Abstract
Objectives
We analysed the prevalence of M184V/I and/or K65R/E/N mutations archived in proviral DNA (pDNA) in youths with perinatal HIV, virological control and who previously carried these resistance mutations in historic plasma samples.
Methods
We included vertically HIV-infected youths/young adults aged ≥10 years in the Madrid Cohort of HIV-1 Infected Children and Adolescents, exposed to lamivudine and/or emtricitabine, with M184V/I and/or K65R/E/N in historic plasma samples, on antiretroviral therapy (ART), virologically suppressed (HIV-1 RNA <50 copies/mL), and with available PBMCs in the Spanish HIV BioBank. Genomic DNA was extracted from PBMCs and HIV-1 RT gene was amplified and sequenced for resistance testing by Stanford HIV Resistance tool.
Results
Among the 225 patients under follow-up in the study cohort, 13 (5.8%) met selection criteria, and RT sequences were recovered in 12 (92.3%) of them. All but one were Spaniards, carrying subtype B, with a median age at PBMCs sampling of 21.3 years (IQR: 15.6–23.1) with 4 years (IQR 2.1–6.5) of suppressed viral load (VL). Nine (75%) youths did not present M184V/I in pDNA after at least 1 year of viral suppression. In December 2019, the remaining three subjects carrying M184V/I in pDNA maintained suppressed viraemia, and two still used emtricitabine in ART.
Conclusions
The prevalence of resistance mutations to lamivudine and emtricitabine in pDNA in a cohort of youths perinatally infected with HIV who remain with undetectable VL, previously lamivudine and/or emtricitabine experienced, was infrequent. Our results indicate that ART including lamivudine or emtricitabine may also be safe and successful in youths with perinatal HIV with previous experience of and resistances to these drugs detected in plasma.
Introduction
Vertically HIV-infected patients who have survived and who are currently adolescents and young adults have been exposed to various ART regimens during their lifetime and treated with suboptimal ART regimens. As a consequence, they have a higher risk of selecting and accumulating resistance mutations, among others, M184V/I and/or K65R/E/N in the HIV reverse transcriptase.1,2 Treatment of these patients is often challenging; the use of combinations including multiple drugs increases drug-related toxicity and increases adherence problems, making it difficult to achieve and maintain virological suppression.
Simplification of ART improves adherence and quality of life in virologically suppressed patients, and is a key aspect in the management of the HIV-infected youth population. Dual integrase-inhibitors-based therapy with dolutegravir (DTG) plus lamivudine (3TC) can be a very attractive antiretroviral treatment strategy, showing durable efficacy, safety, low toxicity, and in a single tablet facilitating adherence. Dolutegravir/lamivudine dual therapy has been shown non-inferior to dolutegravir/tenofovir disoproxil fumarate/emtricitabine in treatment-naive adult patients and also as switch therapy.3,4 However, the development of resistance mutations is a concern, since in patients with previous treatments that have included lamivudine or emtricitabine (FTC), there could be a functional monotherapy concealed in simplification to two-drug combinations.5–7 Furthermore, patients with a long history of ART exposure, such as vertically HIV-infected adolescents who have received many regimens throughout their lives, often present resistance mutations to these drugs (M184V/I and/or K65R/E/N),1,2 and it is unknown whether these simplification strategies are safe in patients with many lines of treatment and previous failures, e.g. youths with perinatal HIV. Some studies in adults have found that M184V/I, as a unique resistance mutation, did not increase the risk of virological failure in an initial follow-up period in well-controlled patients who switched to a regimen of the dual combination of dolutegravir/lamivudine.8,9 There are, however, no studies performed in vertically HIV-infected patients.
The objective of our study was to determine the prevalence of resistance mutations to lamivudine and emtricitabine in proviral DNA (pDNA) in youths with perinatal HIV under ART who were lamivudine and/or emtricitabine experienced, virologically suppressed for at least 1 year before sampling and carrying M184V/I and/or K65R/E/N in their historic plasma genotypes. We also reported their virological situation at 31 December 2019.
Patients and methods
Ethics
The study was conducted according to the Declaration of Helsinki and was approved by the Clinical Research Ethical Committee of the Hospital Universitario Gregorio Marañón (06/2019). Written informed consent was obtained from all participants, as well as from the parents/guardians of children <12 years old.
