Abstract
Background
The neuropsychiatric disorders due to post‐streptococcal autoimmune complications such as Sydenham's chorea (SC) are associated with acute rheumatic fever and rheumatic heart disease (ARF/RHD). An animal model that exhibits characteristics of both cardiac and neurobehavioral defects in ARF/RHD would be an important adjunct for future studies. Since age, gender, strain differences, and genotypes impact on the development of autoimmunity, we investigated the behavior of male and female Wistar and Lewis rat strains in two age cohorts (<6 weeks and >12 weeks) under normal husbandry conditions and following exposure to group A streptococcus (GAS).
Methods
Standard behavioral assessments were performed to determine the impairments in fine motor control (food manipulation test), gait and balance (beam walking test), and obsessive‐compulsive behavior (grooming and marble burying tests). Furthermore, electrocardiography, histology, and behavioral assessments were performed on male and female Lewis rats injected with GAS antigens.
Results
For control Lewis rats there were no significant age and gender dependent differences in marble burying, food manipulation, beam walking and grooming behaviors. In contrast significant age‐dependent differences were observed in Wistar rats in all the behavioral tests except for food manipulation. Therefore, Lewis rats were selected for further experiments to determine the effect of GAS. After exposure to GAS, Lewis rats demonstrated neurobehavioral abnormalities and cardiac pathology akin to SC and ARF/RHD, respectively.
Conclusion
We have characterised a new model that provides longitudinal stability of age‐dependent behavior, to simultaneously investigate both neurobehavioral and cardiac abnormalities associated with post‐streptococcal complications.
Keywords: animal model, group A streptococcus, Lewis rats, paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS), rheumatic fever, rheumatic heart disease, sydenham chorea (SC)
1. INTRODUCTION
Human infections with Streptococcus pyogenes (group A streptococcus, GAS) constitute up to 700 million cases annually. 1 Repeated or untreated GAS infections may trigger autoimmune diseases including acute rheumatic fever (ARF), which could lead to rheumatic heart disease (RHD), acute post‐streptococcal glomerulonephritis (APSGN) and paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) in children and young adults. 2 , 3 Sydenham chorea (SC), a neurological manifestation observed in a proportion of patients with ARF is also presumed to be immune‐mediated. 3 Manifestations of ARF/RHD occur due to inflammatory changes to the heart, brain, joint, and connective tissue. 2 A patient with ARF can have both carditis and neurobehavioral complications simultaneously or these can be separate. Indeed SC, which manifests in movement disorder, is one of the major criteria for ARF diagnosis. 4 , 5 In contrast PANDAS is characterised by a sudden onset of obsessive‐compulsive disorder (OCD) associated with GAS infection. 6 The mechanisms behind these neurobehavioral syndromes remain unclear. 7 However, it has been shown that antibodies against GAS cross‐react with neurotransmitter receptor (D1 and D2 dopamine receptors) signaling kinases and ion channels, located primarily in striatal regions of the basal ganglia in susceptible hosts, due to antigen molecular mimicry. 8
Post‐streptococcal autoimmune complications are uniquely human conditions and modeling of these symptoms in animals is challenging. To date, a limited number of animal models of GAS related neurobehavioral disorders have been reported. 9 , 10 , 11 , 12 , 13 , 14 Rodent models have been invaluable in studying mechanism of complex pathologies of post‐streptococcal sequelae including RHD, 15 , 16 , 17 , 18 , 19 , 20 APSGN 21 , 22 , 23 and SC/PANDAS. 9 , 10 , 11 , 12 , 13 , 14 Both Wistar and Lewis rats have been used to examine autoimmune diseases and behavioral changes. For example, Wistar rats have been used to investigate autoimmune diseases including spontaneous thyroiditis, 24 type II collagen‐induced arthritis, 25 autoimmune pancreatitis 26 and autoimmune encephalomyelitis, 27 and neurobehavioral changes such as anxiety, 28 epilepsy, 29 post‐traumatic stress 30 and complications of meningitis. 31 Lewis rats have been used to investigate experimental autoimmune encephalitis, 32 multiple sclerosis. 33 and post‐streptococcal autoimmune diseases., 11 , 12 , 15 , 16 , 17 , 18 , 19 , 20 However, no animal models have been used to simultaneously assess neurobehavioral and cardiac complications associated with post‐streptococcal autoimmune complications. Since genotypes, gender and age affect the development of autoimmune complications, 34 identification of an appropriate animal model is important to investigate the pathogenesis of post‐streptococcal complications. Hence, firstly, we assessed Wistar and Lewis rat strains to determine whether one or both are suitable to study GAS associated neurobehavioral disorders by assessing the longitudinal responses of rats that were raised under normal husbandry conditions using standardised behavioral tests. We found that Lewis rats demonstrate no age‐related effects and hence are more suitable for our study. Then, using both male and female Lewis rats, we simultaneously demonstrated both neurobehavioral changes and cardiac abnormalities subsequent to GAS injection. We herein also demonstrate that our Lewis rat model is well suited to investigate neurobehavioral and electrocardiographic and inflammatory changes in cardiac tissues, enabling future longitudinal studies on immunopathogenesis and investigation of novel treatment modalities, and therefore provides a realistic model for testing vaccine safety and efficacy.
2. METHODS
All animal experiments were conducted in accordance with national guidelines for the care and use of laboratory animals. These experimental protocols were approved by the Animal Ethics Committee of University of New England (AEC18‐092; 19‐013).
2.1. Animals and behavioral tests
Six‐ and twelve‐week‐old Wistar (Crl:WI) and Lewis rats (LEW/Crl) (both male and female) were purchased from the Centre for Animal Research and Teaching, University of New England. All animals used in this study were experimentally naive; they were housed three per cage and kept in a controlled environment maintained at a constant temperature (20‐22°C) and humidity (50%) with access to laboratory rodent chow (Gordon's Premium Rat & Mouse Pellets, Australia) and water ad libitum. The following behavioral tests were performed using 5 male and 5 female 6‐ and 12‐week‐old Wistar and Lewis rats. The grooming test and food manipulation tests were conducted under infrared light while the beam walking and marble burying test were conducted in normal light. The marble burying test was manually analysed by a researcher. All other sessions were manually recorded with a HD digital video recorder (Swann, Australia).
