Abstract
Abstract
Tegoprazan, a novel potassium‐competitive acid blocker, is used to treat acid‐related diseases. However, there is no information on the pharmacokinetic (PK) and pharmacodynamic (PD) profiles of the marketed dosage of tegoprazan under various meal timings in a fed and fasted state. The study aimed to assess the effect of meal timing on PKs and PDs of tegoprazan 50 mg after a single administration in healthy male subjects. An open‐label, single‐dose, three‐treatment, three‐period crossover study was conducted. A total of 12 subjects were orally administered a single dose of tegoprazan 50 mg among various conditions: in a fasted state, at 30 min before or 30 min after a high‐fat meal. PK parameters were estimated by the noncompartmental method. Continuous 24‐h intragastric pH monitoring was done for PD analysis. The PKs and PDs of tegoprazan were compared among the various meal timings. Compared with the fasting condition, the PK profile of tegoprazan was similar when administered 30 min before a high‐fat meal; however, delayed absorption with similar systemic exposure was observed when administered 30 min after a high‐fat meal. The magnitude of acid suppression evaluated through the PD parameters increased when administered 30 min after a high‐fat meal compared with fasting the condition and when administered 30 min before a high‐fat meal. However, the increased difference in acid suppression was not clinically significant. Meal timing had no clinically significant effect on the PKs and PDs of tegoprazan 50 mg. Therefore, the marketed dosage of tegoprazan could be administered regardless of the meal timing.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Tegoprazan, a novel potassium‐competitive acid blocker, is used to treat acid‐related diseases.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study evaluated the effect of food on pharmacokinetics (PKs) and pharmacodynamics (PDs) of tegoprazan under various mealtime conditions.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study showed that delayed absorption of tegoprazan was observed at “after meal condition,” however, the amount of systemic exposure of “after meal condition” was similar to “fasting condition” and “before meal condition.” In addition, gastric acid suppression of tegoprazan was similar between fasting condition and before meal condition, whereas increased gastric acid suppression was observed at after meal condition.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
In the actual clinical environment, patients take medicine under various fed conditions. This study evaluated the effect of food on PKs and PDs of tegoprazan in various clinical conditions, and provided the important information about meal timing when administering tegoprazan.
INTRODUCTION
Gastroesophageal reflux disease (GERD) and peptic ulcer disease (PUD) are common acid‐related diseases, and both diseases are generally treated by acid suppression drugs, which reduce gastric acid secretion. 1 Among several acid suppression drugs, proton pump inhibitors (PPIs) are most commonly prescribed to treat acid‐related diseases over the past several decades. 2 However, PPIs have several limitations in clinical use due to their own characteristics, including mechanism of action and pharmacokinetics (PKs).
PPIs are converted by acid to active forms which irreversibly inhibit H+/K+ ATPase located on the apical membrane of the gastric parietal cell. 3 PPIs have relatively short half‐lives of about 1 h. 4 Because of these characteristics, the onset of acid suppression and the recovery after drug cessation are relatively late, and nocturnal acid breakthrough easily occurs even with a twice‐daily dosing regimen. 4 , 5 , 6
Moreover, noncompliance with PPIs led by a limited administration time is another drawback of PPIs. 7 , 8 Considering the mechanism of action requiring acid‐activation, it is necessary to intake food when taking PPIs. However, most PPIs cannot be administered in the fed state due to food effects. According to the previous food effect studies, the bioavailability of esomeprazole is affected by food in that systemic drug exposure is significantly diminished in the fed condition. 9 In addition, the median percentage of time of pH greater than 4 was 42.0% when the PPI was administered in the fasting condition; however, it was 17.2% when administered in the fed condition. 10 Therefore, most PPIs, including omeprazole and lansoprazole, are recommended to be administered about 1 h before a meal. 11 These limited administration times of most PPIs could trigger treatment failure of acid‐related diseases, although some PPIs, like dexlansoprazole, modified‐release formulation can be administered regardless of meal timing. 12
Recently, potassium‐competitive acid blockers (P‐CABs) have been developed as novel acid suppression drugs. Tegoprazan, one of the P‐CABs, was approved for the treatment of GERD, gastric ulcers and Helicobacter pylori eradication in South Korea. Tegoprazan has a linear PK profile and dose‐dependent pharmacodynamic (PD) profiles in a dose range of 50–400 mg, and the time to reach maximum plasma concentration (Tmax) and the terminal elimination half‐life (t1/2) of tegoprazan are about 0.5–1 h and 3–5 h, respectively. 13 Tegoprazan is mainly metabolized by CYP3A4 to the active metabolite (M1).
