Abstract
Despite the impressive efficacies demonstrated in preclinical research, hundreds of potentially neuroprotective drugs have failed to provide effective neuroprotection for ischemic stroke in human clinical trials. Lack of a powerful animal model for human ischemic stroke could be a major reason for the failure to develop successful neuroprotective drugs for ischemic stroke. This review recapitulates the available cerebral ischemia animal models, provides an anatomical comparison of the circle of Willis of each species, and describes the functional assessment tests used in these ischemic stroke models. The distinct differences between human ischemic stroke and experimental stroke in available animal models is explored. Innovative animal models more closely resembling human strokes, better techniques in functional outcome assessment and better experimental designs generating clearer and stronger evidence may help realise the development of truly neuroprotective drugs that will benefit human ischemic stroke patients. This may involve use of newer molecules or revisiting earlier studies with new experimental designs. Translation of any resultant successes may then be tested in human clinical trials with greater confidence and optimism.
Keywords: cerebral ischemia, circle of Willis, focal ischemic models, functional assessment tests, neuroprotection, preclinical model
Proposed modifications in animal laboratory methods to bridge the gaps between interventional studies in animal models and human trials.
Abbreviations
- BA
basilar artery
- CoW
circle of Willis
- ICA
internal carotid artery
- MCA
middle cerebral artery
- MCAo
middle cerebral artery occlusion
- NHPs
non‐human primates
- NIHSS
The National institute of health Stroke Scale
- NTR
normotensive rats
- OlfA
olfactory artery
- PCA
posterior cerebral artery
- rTPA
Recombinant Tissue Plasminogen Activator
- SHR
spontaneously hypertensive rats
- STAIR
Stroke Therapy Academic Industry Roundtable Preclinical Recommendations
1. INTRODUCTION
Stroke is a condition of rapidly developing loss of brain function due to disturbance in the blood supply to the brain causing neurologic impairments. Stroke presents as neurological deficits of sudden onset depending on the region of brain affected and the functional aspects of the affected part. The interference in the cerebral blood supply may be due to ischemia (in 85% of cases) or haemorrhage (in 15%). According to the American Stroke Association, stroke ranks as the fifth commonest cause of all mortality in industrialized nations and is the leading cause of long term disability in the elderly. 1 The reduction in the cerebral blood flow in ischemic stroke is due to a stenosis or occlusion of a cerebral artery by mechanisms such as thrombosis, embolism, vasculitis or vasospasm and leads to a hypoxic condition followed by the death of brain cells in the region. This ischemic‐hypoxic condition leads to excitotoxicity, peri‐infarct depolarization, production of reactive oxygen and nitrogen species, lipid peroxidation, calcium influx, inflammation, apoptosis and necrosis resulting in tissue damage. 2
Even though the pathophysiology of stroke is largely understood, the therapeutic options for treating acute ischemic stroke remain scarce, currently limited to antiplatelet drugs, thrombolytic therapy, mechanical thrombectomy, surgical decompressions, care in stroke units and rehabilitation. 3 , 4 For several years, the use of the clot busting drug recombinant tissue plasminogen activator (rTPA) to promote recanalization was the standard of care for the treatment of ischemic stroke, but recently acute mechanical thrombectomy has become a more effective but difficult option. However, the proportion of eligible ischemic stroke patients receiving rTPA or mechanical thrombectomy is small because of the limited time window of 3‐6 hours and the sophisticated infrastructure required for these treatments. 5 , 6 The various neuroprotective agents other than the clot thrombolysing drugs target specific pathways in the series of cascade events. Since, the mechanisms of action of these drugs are different, a combination of several agents targeting multiple pathways can provide a more effective therapeutic intervention for stroke. 7 Some of the common neuroprotective agents studied in preclinical research are summarized in Table 1.
TABLE 1.
Common neuroprotective agents used in stroke preclinical research
| Neuroprotective agents | Mechanism of action | Drugs | Outcome |
|---|---|---|---|
| Free‐radical scavengers/antioxidants | Scavenges the oxygen free radicals that cause destruction of cellular membranes | Tirilazad | Effective but potential source of bias 90 |
| Citicoline | Effective 91 | ||
| NXY‐059 | Effective but confounded by study quality 29 | ||
| Edaravone | Effective but study quality issue 92 | ||
| Ebselen | Effective but narrow therapeutic window 93 | ||
| NSP‐116 | Effective 94 | ||
| Calcium channel blockers | Blocks the abnormal increase in intercellular calcium levels, which activates several destructive enzymes | Nimidopine | No convincing evidence 95 |
| Flunarizine | Effective 96 | ||
| Glutamate antagonists | Blocks the activation of of glutamate receptors, increases the calcium ion influx leading to cell death | YM872 | Effective 97 |
| ZK200775 | Effective 98 | ||
| Aptiganel | Effective 99 | ||
| MK‐801 | Effective 100 | ||
| Anti‐inflammatory agents | Targets the inflammatory agents and mediators | Enlimomab | Effective 101 |
| LeukArrest | Effective 67 | ||
| rNIF | Effective 102 | ||
| Anti‐thrombotic agents | Prevents the formation of clots | Enoxaparin | Effective 103 |
| Heparin | Effective 104 | ||
| Aspirin | Effective 105 | ||
| Thrombolytic agent | Helps in lysing the clot that occludes the vessel | rTPA | Effective but side effects 106 |
| Multiple mechanisms | Cerebrolysins | Helps recovery of brain tissue surrounding infarction with synaptogenesis | |
| Other agents | Multiple targets | Statins | Effective (study quality issue) 107 |
| Hypothermia | Effective 108 | ||
| Human urinary kallidinogenase | Effective 109 | ||
| Granulocyte‐Colony Stimulating Factor | Effective 110 | ||
| Stem cells | Effective (study quality and publication bias) 111 |
Abbreviation: rTPA, Recombinant Tissue Plasminogen Activator.
Preclinical studies in animal models have a major role in contributing towards a better understanding of the pathophysiology and therapeutic options for stroke. Over the past few decades, this has resulted in the development of a number of experimental stroke animal models that resemble human stroke. Though neuroprotective strategies tried in these models have been theoretically very promising, there is a loss of translation in neuroprotection from preclinical to clinical studies. 8 , 9 , 10 Apart from the effects of species differences, this has been attributed to suboptimal ischemic stroke models and experimental designs, including outcome assessment methods. This narrative review summarizes different cerebral ischemia animal models, behavioural assessment methods used in these models and the confounding factors of preclinical and clinical stroke research.
2. PROBLEMS IN BENCH TO BEDSIDE TRANSLATION
Animal studies have contributed a lot to the understanding of the pathophysiology of stroke, and several animal species such as mice, rabbits and non‐human primates (NHPs) have been used as models to study stroke. Many hundreds of drugs have been tested preclinically in animal ischemic stroke models and found to be effective for neuroprotection, but very few proved to have even a semblance of clinically meaningful therapeutic benefit in human stroke trials. 11 To address the question of what makes an ideal stroke model, we must ascertain to what extent an experimental stroke animal model resembles human stroke.
2.1. Drug administration and the therapeutic window
In a large number of animal models used to investigate the effects of novel neuroprotective agents, these agents were used prior to the induction of ischemia. However, in obvious contradistinction, in human stroke, any practical neuroprotective drug must be used postictally. Therefore any meaningful trial of neuroprotective drugs in animal models needs to ensure the administration of neuroprotective drugs only after induction of ischemia and the appearance of definite features of brain injury. 12 , 13
Similarly, the therapeutic window of neuroprotective drug plays a major role. Most of the neuroprotective agents are known to have a short therapeutic window. Usually, in preclinical studies, neuroprotective drugs are administered immediately after the onset of stroke symptoms, whereas in clinical trials, the effects of exposure are often investigated beyond a thrombolytic/thrombectomy therapeutic time window of 6 hours or more. So while it is essential to administer the drugs as early as possible after the stroke onset, 3 it may also be necessary, from a practical point of view, to test drugs which work beyond such narrow windows.
