Abstract
Sex/gender difference exists in the physiology of multiple organs. Recent epidemiological reports suggest the influence of sex-steroids in modulating a wide variety of disease conditions. Sex-based discrepancies have been reported in pulmonary physiology and various chronic inflammatory responses associated with lung diseases like asthma, chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, and rare lung diseases. Notably, emerging clinical evidence suggests that several respiratory diseases affect women to a greater degree, with increased severity and prevalence than men. Although sex-specific differences in various lung diseases are evident, such differences are inherent to sex-steroids, which are major biological variables in men and women who play a central role to control these differences. The focus of this chapter is to comprehend the sex-steroid biology in inflammatory lung diseases and to understand the mechanistic role of sex-steroids signaling in regulating these diseases. Exploring the roles of sex-steroid signaling in the regulation of lung diseases and inflammation is crucial for the development of novel and effective therapy. Overall, we will illustrate the importance of differential sex-steroid signaling in lung diseases and their possible clinical implications for the development of complementary and alternative medicine to treat lung diseases.
Keywords: Estrogen, Testosterone, Progesterone, Asthma, COPD, Sex difference
14.1. Introduction
Sex is considered the greatest invention of all time: it not only is important in sexual reproduction, but facilitates the evolution of higher life forms and also had a profound impact on human culture, history, and society. In the current health care system, along with social sectors, “sex” (biological basis between females and males) and “gender” (roles in society and behaviors) variables have been considered important parameters for research and action [54, 126]. As a difference in biology among the sexes decides various diseases specific to males and females, however, the role of sex/gender in disease pathophysiology is not yet fully explored. Overall, the knowledge gap is high in many areas like 1) differences in disease prevalence in men and women, 2) reasons behind that difference, 3) is there any difference in signaling mechanisms and 4) how to design preventive and therapeutic treatment approaches concerning the change in disease prevalence. This situation has been reformed continuously with recent breakthrough research work and curiosity in determining and reacting to sex and gender differentials in disease conditions [7, 126]. However, to date, there is no clear evidence about the role of major sex-steroids (sex/gender determining factors) and their signaling in the pathophysiology of respiratory diseases. The goal of this chapter is to delineate the influence of sex-steroids (estrogens, progesterone, and testosterone) and their signaling in lung diseases.
Sex differences in the health and disease have been gaining considerable interest and widely explored in cardiovascular structure/function [15, 17, 194, 260], neurological research [117, 152, 160, 161, 210, 284, 324], and metabolism [30, 38, 127, 338]. Furthermore, the role of sex difference in clinical pharmacology is well evident and provides details about the basic mechanisms to understand their role in drug pharmacokinetics and pharmacodynamics for therapeutic optimization of the frequency of dose or their effects along with possible adverse effects. [30, 38, 111, 211, 305]. There is growing evidence that reports the effect of sex-steroids in different lung components, and how it contributes to various diseases like pulmonary fibrosis, cancer, chronic obstructive pulmonary disease (COPD), asthma, and even pulmonary hypertension [10, 11, 13, 37, 58, 71, 169, 171, 204, 215, 216, 294, 295], but still need more thorough investigations. Intrinsic sex differences in the growth of lungs and function are present even before birth in utero and are visible during the different stages of human life from childhood to old age [140, 257]. Changes in lung physiology and their functions due to sex differences during the various stages of lifespan, such as puberty, pregnancy, menopause, and during aging propose additional modulatory roles of sex-steroids and/or their metabolites [45, 214, 215]. Multiple recent in vitro and in vivo studies established the crucial role of sex-steroids in modulating lung pathophysiology [8, 10, 13, 36, 168–170, 204, 258]. For example, the prevalence of asthma is more common in boys, which is more than double the risk of developing asthma in girls [39, 60, 62, 163, 229], and as age increases interestingly this trend reverses. Adult women, after puberty, tend to show higher chances of asthma occurrence with greater severity compared to men [39, 60, 62, 163, 229]. Also, few female patients with existing asthma experience exacerbated asthma symptoms in their premenstrual or menstrual phases [2, 220]. Higher chances of asthma or poor prognosis in women suggest the importance to explore the role of inherent sex difference versus sex-steroids signaling especially female sex-steroid, estrogen per se in the pathophysiology of lung diseases. Furthermore, studying the comparative effects of sex-steroids and their locally produced metabolites will further improve our understanding of disease pathophysiology [214, 320].
Epidemiological studies demonstrate a critical role of sex-steroid signaling to control the mechanisms associated with the inflammatory response in the lungs [5, 49, 52, 55]. Multiple reports suggest a higher susceptibility in women to an inflammatory response with worse complications of lung disease associated with inflammation compared to men [19, 55, 67, 216, 307]. Besides, earlier in vivo studies showed sex-steroids differentially regulate lung immune responses in the mouse model of asthma [55, 109]. Notably, data from clinical studies have shown that circulating sex-steroid levels may contribute significantly in regulating innate immune responses and affects inflammation and airway tone during the menstrual cycle in female asthmatic patients, however, the exact signaling mechanism of sex-steroids and the associated mechanisms are multifaceted and not fully explored [96, 208, 234, 252, 282]. This chapter explores our attempts to comprehend the influence of sex-steroids signaling in lung diseases and inflammatory response in the lungs. Accordingly, exploring the mechanisms of sex-steroids signaling becomes important in both ways of appreciating sex differences in normal lung physiology, and disease development and its ultimate therapeutic approach. The major goal of this chapter is to highlight the growth in research relating to the differential role of sex-steroids and the lung.
14.2. Sex-Steroids and Their Biology
Sex is the major characteristic by which all individuals are differentiated with respect to reproductive organs and functions as female or male, whereas gender is described as a way of social conduct, manhood, and femaleness [23, 111, 294, 295, 342]. In 2001, the Institute of Medicine (IOM) of the National Academy of Sciences (NAS) demonstrated exclusive evidence showing that “sex matters”; especially “being male or female is an important variable in human that must be contemplated as a biological variable in disease pathophysiology as well, while designing and analyzing studies at all health research spectrum” [56]. As per IOM, biological variations correlates with sex-based differences among males and females, while cultural and social distinction characterized as gender [305].
Sex-based specific effects in the context of disease pathophysiology and occurrence are often connected to the changed sex-steroid milieu in males and females [341]. Sex-steroids, comprising estrogens, progesterone, and testosterone have been traditionally defined by their association in the normal functioning of reproductive system function. However, multiple earlier studies demonstrated the importance of sex-steroids in the field of cardiovascular [187, 194, 205, 260], metabolic [38, 127, 338], and neurological research [152, 161, 210, 225, 284]. Also, emerging clinical and epidemiological data proven the crucial role of sex-steroids in the modulation of various lung diseases [8, 13, 36, 169–171, 278, 294, 324, 337]. Among all sex-steroids, estrogens and progesterone are reflected as key sex-steroids secreted by the ovary, while testosterone by the testes. Moreover, in addition to steroidogenic organs, sex-steroids are also produced by local peripheral tissues [215, 294]. After production, these hormones may act in a paracrine manner or circulate in the bloodstream to act at target tissues in an endocrine fashion [231, 341]. Typically, sex-steroids are produced in the gonads, adrenal glands, and the fetoplacental unit from common precursor cholesterol by well-known biosynthetic pathways (Fig. 14.1). In the ovary, the onset of the steroidogenic pathway is theca cells, where first Pregnenolone from cholesterol using the cytochrome P450 (CYP) side-chain cleavage enzyme (CYP11A) in the mitochondrial membrane. Whereas, a similar process happens Leydig cells of the testes. Pregnenolone further forms progesterone (via 3β-hydroxy-steroid dehydrogenase, 3β-HSD) or dehydro-epiandrosterone (DHEA) via CYP17 and then diverges toward the formation of androstenedione (3β-HSD) and sub-sequent testosterone (17β-HSD). Furthermore, testosterone converts to estrogens by formation of an aromatic ring by utilizing aromatase enzyme (CYP19) [63, 232, 277, 294].
Fig. 14.1.
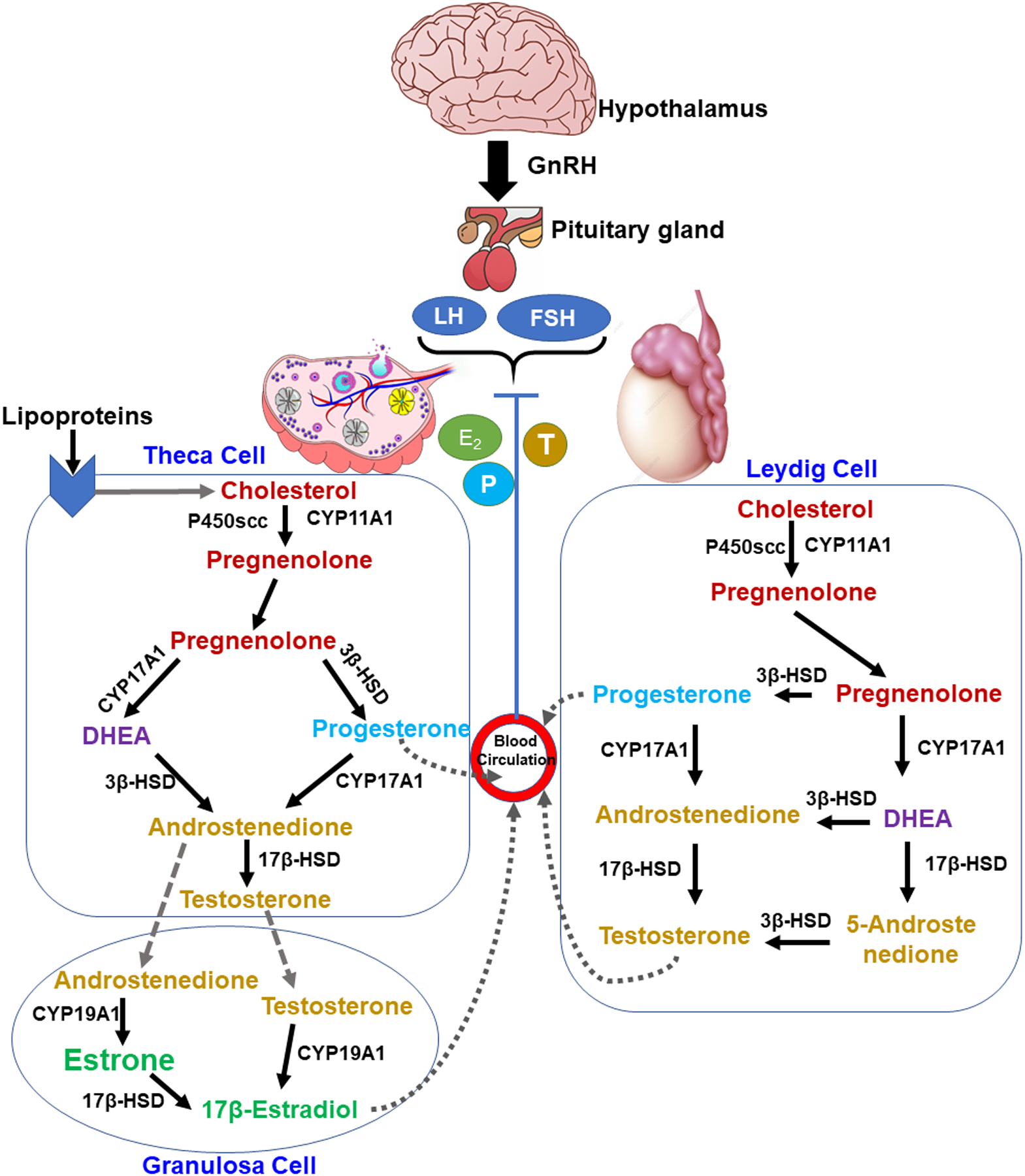
Sex-steroid hormone biosynthesis from cholesterol. In steroidogenesis pathway cholesterol converts to pregnenolone which further cleaved via two different pathways and leads to the production of testosterone and subsequent estrogen. P450scc, P450 side chain cleavage; CYP11A1, cytochrome P-450, family 11, subfamily A, polypeptide 1 gene; 3β-HSD, 3β-hydroxysteroid dehydrogenase; CYP17A1, cytochrome P-450, family 17, subfamily A, polypeptide 1 gene; 17β-HSD, 17β-hydroxysteroid dehydrogenase; CYP19A1, cytochrome P-450, family 19, subfamily A, polypeptide 1 gene.
14.2.1. Estrogen
Estrogens are major female sex-steroids that are important for sexual and reproductive development, especially in women. In women, there are three main steroidal estrogens; estrone (E1), estradiol (17β-estradiol; E2), and estriol (E3) are produced by steroidogenic cells (e.g., ovaries, placenta, and adipose tissue) as well as extrahepatic tissues [84, 189, 192, 206, 294, 295, 324]. Another form of estrogen called estetrol (E4) is produced only during pregnancy [320, 321]. Estrogens are also produced by ovarian androgens, specifically testosterone and androstenedione via the steroidogenesis pathway in the presence of aromatase enzyme [294]. Endogenously, estrogens are metabolized by CYP enzymes CYP1A1 and CYP1B1 into two catechol estrogens, 4-hydroxyestradiol (4-OHE2) and 2-hydroxyestraidol (2-OHE2), while CYP3A4 converts estrogen into 16α-hydroxyestradiol (16α-OHE2) [95, 190, 294, 295, 320, 324]. Furthermore, 4-OHE2 and 2-OHE2 are metabolized to methoxyestrogens via catechol-O-methyltransferase (COMT) and forms 4-methoxyestradiol (4-ME) and 2-methoxyestradiol (2-ME) respectively [22, 95, 190, 199, 202, 207, 288]. Recent reports from our groups and others have described the potential capability of the lung tissue to locally metabolize thereby inactivating sex-steroids and modulating the actions of sex-steroids at a cellular level [8, 108, 324]. Among all estrogens, E2 possesses the highest estrogenic activity and found to be more abundant in blood circulation, particularly during reproductive ages [294]. However, the level of estrogens fluctuates in women throughout their lifetime based on the menstrual period, pregnancy, menopausal periods, ages, and some other factors. In premenopausal conditions, normal estradiol levels, which range from 40 to 400 pg/mL drops considerably to almost 10 to 20 pg/mL after menopause [151, 308, 316]. During the menstrual cycle, estradiol increases in the follicular phase (from days 0 to 14) up to the range of 40 to 100 pg/mL, which can reach up to the highest level 100 to 400 pg/mL on day 14. During the luteal phase, the levels of estradiol drop up to 40 to 250 pg/mL and return to lower levels before starting the next menstrual cycle (Table 14.1) [22, 151, 316]. Men also produce estrogen ranges from 15–50 pg/mL in normal, healthy condition [324], albeit comparatively lower than women. In men, testes form estradiol by converting testosterone by aromatase in Leydig cells and germ cells [151, 316]. Notably, the estradiol levels in elderly males are comparatively high (20–30 pg/mL) than menopausal females (10 to 20 pg/mL), which accord with earlier reports suggesting increased aromatase activity with age converts testosterone to estrogens in males [332]. Values provided in Table 14.1 for estradiol and progesterone in different life stages of human are derived from multiple established reports.
Table 14.1.