The food effect of tegoprazan was already evaluated for 200 mg, and it was verified that tegoprazan had no food effect. The systemic exposure of tegoprazan between fasting and after meal conditions was comparable, and similar gastric acid suppression was observed. 14 Because tegoprazan 200 mg is a higher strength than 50 mg and PKs of tegoprazan is dose proportional, it is estimated that there is no food effect for tegoprazan 50 mg, which is the marketed dosage. However, there is no information on the PK and PD profiles of tegoprazan 50 mg under various mealtime conditions, such as “administration before a meal,” which can easily occur in an actual clinical environment. If such profiles are evaluated, the compliance of tegoprazan will improve as the administration time of tegoprazan becomes clearer. Consequently, this study aimed to assess the effect of various meal timings on the PKs, PDs, and safety of tegoprazan 50 mg after a single administration in healthy male subjects.
METHODS
Subjects and study design
The study was reviewed and approved by the Korean Ministry of Food and Drug Safety (MFDS) and the Institutional Review Board of Seoul National University Hospital (clinicaltrials.gov: NCT03863938). This study was conducted at the Seoul National Hospital Clinical Trials Center in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice.
Healthy male volunteers aged between 19 and 50 years with a body mass index of 18.0–27.0 kg/m2 that were Helicobacter pylori negative participated in the study. The health status of the volunteers was determined based on medical history, physical examination, vital signs, 12‐lead electrocardiogram (ECG), and clinical laboratory test. Written informed consent was obtained from all the subjects before any study‐related procedures.
The study had a randomized, open‐label, single‐dose, three‐treatment, three‐period crossover design. In the first period, 24‐hour intragastric pH monitoring was conducted as the baseline 1 day before the administration of tegoprazan. The measurement was started after at least 10 h of fasting, and only lunch and dinner were provided during the measurement. After the baseline monitoring, all the subjects were orally administered a single dose of tegoprazan 50 mg in the fasted state, at 30 min before or at 30 min after a high‐fat meal according to the assigned sequence. The high‐fat meal contained 900 kcal with over 35% of fat content, and the subjects were supposed to finish eating within 20 min. After the administration of tegoprazan, 24‐h intragastric pH monitoring for PD assessment and serial blood sampling at predose and 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, and 48 h postdose for PK analysis of tegoprazan and M1 were conducted. During the intragastric monitoring, the same types of lunch and dinner were provided, and drinking water was prohibited 1 h before and 2 h after the administration of tegoprazan. In the second and third periods, tegoprazan was administered according to the assigned sequence, and the same procedures, including PK/PD assessment, were conducted (Figure 1).
Figure 1.
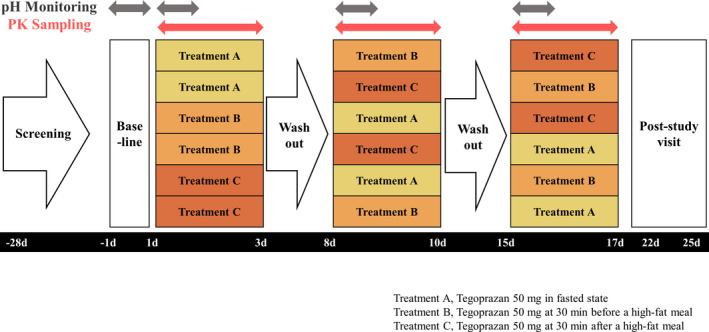
Study design. PK, pharmacokinetic
Bioanalytical method
The plasma concentrations of tegoprazan and M1 were measured by a validated method using ultra‐performance liquid chromatography‐tandem mass spectrometry (UPLC‐MS/MS) consisting of the ACQUITY UPLC System (Waters) and Xevo TQ MS (Waters). 15 The validated concentration ranges for tegoprazan and M1 were 20.0–10,000 ng/ml and 10.0–5000 ng/ml, respectively. The within‐run and between‐run precision for tegoprazan was 0.9%–3.0% and 1.0%–2.8%, respectively. The corresponding values for M1 were 0.8%–5.6% and 1.0%–5.5%, respectively. The within‐run and between‐run accuracy was −2.1% to −0.4% and −3.1% to 1.5%, respectively, for tegoprazan, and −2.5% to 6.5% and −3.3% to 1.7%, respectively, for M1.
Pharmacokinetic assessment
PK parameters were estimated by noncompartmental method using Phoenix WinNonlin version 8.0 (Certara, St. Louis, MO). The primary PK parameters were maximum plasma concentration (Cmax) and area under the concentration–time curve from time zero to the last quantifiable time point (AUClast) of tegoprazan. The secondary PK parameters were Tmax and t1/2 for tegoprazan, and Cmax, AUClast, Tmax, t1/2, and metabolic ratio (MR) for M1. PK analysis was performed using the concentration‐time data collected from the subjects who completed the study without major deviation.
Pharmacodynamic assessment
Continuous 24‐h intragastric pH monitoring was carried out using an ambulatory 24‐h pH recorder, Digitrapper pH 400 (Medtronic, Inc., Fridley, MN) for PD assessment. The PD parameters were evaluated as percentage of time of pH greater than or equal to 4, mean pH, median pH, and baseline corrected parameters including Δ% time pH greater than or equal to 4, Δmean pH, and Δmedian pH. PD analysis was performed using the collected 24‐h intragastric pH data from the subjects who completed the study and had sufficient pH data over 95% of measuring time.
