Abstract
Increased beta band synchrony has been demonstrated to be a biomarker of Parkinson’s disease (PD). This abnormal synchrony can often be prolonged in long bursts of beta activity, which may interfere with normal sensorimotor processing. Previous closed loop deep brain stimulation (DBS) algorithms used averaged beta power to drive neurostimulation, which were indiscriminate to physiological (short) versus pathological (long) beta burst durations. We present a closed-loop DBS algorithm using beta burst duration as the control signal. Benchtop validation results demonstrate the feasibility of the algorithm in real-time by responding to pre-recorded STN data from a PD participant. These results provide the basis for future improved closed-loop algorithms focused on burst durations for in mitigating symptoms of PD.
I. Introduction
Persistent synchrony of beta band oscillations (13–30 Hz) has emerged as a biomarker of Parkinson’s disease (PD) [1], [2]. Recent evidence suggests that short duration bursts of beta oscillations (< ∼200 ms) are an indication of normal sensorimotor processing [3]. However, prolonged beta bursts may interfere with sensorimotor processing and have been correlated with motor disability, gait impairment and freezing of gait (FOG) in Parkinson’s disease [4], [5]. Administration of dopaminergic medication or deep brain stimulation (DBS) attenuated beta burst durations and was associated with improved movement [4]–[6]. These treatments, especially DBS, provide a means for direct neuromodulation of pathological beta bursts.
Closed-loop DBS (clDBS) is an emerging area of therapy where either kinematic or neural signals have been used to adapt the intensity of DBS [7]–[9]. Improvements in bradykinesia and tremor were evident when beta power was the control variable for clDBS in both the acute post-operative setting and using chronically implanted sensing neurostimulators [8], [9]. There is also preliminary evidence of improvement in FOG during clDBS using similar algorithms [10]. These algorithms are limited in their ability to track more resolute changes in beta burst dynamics associated with PD symptoms in real time.
In this paper we provide a platform and preliminary results of a closed-loop DBS system that tracks beta burst durations as the control variable to adapt DBS stimulation intensity. The system was evaluated using a benchtop setup that could play back previously recorded neural data and record output stimulation from the developed system. The results of this demonstration illustrate the system’s ability to adjust stimulation in real time in response to sensed beta-burst activity, a prerequisite for future human-studies with this investigational platform.
II. Methods
A. Burst Duration Calculation
Beta burst durations were identified from raw local field potentials (LFPs). Data was bandpass filtered (specifics of the filters used are in sections B and C) using a 6 Hz band centered on the peak beta power as measured by a power spectral density analysis. A linear envelope was then calculated by squaring the data, finding the peaks in the data, and linearly interpolating between the peaks (Figure 1A) [5]. A burst was defined as the duration of time the linear envelope remained above a participant specific threshold that was determined based on the average trough (minima) power in the linear envelope of the signal in the 45–65 Hz band [11]. A pathological burst was defined as being longer than 300 ms, which is based on the average burst duration of pink noise data that follows a 1/f distribution.
