Abstract
Free‐living amoebae (FLAs) are protozoa developing autonomously in diverse natural or artificial environments. The FLAs Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri represent a risk for human health as they can become pathogenic and cause severe cerebral infections, named granulomatous amoebic encephalitis (GAE), Balamuthia amoebic encephalitis (BAE), and primary amoebic meningoencephalitis (PAM), respectively. Additionally, Acanthamoeba sp. can also rarely disseminate to diverse organs, such as the skin, sinuses, or bones, and cause extracerebral disseminated acanthamebiasis (EDA). No consensus treatment has been established for cerebral FLA infections or EDA. The therapy of cerebral and disseminated FLA infections often empirically associates a large diversity of drugs, all exhibiting a high toxicity. Nevertheless, these pathologies lead to a high mortality, above 90% of the cases, even in the presence of a treatment. In the present work, a total of 474 clinical cases of FLA infections gathered from the literature allowed to determine the frequency of usage, as well as the efficacy of the main drugs and drug combinations used in the treatment of these pathologies. The efficacy of drug usage was determined based on the survival rate after drug administration. The most efficient drugs, drug combinations, and their mechanism of action were discussed in regard to the present recommendations for the treatment of GAE, EDA, BAE, and PAM. At the end, this review aims to provide a useful tool for physicians in their choice to optimize the treatment of FLA infections.
INTRODUCTION
Free‐living amoebae (FLAs) are cosmopolitan unicellular eukaryotic organisms that can be found either in natural environments, such as lakes, rivers, and soil, or artificial ecosystems, like swimming pools, cooling towers, and drinking water networks. These protozoa have a widespread distribution over the world, which does not depend on the climate. In opposition to parasitic amoebae, which need a host to survive (e.g., Entamoeba histolytica), FLAs can develop autonomously in the environment. Because of their phagotrophic nutrition, FLAs can ingest diverse potential pathogenic micro‐organisms, such as viruses, bacteria, fungi, or even protozoa. 1 Some of these micro‐organisms, designated as amoeba‐resisting micro‐organisms, have developed strategies to resist to phagocytosis and use FLA as a replication niche and as a vehicle. Additionally, some FLAs described to be amphizoic are able to live either as a free‐living organism in the environment or as a parasite in a host leading to severe ocular or cerebral infections. This is the case of the amoebae from the genus Acanthamoeba, Balamuthia mandrillaris, and Naegleria fowleri. Therefore, with both a role of “trojan horse” for amoeba‐resisting micro‐organisms and an intrinsic ability for amphizoic amoebae to infect a host, FLAs constitute a considerable risk for human health.
Amoebae belong to a wide polyphyletic group and are mostly found in two super‐groups 2 : Amoebozoa (genus: Acanthamoeba, Entamoeba, Balamuthia…) and Excavata (genus Naegleria...). Therefore, for example, the amoebae from the genus Acanthamoeba and Balamuthia are more closely related to Entamoeba than to Naegleria in a phylogenetic point of view. FLAs from the Amoebozoa super‐group (including Acanthamoeba spp. and Balamuthia mandrillaris) present two stages in their life cycle, a vegetative stage called trophozoite and a resistant nondividing stage called cyst. In the Excavata super‐group, the FLAs present an additional flagellated stage, which is induced by nutritional deprivation and allows the amoebae to find other favorable environmental niches. 3
Amphizoic FLAs can become pathogenic when they enter a mammalian host. Both Acanthamoeba spp. and Balamuthia mandrillaris can cause severe cerebral infections, mostly in immunocompromised individuals, called granulomatous amoebic encephalitis (GAE) or Balamuthia amoebic encephalitis (BAE), respectively. They will reach the central nervous system (CNS) by hematogenous spread from a primary site in wounded skin or lower respiratory tract via nasal passage. In case of entry through wounded skin, they can also rarely cause disseminated infections characterized by widespread granulomatous infiltration of the skin and other organs, such as lungs, bones, or sinuses. 4 In several disseminated infections caused by Acanthamoeba, called disseminated acanthamebiasis (DA), no CNS involvement was observed, rendering these DA exclusively extracerebral. 4 Concerning Naegleria fowleri, this protozoan will penetrate a host through the nasal mucosa, usually following recreational water activities (such as swimming, diving, or water skiing), and further migrates to the brain via the olfactory nerves to cause another severe CNS infection called primary amoebic meningoencephalitis (PAM). With only several hundred cases currently reported worldwide (~ 200 for GAE, ~ 100 for BAE, and more than 300 for PAM), CNS infections caused by FLAs are rare, but the actual disease burden is likely underestimated as up to 60% of encephalitis cases are undiagnosed. 5 Nonetheless, the vast majority of these cerebral infections, ~ 90% for GAE and BAE and above 95% for PAM, are lethal despite antimicrobial therapy. 6
No specific treatment has been set up against CNS infections caused by FLAs. Although the outcome of these treatments greatly depends on the timing of diagnosis, they usually associate a high number of drugs exhibiting diverse mechanisms of action. 6 These drugs include azoles (e.g., fluconazole, ketoconazole, and itraconazole) acting on ergosterol biosynthesis benzimidazole derivatives (e.g., albendazole) inhibiting microtubule polymerization, pyrimidine derivatives (e.g., flucytosine, pyrimethamine, and trimethoprim), pentamidine or sulfamides (e.g., sulfadiazine and sulfamethoxazole) inhibiting DNA synthesis, antileishmanial agents, such as amphotericin B targeting the ergosterol on the amoebal plasma membrane, or miltefosine inducing apoptosis‐like cell death, and antibacterial agents, such as rifampicin or macrolides and derivatives (e.g., azithromycin, clarithromycin, and clindamycin) inhibiting RNA transcription and protein translation, respectively. 7 , 8 , 9 , 10 , 11 , 12
This review aims to establish the present therapeutic state of art gathering clinical data related to cerebral and disseminated FLA infections. As these pathologies are scarce and their treatment empirical, with fragmentary clinical data and various conditions involving different therapeutic schedules, particularly different drugs and drug combinations, it was not possible to apply a statistical analysis of these data that would have carefully deciphered the relative importance of classical parameters, such as characteristics of the patients (age, sex, location, and health status), the treatment dose or schedule, route of administration, drug combination, and the clinical effects of the treatment. As a consequence of this limitation, this review will highlight the most salient treatment characteristics found in the literature. In the present study, we gathered a total of 474 clinical cases of FLA infections (111 BAE, 119 GAE, 209 PAM, and 35 extracerebral DA) available in the literature in order to outline, among the treatments described, the most frequently and/or most efficiently drugs used. The details of each of the 474 clinical cases, in terms of age, sex, country, outcome, and drug combination used, is presented in Table S1. Following a first step examining the proportion of PAM, BAE, GAE, and extracerebral DA survival cases collected in the present work, the treatment regimen used for each FLA pathology was analyzed using two criteria of drug usage: (a) the frequency of drug usage (FDU) corresponding to the percentage of cases treated by the drug among the total number of cases, (b) the efficacy of drug usage (EDU) corresponding to the percentage of survival cases treated by the drug among the total number of cases treated by the drug (Figures 1, 2, 3, 4). In parallel, the main combination therapies used were also identified and compared between Acanthamoeba spp., B. mandrillaris and N. fowleri infections (Table 1). In the aim to clarify the relative importance of the drugs used, this review focuses first on the most commonly used drugs (FDU criterion) and second on the most efficient ones (EDU criterion). The combination of both criteria was further analyzed in a graph plotting the EDU as a function of FDU.
FIGURE 1.
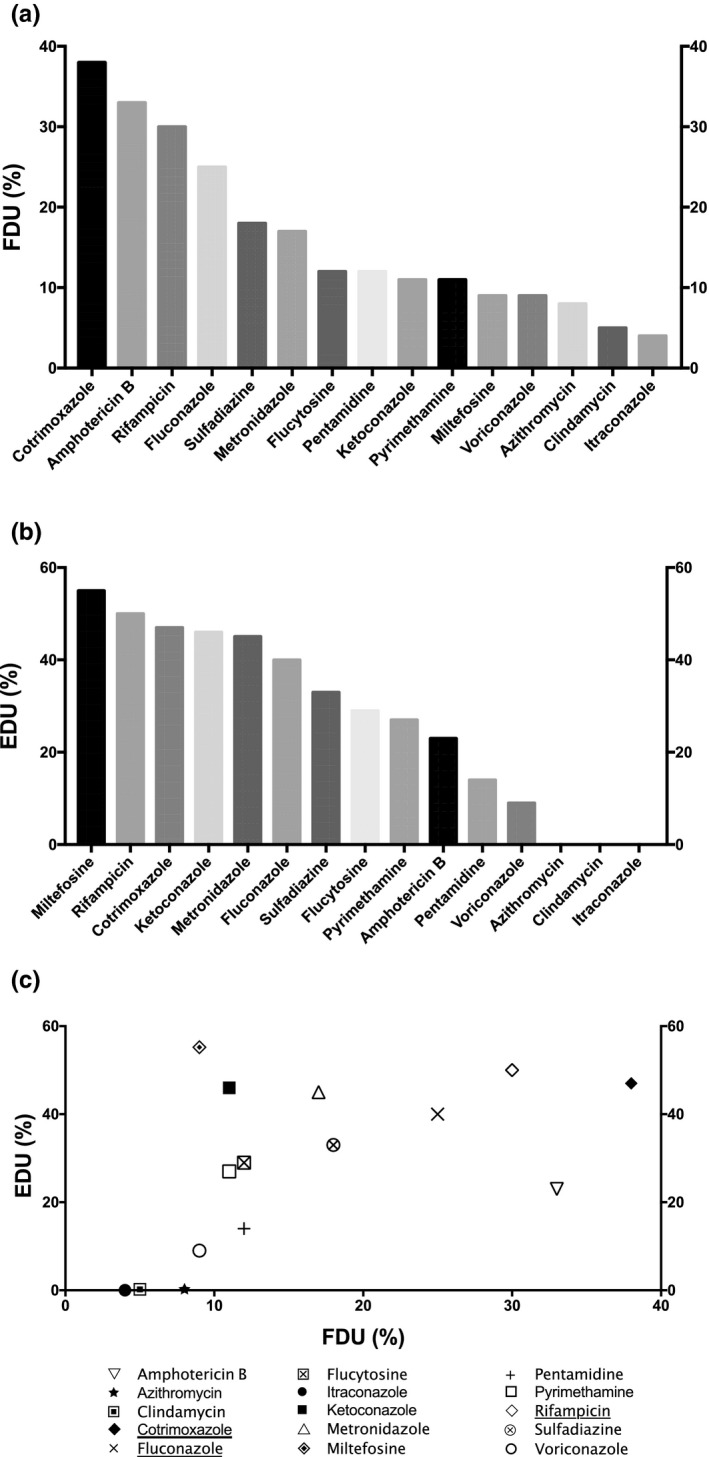
Frequency and efficiencies of drugs used in GAE therapy. (a) Frequency of drug usage (FDU) = percentage of cases treated by the drug among the total number of cases; (b) efficacy of drug usage (EDU) = percentage of survival cases treated by the drug among the total number of cases treated by the drug. (c) Plot of EDU as a function of FDU. The most relevant drugs used in GAE treatment based on both EDU and FDU criteria are underlined in the legend. The drugs selected were used at least in 5 distinct clinical cases among the 119 total GAE clinical cases gathered. Unspecific treatments include general procedures to decrease intracranial pressure (e.g., mannitol), or inflammation (e.g., corticosteroids), or treatment for differential diagnosis, such as antivirals, cephalosporins, carbapenems, or glycopeptides, for viral or bacterial meningitis. GAE, granulomatous amoebic encephalitis caused by Acanthamoeba
FIGURE 2.