Study population
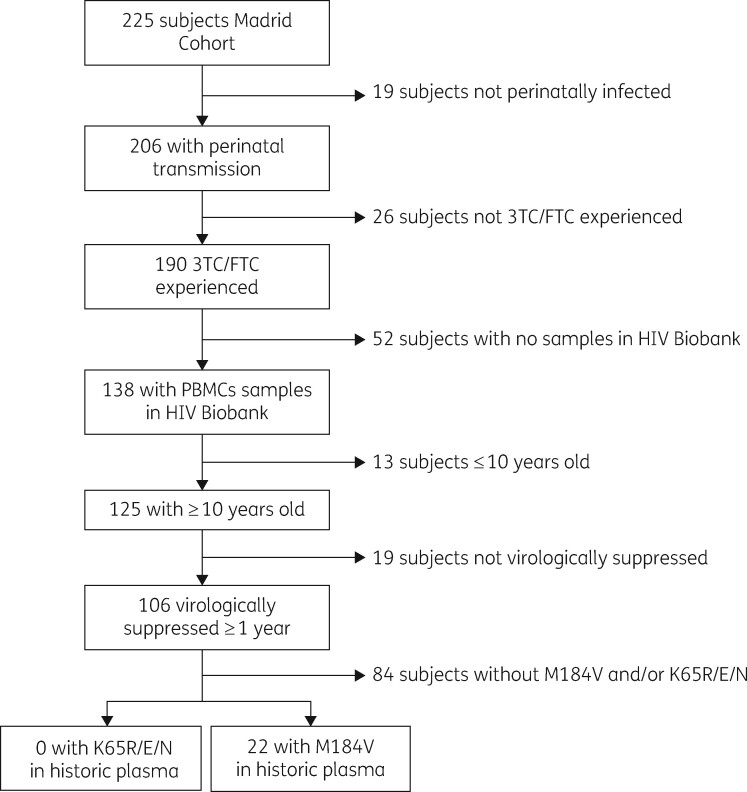
The study was performed within Madrid Cohort of HIV-1-Infected Children and Adolescents, belonging to the Paediatric Cohort of the Spanish National AIDS Network (CoRISpe), which actively collaborates with the Spanish HIV BioBank.10,11 Inclusion criteria included vertically HIV-infected youths and young adults aged 10 years and older at patient selection time, under ART and virologically suppressed with HIV-1 RNA <50 copies/mL for ≥1 year before sampling, lamivudine-and/or emtricitabine-experienced, and carrying M184V/I and/or K65R/E/N in their historic plasma samples, as indicated in Figure 1. We considered three timepoints: point 1 referred to the collection time of historic plasma carrying M184V; point PBMCs referred to PBMCs sampling time; and 31 December 2019 was the last evaluation. Clinical, epidemiological, immunological and virological data, and all available resistance genotypes were collected for each patient.
Figure 1.
Flow chart of patients included in the study.
Nucleic acid extraction and HIV-1 RT amplification
To analyse the prevalence of resistance changes M184V/I and/or K65R/E/N to lamivudine and emtricitabine in pDNA of study population, genomic DNA was extracted from available PBMCs obtained from the Spanish HIV BioBank using QIAamp® DNA Blood Mini Kit (QIAGEN) according to manufacturer’s protocol. Partial reverse transcriptase (RT, codons 1–335) from HIV-1 pol was amplified by RT-PCR and nested-PCR from extracted DNA using primers designed by the WHO (https://www.who.int/hiv/pub/drugresistance/dried_blood_spots/en/). PCR amplicons were purified using the Illustra™ ExoProStar 1-Step™ (GE Healthcare Life Sciences, UK) and sequenced by Macrogen Inc. (Geumchun-gu, Korea) to obtain consensus RT sequences. The presence of M184V/I and K65R/E/N was analysed by the HIVdb Program Genotypic Resistance Interpretation Algorithm v8.9-1 (Stanford University, USA). Genotyping testing in plasma was performed under similar conditions during routine clinical assessment of patients by using Viroseq HIV-1 Genotyping (Abbott).
Statistical analysis
Categorical variables were expressed as counts and proportions and comparisons between them were assessed using the Fisher test. Quantitative variables were reported by medians and IQR and compared using the Mann–Whitney U test. All analyses were performed by using GraphPad Prism version 8.0.1 (San Diego, California, USA). Two-sided P values of <0.05 were considered statistically significant.
Results
Among the 225 patients under follow-up in the Madrid study cohort at study time, 22 (9.8%) met the inclusion criteria. Five males and four females, all born in Spain except three patients who came from sub-Saharan Africa, North Africa and Latin America, were excluded because they did not have available PBMCs in the Spanish HIV BioBank. Thirteen patients (59.1%) had available PBMCs. We were able to amplify HIV and obtain the consensus RT sequence from pDNA in 12 (92.3%) of these 13 patients (Table 1). Among them, five were males (P4, P6, P8, P9, P10) and seven were females (P1, P2, P3, P5, P7, P11, P12). Most were born from Spanish parents and carried HIV-1 subtype B, except one (P12) born to sub-Saharan parents with CRF02_AG recombinant. Only one patient had AIDS stage (P9). All carried M184V mutation in their historic plasma (point 1 in Table 1), but no K65R/N/E mutations. The median time between the start of the lamivudine/emtricitabine regimen and the first detection of historical M184V mutation in plasma was 3.8 years (IQR: 1.8–6.1). At PBMC sampling 9 out of 12 subjects were taking regimens including lamivudine or emtricitabine, and 2 out of 9 carried M184V in pDNA. The subjects’ median age was 21.3 years (IQR: 15.6–23.1), with a median of 906 CD4 T lymphocytes (IQR: 617–1212) and all presented suppressed viral load (VL). PBMCs were collected between 2010 and 2018, after a median of 8 years (IQR: 4.6–10.9) from first M184V detection in plasma genotype and after a median of 4 years (IQR: 2.1–6.5) of virological suppression. At last evaluation (31 December 2019) all patients had VL <50 copies/mL, with a median time of 7.2 years (IQR: 6.1–8.6) of undetectable VL from PBMCs sampling. None of the 12 patients presented K65R/N/E in their pDNA. Nine (75%) subjects did not present M184V/I in pDNA after at least 1 year of viral suppression.