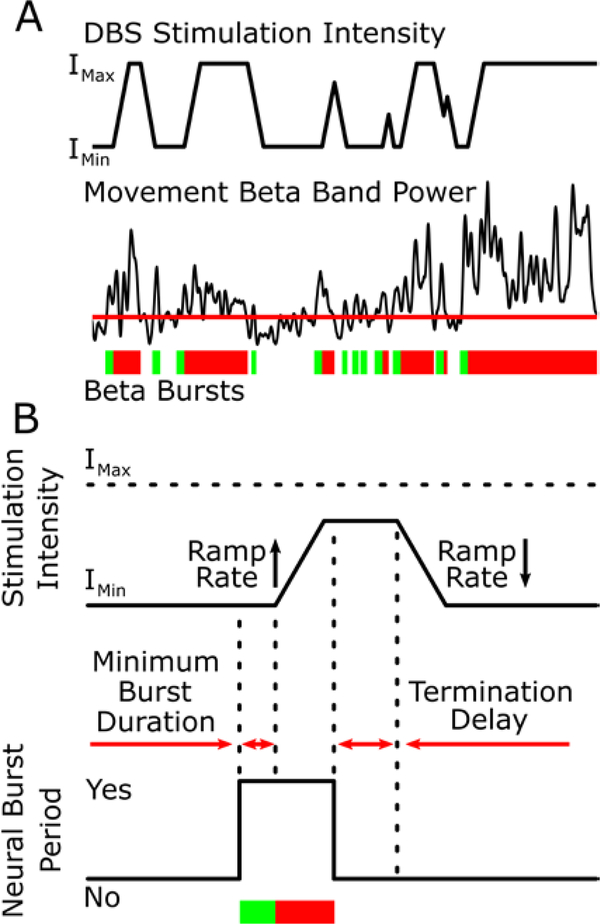
Figure 1:
The burst duration control policy algorithm. (A) Burst durations are calculated from sequential crossings of the envelope of the movement band beta power over a participant specific threshold. Green bars indicate physiological burst durations and the transition to red shows when the burst has become pathological (>300 ms). DBS intensity adapts between Imin and Imax in response to the burst durations. (B) The DBS intensity is set to increase and decrease at tolerable ramp rates that are predetermined to be safe for each participant. DBS intensity increases after the minimum burst duration is detected and decreases in intensity after the burst ends and the termination delay has elapsed.
B. Summit System Development
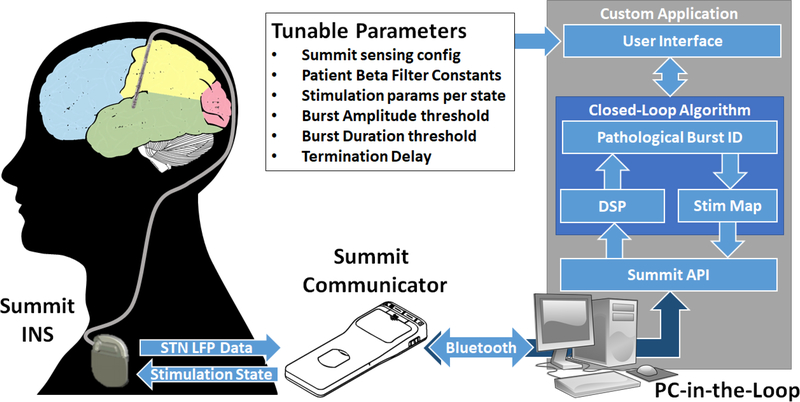
To implement a system capable of delivering beta-burst driven adaptive stimulation for a human patient, we made use of the Medtronic investigational-use Summit System (Summit™ RC+S, Medtronic Inc). The Summit™ RC+S system was developed as a successor to the Medtronic Activa™ PC+S-NexusD/E system, which our group has extensively used in previous closed-loop stimulation work [7], [8] and allows for advanced feasibility testing of new DBS concepts in humans through the FDA investigational device exemption application process. The Summit™ RC+S System is composed of a rechargeable implanted neurostimulator (INS) with sensing and closed-loop stimulation capabilities, a bidirectional communicator, and a C# API [12]. This software API allows for the creation of custom applications to configure the implanted device or, as is done in this case, to perform ‘distributed’ closed-loop stimulation through the processing of neural data on the PC [13]. This distributed functionality was created to provide a means to do advanced prototyping of new algorithms that could not be embodied by the embedded framework as provided by the Summit INS firmware, which is the case with our beta burst algorithm described in Section A.