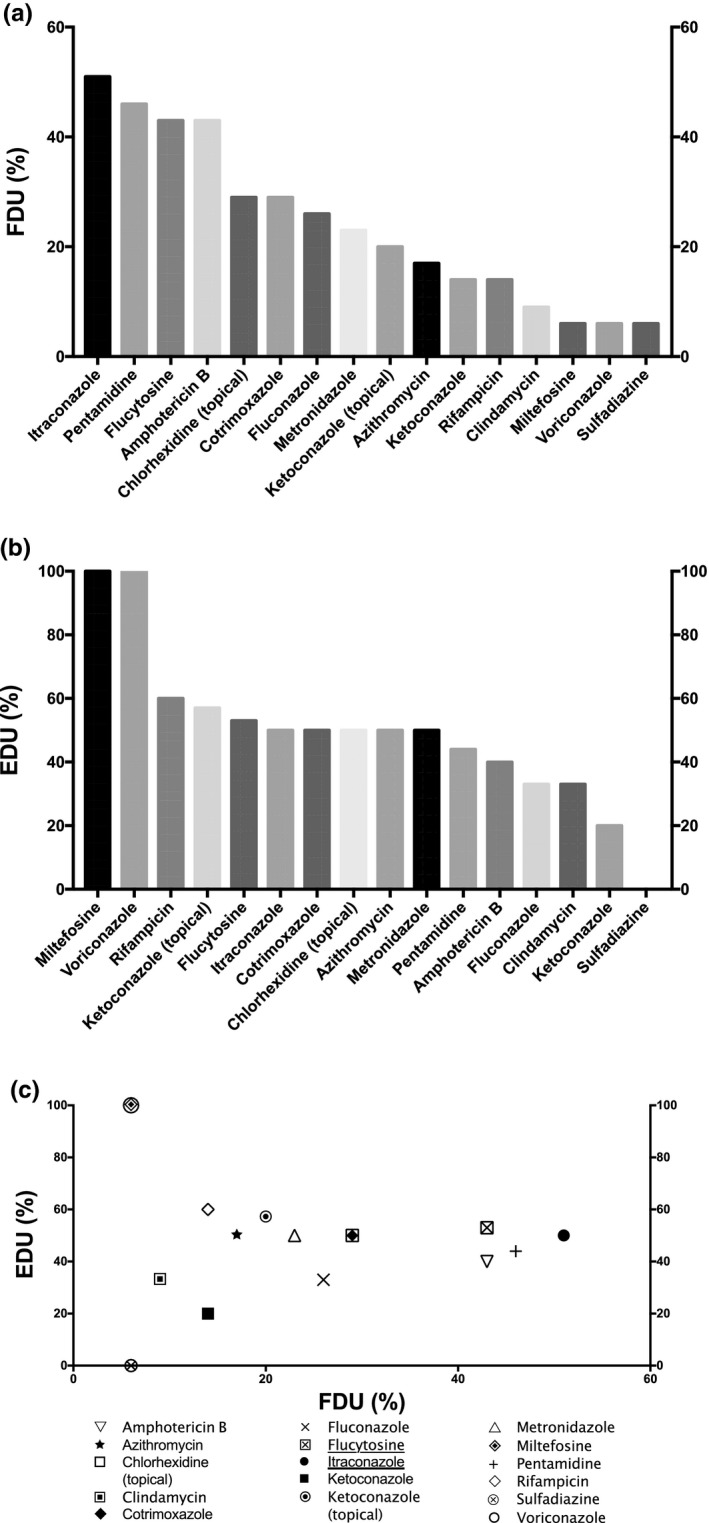
Frequency and efficiencies of drugs used in EDA therapy. (a) Frequency of drug usage (FDU) = percentage of cases treated by the drug among the total number of cases; (b) efficacy of drug usage (EDU) = percentage of survival cases treated by the drug among the total number of cases treated by the drug. (c) Plot of EDU as a function of FDU. The most relevant drugs used in EDA treatment based on both EDU and FDU criteria are underlined in the legend. The drugs selected were used at least in 5 distinct clinical cases among the 35 EDA clinical cases gathered. Unspecific treatments include general procedures to decrease intracranial pressure (e.g., mannitol), or inflammation (e.g., corticosteroids), or treatment for differential diagnosis, such as antivirals, cephalosporins, carbapenems, or glycopeptides, for viral or bacterial meningitis. EDA, extracerebral disseminated acanthamoebiasis
FIGURE 3.
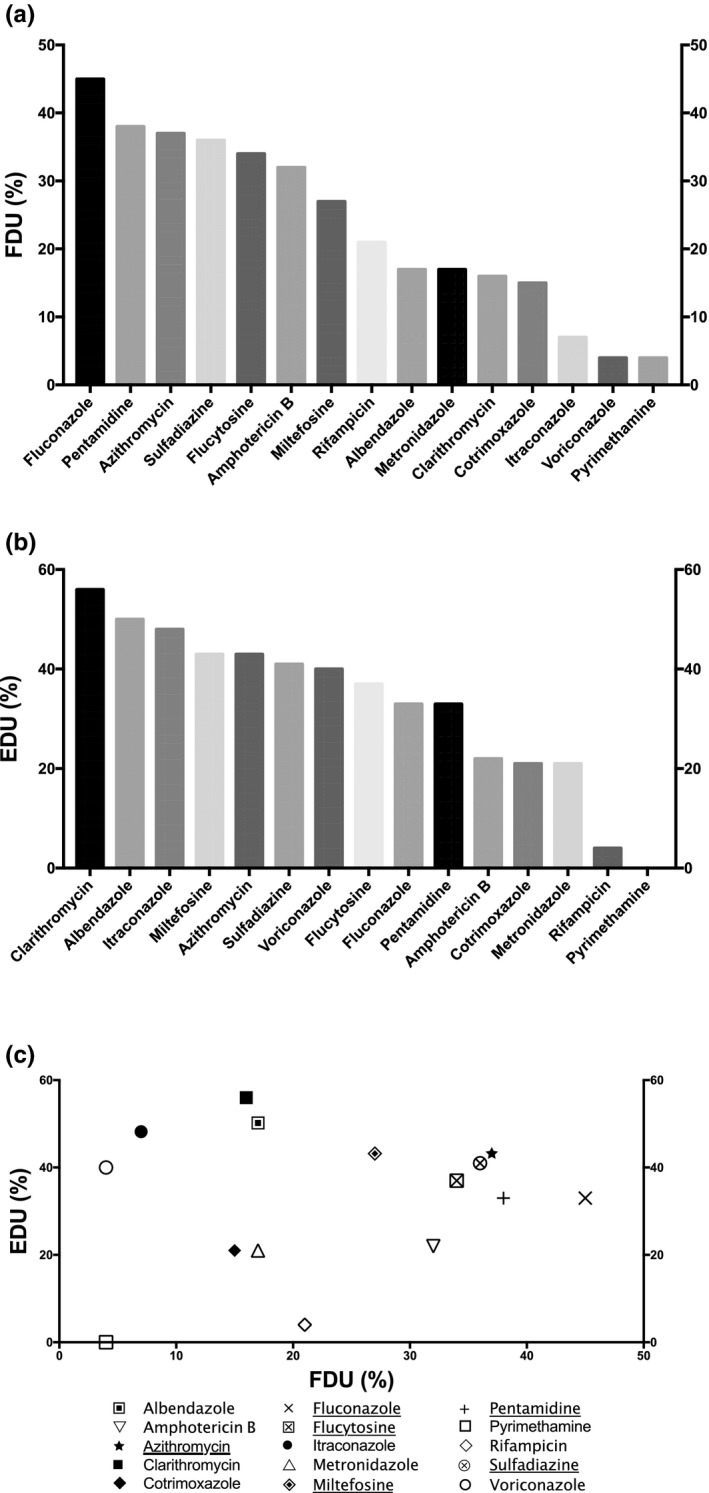
Frequency and efficiencies of drugs used in BAE therapy. (a) Frequency of drug usage (FDU) = percentage of cases treated by the drug among the total number of cases; (b) efficacy of drug usage (EDU) = percentage of survival cases treated by the drug among the total number of cases treated by the drug. (c) Plot of EDU as a function of FDU. The most relevant drugs used in BAE treatment based on both EDU and FDU criteria are underlined in the legend. The drugs selected were used at least in 5 distinct clinical cases among the 111 BAE clinical cases gathered. Unspecific treatments include general procedures to decrease intracranial pressure (e.g., mannitol), or inflammation (e.g., corticosteroids), or treatment for differential diagnosis, such as antivirals, cephalosporins, carbapenems, or glycopeptides, for viral or bacterial meningitis. BAE, Balamuthia amoebic encephalitis
FIGURE 4.
