Abstract
Streptococcus mutans is a colonizer of the human dentition, and under conditions of dysbiosis is the primary causative agent of dental caries. The pathogenic potential of S. mutans depends, in part, on its ability to regulate the transport of metal ions across the plasma membrane to maintain intracellular metal ion homeostasis. Research in our laboratory has focused on the Mn2+-specific SloC lipoprotein importer and its regulator encoded by the S. mutans sloR gene. Herein, we used a bioinformatics approach to identify a gene on the S. mutans UA159 chromosome, SMU_1176, as a metal ion efflux transporter that contributes to S. mutans manganese ion homeostasis. Metal ion sensitivity assays performed with the wild-type S. mutans UA159 strain and an isogenic SMU_1176 insertion-deletion mutant, called GMS3000, revealed significantly heightened sensitivity of GMS3000 to MnSO4 challenge. 54Mn uptake experiments support the accumulation of 54Mn in GMS3000 cell pellets when compared to 54Mn concentrations in UA159 or in a complemented strain of GMS3000, called GMS3001. Inductively coupled plasma mass spectrometry (ICP-MS) studies were performed in parallel to quantify intracellular manganese concentrations in these strains, the results of which corroborate the 54Mn uptake studies, and support the SMU_1176 gene product as a Mn2+ efflux protein. Expression profiling experiments revealed de-repression of SMU_1176 gene transcription in the SloR-deficient GMS584 strain of S. mutans, especially under high manganese conditions. In conclusion, the S. mutans SMU_1176 gene, which we renamed mntE, is a manganese efflux transporter that contributes to essential metal ion homeostasis as part of the SloR regulon.
Keywords: manganese export, metal ion homeostasis, streptococci
1 |. INTRODUCTION
Dental caries is the most common non-communicable disease worldwide, affecting approximately half of the world’s population (Vos et al., 2017). Among Americans between the ages of 5 and 17, the incidence of dental caries is five times greater than that of asthma, and seven times more prevalent than hay fever (Surgeon General Report, 2000). Dental caries is especially prevalent among families in America with low income and limited education (Marthaler, 2004), with individuals living at or below the poverty line showing a more than twofold increase in caries incidence compared to wealthier individuals (Surgeon General Report, 2000). In addition, approximately 20% of children under the age of 5 and adults over the age of 65 experience untreated tooth decay which, taken together with an aging population, emphasizes the enormity of this public health issue (Albino, Dye, & Ricks, 2019; Dye, Thornton-Evans, Li, & Iafolla, 2015; NIDCR: Dental Caries in Children). In fact, severe caries can ultimately impair quality of life, and in its advanced stages can lead to chronic systemic infection.
The oral microbiome plays a major role in overall health and well-being. Among the many colonizers of the tooth surface is Streptococcus mutans, a Gram positive, acidogenic and aciduric bacterium, and the primary causative agent of caries in humans. As an obligate biofilm former, S. mutans accumulates on the surface of the dentition where it can convert dietary sugars into lactic acid as a metabolic byproduct of fermentation (Loesche, 1986). The accumulation of lactic acid can lead to the demineralization of the enamel and perpetuate decay that, if untreated, could involve the underlying dentin. Should S. mutans gain access to the general circulation, it can home to the heart valves where it can instigate infective endocarditis (McGhie, Hutchison, Nye, & Ball, 1977).
Metal ions play key roles in cellular and subcellular processes, acting as essential cofactors in numerous enzymatic reactions. The metal ion withholding system in the mammalian host sequesters transition metals like iron, zinc, and manganese, as a form of nutritional immunity that can hinder bacterial growth and subsequent infection. As a result, the survival and persistence of oral microbes relies on their ability to procure divalent cations as essential micronutrients (Palmer & Skaar, 2016). Metalloregulatory proteins play a central role in mediating the expression of genes whose products are required for running these micronutrient acquisition systems (Merchant & Spatafora, 2014). These metalloregulators can serve as transcriptional repressors or activators, and their activity is coordinated upon binding to specific metal ions. Like other successful pathogens, S. mutans expresses metal ion-dependent regulatory proteins that maintain appropriate cytosolic concentrations of Mn2+ and Fe2. In this way, cellular and subcellular processes may proceed so as to foster S. mutans survival and persistence in the host. These regulatory proteins are especially crucial during periods of feast and famine when the concentrations of Mn2+ in the oral cavity are highly transient.
In S. mutans, the sloABCR operon plays a crucial role in metal ion uptake across the bacterial cell membrane. The operon encodes a SloABC transport system for Mn2+/Fe2+ that is regulated by a 25 kDa SloR metalloregulatory protein expressed immediately downstream (Merchant & Spatafora, 2014; Monette et al., 2018). Specifically, the sloABC genes encode an ATP-binding cassette (ABC) transport system that enables the acquisition of Mn2+ and Fe2+ via a membrane spanning SloC lipoprotein (Haswell et al., 2013). Uptake is especially prevalent during a mealtime when free metal ions are readily available in the oral cavity. In response, S. mutans downregulates the expression of its sloABC gene products so as to prevent the over-accumulation of metal ions which could have cytotoxic effects, owing to the generation of reactive oxygen species (ROS) (Crepps et al., 2016; Monette et al., 2018). During periods of fasting, however, the bacterium upregulates the expression of the transmembrane SloC lipoprotein along with its accompanying ATP-binding (sloA) and -hydrolyzing (sloB) proteins, all of which are pivotal for metal ion uptake. The expression of this uptake machinery is governed, in large part, by SloR-mediated control. Previous work in our laboratory indicates that the 25kDa SloR protein binds to DNA as homodimers, and that SloR dimerization requires the binding of three Mn2+ ions to each monomeric subunit (Monette et al., 2018).
To date, reports in the literature describe a plethora of experiments that center on bacterial metal ion uptake mechanisms and their role in maintaining intracellular metal ion homeostasis. Relatively little is known however, about the role of Mn2+ export in this maintenance strategy. While potential Mn2+ exporters have yet to be identified in S. mutans, efflux proteins such as YeaB and MntE have been reported in Bacillus subtilis and Streptococcus pneumoniae, respectively (Huang, Shin, Pinochet-Barros, Su, & Helmann, 2017; Rosch, Gao, Ridout, Wang, & Tuomanen, 2009). In this study, we identified the S. mutans SMU_1176 gene as a homolog of the yeaB and mntE genes, and present evidence that implicates the SMU_1176 gene product in manganese efflux.