Certain neuroprotective drugs such as citicoline, edaravone and ozagrel have been shown to be successful in phase II/III clinical trials. Edaravone, a free radical scavenger, received regulatory approval in Japan after a multicentre, randomised, placebo‐controlled, and double‐blind trial by the EAIS group in 2003 (N = 250). Even though the trial found the drug to be efficacious, adverse events in the form of acute renal failure were reported. 14 , 15 Similarly, the ICTUS multi‐centre trial conducted in Germany, Spain and Portugal to test the effectiveness of citicholine (N = 2298), was terminated after the results from an interim analysis showed no efficacy in moderate to severe stroke. 16 This was further supported by the 2020 Cochrane review by Martí‐Carvajal et al, which showed that citicoline made little or no difference to a favourable outcome when compared to controls. In addition, the authors reported that several studies included in the review had a high risk of bias, having been sponsored by pharmaceutical companies. Author neutrality is also scientifically questionable since some of the authors of the studies were receiving honoraria and consultation fees from drug firms. 17 A meta‐analysis of clinical trials on Sodium Ozagrel in treatment of acute ischemic stroke showed its effectiveness in improving short‐term impairment, but large scale, high quality studies are needed to prove beneficial effects on long‐term disability. 18 Cerebrolysin is another preparation that has been claimed to be effective in stroke therapy but the interpretations of the studies are far from uniform. 19 , 20 A meta‐analysis of 9 trials of cerebrolysin by Bornstein et al in 2018 showed that it is safe and efficacious in stroke therapy, 21 but the Cochrane review of cerebrolysin trials (2015) had failed to find similar result. 22
It is against this background that the Stroke Therapy Academic Industry Roundtable (STAIR) committee was formed and they recommended that certain preclinical criteria should be met before going on to large clinical trials. The committee was formed to provide recommendations for the preclinical evaluation of stroke therapies, phase II and phase III trial design, enhancing trial implementation and completion, novel approaches for measuring outcome, and regulatory considerations. 23
The first drug that was approved by the STAIR committee was the free radical scavenging drug NXY‐059. The drug was extensively studied in several animal models for transient and permanent stroke, from the rat to the marmoset primate including dose‐response studies which revealed neuroprotective effects of NXY‐059. 24 , 25 The neuroprotective role was also demonstrated as reduction in the infarct volume as a surrogate marker in thrombotic models of stroke in rabbits. 26 The effects of physiological parameters were also taken into account in preclinical studies when functional assessments were done for short and long term impact. 27 In order to minimise experimental biases, preclinical studies of NXY‐059 also incorporated newer clinical trial methods such as randomisation and blinding. In the larger SAINT‐II clinical trial that followed, NXY‐059 was inefficacious for the treatment of acute ischemic stroke when given within 6 hours of onset of symptoms. Even though NXY‐059 studies successfully fulfilled investigative criteria laid down by STAIR, the drug failed in these phase II‐III clinical trials to show its efficacy. 28 Subsequently no larger trials have reproduced these results. A meta‐analysis of the efficacy of NXY‐059 in animal models revealed that the results were confounded by low study quality. 29 Therefore the study design, conduct and reporting of results of preclinical studies play an important role.
2.2. The ideal stroke model
In preclinical research, the selection of the proper stroke model can be a critical step. Since the human stroke is heterogeneous, it is very difficult to produce a single perfect stroke model that mimics the human stroke completely. Depending upon the species and method of induction of stroke, the size, severity and the location of infarcts varies. 30 , 31 , 32 For instance, some techniques involve craniectomy‐like procedures, resulting in skull trauma, which is very unlikely in human stroke.
Other embolic techniques are less invasive but allow less control over the location and extent of the cerebral infarctions. 33 Hence an induction method that mimics the human clinical situation as closely as possible should be selected.
The complexity of the nervous system increases from one species to another in the hierarchy produced during the course of evolution. Rodents are the preferred species for preclinical studies of stroke but there are major differences in the brain composition of rodent and humans. Gyrencephaly is a mammalian specific trait present in humans, whereas rodents possess lissencephalic brains. 34 About 90% of rodent brain tissue consists of gray matter while human brains are only 50% gray matter. In the clinical situation, subcortical white matter strokes account for almost 25% of stroke subtypes but it is not easy to duplicate white matter strokes in rodents given their low white matter content. 35 The differences between clinical human ischemic stroke and animal models of ischemic stroke are summarised in Table 2.
TABLE 2.
Physio‐anatomical comparison of animal stroke models with human stroke victims
| Characteristics | Animal stroke models | Human stroke victims |
|---|---|---|
| Origin of stroke |
|
|
| Region affected |
|
|
| Population |
|
|
| Anatomy |
|
|
| Nature of stroke |
|
|
| Drug administration |
|
|
Abbreviation: MCA, middle cerebral artery.
Circle of Willis (CoW) is an anastomotic vascular network which connects the anterior and posterior parts as well as the left and right sides of the brain, providing a collateral blood flow when there is ischemia, vessel damage or any kind of occlusion. The status of collateral circulation is of utmost importance to stroke risk and its variations can influence the severity and progression of ischemic stroke. CoW is normal only in less than 50% of the population and is more commonly an incomplete circle in its anastomotic configuration. 36 Earlier studies have shown that patients with complete CoW have a lower National institute of health Stroke Scale (NIHSS) score, fewer neurological impairments and a better chance at recovery. 37 Similarly, in a study done in 2015 Seeters et al from the SMART group reported that patients with an incomplete anterior CoW had a higher risk of getting an anterior circulation stroke (Figure 1). 38
FIGURE 1.
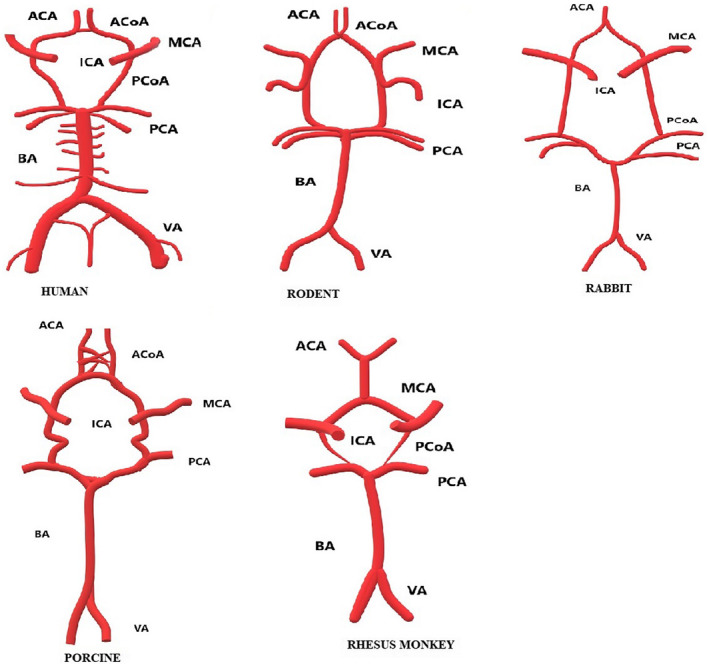
Circle of Willis (CoW) of mammalian stroke models. ACA, anterior cerebral artery; ACoA, anterior communicating artery; BA, basilar artery; ICA, internal carotid artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; PCoA, posterior communicating artery; VA, vertebral artery. (Hand drawn using Paint 3D based on the schematic representations in Sorby‐Adams, A. J., Vink, R., & Turner, R. J. (2018). Large animal models of stroke and traumatic brain injury as translational tools. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 315(2), R165‐R190. https://doi.org/10.1152/ajpregu.00163.201
In animals, for instance, the diameters of the posterior communicating arteries (CoA) of both sheep and pig are similar to the anterior cerebral artery. But in humans, the diameter of the posterior CoA is half that of the anterior cerebral artery, which leads to a reduced blood flow in posterior CoA in humans compared to animals. 39 Similarly in baboons, the bilateral anterior cerebral arteries are connected by a network of arteries, whereas in humans they are connected by a single vessel. 40 These variations play a major role in modelling stroke in animals as they can affect the collateral blood flow in CoW. Since the techniques used to induce stroke in animals depend solely on the collateral blood flow, they can negatively influence the efficacy of drugs.