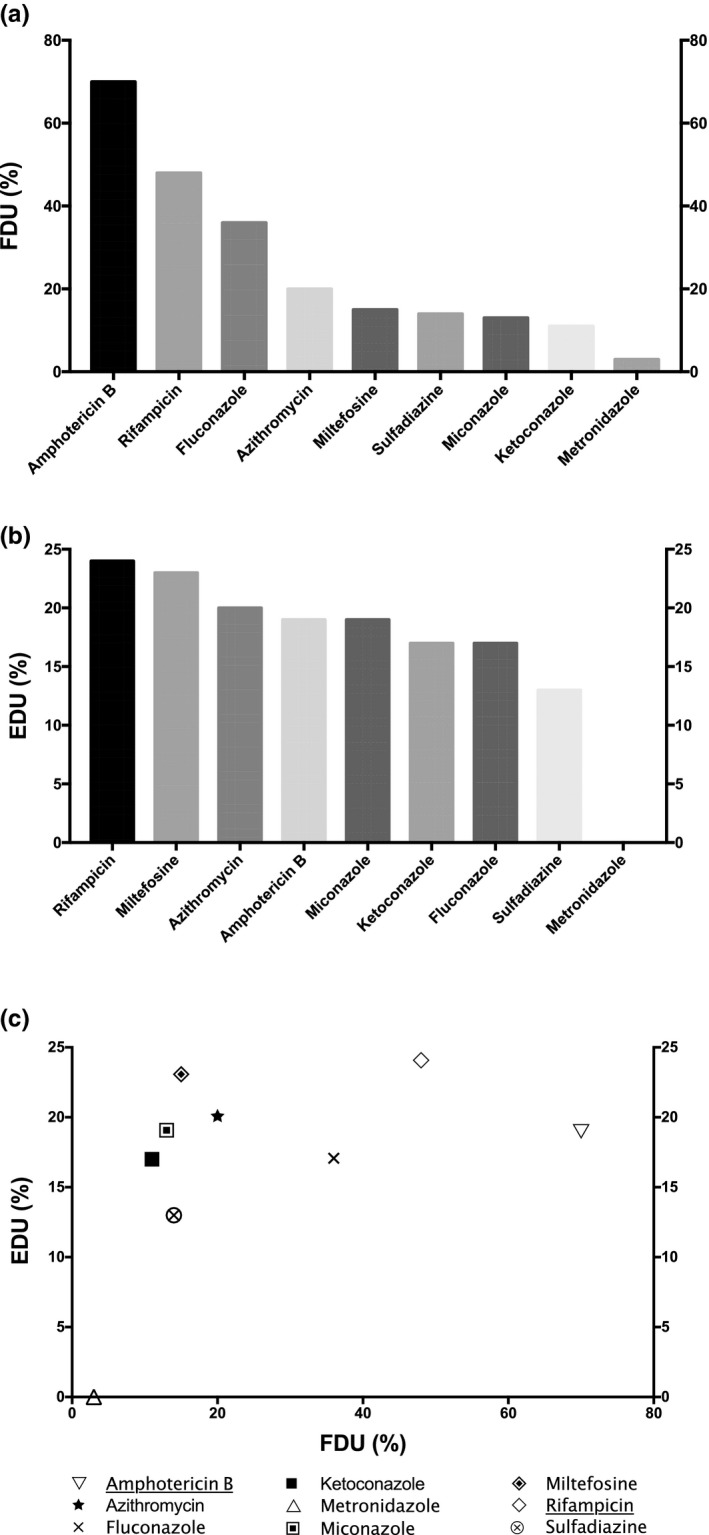
Frequency and efficiencies of drugs used in PAM therapy. (a) Frequency of drug usage (FDU) = percentage of cases treated by the drug among the total number of cases; (b) efficacy of drug usage (EDU) = percentage of survival cases treated by the drug among the total number of cases treated by the drug. (c) Plot of EDU as a function of FDU. The most relevant drugs used in PAM treatment based on both EDU and FDU criteria are underlined in the legend. The drugs selected were used at least in 5 distinct clinical cases among the 209 PAM clinical cases gathered. Unspecific treatments include general procedures to decrease intracranial pressure (e.g., mannitol), or inflammation (e.g., corticosteroids), or treatment for differential diagnosis, such as antivirals, cephalosporins, carbapenems, or glycopeptides, for viral or bacterial meningitis. PAM, primary amoebic meningoencephalitis
TABLE 1.
Main drug combinations used in the treatment of FLA infections
| Pathology | Drug combination | EDU a | References |
|---|---|---|---|
| GAE |
AGAE Rifampicin + Cotrimoxazole + (Fluconazole or Ketoconazole) |
5/6 (83%) | 24, 25, 26, 27, 28, 29 |
|
BGAE Rifampicin + Cotrimoxazole + Amphotericin B |
3/6 (50%) | 30 | |
|
CGAE Fluconazole + Metronidazole + Cotrimoxazole + (Miltefosine and/or Rifampicin) |
3/3 (100%) | 28, 31, 32 | |
| EDA | Chlorhexidine gluconate + Itraconazole +Ketoconazole (topical) + Pentamidine +/‐ Flucytosine | 2/4 (50%) | 34, 35, 36, 37 |
| BAE |
ABAE Azithromycin + Fluconazole + (Flucytosine or Albendazole) + Miltefosine + Pentamidine + Sulfadiazine |
4/9 (44%) | 44, 47, 48, 49, 50, 51 |
|
BBAE Albendazole + Amphotericin B + Azithromycin + Clarithromycin + Fluconazole + Flucytosine + Metronidazole + Miltefosine + Pentamidine + Sulfadiazine + Cotrimoxazole + Voriconazole |
2/2 (100%) | 44, 45 | |
|
CBAE Azithromycin + Flucytosine + Sulfadiazine |
2/2 (100%) | 44, 46 | |
| PAM |
APAM Amphotericin B + Azithromycin + Fluconazole + Rifampicin +/‐ Miltefosine |
6/34 (18%) | 60, 61, 62, 63, 64, 65, 76 |
|
BPAM Amphotericin B + (Fluconazole or Ketoconazole or Miconazole) +/‐ Rifampicin |
8/45 (18%) | 57, 83 | |
|
CPAM Amphotericin B + Rifampicin |
8/21 (38%) | 59, 98 |
The drug combinations selected were used at least in two survival cases.
Abbreviations: BAE, Balamuthia amoebic encephalitis; EDA, extracerebral disseminated acanthamoebiasis; EDU, efficacy of drug usage; FLA, free‐living amoebae; GAE, granulomatous amoebic encephalitis caused by Acanthamoeba; PAM, primary amoebic meningoencephalitis.
EDU = percentage of survival cases treated by the drug among the total number of cases treated by the drug.
METHODS OF DATA SELECTION
The case reports were collected in the literature by using PubMed database and the following keywords: “Acanthamoeba,” “Naegleria,” “Balamuthia,” “Granulomatous Amoebic Encephalitis,” “Balamuthia Amoebic Encephalitis,” and “Primary Amoebic Meningoencephalitis” (date of last search: June 15, 2020). Articles published from 1965 to 2020 with at least an abstract available in English were selected for the current study. Articles where treatment details were absent were excluded from the study, giving a total of 9 excluded articles out of the 303 analyzed. In the aim of providing homogeneous data in this review, treatment durations or doses as well as routes of administration were not extracted from the literature as these data are most often not described in the publications selected.
PROPORTION OF SURVIVAL CASES IN PAM, BAE, GAE, AND EXTRACEREBRAL DA
Among the clinical cases of PAM (209), BAE (111), and GAE (119) gathered, 29 (~ 14%), 22 (~ 20%), and 31 (~ 20%) survived, respectively, following diverse combination therapies (Table S1). The higher proportion of survival cases obtained in the present study compared with the survival rate reported in the literature (around 10% for BAE and GAE and below 5% for PAM) 6 could be ascribed to the fact that only the clinical cases where the treatment or the absence of treatment was clearly denoted were taken into account.
Moreover, extracerebral DA (EDA) are extremely rare Acanthamoeba infections and occur almost exclusively in immunocompromised patients. 4 , 6 Indeed, among the 35 clinical cases of EDA that we gathered in the current study, only one was immunocompetent, and 14 survived (Table S1). In spite of the low number of cases, the higher proportion of survivals compared to GAE (40% for EDA vs. 20% for GAE) could be due to the fact that EDA is a preliminary form of Acanthamoeba infection, 4 and could therefore be cured less infrequently than GAE as both diagnosis and treatment are established earlier in the pathology. Moreover, as for PAM, BAE, and GAE, the proportion of EDA survival cases is also higher than the percentage of 27% reported in the literature, 4 presumably due to a selection of reports excluding clinical cases with undefined treatment. Therefore, as the proportions of survival cases of each FLA infection are higher than the percentages previously described, 4 , 6 it is likely that the clinical cases omitted in the present work, because of a lack of treatment description, are mostly death cases. Another possibility would be that, since 2004, new efficient drugs, such as voriconazole or miltefosine, would have increased the general proportion of survival cases.
COMBINATION THERAPIES USED FOR THE TREATMENT OF GAE
In this pathology, the combination of trimethoprim and sulfamethoxazole (cotrimoxazole) has the highest FDU showing that this drug is the most frequently used drug (Figure 1a). However, only 47% of patients with GAE treated with cotrimoxazole survived (Figure 1b). Despite this appreciable efficacy in vivo, this drug combination did not show amoebicidal activity below 100 µg/ml in vitro. 13
Although amphotericin B was the second most frequently used drug for GAE treatment (Figure 1a), it exhibits a low efficacy (Figure 1b): only 23% of the patients treated by amphotericin B survived (vs. 55% for miltefosine). Despite a poor penetration through the blood‐brain barrier (BBB; below 1% of the serum concentration), amphotericin B has been successfully used for the treatment of CNS infections, such as C. albicans meningoencephalitis or cryptococcal meningitis. 14 Nevertheless, in Acanthamoeba spp., a natural mechanism of resilience to amphotericin B allowing the amoeba to return to an initial level of susceptibility at each generation has been evidenced in vitro. 13 Therefore, according to its low efficacy both in vitro and in vivo, the relevance of using amphotericin B may be further reconsidered in patients with GAE.
With an FDU of 30%, and an EDU at 50% (just after miltefosine; Figure 1b), rifampicin is also one of the most frequent and efficient drug used to treat GAE. Nonetheless, this antibacterial agent did not show any significant antiacanthamoebal activity in vitro. 13 , 15 Despite a nonsignificant in vitro activity of both cotrimoxazole and rifampicin on Acanthamoeba spp., these drugs can penetrate well the BBB due to their lipophilic properties and are often used for the treatment of CNS infections. 14 Therefore, they would be good candidates for in vivo additive effects in GAE treatment as they could have a synergistic activity with another drug used in the combination therapy.