2 |. MATERIALS AND METHODS
2.1 |. Bacterial strains, plasmids, and primers
The bacterial strains and plasmids used in this study are shown in Table 1. Working stocks of bacterial strains were obtained from overnight cultures and stored in sterile glycerol at −20°C or −80°C, respectively. The primers used in this study are listed in Table 2 and were generated using the NCBI Primer BLAST tool. Sigma’s Oligo Evaluator tool was used to confirm efficient primer design, and primers were tested for specificity against the S. mutans UA159 genome through an NCBI BLAST. Primers were ordered and obtained from Eurofins Genomics.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description or relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | F- supE44 ΔlacU169Φ80dlacZΔM15 hsdR17 recA1 endA1 gyrA96 thi-1 relA | Hanahan (1983) |
| S. gordonii DL1 | Wild-type H2O2-producing primary colonizer | Kreth, Zhang, and Herzberg (2008) |
| S. mutans UA159 | Wild-type, serotype c | ATCC 700610 |
| S. mutans GMS284 | UA159-derived, sloC-deficient, Emr | Crepps et al. (2016) |
| S. mutans GMS3000 | UA159-derived, SMU_1176- deficient, Emr | This study |
| S. mutans GMS3001 | GMS3000 containing wild-type SMU_1176 gene in trans on plasmid pJO, Emr, Spr | This study |
| S. mutans GMS584 | UA159-derived, sloR-deficient, Emr | Rolerson et al. (2006) |
| S. mutans SMCitM | UA159-derived, CitM-deficient, Emr | Korithoski, Krastel, and Cvitkovitch (2005) |
| Plasmids | ||
| pDL277 | E. coli-streptococcal shuttle vector, Spr | LeBlanc, Lee, and Abu-Al-Jaibat (1992) |
| pJO | pDL277 derived, harbors wild-type SMU_1176 gene, Spr | This study |
TABLE 2.
List of oligonucleotide primers used in this study
| Assay and primer name | Nucleotide sequence (5′ to 3′) | Annealing temp (°C) | Amplicon size (bp) |
|---|---|---|---|
| PCR ligation mutagenesis | |||
| YeaB.P1.F | GCTGCAGCTTACAT CATCGC | 65 | 863 |
| YeaB.P2.R | GGCGCGCCGACGCCGAGCAAGTTTAAGGT | ||
| YeaB.P3.F | GGCCGGCCGGTCGCTCAAGTGAAGCAAA | 64 | 686 |
| YeaB.P4.R | GGCAAAGCTTTAGGTGCTCA | ||
| Cloning | |||
| YeaBP-1F. EcoRI | CGAGTGGAATTCGCTGCAGCTTACATCATCGC | 68 | 2,560 |
| YeaBP-4R.SacI | ATAATAGAGCTCGGCAAAGCTTTAGGTGCTCAGTCA | ||
| qRT-PCR | |||
| YeaB. qRT-PCR.F | CGTGGTCCAACAATCGCTATT | 63 | 90 |
| YeaB. qRT-PCR.R | TGTTAGTGAAGCAGAATGCAA | ||
| 16S.F | GCTTACCAAGGCGACGATACA | 65 | 135 |
| 16S.R | GCGTTGCTCGGTCAGACTTTC | ||
2.2 |. Bacteriological media and reagents
S. mutans UA159, GMS284, GMS3000, and GMS3001 and S. gordonii DL1 cultures were grown standing at 37°C and 5% CO2 in Todd-Hewitt broth (THB) supplemented with 0.3% yeast extract (THYE). THYE broth was supplemented with erythromycin (10 μg/ml) when growing S. mutans GMS284, GMS3000, and GMS3001, and with spectinomycin (1,200 μg/ml) when growing GMS3001.
2.3 |. In silico search of the S. mutans UA159 genome for manganese exporters
We used a gene discovery approach to reveal the presence of potential metal ion efflux systems encoded by the S. mutans UA159 genome. Specifically, a BLAST search of the NCBI database (https://www.ncbi.nlm.nih.gov/) was performed with the mntP and mntS (formerly ydfM and yeaB) manganese export genes from Bacillus sp. and Streptococcus sp., respectively, as query sequences. Homologous sequences in S. mutans that shared at least 30% amino acid identity with the B. subtilis MntP (formerly YdfM) and S. pneumococcus MntS (formerly YeaB) manganese exporters were subsequently used to query the Interpro consortium (https://www.ebi.ac.uk/interpro/) in search of predicted protein domains and signature sites.
2.4 |. Construction of a SMU_1176 mutation in S. mutans
A PCR ligation mutagenesis approach was used to disrupt the 1,190 bp SMU_1176 coding sequence on the S. mutans UA159 chromosome (Lau, Sung, Lee, Morrison, & Cvitkovitch, 2002). Specifically, primers YeaB.P1.F and YeaB.P4.R (Table 2) were used to amplify the 5’ and 3’ ends of the SMU_1176 gene from the S. mutans UA159 chromosome, generating an 863bp gap in the coding sequence. A YeaB. P2.R and YeaB.P3.F primer pair (Table 2) was used in parallel to amplify an 860bp erythromycin resistance cassette from the genome of SMCitM, a citM deficient strain of UA159 (Table 1). The resulting amplicons were digested with AscI and FseI and ligated so as to position the antibiotic cassette downstream of the SMU_1176 start codon. The ligation mixture was used to transform S. mutans UA159 in the presence of 750μg/ml of competence-stimulating peptide (CSP) as previously described (Li, Lau, Lee, Ellen, & Cvitkovitch, 2001). Chromosomal DNA, isolated from select transformants grown on THYE-erm plates, was used as a template for PCR and subsequent nucleotide sequencing with primers YeaB.P1.F and YeaB.P4.R (Table 2) to confirm SMU_1176 disruption by allelic exchange. The resulting SMU_1176 insertion-deletion mutant was named GMS3000.
2.5 |. Complementation of SMU_1176 in S. mutans GMS3000
Plasmid pJO (Table 1) was constructed by cloning a 2.56kb amplicon containing the SMU_1176 coding sequence and its upstream regulatory region into the EcoRI and SacI site of plasmid pDL277 using primers YeaBP-1F.EcoRI and YeaBP-4R.SacI (Table 2). The resulting recombinant plasmid was transformed into E. coli DH5α and transformants were selected on Luria agar plates supplemented with spectinomycin. Plasmid DNA isolated with a mini-prep spin kit (Qiagen) was used to complement the SMU_1176 mutation in S. mutans GMS3000 in trans. Transformation occurred in the presence of CSP as described above, and transformants resistant to spectinomycin were selected on THYE-spec agar plates. The complemented strain was named GMS3001 and confirmed through off-site (Eurofins Genomics) Sanger sequencing.