Table 1.
Main clinical features of the 12 vertically HIV-infected patients included in the study
| With M184V in pDNA |
Without M184V in pDNA |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P value | |
| Timepoint PBMCs | |||||||||||||
| Time on ART | 17.2 | 14.3 | 12.4 | 9.8 | 18.8 | 14.7 | 8.8 | 13.7 | 13.8 | 17.9 | 13.5 | 10.8 | 0.7 |
| Time from point 1 | 11 | 5.1 | 1.5 | 8.8 | 7.6 | 1.8 | 8.3 | 13.4 | 2.9 | 12.1 | 6.6 | 10.8 | 0.5 |
| Time with undetectable VL since point 1 | 10.2 | 5.1 | 1.5 | 6.2 | 7.5 | 1.8 | 2.1 | 3 | 1.9 | 3.6 | 4.4 | 9 | 0.9 |
| Total time with 3TC/FTC exposure | 6.6 | 6.9 | 7.5 | 1.1 | 15 | 6.8 | 5.9 | 6.7 | 12.9 | 10.2 | 12 | 10.8 | 0.6 |
| Time with 3TC/FTC exposure before point 1 | 6.2 | 1.8 | 6.1 | 1.1 | 7.4 | 5.1 | 2.4 | 2.3 | 10.7 | 1.8 | 5.8 | 0 | 0.6 |
| Time with 3TC/FTC exposure since point 1 | 0.4 | 5.1 | 1.4 | 0 | 7.6 | 1.7 | 3.5 | 4.4 | 2.2 | 8.4 | 6.2 | 10.8 | 0.3 |
| ART regimen | RPV/DTG | FTC/TDF/ EFV | FTC/TDF/ EFV | ZDV/ABC/ LPV/r | FTC/TDF/ DRV/r | FTC/TDF/ DRV/r | 3TC/ABC/ ATV/r | FTC/TDF/ DRV/r | 3TC/ABC/ EFV | DRV/r | FTC/TDF/ LPV/r | 3TC/ABC EFV | – |
| Duration ART regimen | 3.5 | 2.5 | 1.1 | 3.7 | 3.8 | 1.7 | 3.6 | 3.1 | 2.1 | 2.8 | 4.6 | 1.2 | 0.4 |
| 31 December 2019 | |||||||||||||
| Time from PBMC sampling | 1.2 | 8.5 | 9.2 | 7.5 | 5 | 8.2 | 8.8 | 6.5 | 6.8 | 6.7 | 9.2 | 1.7 | 0.7 |
| ART regimen | RPV/DTG | FTC/TAF/ RPV | FTC/TAF/ RPV | 3TC/ABC/ DTG | 3TC/ABC/ DTG | 3TC/ABC/ DTG | 3TC/ABC/ DTG | 3TC/ABC/ DTG | 3TC/DRV/c | DRV/c | DRV/r DTG | 3TC/ABC/ EFV | – |
All times and ages are expressed in years. Point 1 refers to collection time of historic plasma carrying M184V. Timepoint PBMCs refers to PBMCs sampling time. 31 December 2019, last evaluation; total time 3TC/FTC exposure refers to cumulative exposure to 3TC/FTC. P, patient; ART, antiretroviral therapy. VL, viral load (RNA-HIV-1 copies/mL); pDNA, proviral DNA. 3TC, lamivudine; FTC, emtricitabine; ABC, abacavir; RPV, rilpivirine; TDF, tenofovir disoproxil fumarate; TAF, tenofovir alafenamide; EFV, efavirenz; ZDV, zidovudine; LPV/r, lopinavir/ritonavir; DRV/r, darunavir/ritonavir; ATV/r, atazanavir/ritonavir; DRV/c, darunavir/cobicistat; DTG, dolutegravir.
No statistically significant differences were found between patients with or without M184V in pDNA (Table 1). Youths with and without M184V at pDNA showed similar time (median years) under ART to PBMCs collection [14.3 (IQR: 13.4–15.8) versus 13.7 (IQR: 10.8–14.7), P = 0.7]. However, youths with M184V tended to have more time with undetectable VL since M184V appearance in the last positive historical genotype in plasma to PBMCs sampling [5.1 (IQR: 3.3–7.7) versus 3.6 (IQR: 2.1–6.2), P = 0.9] and tended to have greater time under lamivudine or emtricitabine exposure before historical genotype in plasma [6.1 (IQR: 4–6.2) versus 2.4 (IQR: 1.8–5.8), P = 0.6] but lower time under lamivudine or emtricitabine from the first M184V detection in plasma to PBMCs sampling [1.4 (IQR: 0.9–3.3) versus 4.4 (IQR: 2.2–7.6), P = 0.3].