Among the azoles, fluconazole was the most frequently used in GAE treatment with an FDU of 25% (Figure 1a). The other azoles, namely ketoconazole, voriconazole, and itraconazole, are more scarcely used with FDU at 11%, 9%, and 4%, respectively (Figure 1a). Despite a low FDU, ketoconazole was the most efficient azole with an EDU at 46%, and 40% of the patients with GAE treated by fluconazole survived (Figure 1b). On the other hand, the efficiencies of both voriconazole and itraconazole were among the lowest observed in GAE treatment (Figure 1b), despite interesting in vitro activities on several Acanthamoeba strains previously reported. 16 Although ketoconazole crosses only weakly the BBB but exhibited an interesting in vitro activity on Acanthamoeba spp. with an half‐maximal inhibitory concentration at ~ 8 µM, 13 , 17 fluconazole can penetrate readily in the cerebrospinal fluid (CSF) despite a weak in vitro activity on few acanthamoebal strains. 14 , 16 The efficiencies observed for fluconazole and ketoconazole in GAE treatment could be ascribed to a high propensity to penetrate the BBB despite a low anti‐Acanthamoeba activity and to a prominent antiacanthamoebal activity in spite of a low BBB passage, respectively. Thus, according to their similar EDU ranking, fluconazole and ketoconazole are the most efficient azoles used in GAE therapy, and might be used at the same level of preference in the treatment of this cerebral pathology.
Sulfadiazine displayed a low FDU at 18% and an intermediate EDU at 33% (Figure 1a,b). Moreover, a single patient with GAE survived with a monotherapy of sulfadimidine, a sulfadiazine derivative that was used only once among the clinical cases gathered in the present work. 18 Furthermore, despite a low FDU at 17%, metronidazole exhibited an interesting EDU at 45% (Figure 1).
Interestingly, despite that miltefosine has been infrequently used (only 11 cases among the 119), it presents the highest EDU in patients with GAE: 6 patients survived among the 11 patients with GAE treated by miltefosine (Table S2; Figure 1b). Miltefosine has been reported to penetrate the BBB of a patient with GAE but was measured at only low concentration in the CSF. 19 Furthermore, antiacanthamoebal activity was reported for this drug in vitro with a half‐maximal inhibitory concentration at ~ 8 µM on A. castellanii and 40 µM on A. polyphaga. 13 , 20 , 21 Therefore, regarding its high EDU value, the low level of miltefosine penetrating into the CSF would be sufficient for an antiacanthamoebal activity. Consequently, miltefosine might be considered more frequently for the treatment of GAE in the future. All the other drugs displayed low FDU and EDU. Especially azithromycin and clindamycin, which were the most inefficient drugs used in GAE treatment with an EDU at 0% (Figure 1b).
According to the plot of EDU as a function of FDU (Figure 1c), the most frequently used (FDU ≥ 25%) and the most efficient (EDU > 30%) drugs in GAE treatment are: cotrimoxazole, rifampicin, and fluconazole. Therefore, despite the lack of in vitro antiacanthamoebal activity, these drugs might be used by practitioners to treat GAE as they display the highest FDU and EDU criteria. Their in vivo activity could be attributed to a synergistic effect when used in combination therapy.
The Infectious Disease Society of America (IDSA) currently has a C‐III‐level recommendation (from clinical experience, descriptive studies, or reports of expert committees, but with low evidence) for two drug combinations: (a) cotrimoxazole, rifampicin, and ketoconazole, or (b) fluconazole, sulfadiazine, and pyrimethamine. 22 Moreover, the Center for Disease Control and Prevention (CDC) rendered miltefosine available as an investigational drug for the treatment of cerebral FLA infections in the United States. 23 According to our study, cotrimoxazole, rifampicin, and fluconazole appear to be the most frequent and efficient drugs used in GAE treatment. Amphotericin B is frequently used, but its EDU is low, in opposition to miltefosine and ketoconazole, which are not frequently used but present very interesting EDU (Figure 1b). In line with these observations, the most common and efficient drug combination used among the 119 clinical cases studied in the current work is the combination AGAE associating rifampicin with cotrimoxazole and with fluconazole or ketoconazole (Table 1). 24 , 25 , 26 , 27 , 28 , 29 This combination was successful in 83% of the cases (Table 1), and is very close to the regimen advised by the IDSA for GAE treatment. 22 In particular, the combination associating rifampicin, cotrimoxazole, and ketoconazole was successful in three cases of four, 24 , 25 , 27 , 29 and the one combining rifampicin, cotrimoxazole, and fluconazole had a positive outcome in the two cases where it was used. 26 , 28 In our study, a similar combination associating amphotericin B with rifampicin and cotrimoxazole was also often used, but its efficacy was lower, at 50% (combination BGAE in Table 1). 30 Additionally, an association of fluconazole, metronidazole, and cotrimoxazole with miltefosine and/or rifampicin, named combination CGAE, was also used with a positive outcome in 100% of the cases (Table 1). 28 , 31 , 32 This promising latter combination includes drugs which are among the most efficient emphasized in the current work (Figure 1b).
COMBINATION THERAPIES USED FOR THE TREATMENT OF EDA
The most frequently used drug for EDA treatment was itraconazole (Figure 2a). However, this azole presented an EDU value at only 50% (Figure 2b). The other azoles were more scarcely used among patients with EDA, with an FDU between 6% for voriconazole and 26% for fluconazole (Figure 2a). Fluconazole and ketoconazole had also one of the lowest EDU at 33% and 20%, respectively. However, despite an uncommon usage in EDA therapy (only 2 patients), voriconazole was always associated with a treatment leading to a successful outcome (Figure 2b).
Pentamidine and amphotericin B were among the most frequently used drugs for EDA treatment with FDU at 46% and 43%, respectively (Figure 2a). However, the EDU of both drugs was among the lowest in EDA treatment, below 50% (Figure 2b). Therefore, the use of pentamidine or amphotericin B in EDA therapy might be reconsidered. These observations are in line with the GAE treatment where low efficacy of usage of both drugs were noticed (Figure 1b).
Flucytosine was also among the most frequently used drug for EDA treatment with an FDU at 43% and exhibited an interesting EDU at 53% (Figure 2a,b). Moreover, this pyrimidine analog showed attractive in vitro activity on A. castellanii despite a capacity to develop resistance for some virulent strains. 13 , 33 Therefore, in spite of a good efficacy, the relevance of using flucytosine in EDA therapy might be limited by the capacity of some Acanthamoeba strains to develop resistance.
Most EDA clinical manifestations are cutaneous and are therefore treated by using topical medications. Among these topical treatments, two drug formulations are usually used: chlorhexidine solution and ketoconazole cream. Among these topical treatments, chlorhexidine was the most frequently used with an FDU value at 29%, but is efficient in only half of the cases (Figure 2). Conversely, ketoconazole cream was less frequently used (20% of the cases) but displays a slightly better EDU at 57% than chlorhexidine (Figure 2). Therefore, according to their efficacy of usage, ketoconazole cream might be selected as a first‐line drug, before chlorhexidine, among topical treatments in EDA therapy.
In opposition to GAE treatment, cotrimoxazole was not the most frequently used drug in EDA therapy. Conversely, this drug exhibited the same FDU (29%) and EDU (50%) values as chlorhexidine (Figure 2). Likewise, both metronidazole and azithromycin were efficient in half of the cases despite an FDU below 25% (Figure 2). Therefore, cotrimoxazole, metronidazole, and azithromycin might be used, but not as a first‐line treatment, in EDA therapy. Furthermore, clindamycin and sulfadiazine are among the least frequently used and efficient drugs (Figure 2). Therefore, the usage of these drugs in EDA therapy seems to be hazardous.
Like voriconazole, the only 2 patients with EDA treated by miltefosine were cured, giving also to this drug a maximal EDU value at 100% (Figure 2b). Therefore, despite a low FDU value at 6%, voriconazole and miltefosine have always been successfully used, in opposition to itraconazole, which was the most frequently used drug in EDA treatment, but was efficient in only half of the cases (Figure 2). Moreover, despite a low frequency of usage (14%), rifampicin presented also an encouraging EDU at 60% in EDA treatment (Figure 2). Consequently, consistent with their high EDU, voriconazole, miltefosine, and rifampicin might be used more frequently for EDA treatment.
As illustrated in Figure 2c, flucytosine and itraconazole display the highest FDU (> 40%) and EDU (≥ 50%) criteria in EDA treatment. Therefore, these most efficient and frequently used drugs might be primarily used by practitioners in EDA treatment. In spite of their high FDU values, amphotericin B and pentamidine were not among the most efficient drugs, as their EDU values were among the lowest obtained in EDA treatment (Figure 2b).
The most frequently used combination therapy among the 35 clinical cases collected were associated with chlorhexidine, itraconazole, ketoconazole cream, and pentamidine, with or without sulfadiazine (Table 1). 34 , 35 , 36 , 37 This combination was successful in 50% of the cases. Nevertheless, according to the EDU criterion analyzed in the current work (Figure 2b), the substitution of pentamidine and/or itraconazole in this combination by voriconazole and/or miltefosine could increase the efficacy of treatment.
COMBINATION THERAPIES USED FOR THE TREATMENT OF BAE
With an FDU at 45%, fluconazole was the most frequently used drug in BAE therapy (Figure 3a). However, only one third of the patients with BAE treated by this drug survived (Figure 3b). On the opposite, another azole, itraconazole, was among the least frequently used drug with an FDU at 9%, but showed one of the highest efficacy of usage among the drugs used in BAE therapy: 48% of the patients with BAE treated by itraconazole survived (Figure 3). Likewise, voriconazole was one of the least frequently used drugs in BAE treatment but presented an intermediate EDU value at 40% (Figure 3b). Therefore, based on the efficacy of usage of both azoles, itraconazole might be preferred to voriconazole or fluconazole in BAE therapy. Moreover, as for GAE, although a low anti‐Balamuthia activity was previously reported for fluconazole, 38 the in vivo efficacy of this drug could be assigned to its high capacity to penetrate the BBB. 14 Likewise, voriconazole can readily penetrate the BBB/CSF but displayed little to no inhibitory effect in vitro on B. mandrillaris. 14 , 21 Conversely, itraconazole shows a low BBB passage, 14 and, to our knowledge, its in vitro activity on Balamuthia mandrillaris has not been studied yet.
Furthermore, pentamidine was the second most frequently used drug in BAE therapy with an FDU value at 38% (Figure 3a). However, this drug displayed the same modest EDU value as fluconazole at 33% (Figure 3b). Nonetheless, this diamidine can cross the BBB and displayed in vitro anti‐Balamuthia activity with 82% amoebal growth inhibition at 1 µg/ml. 38 , 39 Therefore, pentamidine might be used, but not as a first‐line drug in BAE therapy.