2.6 |. Metal ion sensitivity assay
Cultures of S. mutans UA159, GMS284, GMS3000, and GMS3001 were grown for 16–18 hr in 14ml of THYE with appropriate antibiotic selection at 37°C and 5% CO2. The cultures were normalized to within ±0.005 Absorbance units. 20 μl of each strain was plated as contiguous lawns on THYE Agar. Sterile Whatman paper filter disks placed on the agar surface with sterile forceps were impregnated with 8 μl of either 1 M ZnSO4, 1 M MnSO4, 1 M FeSO4, or 1 M CoSO4. The plates were incubated for 16–18 hr at 37°C with 5% CO2, and the zones of inhibition were measured.
2.7 |. Manganese sensitivity assay
Overnight cultures of S. mutans UA159, GMS284, GMS3000, and GMS3001 were grown as described above. The cell suspensions were centrifuged at 4,700 g for 7 min and resuspended in 1ml of prewarmed THYE after which they were diluted with sterile prewarmed THYE to standardize cell density (OD600 = 0.1 ± 0.005A). 0.5 ml of the cell suspensions were added to microcentrifuge tubes supplemented with MnSO4 to a final concentration of 0, 125, or 250 μM MnSO4. The cell suspensions were vortexed and 8 μl of each was spotted onto THYE plates. The cells were allowed to grow for 16–18 hr overnight at 37°C with 5% CO2.
2.8 |. 54Mn Metal Ion Uptake Assays
S. mutans wild-type and mutant strains were grown as described previously. 250 μl of each cell suspension was aliquoted into 45ml of prewarmed THB and incubated for 3 hr at 37°C with 5% CO2. Cell density was standardized for each culture to 0.1 OD600 units and then adjusted to within 0.005 OD600 units of each other. 1 ml aliquots of each cell suspension were prepared as control and treatment groups. 1.8 μl of a 54Mn working stock (7.18 μCi/μl) was added to the treatment group, while 1.8 μl of 0.5 M HCl was added to the control group. All samples were incubated for 16–18 hr at 37°C with 5% CO2 and pelleted by centrifugation at 9,600 g for 3 min. For the treatment groups, 100 μl of supernatant was transferred to separate liquid scintillation vials containing 3 ml ScintiVerse. Cell pellets for both the control and treatment samples were generated by centrifugation and washed upon resuspension in 1 ml THB. This washing step was repeated two times after which the cell pellets were resuspended in 100 μl THB. Samples from the treatment group were transferred to liquid scintillation vials and cell-associated 54Mn was assessed in a liquid scintillation counter (Hidex). For the control samples, 10-fold serial dilutions were prepared and 20 μl of each dilution was spread-plated onto Todd Hewitt Agar. The plates were incubated at 37°C with 5% CO2 for 16–18 hr and a viable plate count was determined. The number of colony-forming units for each culture was used to normalize the intracellular 54Mn data expressed in counts per minute for each treatment sample.
2.9 |. Inductively coupled plasma-mass spectrometry (ICP-MS)
S. mutans UA159, GMS284, GMS3000, and GMS3001 were grown in 14ml of THB, or in THB supplemented with MnSO4 to a final concentration of 10 μM. The cultures were incubated for 16–18 hr at 37°C with 5% CO2, centrifuged at 4,700 g for 7 min, and the cell pellets resuspended in either 1ml THB or THB supplemented with Mn2+ for the control and treatment groups, respectively. The samples were transferred to pre-weighed microcentrifuge tubes and the cells were washed as described above except with pelleting for 5 min at 21,000 g at 4°C. Two additional washes were performed followed by overnight drying in an Eppendorf Vacufuge Concentrator (ThermoFisher). A dry weight for each pellet was obtained and the pellets were resuspended in 500 μl of 2.8% w/v nitric acid and incubated at 98°C for 20 min, with intermittent agitation. The samples were centrifuged to remove debris and the supernatants were transferred to 14 ml sterile Falcon tubes and further diluted to 5 ml using 5% w/v Nitric Acid. The supernatants, along with a series of Mn2+-containing standards were analyzed for metal ion content on an iCAP TQ ICP-MS (ThermoFisher) with a CETAC Asx-520 Autosampler (ThermoFisher). The standards were used to generate a calibration curve, which was then used to determine the concentration of Mn in each of the samples. Each tube was sampled in triplicate, and an average concentration was determined. The data were normalized using the dry pellet weights and corrected for machine drift.
2.10 |. Hydrogen peroxide sensitivity assays
An overnight culture of Streptococcus gordonii was prepared in 14ml of THYE. The culture was incubated at 37°C with 5% CO2 for 16–18 hr and 8 μl of cell culture were spotted, in triplicate, onto the surface of THYE agar plates which were incubated at 37°C with 5% CO2 for 16 hr. In parallel, S. mutans UA159, GMS284, GMS3000, and GMS3001 cultures were prepared in 14ml THYE, with appropriate antibiotic selection, and incubated at 37°C with 5% CO2 for 16–18 hr. The cultures were centrifuged at 4,700 g for 5 min, the cell pellets were resuspended in 1ml of fresh THYE, and the cell suspensions were further adjusted for cell density with fresh THYE (OD600 = 0.1 ± 0.005A). Eight microliter of each culture was then spotted, in triplicate, adjacent to a series of S. gordonii spots that had been pre-inoculated on the THYE agar plates and grown for 16–18 hr. The plates were incubated at 37°C with 5% CO2 for approximately 22 hr. Growth of S. mutans strains in the presence of S. gordonii was normalized by dividing the vertical diameter of the S. mutans area of growth by the vertical diameter of the S. gordonii area of growth and averaging the result across three independent experiments.
