ABSTRACT
Importance
Vitamin A (or retinol) has considerable antioxidative and anti‐inflammatory attributes and it may have protective effects on the respiratory health of patients with cystic fibrosis (CF). This issue, however, remains controversial.
Objective
The purpose of the present study was to investigate the relationship between serum retinol levels (SRL) and force expiratory volume in 1 second (FEV1) in patients with CF.
Methods
All patients with pancreatic insufficiency attending the CF Department of “Agia Sofia” Children’s Hospital in Athens, Greece, aged 6 to 19 years during the 2012–2016 period, who could perform spirometry effectively, were included in the study. The impact of SRL on FEV1 was examined longitudinally and analyzed with generalized estimating equations.
Results
The study included 231 patients and 851 SRL measurements were performed. In 25 (3.2%) cases the SRL were below the 5th percentile of reference distribution; none was above the 95th percentile. The analysis showed that SRL was positively correlated with the FEV1 (P < 0.001).
Interpretation
In this sample of children and adolescents with CF, vitamin A deficiency was rare. Our results suggest a positive relationship between SRL and FEV1.
Keywords: Cystic fibrosis, Vitamin A, Retinol, Lung function
Vitamin A serum levels are correlated with pulmonary function in children and adolescents with cystic fibrosis.

INTRODUCTION
Most patients with cystic fibrosis (CF) suffer from exocrine pancreatic insufficiency (PI) with maldigestion and malabsorption of fat and proteins. Pancreatic enzyme replacement therapy can prevent steatorrhea but cannot normalize fat‐soluble vitamin absorption. As a result, patients with CF with PI must be supplemented daily with fat‐soluble vitamin preparations without, however, being always able to achieve the optimal levels. 1
Vitamin A is the generic term for all β‐ionone derivatives (other than carotenoids) that have the biological activity of all‐trans‐retinol and include retinol, retinal, and retinoic acid. 2 The terms vitamin A and retinol will hereafter be used interchangeably. Vitamin A is supplemented in all patients with CF with PI, but the recommended doses vary between centres. 3 During pulmonary exacerbations, the serum retinol levels (SRL) are reduced and they are restored after the acute inflammation is resolved. 4
In CF lung disease, the chronic bacterial infection and the resultant neutrophil dominated inflammation lead to a massive release of proteases and reactive oxygen species into the airways. Vitamin A appears to have some noticeable properties that may contribute to the amelioration of respiratory inflammation. Indeed, as it has been shown in vitro in bronchial epithelial cells, vitamin A has anti‐inflammatory properties, namely anti‐protease and anti‐oxidant activities, that protect the lung from damage. 5 , 6 Also, animal studies have illustrated that vitamin A supplementation might augment anti‐inflammatory activity. 7 , 8 So far, however, the available data remain controversial with some studies supporting a potential contributing role in lung health 9 , 10 , 11 , 12 while others have found that no such association exists. 13 , 14 , 15
In the present longitudinal study, we investigated the relationship between vitamin A status and forced expiratory volume in 1 second (FEV1) in patients with CF. In examining this relationship, we took into account various factors known to affect lung function in CF.
METHODS
Ethical approval
The study protocol was approved by the “Agia Sofia” Children’s Hospital Ethics Committee (approval number 3685/24‐05‐17). Due to the retrospective character of the study and the anonymous data analysis informed consent was not required.
Patients selection
This was part of a retrospective longitudinal observational study on the effects of the lipid‐soluble vitamins on respiratory health of patients with CF. 16 , 17 It was performed in the CF department of “Agia Sofia” Children’s Hospital in Athens, whose catchment area covers about threefourths of the country’s paediatric population with CF. Patients with PI attending the CF department, who were 6 to 19 years old during the five years of the study and could perform spirometry effectively, were eligible for inclusion. Patients with lung and/or liver transplantation were excluded to avoid creating undue heterogeneity in the study population.