2.2. Food manipulation test (fine motor control)
One day after a 10 minute exposure to a 38 × 21 cm plexiglass observation box (habituation), 24‐hour food‐deprived rats were placed in the box, and 5 minutes later two pellets of (approximately 2 g each) rodent laboratory chow were placed in the box. Rats were given 10 minutes to consume the food pellet, and their ability to manipulate the food was blind‐rated independently by two observers, using the Kolb and Holmes scale 35 : (0) unable to manipulate the food pellet with the forepaws; (1) holds the food pellet against the floor with its forepaws when it eats; (2) eats the food pellet from the floor and sometimes hold the food pellet in its forepaws; (3) picks the food pellet up in its forepaws and partially eats it but drops the food pellet before it is all consumed; and (4) sits up on its hind paws and holds the food pellet in its front paws until it is finished. 36
2.3. Beam walking test (gait and balance)
Motor coordination and balance were assessed by measuring the ability of rats to traverse a narrow (2.5 cm) square wood beam mounted on a narrow support that was elevated 30 cm above the ground. On day 1 each rat was given three training trials on a 5 cm wide beam. On day 2, after one training trial on the 5 cm beam, rats were given three training trials on a 2.5 cm wide beam and the time it took each rat to traverse the 2.5 cm beam on the third trial and the number of foot slips were recorded. 37
2.4. Grooming (OCD)
Each rat was videotaped individually for 30 minutes on three consecutive days in a 38 × 21 cm plexiglass observation box. Ten minutes after the beginning of each session, the rat was misted with water to induce grooming. The duration of grooming behavior in terms of number of grooming bouts and body region of grooming was assessed for 20 minutes immediately after misting. 38
2.5. Marble burying (OCD)
Rats were placed individually in a cage measuring 37 cm long × 21 cm wide × 18 cm high, containing Corncob bedding at a depth of 5 cm that contained nine marbles 2.3 cm in diameter arranged in two rows along the short wall of the cage. The number of marbles buried or covered half or more by the bedding by each rat over a 15 minute period was determined.
2.6. Preparation of formalin‐killed group A streptococcus
Streptococcus pyogenes (GAS) M type 5 strain (stock: M5 GAS, M5T5.2A, 01.02.2008) was plated on sheep blood agar (SBA) and incubated overnight at 37°C with 5% CO2. A single colony of GAS from the SBA plate was inoculated into 1 L of Todd Hewitt yeast broth and incubated overnight at 37°C with 5% CO2. The total colony forming unit (CFU) in 1 L of Todd Hewitt yeast broth was determined by serially diluting the culture from 1:10 to 1:1012 and then plating in triplicate on SBA, and incubating overnight at 37°C with 5% CO2. After counting the number of colonies in each dilution plate the bacterial concentration was adjusted to 1 × 1011 CFU/mL, allowing 1 × 1010 CFU in 100 μL for injections. The bacteria in the broth culture were pelleted by centrifuging at 4000× g for 10 minutes. The bacterial pellet was washed three times with PBS, resuspended in 10 ml PBS plus 1% (of total volume) neutral buffered formalin, and incubated at 4°C for 48 hours to prepare whole formalin‐killed GAS antigens. To confirm the sterility of the preparations, they were plated on to SBA plates and incubated for another 48 hours at 37°C with 5% CO2. No viable bacterial colonies were detected.
2.7. Injection of rats
Following anesthesia with isofluorane (5% induction and 2% maintenance) and oxygen, Lewis rats (both male and female) were injected subcutaneously (s.c.) with 1 × 1010 CFU in 100 μL of formalin‐killed GAS or 100 μL of PBS for controls, in complete Freund's adjuvant (Sigma, Australia) in the hock. 15 , 16 , 17 , 19 , 20 In addition on days 1 and 3, rats were intraperitoneally administered 0.3 μg of Bordetella pertussis toxin (Gibco, USA). On day 7, 14, 21, 35, and 56 after the primary injection, both rat groups received booster injections of 1 × 1010 CFU in 100 μL of formalin‐killed GAS or PBS in incomplete Freund's adjuvant (Sigma, Australia) s.c. in the flank. All rats were euthanized 70 days after primary injection. Hearts and brains were dissected following transcardial perfusion and tissues were fixed in 10% neutral‐buffered formalin for further analysis. 15 , 16 , 17 , 19 , 20
2.8. Electrocardiography
As a functional test for heart, electrocardiography (ECG) was performed on all experimental rats before injection and prior to euthanasia. Rats were anesthetised using 5% isofluorane and maintained at 2% during the ECG with 1‐1.5 L/min of oxygen. The ECG traces were recorded using AD Instruments (Power Laboratories). The electrical potentials were recorded for 1‐2 minutes with LabChart 8 software provided by AD Instruments (Power Laboratories). After performing ECG, the data were visualised using LabChart 8 software. The peak values of P and R points at three different segments of ECG from each rat were individually extracted and analysed.
2.9. Histology
Formalin‐fixed paraffin‐embedded (FFPE) sections of rat heart sections were stained with Harris Haematoxylin and Eosin (H&E) and examined microscopically for infiltration of inflammatory cells, fibrosis or necrosis as evidence of myocarditis or valvulitis. The heart sections were examined using a light microscope (Olympus, Germany). The mitral valvulitis and myocarditis scores from each rat were summed to achieve a “carditis” score based on the number of inflammatory cells, focal lesions and Aschoff‐type lesions according to previously published methods. 20
2.10. Statistical analysis
A three‐way analysis of variance (ANOVA) with post hoc analysis using Tukey's test (Prism version 8 software GraphPad, USA) was used to assess statistically significant differences between the age (<6 weeks vs >12 weeks), gender (male vs female) and strain (Lewis vs Wistar). A two‐way ANOVA was used to assess behavioral and ECG differences between rats treated with GAS and PBS. All data were reported as means ± SEM and P values < .05 were considered significant.
3. RESULTS
3.1. Comparison of rat behavioral phenotypes
To define the age‐ and gender‐dependent behavioral stability of Lewis and Wistar rat strains under normal husbandry conditions, both strains were subjected to a series of standardised behavioral tests (food manipulation, beam walking, grooming and marble burying). No significant differences were observed between the two strains (P = .9519), gender (P = .7418) and the two age groups (P = .1066) in the food manipulation test (Figure 1A). Similarly, no significant differences were observed in the time taken to traverse the beam in the beam walking test between the two strains of rats or between genders at 6 weeks of age. However, male Wistar rats older than 12 weeks took significantly more time to transverse the narrow beam compared to both male (P < .01) and female (P < .001) Lewis rats older than 12 weeks (Figure 1B). Six‐week‐old Lewis and Wistar rats displayed no significant differences in marble burying behavior. In contrast, male Wistar rats older than 12 weeks buried significantly more marbles than either male (P < .01) or female Lewis rats older than 12 weeks (P = .01; Figure 1C). Significant differences were observed between strain, age and gender in the grooming test. Male Lewis rats less than 6 weeks old and more than 12 weeks old spent significantly (P < .001) more time grooming than male and female Wistar rats on all three observation days (Figure 1D‐F). However, no significant differences were observed in grooming behavior within the strain, age and gender parameters (Figure 1D‐F). There were no significant changes observed between days 1 to 3 in grooming behavior of both Wistar and Lewis rats (P > .001). However, the grooming behavior of male Lewis rats younger than 6 weeks was significantly reduced (P = .0217; Figure 1D‐F). Thus, under normal husbandry conditions, Lewis rats had a more stable age‐ and gender‐dependant behavioral phenotype than Wistar rats as determined by standardised food manipulation, beam walking, marble burying and grooming behavioral tests. Therefore, due to the stability of their behavioral phenotype, the Lewis rat strain was chosen as the preferred animal model to investigate GAS‐induced neurobehavioral changes and cardiac pathology.