Discussion
This is the first study analysing the existence of lamivudine- or emtricitabine resistance-conferring mutations in pDNA (M184V/I and/or K65R/E/N) in historic plasma genotypes in a cohort of vertically HIV-infected patients under ART after at least 1 year of viraemia suppression. We observed long viral suppression [4 years median (IQR: 2.1–6.5)] in the study patients with historic resistance to lamivudine in plasma, as previously reported in adult cohorts.8,9 We also emphasize the absence of detection of M184V by population sequencing of PBMCs after years of complete virological suppression. Only 3/12 patients (25%) with pDNA sequence under study presented the M184V mutation in their pDNA and none presented K65R/E/N.
Previous studies have suggested that a prolonged time with undetectable VL is associated with clearance of M184V in PBMCs in some adult cohorts.4,12,13 However, the nine youths in our study without M184V in their pDNA presented lower time under undetectable VL than those with M184V. We also observed that these three youths had more time exposed to lamivudine/emtricitabine before the first detection of M184V in their historic plasma, but less time exposed to these drugs from the first detection in plasma to the PBMCs sampling, compared with the nine youths without M184V in their pDNA. It may be possible that the loss of M184V in PBMCs might be associated with the longer time under lamivudine/emtricitabine exposure, although this should be confirmed in larger study cohorts.
It is of interest to confirm whether lamivudine or emtricitabine could have any role in viral replication control in youths with perinatal HIV with previously documented resistance to these drugs in their historical genotype in plasma, since 9 (75%) out of 12 patients were still using lamivudine or emtricitabine in their ART regimen on 31 December 2019, maintaining undetectable VL. A previous study showed an increase in VL after discontinuing lamivudine despite the presence of the M184V mutation in plasma, suggesting a residual antiviral effect of this drug.14 Other studies conducted in HIV-infected adults have shown that lamivudine remained effective for virological suppression despite the existence of documented genotypic mutations.15,16
Studies in adults have gone a step further and have used dual therapies based on lamivudine and an integrase inhibitor as suppressive simplification regimens in patients with archived mutations in the historical genotype. In a pilot clinical trial (ART-PRO) performed in an HIV-infected adult cohort, dolutegravir/lamivudine demonstrated efficacy for maintenance of HIV viral suppression at 48 weeks in 21 patients with archived lamivudine-resistant viruses present in pDNA at low frequency, since they were detected by next generation sequencing, but not by population sequencing. However, some patients included in ART-PRO had a low minority of M184V detectable by ultra-deep sequencing, although the long-term clinical impact of this low burden of archived resistance is unknown and deserves further study.8,9
ART switching is a therapeutic strategy used to improve quality of life and avoid toxicity in patients under ART. Some publications have showed that lamivudine/emtricitabine-based regimens might work in the presence of M184V in historical Sanger baseline sequences. In the MOBIDIP cohort, dual maintenance therapy with boosted protease inhibitor plus lamivudine was associated with a high rate of success, despite the presence of M184V at first-line treatment failure. However, boosted protease inhibitor monotherapy should not be recommended for these patients.17 Other studies showed that switch regimens, as bictegravir/emtricitabine/tenofovir alafenamide and abacavir/lamivudine/dolutegravir triple therapy and ART-PRO for dual therapy, were successful in the absence of historical M184V in baseline PBMCs.18,19 The choice of ART in the clinic has always been based on the study of genotype mutations by Sanger sequencing. Some studies have showed that choosing treatment based on ultradeep mutations has a limited impact on virological outcomes.20 We do not know whether in perinatally infected youths with accumulated resistances and in whom adherence is sometimes compromised, treatment based on PBMCs resistance may be helpful or not, compromising the success of virological control and future treatments in this vulnerable population. In clinical practice, to improve adherence and to avoid toxicity, there are studies showing that switching to a dual therapy strategy is effective for maintaining viral suppression in this population.21 In our study cohort, it can be seen that previously archived mutations such as M184V are lost over time and after virological suppression. pDNA measurement may then be a useful decision-making tool for switching to simpler and less-toxic treatment regimens.
Our study has some limitations. The first one was the sample size, since only 12 subjects met all the inclusion criteria for the study due to PBMCs availability. Secondly, we used historical plasma genotypes and PBMCs samples in the study. Frozen samples might just by chance have PBMCs that do not contain archived mutations, in contrast with plasma genotyping, performed in the setting of viral replication. Thirdly, only one patient switched to a lamivudine/emtricitabine dual regimen, and none switched to dolutegravir/lamivudine/emtricitabine. However, our study, together with the one published in adult cohorts on this same topic, may open the possibility of evaluating the use of regimens that include lamivudine or emtricitabine, especially dolutegravir-based regimens, in the treatment of vertically HIV-1-infected patients with a long period of infection and previous experience of and genotypic resistance to lamivudine/emtricitabine.
To sum up, in the study cohort, most (9/12) vertically HIV-infected youths with previous M184-bearing resistant viruses in plasma, did not present this mutation in pDNA after a long period of virological suppression. These results suggest that measurement of pDNA may be useful to detect the presence of M184V/I mutation in vertically HIV-infected youths with this mutation previously detected in plasma. Our results also suggest that pDNA measurement may also be useful to select possible simplification therapies including lamivudine or emtricitabine in vertically HIV-infected patients with previous experience of and resistance to these drugs.