Safety assessment
Safety was assessed through physical examinations, vital signs, ECGs, clinical laboratory tests, and adverse event monitoring.
Statistical analysis
To assess how meal timing affects the PKs of tegoprazan in a fasted state basis, analysis of variance (ANOVA) was conducted with the treatment, period, and sequence as the fixed effects and the subject as a random effect. The geometric mean ratios (GMRs) and their corresponding 90% confidence intervals (CIs) of the Cmax and AUClast for 30 min before a high‐fat meal to the fasted state and 30 min after a high‐fat meal to the fasted state were calculated. To assess the effect of meal timing on PDs, the PD parameters were analyzed with ANOVA in the same manner as the PKs. The statistical analysis was conducted with the SAS software version 9.4 (SAS Institute, Cary, NC).
RESULTS
Study population
A total of 12 subjects were enrolled and randomly assigned to 6 sequences. All of them completed the entire study schedule without any major deviations, so they were included in the PK, PD, and safety analysis. The demographics of the subjects were as follows: age, 29.8 ± 4.8 (mean ± SD) years; height, 174.0 ± 3.3 cm; weight, 71.1 ± 7.9 kg; and body mass index (BMI), 23.5 ± 2.1 kg/m2.
Pharmacokinetics
When administered 30 min before a high‐fat meal, the PK profile of tegoprazan was similar to the PK profile of tegoprazan when administered in the fasted state (Figures 2, 3). The Cmax of tegoprazan was observed as 775.92 μg/L and 803.00 μg/L when administered 30 min before a high‐fat meal and in the fasted state, respectively, and the corresponding AUClast was calculated as 2669.82 h∙μg/L and 2873.32 h∙μg/L, respectively. The GMRs (90% CIs) for the Cmax and AUClast for the 30 min before a high‐fat meal to the fasted state were calculated as 0.9631 (0.7517–1.234) and 0.9388 (0.8674–1.016), respectively (Table 1).
Figure 2.
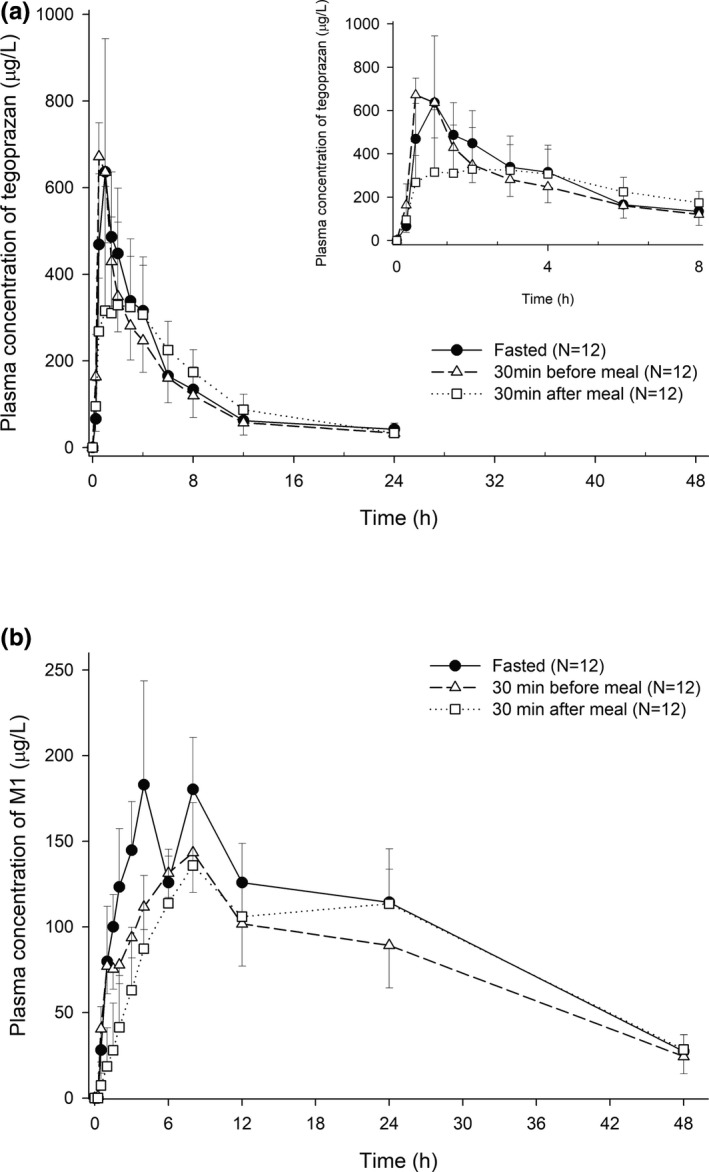
Mean plasma concentration of tegoprazan and its metabolite (M1) after a single oral administration of tegoprazan 50 mg in the fasted state, at 30 min before a high‐fat meal or at 30 min after a high‐fat meal. (a) Tegoprazan and (b) M1
Figure 3.