All patients were diagnosed with CF based on the presence of two disease‐causing mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene in trans, and a positive (Cl ≥ 60 mEq/L) sweat test. The data were collected longitudinally and retrospectively from 2012 to 2016 and the following variables were recorded and used in the analysis: weight, height, SRL, liver disease, presence of CF‐related diabetes (CFRD), isolation of Pseudomonas aeruginosa (Pa), Staphylococcus aureus (Sa), and Stenotrophomonas maltophilia (Sm) in cultures, treatment with CFTR modulators, and values of percentpredicted forced expiratory volume in 1 second (ppFEV1). Children were considered positive for Pa, Sa, or Sm, in each calendar year of the study if these bacteria were isolated in sputum, deep throat swabs, or bronchoalveolar lavage fluid at least once in this particular year.
Concerning liver involvement, patients were allocated in 3 groups in concordance with the recently agreed phenotypic characterization, 18 namely, no liver disease, non‐cirrhotic, and cirrhotic. All measurements of SRL were performed with high‐performance liquid chromatography. Weight, height, SLR and FEV1 were measured during the annual reviews with the patients being clinically stable i.e., without exacerbation in the past month. Spirometry results were considered acceptable if there was a blunt peak in the flow‐volume loop, the expiration did not stop prematurely, and there were no interruptions during the expiratory flow. 19 The ppFEV1 was calculated with the National Health and Nutrition Examination Survey (NHANES) III reference equations. We used the 5th and 95th percentile of the SRL distribution in healthy populations 20 to define the lower and upper limits of normal (32.5 μg/dL and 87.8 μg/dL, respectively).
Subjects were also characterized by predicted CFTR function as being either “residual” namely, carrying at least one CFTR mutation with partial function (class IV or V), or “minimal” namely, those who did not carry a class IV or V mutation. 21 , 22 Calculation of body mass index (BMI) z‐score was based on the growth reference data provided by the World Health Organization. 23
Statistical analysis
The distribution of all continuous variables was checked with the Shapiro‐Wilks test. Variables that deviated from normality were presented as median with interquartile range (IQR). To examine the univariate correlation between SRL and ppFEV1 we performed the Spearman’s rank test and used the corresponding correlation coefficient (r). The impact of SRL on ppFEV1 was examined with a linear generalized estimating equations model (GEE) where repeated observations on patients with CF were modelled over time. We controlled for BMI z‐score, liver involvement, CFRD, isolation of Pa, Sa, and Sm in cultures, and treatment with CFTR modulators. The 3 liver involvement groups were combined in a single ordered variable with 3 levels. Treatment with CFTR modulators was considered a categorical, non‐ordered variable with 3 levels, namely no treatment, treatment with ivacaftor, and treatment with ivacaftor/lumacaftor combination. To ensure that the GEE model could handle the missing data appropriately, we used the Little’s test for data missing completely at random (MCAR). 24 Stata 13 (StataCorp, College Station, TX) was used for the data analysis.
RESULTS
The overall number of patients 6 to 19 years old attending the CF department during the study period was 231 and were all included in the study. Patients characteristics are presented in Table 1. All subjects belonged to the “minimal” predicted CFTR function category. More specifically, 92 (39.8%) patients were homozygous for F508del‐CFTR, 70 (30.3%) were compound heterozygous for F508del with another mutation, and 69 (29.8%) were compound heterozygous for various class I or II or III mutations.
TABLE 1.
Patients’ characteristics (n = 231)
| Parameters | Characteristics |
|---|---|
| Age at entry into the study (years) | 11 (7, 15) |
| Male | 107 (46.3) |
| Presence of CFRD | 119 (3.9) |
| Vitamin A serum levels (μg/dL) | 37.0 (29.0, 43.0) |
| BMI z‐score | 0.4 (−0.4, 1.1) |
| ppFEV1 | 97.0 (81.7, 107.2) |
| Liver involvement | |
| No involvement | 189 (81.8) |
| Non‐cirrhotic | 36 (15.6) |
| Cirrhosis | 6 (2.6) |
| Patients with CFTR modulators use | |
| Ivacaftor | 4 (1.7) |
| Lumacaftor/Ivacaftor | 23 (10.0) |
Data are shown as median (interquartile range) or n (%). CFRD, cystic fibrosis related diabetes; BMI, body mass index; ppFEV1, percent‐predicted forced expiratory volume in 1 second.