FIGURE 1.
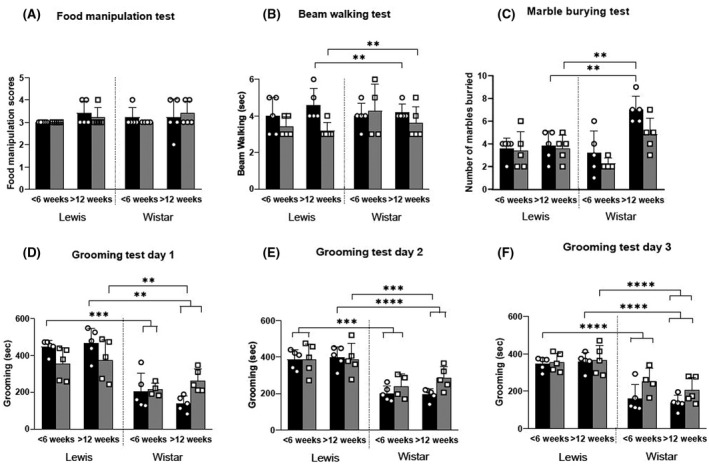
Longitudinal neurobehavioral changes in Lewis and Wistar rats. Neurobehavioral changes were assessed in male (■) and female ( ) Lewis and Wistar rats less than 6 weeks and more than 12 weeks of age. The following tests were carried out: food manipulation (A), beam walking (B), marble burying (C), and grooming tests on days 1‐3 (D‐F). In each group (n = 5), age‐matched rats were used. These tests indicate that there are no significant neurobehavioral differences in the Lewis rats at the two different ages tested. *Significantly different from the control group (*P < .05, **P < .01, ***P < .001, ****P < .0001)
) Lewis and Wistar rats less than 6 weeks and more than 12 weeks of age. The following tests were carried out: food manipulation (A), beam walking (B), marble burying (C), and grooming tests on days 1‐3 (D‐F). In each group (n = 5), age‐matched rats were used. These tests indicate that there are no significant neurobehavioral differences in the Lewis rats at the two different ages tested. *Significantly different from the control group (*P < .05, **P < .01, ***P < .001, ****P < .0001)
3.2. Exposure to GAS is associated with an altered neurobehavioral phenotype in Lewis rats
To determine if a GAS injection significantly altered the baseline neurobehavioral phenotype of Lewis rats, the animals were subjected to GAS injection in their hock and their behaviors monitored using the same suite of standardised behavioral tests. Food manipulation scores, time taken to traverse the narrow beam, total grooming time and number of marbles buried by male and female Lewis rats injected with formalin‐killed GAS or PBS are provided in Figure 2. Both male and female rats injected with GAS showed difficulties in handling food pellets (P = .0066 and P = .0086) and took more time in traversing the narrow (2.5 cm) beam without any foot slips (P < .0001 and P = .0101). Male and female rats injected with GAS spent more time grooming in the induced grooming test than control rats on all three days without any loss of hair and skin lesions (P < .0001 and P < .0001). GAS‐injected rats (male and female) buried more marbles than control rats (P < .0001 and P = .003).
FIGURE 2.
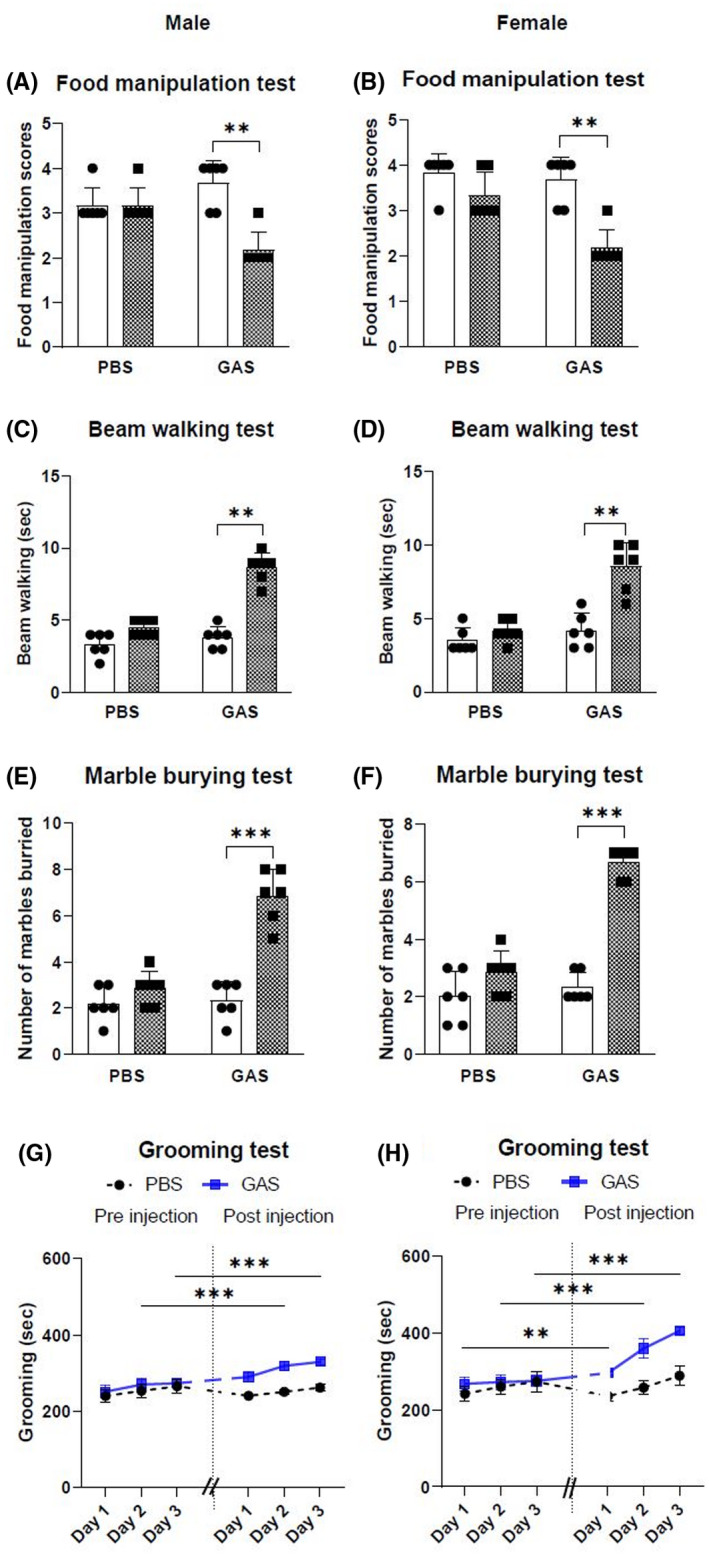
Neurobehavioral changes in Lewis rats of both sexes following injection with GAS. Neurobehavioral changes were assessed in male and female Lewis rats before injection (□) and after injection ( ) using the following tests: food manipulation (A, B), beam walking (C, D), marble burying (E, F) and grooming (G, H). Following GAS injection impairment in manipulating the food pellets (A, B) and traversing the narrow beam (C, D) were observed in both male and female rats compared to controls, and GAS‐injected rats buried more marbles than control rats (E, F). Increased grooming behavior was observed in rats of both sexes following GAS injection compared to controls (G, H). In each experiment (n = 6), age‐matched rats were used. *Significantly different from the control group, (*P < .05, **P < .01, ***P < .001)