Studies show that cerebral lesions that develop in hypertensive and normotensive patients can be significantly different. A pathological study was conducted on spontaneously hypertensive rats (SHR) and normotensive rats (NTR) of the Wistar strain, in which the cerebral infarction after a bilateral carotid artery ligation was examined. Only small cerebral infarctions appeared in 39% of NTR, whereas 82% of SHR developed diffused and extensive cerebral infarctions. 41 In many study series, the subjects preferred for preclinical research will be mostly young and healthy without comorbidities, whereas in reality a large proportion of affected stroke patients are aged with pre‐existing comorbidities and on one or more prescription drugs. 12
2.3. Study design and confounding factors
A variable which should be considered in study design is the use of anesthesia while inducing stroke in animal models. Anesthesia has been found to have some sort of neuroprotective role because the anesthetic changes certain metabolic parameters in the brain but none of them has proven to be a potential neuroprotective drug. Hence the use of anesthesia could hinder studies of neuroprotection. 42 , 43 In order to circumvent this possibility, studies tend to avoid anesthesia while inducing stroke models, which leads to another confounding factor whereby the animals suffer stress, which can adversely affect the results. 44
Another flaw which could partly account for the failure of translation research could be the presence of bias. Only a minority of the articles on preclinical studies published so far, were found to have mentioned details of study methods such as allocation concealment, randomisation and blinding, which are recommended by STAIR. 45 These study design features are essential to ensure a high standard of scientific inquiry. To limit bias in the conduct of the studies and to ensure the proper reporting of the experimental data, it is recommended to follow established guidelines such as ARRIVE or CAMARADES. 46
2.4. Outcome measures and functional assessment
Assessment of cognitive and behavioural responses to interventions in animal models is challenging because of obvious difficulties in communications between the investigators and the tested animals, and in deciphering what could be the equivalents of certain cognitive domains such as reasoning and language. The measuring of appropriate parameters of short and long‐term functional status are important in stroke recovery studies in clinical scenarios. Functional outcome measures such as the Barthel index, the Modified Rankin Scale and the NIHSS are widely used in human stroke trials. 47 , 48 Perhaps conceptually similar functional outcome measures need to be incorporated more in animal experimental studies, rather than measuring only surrogate outcomes like cerebral infarct size, since even small infarctions in certain locations can result in major functional deficits and vice versa. Currently, there are, of course, many behaviour assessment tests currently being used to analyse the post‐stroke functional status effects in animal models, which will continue to help in monitoring functional outcomes over time.
In addition, the minimum and maximum tolerated drug doses should be determined in preclinical studies in order to use the drugs successfully in clinical trials. In general, there is a tendency to lower the tolerated dose in clinical trials to avoid toxicity. On the other hand, in preclinical studies, drugs are generally administered for short periods after the ischemic stroke, so there is huge variability in the dosing schedule of clinical trials. Sometimes a single dose will not be sufficient to give the targeted response and excessive drug accumulation can also cause deleterious effects and reduced efficacy. 3 , 49
Taking account of these factors, the different mammalian models of focal ischemic stroke are discussed below.
3. MAMMALIAN MODELS OF FOCAL ISCHEMIC STROKE
3.1. Rodent models
Numerous animals have been used for preclinical stroke research but the choice of species for ischemic stroke studies has mostly been limited to rodents because they are relatively inexpensive and their cranial circulatory anatomy is reasonably similar to the human neurovascular anatomy. The ease with which the ischemic model can be controlled in terms of severity, duration and location of occlusion also makes rodents a preferred species. 50 , 51
3.1.1. Method of stroke induction
Rodents are suitable candidates for the different methods of induction available. The intraluminal filament model of middle cerebral artery occlusion (MCAo) is one the most widely used and established methods of stroke induction in rats and mice. These models exhibit a similar kind of penumbra, and large and reproducible infarcts that mimic human ischemic stroke. The models are also used for studying neuronal cell death and blood‐barrier damage, making them suitable candidates for neuroprotection studies. 52 The disadvantage of models developed through this method is the extent of tissue damage caused in the brain. In some studies, damage extended to the thalamus, substantia nigra and hippocampus. There is a chance of retinal ischemia in rodents given filamentous MCAo because of the close proximity of the ophthalmic artery to the middle cerebral artery (MCA). 53 The extensive damage can also result in subarachnoid haemorrhages. Such an induction method is not suitable for thrombolysis studies because, thrombolysis acts by dissolution of clots and re‐establishment of perfusion as in the human stroke scenario, whereas in the said technique, infarction is not induced by occlusion of vessels by clots. The preferred models for studying thrombolysis drugs are the photothrombosis and thromboembolic stroke models. Both these models induce stroke by occluding the vessels using clots and can be controlled in their severity and duration of occlusion. The former model induces vasogenic edema, 54 whereas the latter produces a small and variable cerebral infarct volume, 55 which is unlikely in human cases. The ischemic lesions formed by endothelin‐I administration are reproducible and there is also a marked reduction in the cerebral blood flow but the ischemia or hypoxia condition develops very slowly, with less edema, which does not resemble human ischemic stroke. 56
3.1.2. Anatomical comparison
Rodents are lissencephalic animals and their brains contain less than 10% white matter, unlike humans brains with more than 60%. 57 The CoW is collateral in both humans and rats, unlike some strains of mice. In the ddY strain of mice, the internal carotid artery (ICA) joins the CoW structure in between the MCA and the posterior cerebral artery (PCA). Hence, the CoW does not complete a circle: the CoW nourishes the anterior portion which branches out to the olfactory artery (OlfA), and the PCA, along with the MCA, and the basilar artery (BA) does not connect to the PCA. Thus, in humans, the ICA drains blood directly into the MCA, while in mouse, it is drained into the OlfA. 58 It is very important to note that a complete mouse CoW is present in only 10% of mice, while the posterior communicating artery is lacking unilaterally in 60% and bilaterally in 30% of the animals. 59
3.1.3. Neurobehavioral outcomes