Given that our beta burst algorithm could not be implemented using the embedded functionality of the Summit INS, we opted to develop a software application using the Summit API to create a PC-in-the-loop approach as shown in Figure 2. The Summit INS, which will be implanted in PD patients with electrical leads in the subthalamic nucleus (STN) for stimulation, has a separate (not shown) clinical programmer for the configuration of all safety-related parameters, such as active stimulation contacts (electrodes) and amplitude limits. After clinical programming, our application makes use of the Summit Communicator to establish a bidirectional communication channel with the INS. Researchers are then able to make use of the user interface to configure the sensing and streaming parameters for experimental use and set up patient-customized distributed algorithm parameters before initiating a trial.
Figure 2:
System Block Diagram. PC-in-the-loop architecture utilizing the Summit communicator to provide STN beta-burst based adaptive stimulation. Summit Communicator and INS images are both credited to Medtronic.
Upon initiation of the experiment, we begin shifting data from new neural packets into two buffers for digital signal processing. Each buffer contains the LFP data from each of the patient’s two STNs. We then apply a 128th-order bandpass FIR filter to the data using patient-specific coefficients to isolate the beta-band of interest. Unlike prior work performed by this group which relied on an IIR filter during post-hoc analysis of beta burst activity [5], a FIR filter was chosen to improve robustness against possible dropped packets, which would create discontinuities in buffered data. We then square the output and find the peaks before enveloping the data using linear interpolation between peaks. The most recent beta burst is identified by finding periods of time in buffered data where the enveloped data is above the patient identified threshold (Figure 1). The duration of this burst is checked against the burst duration threshold to determine if the burst is “pathological” or “normal”. This determination of the burst nature is used to determine the desired stimulation state of the device. A termination delay, or amount of time after the burst has ended before changing stimulation again, can be set on a participant specific basis based on their neural dynamics.
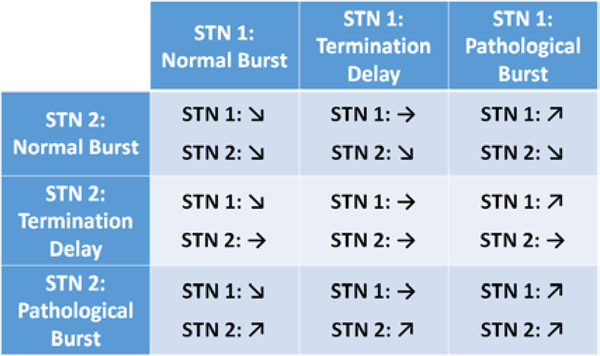
Adjustment of stimulation based on the identified beta burst activity is performed by making use of the Summit INS on-board state table, which can be used to ramp stimulation parameters to desired setpoints based on an embedded stimulation table [14]. In this project, we make use of test functionality built into the API that forces the Summit INS to manually change between state table entries based on our external analysis, which has the effect of the device ramping parameters automatically in response to our commands. The three-by-three state table, illustrated in Figure 3, is configured to ramp stimulation up in a specific STN in the case of a pathological burst being detected, hold stimulation constant during the termination delay, and slowly ramp stimulation down when normal beta burst activity is detected (Figure 1A and B).
Figure 3:
Burst to Stimulation action map
C. Benchtop Evaluation of System Functionality
To test this system, we made use of an un-implanted Summit INS, which was connected to a signal generator and resistor network. STN LFPs were used that had been previously recorded from a participant with an implanted Medtronic Activa™ PC+S neurostimulator and model 3389 DBS leads (male, 58 years old, 9 year disease duration). The participant provided informed written consent and all study procedures were approved by the Stanford University Institutional Review Board. In post-processing, data recorded from the Summit INS was then filtered using a zero-lag FIR filter (128th order Butterworth) and control decisions were determined based on the zero-lag FIR filtered data. The control decisions based on the zero-lag FIR filtered data were compared to the control decisions of the C# application that were made in real-time. For this simulation, the termination delay was set to 10 ms, the stimulation intensity was bounded between 2 mA (Imin) and 5 mA (Imax), and the ramp up and down times were set to 1 mA/sec. Given the fact that we were using data previously collected from a patient to assess system functionality, we increased the threshold beyond what would normally be used based on prior work [11] to compensate for the lack of stimulation impact on beta bursting activity.
