggests significance of DNMT3A mutations. Oncotarget. 2016;7:54825‐54837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yoshizato T, Nannya Y, Atsuta Y, et al. Genetic abnormalities in myelodysplasia and secondary acute myeloid leukemia: impact on outcome of stem cell transplantation. Blood. 2017;129:2347‐2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