However, not all patients had 5 yearly follow‐ups; in 136 patients either one or more of their annual review appointments was missed or were not six years old at the beginning of the study. In total 896 annual estimations were performed though in 45 (5.0%) of them the SRL were not measured for technical reasons. Also, in 92 (11.0%) cases, the spirometric flow‐volume loops were not acceptable and their corresponding ppFEV1 values were considered as missing in the analysis. The distribution of SRL was skewed to the right (P < 0.001). In 25 (3.2%) cases the SRL were below the 5th percentile; none was above the 95th percentile.
Little’s test suggested that the missing values in our analysis were MCAR (P > 0.05) and could be handled efficiently by the GEE models.
Univariate analysis showed that SRL were weakly but significantly associated with ppFEV (r = 0.31, P < 0.001). The longitudinal analysis with the GEE model also showed that SRL was positively correlated with the ppFEV1 (beta coefficient: 0.39, 95% Confidence Interval: 0.24–0.54, P < 0.001) (Figure 1). This result implies that for each 1 μg/dL increase in SRL the mean increase in ppFEV1 is 0.39.
FIGURE 1.
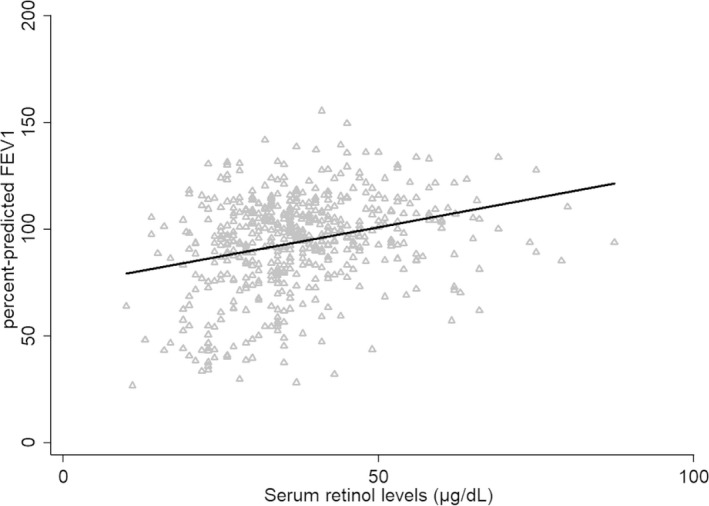
Graphic representation of the correlation between the SRL and ppFEV1 as predicted by the GEE model. The triangles represent measured values. SRL, serum retinol levels; ppFEV1, percent‐predicted forced expiratory volume in 1 second; GEE, generalized estimating equations.
DISCUSSION
In the current study, we found that vitamin A serum levels were correlated with pulmonary function. There were no measurements with abnormally high SRL and only a small proportion were below the low normal levels. The use of the 5th percentile to define hypovitaminosis A was dictated by a statistical and not biological reasoning and we cannot support that this value is the optimum cutoff point to determine low SRL in patients with CF.
Many authors have addressed the association between SRL and pulmonary function with contradictory results. In a small study of 38 patients with CF (6.1–25.2 years old), all of whom were in stable clinical condition, a significant correlation between SRL and ppFEV1 was observed. 9 In another cross‐sectional study that included 98 young patients with CF (6.8–22.3 years old) without pulmonary exacerbation, the authors found a positive relationship between ppFEV1 and SRL. 10 In two other studies, the relationship between the SRL and ppFEV1 was examined univariably, namely, without controlling for any confounding factors. The first study, which included 312 patients with CF aged from 6 months to 45 years, found a significant positive relationship between SRL and ppFEV1, 11 whereas the second one that was performed in 32 patients with CF aged from 4.3 to 27.3 years old, did not find any correlation. 14
In a more recent and more comprehensive study from Holland, authors investigated longitudinally the relationship between SRL and ppFEV1 in 228 children and adolescents with CF. Serum IgG was also measured and used as an indicator of chronic inflammation in the analysis. The authors found no association between ppFEV1 and SRL. 13 The discrepancy with our results is probably due to the different design of the two studies. Specifically, we did not take into account any marker of inflammation but, on the contrary, we controlled for many confounding factors being able to affect pulmonary function in patients with CF. Also, it has to be noted that SRL and serum IgG levels were both used as predictor covariates in their analysis. However, these two variables are not independent, as the same group has shown, 25 due to their involvement in the inflammatory process.