Azithromycin exhibited an FDU value similar to the one of pentamidine (37%), and was used with success in 43% of the cases (Figure 3). Among the macrolides used in BAE treatment, clarithromycin was the least used drug with an FDU at 16% (Figure 3a). Nevertheless, this drug displayed the highest efficacy of usage with an EDU at 56%: 10 patients with BAE survived out of 18 treated by clarithromycin (Figure 3b; Table S3). Therefore, according to the individual EDU criterion, clarithromycin might be preferred to azithromycin among the macrolides used in BAE therapy. Nevertheless, if both EDU and FDU criteria are considered, azithromycin should be preferred to clarithromycin, as this latter drug was only scarcely used up to now in BAE treatment (Figure 3c). Moreover, although both azithromycin and clarithromycin display a low in vitro anti‐Balamuthia activity at 10 µg/ml, 38 these macrolides can cross the BBB and, in the case of azithromycin, can highly concentrate in the brain with a cerebral concentration up to 200 times higher than the one in the serum. 14 , 40
Sulfadiazine and flucytosine were used in 36% and 34% of the BAE cases, and displayed an intermediate EDU value of 41% and 37%, respectively (Figure 3). Therefore, according to their efficiencies, both sulfadiazine and flucytosine might be good candidates for their in vivo additive effect in BAE therapy. Moreover, both drugs can penetrate readily the BBB, 14 but their antiamoebal activity on B. mandrillaris has been previously reported to be low to inexistent at up to 10 µg/ml. 41
Despite an intermediate FDU value at 32%, amphotericin B exhibited a low efficacy with an EDU value at 22% (Figure 3). Other drugs, such as pyrimethamine, cotrimoxazole, metronidazole, and rifampicin have low to intermediate frequency of usage (from 4% to 21%), and are all among the least efficient drugs (Figure 3). Therefore, these drugs might not be further used in BAE therapy.
Miltefosine displayed the same EDU value as azithromycin at 43%, despite a low FDU at 27% (Figure 3). Consequently, miltefosine might be used more frequently in BAE treatment (Figure 3b). Although this drug has been described to penetrate poorly the BBB in a patient with BAE with a low concentration in CSF at the micromolar level, it displayed an in vitro amoebicidal activity on B. mandrillaris at 40 µM. 21 , 42 Therefore, the interesting EDU observed for miltefosine could be ascribed to a synergy of action with one of the drugs used in BAE combination therapy, leading to a decrease of the active concentration of the alkyl‐phosphocholine in the CSF and the brain.
With an EDU value at 50%, albendazole was the second most efficient drug used in BAE therapy, despite a low FDU at only 17% (Figure 3). Therefore, albendazole might be used more frequently as it displays one of the highest efficacies of usage in BAE therapy. Furthermore, albendazole is a small lipophilic drug metabolized in the liver in an active metabolite, albendazole sulfoxide, which readily penetrates the BBB. 14 However, to our knowledge, the activity of albendazole, or its active metabolite, on B. mandrillaris has not been analyzed yet.
According to both EDU and FDU criteria, sulfadiazine and azithromycin are the most efficient (EDU > 40%) and frequently used (FDU > 35%) drugs in BAE treatment (Figure 3c). Therefore, these drugs might be primarily used in BAE treatment, presumably in combination therapy in order to promote synergistic effects as their in vitro antiamoebal activity on B. mandrillaris is low. 38 , 41 Among macrolides, clarithromycin has a better EDU than azithromycin but was used in only 16% of the patients with BAE and would therefore presently be more uncertain to be used in BAE treatment. Moreover, despite a noticeable FDU at 27%, miltefosine displayed an encouraging EDU above 40% (Figure 3c), and its use might thus be considered in BAE therapy.
The IDSA has currently a C‐III level recommendation for the following drug combination for BAE treatment: pentamidine, macrolide (azithromycin or clarithromycin), fluconazole, sulfadiazine, and flucytosine. 22 The CDC also recommends this combination in addition to miltefosine, which showed some promising results in BAE therapy. 43 , 44 Most of these drugs showed a high to intermediate efficacy according to the present analysis, except for pentamidine which had a modest EDU at 33% (Figure 3b). The current analysis also showed that albendazole and itraconazole displayed one of the highest efficacies and might therefore be used in the BAE drug combination therapy. As for GAE, no consensus combination has been set up yet for BAE treatment, 44 and several other combinations have also been used successfully, associating up to 12 different molecules, such as in combination BBAE (Table 1). Indeed, this combination composed of albendazole, amphotericin B, azithromycin, clarithromycin, fluconazole, flucytosine, metronidazole, miltefosine, pentamidine, sulfadiazine, cotrimoxazole, and voriconazole has been used each time with success in two BAE clinical cases (Table 1). 44 , 45 Conversely, a combination associating a few number of drugs (combination CBAE: azithromycin, flucytosine, and sulfadiazine) was also 100% successful in two distinct patients with BAE (Table 1). 44 , 46 The most relevant combination therapy used among the 111 BAE clinical cases collected is the combination ABAE, associating 6 distinct drugs: azithromycin, fluconazole, miltefosine, pentamidine, sulfadiazine, with flucytosine or albendazole. 44 , 47 , 48 , 49 , 50 , 51 This combination was successful in 44% of the cases (Table 1) and was very close to the one recommended by the CDC, except that flucytosine could be substituted by albendazole. Moreover, among the 111 BAE clinical cases gathered, the combination recommended by the CDC sensu stricto was used in 7 patients, 48 , 49 and was successful in only in 2 of them (29% of success). Therefore, the presence of albendazole in the drug combination could increase the efficacy of BAE therapy.
COMBINATION THERAPIES USED FOR THE TREATMENT OF PAM
Among the 209 PAM clinical cases gathered, all drugs used exhibited low EDU, with a maximal value for rifampicin at 24%, considerably below the highest EDU observed at between 50 and 60% for the other FLA cerebral infections. This difference could be ascribed to the shorter survival time of PAM (few days), compared with GAE or BAE (several weeks to several months), 4 rendering more difficult the establishment of an accurate diagnosis, and thus of an efficient drug combination therapy. Indeed, the percentage of survival cases was lower for PAM (14%) than for GAE or BAE (20%).
Amphotericin B was the most frequently used drug in PAM treatment with a high FDU value at 70% (Figure 4a). Interestingly, all PAM survival cases (29 out of 29) were treated by amphotericin B (Table S4). Therefore, the efficacy among survival cases (ESCs) of this polyene drug is 100% in PAM therapy. However, only 19% of the patients with PAM treated by amphotericin B survived. The maximal ESC of amphotericin B on PAM could be due to a high amoebicidal activity at the submicromolar range previously described on N. fowleri, despite a poor penetration through the BBB. 14 , 52 Therefore, according to its ESC, amphotericin B should be used in PAM treatment, but in a synergistic combination with other drugs, as its EDU is modest and only one PAM survival case has been described with a monotherapy of amphotericin B among the 29 analyzed in the current study. 53 Indeed, several drugs used in PAM combination therapy, such as rifampicin, miconazole, or azithromycin, were described to display a synergistic activity with amphotericin B. 54 , 55 , 56
Rifampicin displayed the highest efficacy in PAM therapy (24%) and was the second most frequently used drug with an FDU at 48% (Figure 4). Nevertheless, rifampicin did not display a significant in vitro antiamoebal activity on N. fowleri, despite a noticeable passage through the BBB. 14 , 57 Therefore, the efficacy of rifampicin in PAM treatment could be attributed to a synergy of action with another drug used in the combination therapy, such as amphotericin B, as previously described in a mouse PAM model in vivo. 54
Fluconazole was the most used azole in PAM therapy with an FDU value at 36% (Figure 4a). However, its EDU was only at 17% (Figure 4b). Two other azoles, namely miconazole and ketoconazole, were infrequently used in PAM therapy, with FDU value at 13% and 11%, respectively (Figure 4a). Nonetheless, the EDU value of miconazole and ketoconazole is the same as amphotericin B and fluconazole, at 19% and 17%, respectively (Figure 4b). Therefore, as miconazole exhibited the best EDU of usage among azoles, this drug might be preferred to fluconazole or ketoconazole in PAM treatment, despite its sparse use. Moreover, miconazole has been previously described to display a synergistic antiamoebal activity against N. fowleri with amphotericin B. 56
Azithromycin was used in 20% of the PAM cases, and 20% of the patients treated by this macrolide survived (Figure 4). Besides its capacity to cross the BBB and its interesting in vitro antiamoebal activity on Naegleria fowleri (minimal inhibitory concentration at 13.4 µM), azithromycin exhibited a synergistic activity with amphotericin B both in vitro on N. fowleri and in vivo in a mouse PAM model. 14 , 55 Therefore, azithromycin might be used in PAM therapy, preferably in combination with amphotericin B.
Miltefosine was used only in 15% of the PAM cases, but exhibited an EDU at 23%, showing that this alkyl‐phosphocholine is the second most efficient drug used in PAM therapy (Figure 4). However, this drug was found at low concentrations in the CSF showing a poor penetration through the BBB and exhibited only a mild in vitro activity on N. fowleri. 21 , 42 Therefore, the efficacy of usage of miltefosine could be ascribed, as for amphotericin B, to a synergy of activity with another drug used in PAM combination therapy. However, to our knowledge, no drug used in PAM therapy has been described yet to display a synergistic activity with miltefosine.
Sulfadiazine and metronidazole are the least efficient and among the least frequently used drugs in PAM therapy (Figure 4). Therefore, the relevance of using these drugs in PAM therapy might be reconsidered.
Considering both FDU and EDU criteria, amphotericin B and rifampicin are the most efficient and frequently used drugs in PAM treatment (Figure 4c). Indeed, despite an EDU below 20%, amphotericin B was used in 100% PAM survivors. Moreover, rifampicin displayed the highest EDU (24%) and was frequently used (48% of the cases) in PAM treatment. This latter drug might act in synergy with amphotericin B against N. fowleri, as previously described in a PAM mouse model in vivo. 54 Therefore, both amphotericin B and rifampicin might be primarily used by practitioners in PAM treatment.