The majority of ischemic stroke studies have been done in rodents and include several functional assessment tests such as neurological scales, sensorimotor tests and cognitive tests. The staircase, lateralized stepping, rotarod and apomorphine‐induced rotations are the most robust and reliable tests for assessing long‐term deficits in the 30 minute transient MCAo model of stroke. The Pasta test helps in assessment of fine motor coordination. Modified neurological score are helpful in measuring short‐term deficits and recovery. 60 Generally, stroke patients often suffer language disability, aphasia, and visual deficit along with other physical disabilities. But the majority of preclinical studies do not take this into account due to the difficulty in assessing these deficits in animals. Studies had shown the involvement of the cognitive area, suggesting that there is possibility of cognitive impairment after stroke in rodent models. 61 , 62 Radial Maze and Morris watermaze tests are used to assess spatial learning and memory impairments in rodents. 63 , 64
3.2. Rabbit model of ischemic stroke
The rabbit has been used extensively for embolic models of ischemic stroke. Thrombolysis studies done in rabbit embolic stroke models, in contrast to the rodent ischemic stroke models, have been more successful in translations to human therapeutic use. The current standard of care TPA treatment was evaluated early in rabbit embolic stroke models. 65
3.2.1. Method of stroke induction
The rabbit model of focal ischemia is induced by the occlusion of arteries by administering clots into the common carotid artery via a catheter. This can be achieved with both small and large clots. The rabbit small clot embolus model (RSCEM) results in the occlusion of random smaller arteries, resulting in heterogeneous stroke, while the rabbit large clot embolus model creates an obstructive embolus in the MCA. 65 , 66 Other approaches used for inducing focal ischemic stroke in rabbits are transorbital occlusion of MCA without craniectomy and transfemoral micro‐catheterization of occlusion of PCA, which produce large vessel ischemic stroke in rabbits, resulting in reproducible and stable infarcts. 67 , 68
3.2.2. Anatomical comparison
Rabbits are gyrencephalic and contain a significant amount of white matter, unlike rodents. They possess a well‐developed CoW, like humans. New Zealand white rabbits are the most widely used rabbit in embolic model of stroke studies. Like humans, these animals show individual variation in CoW. Around 29% of rabbits had a complete classical symmetrical CoW in a cohort of 100 rabbits having duplication of the MCA. Hence duplication of the MCA can skew the results of MCA targeted stroke studies. 69 Earlier models infused embolic agents using a catheter via a vertebral approach, but this approach can be complicated, if vertebral arteries are absent or hypoblastic. 65 , 69
3.2.3. Neurobehavioral outcomes
Neurological assessments in rabbit are still not established and standardized. The Wryneck model of neurological assessment includes tests for behaviour, reflex, stimuli reflex and posture. The animals are scored from 0 to 10, with normal animals having a score of 0 and dead animals, a score of 10. 70 A modified Bederson scoring has been used to evaluate neurological impairments in rabbits. The total score is 4, with a higher score indicating a more serious defect. 71
3.3. Pig model of ischemic stroke
The emergence of pig experimental models has increased dramatically because of the close resemblance between pig and human anatomy, physiology, and metabolic profile, and the pig's longer life span. Pigs are also widely used because they are commercially available, and are less costly to maintain and present fewer ethical issues than primate models.
3.3.1. Method of stroke induction
The occlusion of MCA in pigs is complicated compared to rodents and rabbits. Transorbital approaches have been used predominantly for MCA occlusion but there are disadvantages such as intraocular decompression and coagulation of the optic nerve or the enucleation of one eyeball to provide sufficient surgical space and adequate exposure of the MCA. 72 Hence, a transcranial technique has been developed which involved performance of a frontotemporal craniectomy along with orbital rim ostectomy and zygomatic arch resection, which consistently resulted in cerebral infarction with gray and white matter involvement but with an acceptably low morbidity. Unlike other animal models, using other approaches like embolus, catheter or ligation to induce stroke in pigs is difficult as they have a network of blood vessels called rete mirable in the skull. This collateralization of blood within the rete mirable limits the use of occlusion along with approaches. However a distinct disadvantage observed with this type of approach was a reduction in feeding behaviour in the animals. 57
3.3.2. Anatomical comparison
The gyrencephalic pig brain consists of more than 60% white matter, like humans, making them an easily translatable model of human neurological disorders. Human brains are 7.5 times the size of an average pig's brain. 57 The CoW structure of pigs is considered similar to humans, but the presence of rete mirable should be taken into consideration when developing models for cerebral ischemia. In addition, the ICA forms an integral part of the CoW before dividing into the terminal branches, namely the anterior and the middle cerebral arteries, while in humans it is not an integral part of the CoW, rather a connection point between the anterior and middle cerebral arteries. Similarly, the anterior communicating artery forms a network in the pig whereas in man it is a single artery. The PCA is a branch of the BA in man, forming an integral part of the CoW, whereas in pigs, posterior cerebrals do not take part in the formation of the circle as they are branches of posterior communicating arteries. 39
3.3.3. Neurobehavioral outcomes
Because of the similarity in brain structure of pigs and humans, pigs have been used to develop promising stroke animal models. A commonly used functional assessment is the open‐field test which helps to assess the locomotor activity and emotional reactivity of the animal. Studies have also used gait analysis which has shown significant asymmetries in both temporal and spatial variables. Pigs induced with stroke showed asymmetries in temporal parameters of the hind limb as well as maximum height of front hoof. 74 Some studies have used adaptations from neurological assessment performed on dogs to assess the post‐stroke injury. The tests include menace response, pupillary light reflex, qualitative gait assessment, postural reactions. Functional impairments in stroke models also include levels of consciousness, cranial nerve activity, changes in appetite, and circling behaviour. 57 , 75
3.4. Non‐human primate model of ischemic stroke
Non‐human primates, as in humans have a larger cerebral volume, and more white matter content in their gyrencephalic brains. The common species used for stroke research are marmosets of the Papio and Macaca species. The neurological examinations that are used for functional status and recovery in human studies can be applied to these animals. This is because they mimic human stroke more closely than other models. There are indeed subtle differences between species of primates in terms of brain, vascular anatomy, physiology and behaviour.