III. Results
Benchtop testing demonstrated the ability of the system to respond to beta burst duration in real time (Figure 4). In this particular example, the Summit responded to pathological burst durations by increasing stimulation and when bursts were within the physiological range it decreased stimulation. The control decisions in real time were slightly delayed compared to the ideal zero-lag FIR filtered data decisions, but were still made within a reasonable time window, with a delay measured of approximately 300–400 ms. When inserting time measuring stopwatches into the application, we measured our data processing (FIR, envelope, threshold, and control decision logic) at approximately 70 milliseconds. The remainder of the delay was due to Summit communication through the APIs and bidirectional communication bridge.
Figure 4:
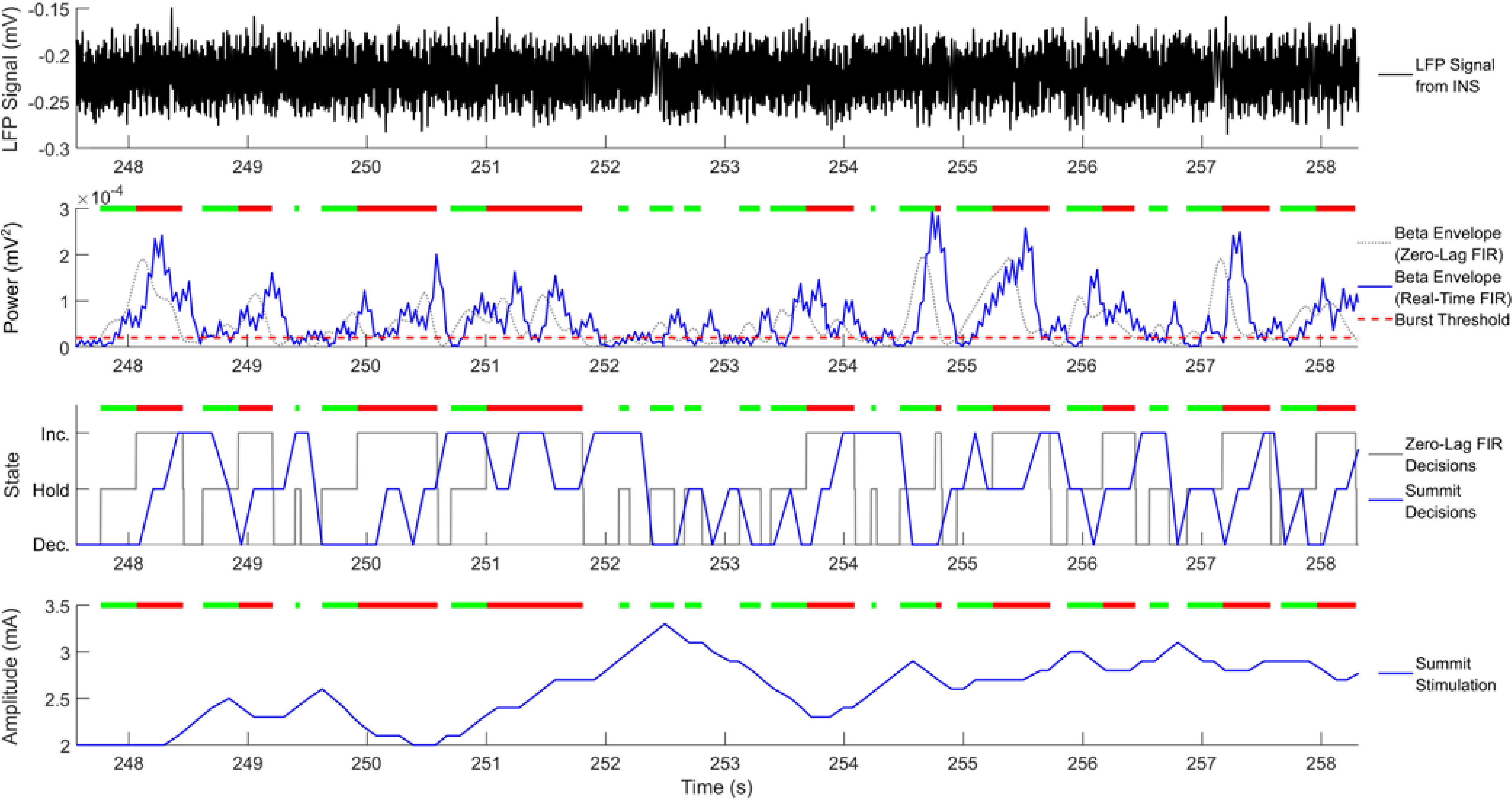
Real-time System demonstration of developed system: Top plot is sensed raw signals collected from INS through benchtop playback. Plots 2 and 3 illustrate the calculated beta burst envelope and on-board stimulation state decisions respectively given a tuned threshold. Gray traces correspond to offline MATLAB calculations of envelop and stimulation using zero-lag FIR filter, while real-time system in blue illustrate lag due to processing and communication to Summit System and minor differences due to different filter coefficients, in general real-time system results match. Bottom plot illustrates real time stimulation results as logged by the Summit System logs, which are a verified and validated record of stimulation changes for the device. Bars above the bottom 3 plots indicate bursts that were calculated using the zero-lag FIR filter, where the transition of from green to red indicates that a burst has become pathological (>300 ms).
IV. Discussion
In this paper we propose and demonstrate a novel control algorithm for monitoring beta burst durations from LFPs of the brain. Using benchtop testing, we demonstrated the feasibility of using this novel algorithm in real time using previously recorded STN neural data. Tests revealed a reasonable delay in the control decisions to the beta bursts, primarily due to Summit System communication latency. These results demonstrate that beta burst duration is a feasible control signal for closed loop DBS.