Acknowledgements
We thank all patients and their families for their participation in paediatric research or in CoRISpe, to all professionals participating in CoRISpe and CoRISpe-FARO Cohorts and to the Paediatric HIV BioBank integrated in the Spanish AIDS Research Network (RIS) and collaborating centres, for the generous gifts of clinical samples used in this work. We also thank Paul Devlin for his English editing of manuscript.
Members of the paediatric cohort of the Spanish national AIDS network (CoRISpe and CoRISpe-Faro)
CoRISpe Cohort Working Group: María José Mellado, Luis Escosa, Milagros García Hortelano, Talía Sainz (Hospital Universitario La Paz, Madrid); Pablo Rojo, Luis Prieto-Tato, Cristina Epalza (Hospital Universitario Doce de Octubre, Madrid); José Tomás Ramos, Marta Illán (Hospital Clínico San Carlos, Madrid); Sara Guillén (Hospital Universitario de Getafe, Madrid); María Luisa Navarro, Jesús Saavedra, Mar Santos, Begoña Santiago, Santiago Jimenez de Ory, Itzíar Carrasco, Arantxa Berzosa, David Aguilera, Maria Angeles Muñoz-Fernández (Hospital Universitario Gregorio Marañón, Madrid); Miguel Ángel Roa (Hospital Universitario de Móstoles, Madrid); María Penín (Hospital Universitario Príncipe de Asturias de Alcalá de Henares, Madrid); Jorge Martínez (Hospital Infantil Universitario Niño Jesús, Madrid); Katie Badillo (Hospital Universitario de Torrejón, Madrid); Ana Belén Jiménez (Hospital Fundación Jiménez Díaz, Madrid); Adriana Navas (Hospital Universitario Infanta Leonor, Madrid); Eider Oñate (Hospital Universitario Donostia, Guipúzcoa); Itziar Pocheville (Hospital Universitario Cruces, Vizcaya); Elisa Garrote (Hospital Universitario Basurto, Vizcaya); Elena Colino (Hospital Insular Materno Infantil, Gran Canaria); Jorge Gómez Sirvent (Hospital Universitario Virgen de la Candelaria, Tenerife); Mónica Garzón, Vicente Román (Hospital General, Lanzarote); Raquel Angulo (Hospital de Poniente de El Ejido, Almería); Olaf Neth, Lola Falcón (Hospital Universitario Virgen del Rocío, Sevilla); Pedro Terol (Hospital Universitario Virgen de la Macarena, Sevilla); Juan Luis Santos, Álvaro Vázquez (Hospital Universitario Virgen de las Nieves, Granada); David Moreno (Hospital Regional Universitario Carlos Haya, Málaga); Francisco Lendínez (Complejo Hospitalario Torrecárdenas, Almería); Estrella Peromingo (Hospital Universitario Puerta del Mar, Cádiz); Beatriz Ruiz (Hospital Universitario Reina Sofía de Córdoba); Ana Grande (Complejo Hospitalario Universitario Infanta Cristina, Badajoz); Francisco José Romero (Complejo Hospitalario, Cáceres); Carlos Pérez (Hospital de Cabueñes, Asturias); Miguel Lillo (Complejo Hospitalario Universitario, Albacete); Begoña Losada (Hospital Virgen de la Salud, Toledo); Mercedes Herranz (Hospital Virgen del Camino, Navarra); Matilde Bustillo, Miguel Lafuente (Hospital Universitario Miguel Servet, Zaragoza); Pilar Collado (Hospital Clínico Universitario Lozano Blesa, Zaragoza); José Antonio Couceiro (Complejo Hospitalario Universitario, Pontevedra); Leticia Vila (Complejo Hospitalario Universitario, La Coruña); Consuelo Calviño (Hospital Universitario Lucus Augusti, Lugo); Ana Isabel Piqueras, Manuel Oltra (Hospital Universitario La Fe, Valencia); César Gavilán (Hospital Universitario de San Juan de Alicante, Alicante); Elena Montesinos (Hospital General Universitario, Valencia); Marta Dapena (Hospital General, Castellón); Cristina Álvarez, Beatriz Jiménez (Hospital Universitario Marqués de Valdecilla, Cantabria); Ana Gloria Andrés (Complejo Hospitalario, León); Víctor Marugán, Carlos Ochoa (Complejo Hospitalario, Zamora); Ana Isabel Menasalvas, Eloísa Cervantes (Hospital Universitario Virgen de la Arrixaca, Murcia); Pere Soler-Palacín, Maria Antoinette Frick (Hospital Universitari Materno Infantil Vall d’ Hebron, Barcelona); Antonio Mur, Nuria Lopez (Hospital Universitari del Mar, Barcelona); María Mendez (Hospital Universitari Germans Trias i Pujol, Barcelona); Lluıs Mayol (Hospital Universitari Josep Trueta, Girona); Teresa Vallmanya (Hospital Universitari Arnau de Vilanova, Lleida); Olga Calavia (Hospital Universitari Joan XXIII, Tarragona); Lourdes García (Consorci Sanitari del Maresme de Mataro, Barcelona), María Teresa Coll (Hospital General de Granollers, Barcelona); Valentí Pineda (Corporacio Sanitària Parc Taulı de Sabadell, Barcelona); Neus Rius (Hospital Universitari Sant Joan de Reus, Tarragona); Joaquín