The method of stroke induction plays a major role in mimicking human stroke. The most common approaches used have been pterional craniotomy, craniotomy followed by electrocoagulation and the transorbital approach. The transorbital method, however, requires the enucleation of one eye, which will affect the physiology, psychology and post‐stroke neurological assessments. 76 These are invasive procedures that inflict pain and stress on the animal. 77 In order to overcome these disadvantages, an image guided endovascular technique was developed. In this technique, a guiding catheter is inserted into the femoral artery and is extended to reach the ICA. With the help of a microcatheter, an embolus (such as a balloon) is delivered to the MCA or an endovascular suture is applied to induce cerebral ischemia. This results in successful establishment of focal cerebral ischemia. 78 , 79
3.4.1. Anatomical comparison
Both gyrencephalic and lissencephalic non‐human primate species are used as focal ischemic stroke models. Callithrix jacchus (common marmoset) and Saimiri sciureus (squirrel monkey) are the most commonly used lissencephalic NHPs in stroke research. 80 The free radical trapping agent NXY‐059 was found to be a successful neuroprotectant in a common marmoset model of ischemic stroke, but was proven to be inefficacious in human clinical trials. 28 , 81 The structural architecture of a brain without convolutions could be one of the reasons behind its failure in humans. Gyrencephalic species possess larger brains that exhibit cortical organization, deep gray nuclei and white matter tracts like the internal capsule, white/gray matter ratios, and vascular distributions similar to humans. Macaca and Papio species are the most commonly used gyrencephalic NHPs. 80 The structure of CoWs of Macaca species look similar, with the absence of an anterior communicating artery. 82 Another study done in Macaca mullata has shown that there is a single distal anterior cerebral artery, unlike in humans. 83
3.4.2. Neurobehavioral outcomes
Functional recovery for non‐human primate animals depends upon the species of animal. Animals such as baboons, which are aggressive in nature, are only observed using a specialised non‐human primate stroke scale. This is a qualitative variant of the NIHSS in NHPs. This sensorimotor test assesses the motor coordination, visual field defects and cognitive defects. 84 The two‐tube choice task reveals the spatial neglect and the hand preference of stroke induced NHPs. 85 The hill and valley staircase task helps to assess the motor impairment of the contralateral arm and the degree of spatial neglect associated with cerebral ischemia and it is also helpful in separating motor deficits from spatial neglect. Marshall and Ridley (2000) developed a test called the six‐tube search task to understand spatial deficits in stroke induced NHPs. 86 The Kluver board task helps to assess the overall strength of the extremity, fine motor function and hemineglect or visual field cuts. 87 Cognitive tasks help to assess memory and learning deficits. The object retrieval detour task has been used in a chronic ischemic stroke model in cynomogolous macaques, and helps to evaluate cognitive deficits associated with motor function in reaching movements. 77 The Wisconsin General Testing apparatus consists of a battery of behavioural tests like the delayed non‐matching‐to‐sample test, the spatial and color conditions of the delayed recognition span test, spatial reversal learning, and object reversal learning, which have been used in aged rhesus monkeys to study the pattern of cognitive decline. 88
3.5. Other non‐primate mammalian models
The pursuit on models closer to the human species has led to experiments in other species. These include sheep, cats and dogs. The STAIR guidelines recommend using two different species of animals for studying the efficacy of a drug, with initial studies preferably on rodents and further studies on larger animals, before going on to clinical trials. 46 , 89
4. CONCLUSION
Table 3 summarises the major differences between rodent and larger animal ischemic models and gives an overview of these models. Though models using larger and more evolved animal species more closely resemble human strokes, experiments in these models are plagued by a number of practical considerations and ethical questions that have significantly hampered their use in animal studies. Whether their use may lead to greater scientific advancement and development of drugs and devices useful in human stroke treatment interventions is a matter of intense debate. Better design of animal experiments and better publishing guidelines for animal studies to minimise publications of biased reports are also absolutely essential. Though discussion on this subject is not within the scope of this review, serious deliberations aimed at developing a clear scientific consensus and its support by national governments round the globe are urgently needed. The neuroprotective strategy will continue to remain a vague but achievable goal until then. Experimental studies and therapeutic interventions should be designed in such a way that they resemble and have relevance to the human condition. With the help of optimised, ethically sound and scientifically robust animal research models and study designs, we can bridge the gap between bench and bedside in stroke therapy.
TABLE 3.
Comparison of rodent and large animal models
| Rodents | Large animals | |
|---|---|---|
| Physiological characteristics | Dissimilar | Similar to humans |
| Mimicking human stroke | Poorly | Better |
| Anatomical similarity | Lissencephalic and less white matter | Gyrencephalic and more white matter like humans |
| Induction of stroke involving craniectomy | Difficult to perform as they have small brain | Easy to perform neurosurgery to induce stroke |
| MCAo by suture | Easy to perform | Difficult |
| Facilities required for the surgery | Doesn't need bigger equipment to induce stroke | Need facility and equipment similar to humans to perform surgery |
| MCAo procedure, anesthesia and monitoring | Easy | Complicated |
| Cerebral infarctions | Stable and can be controlled | Unstable |
| In vivo structural and functional imaging of brain such as MRI, PET and CT | Yes, but specially designed equipment is needed | Yes, the equipment used for human studies can be used for the imaging |
| Neurologic evaluation and neurological behaviours | Contain numerous tests which account for different parameters in rodents | Similar to humans |
| Post‐operative injuries | Mild | Severe |
| Transgenic manipulations | Easy | Difficult |
| Ethical issues | Low | High |
| Care and maintenance | Easy | Difficult |
| Reproductive period | Fast | Slow |
| Cost | Less | High |
Abbreviation: MCAo, middle cerebral artery occlusion.
CONFLICT OF INTEREST
The authors have no conflict of interest.
AUTHOR SPECIFICS
Dr Sunil Narayan was a former Commonwealth split‐site PhD fellow at the Centre for Life and Institute of Biomedicine at the University of Newcastle and is currently the Professor in charge of Neurobiology programme and a Senior Professor in the Department of Neurology at the Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry. Ms Simy Grace is a PhD Scholar and Junior Research Fellow (CSIR), and Dr A Murugesan is a Post‐doctoral Fellow, Scientists’ pool (CSIR) being trained at the same facility, in JIPMER. Dr Prakash Babu is currently Dean of the School of Medical Science, University of Hyderabad and is also working on animal models of ischemic stroke and other neurodegenerative disorders. Dr C Saravana Babu is currently working as Associate Professor in the Department of Pharmacology, JSS Mysuru and Dr A. Hannah Rachel Vasanthi was a former CV Raman Fellow, now working as Professor and Head in the Department of Biotechnology, at the University of Pondicherry, India.