2.11 |. RNA isolation and cDNA synthesis
Total intact RNA was isolated according to a modified protocol of Crepps et al. (2016). Cultures of S. mutans UA159 and GMS584 were grown to mid-logarithmic-phase (OD600 = 0.50) in semi-defined medium (SDM) supplemented to a final concentration of either 5 or 500 μM MnSO4, then centrifuged at 4,700 g for 5 min prior to resuspending in RNAprotect Bacterial Reagent (Qiagen). The cells were pelleted again as described above and the pellets were resuspended in 250 μl of 50 mM Tris-10 mM EDTA buffer. Samples were transferred to separate lysing matrix B tubes (MP Biomedicals) containing zirconium beads, 10 μl of 10% SDS and 300 μl of acid phenol. Cells were lysed in a FastPrep-24 mechanical disrupter (MP Biomedicals) for two rounds of 30 s disruptions with intermittent chilling on ice. The cell slurry was centrifuged at 19,000 g for 10 min at 4°C in a Sorvall Legend Micro 21R centrifuge (Thermo-Scientific). 180 μl of the supernatant was DNase I-treated and purified on an RNeasy column (Qiagen) according to the protocols provided by the manufacturer. Total intact RNA was examined for integrity on a 1% agarose gel and adjusted to a final concentration of 100 ng/μl using a NanoDrop Lite spectrophotometer (Thermo Fisher Scientific). Total intact RNAs were then reverse transcribed using a Revert-Aid First-Strand cDNA Synthesis Kit (Thermo-Scientific) according to the manufacturer’s instructions. Reaction mixtures without RNA served as no-template controls (NTC), and those without reverse transcriptase were included as negative controls (RT−).
2.12 |. Semi-quantitative real-time PCR (qRT-PCR)
Semi-quantitative real-time PCR was performed with the cDNA products described above (diluted 1:39) as templates (Crepps et al., 2016). Amplification was performed in a CFX96 real-time system with SsoFast EvaGreen Supermix (Bio-Rad) as the intercalating dye and 500 nM SMU_1176 or 16S primers (Table 2). The thermocycling conditions were 95°C for 3 min followed by 40 cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 15 s. Each amplification was performed in technical triplicate. The expression of the SMU_1176 gene was normalized against that of 16S, an endogenous control gene with steady-state expression. No template controls (NTCs) and RT-negative controls were included and processed in parallel with the experimental test samples.
3 |. RESULTS
3.1 |. The SMU_1176 gene product shares homology with other known Mn2+ exporters
We used a combination of bioinformatic and genetic approaches to determine whether the S. mutans SMU_1176 gene product shares homology with known Mn2+ efflux proteins in Bacillus subtilis (YeaB) and Streptococcus pneumoniae (MntE). Multiple protein sequence alignments reveal conservation across all three bacterial species (Figure 1). In pairwise comparisons we noted that the S. mutans SMU_1176 protein shares 46% amino acid identity with YeaB and 62% amino acid identity with MntE.
FIGURE 1.
The SMU_1176 gene shares homology with the known Mn2+ exporters YeaB and MntE in Bacillus subtilis and Streptococcus pneumoniae, respectively. Asterisks (*) represent amino acid positions which have a single fully conserved residue. A colon (:) indicates conservation between amino acids with strongly similar properties, scoring >0.5 in a PAM250 Matrix. A period (.) signifies conservation between amino acid residues with weakly similar properties, scoring <0.5 in a PAM250 Matrix. Amino acid sequences for all three proteins were obtained from NCBI using the following accession numbers: SMU_1176: NP_721561.1; YeaB: AAB62307.1; MntE: WP_140223066.1
3.2 |. An SMU_1176 insertion-deletion mutant, GMS3000, is hyper-sensitive to manganese challenge
To explore a potential role for SMU_1176 in manganese transport, we performed spot inoculation assays with an SMU_1176 insertion-deletion mutant that we generated from the UA159 wild-type strain using a PCR ligation mutagenesis approach (Lau et al., 2002). The resulting strain, called GMS3000, was complemented in trans with the wild-type SMU_1176 gene cloned on plasmid pDL277, generating strain GMS3001. The UA159, GMS3000, and GMS3001 strains were assessed for manganese sensitivity in spot inoculation assays, along with a sloC manganese import mutant, GMS284 constructed previously in our laboratory (Crepps et al., 2016). The experimental results indicate that growth of GMS3000 is compromised when challenged with 125uM MnSO4, and inhibited in the presence of 250 μM MnSO4 (Figure 2), consistent with its heightened sensitivity to manganese. Importantly, neither the UA159 wild-type strain, the complemented GMS3001 strain, nor the GMS284 import mutant were sensitive to manganese exposure at any of the test conditions.
FIGURE 2.
SMU_1176-deficient GMS3000 is sensitive to MnSO4 challenge. Notably, the wild-type UA159 strain, the SloC-deficient GMS284 manganese import mutant, and the SMU_1176-complemented GMS3001 strain show no sensitivity to MnSO4 challenge at any of the test concentrations. In contrast, growth of the GMS3000 SMU_1176 insertion-deletion mutant is compromised in the presence of 125 μM MnSO4, and inhibited at 250 μM, consistent with the sensitivity of this mutant to manganese. All cultures were grown for 16–18 hr in 14 ml of THYE and appropriate antibiotic selection at 37°C with 5% CO2. The cells were pelleted, resuspended, and normalized by adjusting the OD600nm to 0.1A ± 0.005A. The cell suspensions were then supplemented with MnSO4 after which 8 μl of each culture was plated onto the surface of THYE agar and incubated for 16–18 hr at 37°C and 5% CO2
3.3 |. Sensitivity of S. mutans GMS3000 is manganese-specific
To investigate the metal ion specificity of SMU_1176, we tested GMS3000, GMS3001, GMS584, GMS284 and their wild-type UA159 progenitor in disk diffusion assays with MnSO4, ZnSO4, CoSO4, and FeSO4 as test cations. Interestingly, the GMS3000 insertion-deletion mutant displayed a large zone of inhibition (ZOI) when challenged with MnSO4, but no heightened sensitivity to any of the other test cations (Table 3). Specifically, the ZOI surrounding GMS3000 was significantly greater (p < .001 ANOVA with Tukey’s test) than that of the other test strains at manganese concentrations ranging from 0.125 to 1 M MnSO4. In contrast, no ZOIs were observed for S. mutans UA159, GMS284, GMS584, or GMS3001 when exposed to manganese concentrations up to 1 M MnSO4 (Table 3 and data not shown). We did observe heightened sensitivity of the sloR-deficient GMS584 strain to cobalt, however. Taken together, these findings support heightened manganese sensitivity for the mutation-bearing SMU_1176 strain, as well as specificity of the SMU_1176 gene product in manganese transport.
TABLE 3.