Dueñas (Hospital Universitari Son Espases, Mallorca); Clàudia Fortuny, Antoni Noguera-Julian (Hospital Sant Joan de Deu de Esplugues de Llobregat, Barcelona). CoRISpe-FARO Cohort Working Group: Ignacio Bernardino, María Luisa Montes, Eulalia Valencia (Hospital Universitario La Paz, Madrid); Rafael Rubio, Federico Pulido, Otilia Bisbal (Hospital Universitario Doce de Octubre, Madrid); Gabriel Gaspar Alonso (Hospital Universitario de Getafe, Madrid); Juan Berenguer, Cristina Díez, Teresa Aldamiz, Pedro Montilla, Elena Bermúdez, Maricela Valerio (Hospital Universitario Gregorio Marañón, Madrid); José Sanz (Hospital Universitario Príncipe de Asturias de Alcalá de Henares, Madrid); Sari Arponen, Alejandra Gimeno (Hospital Universitario de Torrejón, Madrid); Miguel Cervero, Rafael Torres (Hospital Universitario Severo Ochoa de Leganés, Madrid); Santiago Moreno, Ma Jesús Pérez, Santos del Campo (Hospital Universitario Ramón y Cajal, Madrid); Pablo Ryan, Jesús Troya (Hospital Universitario Infanta Leonor, Madrid); Jesús Sanz (Hospital Universitario La Princesa, Madrid); Juan Losa, Rafael Gómez (Hospital Universitario Fundación Alcorcón, Madrid); Miguel Górgolas (Hospital Fundación Jiménez Díaz, Madrid); José Antonio Iribarren, Francisco Rodríguez, Lydia Pascual, María José Aramburu (Hospital Universitario Donostia, Guipúzcoa); Ane Josune Goikoetxea (Hospital Universitario Cruces, Vizcaya); Josefa Muñoz, Sofía Ibarra (Hospital Universitario Basurto, Vizcaya); Michele Hernández (Hospital Universitario Insular, Gran Canaria); Juan Luis Gómez Sirvent, Jehovana Rodríguez (Hospital Universitario de Canarias, Tenerife); Miguel Ángel Cárdenes (Hospital Universitario Doctor Negrín, Gran Canaria); Luis Fernando López-Cortés, Cristina Roca, Silvia Llaves (Hospital Universitario Virgen del Rocío, Sevilla); María José Ríos, Jesús Rodríguez, Virginia Palomo (Hospital Universitario Virgen de la Macarena, Sevilla); Juan Pasquau, Coral García (Hospital Universitario Virgen de las Nieves, Granada); José Hernández, Clara Martínez (Hospital Universitario Clínico San Cecilio, Granada); Antonio Rivero, Ángela Camacho (Hospital Universitario Reina Sofía, Córdoba); Dolores Merino, Laura Corpa (Hospital Universitario Juan Ramón Jiménez, Huelva); Elisa Martínez, Fernando Mateos, José Javier Blanch (Complejo Hospitalario Universitario, Albacete); Miguel Torralba (Hospital Universitario, Guadalajara); Piedad Arazo, Gloria Samperiz (Hospital Universitario Miguel Servet, Zaragoza); Celia Miralles, Antonio Ocampo, Guille Pousada (Hospital Álvaro Cunqueiro, Pontevedra); Álvaro Mena (Complejo Hospitalario Universitario, La Coruña); Marta Montero, Miguel Salavert, Iván Castro, Sandra Cuéllar (Hospital Universitario La Fe, Valencia); María José Galindo, Ramón Ferrando (Hospital Clínico Universitario, Valencia); Joaquín Portilla, Irene Portilla (Hospital General Universitario, Alicante); Félix Gutiérrez, Mar Masiá, Cati Robledano, Araceli Adsuar (Hospital General Universitario de Elche, Alicante); Carmen Hinojosa (Hospital Clínico, Valladolid); Jésica Abadía (Hospital Universitario Río Hortega, Valladolid); Carlos Galera, Helena Albendín, Marian Fernández (Hospital Universitario Virgen de la Arrixaca, Murcia); José Ramón Blanco (Complejo Hospitalario San Millán-San Pedro, la Rioja); Joaquín Burgos (Hospital Universitari Vall d’ Hebron, Barcelona); Berta Torres, Elisa de Lazzari (Hospital Clinic, Barcelona); and Paediatric HIV-BioBank integrated in the Spanish AIDS Research Network and collaborating Centers.
Funding
This study was fully supported by the Instituto de Salud Carlos III of Spain (PI19/01530 –RIS_EPICLIN_14/2017), PI15/01005–RIS_EPICLIN-02/2016) co-financed by the European Development Regional Fund (‘A Way to Achieve Europe’). The study was integrated in the research supported by the Paediatric Cohort of the Spanish National AIDS Network (CoRISpe), and by Instituto de Salud Carlos III, Spanish Health Ministry (Grant no. RD06/0025-ISCIII-FEDER). The study is also included in the ‘Subprograma de Inmigración y Salud’ from CIBERESP (Spain) and in Red de Investigación Translacional en Infectología Pediátrica (RITIP) and results also complement RIS-EPICLIN 10_2019 and RIS-EPICLIN-06/2013 projects. D.A.A. is funded by the Spanish Ministry of Health—Instituto de Salud Carlos III (ISCIII) and co-funded by the European Union (FEDER) (Contrato Río Hortega CM18/00100).