) using the following tests: food manipulation (A, B), beam walking (C, D), marble burying (E, F) and grooming (G, H). Following GAS injection impairment in manipulating the food pellets (A, B) and traversing the narrow beam (C, D) were observed in both male and female rats compared to controls, and GAS‐injected rats buried more marbles than control rats (E, F). Increased grooming behavior was observed in rats of both sexes following GAS injection compared to controls (G, H). In each experiment (n = 6), age‐matched rats were used. *Significantly different from the control group, (*P < .05, **P < .01, ***P < .001)
3.3. Exposure to GAS induces electrocardiographic and histological changes
In this study, conduction abnormalities were detected by ECG. Prolonged P‐R intervals were observed in rats injected with GAS (Figure 3) compared with findings for the control rats. Prolongation of the P‐R interval on ECG is a hallmark of conduction abnormalities observed in ARF/RHD. H&E‐stained sections of hearts from rats injected with GAS were examined for evidence of cardiac lesions and compared to heart tissue from control rats immunized with PBS. Histological examination of heart sections demonstrated infiltration of mononuclear cells with variable degrees of inflammatory changes (Figure 4). In comparison, the heart tissues of PBS‐treated control rats showed little or no evidence of inflammation. All rats injected with GAS had mononuclear cell infiltration characteristic of inflammatory changes and patchy granulomatous cells were observed in the myocardium (Figure 4). Mononuclear cell infiltration into valves of GAS‐injected rats was also observed.
FIGURE 3.
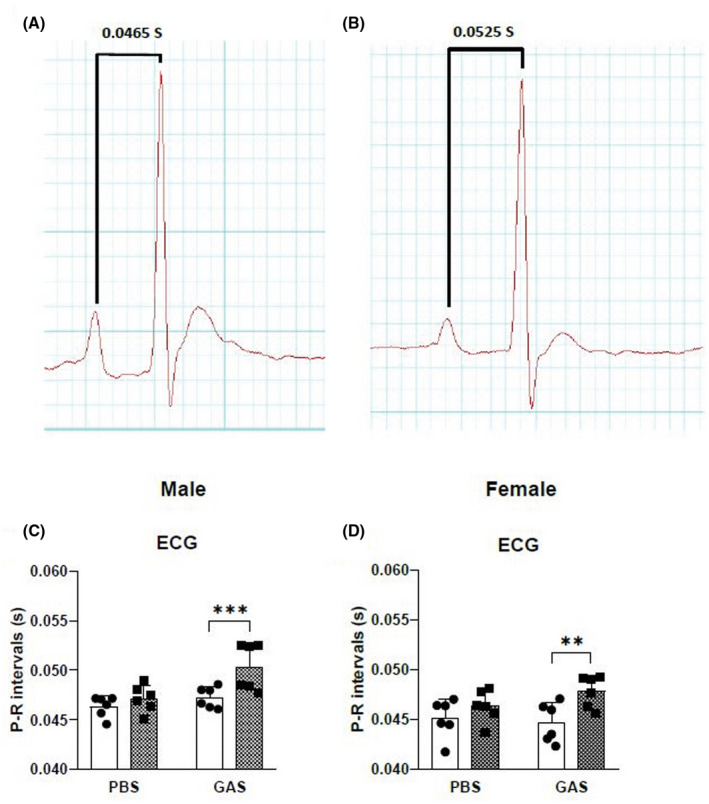
Electrocardiographic changes in Lewis rats following GAS injection. Representative ECG traces from a PBS‐ (A) and a GAS‐injected (B) rat demonstrate a prolongation of P‐R interval consistent with conduction abnormalities due to carditis. (C and D) Prolongation of P‐R interval in ECG was observed in male (C) and female rats (D) following injection with GAS after 70 days post primary injection. □, Pre injection;  , Post injection. In each group (n = 6), age‐matched rats were used. *Significantly different from the control group, (*P < .05, **P < .01, ***P < .001, Two‐way ANOVA)
, Post injection. In each group (n = 6), age‐matched rats were used. *Significantly different from the control group, (*P < .05, **P < .01, ***P < .001, Two‐way ANOVA)
FIGURE 4.