The inherent delays in the real time system were expected with the application of the real-time FIR filter vs. the zero-lag FIR filter. In order to get a similar output in terms of signal shape, the real-time FIR filter would have to be increased even further than the 128th order used in this test setup. However, this would introduce an increased delay in data processing, and the control decisions would be made well after the burst had occurred. The 128th order filter that was used in the test bench setup was a compromise between signal smoothness and FIR delay (< than the data packet length). Future iterations of the system could consider different real-time FIR filter designs to minimize delay and increase signal quality.
Other parameters that were not explored in this test bench that could affect the therapeutic effect of the system include the termination delay and the ramp rate of stimulation. Increasing the ramp rate would make the system more responsive, but it would need to be kept within a range that is deemed safe and tolerable for the patient. Increasing the termination delay would make the system less responsive, but this may be necessary for the initiation of movement when beta desynchronizes and stimulation needs to stay high to maintain a therapeutic effect [7].
Overall, these results provide the basis for future closed-loop controllers that use beta burst durations as the control variable. Future work should focus on applying these algorithms in chronically implanted patients with PD to assess the efficacy of these algorithms in shortening burst durations and improving signs and symptoms.
Acknowledgment
The authors would like to thank the members of the Bronte-Stewart Lab and the participant in the study. This work was supported by the Parkinson’s Foundation, the Robert and Ruth Halperin Foundation, John A. Blume Foundation, Helen M. Cahill Award for Research in Parkinson’s Disease, the Stanford Bio-X Graduate Fellowship. Medtronic Inc., provided the devices at no charge to the patients and provided the bench system used in this study but no additional financial support.
Contributor Information
Matthew N. Petrucci, Department of Neurology and Neurological Sciences at Stanford University, Stanford, CA, 94305 USA.