ACKNOWLEDGEMENT
Ms Simy Grace Cherian and Dr Murugesan Arumugam were funded by Council of Scientific and Industrial Research (CSIR), Government of India through a Junior Research Fellowship and the Scientist's Pool Scheme, respectively. This work was funded by the Core Research Grant scheme of the SERB division of the Department of Science and Technology, Government of India. (Grant No. CRG/2019/002076). The author would like to thank JIPMER e‐library facility and national knowledge network, India for providing access to the NCBI scientific journals and other relevant e‐data.
Narayan SK, Cherian SG, Babu PP, Babu CS, Vasanthi AHR, Arumugam M. Preclinical animal studies in ischemic stroke: Challenges and some solutions. Anim Models Exp Med. 2021;4:104–115. 10.1002/ame2.12166
REFERENCES
- 1. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):E139‐E596. [DOI] [PubMed] [Google Scholar]
- 2. Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391‐397. [DOI] [PubMed] [Google Scholar]
- 3. Cheng YD, Al‐Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx. 2004;1(1):36‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Min J, Farooq MU, Gorelick PB. Neuroprotective agents in ischemic stroke: past failures and future opportunities. Clin Investig (Lond). 2013;3(12):1167‐1177. [Google Scholar]
- 5. The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581‐1587. [DOI] [PubMed] [Google Scholar]
- 6. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317‐1329. [DOI] [PubMed] [Google Scholar]
- 7. Moretti A, Ferrari F, Villa RF. Neuroprotection for ischaemic stroke: current status and challenges. Pharmacol Ther. 2015;146:23‐34. [DOI] [PubMed] [Google Scholar]
- 8. Green AR, Shuaib A. Therapeutic strategies for the treatment of stroke. Drug Discovery Today. 2006;11(15‐16):681‐693. [DOI] [PubMed] [Google Scholar]
- 9. Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87(1):179‐197. [DOI] [PubMed] [Google Scholar]
- 10. Casals JB, Pieri NCG, Feitosa MLT, et al. The use of animal models for stroke research: a review ‐ PubMed. Comp Med. 2011;61(4):305‐313. https://pubmed.ncbi.nlm.nih.gov/22330245/ [PMC free article] [PubMed] [Google Scholar]
- 11. O’Collins VE, Macleod MR, Donnan GA, Horky LL, Van Der Worp BH, Howells DW. 1,026 Experimental treatments in acute stroke. Ann Neurol. 2006;59(3):467‐477. [DOI] [PubMed] [Google Scholar]
- 12. O’Collins VE, Donnan GA, MacLeod MR, Howells DW. Hypertension and experimental stroke therapies. J Cereb Blood Flow Metab. 2013;33(8):1141‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kotoda M, Ishiyama T, Mitsui K, Hishiyama S, Matsukawa T. Neuroprotective effects of amiodarone in a mouse model of ischemic stroke. BMC Anesthesiol. 2017;17(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edaravone Acute Infarction Study Group . Effect of a novel free radical scavenger, edaravone (MCI‐186), on acute brain infarction. Cerebrovasc Dis. 2003;15(3):222‐229. [DOI] [PubMed] [Google Scholar]
- 15. Feng S, Yang Q, Liu M, et al. Edaravone for acute ischaemic stroke. Cochrane Database Syst Rev. 2011(12). CD007230. 10.1002/14651858.CD007230.pub2 [DOI] [PubMed] [Google Scholar]
- 16. Dávalos A, Alvarez‐Sabín J, Castillo J, et al. Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo‐controlled study (ICTUS trial). Lancet. 2012;380(9839):349‐357. [DOI] [PubMed] [Google Scholar]
- 17. Martí‐Carvajal AJ, Valli C, Martí‐Amarista CE, Solà I, Martí‐Fàbregas J, Bonfill CX. Citicoline for treating people with acute ischemic stroke. Cochrane Database Syst Rev. 2020;8:CD013066. 10.1002/14651858.CD013066.pub2. PMID: 32860632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang J, Yang J, Chang X, Zhang C, Zhou H, Liu M. Ozagrel for acute ischemic stroke: a metaanalysis of data from randomized controlled trials. Neurol Res. 2012;34(4):346‐353. [DOI] [PubMed] [Google Scholar]
- 19. Heiss WD, Brainin M, Bornstein NM, Tuomilehto J, Hong Z. Cerebrolysin in patients with acute ischemic stroke in Asia: Results of a double‐blind, placebo‐controlled randomized trial. Stroke. 2012;43(3):630‐636. [DOI] [PubMed] [Google Scholar]
- 20. Gharagozli K, Harandi AA, Houshmand S, et al. Efficacy and safety of Cerebrolysin treatment in early recovery after acute ischemic stroke: a randomized, placebo‐controlled, double‐blinded, multicenter clinical trial. J Med Life. 2017;10(3):153‐160. ncbi.nlm.nih.gov/pmc/articles/PMC5652261/ [PMC free article] [PubMed] [Google Scholar]
- 21. Bornstein NM, Guekht A, Vester J, et al. Safety and efficacy of Cerebrolysin in early post‐stroke recovery: a meta‐analysis of nine randomized clinical trials. Neurol Sci. 2018;39(4):629‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ziganshina LE, Abakumova T. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2015;(6):CD007026. [DOI] [PubMed] [Google Scholar]
- 23. Albers GW, Goldstein LB, Hess DC, et al. Stroke treatment academic industry roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra‐arterial and neuroprotective therapies. Stroke. 2011;42(9):2645‐2650. [DOI] [PubMed] [Google Scholar]
- 24. Marshall JWB, Cummings RM, Bowes LJ, Ridley RM, Green AR. Functional and histological evidence for the protective effect of NXY‐059 in a primate model of stroke when given 4 hours after occlusion. Stroke. 2003;34(9):2228‐2233. [DOI] [PubMed] [Google Scholar]
- 25. Sydserff SG, Borelli AR, Green AR, Cross AJ. Effect of NXY‐059 on infarct volume after transient or permanent middle cerebral artery occlusion in the rat; studies on dose, plasma concentration and therapeutic time window. Br J Pharmacol. 2002;135(1):103‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lapchak PA, Araujo DM, Song D, Wei J, Zivin JA. Neuroprotective effects of the spin trap agent disodium‐[(tert‐butylimino)methyl]benzene‐1,3‐disulfonate N‐oxide (generic NXY‐059) in a rabbit small clot embolic stroke model: combination studies with the thrombolytic tissue plasminogen activator. Stroke. 2002;33(5):1411‐1415. [DOI] [PubMed] [Google Scholar]
- 27. Kuroda S, Tsuchidate R, Smith ML, Maples KR, Siesjö BK. Neuroprotective effects of a novel nitrone, NXY‐059, after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1999;19(7):778‐787. [DOI] [PubMed] [Google Scholar]
- 28. Shuaib A, Lees KR, Lyden P, et al. NXY‐059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357(6):562‐571. [DOI] [PubMed] [Google Scholar]
- 29. MacLeod MR, Van Der Worp HB, Sena ES, Howells DW, Dirnagl U, Donnan GA. Evidence for the efficacy of NXY‐059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke. 2008;39(10):2824‐2829. [DOI] [PubMed] [Google Scholar]
- 30. Robinson MJ, Macrae IM, Todd M, Reid JL, McCulloch J. Reduction of local cerebral blood flow to pathological levels by endothelin‐1 applied to the middle cerebral artery in the rat. Neurosci Lett. 1990;118(2):269‐272. [DOI] [PubMed] [Google Scholar]
- 31. Busch E, Krüger K, Hossmann K‐A. Improved model of thromboembolic stroke and rt‐PA induced reperfusion in the rat. Brain Res. 1997;778(1):16‐24. [DOI] [PubMed] [Google Scholar]
- 32. Schroeter M, Jander S, Stoll G. Non‐invasive induction of focal cerebral ischemia in mice by photothrombosis of cortical microvessels: characterization of inflammatory responses. J Neurosci Methods. 2002;117(1):43‐49. [DOI] [PubMed] [Google Scholar]
- 33. Sommer CJ. Ischemic stroke: experimental models and reality. Acta Neuropathol. 2017;133(2):245‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kelava I, Lewitus E, Huttner WB. The secondary loss of gyrencephaly as an example of evolutionary phenotypical reversal. Front Neuroanat. 2013;7(June):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sozmen EG, Hinman JD, Carmichael ST. Models that matter: white matter stroke models. Neurotherapeutics. 2012;9(2):349‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kapoor K, Singh B, Dewan LIJ. Variations in the configuration of the circle of Willis. Anat Sci Int. 2008;83(2):96‐106. [DOI] [PubMed] [Google Scholar]