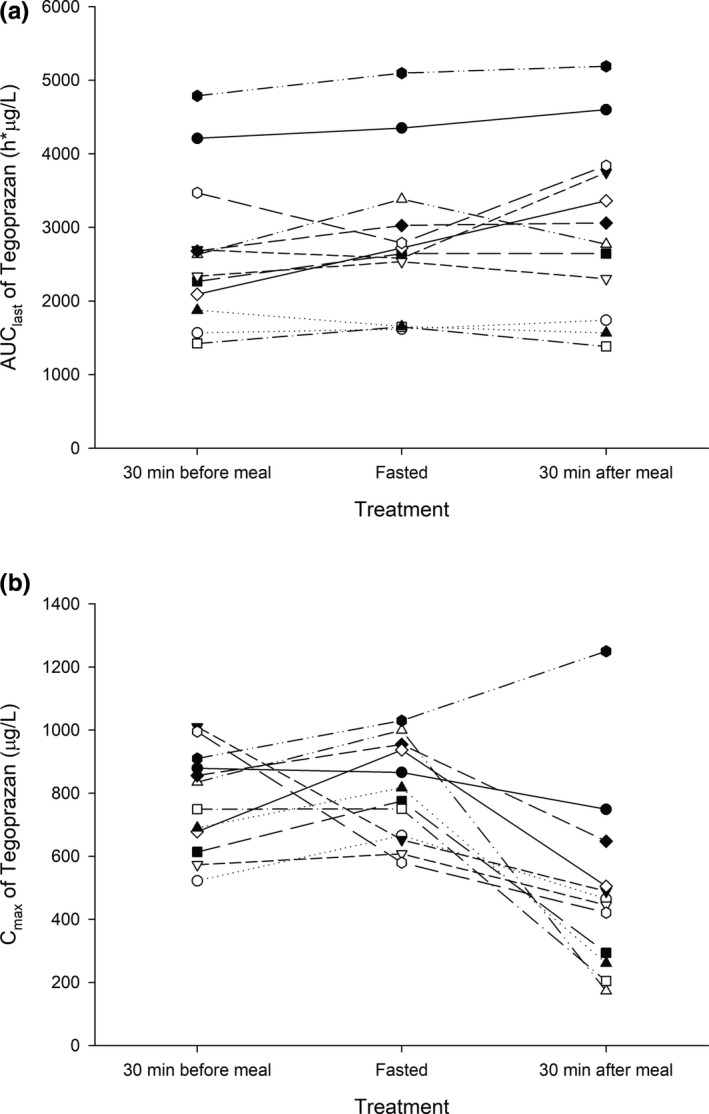
Individual pharmacokinetic parameters after a single oral administration of tegoprazan 50 mg in the fasted state, at 30 min before a high‐fat meal or at 30 min after a high‐fat meal. (a) AUClast, (b) Cmax. AUClast, area under the concentration–time curve from time zero to the last quantifiable time point; Cmax, maximum plasma concentration
Table 1.
Summary of pharmacokinetic parameters of tegoprazan and M1 after a single oral administration of tegoprazan 50 mg in the fasted state, at 30 min before a high‐fat meal or at 30 min after a high‐fat meal
|
Fasted (N = 12) |
30 min before meal (N = 12) |
30 min after meal (N = 12) |
|||
|---|---|---|---|---|---|
| Parameter a | Parameter a |
GMR b (90% CI) |
Parameter a |
GMR c (90% CI) |
|
| Tegoprazan | |||||
| Cmax, μg/L |
803.00 ± 156.23 [580.00–1698.10] |
775.92 ± 162.61 [522.00–1010.00] |
0.9631 (0.7517–1.2340) |
492.00 ± 293.94 [173.00–1250.00] |
0.5396 (0.4211–0.6914) |
| AUClast, h∙μg/L |
2837.32 ± 1054.64 [1618.61–5095.97] |
2669.82 ± 1022.94 [1424.13–4788.40] |
0.9388 (0.8674–1.0160) |
3017.05 ± 1193.95 [2915.98–5190.38] |
1.0455 (0.9661–1.1316) |
| AUCinf, h∙μg/L |
3137.75 ± 1166.15 [1702.07–5657.24] |
2917.65 ± 1084.47 [1521.26–5162.56] |
3321.58 ± 1219.48 [1784.05–5620.45] |
||
| Tmax, h | 1.00 [0.50–2.00] | 0.48 [0.48–1.00] | 3.00 [0.50–8.00] | ||
| t1/2, h |
4.10 ± 1.38 [2.78–7.47] |
4.16 ± 1.06 [3.02–6.10] |
5.00 ± 1.55 [3.00–8.02] |
||
| M1 | |||||
| Cmax, μg/L |
198.83 ± 58.26 [122.00–359.00] |
146.42 ± 19.41 [122.00–187.00] |
0.7551 (0.6609–0.8627) |
142.16 ± 30.21 [78.90–181.00] |
0.7214 (0.6314–0.8242) |
| AUClast, h∙μg/L |
4461.94 ± 1008.27 [2061.41–5908.94] |
3497.93 ± 951.53 [1685.18–4614.80] |
0.7743 (0.7176–0.8355) |
3796.57 ± 915.63 [1429.14–4902.04] |
0.8432 (0.7815–0.9099) |
| AUCinf, h∙μg/L |
5133.81 ± 1207.21 [2939.16–7726.26] |
4178.02 ± 1055.77 [2633.51–6116.44] |
5324.01 ± 2138.92 [3595.68–11,648.14] |
||
| Tmax, h | 8.00 [3.83–8.00] | 8.00 [6.00–8.02] | 8.00 [6.00–24.00] | ||
| t1/2, h |
15.36 ± 3.28 [11.95–23.86] |
15.75 ± 3.37 [11.49–24.26] |
24.82 ± 26.25 [12.74–107.65] |
||
| MR d |
1.74 ± 0.44 [1.20–2.79] |
1.51 ± 0.35 [1.11–2.31] |
1.90 ± 1.51 [1.00–6.53] |
||
Abbreviations: AUCinf, area under the concentration–time curve from time zero to infinity; AUClast, area under the concentration–time curve from time zero to the last quantifiable time point; CI, confidence interval; Cmax, maximum plasma concentration; GMR, geometric mean ratio; M1, active metabolite of tegoprazan; MR, metabolic ratio; Tmax, the time to reach maximum plasma concentration; t1/2, terminal elimination half‐life.