SRL are associated with the degree of inflammation. Actually, SRL are depressed and may even reach the deficient range in acute pulmonary exacerbations of CF; they increase spontaneously after recovery. 11 , 15 Reduced SRL, even if they are in the normal range, are associated with an increased risk of pulmonary exacerbation in CF although it is not known if it is the low SRL that predispose to exacerbations or it is the imminent exacerbation that has commenced to consume vitamin A. 12 Our results showed an association between vitamin A and pulmonary function in CF but unfortunately, that could not prove a cause and effect relationship and illuminate the nature of the underlying pathophysiology. In view of the well‐known relationship between inflammation and lung function, 26 we believe that our results reflect the involvement of vitamin A in the inflammatory process.
Our study has certain limitation. First of all, the data were collected retrospectively and came from a single centre which limits the generalizability of the results. There are quite a few missing values of SLR but because this was not the result of a systematic procedure it is unlikely to have introduced bias in our results. We avoided the pitfall of having SRL measurements during acute exacerbation since SRL were measured when patients were clinically stable. However, as acute pulmonary exacerbation in CF is a non‐well‐defined term, we cannot comprehensively rule out that some of our patients may have been experiencing a subtle form of exacerbation. Finally, we have not included markers of bronchial inflammation in our analysis nor we accounted for other respiratory microorganisms that might relate to disease severity, such as Achromobacter xylosoxidans or nontuberculous mycobacteria.
In conclusion, this study suggests that vitamin A status is associated with lung health in patients with CF and therefore serious consideration should always be given to the optimization of the SRL especially in patients with impaired lung function.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
Thank you to Tzeni Troupi and Kimon Sfingos for performing the spirometry tests.
Loukou I, Moustaki M, Sardeli O, Plyta M, Katsagoni CN, Douros K. Association of vitamin A status with lung function in children and adolescents with cystic fibrosis. Pediatr Invest. 2021;5:125‐129. 10.1002/ped4.12270
REFERENCES
- 1. Lancellotti L, D’Orazio C, Mastella G, Mazzi G, Lippi U. Deficiency of vitamins E and A in cystic fibrosis is independent of pancreatic function and current enzyme and vitamin supplementation. Eur J Pediatr. 1996;155:281–285. [DOI] [PubMed] [Google Scholar]
- 2. Bendich A, Langseth L. Safety of vitamin A. Am J Clin Nutr. 1989;49:358–371. [DOI] [PubMed] [Google Scholar]
- 3. de Vries JJ , Chang AB, Bonifant CM, Shevill E, Marchant JM. Vitamin A and beta (β)‐carotene supplementation for cystic fibrosis. Cochrane Database of Syst Rev. 2018;8:CD006751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greer RM, Buntain HM, Lewindon PJ, Wainwright CE, Potter JM, Wong JC, et al. Vitamin A levels in patients with CF are influenced by the inflammatory response. J Cyst Fibros. 2004;3:143–149. [DOI] [PubMed] [Google Scholar]
- 5. Nakajoh M, Fukushima T, Suzuki T, Yamaya M, Nakayama K, Sekizawa K, et al. Retinoic acid inhibits elastase‐induced injury in human lung epithelial cell lines. Am J Respir Cell Mol Biol. 2003;28:296–304. [DOI] [PubMed] [Google Scholar]
- 6. Nabeyrat E, Corroyer S, Besnard V, Cazals‐Laville V, Bourbon J, Clement A. Retinoic acid protects against hyperoxia‐mediated cell‐cycle arrest of lung alveolar epithelial cells by preserving late G1 cyclin activities. Am J Respir Cell Mol Biol. 2001;25:507–514. [DOI] [PubMed] [Google Scholar]
- 7. Redlich CA, Rockwell S, Chung JS, Sikora AG, Kelley M, Mayne ST. Vitamin A inhibits radiation‐induced pneumonitis in rats. J Nutr. 1998;128:1661–1664. [DOI] [PubMed] [Google Scholar]