The results of metal ion sensitivity assays support a role for the SMU_1176 gene product in Mn2+ homeostasis
| Ion | Average Δ Zone of Inhibition Relative to UA159 (cm) |
|||
|---|---|---|---|---|
| GMS284 | GMS584 | GMS3000 | GMS3001 | |
| Zn2+ | n.d. | −0.03333 ± 0.05774 | −0.06667 ± 0.11547 | −0.06667 ± 0.11547 |
| Mn2+ | 0 ± 0 | 0 ± 0 | 0.933333 ± 0.057735 | 0 ± 0 |
| Fe2+ | n.d. | 0.066667 ± 0.057735 | 0 ± 0 | 0 ± 0 |
| Co2+ | −0.1 ± 0.173205 | 0.333333 ± 0.305505 | −0.26667 ± 0.057735 | −0.26667 ± 0.057735 |
Note: The SMU_1176-deficient GMS3000 strain was the only test strain demonstrating high sensitivity to Mn2+ challenge as revealed by a large zone of inhibition (ZOI) compared to that of the UA159 wild-type progenitor strain. Overnight cultures of S. mutans strains UA159, GMS284, GMS584, GMS3000, and GMS3001 were standardized to an OD600nm of 0.1 ± 0.005A and plated as lawns on THYE agar. Sterile Whatman paper filter disks impregnated with 8 μl of either 1 M ZnSO4, 1 M MnSO4, 1 M FeSO4, or 1 M CoSO4 were placed on the agar surface. After overnight incubation, differences in ZOI were determined by subtracting the ZOI of a particular divalent cation/strain combination from the ZOI for the same cation in UA159 (N = 3, except where indicated as n.d. = “not determined.” Shown is the average difference in ZOI for each test strain and the standard deviation for each experimental condition.
3.4 |. Mn2+ accumulates in GMS3000 cell pellets in 54Mn uptake experiments
To investigate whether SMU_1176 is responsible for manganese import or export, we conducted 54Mn uptake assays, the results of which reveal an accumulation of 54Mn in GMS3000 cell pellets (Figure 3). That is, intracellular 54Mn accumulation was significantly greater in GMS3000 compared to that in UA159 (p < .001 ANOVA with Tukey’s test) and GMS3001 (p < .01 by ANOVA with Tukey’s test). As expected, S. mutans GMS284 which harbors a mutation in the sloC manganese importer accumulated 54Mn in the supernatant. Interestingly, both UA159 and GMS284 accumulated comparable levels of intracellular 54Mn, suggesting the presence of additional Mn2+ importers in S. mutans. Altogether, these findings support SMU_1176 as a manganese exporter.
FIGURE 3.
54Mn accumulates in S. mutans GMS3000, an SMU_1176 insertion-deletion mutant. Intracellular concentrations of 54Mn were significantly greater in GMS3000 compared to those in UA159, GMS284, and GMS3001, consistent with a role for the SMU_1176 gene product as Mn2+ efflux protein. Overnight cultures of UA159, GMS284, GMS3000, and GMS3001 were normalized to ±0.005A, and grown in the presence of 54Mn (experimental) or 0.5 M HCl (control) for 16–18 hr. The cell pellets and supernatants were separated by centrifugation, and counts per minute (CPM) were determined by liquid scintillation counting. The number of colony forming units (CFUs) was determined by plating serial dilutions of the control cultures in parallel. The CPM of the cell pellets were divided by the CPM of the supernatants, and that value was divided by the number of CFUs to normalize the 54Mn transport data. Error bars denote the standard deviation about the mean. Variation in the experimental design includes some inevitable loss of cell associated 54Mn during the wash steps. Nevertheless, ANOVA analysis with Tukey’s test revealed significant differences between GMS3000 and each of the other three test strains (p < .01). N = 3
3.5 |. S. mutans GMS3000 accumulates intracellular manganese in ICP-MS experiments
To confirm SMU_1176 as a manganese efflux protein, we performed inductively coupled plasma mass spectrometry (ICP-MS) experiments on the aforementioned S. mutans strains in the presence and absence of 10 μM MnSO4. As expected, intracellular manganese concentrations increased significantly in all four S. mutans strains when grown in the presence of 10 μM MnSO4 versus in its absence (p < .000001 by ANOVA with Tukey’s test) (Figure 4). Notably however, the increase in intracellular Mn2+ that we observed for GMS3000 grown in the presence of MnSO4 was significantly greater than that for UA159 (p < .0000001 by ANOVA with Tukey’s test), GMS284 (p < .00001 by ANOVA with Tukey’s test), and GMS3001 (p < .000001 by ANOVA with Tukey’s test) grown in the presence of MnSO4. The accumulation of Mn2+ in the GMS3000 strain coupled with the relatively low intracellular Mn2+ concentrations that we noted in the SMU_1176-proficient UA159 and GM3001 strains, provide strong evidence that SMU_1176 functions as a Mn2+-specific efflux protein in S. mutans. Moreover, the results of the ICP-MS experiments corroborate those of the 54Mn uptake assays, lending further support to an efflux role for SMU_1176.
FIGURE 4.
The results of ICP-MS support significantly heightened intracellular Mn2+ concentrations in S. mutans GMS3000 compared to those in the UA159 wild-type progenitor, the GMS3001 complemented strain, and the GMS284 Mn2+ import mutant. These findings further implicate SMU_1176 as a Mn2+ efflux protein. Each strain was grown overnight in THB or THB supplemented with 10 μM MnSO4, pelleted, and washed three times. Pellets were dried overnight in a Vacufuge, and the dry weights were determined for normalization. The pellets were digested in 2.8% Nitric Acid at 98°C with vigorous, intermediate vortexing. Supernatants containing the cytosolic contents were diluted to a total volume of 5 ml using 5% Nitric Acid, and then analyzed on the ICP-MS. The ICP-MS measurements were corrected for machine drift and normalized using the cell pellet weights to generate a ppb/mg value for each strain. Error bars denote standard deviation about the mean. ANOVA analysis with Tukey’s test revealed significant differences between GMS3000 and each of the other three strains in the presence of 10 μM MnSO4 (p < .00001). N = 3
3.6 |. Increased susceptibility of GMS3000 to peroxigenic S. gordonii
To investigate a role for SMU_1176 in S. mutans oxidative stress tolerance, we spot-inoculated UA159, GMS284, GMS3000, and GMS3001 cells next to H2O2-producing S. gordonii. As described in a previous report (Crepps et al., 2016), the sloC-deficient GMS284 strain, which is compromised for essential manganese uptake, demonstrates decreased survivorship when challenged with S. gordonii H2O2-production. In this study, we noted reduced survivorship of GMS3000 compared to that of the UA159 and GMS3001 strains (Figure 5), indicating that GMS3000, like GMS284, is more sensitive to physiological levels of peroxide stress. While this GMS284 phenotype likely derives from low intracellular levels of essential Mn2+, we propose that the GMS3000 phenotype is the result of over-accumulation of intracellular manganese owing to compromised SMU_1176-mediated metal ion export.