The IDSA has a current C‐III level recommendation for the following drug combination for PAM treatment: amphotericin B, rifampicin combined with another agent. 22 In complement with these guidelines, the CDC recommends using the following association for PAM therapy: amphotericin B, rifampicin, azithromycin, miltefosine, and fluconazole or miconazole. 58 All these drugs are the most efficient used in the 209 clinical cases gathered in the current work, except for fluconazole, which was frequently used but exhibited only a modest EDU and might be substituted by miconazole. The most relevant drug combinations gathered in the current work for PAM treatment associated either (a) amphotericin B and an azole (fluconazole, ketoconazole or miconazole), with or without rifampicin (combination BPAM), or (b) amphotericin B, azithromycin, fluconazole, or rifampicin with or without miltefosine (combination APAM; Table 1). 56 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 In the latter combination, miltefosine was associated with rifampicin in 28 cases out of 34, 60 , 61 , 62 , 63 , 64 , 70 , 73 , 76 both drugs presenting the highest EDU according to our analysis (Figure 4b). The combination APAM, which was the closest to the one recommended by the CDC, was successful in 18% of the 34 clinical cases treated by this drug association. Moreover, in the combination APAM associating both rifampicin and miltefosine with amphotericin B, azithromycin, and fluconazole, the outcome was also successful in 18% of 28 cases. 60 , 61 , 62 , 63 , 64 Nevertheless, the combination BPAM was used in a larger number of cases (45) and exhibited the same EDU value at 18% (Table 1). Therefore, this simplified combination associating only two or three molecules might also be recommended for PAM therapy, with a lower risk of toxicity for the patient. Moreover, another simplified association using only 2 molecules, amphotericin B and rifampicin, named combination CPAM, has also been highlighted in the current work with higher efficiency compared with the combinations APAM and BPAM: 38% for EDU value (Table 1). 59 , 98 Therefore, the combination CPAM, which would be less toxic than the other combinations as it contains only two drugs, might also be recommended in PAM treatment, more probably at early stages of the pathology. Indeed, the celerity to give an accurate diagnosis during the pathology influences the complexity and the toxicity of the drug combination used. Therefore, amphotericin B and rifampicin, which compose the combination CPAM, might be used in priority after PAM diagnosis, and the other drugs constituting combinations APAM or BPAM (azithromycin, azoles, and miltefosine) might be used subsequently during the pathology.
EFFICACY OF AN ABSENCE OF THERAPY OR UNSPECIFIC TREATMENTS ON FLA INFECTIONS
In all FLA infections, the outcome was always fatal in the absence of treatment, representing 3–5% of the cases collected (Tables S2–S4). Moreover, a large majority of the cases, ~ 60–85%, received unspecific treatments (Tables S2–S4). Nevertheless, some antibacterial drugs, such as rifampicin or macrolides, were not included in these unspecific treatments as they were very frequently used in FLA infections and exhibited a high efficacy (Figures 1, 2, 3, 4). Furthermore, a small proportion of the patients, 15–25%, affected by cerebral FLA infection and treated by unspecific drugs, survived (Tables S2–S4). However, among the FLA‐infected patients who received exclusively unspecific treatments, representing 10–16% of the FLA infection cases, only 2 patients with GAE survived. 99 , 100 Therefore, the added‐value of unspecific drugs used in FLA infection therapy might be analyzed further in order to improve and optimize the drug combinations used to treat these pathologies.
CONCLUSION
Currently, 10–15 different antiprotozoal and antibiotic repurposed drugs, acting on diverse cellular pathways (Figure 5), are empirically used for the treatment of GAE, EDA, BAE, or PAM. This review highlights the relative interest of the different drugs used, through the plots of EDU as a function of FDU for FLA infections (Figures 1c, 2c, 3c, 4c), allowing the identification of the most promising drugs, worthy of clinical use by practitioners. Although the activity and the mechanism of action of some of these drugs has been described in FLA (e.g., azoles), 18 some others have weak in vitro antiamoebal activity, and/or their mechanism of action has not been deciphered yet in these protozoa (e.g., rifampicin, macrolides, or cotrimoxazole; Figure 5). 13 , 38 , 57 Nevertheless, several of these latter drugs have been shown to display a synergistic action on FLA with some antiamoebal drugs, such as azithromycin or rifampicin with amphotericin B. 54 , 55 Therefore, despite a poor rationale of usage, the clinicians use these drugs in combination in order to gain a therapeutic advantage. Among the drug associations emphasized in the current work, the most relevant combinations used for GAE, BAE, and PAM therapy are named AGAE, ABAE, and APAM, respectively (Table 1), and are very close to the ones recommended by the IDSA and CDC. 22 , 23 , 43 , 44 , 58 Indeed, many drug combinations were used successfully in the treatment of FLA infections, but as many distinct molecules can be used (up to 12 for BAE, for example), their combinations are complex and often unique. This is especially the case for GAE and BAE treatment where the most relevant drug combination was used only in 6 cases out of a total of 119 (5%; AGAE) and in 9 cases out of total of 111 (8%; ABAE), respectively. The other combinations were either unique or very scarcely used. Among these associations, some have shown a high efficacy despite a low frequency of usage, such as combinations CGAE, BBAE, and CBAE (Table 1). These combinations might be further used in order to determine their efficacy and their relevance of usage in therapy. Concerning EDA, as the number of total cases is low (35), the drug associations used were often unique and only one relevant association was revealed for this pathology, using notably topical treatments for cutaneous infections (chlorhexidine and/or ketoconazole cream). For PAM, as the number of cases is more important than for GAE or BAE, better consensus treatments, with less diversified associations, and more clinical cases per drug combination, can be established (Table 1).
FIGURE 5.
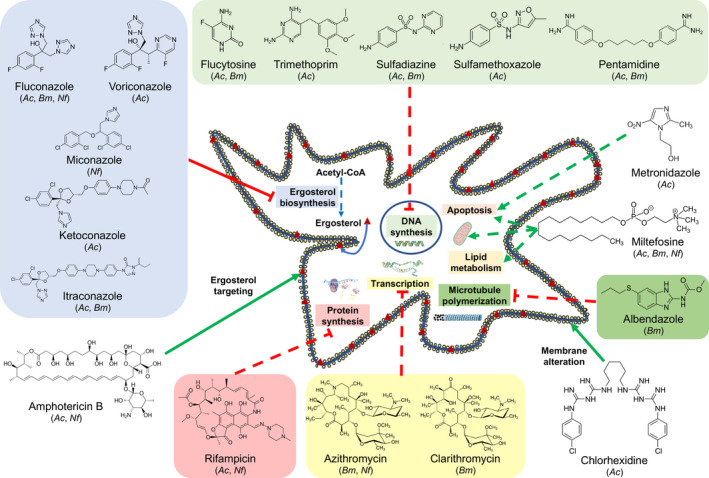
Cellular pathways disrupted by drugs used for the treatment of FLA infections. Drugs used for the treatment of FLA infection can target ergosterol in the amoebal membrane (amphotericin B), cause membrane disruption (chlorhexidine), inhibit microtubule polymerization (albendazole), induce DNA fragmentation by apoptosis‐like pathways (miltefosine and metronidazole), or affect lipid metabolism and mitochondrial membrane potential (miltefosine). Some other drugs can also affect ergosterol (azoles), DNA (flucytosine, trimethoprim, pentamidine, and sulfamides), RNA (macrolides), or protein (rifampicin) biosynthesis. All drugs represented in the figure are in the top five most frequently or most efficiently used drugs to treat Acanthamoeba spp. (Ac), Balamuthia mandrillaris (Bm), or Naegleria fowleri (Nf) infections. Green arrows and red bar‐headed lines represent an activation/alteration and an inhibition of a pathway, respectively. Full lines and dashed‐lines represent a mechanism of action previously described in FLA and in microorganisms other than FLA, respectively. For this latter groups of drugs, their mechanism of action has been previously described in bacteria for rifampicin and macrolides, Leishmania sp. for miltefosine, or Giardia intestinalis for albendazole and metronidazole. FLA, free‐living amoebae
Amphotericin B was present in all relevant PAM drug combinations, and therefore might be used in the treatment of this pathology. However, the efficacy of this drug in the other FLA infections was more uncertain. Azoles (fluconazole, ketoconazole, itraconazole, or miconazole) are also molecules that are often used for the treatment of FLA infections. Miltefosine, which was recently added in the drug combinations, is also a promising drug as it has shown a high efficacy for the treatment of GAE, BAE, or PAM. Antibacterial drugs, such as rifampicin for GAE or PAM, macrolides for BAE, or cotrimoxazole for GAE, might also be used as additive drugs in the combinations as they exhibited high efficiencies. In BAE therapy, albendazole also displayed high efficiencies despite a low frequency of usage and might therefore be included more often in the drug combination.
Because FLA infections constitute rare pathologies, it is clearly underdiagnosed properly because of a lack of experience of some physicians in affected areas. As the mortality rate of FLA infection is very high, an early diagnosis is essential for the efficacy of the treatment used. This situation leads to empirical decisions at the therapeutic level. Thus, the number of cases is not presently exploitable for a solid statistical analysis of classical parameters (age, sex, geographical location, dose, route of administration, treatment duration…) from the clinical data available. Concerning the efficacy of drug combinations, the various drug combination parameters (i.e., number of combined drugs, nature of active principle, and the lack of information about drug interactions) did not allow to highlight decisive criteria exhibiting the best balance efficacy/tolerance. As FLA infections are scarce, because misdiagnosed, the knowledge of the balance benefit/risk of these drug combinations needs several more years of careful clinical investigations. Therefore, it is necessary to develop animal models to evaluate the most promising drug combinations used in clinics. Nonetheless, in vitro studies are required to evaluate drug interaction in FLA leading to synergy, additive effect or antagonism, as well as the mechanism of action involved in these interactions. Moreover, new specific antiamoebal drugs need to be developed in order to treat with more accuracy and less toxicity these FLA infections.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
AT analyzed the data and wrote the first draft, ZFM edited the manuscript, PML edited the manuscript, SP analyzed the data and wrote the article.
Supporting information
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGMENTS
This work was funded by the Syndicat des Eaux d'Île‐de‐France (grant N 9655 0). The funder has no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
The authors declare that this work was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Funding information
This work was funded by the Syndicat des Eaux d'Île‐de‐France (grant N 9655 0).