Circulating levels of sex-steroid in males and females during the various stages of life
| Stages | Estradiol (pg/mL) | Progesterone (pg/mL) | |||
|---|---|---|---|---|---|
| Female | Male | Female | Male | ||
| Normal | 40 – 400 | 15 – 50 | <890 | 0 – 480 | |
| During menstrual cycle | Follicular phase (day 0 to 14) |
40 – 100 | N/A |
200 – 1200 | N/A |
| Ovulation (day 14) | 100 – 400 | 200 – 2000 | |||
| Luteal phase (day 15 to 28) |
40 – 250 | 2000 – 10,000 | |||
| After menopause/elderly men | 10 – 20 | 20 – 30 | <400 | 145 | |
14.2.2. Progesterone
Progesterone, is considered as a second major female sex-steroid hormone that regulates important cellular pathways of the inner lining of the uterus to maintain the pregnancy [317]. Progesterone helps the transition of endometrial from a proliferative to the secretory stage successively, which forms blastocyst nesting, and provides essential support during the preservation of pregnancy [77, 263, 327]. Ovaries, placenta, and adrenal glands are main gonadal organs which produce progesterone, additionally, brain and adipose tissue also produce a small amount of progesterone in both males and females [306, 317]. Progesterone also is a part of various metabolic and physiological modifications in different stages of life which includes puberty [40, 242], menstrual cycle [287, 336], and embryogenesis [133, 248, 313]. In addition to the reproductive system, progesterone also has a critical role in other tissue systems, such as the mammary gland in preparation for breastfeeding [51, 156], the cardiovascular system [314, 319], neurodevelopment process in CNS [124, 132, 158], and bones [274]. In the bloodstream, progesterone circulates bound form by attaching to cortisol-binding globulin and serum albumin. Circulatory progesterone in bound form possesses very short half-life (t1/2) of about only 5 minutes. After metabolism mainly in the liver, progesterone gets converted into sulfates and glucuronides and eliminated from the body through urine [317].
Progesterone levels are comparatively low in women during the follicular phase of preovulation of the menstrual cycle, which increases significantly after the ovulation phase and elevated further in the luteal phase of menstruation [296]. In females the progesterone levels in preovulation were observed <890 ng/mL [300] which alters during ovulations stages. During the ovulation period the levels of progesterone changes are episodic, prior ovulation, it tends to decrease to about 200–1200 pg/mL which increases up to 2000–10,000 pg/mL after ovulation. However, in normal, healthy males the level of progesterone is 0–480 pg/mL [296], which declines with age up to 145 pg/mL (Table 14.1) [32]. During pregnancy, the levels of progesterone maintained steadily by human chorionic gonadotropin (HCG) dependent corpus luteum. After 7 weeks of pregnancy, the placental membrane starts producing progesterone instead of a corpus luteum, and this phase is called the luteal-placental shift. After this phase, the levels of progesterone further increase high and maintained throughout the pregnancy [83, 131, 324].
14.2.3. Testosterone
Testosterone which is the main male circulating hormone produced by the Leydig cells of the testes. Testosterone regulates various sex-associated functions such as fabricating male sex characters, sex differentiation, production of mature spermatozoa, and fertility [115, 294]. Traditionally testosterone was considered as a male hormone, albeit it is also produced in theca cells of the ovaries and by the adrenal gland in minuscule amounts [173, 235]. The initial effect of testosterone in human life is observed as early as in the uterine fetus during the second trimester of pregnancy when the reproductive organs are identical in the fetus [78]. In addition to the major reproductive role, testosterone also employs a wide range of physiological effects in regulating the structure and function of nonreproductive organs, including the heart [78, 283], lungs [195], bone [159], liver [3, 250, 251, 299], intestine [255], kidney [105, 318], adipocytes [221], anabolism [75, 186], metabolism [115, 268, 310, 333], and cognition [25, 162] both in men and women.
Recent studies had been reported a reduced serum testosterone levels in the elderly men, while the mechanistic approach is disputed among researchers, has this naturally occurred process or secondary comorbidities and associated factors play any role [331]. Moreover, comprehensive prospective trials, such as the Massachusetts Male Aging Study, evidently demonstrated a decrease in circulating testosterone is associated with aging [125]. This was further confirmed with some other longitudinal studies showing continuous drops in serum testosterone levels independent of any associated comorbidity and risk factors [247]. The gradual increase in age has shown to decline in free and total serum testosterone levels, with rises in gonadotropins, LH, and FSH. Although the levels of LH increase with age, it does not correlate with testosterone secretion, which points to change in feedback mechanisms of these hormones with the unknown reason [287]. Furthermore, a decrease in free testosterone levels is greater than total testosterone, which eventually leads to reducing the biological activity of testosterone [247, 329]. The “normal” or healthy serum total testosterone levels vary widely among individuals over time, contingent to the normal functioning of the thyroid gland, serum protein levels, and other factors. But in general, men tend to have a higher testosterone level than women. In both males and females the peak level of total serum testosterone reaches around the age of 18 or 19 up to 3000–12,000 pg/mL and 80–330 pg/mL, respectively, then it gets declining throughout the remaining age (Table 14.2; values derived from earlier published reports) [41, 178, 290]. Interestingly, earlier reports also suggest that testosterone levels decrease with age as the aromatase activity increases, which converts testosterone to estrogen [332]. As per the American Urological Association’s (AUA) recent guidelines, the serum testosterone levels of equal to 3000 pg/mL are considered to be normal in males. While for women at a young age (19 years and up), the serum testosterone from 80–330 pg/mL are considered to be normal [41]. Other androgens, including the three pro-androgens: dehydro-epiandrosterone-sulfate (DHEAS), dehydro-epiandrosterone (DHEA), and androstenedione (A) are followed by conversion to active sex-steroids; testosterone and dihydrotestosterone (5α-DHT/DHT) [53]. Here, estrogen or DHT can be formed upon irreversible aromatization of testosterone [66, 113].
Table 14.2.
Average total testosterone levels in male and females through various ages of life
| Age | Male | Female |
|---|---|---|
| 18 years or below | 50–9750 pg/mL | 80–330 pg/mL |
| 18 to 19 years | 3000–12,000 pg/mL | 200–750 pg/mL |
| 19 years and above | 2400–9500 pg/mL | 40–480 pg/mL |
14.2.4. Crosstalk Between Sex-Steroids
Sex steroid hormones include estrogens, androgens, and progesterone, overall which are synthesized from cholesterol via steroidogenic pathways [311] and are present in both sexes (males and females) from the time of birth, while the levels in circulation vary greatly [93, 104, 340]. It is always believed that crosstalk among sex-steroids may occur only in women due to predominant levels of estrogens and progesterone [295]. However, multiple clinical and epidemiological studies reported the presence of all three hormones in both males and females at a variable concentration [90], which provides the possibility for local metabolism and interaction between these hormones in both sexes.
Sex-steroids control cellular mechanisms and functions by through the membrane, intracellular, and/or nuclear receptors, which further interact with discrete nucleotide sequences to alter gene expression [294]. Sex-steroid receptors such as estrogen receptors (ERs; ERα and ERβ), progesterone receptors (PRs; PR-A and PR-B), and androgen receptor (AR) are typically considered as nuclear receptors [294] and studies revealed that sex-steroid receptors generally have some common structural domains: C-terminus ligand binding domain (LBD), variable N-terminus with transcription activation function (AF-1) domain, mid-region DNA-binding domain (DBD) and hinge region. The LBD region of C-terminus carries another AF-2 domain which acts as a second region for transcriptional activity and various functions depends upon the interacting ligands [28, 227]. These similarities between the sex-steroid receptors improve the chances of interaction/crosstalk at multiple signaling levels in the target cells. Whereas specific ERβ activation shows opposite effects to ERα in cell proliferation [9, 11–13, 144], extracellular-matrix (ECM) modulation, and calcium handling. In the nucleus, ERα and ERβ can act as heterodimers or homodimers. Notably, emerging reports also propose the change in transcriptional activity due to heterodimer formation between ERα and AR [34, 294]. Unfortunately, limited data are available suggesting the interaction between sex-steroids, whereas minimal data showing the effects in the lung [294]. It would be very interesting to study the interaction and signaling mechanisms of combined sex-steroids at a different time of lifespan in both males and females. Additionally, to understand the contribution of individual sex-steroids signaling at the cellular and molecular level with their site of action will provide future prospective to better understand the pathophysiology of the disease.
14.3. Role of Sex-Steroid Signaling in Lung Diseases
Sex-steroid signaling and their effects are very complex, and cell-, tissue-, and context-dependent. Although sex-steroids are primarily gonadally derived, they are also locally produced in many tissues, and mediate their cellular actions via genomic and nongenomic receptor activation, which further complicates the overall interpretation. The nongenomic and genomic effects of sex-steroids have been extensively reviewed in various tissues including lung tissues [8, 13, 37, 168, 293–295, 323–326]. Conventional thinking was steroid receptors are generally located in the cytoplasm of target cells, which upon activation translocate into the nucleus to change the gene expression, which needs at least 30 to 60 minutes. Conversely, additional signaling regulatory actions of sex-steroids are displayed in seconds to a few minutes. This time is too rapid to produce genomic changes due to which they are typically termed nongenomic or rapid actions, which distinguish them, from classical sex-steroid dependent genomic actions of regulating gene expression [215, 294, 295]. Nongenomic effects of sex-steroids typically are diverse, from activation of adenylyl cyclase (AC), protein kinase C and A (PKC, PKA), mitogen-activated protein kinases (MAPKs), and heterotrimeric guanosine triphosphate-binding proteins (G proteins) [341]. These nongenomic effects of sex-steroids sometimes are mediated via the classical steroid receptors, which can act as ligand-activated transcription factors, while in other occasion’s classical sex-steroid receptors do not involve in these rapid effects [129, 341, 346]. Emerging indication suggests that the classical sex-steroid receptors can be present at the plasma membrane, which upon activation may trigger a chain of reactions in the cytoplasm [43, 196, 298]. Identification of interaction domains on the classical sex-steroid receptors responsible for the nongenomic effects or functions, and separation of this function from the genomic effects, should pave the way to a better understanding of the receptor-specific action of sex-steroids for therapeutic management of lung diseases.
14.3.1. Asthma
Asthma is a respiratory disorder associated with chronic inflammation causing significant morbidity and mortality worldwide [13, 20, 89, 191, 269–271, 273, 279, 292]. It is an intricate disorder that involves diverse pathophysiology affecting the airway structure and function in the lungs [270, 272, 280, 324]. The most substantial characteristic of asthma is a chronic inflammation of conducting airways, which is often associated with airway hyperresponsiveness (AHR) and remodeling [89, 191, 269–271, 292]. Although multiple genetic and environmental factors play a crucial role in the prevalence of asthma, sex/gender difference also acts as a notable driving force [62, 63, 294, 295]. It is challenging to determine the role of sex-steroids and their signaling mechanisms involved in asthma considering the wide array of factors affecting asthma pathophysiology. Here, intrinsic sex differences play an important role in lung physiology [294, 324], which concurs to apparent epidemiological evidence suggesting the impact of sex differences in asthma incidence, prevalence, and severity [39, 63, 293, 294, 303]. Clinical evidence suggests the prevalence of asthma is more common in boys, which is more than double the risk of developing asthma in girls [39, 60, 62, 163, 229], and as age increases this trend reverses. Adult women, after puberty, show greater incidence, frequency, and severity of asthma compared to men [39, 60, 62, 163, 229]. Similarly, few female patients with asthma experienced an exacerbation of asthmatic symptoms during premenstrual or menstrual phases of their cycle, suggesting a potentially crucial role of sex-steroids, especially estrogen [2, 42, 60, 62, 86, 179, 220, 293, 294]. Sex differences in the development of fetal lungs, as well as the maturation of lung tissues in adults, have been recognized to estrogen [29]. The alveoli development in females is estrogens dependent and control alveologenesis by ERα and ERβ activations [217, 259]. Estrogen treatment tends to show increased surfactant production in the fetal lung [74], which correlates to more rapid lung maturation in the female fetus than in the male fetus [322]. Although the number of alveoli per unit area and alveolar volume has no difference between males and females, males develop larger lungs with larger conducting airways in adulthood [212]. However, women are more vulnerable than men to the occurrence of several lung diseases; further implicates an estrogen’s role in this scenario. In connection to this, studies also reported that the levels of estrogen, in particular, E2 tend to increase in the bloodstream in pregnancy and may worsen lung disease such as asthma [22, 65].
Divergent sex-steroid signaling is described in the healthy and diseased condition of the lungs [294, 295, 328, 337]. Notably, female sex-steroid estrogen has a well-recognized function outside the reproductive system in different organs and tissues in both males and females, which includes cell growth, synthesis, and differentiation [81]. Recent advances in estrogen biology and a wide range of potential effects of estrogen on lung physiology in diseased conditions considerably increased our interest in exploring divergent estrogen signaling and its role in influencing the airway structure and function [325, 326, 328]. In humans, estrogen demonstrates its effect mainly via full-length ERα and ERβ, which comes under the nuclear receptor family of transcription factors [180, 238]. Earlier studies, including our own have demonstrated the increased expression of both ERα and ERβ in asthmatic and nonasthmatic human airway smooth muscle (ASM) cells and found that ERβ expression is significantly greater in the inflammatory/asthmatic condition in human ASM from males and females [18, 85, 155, 164, 325].
The genomic effects of nuclear ERs activation or those translocating from the cytosol to the nucleus are well reported. But, the role of membrane-bound activated ERs, their nongenomic activities, and associated downstream transcription factors are not fully explored [18]. In addition to full-length ERα (ERα-FL), there are two shorter (truncated) isoforms of ERα (ERα–36 and ERα–46) which do not have the AF-1 domain and show complex effects on ERα-FL [18]. ERβ, on the other hand, has five known variants (V1–5), while the modulatory signaling mechanism of ERβ splice variants during inflammation/asthma [18, 144] remains unclear. The overall cellular effects of estrogens vary depending on the nature and effects of the ligand binding (genomic vs. non-genomic), which makes for more complicated signaling within the tissue [91, 137, 139, 312]. Recent studies, including our own in ASM, have shown that acute exposure of E2 at physiological concentration inhibits plasma membrane influx via ERα, resulting in reduced ASM intracellular calcium ([Ca2+]i) [326]. Additionally, PKA and cAMP levels in ASM were increased due to acute exposure of E2 [325]. Further, long term exposure of E2 revealed the divergent effects of ERs in regulating [Ca2+]i in normal and asthmatic conditions. ERα activation showed increased [Ca2+]i response, while ERβ activation tends to decrease [Ca2+]i response in normal as well as asthmatic conditions. Here, ERβ activation effectively reduces [Ca2+]i responses, particularly in asthmatic ASM, via enhanced sarcoplasmic reticulum Ca2+ ATPase 2 SERCA2 function and inhibition of pathways involved in activating voltage-gated L-type Ca2+ channels (LTCC) [36, 37]. Sex-steroid signaling on different calcium regulatory channels in ASM represented schematically in Fig. 14.2. In addition to [Ca2+]i regulation, studies showed differential ER signaling in regulating ASM proliferation and airway remodeling in the context of asthma [11, 13]. Here, ERβ activation downregulated PDGF-induced proliferation via ERK/Akt/p38 MAPK pathways in human ASM cells, while ERα did not show any significant changes [13]. Additionally, ERβ activation also dampened TNFα or PDGF-induced ASM-derived ECM production and deposition via suppressing NFκB signaling [11].
Fig. 14.2.