FIGURE 5.
The SMU1176- deficient S. mutans GMS3000 strain is more sensitive to H2O2 challenge than the UA159 wildtype and complemented GMS3001 strains. (a) THYE agar was spot inoculated with 8 μl of a Streptococcus gordonii DL1 overnight culture and then incubated for 16–18 hr. The next day, overnight cultures of S. mutans UA159, GMS284, or GMS3000 were adjusted to ±0.005A, spotted adjacent to the S. gordonii inocula, and incubated for 16–18 hr. S. mutans growth was subsequently observed for inhibition in the zone of H2O2 diffusion. These data are representative of 9 independent experiments. (b) The growth of the S. mutans strains in the presence of S. gordonii was normalized by dividing the vertical diameter of the S. mutans area of growth by the vertical diameter of the S. gordonii area of growth and taking the average across three independent experiments
3.7 |. SMU_1176 transcription is elevated in a S. mutans GMS584 SloR-deficient mutant, and when grown in high manganese conditions
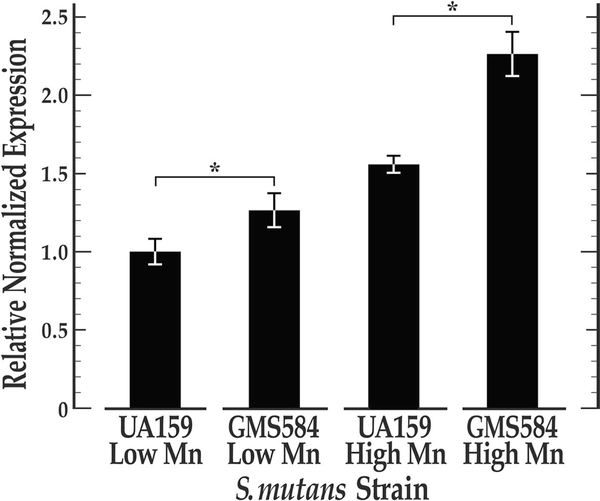
To elucidate SMU_1176 gene regulation and whether it is subject to control by the S. mutans SloR metalloregulator, we performed semi-quantitative real-time PCR (qRT-PCR) experiments to reveal SMU_1176 transcription in UA159 and its isogenic sloR-deficient mutant (GMS584) when grown in the presence of low (5 μM) versus high (500 μM) manganese. While transcription of SMU_1176 was significantly elevated in GMS584 compared to that in UA159 for cells grown at both low and high manganese concentrations (p < .05 and p < .001, respectively, by ANOVA with Tukey’s test), transcription of SMU_1176 was significantly more robust overall when these strains were grown at the higher manganese concentration (Figure 6). These findings support SloR as a repressor of SMU_1176 expression, and elucidate SloR-mediated gene repression that is manganese-dependent.
FIGURE 6.
The results of qRT-PCR experiments reveal increased transcription of the S. mutans SMU_1176 genes in the SloR-deficient GMS584 strain compared to the UA159 wildtype strain, and at high manganese concentration. SMU_1176 expression levels were monitored in the SloRdeficient GMS584 strain and its SloR-proficient UA159 progenitor when grown in a semi-defined medium (SDM). Expression profiles were normalized against the steady state expression of an endogenous 16S control gene, the results of which indicate (via ANOVA analysis with Tukey’s test) significantly heightened SMU_1176 transcription in both S. mutans UA159 and GMS584 when grown in the presence of 500 μM MnSO4 (high) as compared to 5 μM MnSO4 (low) (p < .001). SMU_1176 gene expression was also significantly greater in the SloR-deficient GMS584 strain compared to the wild-type UA159 progenitor for both experimental conditions, but especially at the higher manganese concentration (p < .001). Error bars represent the standard deviation about the mean. N = 3
4 |. DISCUSSION
Manganese is an essential trace nutrient that acts as a cofactor for numerous bacterial enzymes, particularly proteins involved in the oxidative stress response pathway (Hohle & O’Brian, 2014). Manganese superoxide dismutase (MnSOD), present in nearly all facultative anaerobes including S. mutans, can detoxify reactive oxygen species into relatively harmless byproducts, water and hydrogen peroxide (Janssen, Houten, Borm, & Mossman, 1993; Nakayama, 1992). Other studies have demonstrated the exchangeability of manganese for iron as a cofactor in many mononuclear enzymes (Anjem & Imlay, 2012; Sobota, Gu, & Imlay, 2014; Sobota & Imlay, 2011). The selective pressures of manganese limitation—whether via competition from neighboring microbes, metal ion limitation in the local microenvironment, or host metal ion sequestration—have led to the evolutionary development of high affinity uptake systems in bacteria. Many streptococci employ specialized ABC transporters for manganese uptake, like the SloC Mn2+ importer in S. mutans (Eijkelkamp, McDevitt, & Kitten, 2015; Merchant & Spatafora, 2014).
While manganese acquisition is essential for microbial survival, persistence, and pathogenicity in a host organism, too much intracellular manganese can lead to microbial growth arrest and cell death (Huang et al., 2017). The mechanisms by which elevated intracellular manganese induces cell death are not fully understood, though several possibilities have been suggested (Helmann, 2014; Lisher & Giedroc, 2013; Martin, Lisher, Winkler, & Giedroc, 2017). As a result, bacteria have had to evolve mechanisms to maintain intracellular metal ion homeostasis, including manganese exporters, to prevent metal ion over-accumulation and its associated toxicity effects. Indeed, the functional deletion of manganese exporters can significantly reduce virulence as it does in Streptococcus pyogenes and other bacterial pathogens (Grunenwald et al., 2019; Rosch et al., 2009; Turner et al., 2015; Veyrier, Boneca, Cellier, & Taha, 2011).