REFERENCES
- 1. Balczun C, Scheid PL. Free‐living amoebae as hosts for and vectors of intracellular microorganisms with public health significance. Viruses. 2017;9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adl SM, Bass D, Lane CE, et al. Revisions to classification, nomenclature, and diversity of eukaryotes. J Eukaryot Microbiol. 2019;66:4‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marciano‐Cabral F. Biology of Naegleria spp. Microbiol Rev. 1988;52:114‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marciano‐Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16:273‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glaser CA, Gilliam S, Schnurr D, et al. In search of encephalitis etiologies: diagnostic challenges in the California Encephalitis Project, 1998–2000. Clin Infect Dis. 2003;36:731‐742. [DOI] [PubMed] [Google Scholar]
- 6. Schuster FL, Visvesvara GS. Opportunistic amoebae: challenges in prophylaxis and treatment. Drug Resist Updat. 2004;7:41‐51. [DOI] [PubMed] [Google Scholar]
- 7. Prasad R, Shah AH, Rawal MK. Antifungals: mechanism of action and drug resistance. Adv Exp Med Biol. 2016;892:327‐349. [DOI] [PubMed] [Google Scholar]
- 8. McDonald LM, Armson A, Thompson AR, Reynoldson JA. Characterization of benzimidazole binding with recombinant tubulin from Giardia duodenalis, Encephalitozoon intestinalis, and Cryptosporidium parvum. Mol Biochem Parasitol. 2004;138:89‐96. [DOI] [PubMed] [Google Scholar]
- 9. Gangjee A, Kurup S, Namjoshi A. Dihydrofolate reductase as a target for chemotherapy in parasites. Curr Pharm Des. 2007;13:609‐639. [DOI] [PubMed] [Google Scholar]
- 10. Cambau E, Guillard T. Antimicrobials that affect the synthesis and conformation of nucleic acids. Rev Sci Tech. 2012;31:77‐87. [DOI] [PubMed] [Google Scholar]
- 11. Dorlo TPC, Balasegaram M, Beijnen JH, de Vries PJ. Miltefosine: a review of its pharmacology therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother. 2012;67:2576‐2597. [DOI] [PubMed] [Google Scholar]
- 12. Hash JH. Antibiotic mechanisms. Annu Rev Pharmacol. 1972;12:35‐56. [DOI] [PubMed] [Google Scholar]
- 13. Taravaud A, Loiseau PM, Pomel S. In vitro evaluation of antimicrobial agents on Acanthamoeba sp. and evidence of a natural resilience to amphotericin B. Int J Parasitol Drugs Drug Resist. 2017;7:328‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood‐cerebrospinal fluid/blood‐brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23:858‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ondarza RN, Iturbe A, Hernandez E. In vitro antiproliferative effects of neuroleptics, antimycotics and antibiotics on the human pathogens Acanthamoeba polyphaga and Naegleria fowleri. Arch Med Res. 2006;37:723‐729. [DOI] [PubMed] [Google Scholar]
- 16. Nakaminami H, Tanuma K, Enomoto K, et al. Evaluation of in vitro antimaoebic activity of antimicrobial agents against clinical Acanthamoeba isolates. J Ocul Pharmacol Ther. 2017;33:629‐634. [DOI] [PubMed] [Google Scholar]
- 17. Iwen PC, Miller NG. Enhancement of ketoconazole penetration across the blood‐brain barrier of mice by dimethyl sulfoxide. Antimicrob Agents Chemother. 1986;30:617‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cleland PG, Lawande RV, Onyemelukwe G, Whittle HC. Chronic amoebic meningoencephalitis. Arch Neurol. 1982;39:56‐57. [DOI] [PubMed] [Google Scholar]
- 19. Monogue ML, Watson D, Alexander JS, Cavuoti D, Doyle LM, Wang MZ. Minimal cerebrospinal fluid concentration of miltefosine despite therapeutic plasma levels during the treatment of amoebic encephalitis. Antimicrob Agents Chemother. 2019;64:e01127‐e01219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McBride J, Ingram PR, Henriquez FL, Robert CW. Development of colorimetric microtiter plate assay for assessment of antimicrobials against Acanthamoeba. J Clin Microbiol. 2005;43:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schuster FL, Guglielmo BL, Visvesvara GS. In‐vitro activity of miltefosine and voriconazole on clinical isolates of free‐living amoebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol. 2006;53:121‐126. [DOI] [PubMed] [Google Scholar]
- 22. Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2008;47:303‐327. [DOI] [PubMed] [Google Scholar]
- 23. Cope JR. Investigational drug available directly from CDC for the treatment of infections with free‐living amoebae. MMWR Morb Mortal Wkly Rep. 2013;62:666. [PMC free article] [PubMed] [Google Scholar]
- 24. Singhal T, Bajpai A, Kalra V, et al. Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials. Pediatr Infect Dis. 2001;20:623‐627. [DOI] [PubMed] [Google Scholar]
- 25. Khurana S, Mewara S, Verma S, Totadri SK. Central nervous system infection with Acanthamoeba in a malnourished child. BMJ Case Rep. 2012;2012:bcr2012007449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ranjan R, Handa A, Choudhary A, Kumar S. Acanthamoeba infection in an interhemispheric ependymal cyst: a case report. Surg Neurol. 2009;72:185‐189. [DOI] [PubMed] [Google Scholar]
- 27. Saxena A, Mittal S, Burman P, Garg P. Acanthamoeba meningitis with successful outcome. Indian J Pediatr. 2009;76:1063‐1064. [DOI] [PubMed] [Google Scholar]
- 28. Das S, Gunasekaran K, Ajjampur SS, et al. Acanthamoeba encephalitis in immunocompetent hosts: a report of two cases. J Family Med Prim Care. 2020;9:1240‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gupta D, Panda GS, Bakhshi S. Successful treatment of Acanthamoeba meningoencephalitis during induction therapy of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;50:1292‐1293. [DOI] [PubMed] [Google Scholar]
- 30. Das S, Saha R, Rani M, Goyal R, Shah D, Asish JK. Central nervous system infection due to Acanthamoeba: a case series. Trop Parasitol. 2016;6:88‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Modica S, Miracco C, Cusi MG, et al. Non‐granulomatous cerebellar infection by Acanthamoeba spp. in an immunocompetent host. Infection. 2018;46:885‐889. [DOI] [PubMed] [Google Scholar]
- 32. Lackner P, Beer R, Broessner G, et al. Acute granulomatous Acanthamoeba encephalitis in an immunocompetent patient. Neurocrit Care. 2010;12:91‐94. [DOI] [PubMed] [Google Scholar]
- 33. Stevens AR, O'Dell WD. In vitro and in vivo activity of 5‐fluorocytocine on Acanthamoeba. Antimicrob Agents Chemother. 1974;6:282‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Slater CA, Sickel JZ, Visvesvara GS, Pabico RC, Gaspari AA. Brief report: successful treatment of disseminated Acanthamoeba infection in an immunocompromised patient. N Engl J Med. 1994;331:85‐87. [DOI] [PubMed] [Google Scholar]
- 35. Teknos TN, Poulin MD, Laruentano AM, Li KK. Acanthamoeba rhinosinusitis; characterization, diagnosis, and treatment. Am J Rhinol. 2000;14:387‐391. [DOI] [PubMed] [Google Scholar]
- 36. Van Hamme C, Dumont M, Delos M, Lachapelle JM. Cutaneous acanthamoebiasis in a lung transplant patient. Ann Dermatol Venereol. 2001;128:1237‐1240. [PubMed] [Google Scholar]
- 37. Migueles S, Kumar P. Primary cutaneous Acanthamoeba infection in a patient with AIDS. Clin Infect Dis. 1998;27:1547‐1548. [DOI] [PubMed] [Google Scholar]
- 38. Schuster FL, Visvesvara GS. Axenic growth and drug sensitivity studies of Balamuthia mandrillaris, an agent of amoebic meningoencephalitis in humans and other animals. J Clin Microbiol. 1996;34:385‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanderson L, Droguel M, Rodgers J, De Koning HP, Thomas SA. Pentamidine movement across the murine blood‐brain and blood‐cerebrospinal fluid barriers: effect of trypanosome infection, combination therapy, P‐glycoprotein, and multidrug resistance‐associated protein. J Pharmacol Exp Ther. 2009;329:967‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jaruratanasirikul S, Hortiwakul R, Tantisarasart T, Phuenpathom N, Tussanasunthornwong S. Distribution of azithromycin into brain tissue, cerebrospinal fluid, and aqueous humor of the eye. Antimicrob Agents Chemother. 1996;40:825‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dunnebacke TH, Schuster FL, Yagi S, Booton GC. Balamuthia mandrillaris from soil samples. Microbiology. 2004;150:2837‐2842. [DOI] [PubMed] [Google Scholar]
- 42. Roy SL, Atkins JT, Gennuso R, et al. Assessment of blood‐brain barrier penetration of miltefosine used to treat a fatal case of granulomatous amoebic encephalitis possibly caused by an unusual Balamuthia mandrillaris strain. Parasitol Res. 2015;114:4431‐4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Centers for Disease Control and Prevention . (2019)Balamuthia mandrillaris – Granulomatous Amoebic Encephalitis (GAE) treatment. https://www.cdc.gov/parasites/balamuthia/treatment.html. Accessed September 22, 2020.