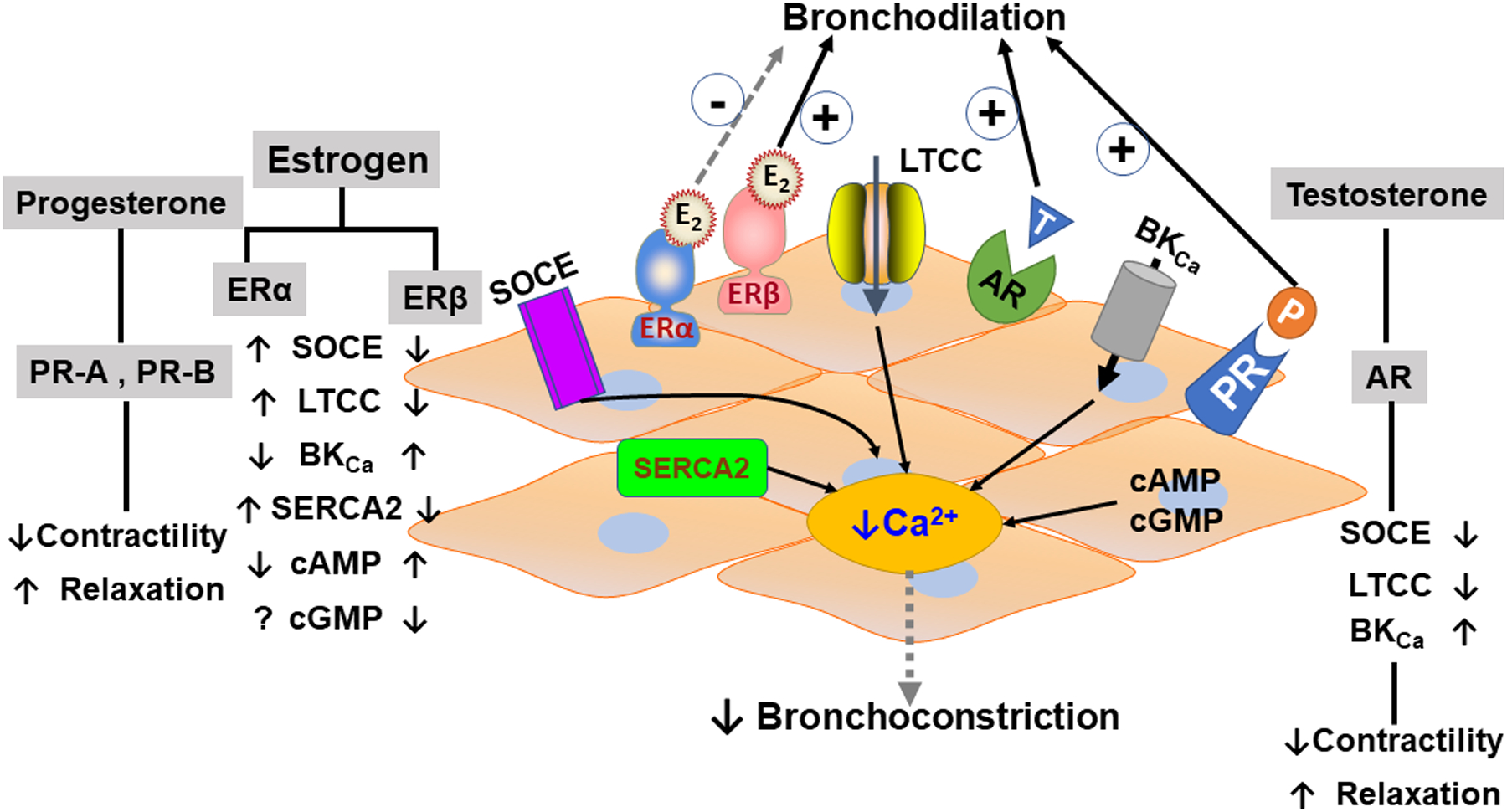
Sex-steroid signaling and their airway smooth muscle cellular mechanism in the regulation of intracellular calcium in lung disease.
Less is known about the role of progesterone in asthma, as most of the studies related to women’s sex-steroids are considerably focused on the regulatory effects of estrogen in lung diseases specific to asthma. Progesterone exerts its effect via activation of PR-A and PR-B with subsequent gene transcription [108, 294]. Both PR receptors are transcribed from the same gene, with minor changes in the truncated N-terminal domain of PR-A [97, 116, 249]. Typically, progesterone has a similar affinity to both PRs, with varied transcriptional activation. Here, PR-B is a strong promoter of transcriptional activities as reported in multiple cell types [116]. It has been shown that activation of PRs leads to recruitment of steroid receptor coactivator (SRCs; SRC-1, SRC-2, SRC-3), and CBP/p300, which are crucial regulatory proteins [226] that modulate histone acetylation/deacetylation and chromatin remodeling. In addition to nuclear receptor activation, PRs also promote and regulate numerous cellular signaling mechanisms independent of nuclear activation [294]. Limited studies have shown that progesterone decreases contractility and induces relaxation of bronchial smooth muscle [262]. Furthermore, progesterone has shown potent vasodilator effect than estrogen and testosterone in pulmonary arteries of both male and female rats [98]. Clinical studies reported a positive association of serum progesterone with peak expiratory flow rate during the luteal phase of the menstrual cycle, when the levels of progesterone are high [209] (Fig. 14.3).
Fig. 14.3.
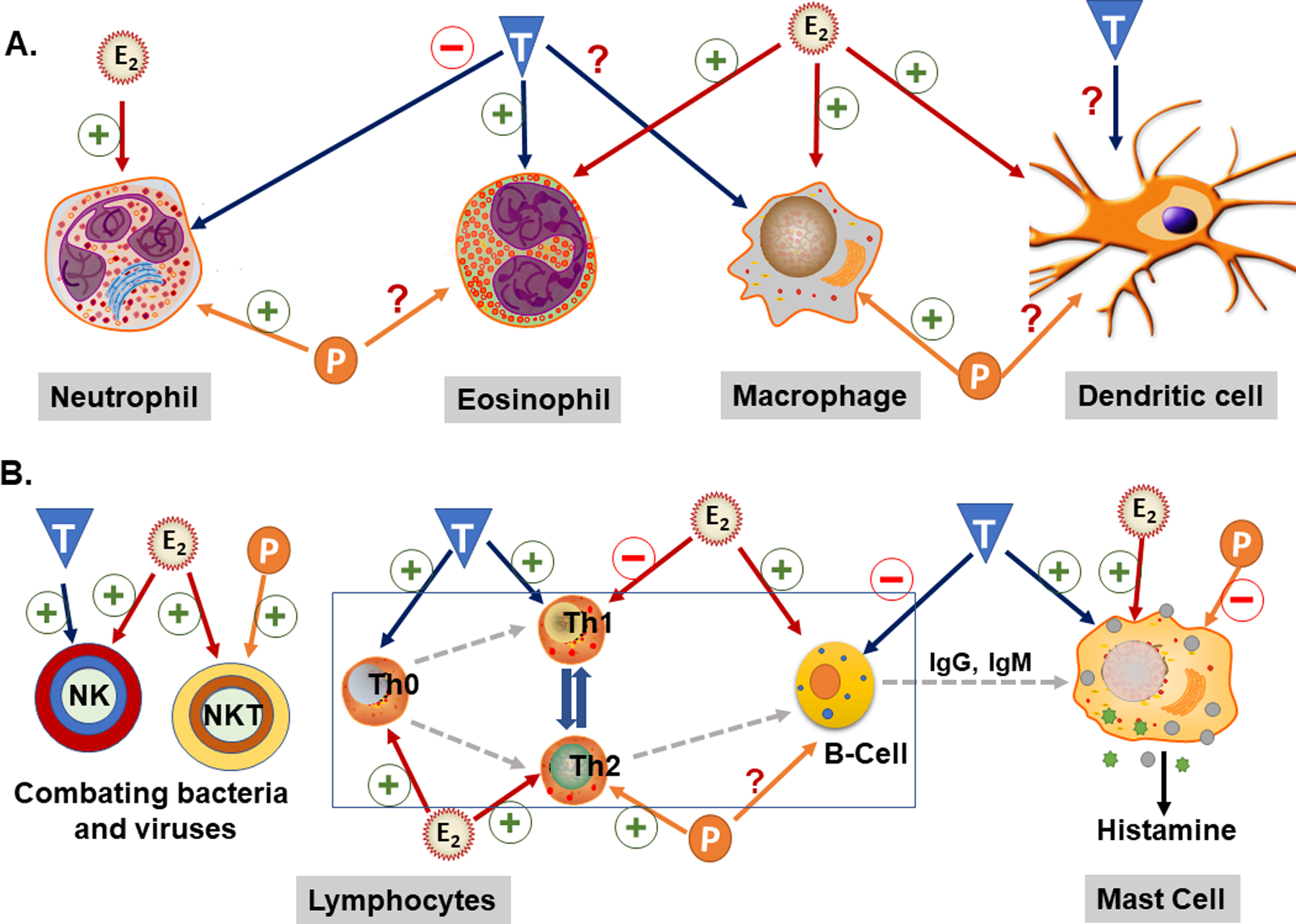
Sex-steroid effects on the immune cells. The effects of estrogen (E), progesterone (P), or testosterone (T) on dendritic cell, neutrophils, eosinophils and macrophages depicted in A. Many substantial inflammatory components like dendritic cells and macrophages/monocytes involved in various inflammatory lung diseases. These cells, particularly initiated after the first response of antigens, CD4+ lymphocytes, regulatory T-cells (Th0), B-lymphocytes, and other immune cells depicted in B.
In comparison to female sex-steroid hormones, the association of androgens in asthma prevalence is more apparent, albeit only by limited data that is available. Multiple studies involving androgens have shown anti-inflammatory activity by decreasing the Th2 cell response [69]. On the contrary castration in males exacerbates asthmatic symptoms [69]. Besides, the severity of asthma in men remains relatively constant from puberty to aging. However, when serum testosterone levels are declined due to aging, the increased asthma severity is observed [59, 294, 324], signifying the positive role for testosterone. Interestingly, even in women, testosterone improves asthma symptoms [344]. Androgens (testosterone and DHT) are well reported to modulate the contractility of various smooth muscle types by regulating the [Ca2+]i levels [44, 100, 245, 261, 289, 293, 324]. DHT, an active metabolite of testosterone at acute exposure (nongenomically) has been found to relax the smooth muscle by decreasing Ca2+ influx through large-conductance Ca2+-activated potassium (BKCa) channels, store-operated Ca2+ entry (SOCE) channels, LTCC and myosin light chain (MLC) phosphorylation [106, 165, 188, 245, 261, 291, 304]. In a mouse model of asthma, testosterone and DHT have shown relaxant effects on tracheal smooth muscle cells via AR [69, 110, 224, 236]. Studies using guinea pig airway tissues showed that AR activation modifies SOCE and LTCCs [165] along with IP3 (inositol 1,4,5-trisphosphate) receptors [246], independent of epithelium and potassium channel [107]. Similarly, the dehydro-epiandrosterone (DHEA) was also shown the same types of effects in the asthma model of guinea pig [110]. Further studies also reported the relaxant effect of testosterone on the epithelium in the rabbit trachea [188]. Studies using human ASM showed functional expression of AR in both males and females, which is upregulated during inflammation/asthmatic condition [171]. Furthermore, activation of AR significantly reduced Ca2+ influx via LTCCs and SOCE [171].
In contrast to human epidemiological data, animal studies relating to sex-steroids and asthma have been shown conflicting results. In a mouse model of asthma, estrogen appears to protect against airway hyperresponsiveness [92, 219], while progesterone aggravates inflammatory airway disease [146]. Furthermore, studies using male and female mice along with ovariectomization (OVX) mice, have reported that estrogen decreases AHR in an ovalbumin-induced model of allergic asthma [219]. Furthermore, spontaneous airway hyperresponsiveness has been reported in ERα knockout mice [61]. Additionally, in vivo studies from our group using a murine model of asthma showed that ERβ activation with specific pharmacological agonists downregulated AHR and airway remodeling [10]. These results were further confirmed by our subsequent study, where we evaluated receptor-specific effects of circulating endogenous estrogen in regulating AHR and remodeling using ERα and ERβ KO mice and found that ERβ KO upregulates airway remodeling and AHR [169, 170]. Interestingly, we found that ERα was either less effective in modulating airway structure/function in the mouse model, or in fact, worsened AHR and remodeling. Studies suggest progesterone has a stimulatory effect in the development of Th2 cells and inflammation with subsequent airway hyperresponsiveness in an allergic model of lung inflammation [87, 138]. In a study using a mouse model of influenza, progesterone treatment declined inflammation and improved lung function by restoring lung tissue homeostasis [138]. Overall, the above data, although limited, suggest very complex, somewhat synergistic, and opposing effects of female sex-steroids, with totally contrasting effects of male sex-steroids in asthma. In order to better understand the role of specific sex-steroids and their crosstalk in the context of asthma pathophysiology, detailed studies are warranted concerning specific cell types, life stages of a person, comorbidities, and the duration and concentration of exposure.
14.3.2. COPD
COPD is a chronic inflammatory lung disease that causes airflow blockage and difficulty in breathing [63]. The common symptoms associated with COPD are excess mucus (sputum) production, frequent coughing, wheezing, and shortness of breath [21]. In most cases, COPD is caused due to long-term exposure to irritating gases or particulate matter, most often from cigarette smoke [294]. Emphysema and chronic bronchitis are the most common conditions, which contribute to the progression of COPD. Emphysema is associated with damage to the alveoli at the end of smaller air passages (bronchioles) in the lungs. While chronic bronchitis is characterized by inflammation of the lining of the airways and subsequent cough and excess mucus production [26, 172, 278, 294, 295]. Historically, COPD was always considered to be a disease that mainly affects males due to the higher prevalence of cigarette smoking [26, 278]. However, epidemiological data in the last few decades showed steadily increasing rates of COPD in females [316, 343]; this could be due to increased smoking by females or other modulators.
In the past from 1999 to 2007, the rate of hospitalizations due to COPD significantly dropped for both men and women, but death rates associated with COPD were only lowered in men suggesting a relation between sex-steroids and pathophysiology of COPD [278]. The age-related deaths in U.S. men with COPD are approximately 30% higher than the rates for women [278]. However, as per the updated Centers for Disease Control and Prevention (CDC) data in the United States suggest that the overall death rates due to COPD in women are greater since the year 2000 [294]. This thinning gap in death rates represents a continuing shift of paradigm in the relative burden of COPD in women [4, 21]. In a recent clinical study with a large population of patients with COPD, females demonstrated more severe COPD with early-onset disease (COPD at age <60 years) and greater susceptibility to COPD with lower tobacco exposure [237, 278, 307]. Additional evidence suggests a greater prevalence of emphysema in men than women [278]. However, the reason for the change in disease patterns in men and women is largely unknown. The airways of female lungs are relatively smaller than that of males with the same lung volume, so there may be a chance that women’s lungs are exposed to a higher concentration of cigarette smoke per unit area of small airway surface [26, 233]. Additionally, the effect of sex-steroids on the prevalence of COPD is not yet explored. Reports exhibit that hormone replacement therapy in women did not affect the incidence of COPD [27]; however, another study showed improved lung function in postmenopausal women after hormone replacement therapy [64]. Interestingly, the metabolism of cigarette smoke may change in men and women since sex differences control the expression and activity of cytochrome P450 enzymes [305]. It is still not clear whether sex-steroids have a protective or detrimental role in COPD. As, studies reported estrogen via ERα activation promotes proliferation and thus potentiates the effect of cigarette smoke, toxic gases, and particulate matters on airway cells, leading to airway narrowing which is more common in women [294]. On the contrary, male sex-steroid testosterone relates to occurrence of greater emphysema due to alveolar destruction, while testosterone replacement therapy (TRT) has shown to decline disease progression in men with COPD [24]. Furthermore, sex-steroids can also modulate various immune responses in a very complex way and contribute to the overall response of the lungs to cigarette smoke in the context of COPD. Overall, these data suggest that women may be more susceptible to developing COPD in response to less cigarette smoke exposure in their lifetime compared to men [26, 294].
14.3.3. Pulmonary Fibrosis
Pulmonary fibrosis (PF) is a progressive fibrotic lung disease-causing scarring in the lungs with unknown etiology [294]. When PF persists for a long time, the scar tissue starts destroying the normal lung tissue and makes it hard for oxygen consumption in the bloodstream. Eventually, low blood oxygen levels and the stiffness in lung tissue due to scarring can lead to shortness of breath, particularly when walking and exercising [138, 317]. Substantial epidemiological reports suggest that sexual dimorphism impact the prevalence and severity of idiopathic pulmonary fibrosis (IPF) [62, 140]. The prevalence of IPF is more common in men with a worse prognosis than women [128, 256, 330]. However, women showed a higher symptomatic burden of IPF when compared to men with worse health-related quality of life [140]. Yet, the role of sex-steroids in modifying these differences in prevalence and symptomatic changes of IPF in men and women are currently unknown.