Ross W. Anderson, Department of Neurology and Neurological Sciences at Stanford University, Stanford, CA, 94305 USA
Johanna J. O’Day, Department of Bioengineering at Stanford University, Stanford, CA, 94305 USA.
Yasmine M. Kehnemouyi, Department of Neurology at Stanford University, Stanford, CA, 94305 USA
Jeffrey A. Herron, Department of Neurological Surgery at the University of Washington, Seattle, WA, 98104 USA.
Helen M. Bronte-Stewart, Department of Neurology at Stanford University, Stanford, CA, 94305 USA
References
- [1].Hammond C, Bergman H, and Brown P, “Pathological synchronization in Parkinson’s disease: networks, models and treatments,” Trends in Neurosciences. 2007. [DOI] [PubMed] [Google Scholar]
- [2].de Solages C, Hill BC, Koop MM, Henderson JM, and Bronte-Stewart H, “Bilateral symmetry and coherence of subthalamic nuclei beta band activity in Parkinson’s disease,” Exp. Neurol, 2010. [DOI] [PubMed] [Google Scholar]
- [3].Feingold J, Gibson DJ, Depasquale B, and Graybiel AM, “Bursts of beta oscillation differentiate postperformance activity in the striatum and motor cortex of monkeys performing movement tasks,” Proc. Natl. Acad. Sci. U. S. A, vol. 112, no. 44, pp. 13687–13692, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tinkhauser G, Pogosyan A, Tan H, Herz DM, Kühn AA, and Brown P, “Beta burst dynamics in Parkinson’s disease off and on dopaminergic medication,” Brain, vol. 140, no. 11, pp. 2968–2981, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Anidi C et al. , “Neuromodulation targets pathological not physiological beta bursts during gait in Parkinson’s disease,” Neurobiol. Dis, vol. 120, no. May, pp. 107–117, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tinkhauser G et al. , “The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson’s disease,” Brain, vol. 140, no. 4, pp. 1053–1067, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Afzal MF, Velisar A, Anidi C, Neuville R, Prabhakar V, and Bronte-Stewart H, “Proceedings #61: Subthalamic Neural Closed-loop Deep Brain Stimulation for Bradykinesia in Parkinson’s Disease,” Brain Stimul, vol. 12, no. 4, pp. e152–e154, 2019. [Google Scholar]
- [8].Velisar A et al. , “Dual threshold neural closed loop deep brain stimulation in Parkinson disease patients,” Brain Stimul, no. xxxx, 2019. [DOI] [PubMed] [Google Scholar]
- [9].Little S et al. , “Adaptive deep brain stimulation in advanced Parkinson disease,” Ann. Neurol, vol. 74, no. 3, pp. 449–457, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Petrucci MN et al. , “Closed Loop Deep Brain Stimulation for Freezing of Gait,” medRxiv, p. 19011858, January. 2019. [DOI] [PMC free article] [PubMed]
- [11].Anderson R et al. , “A Novel Method for Calculating Beta Band Burst Durations in Parkinson’s Disease Using a Physiological Baseline,” bioRxiv, p. 2020.03.25.008185, 2020. [DOI] [PMC free article] [PubMed]
- [12].Herron J, Stanslaski S, Chouinard T, Corey R, Denison T, and Orser H, “Bi-directional brain interfacing instrumentation,” I2MTC 2018 – 2018 IEEE Int. Instrum. Meas. Technol. Conf. Discov. New Horizons Instrum. Meas. Proc., pp. 1–6, 2018. [Google Scholar]
- [13].Stanslaski S et al. , “A Chronically Implantable Neural Coprocessor for Investigating the Treatment of Neurological Disorders,” IEEE Trans. Biomed. Circuits Syst, vol. 12, no. 6, pp. 1230–1245, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Herron J et al. , “Embedding adaptive stimulation algorithms for a new implantable deep-brain stimulation research tool,” 2018 IEEE Biomed. Circuits Syst. Conf. BioCAS 2018 - Proc., pp. 1–4, 2018.