Transparency declarations
None to declare.
Contributor Information
the Paediatric Cohort of the Spanish National AIDS Network (CoRISpe and CoRISpe-FARO):
María José Mellado, Luis Escosa, Milagros García Hortelano, Talía Sainz, Pablo Rojo, Luis Prieto-Tato, Cristina Epalza, José Tomás Ramos, Marta Illán, Sara Guillén, María Luisa Navarro, Jesús Saavedra, Mar Santos, Begoña Santiago, Santiago Jimenez de Ory, Itzíar Carrasco, Arantxa Berzosa, David Aguilera, Maria Angeles Muñoz-Fernández, Miguel Ángel Roa, María Penín, Jorge Martínez, Katie Badillo, Ana Belén Jiménez, Adriana Navas, Eider Oñate, Itziar Pocheville, Elisa Garrote, Elena Colino, Jorge Gómez Sirvent, Mónica Garzón, Vicente Román, Raquel Angulo, Olaf Neth, Lola Falcón, Pedro Terol, Juan Luis Santos, Álvaro Vázquez, David Moreno, Francisco Lendínez, Estrella Peromingo, Beatriz Ruiz, Ana Grande, Francisco José Romero, Carlos Pérez, Miguel Lillo, Begoña Losada, Mercedes Herranz, Matilde Bustillo, Miguel Lafuente, Pilar Collado, José Antonio Couceiro, Leticia Vila, Consuelo Calviño, Ana Isabel Piqueras, Manuel Oltra, César Gavilán, Elena Montesinos, Marta Dapena, Cristina Álvarez, Beatriz Jiménez, Ana Gloria Andrés, Víctor Marugán, Carlos Ochoa, Ana Isabel Menasalvas, Eloísa Cervantes, Pere Soler-Palacín, Maria Antoinette Frick, Antonio Mur, Nuria Lopez, María Mendez, Lluıs Mayol, Teresa Vallmanya, Olga Calavia, Lourdes García, María Teresa Coll, Valentí Pineda, Neus Rius, Joaquín Dueñas, Clàudia Fortuny, Antoni Noguera-Julian Ignacio Bernardino, María Luisa Montes, Eulalia Valencia, Rafael Rubio, Federico Pulido, Otilia Bisbal, Gabriel Gaspar Alonso, Juan Berenguer, Cristina Díez, Teresa Aldamiz, Pedro Montilla, Elena Bermúdez, Maricela Valerio, José Sanz, Sari Arponen, Alejandra Gimeno, Miguel Cervero, Rafael Torres, Santiago Moreno, Ma Jesús Pérez, Santos del Campo, Pablo Ryan, Jesús Troya, Jesús Sanz, Juan Losa, Rafael Gómez, Miguel Górgolas, José Antonio Iribarren, Francisco Rodríguez, Lydia Pascual, María José Aramburu, Ane Josune Goikoetxea, Josefa Muñoz, Sofía Ibarra, Michele Hernández, Juan Luis Gómez Sirvent, Jehovana Rodríguez, Miguel Ángel Cárdenes, Luis Fernando López-Cortés, Cristina Roca, Silvia Llaves, María José Ríos, Jesús Rodríguez, Virginia Palomo, Juan Pasquau, Coral García, José Hernández, Clara Martínez, Antonio Rivero, Ángela Camacho, Dolores Merino, Laura Corpa, Elisa Martínez, Fernando Mateos, José Javier Blanch, Miguel Torralba, Piedad Arazo, Gloria Samperiz, Celia Miralles, Antonio Ocampo, Guille Pousada, Álvaro Mena, Marta Montero, Miguel Salavert, Iván Castro, Sandra Cuéllar, María José Galindo, Ramón Ferrando, Joaquín Portilla, Irene Portilla, Félix Gutiérrez, Mar Masiá, Cati Robledano, Araceli Adsuar, Carmen Hinojosa, Jésica Abadía, Carlos Galera, Helena Albendín, Marian Fernández, José Ramón Blanco, Joaquín Burgos, Berta Torres, and Elisa de Lazzari
References
- 1. Charpentier C, Lambert-Niclot S, Visseaux B. et al. Evolution of the K65R, K103N and M184V/I reverse transcriptase mutations in HIV-1-infected patients experiencing virological failure between 2005 and 2010. J Antimicrob Chemother 2013; 68: 2197–8. [DOI] [PubMed] [Google Scholar]
- 2. Rojas Sánchez P, de Mulder M, Fernandez-Cooke E. et al. Clinical and virologic follow-up in perinatally HIV-1-infected children and adolescents in Madrid with triple-class antiretroviral drug-resistant viruses. Clin Microbiol Infect 2015; 21: 605.e1–9. [DOI] [PubMed] [Google Scholar]
- 3. Cahn P, Madero JS, Arribas JR. et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019; 393: 143–55. [DOI] [PubMed] [Google Scholar]
- 4. van Wyk J, Ajana F, Bisshop F. et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO study. Clin Infect Dis 2020; 71: 1920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Miguel Buckley R, Montejano R, Stella-Ascariz N. et al. New strategies of ARV: the road to simplification. Curr HIV/AIDS Rep 2018; 15: 11–9. [DOI] [PubMed] [Google Scholar]
- 6. Baril JG, Angel JB, Gill MJ. et al. Dual therapy treatment strategies for the management of patients infected with HIV: a systematic review of current evidence in ARV-Naive or ARV-experienced, virologically suppressed patients. PLoS One 2016; 11: e0148231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panel de expertos de la Sociedad Española de Infectología Pediátrica (SEIP) y del Plan Nacional sobre el SIDA (PNS). Documento de consenso sobre tratamiento antirretroviral en niños y adolescentes con infección por el virus de la inmunodeficiencia humana. https://www.seipweb.es/wp-content/uploads/2019/07/GuiasTARpie24may2019-1.pdf.