All parameters are expressed as mean ± SD [range], except Tmax, which is expressed as median [range].
GMR indicates 30 min before a high‐fat meal/fasted.
GMR indicates 30 min after a high‐fat meal/fasted.
MR represents metabolic ratio of tegoprazan (AUCinf, M1/AUCinf, Tegoprazan).
When administered 30 min after a high‐fat meal, a delayed absorption profile with a decreased Cmax and delayed Tmax was observed compared with when administered in the fasted state, but the AUClast was similar (Figures 2, 3). The Cmax and AUClast of tegoprazan were calculated as 492.00 μg/L and 3017.05 h∙μg/L, respectively, when administered 30 min after a high‐fat meal. The GMRs (90% CIs) for the Cmax and AUClast for 30 min after a high‐fat meal to the fasted state were calculated as 0.5396 (0.4211–0.6914) and 1.0455 (0.9661–1.1316), respectively (Table 1).
Compared with the fasted state, in 30 min before a high fat‐meal both Cmax and AUClast of M1 were decreased ~25%, and in 30 min before a high fat‐meal the Cmax and AUClast of M1 were decreased ~30% and 15%, respectively. However, MR was similar among the three conditions (Figure 2, Table 1).
Pharmacodynamics
The profiles of intragastric acidity after tegoprazan administration were similar among the three conditions in that the intragastric pH increased quickly and reached pH 4. However, when administered 30 min after a high‐fat meal, the intragastric acidity already reached around pH 4 because of the food neutralized intragastric acid.
Compared with the fasted state, the PD parameters of tegoprazan were similar when administered 30 min before or 30 min after a high‐fat meal (Figure 4). The percentage of time pH was greater than or equal to 4 was 49.1 ± 11.6%, 53.8 ± 13.4%, and 61.1 ± 7.6% when administered in the fasted state, 30 min before and 30 min after a high‐fat meal, respectively (Table 2). Statistically significant differences of percentage of time pH was greater than or equal to 4, mean pH and median pH were observed by ANOVA test (p = 0.0018, p = 0.0147, and p = 0.0149, respectively). As a result of Bonferroni post hoc, a statistically significant difference in the PD parameters was observed between the conditions when administered in the fasted state and 30 min after a high‐fat meal. The adjusted p values were 0.0015, 0.0141, and 0.0127, respectively.
Figure 4.