- 8. Swamidas GP, Basaraba RJ, Baybutt RC. Dietary retinol inhibits inflammatory responses of rats treated with monocrotaline. J Nutr. 1999;129:1285–1290. [DOI] [PubMed] [Google Scholar]
- 9. Aird FK, Greene SA, Ogston SA, Macdonald TM, Mukhopadhyay S. Vitamin A and lung function in CF. J Cyst Fibros. 2006;5:129–131. [DOI] [PubMed] [Google Scholar]
- 10. Rivas‐Crespo MF, González Jiménez D, Acuña Quirós MD, Sojo Aguirre A, Heredia González S, Díaz Martín JJ, et al. High serum retinol and lung function in young patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2013;56:657–662. [DOI] [PubMed] [Google Scholar]
- 11. Lagrange‐Puget M, Durieu I, Ecochard R, Abbas‐Chorfa F, Drai J, Steghens JP, et al. Longitudinal study of oxidative status in 312 cystic fibrosis patients in stable state and during bronchial exacerbation. Pediatr Pulmonol. 2004;38:43–49. [DOI] [PubMed] [Google Scholar]
- 12. Hakim F, Kerem E, Rivlin J, Bentur L, Stankiewicz H, Bdolach‐Abram T, et al. Vitamins A and E and pulmonary exacerbations in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2007;45:347–353. [DOI] [PubMed] [Google Scholar]
- 13. Woestenenk JW, Broos N, Stellato RK, Arets HG, van der Ent CK , Houwen RH. Serum retinol levels and pulmonary function in children and adolescents with cystic fibrosis. J Cyst Fibros. 2015;14:392–397. [DOI] [PubMed] [Google Scholar]
- 14. Brei C, Simon A, Krawinkel MB, Naehrlich L. Individualized vitamin A supplementation for patients with cystic fibrosis. Clin Nutr. 2013;32:805–810. [DOI] [PubMed] [Google Scholar]
- 15. Duggan C, Colin AA, Agil A, Higgins L, Rifai N. Vitamin A status in acute exacerbations of cystic fibrosis. Am J Clin Nutr. 1996;64:635–639. [DOI] [PubMed] [Google Scholar]
- 16. Loukou I, Moustaki M, Sardeli O, Plyta M, Douros K. Association of vitamin D status with lung function measurements in children and adolescents with cystic fibrosis. Pediatr Pulmonol. 2020;55:1375–1380. [DOI] [PubMed] [Google Scholar]
- 17. Loukou I, Moustaki M, Sardeli O, Plyta M, Douros K. No association between alpha‐tocopherol levels with pulmonary function or exacerbations in cystic fibrosis. Acta Paediatr. 2020;109:1489–1490. [DOI] [PubMed] [Google Scholar]
- 18. Debray D, Narkewicz MR, Bodewes F, Colombo C, Housset C, de Jonge HR , et al. Cystic fibrosis‐related liver disease: research challenges and future perspectives. J Pediatr Gastroenterol Nutr. 2017;65:443–448. [DOI] [PubMed] [Google Scholar]
- 19. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Eur Respir J. 1993;6 Suppl 16:5–40. [DOI] [PubMed] [Google Scholar]
- 20. U.S. Centers for Disease Control and Prevention . National Center for Environmental Health Second national report on biochemical indicators of diet and nutrition in the US Population (2012). https://www.cdc.gov/nutritionreport/pdf/Nutrition_Book_complete508_final.pdf. Accessed March 12, 2021.
- 21. McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet. 2003;361:1671–1676. [DOI] [PubMed] [Google Scholar]
- 22. Green DM, McDougal KE, Blackman SM, Sosnay PR, Henderson LB, Naughton KM, et al. Mutations that permit residual CFTR function delay acquisition of multiple respiratory pathogens in CF patients. Respir Res. 2010;11:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Butte NF, Garza C, de Onis M . Evaluation of the feasibility of international growth standards for school‐aged children and adolescents. J Nutr. 2007;137:153–157. [DOI] [PubMed] [Google Scholar]
- 24. Li C. Little’s Test of missing completely at random. Stata J. 2013;13:795–809. [Google Scholar]
- 25. Woestenenk JW, Broos N, Stellato RK, Arets HG, van der Ent CK , Houwen RH. Vitamin A intake and serum retinol levels in children and adolescents with cystic fibrosis. Clin Nutr. 2016;35:654–659. [DOI] [PubMed] [Google Scholar]
- 26. Cantin AM, Hartl D, Konstan MW, Chmiel JF. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J Cyst Fibros. 2015;14:419–430. [DOI] [PubMed] [Google Scholar]