- 37. Zhou H, Sun J, Ji X, et al. Correlation between the integrity of the circle of willis and the severity of initial noncardiac cerebral infarction and clinical prognosis. Med (United States). 2016;95(10):e2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Seeters T, Hendrikse J, Biessels GJ, et al. Completeness of the circle of Willis and risk of ischemic stroke in patients without cerebrovascular disease. Neuroradiology. 2015;57(12):1247‐1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ashwini CA, Shubha R, Jayanthi KS. Comparative anatomy of the circle of Willis in man, cow, sheep, goat, and pig. Neuroanatomy. 2008;7:54‐65. [Google Scholar]
- 40. Kapoor K, Kak VK, Singh B. Morphology and comparative anatomy of circulus arteriosus cerebri in mammals. Anat Histol Embryol. 2003;32(6):347‐355. [DOI] [PubMed] [Google Scholar]
- 41. Ogata J, Fujishima M, Morotomi Y, Omae T. Cerebral infarction following bilateral carotid artery ligation in normotensive and spontaneously hypertensive rats: a pathological study. Stroke. 1976;7(1):54‐60. [DOI] [PubMed] [Google Scholar]
- 42. Kawaguchi M, Furuya H, Patel PM. Neuroprotective effects of anesthetic agents. J Anesth. 2005;19(2):150‐156. [DOI] [PubMed] [Google Scholar]
- 43. Seto A, Taylor S, Trudeau D, et al. Induction of ischemic stroke in awake freely moving mice reveals that isoflurane anesthesia can mask the benefits of a neuroprotection therapy. Front Neuroenergetics. 2014;6:1. 10.3389/fnene.2014.00001. PMID: 24765075; PMCID: PMC3982055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walker FR, Jones KA, Patience MJ, Zhao Z, Nilsson M. Stress as necessary component of realistic recovery in animal models of experimental stroke. J Cereb Blood Flow Metab. 2014;34(2):208‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hirst JA, Howick J, Aronson JK, et al. The need for randomization in animal trials: an overview of systematic reviews. PLoS One. 2014;9(6):e98856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marc F, Giora F, Howells David W, Hurn Patricia D, KentThomas A, Savitz Sean ILEH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244‐2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Banks JL, Marotta CA. Outcomes validity and reliability of the modified rankin scale: implications for stroke clinical trials. Stroke. 2007;38(3):1091‐1096. [DOI] [PubMed] [Google Scholar]
- 48. Harrison JK, McArthur KS, Quinn TJ. Assessment scales in stroke: clinimetric and clinical considerations. Clin Interv Aging. 2013;8:201‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jonas S, Aiyagari V, Vieira D, Figueroa M. The failure of neuronal protective agents versus the success of thrombolysis in the treatment of ischemic stroke. The predictive value of animal models. Ann NY Acad Sci. 2001;939:257‐267.ncbi.nlm.nih.gov/pubmed/11462778 [DOI] [PubMed] [Google Scholar]
- 50. Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Exp Transl Stroke Med. 2010;2(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener. 2011;6(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clark W, Lessov N, Dixon M, Eckenstein F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res. 1997;19(6):641‐648. [DOI] [PubMed] [Google Scholar]
- 53. Steele EC, Guo Q, Namura S. Filamentous middle cerebral artery occlusion causes ischemic damage to the retina in mice. Stroke. 2008;39(7):2099‐2104. [DOI] [PubMed] [Google Scholar]
- 54. Dietrich WD, Watson BD, Busto R, Ginsberg MD, Bethea JR. Photochemically induced cerebral infarction. I. Early microvascular alterations. Acta Neuropathol. 1987;72(4):315‐325. [DOI] [PubMed] [Google Scholar]
- 55. Chen Y, Zhu W, Zhang W, et al. A novel mouse model of thromboembolic stroke. J Neurosci Methods. 2015;256:203‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schirrmacher R, Dea M, Heiss WD, et al. Which aspects of stroke do animal models capture? A multitracer micro‐PET study of focal ischemia with endothelin‐1. Cerebrovasc Dis. 2016;41(3‐4):139‐147. [DOI] [PubMed] [Google Scholar]
- 57. Platt SR, Holmes SP, Howerth EW, et al. Development and characterization of a Yucatan miniature biomedical pig permanent middle cerebral artery occlusion stroke model. Exp Transl Stroke Med. 2014;6(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Okuyama S, Okuyama J, Okuyama J, et al. The arterial circle of Willis of the mouse helps to decipher secrets of cerebral vascular accidents in the human. Med Hypotheses. 2004;63(6):997‐1009. [DOI] [PubMed] [Google Scholar]
- 59. Schröder H, Moser N, Huggenberger S. Neuroanatomy of the Mouse. Cham, Switzerland: Springer International Publishing; 2020. [Google Scholar]
- 60. Trueman RC, Diaz C, Farr TD, et al. Systematic and detailed analysis of behavioural tests in the rat middle cerebral artery occlusion model of stroke: tests for long‐term assessment. J Cereb Blood Flow Metab. 2017;37(4):1349‐1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Markgraf CG, Green EJ, Hurwitz BE, et al. Sensorimotor and cognitive consequences of middle cerebral artery occlusion in rats. Brain Res. 1992;575(2):238‐246. [DOI] [PubMed] [Google Scholar]
- 62. Zvejniece L, Svalbe B, Liepinsh E, Pulks E, Dambrova M. The sensorimotor and cognitive deficits in rats following 90‐ and 120‐min transient occlusion of the middle cerebral artery. J Neurosci Methods. 2012;208(2):197‐204. [DOI] [PubMed] [Google Scholar]
- 63. Deacon RMJ, Rawlins JNP. T‐maze alternation in the rodent. Nat Protoc. 2006;1(1):7‐12. [DOI] [PubMed] [Google Scholar]
- 64. Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zivin JA, Lyden PD, Degirolami U, et al. Tissue plasminogen activator: reduction of neurologic damage after experimental embolic stroke. Arch Neurol. 1988;45(4):387‐391. [DOI] [PubMed] [Google Scholar]
- 66. Lapchak PA, Araujo DM, Pakola S, Song D, Wei J, Zivin JA. Microplasmin: a novel thrombolytic that improves behavioral outcome after embolic strokes in rabbits. Stroke. 2002;33(9):2279‐2284. [DOI] [PubMed] [Google Scholar]
- 67. Yenari MA, Kunis D, Sun GH, et al. Hu23F2G, an antibody recognizing the leukocyte CD 11/CD 18 integrin, reduces injury in a rabbit model of transient focal cerebral ischemia. Exp Neurol. 1998;153(2):223‐233. [DOI] [PubMed] [Google Scholar]
- 68. English JD, Hetts SW, El‐Ali A, et al. A novel model of large vessel ischemic stroke in rabbits: microcatheter occlusion of the posterior cerebral artery. J Neurointerv Surg. 2015;7(5):363‐366. [DOI] [PubMed] [Google Scholar]
- 69. Caldwell B, Flores R, Lowery J, Brown AT, Culp WC. Variations in the circle of Willis in the New Zealand White rabbit. J Vasc Interv Radiol. 2011;22(8):1188‐1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brown A, Woods S, Skinner R, et al. Neurological assessment scores in rabbit embolic stroke models. Open Neurol J. 2013;7(1):38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Feng L, Liu J, Chen J, Pan L, Feng G. Establishing a model of middle cerebral artery occlusion in rabbits using endovascular interventional techniques. Exp Ther Med. 2013;6(4):947‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Imai H, Konno K, Nakamura M, et al. A new model of focal cerebral ischemia in the miniature pig. J Neurosurg Pediatr. 2006;104(2):123‐132. [DOI] [PubMed] [Google Scholar]
- 73. Webb RL, Kaiser EE, Jurgielewicz BJ, et al. Human neural stem cell extracellular vesicles improve recovery in a porcine model of ischemic stroke. Stroke. 2018;49(5):1248‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Duberstein KJ, Platt SR, Holmes SP, et al. Gait analysis in a pre‐ and post‐ischemic stroke biomedical pig model. Physiol Behav. 2014;125:8‐16. [DOI] [PubMed] [Google Scholar]