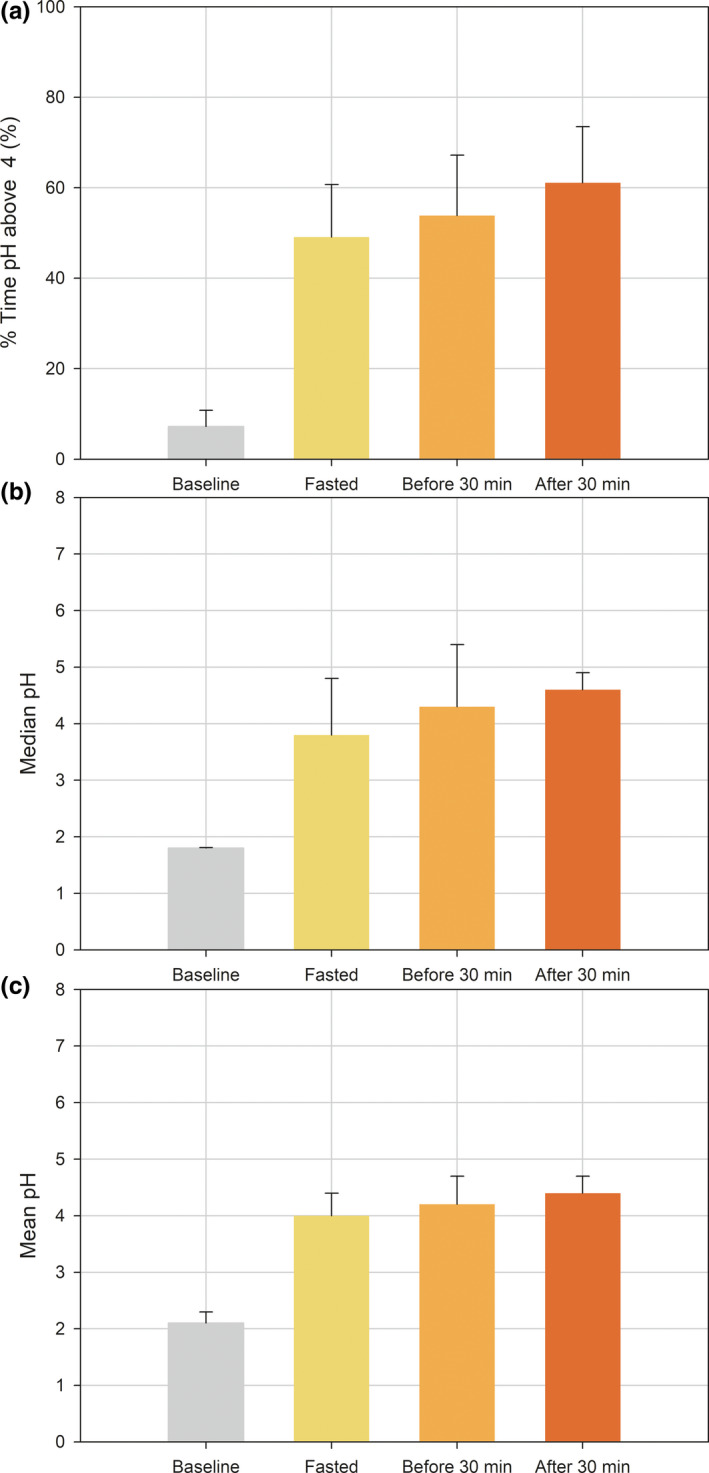
Pharmacodynamic parameters before and after a single oral administration of tegoprazan 50 mg in the fasted state, at 30 min before a high‐fat meal or at 30 min after a high‐fat meal. (a) percentage of time pH ≥4, (b) median pH, and (c) mean pH
Table 2.
Summary of pharmacokinetic parameters of tegoprazan after a single oral administration of tegoprazan 50 mg in the fasted state, at 30 min before a high‐fat meal or at 30 min after a high‐fat meal
| PD parameter a |
Baseline (N = 12) |
Fasted (N = 12) |
30 min before meal (N = 12) |
30 min after meal (N = 12) |
|---|---|---|---|---|
| % Time pH ≥4, % | 7.2 ± 3.6 | 49.1 ± 11.6 | 53.8 ± 13.4 | 61.1 ± 7.6 |
| Median pH | 1.8 ± 0.1 | 3.8 ± 1.0 | 4.3 ± 1.1 | 4.6 ± 0.3 |
| Mean pH | 2.1 ± 0.2 | 4.0 ± 0.4 | 4.2 ± 0.5 | 4.4 ± 0.3 |
| Δ% Time pH ≥4, % | ‐ | 42.0 ± 12.1 | 46.6 ± 13.3 | 54.0 ± 7.1 |
| ΔMedian pH | ‐ | 1.9 ± 1.0 | 2.5 ± 1.1 | 2.7 ± 0.4 |
| ΔMean pH | ‐ | 1.9 ± 0.4 | 2.0 ± 0.5 | 2.2 ± 0.3 |
Abbreviation: PD, pharmacodynamic.
All PD parameters are expressed as mean ± SD; Δ represents baseline corrected pharmacodynamic parameters.
Safety
After the treatment of 30 min before a high‐fat meal, two treatment emergent adverse events (TEAEs) occurred (cough and productive cough), and after the treatment of 30 min after a high‐fat meal, one TEAE occurred (abdominal pain). The relationships between the TEAE and tegoprazan were assessed as “possible” for abdominal pain and “unlikely” for cough and productive cough. There was no serious adverse event during the entire study. There were no clinically significant findings in the physical examinations, vital signs, ECGs, and laboratory tests in all the treatments.