In this study, we proposed a role for the S. mutans SMU_1176 protein in Mn2+ efflux given the significant amino acid identity it shares with other known Mn2+ exporters, such as MntE and YeaB in S. pneumoniae and B. subtilus, respectively. Herein, we presented experimental evidence that supports SMU_1176 as a Mn2+-specific export protein, consistent with the increased sensitivity of GMS3000 to manganese challenge in disk diffusion and spot inoculation assays. Moreover, the results of metal ion uptake and ICP-MS experiments revealed an accumulation of Mn2+ in GMS3000 cell pellets, lending further support to SMU_1176 as a Mn2+ exporter. The results of disk diffusion assays indicate that GMS3000 is uniquely sensitive to manganese, and not to zinc, cobalt, or iron. Together, these results support an SMU_1176 gene that encodes a Mn2+-specific efflux transporter. We therefore propose a change to the SMU_1176 nomenclature, and hereafter refer to SMU_1176 as MntE to reflect the structural and functional similarities it shares with mntE and its gene product in S. pneumoniae.
We went on to hypothesize that mntE-mediated Mn2+ export is likely subject to metalloregulation by the S. mutans SloR protein, and that this regulation might coordinate with that of the sloC manganese importer when metal ions are readily plentiful (Rolerson et al., 2006). Our experimental findings support this hypothesis since mntE expression was significantly de-repressed in a SloR-deficient mutant, especially when grown in a medium containing excess manganese. Metal ion export proteins are typically active when metals are abundant in the surrounding milieu, since their heightened uptake and accumulation can prove toxic owing to Fenton chemistry (Janssen et al., 1993; Yesilkaya et al., 2000).
Yet another source of ROS in the oral cavity can derive from the early colonizing peroxigenic streptococci, namely S. gordonii and S. sanguinis, that elaborate hydrogen peroxide as a competitive strategy in the plaque environment (Crepps et al., 2016; Kreth, Merritt, Shi, & Qi, 2005). S. mutans has evolved mechanisms to detoxify the ROS arsenal of its neighbors, however, including one that relies on the enzymatic activity of its Mn-dependent superoxide dismutase (Miller, 2012; Yesilkaya et al., 2000). Hence, oxidative stress tolerance in S. mutans is dependent, in part, on its superoxide dismutase activity, and by extension, its ability to modulate the import and export of Mn2+ across the plasma membrane (Crepps et al., 2016).
In summary, the S. mutans mntE gene encodes a Mn2+-specific efflux transporter that is under negative transcriptional control by SloR. De-repression of the mntE gene occurs under conditions of high manganese, perhaps via a mechanism that excludes access of RNA polymerase to the mntE promoter, much like the SloR-mediated control we described previously at the S. mutans sloABC locus (Monette et al., 2018). In fact, there is a predicted SloR recognition element or “SRE” (5’-TAATAAGCCTATTATAAT-3’) that shares overlap with the predicted −10 promoter that drives mntE transcription. Gel mobility shift assays are underway to reveal whether the impact of SloR on mntE gene transcription is direct. In addition, manganese ion homeostasis plays an especially critical role in the S. mutans response to oxidative stress. We propose that the functional loss of MntE in S. mutans can lead to reduced fitness and decreased virulence, as it does in streptococcal MntE mutants that are attenuated in a murine model (Rosch et al., 2009; Turner et al., 2015). It is possible that regulation of both manganese import and export activity, perhaps via a master metalloregulator like SloR, is required to achieve homeostatic conditions. Future studies in our lab will include monitoring the transcription of manganese importers like SloC and MntH, as well as that of the MntE exporter over time, and when the bacterium is exposed to excess manganese to address this hypothesis. We also plan to investigate the cariogenic potential of GMS3000 in an animal model of caries. Taken together, these studies can benefit efforts towards rational drug design aimed at mitigating or preventing caries and its associated complications.
ACKNOWLEDGMENTS
This research was funded by NIH/NIDCR R01 Grant #DE014711-10 to G.A.S. and the Middlebury College Department of Biology. We thank Gary Nelson for figure preparation, Robert Haney for help with bioinformatics, and Timothy Allen, Frank Spatafora, and Jody Smith for technical assistance. We declare that we have no conflicts to report.
Funding information
NIH/NIDCR R01, Grant/Award Number: R01 Grant #DE014711-10; Middlebury College Department of Biology
REFERENCES
- Albino J, Dye BA, & Ricks T (2019). 2020 Surgeon General’s Report: Oral Health in America: Advances and Challenges. Retrieved from https://www.nidcr.nih.gov/sites/default/files/2019-08/SurgeonGeneralsReport-2020_IADR_June%202019-508.pdf
- Anjem A, & Imlay JA (2012). Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. Journal of Biological Chemistry, 287(19), 15544–15556. 10.1074/jbc.M111.330365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepps SC, Fields EE, Galan D, Corbett JP, Von Hasseln ER, & Spatafora GA (2016). The SloR metalloregulator is involved in the Streptococcus mutans oxidative stress response. Molecular Oral Microbiology, 31(6), 526–539. 10.1111/omi.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Dental and Craniofacial Research. (n.d.). Dental Caries in Children (Age 2 to 11), Data & Statistics. Retrieved October 5, 2019, from https://www.nidcr.nih.gov/research/data-statistics/dental-caries/children
- Dye B, Thornton-Evans G, Li X, & Iafolla T (2015). Dental caries and tooth loss in adults in the United States, 2011–2012. NCHS Data Brief, 197, 197. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25973996 [PubMed] [Google Scholar]
- Eijkelkamp BA, McDevitt CA, & Kitten T (2015). Manganese uptake and streptococcal virulence. BioMetals, 28(3), 491–508. 10.1007/s10534-015-9826-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunenwald CM, Choby JE, Juttukonda LJ, Beavers WN, Weiss A, Torres VJ, & Skaar EP (2019). Manganese detoxification by MntE is critical for resistance to oxidative stress and virulence of Staphylococcus aureus. Mbio, 10(1), e02915–e02918. 10.1128/mBio.02915-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D (1983). Studies on transformation of Escherichia coli with plasmids. Journal of Molecular Biology, 166(4), 557–580. 10.1016/s0022-2836(83)80284?-8 [DOI] [PubMed] [Google Scholar]