Although in the recent period, the understanding of IPF biology has improved, few animal studies sought to understand the role of sex differences in animal models [114, 335]. Studies using a rat model of PF reported the role of gender difference in experimental PF, where female rats displayed a higher degree of fibrosis than males in bleomycin models of PF. Upon OVX, female rats showed improvement in fibrosis while estrogen treatment worsened the response, which concludes the detrimental effect of estrogen in PF [114]. Interestingly, these changes in PF severity due to sex differences are reversed in a mouse model of bleomycin-induced fibrosis [142]. From these conflicting data it’s hard to interpret the exact role of sex-steroids in PF. A recent study showed the expression of PRs in the fibrotic areas of patients with usual interstitial pneumonia, but whether the PRs signaling mechanism exerts any role in these effects is still unclear [228].
14.3.4. Role of Sex-Steroids in the Pathophysiology of Rare Lung Diseases
Rare lung diseases (RLD) affect an estimated 2.5–5.5 million people in North America and Europe [222]. RLD includes a broad spectrum of pathophysiological conditions occurring either individually or as a cluster affecting every 1 in 2000 individuals [222]. Few of the widely known RLD’s include alpha-1 antitrypsin deficiency (AATD), pulmonary lymphangioleiomyomatosis (LAM), pulmonary alveolar proteinosis (PAP) and lung sarcoidosis (LS). Research on RLD’s is comparatively less due to their rare incidence and prevalence. Among the several RLDs, few have been extensively researched in LAM and AATD [222], while others are yet to be explored. Identifying the pathophysiology and epidemiology of these RLDs is quintessential to better understand the disease manifestation and to be able to manage these diseases with appropriate therapeutics. Few among these RLDs such as LAM, AATD, and LS show a gender disparity suggesting a plausible role for sex-steroids (Table 14.3). Here, we describe the role of sex-steroids in RLDs that have been evidenced to show gender disparities.
Table 3:
Reported roles of sex-steroids in rare lung diseases.
| LAM | AATD | LS | Other RLD’s | |
|---|---|---|---|---|
| Estrogen | ↑Disease progression ↑Proliferation Mechanistic evidence available (ERα ↑ severity and progression) |
↑ A1AT levels Limited data No mechanistic studies |
Females have higher risk, suggesting a role for estrogen and progesterone | No apparent gender differences. Sex-steroid mechanisms yet to be explored |
| Progesterone | ↑Disease progression ↑Proliferation Limited data with no concrete mechanistic evidence |
- | ||
| Testosterone | - | - | - |
14.3.4.1. Pulmonary Lymphangioleiomyomatosis
The pathophysiology of lymphangioleiomyomatosis (LAM) involves abnormal invasion and proliferation of smooth muscle-like cells, leading to the destruction of lung parenchyma [148, 150, 223]. LAM almost exclusively affects women, especially during childbearing age, suggesting a crucial role of female sex-steroids [80, 123, 183]. Several studies demonstrated the expression of ER’s (α and β) and PRs in LAM cells [35, 182], with no information on the expression of ARs. Here, several clinical studies indicate estrogen plays a predominant role in the development and progression of LAM. The progression of LAM is higher in pregnant women and in women taking exogenous estrogen via HRT, while it slows down after menopause. Another female predominant steroid that might contribute to LAM is progesterone. Furthermore, evidence from multiple studies suggests that estrogen positively regulates proliferation in TSC2-null AML cells, ELT3 cells, and 621101 AML cells via c-myc and ERK pathways [130, 149, 197]. Also, MMP-2 expression and activity are upregulated in LAM cells in the presence of estradiol [122, 197]. In vivo studies with TSC2 null cells as xenografts in mice showed enhanced tumor growth in the presence of estrogen, whereas in oophorectomy and aromatase inhibited mice, the tumor growth slowed down indicating the importance of estrogen [76, 275, 276].
Although the theory of estrogen being the major cause of LAM is being aggressively pursued, one major caveat to consider is males produce progesterone albeit significantly less compared to females [218]. The fact that progesterone is high during the luteal phase of the menstrual cycle, during pregnancy and with oral contraceptives and its consistency with situations associated with LAM progression further strengthen the need for exploring progesterone [276]. Furthermore, few studies suggest a higher PR to ER expression ratio in LAM cells [112]. The overall role of progesterone in LAM is still inconclusive as research is still in early stages with contradicting findings. Another study suggests progesterone along with estrogen synergistically potentiates proliferation in ELT3 cells [315], while other studies suggest progesterone inhibits estrogen-mediated proliferation in the same cell type [121, 153]. Further, in vivo murine studies suggest, estrogen is required for tumor growth, while progesterone alone had no effect on the tumor or did not modulate estradiol-induced growth [275]. Overall, the role of estrogen in LAM has been extensively studied by multiple groups establishing its importance in the progression of LAM. However, the role of progesterone and testosterone in LAM is yet to be identified and will probably shed valuable insights into the mechanisms behind the incidence and progression of LAM and its gender specificity.
14.3.4.2. Alpha1-Antitrypsin Deficiency:
Alpha1-antitrypsin (A1AT) deficiency (AATD) is a rare lung disease associated with declining levels of A1AT resulting in the destruction of the lungs by neutrophil elastase [88, 99, 266]. In a few cases, A1AT is produced, but functionally inactive due to abnormal folding of the proteins during their synthesis. Due to its rare incidence and prevalence, there is very limited progress in terms of understanding the epidemiology and pathophysiology of AATD. However, one study reported disparities in gender, age-related hormonal changes, and oral contraceptive use in regulating A1AT levels. According to this study, premenopausal women and women undergoing HRT or using oral contraceptives had higher circulating levels of A1AT, which was reversed in postmenopausal women implicating a potential role for female sex-steroids in controlling circulatory levels of A1AT [301]. Here, the role of progesterone and androgens is yet to be explored. Overall, the direct association of female sex-steroids and A1AT levels suggest a protective role for female sex-steroids in AATD; however, the exact mechanisms are still not known.
14.3.4.3. Lung Sarcoidosis
Sarcoidosis is a systemic inflammatory disease that most commonly targets lymph nodes, lungs, skin, and eyes [157]. Studies suggest the risk of sarcoidosis in slightly higher in females than in males [147, 239, 345]. The current study at a tertiary referral center found that 65.5% of the patients were females, which suggests a plausible role for sex-steroids [166]. This percentage goes upward to 70.3% in the age group of 70 years or older [72]. Although, one study in the African American race suggests increased incidence and risk of sarcoidosis during menarche, menopause, and parity; however, it is still inconclusive when it comes to a wider population [82]. Currently, there is no information available on the role of estrogen, progesterone, and testosterone in sarcoidosis and mechanisms involved.
14.4. Role of Sex-Steroids Signaling in Influencing Immune Responses in the Lungs
Being the most exposed organ, lungs are in a constant battle against exposure to a wide array of pathogens, allergens, toxins, air pollutants, and irritants [243]. Inflammation is the primary and key response to a multitude of insults such as hypersensitivity, infection, and trauma [243]. The constituents of the inflammatory response in the lungs vary depending on the type of pathogen mediated infection, allergens, or injury [1, 309]. During inflammation, numerous types of inflammatory cells are recruited in the lungs, which along with the resident lung cells orchestrate a plethora of inflammatory responses to address the specific need [33, 103, 201]. This orchestrated inflammatory milieu is skewed during disease conditions leading to an imbalance in T-cell mediated response [201]. Since, lungs are vital organs for gaseous exchange, prolonged or excessive inflammation leads to obstruction of the airways resulting in life-threatening conditions [243].
Inflammation is a direct result of the immune responses developed against a specific stimulus, either external or internal. These immune responses are divided largely into two categories, innate and adaptive, which play a pivotal role in host defense mechanisms [213]. Innate mechanisms include leukocytes and epithelium, which are the primary first-line barriers in the lung. On the other hand, adaptive immune mechanisms are far more complex and are driven and influenced by innate immune responses [213]. Here, we present the facts based on earlier and ongoing studies on the role of sex-steroids in regulating airway inflammation by modulating the function of various immune cells and resident lung cells.
14.4.1. Role of Sex-Steroids in Immune Cells
Epidemiological and clinical evidence suggests sex-steroids play a crucial role in regulating inflammatory milieu, especially in the lungs. The female predominance in allergic and autoimmune diseases has created a need for exploring sexual dimorphism and the role of sex-steroids in the immune system. This phenomenon is especially even more complicated in females as reproductive status affects the progression and severity of many diseases. Studies suggest sex-steroids have the potential to bind to various immune cells and resident lung cells influencing the overall immune responses and thereby inflammation [213]. Estrogen plays a pivotal role in regulating inflammation by regulating the immune responses in multiple cell types. The exact role of estrogen in inflammation is complex, as studies suggest it suppresses inflammation in chronic inflammatory diseases, while on the other hand, it produces pro-inflammatory cytokines in autoimmune diseases [22]. In addition, the effect of estrogen on inflammation is highly varied based on the concentration. Allergic airway diseases often exhibit exaggerated inflammation as a result of immune responses elicited by T-cells, B-cells, dendritic cells, macrophages, eosinophils, and neutrophils. These cells release a complex network of inflammatory milieu, which is Th2 biased in allergic diseases such as asthma, resulting in eosinophilia, airway inflammation, and AHR. Here, we limit the discussion to characteristic roles of different immune cells and existing findings on the role of different sex-steroids on regulating the activity of these cells.
14.4.1.1. Dendritic Cells
Dendritic cells (DCs) originate in the bone marrow and reach the lung via circulation [201]. They typically reside around the airway epithelium, alveolar septa, pulmonary capillaries, and airway spaces [201]. DCs along with macrophages serve as the first line of defense against inhaled agents [243]. Further, DCs are antigen-presenting cells (APCs) that have the potential to stimulate naïve T cell proliferation and differentiation. The mechanism of DCs includes identification, ingestion, and processing of antigen followed by migration to lymph nodes leading to a specific immune response [201]. Studies suggest DCs express both ERα/β as well as PR-A and their activity is modified by sex-steroids [118, 119]. One study in a mouse model of asthma suggests, female mice showed an increase in migratory DCs compared to males [230]. In studies not involving lungs, supraphysiological estradiol levels (pregnancy levels) downregulated the production of TNFα and IL-12 in mouse DCs [203].
14.4.1.2. Macrophages
Macrophages are essential in modulating inflammatory responses (acute vs. chronic) [184]. Although macrophages can proliferate in the lungs, their number is inadequate to fight pathogens, which is augmented by DCs [253]. Furthermore, macrophages are the mainstream source for cytokines, chemokines, and other inflammatory mediators, which collectively either aggravate or suppress immune response depending on the pathophysiological scenario [243]. Evidence suggests, macrophages express all 3 steroid receptors (ERs, PRs, and AR) and are modulated by sex-steroids [94, 118, 230]. Studies using murine models of asthma reported that female mice show exacerbated AHR and inflammation compared to male mice [10, 55, 169]. In a separate study, asthmatic mouse models have also shown elevated numbers of macrophages in the lungs of female mice compared to male mice, suggesting a potential role for female sex-steroids in modulating macrophage activity [230]. In addition, sex-steroids have also been implicated in influencing the polarization state of macrophages [334]. Here, estrogen shortened the pro-inflammatory phase of macrophages, while increasing the gene expression of proteins responsible for the resolution phase during asthma [334]. Our own in vivo studies in a mouse model of asthma showed elevated macrophages in females compared to males, which was abrogated in OVX mice, suggesting the importance of estrogen in regulating airway inflammation [10]. Further, we found that this change in macrophage numbers in lungs was largely depended on specific ER, where ERα KO mice showed lower numbers of macrophages compared to ERβ KO mice [169]. In studies from other systems, estradiol inhibited LPS induced TNFα release and nitrite production in RAW 264.7 cells [350], while progesterone inhibits the release of pro-inflammatory membrane-bound vesicular microparticles from macrophages [267]. In contrast, limited studies on androgens suggest, testosterone downregulated TNFα and NO production in macrophages in vitro [285, 297] and downregulated airway inflammation in vivo in mouse model of asthma [69, 110]. Considering these findings, it is evident that sex-steroids regulate macrophage function in inflammation, and to understand the underlying mechanisms involved, further studies are warranted.
14.4.1.3. Neutrophils
Most forms of acute lung injuries showed neutrophils to play a central role in their pathogenesis [193]. They are the first type of cells to be recruited in the event of inflammatory stimuli, which serve as the second line of defense following DCs and macrophages [103, 118, 230]. Neutrophils ingest the foreign particles or debris via phagocytosis, followed by killing them with reactive oxygen species (ROS), antimicrobial proteins, and neutrophil elastase [243]. Furthermore, neutrophils are terminally differentiated cells, which are nonproliferative and synthesize minimal RNA and protein. Any deficiency in the amount or activity of neutrophils in the lungs predisposes individuals to lung infections.
Although the expression studies of sex steroid receptors in neutrophils are limited, multiple studies confirmed the role of gender and sex-steroids in regulating neutrophil numbers and activity [240, 244]. In studies from other systems, pregnancy and luteal phase showed increased granulocyte numbers compared to the follicular phase and normal ovarian cycle [16, 46–48, 101, 102, 254], suggesting a role for progesterone and estrogen in regulating neutrophil activity. However, the neutrophil count in males is similar to females in their menstrual cycle, which raises a question on the role of estrogen in neutrophil activity [48, 348]. In a separate study, estrogens decreased the chemotactic activity of neutrophils, while progesterone enhanced this activity [240]. The role of sex-steroids in regulating the free radical production activity of neutrophils is contradicting and needs further studies [31, 68, 244]. Overall, from the existing literature, estrogen seems to have an anti-inflammatory effect on neutrophils, while progesterone shows a pro-inflammatory effect. Finally, both gender and the reproductive condition seem to affect neutrophil numbers and activity, albeit the underlying mechanisms remain elusive [47].
14.4.1.4. Eosinophils
Eosinophil infiltration is a characteristic feature of asthma and serves as a key cellular component development of allergic airway diseases [57]. Eosinophils are the least common white blood cells in normal lung, which is changed during disease conditions such as asthma, where the increased number of infiltrated eosinophils is found in the lungs from murine models of asthma and in lungs from human asthmatic patients [10, 57, 134, 169, 280, 281, 347]. Further, it is an important source for many cytokines, lipid mediators, growth factors, and chemokines [177].
Studies suggest, estradiol binds to eosinophils [185] and enhances the adhesiveness and degranulation of eosinophils [118, 119]. There are no reports available on the expression of PR’s and AR on eosinophils; however, one study reported that progesterone increases eosinophil infiltration and AHR in a murine model of asthma [286]. Here, an important fact to know is that progesterone is converted to estrogen, which might be the reason for this particular activity. On the contrary, limited studies suggest that androgens downregulate eosinophil adhesiveness and degranulation; however, the mechanisms behind this action are still unclear [145, 146]. Here, our recent studies suggested an elevated eosinophil levels in BAL from female mice compared to male and OVX mice, suggesting a crucial estrogen in eosinophil recruitment [10, 169]. However, the mechanisms behind this effect are still unclear. Overall, further research into the mechanisms behind these effects is warranted to understand the comprehensive role of sex-steroids on eosinophils.
14.4.1.5. Lymphocytes
Lymphocytes are distributed across the lungs, starting from the airways all the way into the lung parenchyma. There are two major subsets of lymphocytes, thymus-dependent T-lymphocytes and bone marrow dependent B-lymphocytes, which are discussed in detail in the following subsections. Lymphocytes (Both B and T) play a pivotal role in autoimmune and allergic disorders due to their immune response regulatory role [48, 167, 218, 264, 339]. It is well documented that sex-steroids, especially estrogen and testosterone bind to lymphocytes and regulate their activity. Peripheral blood mononuclear cells (PBMCs) and thymic cells respond to estradiol, whereas only thymic cells are regulated by androgens in humans [14, 73, 285, 349]. Here, we discuss the role of sex-steroids in regulating the function of T and B lymphocytes, thereby affecting the overall immune response in the lungs.