DISCUSSION
This study was conducted to evaluate the PK and PD profiles of the marketed dosage of tegoprazan under various fed conditions compared with the fasting condition. According to the guidelines of Food‐Effect Bioavailability and Fed Bioequivalence Studies from the US Food and Drug Administration and MFDS, food effect can be evaluated by comparing the fasted and fed (at 30 min after a high‐fat meal) conditions. 16 , 17 However, in the actual clinical environment, patients take medicine under various fed conditions, even if they are instructed on when the medicine should be taken. Therefore, in this study, the effect of meal timing was divided into two conditions, including before and after meals, in order to reflect the actual clinical environment.
In this study, the PK and PD profiles were similar between the fasting and before meal conditions. However, a different PK profile was observed for the after‐meal condition. The Cmax was decreased, and Tmax was delayed, whereas AUClast was similar in that the GMRs of AUClast for before meal condition to the fasting condition and after meal condition to the fasting condition were 0.9388 and 1.0455, respectively. The result of the reduced Cmax and delayed Tmax with a similar AUC was in line with a previous food effect study on tegoprazan 200 mg. 14 The phenomenon was possibly caused by physicochemical property of tegoprazan (pH dependent solubility and high permeability) and the delayed time taken by the drug to reach the small intestine where the majority of substances are absorbed. The delayed absorption of the drug tends to occur when taken with food because the volume of the food delays the gastric emptying time so that the drug reaches the small intestine late. 18 , 19 However, the AUC representing the amount of systemic drug exposure of after meal condition was similar to the other conditions. Considering that the PDs of P‐CAB was well correlated to the AUC, there was no clinically significant difference in the PK profiles of tegoprazan 50 mg among the three conditions. 20
No clinically different efficacy is expected for tegoprazan 50 mg among fasting or fed conditions despite the decreased systemic exposure of M1 in the fed conditions. M1 has a potency of about one‐tenth of its parent compound, and plasma protein binding is about 99% according to an in vitro experiment (unpublished). Considering the molarity calculated based on the Cmax basis and the plasma protein binding of M1, the tissue concentration of M1 affecting acid suppression was expected to be negligible. In addition, the pharmacologically active moiety inferred by the sum of AUC of tegoprazan and one‐tenth AUC of M1, taking into consideration of the difference in the potency was comparable among the different fed conditions. Therefore, the difference in M1 among the three conditions did not clinically affect the magnitude of the acid suppression due to the significant difference in the potency.
As expected from the result of the PK, there was no clinically significant difference in PDs of tegoprazan 50 mg among the three conditions. The efficacy of tegoprazan was evaluated by the PD parameters, which reflected the change in intragastric pH. Among the various PD parameters, maintaining intragastric pH was greater than or equal to 4 is representatively used as a clinical threshold for tissue damage and symptoms caused by esophageal refluxate. 21 , 22 In this study, all PD parameters were significantly increased after the administration of tegoprazan regardless of the fasting or fed condition, and the maximum gastric pH was observed as approximately pH 6, which means meal timing did not affect the acid suppression of tegoprazan. However, there was a statistically significant difference in the PD parameters between the fasting and after meal conditions such that increased PD parameters were observed after meal conditions. It is believed that the increased PD parameters are caused by the neutralization of the pH by the food rather than by the drug effect, considering the fasting and after‐meal conditions have a similar AUC and the correlation between AUC and PD.
This study has a limitation in that the baseline intragastric pH measurement was conducted only once at the fasted state in the first period. Considering inconvenience of the subjects during 24‐h intragastric pH monitoring, baselines for the second and third periods with the fed conditions were not measured. Therefore, if further food effect studies are conducted, more accurate results will be obtained if both baselines for the fasting and fed conditions with every period are evaluated.
In conclusion, meal timing had no clinically significant effect on the PK, efficacy, and safety of tegoprazan 50 mg. Therefore, tegoprazan 50 mg is expected to be administered regardless of the meal timing.
CONFLICT OF INTEREST
N.S., A.R.K., B.T.K., and G.S.S. are employees of HK inno.N Corp, Seoul, Korea. All other authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
D.Y.Y. and S.L. wrote the manuscript. D.Y.Y., J.S., N.S., A.R.K., B.T.K., G.S.S., I.J., and S.L designed the research. D.Y.Y., J.S., I.J., and S.L performed the research. D.Y.Y., J.S., I.J., and S.L. analyzed the data.
ACKNOWLEDGMENT
Jung Sunwoo, MD, was previously employed at the Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital. Her contribution to this manuscript is based on her prior employment, and the current manuscript does not reflect any position of the Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, University of Ulsan College of Medicine.
Funding Information
This study was fully funded by HK inno.N Corp, Seoul, Korea.