- 44. Cope JR, Landa J, Nethercut H, et al. The epidemiology and clinical features of Balamuthia mandrillaris disease in the Unites States, 1974–2016. Clin Infect Dis. 2019;68:1815‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vollmer ME, Glaser C. A Balamuthia survivor. JMM Case Rep. 2016;3:e005031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lehmer LM, Ulibarri GE, Ragsdale BD, Kunkle J. Cutaneous Balamuthia mandrillaris infection as a precursor to Balamuthia amoebic encephalitis (BAE) in a healthy 84‐year‐old Californian. Dermatol Online. 2017;23:4. [PubMed] [Google Scholar]
- 47. Gupte AA, Hocevar SN, Lea AS, et al. Transmission of Balamuthia mandrillaris through solid organ transplantation: utility of organ recipient serology to guide clinical management. Am J Transplant. 2014;14:1417‐1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moriarty P, Burke C, McCrossin D, et al. Balamuthia mandrillaris encephalitis: survival of a child severe meningoencephalitis and review of the literature. J Pediatr Infect Dis Soc. 2014;3:e4‐e9. [DOI] [PubMed] [Google Scholar]
- 49. Schlessinger S, Kokko K, Fratkin J, et al. Balamuthia mandrillaris transmitted through organ transplantation—Mississippi, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1165‐1170. [PubMed] [Google Scholar]
- 50. Piper KJ, Foster H, Susanto D, Maree CL, Thornton SD, Cobbs CS. Fatal Balamuthia mandrillaris brain infection associated with improper nasal lavage. Int J Infect Dis. 2018;77:18‐22. [DOI] [PubMed] [Google Scholar]
- 51. Yohannan B, Feldman M. Fatal Balamuthia mandrillaris encephalitis. Case Rep Infect Dis. 2019;2019:9315756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim JH, Jung SY, Lee YJ, et al. Effect of therapeutic chemical agents in vitro and on experimental meningoencephalitis due to Naegleria fowleri. Antimicrob Agents Chemother. 2008;52:4010‐4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Anderson K, Jamieson A. Primary amoebic meningoencephalitis. Lancet. 1972;299:902‐903. [DOI] [PubMed] [Google Scholar]
- 54. Thong YH, Rowan‐Kelly B, Ferrante A. Treatment of experimental Naegleria meningoencephalitis with a combination of amphotericin B and rifamycin. Scand J Infect Dis. 1979;11:151‐153. [DOI] [PubMed] [Google Scholar]
- 55. Soltow SM, Brenner GM. Synergistic activities of azithromycin and amphotericin B against Naegleria fowleri in vitro and in a mouse model of primary amebic meningoencephalitis. Antimicrob Agents Chemother. 2007;51:23‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seidel JS, Harmatz P, Visvesvara GS, Cohen A, Edwards J, Turner J. Successful treatment of primary amoebic meningoencephalitis. N Engl J Med. 1982;306:346‐348. [DOI] [PubMed] [Google Scholar]
- 57. Goswick SM, Brenner GM. Activities of therapeutic agents against Naegleria fowleri in vitro and in a mouse model of primary amoebic meningoencephalitis. J Parasitol. 2003;89:837‐842. [DOI] [PubMed] [Google Scholar]
- 58. Cope JR, Ali IK. Primary amoebic meningoencephalitis: what have we learned in the last 5 years? Curr Infect Dis Rep. 2016;18:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Capewell LG, Harris AM, Yoder JS, et al. Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United Stated, 1937–2013. J Pediatr Infect Dis. 2015;4:e68‐e75. [DOI] [PubMed] [Google Scholar]
- 60. Cope JR, Conrad DA, Cohen N, et al. Use of the novel therapeutic agent miltefosine for the treatment of primary amoebic meningoencephalitis: report of 1 fatal and 1 surviving case. Clin Infect Dis. 2016;62:774‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ghanchi NK, Jamil B, Khan E, et al. Case series of Naegleria fowleri primary amoebic meningoencephalitis from Karachi, Pakistan. Am J Trop Med Hyg. 2017;97:1600‐1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dunn AL, Reed T, Stewart C, Levy RA. Nagleria fowleri that induces primary amoebic meningoencephalitis: rapid diagnosis and rare case of survival in a 12‐year‐old Caucasian girl. Lab Med. 2016;47:149‐154. [DOI] [PubMed] [Google Scholar]
- 63. Heggie TW, Küpper T. Surviving Naegleria fowleri infections: a successful case report and novel therapeutic approach. Travel Med Infect Dis. 2017;16:49‐51. [DOI] [PubMed] [Google Scholar]
- 64. Linam WM, Ahmed M, Cope JR, et al. Successful treatment of an adolescent with Naegleria fowleri amoebic meningoencephalitis. Pediatrics. 2015;135:e744‐e748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sharma A, Sharma A, Guleria S. Successful treatment of a case of primary amoebic meningoencephalitis: how important is history taking. Indian J Crit Care Med. 2015;19:126‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sood A, Chauhan S, Chandel L, Jaryal SC. Prompt diagnosis and extraordinary survival from Naegleria fowleri meningitis: a rare case report. Indian J Med Microbiol. 2014;32:193‐196. [DOI] [PubMed] [Google Scholar]
- 67. Vargas‐Zepeda J, Gomez‐Alcala AV, Vasquez‐Morales JA, Licea‐Amaya L, De Jonckheere JF, Lares‐Villa F. Successful treatment of Naegleria fowleri meningoencephalitis by using intravenous amphotericin B, fluconazole and rifampicin. Arch Med Res. 2005;36:83‐86. [DOI] [PubMed] [Google Scholar]
- 68. Poungvarin N, Jariya P. The fifth nonlethal case of primary amoebic meningoencephalitis. J Med Assoc Thai. 1991;74:112‐115. [PubMed] [Google Scholar]
- 69. Yadav D, Aneja S, Dutta R, Maheshwari A, Seth A. Youngest survivor of Naegleria meningitis. Indian J Pediatr. 2013;80:253‐254. [DOI] [PubMed] [Google Scholar]
- 70. Cetin N, Balckall D. Naegleria fowleri meningoencephalitis. Blood. 2012;119:3658. [DOI] [PubMed] [Google Scholar]
- 71. McLaughlin A, O'Gorman T. A local case of fulminant primary amoebic meningoencephalitis due to Naegleria fowleri. Rural Remote Health. 2019;19:4313. [DOI] [PubMed] [Google Scholar]
- 72. Roy SL, Metzger R, Chen JG, et al. Risk of transmission of Naegleria fowleri from solid organ transplantation. Am J Transplant. 2014;14:163‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stowe RC, Pehlivan D, Friederich KE, Lopez MA, DiCarlo SM, Boerwinkle VL. Primary amebic meningoencephalitis in children: a report of two fatal cases and review on the literature. Pediatr Neurol. 2017;70:75‐79. [DOI] [PubMed] [Google Scholar]
- 74. Tuppeny M. Primary amoebic meningoencephalitis with subsequent organ procurement: a case study. J Neurosci Nurs. 2011;43:274‐279. [DOI] [PubMed] [Google Scholar]
- 75. Centers for Disease Control and Prevention . Primary amebic meningoencephalitis – Arizona, Florida, and Texas, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:573‐577. [PubMed] [Google Scholar]
- 76. Vareechon C, Tarro T, Polanco C, Anand V, Pannaraj PS, Dien Bard J. Eight‐year‐old male with primary amebic meningoencephalitis. Open Forum. Infect Dis. 2019;6:ofz349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Saleem T, Rabbani M, Jamil B. Primary amoebic meningoencephalitis: two new cases from Pakistan. Trop Doct. 2009;39:242‐243. [DOI] [PubMed] [Google Scholar]
- 78. Shakoor S, Beg MA, Mahmood SF, et al. Primary amebic meningoencephalitis caused by Naegleria fowleri, Karachi, Pakistan. Emerg Infect Dis. 2011;17:258‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Centers for Disease Control and Prevention . Primary amebic meningoencephalitis – Georgia, 2002. MMWR Morb. Mortal. Wkly Rep. 2003;52:962‐964. [PubMed] [Google Scholar]
- 80. Stevens AR, Shulman ST, Lansen TA, Cichon MJ, Willaert E. Primary amoebic meningoencephalitis: a report of two cases and antibiotic and immunologic studies. J Infect Dis. 1981;143:193‐199. [DOI] [PubMed] [Google Scholar]
- 81. Schoeman CJ, van der Vyver AE, Visvesvara GS. Primary amebic meningo‐encephalitis in Southern Africa. J Infect. 1993;26:211‐214. [DOI] [PubMed] [Google Scholar]
- 82. Wang Q, Li J, Ji J, et al. A case of Naegleria fowleri related primary amoebic meningoencephalitis in China diagnosed by next‐generation sequencing. BMC Infect Dis. 2018;18:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen M, Ruan W, Zhang L, Hu B, Yang X. Primary amebic meningoencephalitis: a case report. Korean J Parasitol. 2019;57:291‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shamsizadeh A, Zaker N, Maraghi S, Momen AA. Primary amoebic meningoencephalitis. Pak J Med Sci. 2006;22:471‐473. [Google Scholar]
- 85. Singh SN, Patwari AK, Dutta R, Taneja N, Anand VK. Naegleria meningitis. Indian Pediatr. 1998;35:1012‐1015. [PubMed] [Google Scholar]
- 86. Brown RL. Successful treatment of primary amoebic meningoencephalitis. Arch Intern Med. 1991;151:1201‐1202. [PubMed] [Google Scholar]
- 87. Gautam PL, Sharma S, Puri S, Kumar R, Midha V, Bansal R. A rare case of survival from primary amoebic meningoencephalitis. Indian J Crit Care Med. 2012;16:34‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jain R, Prabhakar S, Modi M, Bhatia R, Sehgal R. Naegleria meningitis: a rare survival. Neurol India. 2002;50:470‐472. [PubMed] [Google Scholar]
- 89. Movahedi Z, Shokrollahi MR, Aghaali M, Heydari H. Primary amoebic meningoencephalitis in an Iranian infant. Case Rep Med. 2012;2012:782854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rai R, Singh DK, Srivastava AK, Bhargava A. Primary amoebic meningoencephalitis. Indian Pediatr. 2008;45:1004‐1005. [PubMed] [Google Scholar]
- 91. Wang A, Kay R, Poon WS, Ng HK. Successful treatment of amoebic meningoencephalitis in a Chinese living in Hong‐Kong. Clin Neurol Neurosurg. 1993;95:249‐252. [DOI] [PubMed] [Google Scholar]
- 92. Azam N, Hayat A. Primary amoebic meningo encephalitis – a case study. Pak Armed Forces Med J. 2013;63:282‐284. [Google Scholar]
- 93. Fuda FS, Sigauke E, Cavuoti D, Gander R, Kroft S, Willis MS. Primary amoebic meningoencephalitis. Lab Med. 2002;33:57‐60. [Google Scholar]
- 94. Gupta N, Bhaskar N, Duggal S, Ghalaut PS, Kundra S, Arora DR. Primary amoebic menigoencephalitis: first reported case from Rohtak, North India. Braz J Infect Dis. 2009;13:236‐237. [DOI] [PubMed] [Google Scholar]
- 95. Phu NH, Hoang Mai NT, Nghia HDT, et al. Fatal consequences of freshwater pearl diving. Lancet. 2013;381:176. [DOI] [PubMed] [Google Scholar]
- 96. Yoder JS, Straif‐Bourgeois S, Roy SL, et al. Primary amebic meningoencephalitis deaths associated with sinus irrigation using contaminated tap water. Clin Infect Dis. 2012;55:e79‐e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kaushal V, Chhina DK, Ram S, Singh G, Kaushal RK, Kumar R. Primary amoebic meningoencephalitis due to Naegleria fowleri. J Assoc Physicians India. 2008;56:459‐462. [PubMed] [Google Scholar]
- 98. Grate I Jr. Primary amoebic menigoencephalitis: a silent killer. CJEM. 2006;8:365‐369. [DOI] [PubMed] [Google Scholar]
- 99. Ghadage DP, Choure AC, Wankhade AB, Bhore AV. Opportunistic free: living amoeba now becoming a usual pathogen? Indian J Pathol Microbiol. 2017;60:601‐603. [DOI] [PubMed] [Google Scholar]
- 100. Lalitha MK, Anandi V, Srivastava A, Thomas K, Cherian AM, Chandi SM. Isolation of Acanthamoeba culbertsoni from a patient with meningitis. J Clin Microbiol. 1985;21:666‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4