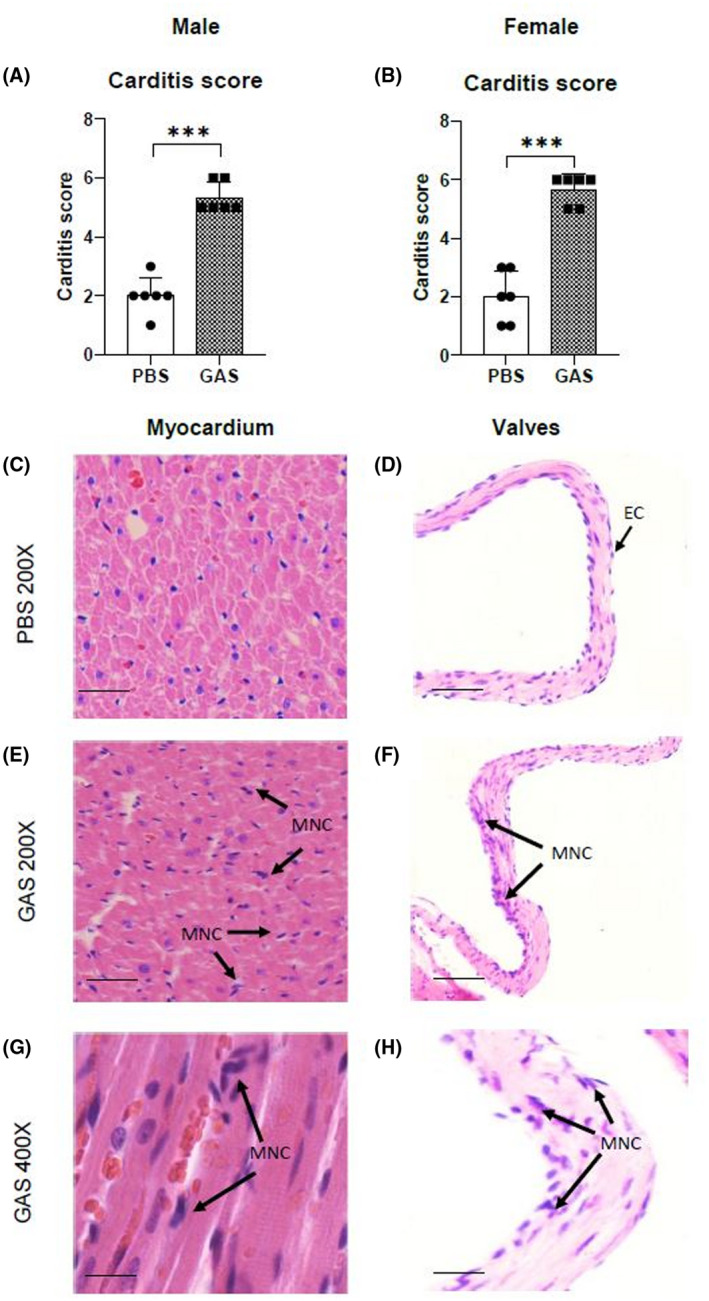
Histological changes in Lewis rats following GAS injection. □, Pre injection;  , Post injection. Carditis scores were significantly higher in Lewis rats injected with GAS (A, B) compared to control rats. In histological sections of cardiac tissue from rats injected with GAS, evidence of inflammatory changes in the myocardium (E, G) and valvular tissue (F, H) characterised by mononuclear cell infiltration (arrows) were observed. Whereas in control rats minimal evidence of mononuclear cell infiltration was observed in the myocardium (C) and in valvular tissue (D). In each group (n = 6), age‐matched rats were used. *Significantly different from the control group, (*P < .05, **P < .01, ***P < .001, Two‐way ANOVA). MNC: mononuclear cells; EC: endothelial cell. Representative images (C‐H); Bars 50 µm
, Post injection. Carditis scores were significantly higher in Lewis rats injected with GAS (A, B) compared to control rats. In histological sections of cardiac tissue from rats injected with GAS, evidence of inflammatory changes in the myocardium (E, G) and valvular tissue (F, H) characterised by mononuclear cell infiltration (arrows) were observed. Whereas in control rats minimal evidence of mononuclear cell infiltration was observed in the myocardium (C) and in valvular tissue (D). In each group (n = 6), age‐matched rats were used. *Significantly different from the control group, (*P < .05, **P < .01, ***P < .001, Two‐way ANOVA). MNC: mononuclear cells; EC: endothelial cell. Representative images (C‐H); Bars 50 µm
4. DISCUSSION
There is now an extensive body of both clinical 8 , 39 , 40 , 41 , 42 and experimental evidence 9 , 10 , 11 , 12 , 13 , 14 that unequivocally suggests that autoimmune mechanisms underpin the development of SC and PANDAS. The unifying feature of both these entities is a preceding infection with GAS. Since the early nineteenth century, 43 SC has been recognised to be closely associated with the symptomatology of ARF/RHD and remains as a major diagnostic criteria for ARF/RHD. 4 , 5 While the manifestations of SC, including the neurological abnormalities of involuntary movements and psychiatric symptomatology meeting the criteria of OCD, have been associated with ARF/RHD, the classical description of PANDAS, an entity that was described in the late twentieth century, 44 , 45 , 46 does not include these involuntary movements. Clinically distinguishing the differences in neurobehavioral abnormalities between SC and PANDAS is problematic. Delineating the immunopathological mechanisms contributing to overlapping and the unrelated symptomatology between these two clinical entities is challenging.
ARF/RHD is a uniquely human disease. Therefore, while developing models for this condition, it is important that both the cardiac and neurobehavioral changes observed in the clinical condition are replicated in the experimental model. To date, the animal models developed to determine the mechanisms of post‐streptococcal sequelae have either investigated the cardiac pathology 15 , 16 , 17 , 19 , 20 , 47 , 48 , 49 or the neurobehavioral aspects of SC. 9 , 10 , 11 , 12 , 13 , 14 Previously no other models have been described that enable the simultaneous investigation of both cardiac and neurobehavioral changes that are known to be pathognomonic in ARF/RHD.
Mice and rats display different behaviors. OCD‐like behaviors such as induced grooming and marble burying and motor deficits such as food manipulation are predominantly observed in rats rather than mice. Based on the antibody deposition sites in the brain, the pathology in rats may more closely resemble human pathology. 50 , 51 , 52 For the first murine model developed to determine neurobehavioral disorders associated with GAS, Hoffman et al in 2004 used female SJL/J mice 9 and demonstrated decreased ambulation during open‐field tests to novel surroundings, 9 , 10 impaired motor coordination in Rotarod tests and increased rearing behavior following exposure to GAS antigens. Immunopathological studies revealed that in SJL/J mice, anti‐GAS antibody deposition was mainly in the hippocampus and deep cerebellar nuclei but very little in basal ganglia. 9 , 10 , 13 The Lewis rat model to determine neurobehavioral changes developed by Brimberg et al 11 showed that male rats injected with killed GAS had impaired food handling ability and motor control behavior. Furthermore, rats showed increased grooming after exposure to GAS antigens when compared to control rats. 11 In addition, adoptive transfer of serum from GAS‐injected rats into the striatum of recipient Lewis rats resulted in impairment in beam walking and obsessive marble burying compared to naive rats. 12 In these rats, autoantibody deposition was predominantly observed in the prefrontal cortex and the basal ganglia. 11 , 12 Although the mechanism whereby the compromised blood‐brain barrier (BBB) enables these antibodies to cross into the brain and deposit in the basal ganglia has been debated, recent studies have clearly demonstrated in mice that following intranasal challenge with GAS, GAS‐specific Th17 cells from the nasal‐associated lymphoid tissue migrate into the brain, breaking the BBB to facilitate microglial activation and IgG deposition, leading to neurobehavioral changes. 14 , 53 Compromised BBB in rodents facilitates autoantibody deposition in deep cerebellar nuclei (DCN), globus pallidus, thalamus, hippocampus and periventricular area, caudate nucleus, lateral hypothalamus and piriform cortex. 9 , 10 , 13 , 14 The studies that investigated the post‐streptococcal neurobehavioral changes did not determine whether the experimental animals developed cardiac pathology.