T-lymphocytes
T-lymphocytes are originated from thymus and are widely classified into two subsets: CD4+ and CD8+ cells [243]. CD4+ cells (T helper cells) are further divided into two subsets, Th1 and Th2 with different cytokine populations. Th1 cytokines (IFNγ, TNFα, etc.) produce pro-inflammatory responses and drive cellular immunity, Th2 cytokines (IL-4, IL-5, IL-9, and IL-13) on the other hand drive humoral immunity resulting in antibody production, IgE and eosinophilic responses [243]. Historically, it is known that Th1 and Th2 mediated immune responses are antagonistic to one another and nullify the overall inflammation. However, in case of an imbalance between these immune responses, it often leads to prolonged inflammation that often translates to a key component of disease pathophysiology. In recent years, our understanding of the interactions and crosstalk between immune cells has significantly increased and has led us to consider, if this concept is far more complex than it is. In this context, recently identified Th17 mediated immune responses have gained considerable significance. Depending on the disease pathophysiology, multiple cell types trigger a coordinated T-cell mediated immune response, which can be widely classified into T-helper 1 (Th1), Th2, and Th17 mediated immune responses [167]. CD8+ cells on the other hand secrete toxic molecules that help in eliminating infected cells and tumor cells and are cytotoxic [167]. In addition to CD4+ and CD8+ cells, there are natural killer cells (NK cells) and natural killer like T cells (NKT cells), which have no specific antigen-specific receptors and are important in combating bacterial and viral infections [50, 181, 182].
All the subpopulations of T-cells express ERs (α and β) [265]; however, the expression profiles of PRs and ARs have not been examined systematically across the human anatomy [141, 154, 241], although studies suggest that sex-steroids (including progesterone and androgens) modulate T-cell numbers and function. Multiple studies suggest lower T-cell numbers in males compared to females [46–48], which might be due to the ability of testosterone to induce apoptosis in T-cells [70]. Evidence indicates higher estrogen levels skew the immune system toward Th2 response, increasing eosinophil recruitment and IL-4 and IL-13 levels in the lung [87, 309, 312]. This may possibly explain the higher incidence and severity of asthma in females compared to males. Further, in vivo studies show a higher allergic response in female mice compare to male mice, while OVX mice tend to develop relatively less airway inflammation [10, 169, 200]. Progesterone inhibits differentiation of Th1 cells [154, 264] while inducing IL-4 and IL-10 production [241, 264] and inhibiting TNFα action by antagonizing NFкB [141].
On the contrary, androgens tend to skew the immune response toward Th1. DHEA, a precursor to testosterone alleviated airway inflammation in murine models [349]. Interestingly, male patients with asthma tend to show relatively lower levels of DHEA compared to healthy controls [73]. Overall, existing evidence suggests female sex-steroids (estrogen and progesterone) are detrimental in allergic airway diseases in the context of T-cells due to their bias toward Th2 response, however, reports on androgens effects on T-Cell are contradicting and warrants further studies. Although the superficial role of sex-steroids on regulating T-cell function in surfacing, the underlying mechanisms responsible for these changes are still obscure.
B-lymphocytes
Activation of B-cells plays a crucial role in increasing the serum IgE levels. B-cells are activated by differentiated Th2 cells. Occasionally, antigen-activated B-cells differentiate into memory cells, which produce prolonged and long-lasting inflammation [135, 136]. More importantly, B-cells are the major mediators of antibody production in response to multiple stimuli [48, 70, 167, 181]. These B-cell mediated antibodies, especially IgE degranulates mast cells resulting in the release of histamine, IL-4, and IL-13, which aggravate inflammation and AHR in the allergic airways.
Evidence from clinical blood panels suggests elevated serum antibody concentrations in females compared to males, suggesting a crucial role for female sex-steroids (estrogen and progesterone) in regulating B-cell activity [6, 120, 198]. Testosterone has been evidenced to inhibit IgG and IgM production, while estrogen increased it in vitro [174–176, 339]. Several studies in mouse models of allergic diseases reported elevated levels of IgE and IgG in female mice compared to male mice [79, 302]. Furthermore, another study in a murine model of allergic asthma reported downregulated bronchial inflammation in males compared to females, which was alleviated in castrated males, suggesting a pivotal role for androgens in allergic airway diseases [143]. Existing evidence suggests an important role for testosterone and estrogen in B-cell mediated antibody production; however, the role of progesterone is yet to be investigated.
14.5. Conclusion and Future Scope
To conclude all the above, the existing knowledge on the intrinsic sex difference and their association in controlling intracellular signaling to whole organ structure and function in normal and disease condition suggest the importance of sex-steroids. Convincing data from an array of studies also demonstrated the differential effects of sex-steroids in the management of lung structure and function, but there is limited knowledge on sex-steroid signaling during normal lung physiology and diseased conditions. Sex-steroids are known to produce their cellular and molecular effects via genomic or non-genomic activation of nuclear and membrane-associated receptors respectively. However, there are multiple additional factors such as gender, age, effector cell types, receptor expression pattern, duration of exposure, disease type, and crosstalk between the sex-steroids, which further directs the sex-steroid signaling and their resultant effects on cellular function. Additionally, the emerging concepts of locally produced versus systemic steroids and their metabolites produced in tissue amplify the complexity of sex-steroid signaling in lung diseases. Another major issue that needs to be a highlight is the role of sex-steroids in regulating inflammatory milieu, especially in lung diseases. As studies exploring the mechanistic role of sex-steroids in airway inflammation by modulating Th1 and Th2 immune cell signaling has supported both protective versus detrimental effects of sex-steroids. Overall, multiple studies have recognized the divergent effects of sex-steroids with respect to their differential receptor expressions in respiratory diseases, but it remains to understand the sex-steroid signaling and how it controls the pathophysiology of the lung in normal and diseased conditions. A detailed exploration of sex-steroid signaling and their mechanisms in airway disease can open the new avenue to the effective management of lung diseases by developing a novel therapeutic approach.
Acknowledgment
Supported by NIH grants R01-HL123494, R01-HL123494-02S1, and R01-HL146705 (Venkatachalem)
References
- 1.Adler KB, Fischer BM, Wright DT, et al. Interactions between respiratory epithelial cells and cytokines: relationships to lung inflammation. Ann N Y Acad Sci. 1994;725:128–45. 10.1111/j.1749-6632.1994.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal AK, Shah A. Menstrual-linked asthma. J Asthma. 1997;34(6):539–45. [DOI] [PubMed] [Google Scholar]
- 3.Aguirre MA, Vélez A, Romero M, et al. Gynecomastia and sexual impotence associated with methotrexate treatment. J Rheumatol. 2002;29(8):1793–4. [PubMed] [Google Scholar]
- 4.Akinbami LJ, Liu X. Chronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998–2009. NCHS Data Brief. 2011;63:1–8. [PubMed] [Google Scholar]
- 5.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012;94:1–8. [PubMed] [Google Scholar]
- 6.Allansmith M, McClellan B, Butterworth M. Stability of human immunoglobulin levels. Proc Soc Exp Biol Med. 1967;125(2):404–7. 10.3181/00379727-125-32104. [DOI] [PubMed] [Google Scholar]
- 7.Altman LK. Action urged on diseases with dangers for women. New York Times, February 28; 2004. [Google Scholar]
- 8.Ambhore NS, Bhallamudi S, Kalidhindi R, et al. Role of estrogen metabolites in regulating intracellular calcium in human airway smooth muscle cell. Am J Respir Crit Care Med. 2020;201:A1237. [Google Scholar]
- 9.Ambhore NS, Kalidhindi R, Pabelick C, et al. Receptor specific estrogen signaling regulates extracellular matrix deposition in human airway smooth muscle remodeling. Am J Respir Crit Care Med. 2019a;199:A2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambhore NS, Kalidhindi RSR, Loganathan J, et al. Role of differential estrogen receptor activation in airway hyperreactivity and remodeling in a murine model of asthma. Am J Respir Cell Mol Biol. 2019b;61(4):469–80. 10.1165/rcmb.2018-0321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambhore NS, Kalidhindi RSR, Pabelick CM, et al. Differential estrogen-receptor activation regulates extracellular matrix deposition in human airway smooth muscle remodeling via NFκB pathway. FASEB J. 2019c;33(12):13935–50. 10.1096/fj.201901340R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambhore NS, Katragadda R, Kalidhindi R, et al. Estrogen receptor beta signaling negatively regulates PDGF induced human airway smooth muscle proliferation. Am J Respir Crit Care Med. 2018a;197:A1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambhore NS, Katragadda R, Kalidhindi RSR, et al. Estrogen receptor beta signaling inhibits PDGF induced human airway smooth muscle proliferation. Mol Cell Endocrinol. 2018b;476:37–47. 10.1016/j.mce.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angele MK, Knöferl MW, Ayala A, et al. Testosterone and estrogen differently effect Th1 and Th2 cytokine release following trauma-haemorrhage. Cytokine. 2001;16(1):22–30. 10.1006/cyto.2001.0945. [DOI] [PubMed] [Google Scholar]
- 15.Appelman Y, van Rijn BB, Monique E, et al. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015;241(1):211–8. [DOI] [PubMed] [Google Scholar]
- 16.Apseloff G, Bao X, LaBoy-Goral L, et al. Practical considerations regarding the influence of the menstrual cycle on leukocyte parameters in clinical trials. Am J Ther. 2000;7(5):297–302. 10.1097/00045391-200007050-00005. [DOI] [PubMed] [Google Scholar]
- 17.Arain FA, Kuniyoshi FH, Abdalrhim AD, et al. Sex/gender medicine. Circ J. 2009;73:1774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aravamudan B, Goorhouse KJ, Unnikrishnan G, et al. Differential expression of estrogen receptor variants in response to inflammation signals in human airway smooth muscle. J Cell Physiol. 2017;232(7):1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34(3):J258–65. [DOI] [PubMed] [Google Scholar]
- 20.Arvizo RR, Miranda OR, Thompson MA, et al. Effect of nanoparticle surface charge at the plasma membrane and beyond. Nano Lett. 2010;10(7):2543–8. 10.1021/nl101140t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aryal S, Diaz-Guzman E, Mannino DM. COPD and gender differences: an update. Transl Res. 2013;162(4):208–18. [DOI] [PubMed] [Google Scholar]
- 22.Assaggaf H, Felty Q. Gender, estrogen, and obliterative lesions in the lung. Int J Endocrinol. 2017;2017: 8475701. 10.1155/2017/8475701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin ED, Lahm T, West J, et al. Gender, sex hormones and pulmonary hypertension. Pulm Circ. 2013;3(2):294–314. 10.4103/2045-8932.114756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baillargeon J, Urban RJ, Zhang W, et al. Testosterone replacement therapy and hospitalization rates in men with COPD. Chron Respir Dis. 2018;16:1479972318793004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bain J The many faces of testosterone. Clin Interv Aging. 2007;2(4):567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes PJ. Sex differences in chronic obstructive pulmonary disease mechanisms. Am J Respir Crit Care Med. 2016;193(8):813–4. [DOI] [PubMed] [Google Scholar]
- 27.Barr RG, Wentowski CC, Grodstein F, et al. Prospective study of postmenopausal hormone use and newly diagnosed asthma and chronic obstructive pulmonary disease. Arch Intern Med. 2004;164(4):379–86. [DOI] [PubMed] [Google Scholar]
- 28.Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000;6(3):225–36. [DOI] [PubMed] [Google Scholar]
- 29.Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. 1999;54(12):1119–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beierle I, Meibohm B, Derendorf H. Gender differences in pharmacokinetics and pharmacodynamics. Int J Clin Pharmacol Ther. 1999;37(11):529–47. [PubMed] [Google Scholar]
- 31.Békési G, Kakucs R, Várbíró S, et al. In vitro effects of different steroid hormones on superoxide anion production of human neutrophil granulocytes. Steroids. 2000;65(12):889–94. 10.1016/s0039-128x(00)00183-5. [DOI] [PubMed] [Google Scholar]
- 32.Belanger A, Candas B, Dupont A, et al. Changes in serum concentrations of conjugated and unconjugated steroids in 40-to 80-year-old men. J Clin Endocrinol Metabol. 1994;79(4):1086–90. [DOI] [PubMed] [Google Scholar]
- 33.Bender AT, Ostenson CL, Wang EH, et al. Selective up-regulation of PDE1B2 upon monocyte-to-macrophage differentiation. Proc Natl Acad Sci U S A. 2005;102(2):497–502. 10.1073/pnas.0408535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett NC, Gardiner RA, Hooper JD, et al. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol. 2010;42(6):813–27. [DOI] [PubMed] [Google Scholar]
- 35.Berger U, Khaghani A, Pomerance A, et al. Pulmonary lymphangioleiomyomatosis and steroid receptors. An immunocytochemical study. Am J Clin Pathol. 1990;93(5):609–14. 10.1093/ajcp/93.5.609. [DOI] [PubMed] [Google Scholar]
- 36.Bhallamudi S, Ambhore NS, Saladi S, et al. Estrogen receptors differentially regulate the overall contractility of human airway smooth muscle. FASEB J. 2020a;34(S1):1–1. [Google Scholar]
- 37.Bhallamudi S, Connell J, Pabelick CM, et al. Estrogen receptors differentially regulates intracellular calcium handling in human non-asthmatic and asthmatic airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2020b;318(1):L112–24. 10.1152/ajplung.00206.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bigos KL, Pollock BG, Stankevich BA, et al. Sex differences in the pharmacokinetics and pharmacodynamics of antidepressants: an updated review. Gend Med. 2009;6(4):522–43. [DOI] [PubMed] [Google Scholar]
- 39.Bjornson CL, Mitchell I. Gender differences in asthma in childhood and adolescence. J Gend Specif Med. 2000;3(8):57–61. [PubMed] [Google Scholar]
- 40.Blaustein JD, Ismail N. Enduring influence of pubertal stressors on behavioral response to hormones in female mice. Horm Behav. 2013;64(2):390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blazer DG, Liverman CT. Testosterone and aging: clinical research directions. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 42.Bonds RS, Midoro-Horiuti T. Estrogen effects in allergy and asthma. Curr Opin Allergy Clin Immunol. 2013;13(1):92–9. 10.1097/ACI.0b013e32835a6dd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boonyaratanakornkit V, Hamilton N, Márquez-Garbán DC, et al. Extranuclear signaling by sex steroid receptors and clinical implications in breast cancer. Mol Cell Endocrinol. 2018;466:51–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bordallo J, de Boto MJ, Meana C, et al. Modulatory role of endogenous androgens on airway smooth muscle tone in isolated guinea-pig and bovine trachea; involvement of beta2-adrenoceptors, the polyamine system and external calcium. Eur J Pharmacol. 2008;601(1–3):154–62. 10.1016/j.ejphar.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 45.Borkar NA, Sathish V. Sex steroids and their influence in lung disease across the lifespan. In: Silveyra P, Tigno X, editors. Sex-based differences in lung physiology. Rockville: American Physiological Society and Springer-Nature; 2021. [Google Scholar]