- 8. Rial-Crestelo D, de Miguel R, Montejano R. et al. Long-term efficacy of dolutegravir plus lamivudine for maintenance of HIV viral suppression in adults with and without historical resistance to lamivudine: week 96 results of ART-PRO pilot study. J Antimicrob Chemother 2021; 76: 738–42. [DOI] [PubMed] [Google Scholar]
- 9. Charpentier C, Montes B, Perrier M. et al. HIV-1 DNA ultra-deep sequencing analysis at initiation of the dual therapy dolutegravir + lamivudine in the maintenance DOLULAM pilot study. J Antimicrob Chemother 2017; 72: 2831–6. [DOI] [PubMed] [Google Scholar]
- 10. de Jose MI, Jiménez de Ory S, Espiau M. et al. A new tool for the paediatric HIV research: general data from the Cohort of the Spanish Paediatric HIV Network (CoRISpe). BMC Infect Dis 2013; 13: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia-Merino I, de Las Cuevas N, Jimenez JL. et al. Paediatric HIV BioBank: a new role of the Spanish HIV BioBank in paediatric HIV research. AIDS Res Hum Retroviruses 2010; 26: 241–4. [DOI] [PubMed] [Google Scholar]
- 12. Reynes J, Meftah N, Tuaillon E. et al. Dual regimen with dolutegravir and lamivudine maintains virologic suppression even in heavily treatment experienced HIV-infected patients: 96 weeks results from maintenance DOLULAM study. Ninth International AIDS Society Conference on HIV Science, Paris, France, 2017. Abstract 2755.
- 13. Nouchi A, Nguyen T, Valantin MA. et al. Dynamics of drug resistance-associated mutations in HIV-1 DNA reverse transcriptase sequence during effective ART. J Antimicrob Chemother 2018; 73: 2141–6. [DOI] [PubMed] [Google Scholar]
- 14. Campbell TB, Shulman NS, Johnson SC. et al. Antiviral activity of lamivudine in salvage therapy for multidrug-resistant HIV-1 infection. Clin Infect Dis 2005; 41: 236–42. [DOI] [PubMed] [Google Scholar]
- 15. La Rosa AM, Harrison LJ, Taiwo B. et al. Raltegravir in second-line antiretroviral therapy in resource-limited settings (SELECT): a randomised, phase 3, non-inferiority study. Lancet HIV 2016; 3: e247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SECOND-LINE Study Group,, Boyd MA, Kumarasamy N et al. Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomized, open-label, non-inferiority study. Lancet 2013; 381: 2091–9. [DOI] [PubMed] [Google Scholar]
- 17. Ciaffi L, Koulla-Shiro S, Sawadogo AB. et al. Boosted protease inhibitor monotherapy versus boosted protease inhibitor plus lamivudine dual therapy as second-line maintenance treatment for HIV- 1-infected patients in sub-Saharan Africa (ANRS12 286/MOBIDIP): a multicentre, randomised, parallel, open-label, superiority trial. Lancet HIV 2017; 4: e384–e392. [DOI] [PubMed] [Google Scholar]
- 18. Andreatta K, Willkom M, Martin R. et al. Switching to bictegravir/emtricitabine/tenofovir alafenamide maintained HIV-1 RNA suppression in participants with archived antiretroviral resistance including M184V/I. J Antimicrob Chemother 2019; 74: 3555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lambert-Niclot S, Allavena C, Grude M. et al. Usefulness of an HIV DNA resistance genotypic test in patients who are candidates for a switch to the rilpivirine/emtricitabine/tenofovir disoproxil fumarate combination. J Antimicrob Chemother 2016; 71: 2248–51. [DOI] [PubMed] [Google Scholar]
- 20. Su B, Zheng X, Liu Y. et al. Detection of pretreatment minority HIV-1 reverse transcriptase inhibitor-resistant variants by ultra-deep sequencing has a limited impact on virological outcomes. J Antimicrob Chemother 2019; 74: 1408–16. [DOI] [PubMed] [Google Scholar]
- 21. Aguilera-Alonso D, Falcón D, Jiménez d. O S. et al. Dual therapy among hiv-infected children and adolescents within the Spanish national cohort of HIV-infected children (CoRISpe). Thirty-seventh Annual Meeting of the European Society for Paediatric Infectious Diseases, Ljubljana, Slovenia,2019. Abstract ESPID19-0718.