The female Lewis rat model has been used to determine autoimmune pathologies such as RHD 15 , 16 , 17 , 18 , 19 , 20 and SC, 11 , 12 and other autoimmune diseases such as experimental autoimmune encephalitis 32 and multiple sclerosis. 33 The Lewis rat autoimmune valvulitis (RAV) model originally described by Quinn et al 48 and later characterised by our group 15 , 16 , 17 , 18 , 19 , 20 has been universally accepted as a reliable model to study cardiac abnormalities associated with ARF/RHD. In the carditis models that have been previously described it was found that: (a) antibodies generated against GAS M protein and GlcNAc (N‐acetyl‐βD‐glucosamine) cross‐react with bacterial and cardiac tissue 2 , 54 , 55 , 56 , 57 ; (b) adoptive transfer of the anti‐streptococcal antibodies causes carditis in recipient animals 58 ; (c) inflammatory cells were present in valvular and myocardial lesions of animals injected with GAS antigens 19 , 49 , 58 , 59 ; (d) that the T cells infiltrating the cardiac tissue were of the Th17 phenotype 20 , 60 ; (e) both anti‐streptococcal antibodies and T cells are able to activate vascular endothelial cells facilitating the transmigration of effector cells into tissue 59 ; and (f) generation of anti‐streptococcal antibodies and T cells leads to cardiac functional abnormalities detectable by ECG and echocardiography. 19 , 20 , 59 However, none of these studies charcterised a rodent model that can be simultaneously used to determine both the cardiac and neurobehavioral changes that are hallmarks of ARF/RHD. Therefore, we set out to determine whether a model that encompasses both cardiac and neurobehavioral changes could be successfully developed to conduct longitudinal immunopathological and mechanistic studies.
Since post‐streptococcal neurobehavioral changes such as are seen in SC are prominent in females, 61 , 62 and PANDAS and tic disorders are predominantly seen in males, 63 , 64 most neurobehavioral studies described in the literature have either been conducted on male and female mice 9 , 10 or exclusively on male rats. 11 , 12 In this study we initially characterised both male and female Wistar and Lewis rats at different ages to determine their suitability for examining longitudinal neurobehavioral changes. Examination of neurobehavioral complications associated with GAS‐induced autoimmunity should use a species in which changes in motor deficits and compulsive and stereotyped behaviors can be easily measured. 7 However, the motor skill and compulsive behavior stability of rat strains must be determined before selecting the most appropriate breed for examining neuropathology induced by GAS autoimmunity. In this study, data from standardised behavioral tests demonstrated that Lewis rats had greater compulsive behavior and motor skill with phenotypic stability compared to Wistar rats.
Both male and female Lewis rats injected with GAS antigens showed neurobehavioral changes similar to those observed in SC and PANDAS. Similar to the studies exclusively carried out on male rats by Brimberg et al, 11 in our studies we observed impairment in handling food and in traversing the narrow beam (2.5 cm width) in both male and female rats. Based on these findings it is evident that both male and female Lewis rats developed impairments in fine motor control and gait similar to patients with SC and PANDAS. 65 In addition, the grooming behavior of rats injected with GAS was remarkably increased compared to control rats and these rats buried more marbles in marble burying tests, reflecting a form of compulsive behavior resembling that of OCD in patients with SC and PANDAS. 61 , 65 , 66
Conduction defects occur in patients with ARF/RHD and prolongation of P‐R interval is observed. 1 , 2 , 3 Our study revealed that in both male and female Lewis rats injected with GAS antigens there was an increase in infiltration of inflammatory cells into the myocardium and valvular tissues. Therefore both male and female Lewis rats injected with GAS developed carditis/valvulitis and prolongation of the P‐R interval similar to that seen in previous experimental animal studies 19 , 20 , 59 and in patients with ARF/RHD. 1 , 2
Collectively, these data suggest that Lewis rats are preferred over Wistar rats when considering an animal model suitable for a longitudinal time course study, to examine the manifestation of post‐streptococcal neurobehavioral and cardiac abnormalities akin to ARF/RHD. Therefore, we believe that the current model can be used in further studies to: (a) simultaneously determine the sequential immunopathological mechanisms that lead to different symptoms and signs in multiple organs in patients with ARF/RHD, (b) test new therapeutic agents for ARF/RHD and complications, and (c) conduct toxicology, efficacy and safety studies of novel anti‐streptococcal vaccine candidates. We conclude that due to the stability of their compulsive and motor behavioral responses and the cardiac functional and histological changes observed following GAS injection, Lewis rats remain the favoured animal model to simultaneously investigate both cardiac and neurobehavioral changes.
CONFLICT OF INTEREST
No conflicts of interest relevant to this manuscript were reported.
AUTHOR CONTRIBUTIONS
All authors meet the requirements for authorship as stipulated. NK, DJM and KSS conceptualized the study. RAMR, NK, ASH and NMA, designed the experiments. RAMR, NK and ASH performed the animal experiments and analysed the data. All authors contributed to the drafting of the manuscript and have approved the final version of the manuscript for submission.
ACKNOWLEDGMENTS
The authors wish to acknowledge Dr Tom Van Der Touw and Craig Lawlor of the University of New England for their assistance in performing electrocardiography on the rats and Dr Daniel Ebert for facilitating the project.
Rafeek RAM, Lobbe CM, Wilkinson EC, et al. Group A streptococcal antigen exposed rat model to investigate neurobehavioral and cardiac complications associated with post‐streptococcal autoimmune sequelae. Anim Models Exp Med. 2021;4:151–161. 10.1002/ame2.12164
Funding information
RAM Rafeek is recipient of International Postgraduate Research Award (IPRA) from University of New England. CM Lobbe and E. Wilkinson are recipients of student scholarship from the Royal College of Pathologists of Australasia (RCPA).