- 75. Lau VW, Platt SR, Grace HE, Baker EW, West FD. Human iNPC therapy leads to improvement in functional neurologic outcomes in a pig ischemic stroke model. Brain Behav. 2018;8(5):e00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Huang J, Mocco J, Choudhri TF, et al. A modified transorbital baboon model of reperfused stroke. Stroke. 2000;31(12):3054‐3063. [DOI] [PubMed] [Google Scholar]
- 77. Roitberg B, Khan N, Tuccar E, et al. Chronic ischemic stroke model in cynomolgus monkeys: behavioral, neuroimaging and anatomical study. Neurol Res. 2003;25(1):68‐78. [DOI] [PubMed] [Google Scholar]
- 78. Wu D, Chen J, Wang B, et al. Endovascular ischemic stroke models of adult rhesus monkeys: a comparison of two endovascular methods. Sci Rep. 2016;6(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Debatisse J, Wateau O, Cho TH, et al. A non‐human primate model of stroke reproducing endovascular thrombectomy and allowing long‐term imaging and neurological read‐outs. J Cereb Blood Flow Metab. 2021;41(4):745–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cook DJ, Tymianski M. Nonhuman primate models of stroke for translational neuroprotection research. Neurotherapeutics. 2012;9(2):371‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Marshall JWB, Duffin KJ, Green AR, Ridley RM. NXY‐059, a free radical‐trapping agent, substantially lessens the functional disability resulting from cerebral ischemia in a primate species. Stroke. 2001;32(1):190‐198. [DOI] [PubMed] [Google Scholar]
- 82. Kumar N, Lee JJ, Perlmutter JS, Derdeyn CP. Cervical carotid and circle of Willis arterial anatomy of macaque monkeys: a comparative anatomy study. Anat Rec. 2009;292(7):976‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kassell NF, Langfitt TW. Variations in the circle of willis in Macaca mulatta. Anat Rec. 1965;152(3):257‐263. [DOI] [PubMed] [Google Scholar]
- 84. Savitz SI, Schäbitz WR. Reviving neuroprotection using a new approach targeting postsynaptic density‐95 to arrest glutamate excitotoxicity. Stroke. 2012;43(12):3411‐3412. [DOI] [PubMed] [Google Scholar]
- 85. Marshall JWB, Cross AJ, Jackson DM, Green AR, Baker HF, Ridley RM. Clomethiazole protects against hemineglect in a primate model of stroke. Brain Res Bull. 2000;52(1):21‐29. [DOI] [PubMed] [Google Scholar]
- 86. Marshall JWB, Ridley RM. Assessment of cognitive and motor deficits in a marmoset model of stroke. ILAR J. 2003;44(2):153‐160. [DOI] [PubMed] [Google Scholar]
- 87. Dai PM, Huang H, Zhang L, et al. A pilot study on transient ischemic stroke induced with endothelin‐1 in the rhesus monkeys. Sci Rep. 2017;7:45097. 10.1038/srep45097. PMID: 28358140; PMCID: PMC5372164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87(1):25‐34. [DOI] [PubMed] [Google Scholar]
- 89. Cai B, Wang N. Large animal stroke models vs. rodent stroke models, pros and cons, and combination? Brain Edema XVI Transl Basic Sci into Clin Pract Acta Neurochir Suppl. 2016;121:77‐81. [DOI] [PubMed] [Google Scholar]
- 90. Sena E, Wheble P, Sandercock P, Macleod M. Systematic review and meta‐analysis of the efficacy of tirilazad in experimental stroke. Stroke. 2007;38(2):388‐394. [DOI] [PubMed] [Google Scholar]
- 91. Bustamante A, Giralt D, Garcia‐Bonilla L, Campos M, Rosell A, Montaner J. Citicoline in pre‐clinical animal models of stroke: a meta‐analysis shows the optimal neuroprotective profile and the missing steps for jumping into a stroke clinical trial. J Neurochem. 2012;123(2):217‐225. [DOI] [PubMed] [Google Scholar]
- 92. Wu S, Sena E, Egan K, Macleod M, Mead G. Edaravone improves functional and structural outcomes in animal models of focal cerebral ischemia: a systematic review. Int J Stroke. 2014;9(1):101‐106. [DOI] [PubMed] [Google Scholar]
- 93. Lapchak PA, Zivin JA. Ebselen, a seleno‐organic antioxidant, is neuroprotective after embolic strokes in rabbits: synergism with low‐dose tissue plasminogen activator. Stroke. 2003;34(8):2013‐2018. [DOI] [PubMed] [Google Scholar]
- 94. Imai T, Iwata S, Miyo D, Nakamura S, Shimazawa M, Hara H. A novel free radical scavenger, NSP‐116, ameliorated the brain injury in both ischemic and hemorrhagic stroke models. J Pharmacol Sci. 2019;141(3):119‐126. [DOI] [PubMed] [Google Scholar]
- 95. Horn J, de Haan RJ, Vermeulen M, Luiten PGM, Limburg M. Nimodipine in animal model experiments of focal cerebral ischemia. Stroke. 2001;32(10):2433‐2438. [DOI] [PubMed] [Google Scholar]
- 96. De Ryck M, Van Reempts J, Borgers M, Wauquier A, Janssen PA. Photochemical stroke model: flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke. 1989;20(10):1383‐1390. [DOI] [PubMed] [Google Scholar]
- 97. Wang PF, Zhou Y, Fang H, et al. Treatment of acute cerebral ischemia using animal models: A meta‐analysis. Transl Neurosci. 2015;6(1):47‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Turski L, Huth A, Sheardown M, et al. ZK200775: a phosphonate quinoxalinedione AMPA antagonist for neuroprotection in stroke and trauma | PNAS. PNAS. 1998;95(18):10960‐10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Minematsu K, Fisher M, Li L, et al. Effects of a novel NMDA antagonist on experimental stroke rapidly and quantitatively assessed by diffusion‐weighted MRI. Neurology. 1993;43(2):397‐403. [DOI] [PubMed] [Google Scholar]
- 100. Yi N‐X, Zhou L‐Y, Wang X‐Y, et al. MK‐801 attenuates lesion expansion following acute brain injury in rats: a meta‐analysis. Neural Regen Res. 2019;14(11):1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang RL, Chopp M, Jiang N, et al. Anti‐intercellular adhesion molecule‐1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke. 1995;26(8):1438‐1442. [DOI] [PubMed] [Google Scholar]
- 102. Jiang N, Moyle M, Soule HR, Rote WE, Chopp M. Neutrophil inhibitory factor is neuroprotective after focal Ischemia in rats. Ann Neurol. 1995;38(6):935‐942. [DOI] [PubMed] [Google Scholar]
- 103. Zhang ZG, Sun X, Zhang QZ, Yang H. Neuroprotective effects of ultra‐low‐molecular‐weight heparin on cerebral ischemia/reperfusion injury in rats: Involvement of apoptosis, inflammatory reaction and energy metabolism. Int J Mol Sci. 2013;14(1):1932‐1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mary V, Wahl F, Uzan A, Stutzmann JM. Enoxaparin in experimental stroke: neuroprotection and therapeutic window of opportunity. Stroke. 2001;32(4):993‐999. [DOI] [PubMed] [Google Scholar]
- 105. Yan BC, Park JH, Shin BN, et al. Neuroprotective effect of a new synthetic aspirin‐decursinol adduct in experimental animal models of ischemic stroke. Minnerup J, ed. PLoS One. 2013;8(9):e74886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dong MX, Hu QC, Shen P, et al. Recombinant tissue plasminogen activator induces neurological side effects independent on thrombolysis in mechanical animal models of focal cerebral infarction: A systematic review and meta‐analysis. PLoS One. 2016;11(7):e0158848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. García‐Bonilla L, Campos M, Giralt D, et al. Evidence for the efficacy of statins in animal stroke models: a meta‐analysis. J Neurochem. 2012;122(2):233‐243. [DOI] [PubMed] [Google Scholar]
- 108. van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta‐analysis. Brain. 2007;130(12):3063‐3074. [DOI] [PubMed] [Google Scholar]
- 109. Zhang N, Meng F, Bao W, et al. Efficacy of human urinary kallidinogenase in ischemic stroke in animal models: a meta‐analysis. J Int Med Res. 2020;48(9):3000605209434522019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Minnerup J, Heidrich J, Wellmann J, Rogalewski A, Schneider A, Schabitz WR. Meta‐analysis of the efficacy of granulocyte‐colony stimulating factor in animal models of focal cerebral ischemia. Stroke. 2008;39(6):1855‐1861. [DOI] [PubMed] [Google Scholar]
- 111. Lees JS, Sena ES, Egan KJ, et al. Stem cell‐based therapy for experimental stroke: a systematic review and meta‐analysis. Int J Stroke. 2012;7(7):582‐588. [DOI] [PubMed] [Google Scholar]