- Haswell JR, Pruitt BW, Cornacchione LP, Coe CL, Smith EG, & Spatafora GA (2013). Characterization of the functional domains of the SloR metalloregulatory protein in Streptococcus mutans. Journal of Bacteriology, 195(1), 126–134. 10.1128/JB.01648-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann JD (2014). Specificity of metal sensing: Iron and manganese homeostasis in Bacillus subtilis. The Journal of Biological Chemistry, 289(41), 28112–28120. 10.1074/jbc.R114.587071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohle TH, & O’Brian MR (2014). Magnesium-dependent processes are targets of bacterial manganese toxicity. Molecular Microbiology, 93(4), 736–747. 10.1111/mmi.12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Shin J-H, Pinochet-Barros A, Su TT, & Helmann JD (2017). Bacillus subtilis MntR coordinates the transcriptional regulation of manganese uptake and efflux systems. Molecular Microbiology, 103(2), 253–268. 10.1111/mmi.13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen YM, Van Houten B, Borm PJ, & Mossman BT (1993). Cell and tissue responses to oxidative damage. Laboratory Investigation, 69(3), 261–274. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8377469 [PubMed] [Google Scholar]
- Korithoski B, Krastel K, & Cvitkovitch DG (2005). Transport and metabolism of citrate by Streptococcus mutans. Journal of Bacteriology, 187(13), 4451–4456. 10.1128/JB.187.13.4451-4456.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Merritt J, Shi W, & Qi F (2005). Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. Journal of Bacteriology, 187(21), 7193–7203. 10.1128/JB.187.21.7193-7203.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Zhang Y, & Herzberg MC (2008). Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. Journal of Bacteriology, 190(13), 4632–4640. 10.1128/JB.00276-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau PCY, Sung CK, Lee JH, Morrison DA, & Cvitkovitch DG (2002). PCR ligation mutagenesis in transformable streptococci: Application and efficiency. Journal of Microbiological Methods, 49(2), 193–205. 10.1016/S0167-7012(01)00369-4 [DOI] [PubMed] [Google Scholar]
- LeBlanc DJ, Lee LN, & Abu-Al-Jaibat A (1992). Molecular, genetic, and functional analysis of the basic replicon of pVA380–1, a plasmid of oral streptococcal origin. Plasmid, 28(2), 130–145. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1409970 [DOI] [PubMed] [Google Scholar]
- Li YH, Lau PCY, Lee JH, Ellen RP, & Cvitkovitch DG (2001). Natural genetic transformation of Streptococcus mutans growing in biofilms. Journal of Bacteriology, 183(3), 897–908. 10.1128/JB.183.3.897-908.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisher JP, & Giedroc DP (2013). Manganese acquisition and homeostasis at the host-pathogen interface. Frontiers in Cellular and Infection Microbiology, 3, 91. 10.3389/FCIMB.2013.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche WJ (1986). Role of Streptococcus mutans in human dental decay. Microbiological Reviews, 50(4), 353–380. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3540569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler TM (2004). Changes in dental caries 1953–2003. Caries Research, 38(3), 173–181. 10.1159/000077752 [DOI] [PubMed] [Google Scholar]
- Martin JE, Lisher JP, Winkler ME, & Giedroc DP (2017). Perturbation of manganese metabolism disrupts cell division in Streptococcus pneumoniae. Molecular Microbiology, 104(2), 334. 10.1111/MMI.13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhie D, Hutchison JG, Nye F, & Ball AP (1977). Infective endocarditis caused by Streptococcus mutans. Heart, 39(4), 456–458. 10.1136/hrt.39.4.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant AT, & Spatafora GA (2014). A role for the DtxR family of metalloregulators in gram-positive pathogenesis. Molecular Oral Microbiology, 29(1), 1–10. 10.1111/omi.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A-F (2012). Superoxide dismutases: Ancient enzymes and new insights. FEBS Letters, 586(5), 585–595. 10.1016/j.febslet.2011.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monette P, Brach R, Cowan A, Winters R, Weisman J, Seybert F, … Spatafora G. (2018). Autoregulation of the Streptococcus mutans SloR metalloregulator is constitutive and driven by an independent promoter. Journal of Bacteriology, 200(14), e00214–e00218. 10.1128/JB.00214-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K (1992). Nucleotide sequence of Streptococcus mutans superoxide dismutase gene and isolation of insertion mutants. Journal of Bacteriology, 174(15), 4928–4934. 10.1128/jb.174.15.4928-4934.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LD, & Skaar EP (2016). Transition metals and virulence in bacteria. Annual Review of Genetics, 50(1), 67–91. 10.1146/annurev-genet-120215-035146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolerson E, Swick A, Newlon L, Palmer C, Pan Y, Keeshan B, & Spatafora G (2006). The SloR/Dlg metalloregulator modulates Streptococcus mutans virulence gene expression. Journal of Bacteriology, 188(14), 5033–5044. 10.1128/JB.00155-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch JW, Gao G, Ridout G, Wang Y-D, & Tuomanen EI (2009). Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Molecular Microbiology, 72(1), 12–25. 10.1111/j.1365-2958.2009.06638.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobota JM, Gu M, & Imlay JA (2014). Intracellular hydrogen peroxide and superoxide poison 3-deoxy-D-arabinoheptulosonate 7-phosphate synthase, the first committed enzyme in the aromatic biosynthetic pathway of Escherichia coli. Journal of Bacteriology, 196(11), 1980–1991. 10.1128/JB.01573-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobota JM, & Imlay JA (2011). Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proceedings of the National Academy of Sciences of the United States of America, 108(13), 5402–5407. 10.1073/pnas.1100410108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgeon General Report. (2000). Oral health in America: a report of the Surgeon General. Journal of the California Dental Association, 28(9), 685–695. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11324049 [PubMed] [Google Scholar]
- Turner AG, Ong CLY, Gillen CM, Davies MR, West NP, McEwan AG, & Walker MJ (2015). Manganese homeostasis in Group A Streptococcus Is critical for resistance to oxidative stress and virulence. Mbio, 6(2), e00278–15. 10.1128/mBio.00278-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyrier FJ, Boneca IG, Cellier MF, & Taha M-K (2011). A novel metal transporter mediating manganese export (MntX) regulates the Mn to fe intracellular ratio and Neisseria meningitidis virulence. PLoS Path, 7(9), e1002261. 10.1371/journal.ppat.1002261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, … Murray CJL. (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. The Lancet, 390(10100), 1211–1259. 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesilkaya H, Kadioglu A, Gingles N, Alexander JE, Mitchell TJ, & Andrew PW (2000). Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infection and Immunity, 68(5), 2819–2826. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10768978 [DOI] [PMC free article] [PubMed] [Google Scholar]