- 46.Bouman A, Moes H, Heineman MJ, et al. The immune response during the luteal phase of the ovarian cycle: increasing sensitivity of human monocytes to endotoxin. Fertil Steril. 2001;76(3):555–9. 10.1016/s0015-0282(01)01971-9. [DOI] [PubMed] [Google Scholar]
- 47.Bouman A, Schipper M, Heineman MJ, et al. 17betaestradiol and progesterone do not influence the production of cytokines from lipopolysaccharide-stimulated monocytes in humans. Fertil Steril. 2004a;82(Suppl 3):1212–9. 10.1016/j.fertnstert.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 48.Bouman A, Schipper M, Heineman MJ, et al. Gender difference in the non-specific and specific immune response in humans. Am J Reprod Immunol. 2004b;52(1):19–26. 10.1111/j.1600-0897.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- 49.Braman SS. The global burden of asthma. Chest. 2006;130(1):4S–12S. [DOI] [PubMed] [Google Scholar]
- 50.Brigl M, Bry L, Kent SC, et al. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4(12):1230–7. 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 51.Brisken C, Park S, Vass T, et al. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci. 1998;95(9):5076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brozek G, Lawson J, Szumilas D, et al. Increasing prevalence of asthma, respiratory symptoms, and allergic diseases: four repeated surveys from 1993–2014. Respir Med. 2015;109(8):982–90. [DOI] [PubMed] [Google Scholar]
- 53.Burger HG. Androgen production in women. Fertil Steril. 2002;77:3–5. [DOI] [PubMed] [Google Scholar]
- 54.Buvinic M, Médici A, Fernández E, et al. Gender differentials in health. Dis Contr Priorities Dev Countries. 2006;2:195–210. [Google Scholar]
- 55.Cabello N, Mishra V, Sinha U, et al. Sex differences in the expression of lung inflammatory mediators in response to ozone. Am J Phys Lung Cell Mol Phys. 2015;309(10):L1150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calabrese EJ. Gastrointestinal and dermal absorption: interspecies differences. Drug Metab Rev. 1984;15(5–6):1013–32. [DOI] [PubMed] [Google Scholar]
- 57.Calhoun WJ, Sedgwick J, Busse WW. The role of eosinophils in the pathophysiology of asthma. Ann N Y Acad Sci. 1991;629:62–72. 10.1111/j.1749-6632.1991.tb37961.x. [DOI] [PubMed] [Google Scholar]
- 58.Camp PG, Coxson HO, Levy RD, et al. Sex differences in emphysema and airway disease in smokers. Chest. 2009;136(6):1480–8. [DOI] [PubMed] [Google Scholar]
- 59.Canguven O, Albayrak S. Do low testosterone levels contribute to the pathogenesis of asthma? Med Hypotheses. 2011;76(4):585–8. [DOI] [PubMed] [Google Scholar]
- 60.Caracta CF. Gender differences in pulmonary disease. Mount Sinai J Med. 2003;70(4):215–24. [PubMed] [Google Scholar]
- 61.Carey MA, Card JW, Bradbury JA, et al. Spontaneous airway hyperresponsiveness in estrogen receptor-α–deficient mice. Am J Respir Crit Care Med. 2007a;175(2):126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carey MA, Card JW, Voltz JW, et al. It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab. 2007b;18(8):308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carey MA, Card JW, Voltz JW, et al. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Phys Lung Cell Mol Phys. 2007c;293(2):L272–8. [DOI] [PubMed] [Google Scholar]
- 64.Carlson CL, Cushman M, Enright PL, et al. Hormone replacement therapy is associated with higher FEV1 in elderly women. Am J Respir Crit Care Med. 2001;163(2):423–8. [DOI] [PubMed] [Google Scholar]
- 65.Carranza-Lira S, Hernandez F, Sanchez M, et al. Prolactin secretion in molar and normal pregnancy. Int J Gynecol Obstet. 1998;60(2):137–41. [DOI] [PubMed] [Google Scholar]
- 66.Carretero J, Vázquez G, Martín-Clavijo A, et al. In vivo studies on cytodifferentiation of pituitary aromatase-immunoreactive cells. Eur J Anat. 2020;3(2):79–85. [Google Scholar]
- 67.Casimir GJ, Lefèvre N, Corazza F, et al. Sex and inflammation in respiratory diseases: a clinical viewpoint. Biol Sex Differ. 2013;4(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cassidy RA. Influence of steroids on oxidant generation in activated human granulocytes and mononuclear leukocytes. Shock. 2003;20(1):85–90. 10.1097/01.shk.0000070740.34700.cd. [DOI] [PubMed] [Google Scholar]
- 69.Cephus JY, Stier MT, Fuseini H, et al. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 2017;21(9):2487–99. 10.1016/j.celrep.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Champney TH, Prado J, Youngblood T, et al. Immune responsiveness of splenocytes after chronic daily melatonin administration in male Syrian hamsters. Immunol Lett. 1997;58(2):95–100. 10.1016/s0165-2478(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 71.Chapman KR, Tashkin DP, Pye DJ. Gender bias in the diagnosis of COPD. Chest. 2001;119(6):1691–5. [DOI] [PubMed] [Google Scholar]
- 72.Chevalet P, Clément R, Rodat O, et al. Sarcoidosis diagnosed in elderly subjects: retrospective study of 30 cases. Chest. 2004;126(5):1423–30. 10.1378/chest.126.5.1423. [DOI] [PubMed] [Google Scholar]
- 73.Choi IS, Cui Y, Koh YA, et al. Effects of dehydroepiandrosterone on Th2 cytokine production in peripheral blood mononuclear cells from asthmatics. Korean J Intern Med. 2008;23(4):176–81. 10.3904/kjim.2008.23.4.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chu A, Rooney S. Estrogen stimulation of surfactant synthesis. Pediatr Pulmonol. 1985;1(3 Suppl):S110–4. [PubMed] [Google Scholar]
- 75.Church DD, Pasiakos SM, Wolfe RR, et al. Acute testosterone administration does not affect muscle anabolism. Nutr Metab. 2019;16(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clements D, Asprey SL, McCulloch TA, et al. Analysis of the oestrogen response in an angiomyolipoma derived xenograft model. Endocr Relat Cancer. 2009;16(1):59–72. 10.1677/erc-08-0123. [DOI] [PubMed] [Google Scholar]
- 77.Condon JC, Hardy DB, Kovaric K, et al. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-κB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20(4):764–75. [DOI] [PubMed] [Google Scholar]
- 78.Corona G, Rastrelli G, Vignozzi L, et al. Testosterone, cardiovascular disease and the metabolic syndrome. Best Pract Res Clin Endocrinol Metab. 2011;25(2):337–53. [DOI] [PubMed] [Google Scholar]
- 79.Corteling R, Trifilieff A. Gender comparison in a murine model of allergen-driven airway inflammation and the response to budesonide treatment. BMC Pharmacol. 2004;4:4. 10.1186/1471-2210-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc. 2000;75(6):591–4. 10.4065/75.6.591. [DOI] [PubMed] [Google Scholar]
- 81.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. [DOI] [PubMed] [Google Scholar]
- 82.Cozier YC, Berman JS, Palmer JR, et al. Sarcoidosis in Black women in the United States: data from the Black Women’s Health Study. Chest. 2011;139(1):144–50. 10.1378/chest.10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Csapo AI, Pulkkinen MO, Wiest W. Effects of luteectomy and progesterone replacement therapy in early pregnant patients. Am J Obstet Gynecol. 1973;115(6):759–65. [DOI] [PubMed] [Google Scholar]
- 84.Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med. 2013;19(3):197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dahlman-Wright K, Cavailles V, Fuqua SA, et al. International union of pharmacology. LXIV Estrogen Receptors Pharmacol Rev. 2006;58(4):773–81. [DOI] [PubMed] [Google Scholar]
- 86.de Marco R, Locatelli F, Sunyer J, et al. Differences in incidence of reported asthma related to age in men and women. A retrospective analysis of the data of the European Respiratory Health Survey. Am J Respir Crit Care Med. 2000;162(1):68–74. 10.1164/ajrccm.162.1.9907008. [DOI] [PubMed] [Google Scholar]
- 87.de Oliveira AP, Peron JP, Damazo AS, et al. Female sex hormones mediate the allergic lung reaction by regulating the release of inflammatory mediators and the expression of lung E-selectin in rats. Respir Res. 2010;11(1):115. 10.1186/1465-9921-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Serres FJ, Blanco I, Fernandez-Bustillo E. Genetic epidemiology of alpha-1 antitrypsin deficiency in North America and Australia/New Zealand: Australia, Canada, New Zealand and the United States of America. Clin Genet. 2003;64(5):382–97. 10.1034/j.1399-0004.2003.00143.x. [DOI] [PubMed] [Google Scholar]
- 89.Dekkers BG, Maarsingh H, Meurs H, et al. Airway structural components drive airway smooth muscle remodeling in asthma. Proc Am Thorac Soc.2009;6(8):683–92. [DOI] [PubMed] [Google Scholar]
- 90.Derntl B, Pintzinger N, Kryspin-Exner I, et al. The impact of sex hormone concentrations on decision-making in females and males. Front Neurosci. 2014;8:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116(3):561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dimitropoulou C, White RE, Ownby DR, et al. Estrogen reduces carbachol-induced constriction of asthmatic airways by stimulating large-conductance voltage and calcium-dependent potassium channels. Am J Respir Cell Mol Biol. 2005;32(3):239–47. [DOI] [PubMed] [Google Scholar]
- 93.Döhler K, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin, and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97(4):898–907. [DOI] [PubMed] [Google Scholar]
- 94.Draijer C, Hylkema MN, Boorsma CE, et al. Sexual maturation protects against development of lung inflammation through estrogen. Am J Physiol. 2016;310(2):L166–74. 10.1152/ajplung.00119.2015. [DOI] [PubMed] [Google Scholar]
- 95.Dubey RK, Jackson EK, Gillespie DG, et al. Catecholamines block the antimitogenic effect of estradiol on human coronary artery smooth muscle cells. J Clin Endocrinol Metab. 2004;89(8):3922–31. [DOI] [PubMed] [Google Scholar]
- 96.Durrani F, Phelps DS, Weisz J, et al. Gonadal hormones and oxidative stress interaction differentially affects survival of male and female mice after lung Klebsiella Pneumoniae infection. Exp Lung Res. 2012;38(4):165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–76. [DOI] [PubMed] [Google Scholar]
- 98.English K, Jones R, Jones T, et al. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Horm Metab Res. 2001;33(11):645–52. [DOI] [PubMed] [Google Scholar]
- 99.Eriksson S, Lindell SE, Wiberg R. Effects of smoking and intermediate alpha 1-antitrypsin deficiency (PiMZ) on lung function. Eur J Respir Dis. 1985;67(4):279–85. [PubMed] [Google Scholar]
- 100.Espinoza J, Montano LM, Perusquia M. Nongenomic bronchodilating action elicited by dehydroepiandrosterone (DHEA) in a guinea pig asthma model. J Steroid Biochem Mol Biol. 2013;138:174–82. 10.1016/j.jsbmb.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 101.Faas M, Bouman A, Moesa H, et al. The immune response during the luteal phase of the ovarian cycle: a Th2-type response? Fertil Steril. 2000a;74(5):1008–13. 10.1016/s0015-0282(00)01553-3. [DOI] [PubMed] [Google Scholar]
- 102.Faas MM, Slot K, Koiter TR, et al. Corticosterone treatment of pregnant low dose endotoxin-treated rats: inhibition of the inflammatory response. Am J Reprod Immunol. 2000b;44(3):178–83. 10.1111/j.8755-8920.2000.440308.x. [DOI] [PubMed] [Google Scholar]
- 103.Fadok VA, McDonald PP, Bratton DL, et al. Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem Soc Trans. 1998;26(4):653–6. 10.1042/bst0260653. [DOI] [PubMed] [Google Scholar]
- 104.Feist G, Schreck CB, Fitzpatrick MS, et al. Sex steroid profiles of coho salmon (Oncorhynchus kisutch) during early development and sexual differentiation. Gen Comp Endocrinol. 1990;80(2):299–313. [DOI] [PubMed] [Google Scholar]
- 105.Filler G, Ramsaroop A, Stein R, et al. Is testosterone detrimental to renal function? Kidney Int Rep. 2016;1(4):306–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Flores-Soto E, Reyes-Garcia J, Carbajal-Garcia A, et al. Sex steroids effects on guinea pig airway smooth muscle tone and intracellular Ca(2+) basal levels. Mol Cell Endocrinol. 2017;439:444–56. 10.1016/j.mce.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 107.Foradori CD, Werner SB, Sandau US, et al. Activation of the androgen receptor alters the intracellular calcium response to glutamate in primary hippocampal neurons and modulates sarco/endoplasmic reticulum calcium ATPase 2 transcription. Neuroscience. 2007;149(1):155–64. 10.1016/j.neuroscience.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 108.Fuentes N, Silveyra P. Endocrine regulation of lung disease and inflammation. Exp Biol Med. 2018;243(17–18):1313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep. 2017;17(3):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fuseini H, Yung JA, Cephus JY, et al. Testosterone decreases house dust mite-induced type 2 and IL-17A-mediated airway inflammation. J Immunol. 2018;201(7):1843–54. 10.4049/jimmunol.1800293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gandhi M, Aweeka F, Greenblatt RM, et al. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. [DOI] [PubMed] [Google Scholar]
- 112.Gao L, Yue MM, Davis J, et al. In pulmonary lymphangioleiomyomatosis expression of progesterone receptor is frequently higher than that of estrogen receptor. Virchows Archiv. 2014;464(4):495–503. 10.1007/s00428-014-1559-9. [DOI] [PubMed] [Google Scholar]
- 113.García-Arencibia M, Dávila N, Campión J, et al. Identification of two functional estrogen response elements complexed with AP-1-like sites in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2005;94(1–3):1–14. [DOI] [PubMed] [Google Scholar]
- 114.Gharaee-Kermani M, Hatano K, Nozaki Y, et al. Gender-based differences in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2005;166(6):1593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gianatti E, Grossmann M. Testosterone deficiency in men with type 2 diabetes: pathophysiology and treatment. Diabet Med. 2020;37(2):174–86. [DOI] [PubMed] [Google Scholar]
- 116.Giangrande PH, McDonnell DP. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res. 1999;54:291–313. [PubMed] [Google Scholar]
- 117.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62(2):155–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gilliver SC. Sex steroids as inflammatory regulators. J Steroid Biochem Mol Biol. 2010;120(2–3):105–15. 10.1016/j.jsbmb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 119.Gilliver SC, Emmerson E, Campbell L, et al. 17beta-estradiol inhibits wound healing in male mice via estrogen receptor-alpha. Am J Pathol. 2010;176(6):2707–21. 10.2353/ajpath.2010.090432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Giltay EJ, Fonk JC, von Blomberg BM, et al. In vivo effects of sex steroids on lymphocyte responsiveness and immunoglobulin levels in humans. J Clin Endocrinol Metab. 2000;85(4):1648–57. 10.1210/jcem.85.4.6562. [DOI] [PubMed] [Google Scholar]
- 121.Glace L, Grygielko ET, Boyle R, et al. Estrogen-induced stromal cell-derived factor-1 (SDF-1/Cxcl12) expression is repressed by progesterone and by selective estrogen receptor modulators via estrogen receptor alpha in rat uterine cells and tissues. Steroids. 2009;74(13–14):1015–24. 10.1016/j.steroids.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 122.Glassberg MK, Elliot SJ, Fritz J, et al. Activation of the estrogen receptor contributes to the progression of pulmonary lymphangioleiomyomatosis via matrix metalloproteinase-induced cell invasiveness. J Clin Endocrinol Metab. 2008;93(5):1625–33. 10.1210/jc.2007-1283. [DOI] [PubMed] [Google Scholar]
- 123.Goncharova EA, Krymskaya VP. Pulmonary lymphangioleiomyomatosis (LAM): progress and current challenges. J Cell Biochem. 2008;103(2):369–82. 10.1002/jcb.21419. [DOI] [PubMed] [Google Scholar]