REFERENCES
- 1. Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685‐694. [DOI] [PubMed] [Google Scholar]
- 2. Carapetis JR, Beaton A, Cunningham MW, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. 2016;2:15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walker MJ, Barnett TC, McArthur JD, et al. Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin Microbiol Rev. 2014;27(2):264‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones TD. The diagnosis of rheumatic fever. JAMA. 1944;126(8):481‐484. [Google Scholar]
- 5. Gewitz MH, Baltimore RS, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131(20):1806‐1818. [DOI] [PubMed] [Google Scholar]
- 6. Macerollo A, Martino D. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS): an evolving concept. Tremor Other Hyperkinet Mov (NY). 2013;3. 10.7916/D8ZC81M1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cutforth T, DeMille MM, Agalliu I, Agalliu D. CNS autoimmune disease after Streptococcus pyogenes infections: animal models, cellular mechanisms and genetic factors. Future Neurol. 2016;11(1):63‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody‐mediated neuronal cell signaling in Sydenham chorea. Nat Med. 2003;9(7):914‐920. [DOI] [PubMed] [Google Scholar]
- 9. Hoffman KL, Hornig M, Yaddanapudi K, Jabado O, Lipkin WI. A murine model for neuropsychiatric disorders associated with group A beta‐hemolytic streptococcal infection. J Neurosci. 2004;24(7):1780‐1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yaddanapudi K, Hornig M, Serge R, et al. Passive transfer of streptococcus‐induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol Psychiatry. 2010;15(7):712‐726. [DOI] [PubMed] [Google Scholar]
- 11. Brimberg L, Benhar I, Mascaro‐Blanco A, et al. Behavioral, pharmacological, and immunological abnormalities after streptococcal exposure: a novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology. 2012;37(9):2076‐2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lotan D, Benhar I, Alvarez K, et al. Behavioral and neural effects of intra‐striatal infusion of anti‐streptococcal antibodies in rats. Brain Behav Immun. 2014;38:249‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Macri S, Ceci C, Onori MP, et al. Mice repeatedly exposed to Group‐A beta‐Haemolytic Streptococcus show perseverative behaviors, impaired sensorimotor gating, and immune activation in rostral diencephalon. Sci Rep. 2015;5:13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dileepan T, Smith ED, Knowland D, et al. Group A Streptococcus intranasal infection promotes CNS infiltration by streptococcal‐specific Th17 cells. J Clin Invest. 2016;126(1):303‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lymbury RS, Olive C, Powell KA, et al. Induction of autoimmune valvulitis in Lewis rats following immunization with peptides from the conserved region of group A streptococcal M protein. J Autoimmun. 2003;20(3):211‐217. [DOI] [PubMed] [Google Scholar]
- 16. Gorton D, Govan B, Olive C, Ketheesan N. B‐ and T‐cell responses in group a streptococcus M‐protein‐ or Peptide‐induced experimental carditis. Infect Immun. 2009;77(5):2177‐2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gorton D, Blyth S, Gorton JG, Govan B, Ketheesan N. An alternative technique for the induction of autoimmune valvulitis in a rat model of rheumatic heart disease. J Immunol Methods. 2010;355(1‐2):80‐85. [DOI] [PubMed] [Google Scholar]
- 18. Rush CM, Govan BL, Sikder S, Williams NL, Ketheesan N. Animal models to investigate the pathogenesis of rheumatic heart disease. Front Pediatr. 2014;2:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gorton D, Sikder S, Williams NL, et al. Repeat exposure to group A streptococcal M protein exacerbates cardiac damage in a rat model of rheumatic heart disease. Autoimmunity. 2016;49(8):563‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sikder S, Williams NL, Sorenson AE, et al. Group g streptococcus induces an autoimmune carditis mediated by interleukin 17A and interferon gamma in the Lewis Rat model of rheumatic heart disease. J Infect Dis. 2018;218(2):324‐335. [DOI] [PubMed] [Google Scholar]
- 21. Nordstrand A, Norgren M, Holm SE. An experimental model for acute poststreptococcal glomerulonephritis in mice. APMIS. 1996;104(11):805‐816. [DOI] [PubMed] [Google Scholar]
- 22. Nordstrand A, Norgren M, Ferretti JJ, Holm SE. Streptokinase as a mediator of acute post‐streptococcal glomerulonephritis in an experimental mouse model. Infect Immun. 1998;66(1):315‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nordstrand A, Norgren M, Holm S. An experimental model for acute post‐streptococcal glomerulonephritis in mice. In: Horaud T, Bouvet A, Leclercq R, de Montclos H, Sicard M, eds. Streptococci and the Host. Boston: Springer; 1997:809‐811. [DOI] [PubMed] [Google Scholar]
- 24. Wright JR Jr, Senhauser DA, Yates AJ, Sharma HM, Thibert P. Spontaneous thyroiditis in BB Wistar diabetic rats. Vet Pathol. 1983;20(5):522‐530. [DOI] [PubMed] [Google Scholar]
- 25. Song HP, Li X, Yu R, et al. Phenotypic characterization of type II collagen‐induced arthritis in Wistar rats. Exp Ther Med. 2015;10(4):1483‐1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakaguchi Y, Inaba M, Tsuda M, et al. The Wistar Bonn Kobori rat, a unique animal model for autoimmune pancreatitis with extrapancreatic exocrinopathy. Clin Exp Immunol. 2008;152(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L, Xie H, Cui L. Activation of astrocytes and expression of inflammatory cytokines in rats with experimental autoimmune encephalomyelitis. Exp Ther Med. 2018;16(6):4401‐4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Näslund J, Studer E, Pettersson R, et al. Differences in Anxiety‐Like Behavior within a Batch of Wistar Rats Are Associated with Differences in Serotonergic Transmission, Enhanced by Acute SRI Administration, and Abolished By Serotonin Depletion. Int J Neuropsychopharmacol. 2015;18(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Casillas‐Espinosa PM, Sargsyan A, Melkonian D, O'Brien TJ. A universal automated tool for reliable detection of seizures in rodent models of acquired and genetic epilepsy. Epilepsia. 2019;60(4):783‐791. [DOI] [PubMed] [Google Scholar]
- 30. Albrechet‐Souza L, Gilpin NW. The predator odor avoidance model of post‐traumatic stress disorder in rats. Behav Pharmacol. 2019;30(2 and 3):105‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barichello T, Ceretta RA, Generoso JS, et al. Cannabidiol reduces host immune response and prevents cognitive impairments in Wistar rats submitted to pneumococcal meningitis. Eur J Pharmacol. 2012;697(1‐3):158‐164. [DOI] [PubMed] [Google Scholar]
- 32. Pitarokoili K, Ambrosius B, Gold R. Lewis rat model of experimental autoimmune encephalomyelitis. Curr Protoc Neurosci. 2017;81:9.61.61‐69.61.20. [DOI] [PubMed] [Google Scholar]
- 33. Pouzol L, Piali L, Bernard CC, Martinic MM, Steiner B, Clozel M. Therapeutic potential of ponesimod alone and in combination with dimethyl fumarate in experimental models of multiple sclerosis. Innov Clin Neurosci. 2019;16(3‐4):22‐30. [PMC free article] [PubMed] [Google Scholar]
- 34. Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35(3):347‐369. [DOI] [PubMed] [Google Scholar]