REFERENCES
- 1. Shin JM, Vagin O, Munson K, Kidd M, Modlin IM, Sachs G. Molecular mechanisms in therapy of acid‐related diseases. Cell Mol Life Sci. 2008;65(2):264‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nehra AK, Alexander JA, Loftus CG, Nehra V. Proton pump inhibitors: review of emerging concerns. Mayo Clin Proc. 2018;93(2):240‐246. [DOI] [PubMed] [Google Scholar]
- 3. Shin JM, Munson K, Vagin O, Sachs G. The gastric HK‐ATPase: structure, function, and inhibition. Pflugers Arch. 2009;457(3):609‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sachs G. Improving on PPI‐based therapy of GORD. Eur J Gastroenterol Hepatol. 2001;13(suppl 1):S35‐S41. [PubMed] [Google Scholar]
- 5. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10(6):528‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tytgat GN. Shortcomings of the first‐generation proton pump inhibitors. Eur J Gastroenterol Hepatol. 2001;13(suppl 1):S29‐S33. [PubMed] [Google Scholar]
- 7. Persson K. When do ulcer patients take their acid inhibiting medication? Hassle Information. 1993;7:19‐23. [Google Scholar]
- 8. Chey WD, Mody RR, Izat E. Patient and physician satisfaction with proton pump inhibitors (PPIs): are there opportunities for improvement? Dig Dis Sci. 2010;55(12):3415‐3422. [DOI] [PubMed] [Google Scholar]
- 9. Sostek MB, Chen Y, Andersson T. Effect of timing of dosing in relation to food intake on the pharmacokinetics of esomeprazole. Br J Clin Pharmacol. 2007;64(3):386‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hatlebakk JG, Katz PO, Camacho‐Lobato L, Castell DO. Proton pump inhibitors: better acid suppression when taken before a meal than without a meal. Aliment Pharmacol Ther. 2000;14(10):1267‐1272. [DOI] [PubMed] [Google Scholar]
- 11. Gunaratnam NT, Jessup TP, Inadomi J, Lascewski DP. Sub‐optimal proton pump inhibitor dosing is prevalent in patients with poorly controlled gastro‐oesophageal reflux disease. Aliment Pharmacol Ther. 2006;23(10):1473‐1477. [DOI] [PubMed] [Google Scholar]
- 12. Lee RD, Vakily M, Mulford D, Wu J, Atkinson SN. Clinical trial: the effect and timing of food on the pharmacokinetics and pharmacodynamics of dexlansoprazole MR, a novel dual delayed release formulation of a proton pump inhibitor–evidence for dosing flexibility. Aliment Pharmacol Ther. 2009;29(8):824‐833. [DOI] [PubMed] [Google Scholar]
- 13. Han S, Choi HY, Kim YH, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple oral doses of tegoprazan (CJ‐12420), a novel potassium‐competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2019;50(7):751‐759. [DOI] [PubMed] [Google Scholar]
- 14. Han S, Choi HY, Kim YH. Pharmacokinetics, pharmacodynamics and food‐effect of single oral dose of tegoprazan, a novel potassium‐competitive acid blocker (P‐CAB) in healthy male subjects. Abstract presentation at Korea Digestive Week. 2017.
- 15. Hwang JG, Yoo H, Lee JW, Song GS, Lee SH, Kim M‐G. Comparison of pharmacokinetic characteristics of two tegoprazan (CJ‐12420) formulations in healthy male subjects. Transl Clin Pharmacol. 2019;27(2):80‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration . Food‐effect bioavailability and fed bioequivalence studies. https://www.fda.gov/files/drugs/published/Food‐Effect‐Bioavailability‐and‐Fed‐Bioequivalence‐Studies.pdf. Accessed April 8, 2020.
- 17. Ministry of Food and Drug Safety . Food‐effect bioavailability and fed bioequivalence studies. https://www.mfds.go.kr/brd/m_218/view.do?seq=714 Accessed July 10, 2020.
- 18. Doran S, Jones KL, Andrews JM, Horowitz M. Effects of meal volume and posture on gastric emptying of solids and appetite. Am J Physiol. 1998;275(5):R1712‐1718. [DOI] [PubMed] [Google Scholar]
- 19. Koziolek M, Alcaro S, Augustijns P, et al. The mechanisms of pharmacokinetic food‐drug interactions ‐ a perspective from the UNGAP group. Eur J Pharm Sci. 2019;134:31‐59. [DOI] [PubMed] [Google Scholar]
- 20. Sunwoo J, Oh J, Moon SJ, et al. Safety, tolerability, pharmacodynamics and pharmacokinetics of DWP14012, a novel potassium‐competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2018;48(2):206‐218. [DOI] [PubMed] [Google Scholar]
- 21. Smith JL, Opekun AR, Larkai E, Graham DY. Sensitivity of the esophageal mucosa to pH in gastroesophageal reflux disease. Gastroenterology. 1989;96(3):683‐689. [PubMed] [Google Scholar]
- 22. Pursnani KG, Mohiuddin MA, Geisinger KR, Weinbaum G, Katzka DA, Castell DO Experimental study of acid burden and acute oesophagitis. Br J Surg. 1998;85(5):677‐680. [DOI] [PubMed] [Google Scholar]