- 124.González-Orozco JC, Camacho-ArroyoI. Progesterone actions during central nervous system development. Front Neurosci. 2019;13:503. 10.3389/fnins.2019.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gray A, Feldman HA, McKinlay JB, et al. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metabol. 1991;73(5):1016–25. [DOI] [PubMed] [Google Scholar]
- 126.Green MH. From “diseases of women” to “secrets of women”: the transformation of gynecological literature in the later middle ages. J Medieval Early Modern Stud. 2000;30(1):5–39. [Google Scholar]
- 127.Greenhill C Sex differences in fatty liver disease. Nat Rev Endocrinol. 2011;7(6) [DOI] [PubMed] [Google Scholar]
- 128.Gribbin J, Hubbard RB, Le Jeune I, et al. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61(11):980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gross JM, Yee D. How does the estrogen receptor work? Breast Cancer Res. 2002;4(2):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gu X, Yu JJ, Ilter D, et al. Integration of mTOR and estrogen-ERK2 signaling in lymphangioleiomyomatosis pathogenesis. Proc Natl Acad Sci U S A. 2013;110(37):14960–5. 10.1073/pnas.1309110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gu ZP, Wang WC, Lu RF, et al. Plasma progesterone levels in normal and pregnant Chinese women and effects of contraceptives on them. Chin Med J. 1980;93(8):523–7. [PubMed] [Google Scholar]
- 132.Guennoun R, Labombarda F, Deniselle MG, et al. Progesterone and allopregnanolone in the central nervous system: response to injury and implication for neuroprotection. J Steroid Biochem Mol Biol. 2015;146:48–61. [DOI] [PubMed] [Google Scholar]
- 133.Guennoun R, Reyss-Brion M, Gasc J-M. Progesterone receptors in hypothalamus and pituitary during the embryonic development of the chick: regulation by sex steroid hormones. Dev Brain Res. 1987;37(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 134.Gupta S, Basavan D, Muthureddy Nataraj SK, et al. Assessment of inhibitory potential of Pothos scandens L. on ovalbumin-induced airway hyperresponsiveness in balb/c mice. Int Immunopharmacol. 2014;18(1):151–62. 10.1016/j.intimp.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 135.Gupta S, Nataraj SKM, Raju KRS, et al. Peritoneal mast cell stabilization and free radical scavenging activity of Yucca gloriosa L. J Young Pharm. 2015a;7(4):470. [Google Scholar]
- 136.Gupta S, Raju K, Mulukutla S. Peritoneal mast cell stabilization potential of Ziziphus xylopyrus (Retz) wild extract in rat mesenteric model. Insights Allergy Asthma Bronchitis. 2015b;1:7. [Google Scholar]
- 137.Gustafsson J-Å. What pharmacologists can learn from recent advances in estrogen signalling. Trends Pharmacol Sci. 2003;24(9):479–85. [DOI] [PubMed] [Google Scholar]
- 138.Hall OJ, Limjunyawong N, Vermillion MS, et al. Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLoS Pathog. 2016;12(9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hamilton KJ, Hewitt SC, Arao Y, et al. Estrogen hormone biology. In: Current topics in developmental biology, vol. 125. Amsterdam: Elsevier; 2017. p. 109–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Han MK, Arteaga-Solis E, Blenis J, et al. Female sex and gender in lung/sleep health and disease. Increased understanding of basic biological, pathophysiological, and behavioral mechanisms leading to better health for female patients with lung disease. Am J Respir Crit Care Med. 2018;198(7):850–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hardy DB, Janowski BA, Corey DR, et al. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol (Baltimore, Md). 2006;20(11):2724–33. 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- 142.Haston CK, Wang M, Dejournett RE, et al. Bleomycin hydrolase and a genetic locus within the MHC affect risk for pulmonary fibrosis in mice. Hum Mol Genet. 2002;11(16):1855–63. [DOI] [PubMed] [Google Scholar]
- 143.Hayashi T, Adachi Y, Hasegawa K, et al. Less sensitivity for late airway inflammation in males than females in BALB/c mice. Scand J Immunol. 2003;57(6):562–7. 10.1046/j.1365-3083.2003.01269.x. [DOI] [PubMed] [Google Scholar]
- 144.Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–31. [DOI] [PubMed] [Google Scholar]
- 145.Hellings PW, Kasran A, Liu Z, et al. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003a;28(1):42–50. 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 146.Hellings PW, Vandekerckhove P, Claeys R, et al. Progesterone increases airway eosinophilia and hyper-responsiveness in a murine model of allergic asthma. Clin Exp Allergy. 2003b;33(10):1457–63. 10.1046/j.1365-2222.2003.01743.x. [DOI] [PubMed] [Google Scholar]
- 147.Henke CE, Henke G, Elveback LR, et al. The epidemiology of sarcoidosis in Rochester, Minnesota: a population-based study of incidence and survival. Am J Epidemiol. 1986;123(5):840–5. 10.1093/oxfordjournals.aje.a114313. [DOI] [PubMed] [Google Scholar]
- 148.Henske E Tuberous sclerosis complex and lymphangioleiomyomatosis: miles to go, promises to keep. Ann Intern Med. 2011;154(12):840–1. 10.7326/0003-4819-154-12-201106210-00015. [DOI] [PubMed] [Google Scholar]
- 149.Henske EP. The genetic basis of kidney cancer: why is tuberous sclerosis complex often overlooked? Curr Mol Med. 2004;4(8):825–31. 10.2174/1566524043359610. [DOI] [PubMed] [Google Scholar]
- 150.Henske EP, McCormack FX. Lymphangioleiomyomatosis – a wolf in sheep’s clothing. J Clin Invest. 2012;122(11):3807–16. 10.1172/jci58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hess RA. Estrogen in the adult male reproductive tract: a review. Reprod Biol Endocrinol. 2003;1(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hines M Sex-related variation in human behavior and the brain. Trends Cogn Sci. 2010;14(10):448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hodges LC, Houston KD, Hunter DS, et al. Transdominant suppression of estrogen receptor signaling by progesterone receptor ligands inuterine leiomyoma cells. Mol Cell Endocrinol. 2002;196(1–2):11–20. 10.1016/s0303-7207(02)00230-7. [DOI] [PubMed] [Google Scholar]
- 154.Huck B, Steck T, Habersack M, et al. Pregnancy associated hormones modulate the cytokine production but not the phenotype of PBMC-derived human dendritic cells. Eur J Obstet Gynecol Reprod Biol. 2005;122(1):85–94. 10.1016/j.ejogrb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 155.Hughes RA, Harris T, Altmann E, et al. 2-Methoxyestradiol and analogs as novel antiproliferative agents: analysis of three-dimensional quantitative structure-activity relationships for DNA synthesis inhibition and estrogen receptor binding. Mol Pharmacol. 2002;61(5):1053–69. [DOI] [PubMed] [Google Scholar]
- 156.Humphreys RC, Lydon JP, O’Malley BW, et al. Use of PRKO mice to study the role of progesterone in mammary gland development. J Mammary Gland Biol Neoplasia. 1997;2(4):343–54. [DOI] [PubMed] [Google Scholar]
- 157.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153–65. 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 158.Inoue T, Akahira J-I, Suzuki T, et al. Progesterone production and actions in the human central nervous system and neurogenic tumors. J Clin Endocrinol Metabol. 2002;87(11):5325–31. [DOI] [PubMed] [Google Scholar]
- 159.Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol. 2005;63(3):280–93. [DOI] [PubMed] [Google Scholar]
- 160.Jäncke L Sex/gender differences in cognition, neurophysiology, and neuroanatomy. F1000Res. 2018;7:805. 10.12688/f1000research.13917.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Janicki SC, Schupf N. Hormonal influences on cognition and risk for Alzheimer’s disease. Curr Neurol Neurosci Rep. 2010;10(5):359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Janowsky JS. Thinking with your gonads: testosterone and cognition. Trends Cogn Sci. 2006;10(2):77–82. [DOI] [PubMed] [Google Scholar]
- 163.Jensen-Jarolim E, Untersmayr E. Gender-medicine aspects in allergology. Allergy. 2008;63(5):610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jia S, Zhang X, He DZ, et al. Expression and function of a novel variant of estrogen receptor–α36 in murine airways. Am J Respir Cell Mol Biol. 2011;45(5):1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jones RD, English KM, Pugh PJ, et al. Pulmonary vasodilatory action of testosterone: evidence of a calcium antagonistic action. J Cardiovasc Pharmacol. 2002;39(6):814–23. [DOI] [PubMed] [Google Scholar]
- 166.Judson MA. The treatment of pulmonary sarcoidosis. Respir Med. 2012;106(10):1351–61. 10.1016/j.rmed.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 167.Kaiko GE, Horvat JC, Beagley KW, et al. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123(3):326–38. 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kalidhindi R, Ambhore N, Thompson M, et al. Differential estrogen receptor signaling regulatesstore operated calcium entry in human airway smooth muscle. Am J Respir Crit Care Med. 2018;197:A7259. [Google Scholar]
- 169.Kalidhindi RSR, Ambhore NS, Bhallamudi S, et al. Role of estrogen receptors α and β in a murine model of asthma: exacerbated airway hyperresponsiveness and remodeling in ERβ knockout mice. Front Pharmacol. 2020;10:1499. 10.3389/fphar.2019.01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kalidhindi RSR, Ambhore NS, Loganathan J, et al. Estrogen receptor β knock out exacerbates airway hyperresponsiveness and remodeling in a murine model of asthma. Am J Respir Crit Care Med. 2019b;201:A2838. [Google Scholar]
- 171.Kalidhindi RSR, Katragadda R, Beauchamp KL, et al. Androgen receptor-mediated regulation of intracellular calcium in human airway smooth muscle cells. Cell Physiol Biochem. 2019c;53(1):215–28. 10.33594/000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kamil F, Pinzon I, Foreman MG. Sex and race factors in early-onset COPD. Curr Opin Pulm Med. 2013;19(2):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kamilaris TC, DeBold CR, Manolas KJ, et al. Testosterone-secreting adrenal adenoma in a peripubertal girl. JAMA. 1987;258(18):2558–61. [PubMed] [Google Scholar]
- 174.Kanda N, Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immunol. 1999;103(2 Pt 1):282–8. 10.1016/s0091-6749(99)70503-8. [DOI] [PubMed] [Google Scholar]
- 175.Kanda N, Tsuchida T, Tamaki K. Testosterone suppresses anti-DNA antibody production in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1703–11. 10.1002/art.1780400921. [DOI] [PubMed] [Google Scholar]
- 176.Kanda N, Tsuchida T, Tamaki K. Estrogen enhancement of anti-double-stranded DNA antibody and immunoglobulin G production in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum. 1999;42(2):328–37. . [DOI] [PubMed] [Google Scholar]
- 177.Kariyawasam HH, Robinson DS. The role of eosinophils in airway tissue remodelling in asthma. Curr Opin Immunol. 2007;19(6):681–6. 10.1016/j.coi.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 178.Kelsey TW, Li LQ, Mitchell RT, et al. A validated age-related normative model for male total testosterone shows increasing variance but no decline after age 40 years. PLoS One. 2014;9(10):e109346. 10.1371/journal.pone.0109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Keselman A, Heller N. Estrogen signaling modulates allergic inflammation and contributes to sex differences in asthma. Front Immunol. 2015;6:568. 10.3389/fimmu.2015.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2008;73(9–10):864–9. 10.1016/j.steroids.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major his-tocompatibility complex class I molecules. Nature. 2005;436(7051):709–13. 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 182.Kinoshita M, Yokoyama T, Higuchi E, et al. Hormone receptors in pulmonary lymphangiomyomatosis. Kurume Med J. 1995;42(3):141–4. 10.2739/kurumemedj.42.141. [DOI] [PubMed] [Google Scholar]
- 183.Kitaichi M, Izumi T. Lymphangioleiomyomatosis. Curr Opin Pulm Med. 1995;1(5):417–24. [PubMed] [Google Scholar]
- 184.Kitamura N, Ohkouchi S, Tazawa R, et al. Incidence of autoimmune pulmonary alveolar proteinosis estimated using Poisson distribution. ERJ Open Res. 2019;5(1) 10.1183/23120541.00190-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Klebanoff SJ, Durack DT, Rosen H, et al. Functional studies on human peritoneal eosinophils. Infect Immun. 1977;17(1):167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kochakian CD. Definition of androgens and protein anabolic steroids. Pharmacol Ther Part B. 1975;1(2):149–77. [DOI] [PubMed] [Google Scholar]
- 187.Konhilas JP. What we know and do not know about sex and cardiac disease. J Biomed Biotechnol. 2010;2010:562051. 10.1155/2010/562051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kouloumenta V, Hatziefthimiou A, Paraskeva E, et al. Non-genomic effect of testosterone on airway smooth muscle. Br J Pharmacol. 2006;149(8):1083–91. 10.1038/sj.bjp.0706936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Labrie F, Simard J, Luu-The V, et al. Expression of 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase (3 beta-HSD) and 17 beta-hydroxysteroid dehydrogenase (17 beta-HSD) in adipose tissue. Int J Obes. 1991;15(Suppl 2):91–9. [PubMed] [Google Scholar]
- 190.Lakhani NJ, Sarkar MA, Venitz J, et al. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy. 2003;23(2):165–72. [DOI] [PubMed] [Google Scholar]
- 191.Lazaar AL, Panettieri RA Jr. Airway smooth muscle: a modulator of airway remodeling in asthma. J Allergy Clin Immunol. 2005;116(3):488–95. 10.1016/j.jaci.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 192.Lee AJ, Cai MX, Thomas PE, et al. Characterization of the oxidative metabolites of 17β-estradiol and estrone formed by 15 selectively expressed human cytochrome P450 isoforms. Endocrinology. 2003;144(8):3382–98. [DOI] [PubMed] [Google Scholar]
- 193.Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care. 2001;7(1):1–7. 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 194.Leuzzi C, Sangiorgi GM, Modena MG. Gender-specific aspects in the clinical presentation of cardiovascular disease. Fundam Clin Pharmacol. 2010;24(6):711–7. [DOI] [PubMed] [Google Scholar]
- 195.Levesque BM, Vosatka RJ, Nielsen HC. Dihydrotestosterone stimulates branching morphogenesis, cell proliferation, and programmed cell death in mouse embryonic lung explants. Pediatr Res. 2000;47(4):481–91. [DOI] [PubMed] [Google Scholar]
- 196.Levin ER, Hammes SR. Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors. Nat Rev Mol Cell Biol. 2016;17(12):783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li C, Zhou X, Sun Y, et al. Faslodex inhibits estradiol-induced extracellular matrix dynamics and lung metastasis in a model of lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 2013;49(1):135–42. 10.1165/rcmb.2012-0476OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lichtman MA, Vaughan JH, Hames CG. The distribution of serum immunoglobulins, anti-gamma-G globulins (“rheumatoid factors”) and antinuclear antibodies in White and Negro subjects in Evans County, Georgia. Arthritis Rheum. 1967;10(3):204–15. 10.1002/art.1780100306. [DOI] [PubMed] [Google Scholar]
- 199.Liehr JG, Roy D. Free radical generation by redox cycling of estrogens. Free Radic Biol Med. 1990;8(4):415–23. [DOI] [PubMed] [Google Scholar]
- 200.Ligeiro de Oliveira AP, Oliveira-Filho RM, da Silva ZL, et al. Regulation of allergic lung inflammation in rats: interaction between estradiol and corticosterone. Neuroimmunomodulation. 2004;11(1):20–7. 10.1159/000072965. [DOI] [PubMed] [Google Scholar]
- 201.Lipscomb MF, Bice DE, Lyons CR, et al. The regulation of pulmonary immunity. Adv Immunol. 1995;59:369–455. 10.1016/s0065-2776(08)60634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu D, Bachmann KA. An investigation of the relationship between estrogen, estrogen metabolites and blood cholesterol levels in ovariectomized rats. J Pharmacol Exp Ther. 1998;286(1):561–8. [PubMed] [Google Scholar]