- 35. Kolb B, Holmes C. Neonatal motor cortex lesions in the rat: Absence of sparing of motor behaviors and impaired spatial learning concurrent with abnormal cerebral morphogenesis. Behav Neurosci. 1983;97(5):697. [DOI] [PubMed] [Google Scholar]
- 36. Ayalon L, Doron R, Weiner I, Joel D. Amelioration of behavioral deficits in a rat model of Huntington's disease by an excitotoxic lesion to the globus pallidus. Exp Neurol. 2004;186(1):46‐58. [DOI] [PubMed] [Google Scholar]
- 37. Urakawa S, Hida H, Masuda T, Misumi S, Kim TS, Nishino H. Environmental enrichment brings a beneficial effect on beam walking and enhances the migration of doublecortin‐positive cells following striatal lesions in rats. Neuroscience. 2007;144(3):920‐933. [DOI] [PubMed] [Google Scholar]
- 38. Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33(1):23‐34. [DOI] [PubMed] [Google Scholar]
- 39. Cox CJ, Sharma M, Leckman JF, et al. Brain human monoclonal autoantibody from sydenham chorea targets dopaminergic neurons in transgenic mice and signals dopamine D2 receptor: implications in human disease. J Immunol. 2013;191(11):5524‐5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murphy TK, Kurlan R, Leckman J. The immunobiology of Tourette's disorder, pediatric autoimmune neuropsychiatric disorders associated with Streptococcus, and related disorders: a way forward. J Child Adolesc Psychopharmacol. 2010;20(4):317‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murphy TK, Storch EA, Lewin AB, Edge PJ, Goodman WK. Clinical factors associated with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Pediatr. 2012;160(2):314‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meinl E, Derfuss T, Linington C. Identifying targets for autoantibodies in CNS inflammation: strategies and achievements. Clin Exp Neuroimmunol. 2010;1(2):47‐60. [Google Scholar]
- 43. Orefici G, Cardona F, Cox CJ, Cunningham MW. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). In: Ferretti JJ, Stevens DL, Fischetti VA, eds. Streptococcus Pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City, OK: University of Oklahoma Health Sciences Center (c) The University of Oklahoma Health Sciences Center; 2016. [PubMed] [Google Scholar]
- 44. Swedo SE, Leonard HL, Schapiro MB, et al. Sydenham's chorea: physical and psychological symptoms of St Vitus dance. Pediatrics. 1993;91(4):706‐713. [PubMed] [Google Scholar]
- 45. Swedo SE, Leonard HL, Mittleman BB, et al. Identification of children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections by a marker associated with rheumatic fever. Am J Psychiatry. 1997;154(1):110‐112. [DOI] [PubMed] [Google Scholar]
- 46. Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155(2):264‐271. [DOI] [PubMed] [Google Scholar]
- 47. Galvin JE, Hemric ME, Kosanke SD, Factor SM, Quinn A, Cunningham MW. Induction of myocarditis and valvulitis in lewis rats by different epitopes of cardiac myosin and its implications in rheumatic carditis. Am J Pathol. 2002;160(1):297‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Quinn A, Kosanke S, Fischetti VA, Factor SM, Cunningham MW. Induction of autoimmune valvular heart disease by recombinant streptococcal m protein. Infect Immun. 2001;69(6):4072‐4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kirvan CA, Galvin JE, Hilt S, Kosanke S, Cunningham MW. Identification of streptococcal m‐protein cardiopathogenic epitopes in experimental autoimmune valvulitis. J Cardiovasc Transl Res. 2014;7(2):172‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Singer HS, Mascaro‐Blanco A, Alvarez K, et al. Neuronal antibody biomarkers for Sydenham’s chorea identify a new group of children with chronic recurrent episodic acute exacerbations of tic and obsessive compulsive symptoms following a streptococcal infection. PLoS One. 2015;10(3):e0120499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cox CJ, Zuccolo AJ, Edwards EV, et al. Antineuronal antibodies in a heterogeneous group of youth and young adults with tics and obsessive‐compulsive disorder. J Child Adolesc Psychopharmacol. 2015;25(1):76‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ben‐Pazi H, Stoner JA, Cunningham MW. Dopamine receptor autoantibodies correlate with symptoms in Sydenham's chorea. PLoS One. 2013;8(9):e73516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Platt MP, Bolding KA, Wayne CR, et al. Th17 lymphocytes drive vascular and neuronal deficits in a mouse model of postinfectious autoimmune encephalitis. Proc Natl Acad Sci USA. 2020;117(12):6708‐6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cunningham MW. Molecular mimicry, autoimmunity, and infection: the cross‐reactive antigens of group A streptococci and their sequelae. Microbiol Spectr. 2019;7(4). 10.1128/microbiolspec.GPP3-0045-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cunningham MW. Post‐streptococcal autoimmune sequelae: rheumatic fever and beyond. In Ferretti JJ, Stevens DL, Fischetti VA, eds. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City: University of Oklahoma Health Sciences Center; 2016. https://www.ncbi.nlm.nih.gov/books/NBK333434/ [PubMed] [Google Scholar]
- 56. Cunningham MW. Rheumatic fever, autoimmunity, and molecular mimicry: the streptococcal connection. Int Rev Immunol. 2014;33(4):314‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cunningham MW. Streptococcus and rheumatic fever. Curr Opin Rheumatol. 2012;24(4):408‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sikder S, Price G, Alim MA, et al. Group A streptococcal M‐protein specific antibodies and T‐cells drive the pathology observed in the rat autoimmune valvulitis model. Autoimmunity. 2019;52(2):78‐87. [DOI] [PubMed] [Google Scholar]
- 59. Sikder S, Rush CM, Govan BL, Alim MA, Ketheesan N. Anti‐streptococcal antibody and T‐cell interactions with vascular endothelial cells initiate the development of rheumatic carditis. J Leukoc Biol. 2020;107(2):263‐271. [DOI] [PubMed] [Google Scholar]
- 60. Fae K, Kalil J, Toubert A, Guilherme L. Heart infiltrating T cell clones from a rheumatic heart disease patient display a common TCR usage and a degenerate antigen recognition pattern. Mol Immunol. 2004;40(14‐15):1129‐1135. [DOI] [PubMed] [Google Scholar]
- 61. Swedo SE, Rapoport JL, Cheslow DL, et al. High prevalence of obsessive‐compulsive symptoms in patients with Sydenham's chorea. Am J Psychiatry. 1989;146(2):246‐249. [DOI] [PubMed] [Google Scholar]
- 62. Murphy TK, Parker‐Athill EC, Lewin AB, Storch EA, Mutch PJ. Cefdinir for recent‐onset pediatric neuropsychiatric disorders: a pilot randomized trial. J Child Adolesc Psychopharmacol. 2015;25(1):57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pavone P, Rapisarda V, Serra A, et al. Pediatric autoimmune neuropsychiatric disorder associated with group a streptococcal infection: the role of surgical treatment. Int J Immunopathol Pharmacol. 2014;27(3):371‐378. [DOI] [PubMed] [Google Scholar]
- 64. Snider LA, Swedo SE. PANDAS: current status and directions for research. Mol Psychiatry. 2004;9(10):900‐907. [DOI] [PubMed] [Google Scholar]
- 65. Gordon N. Sydenham's chorea, and its complications affecting the nervous system. Brain Dev. 2009;31(1):11‐14. [DOI] [PubMed] [Google Scholar]
- 66. Murphy TK, Husted DS, Edge PJ. Preclinical/Clinical evidence of central nervous system infectious etiology in PANDAS. Adv Neurol. 2006;99:148. [PubMed] [Google Scholar]