- 203.Liu HY, Buenafe AC, Matejuk A, et al. Estrogen inhibition of EAE involves effects on dendritic cell function. J Neurosci Res. 2002;70(2):238–48. 10.1002/jnr.10409. [DOI] [PubMed] [Google Scholar]
- 204.Loganathan J, Pandey R, Ambhore NS, et al. Laser-capture microdissection of murine lung for differential cellular RNA analysis. Cell Tissue Res. 2019:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lohff B, Rieder A Editorial. Wien Med Wochenschr. 2004:154, 391–393. 10.1007/s10354-004-0092-x [DOI] [PubMed] [Google Scholar]
- 206.Luu-The V, Labrie F. The intracrine sex steroid biosynthesis pathways. Prog Brain Res. 2010;181:177–92. 10.1016/s0079-6123(08)81010-2. [DOI] [PubMed] [Google Scholar]
- 207.MacDonald PC, Madden JD, Brenner PF, et al. Origin of estrogen in normal men and in women with testicular feminization. J Clin Endocrinol Metabol. 1979;49(6):905–16. [DOI] [PubMed] [Google Scholar]
- 208.Mandhane PJ, Hanna SE, Inman MD, et al. Changes in exhaled nitric oxide related to estrogen and progesterone during the menstrual cycle. Chest. 2009;136(5):1301–7. [DOI] [PubMed] [Google Scholar]
- 209.Mannan SR, Begum N. Correlation of serum level of progesterone with peak expiratory flow rate (PEFR) in different phases of menstrual cycle. Anwer Khan Modern Med College J. 2012;3(1):6–9. [Google Scholar]
- 210.Manson JE. Prenatal exposure to sex steroid hormones and behavioral/cognitive outcomes. Metabolism. 2008;57:S16–21. [DOI] [PubMed] [Google Scholar]
- 211.Martin CM. Gender-specific pharmacology: implications for therapy. Consult Pharm. 2006;21(8):620–2. 631–624 [DOI] [PubMed] [Google Scholar]
- 212.Martin TR, Castile RG, Fredberg JJ, et al. Airway size is related to sex but not lung size in normal adults. J Appl Physiol. 1987;63(5):2042–7. [DOI] [PubMed] [Google Scholar]
- 213.Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005;2(5):403–11. 10.1513/pats.200508-090JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Martin YN, Manlove L, Dong J, et al. Hyperoxia-induced changes in estradiol metabolism in postnatal airway smooth muscle. Am J Phys Lung Cell Mol Phys. 2014;308(2):L141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Martin YN, Pabelick CM. Sex differences in the pulmonary circulation: implications for pulmonary hypertension. Am J Phys Heart Circ Phys. 2014;306(9):H1253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Martinez FJ, Curtis JL, Sciurba F, et al. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med. 2007;176(3):243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Massaro D, Clerch LB, Massaro GD. Estrogen receptor-α regulates pulmonary alveolar loss and regeneration in female mice: morphometric and gene expression studies. Am J Phys Lung Cell Mol Phys. 2007;293(1):L222–8. [DOI] [PubMed] [Google Scholar]
- 218.Mathur S, Mathur RS, Goust JM, et al. Cyclic variations in white cell subpopulations in the human menstrual cycle: correlations with progesterone and estradiol. Clin Immunol Immunopathol. 1979;13(3):246–53. 10.1016/0090-1229(79)90069-2. [DOI] [PubMed] [Google Scholar]
- 219.Matsubara S, Swasey CH, Loader JE, et al. Estrogen determines sex differences in airway responsiveness after allergen exposure. Am J Respir Cell Mol Biol. 2008;38(5):501–8. 10.1165/rcmb.2007-0298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Matteis M, Polverino F, Spaziano G, et al. Effects of sex hormones on bronchial reactivity during the menstrual cycle. BMC Pulm Med. 2014;14(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mauras N, Hayes V, Welch S, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metabol. 1998;83(6):1886–92. [DOI] [PubMed] [Google Scholar]
- 222.McCarthy C, Lara Gallego B, Trapnell BC, et al. Epidemiology of rare lung diseases: the challenges and opportunities to improve research and knowledge. Adv Exp Med Biol. 2017;1031:419–42. 10.1007/978-3-319-67144-4_24. [DOI] [PubMed] [Google Scholar]
- 223.McCormack FX, Travis WD, Colby TV, et al. Lymphangioleiomyomatosis: calling it what it is: a low-grade, destructive, metastasizing neoplasm. Am J Respir Crit Care Med. 2012;186(12):1210–2. 10.1164/rccm.201205-0848OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.McEwan IJ, Brinkmann AO. Androgen Physiology: Receptor and Metabolic Disorders. [Updated 2016]. In: Feingold KR, Anawalt B, Boyce A, et al. , editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000 [Google Scholar]
- 225.McEwen BS. Stress, sex and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.McKenna NJ, Lanz RB, O’Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20(3):321–44. [DOI] [PubMed] [Google Scholar]
- 227.McKenna NJ, O’Malley BW. Minireview: nuclear receptor coactivators – an update. Endocrinology. 2002;143(7):2461–5. [DOI] [PubMed] [Google Scholar]
- 228.Mehrad M, Bittar HET, Yousem SA. Sex steroid receptor expression in idiopathic pulmonary fibrosis. Hum Pathol. 2017;66:200–5. [DOI] [PubMed] [Google Scholar]
- 229.Melgert BN, Ray A, Hylkema MN, et al. Are there reasons why adult asthma is more common in females? Curr Allergy Asthma Rep. 2007;7(2):143–50. [DOI] [PubMed] [Google Scholar]
- 230.Melgert BN, ten Hacken NH, Rutgers B, et al. More alternative activation of macrophages in lungs of asthmatic patients. J Allergy Clin Immunol. 2011;127(3):831–3. 10.1016/j.jaci.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 231.Melmed S, Polonsky KS, Larsen PR, et al. Williams textbook of endocrinology. Amsterdam: Elsevier Health Sciences; 2015. [Google Scholar]
- 232.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–7. [DOI] [PubMed] [Google Scholar]
- 233.Merkus P, Have-Opbroek A, Quanjer P. Human lung growth: a review. Pediatr Pulmonol. 1996;21(6):383–97. [DOI] [PubMed] [Google Scholar]
- 234.Mikerov AN, Gan X, Umstead TM, et al. Sex differences in the impact of ozone on survival and alveolar macrophage function of mice after Klebsiella pneumoniae infection. Respir Res. 2008;9(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mikhail G Hormone secretion by the human ovaries. Gynecol Obstet Investig. 1970;1(1):5–20. [DOI] [PubMed] [Google Scholar]
- 236.Mikkonen L, Pihlajamaa P, Sahu B, et al. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol. 2010;317(1–2):14–24. 10.1016/j.mce.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 237.Miller MR, Jordan RE, Adab P. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax. 2011;66(10):921–2. [DOI] [PubMed] [Google Scholar]
- 238.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60(2):210–41. 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Milman N, Selroos O. Pulmonary sarcoidosis in the Nordic countries 1950–1982. Epidemiology and clinical picture. Sarcoidosis. 1990;7(1):50–7. [PubMed] [Google Scholar]
- 240.Miyagi M, Aoyama H, Morishita M, et al. Effects of sex hormones on chemotaxis of human peripheral polymorphonuclear leukocytes and monocytes. J Periodontol. 1992;63(1):28–32. 10.1902/jop.1992.63.1.28. [DOI] [PubMed] [Google Scholar]
- 241.Miyaura H, Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol. 2002;168(3):1087–94. 10.4049/jimmunol.168.3.1087. [DOI] [PubMed] [Google Scholar]
- 242.Mohr M, Wong A, Tomm R, et al. Pubertal development of estradiol-induced hypothalamic progesterone synthesis. Horm Behav. 2019;111:110–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Moldoveanu B, Otmishi P, Jani P, et al. Inflammatory mechanisms in the lung. J Inflamm Res. 2009;2:1–11. [PMC free article] [PubMed] [Google Scholar]
- 244.Molloy EJ, O’Neill AJ, Grantham JJ, et al. Sex-specific alterations in neutrophil apoptosis: the role of estradiol and progesterone. Blood. 2003;102(7):2653–9. 10.1182/blood-2003-02-0649. [DOI] [PubMed] [Google Scholar]
- 245.Montano LM, Espinoza J, Flores-Soto E, et al. Androgens are bronchoactive drugs that act by relaxing airway smooth muscle and preventing bronchospasm. J Endocrinol. 2014;222(1):1–13. 10.1530/JOE-140074. [DOI] [PubMed] [Google Scholar]
- 246.Montano LM, Flores-Soto E, Reyes-Garcia J, et al. Testosterone induces hyporesponsiveness by interfering with IP3 receptors in guinea pig airway smooth muscle. Mol Cell Endocrinol. 2018;473:17–30. 10.1016/j.mce.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 247.Morley JE, Kaiser FE, Perry HM III, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410–3. [DOI] [PubMed] [Google Scholar]
- 248.Morris D, Diskin M. Effect of progesterone on embryo survival. Animal. 2008;2(8):1112–9. [DOI] [PubMed] [Google Scholar]
- 249.Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128(2):139–46. [DOI] [PubMed] [Google Scholar]
- 250.Muthusamy T, Murugesan P, Balasubramanian K. Sex steroids deficiency impairs glucose transporter 4 expression and its translocation through defective Akt phosphorylation in target tissues of adult male rat. Metabolism. 2009;58(11):1581–92. [DOI] [PubMed] [Google Scholar]
- 251.Muthusamy T, Murugesan P, Srinivasan C, et al. Sex steroids influence glucose oxidation through modulation of insulin receptor expression and IRS-1 serine phosphorylation in target tissues of adult male rat. Mol Cell Biochem. 2011;352(1–2):35–45. [DOI] [PubMed] [Google Scholar]
- 252.Myers JR, Sherman CB. Should supplemental estrogens be used as steroid-sparing agents in asthmatic women? Chest. 1994;106(1):318–9. [DOI] [PubMed] [Google Scholar]
- 253.Nakata K, Gotoh H, Watanabe J, et al. Augmented proliferation of human alveolar macrophages after allogeneic bone marrow transplantation. Blood. 1999;93(2):667–73. [PubMed] [Google Scholar]
- 254.Northern AL, Rutter SM, Peterson CM. Cyclic changes in the concentrations of peripheral blood immune cells during the normal menstrual cycle. Proc Soc Exp Biol Med. 1994;207(1):81–8. 10.3181/00379727-207-43795. [DOI] [PubMed] [Google Scholar]
- 255.Olorunshola K, Aliyu O, Achie L. Testosterone and orchidectomy modulates intestinal fluid and glucose transport in albino wistar rat. Eur J Sci Res. 2012;76:281–7. [Google Scholar]
- 256.Olson AL, Swigris JJ, Lezotte DC, et al. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176(3):277–84. [DOI] [PubMed] [Google Scholar]
- 257.Pagtakhan RD, Bjelland JC, Landau LI, et al. Sex differences in growth patterns of the airways and lung parenchyma in children. J Appl Physiol. 1984;56(5):1204–10. [DOI] [PubMed] [Google Scholar]
- 258.Pandey S, Werner R, Ambhore N, et al. Association of DNA-methylation markers and airway remodeling in mouse model of allergic asthma. Am J Respir Crit Care Med. 2020;201:A5656. [Google Scholar]
- 259.Patrone C, Cassel TN, Pettersson K, et al. Regulation of postnatal lung development and homeostasis by estrogen receptor β. Mol Cell Biol. 2003;23(23):8542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Pérez-Lopez FR, Larrad-Mur L, Kallen A, et al. Gender differences in cardiovascular disease: hormonal and biochemical influences. Reprod Sci. 2010;17(6):511–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Perusquia M, Flores-Soto E, Sommer B, et al. Testosterone-induced relaxation involves L-type and store-operated Ca2+ channels blockade, and PGE 2 in guinea pig airway smooth muscle. Pflugers Arch. 2015;467(4):767–77. 10.1007/s00424-014-1534-y. [DOI] [PubMed] [Google Scholar]
- 262.Perusquía M, Hernández R, Montaño LM, et al. Inhibitory effect of sex steroids on guinea-pig airway smooth muscle contractions. Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol. 1997;118(1):5–10. [DOI] [PubMed] [Google Scholar]
- 263.Phung J, Paul J, Smith R. Maintenance of pregnancy and parturition. In: Maternal-fetal and neonatal endocrinology. Amsterdam: Elsevier; 2020. p. 169–87. [Google Scholar]
- 264.Piccinni MP, Giudizi MG, Biagiotti R, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155(1):128–33. [PubMed] [Google Scholar]
- 265.Pierdominici M, Maselli A, Colasanti T, et al. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol Lett. 2010;132(1–2):79–85. 10.1016/j.imlet.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 266.Piitulainen E, Eriksson S. Decline in FEV1 related to smoking status in individuals with severe alpha1-antitrypsin deficiency (PiZZ). Eur Respir J. 1999;13(2):247–51. 10.1183/09031936.99.13224799. [DOI] [PubMed] [Google Scholar]
- 267.Pisetsky DS, Spencer DM. Effects of progesterone and estradiol sex hormones on the release of microparticles by RAW 264.7 macrophages stimulated by Poly(I:C). Clin Vaccine Immunol. 2011;18(9):1420–6. 10.1128/cvi.05110-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Pitteloud N, Hardin M, Dwyer AA, et al. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metabol. 2005;90(5):2636–41. [DOI] [PubMed] [Google Scholar]
- 269.Prakash Y Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Phys Lung Cell Mol Phys. 2013a;305(12):L912–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Prakash Y Emerging concepts in smooth muscle contributions to airway structure and function: implications for health and disease. Am J Phys Lung Cell Mol Phys. 2016;311(6):L1113–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Prakash Y, Martin RJ. Brain-derived neurotrophic factor in the airways. Pharmacol Ther. 2014;143(1):74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Physiol Lung Cell Mol Physiol. 2013b;305(12):L912–33. 10.1152/ajplung.00259.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Prakash YS. Asthma without borders. Am J Physiol. 2020;318(5):L1001–l1003. 10.1152/ajplung.00114.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Prior J Progesterone as a bone-trophic hormone. Endocr Rev. 1990;11(2):386–98. [DOI] [PubMed] [Google Scholar]
- 275.Prizant H, Sen A, Light A, et al. Uterine-specific loss of Tsc2 leads to myometrial tumors in both the uterus and lungs. Mol Endocrinol (Baltimore, Md). 2013;27(9):1403–14. 10.1210/me.2013-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Prizant H, Taya M, Lerman I, et al. Estrogen maintains myometrial tumors in a lymphangioleiomyomatosis model. Endocr Relat Cancer. 2016;23(4):265–80. 10.1530/erc-15-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Provost PR, Blomquist CH, Godin C, et al. Androgen formation and metabolism in the pulmonary epithelial cell line A549: expression of 17β-hydroxysteroid dehydrogenase type 5 and 3α-hydroxysteroid dehydrogenase type 3. Endocrinology. 2000;141(8):2786–94. [DOI] [PubMed] [Google Scholar]
- 278.Raghavan D, Jain R. Increasing awareness of sex differences in airway diseases. Respirology. 2016;21(3):449–59. [DOI] [PubMed] [Google Scholar]
- 279.Raju KR, Ambhore NS, Mulukutla S, et al. Salicylic acid derivatives as potential anti asthmatic agents using disease responsive drug delivery system for prophylactic therapy of allergic asthma. Med Hypotheses. 2016;87:75–9. 10.1016/j.mehy.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 280.Raju KR, Kumar MN, Gupta S, et al. 5-Aminosalicylic acid attenuates allergen-induced airway inflammation and oxidative stress in asthma. Pulm Pharmacol Ther. 2014;29(2):209–16. 10.1016/j.pupt.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 281.Raju RS, Kumar MS, Kannan E, et al. Drug loaded liposomes of mesalamine incorporated into disease responsive microgels (Micro) for treating allergic asthma: An approach using smart drug delivery system. Eur Respiratory Soc. 2015;46(Suppl 59):PA5011. [Google Scholar]
- 282.Rao CK, Moore CG, Bleecker E, et al. Characteristics of perimenstrual asthma and its relation to asthma severity and control: data from the severe asthma research program. Chest. 2013a;143(4):984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol. 2013b;9(8):479. [DOI] [PubMed] [Google Scholar]
- 284.Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. In: Progress in brain research, vol. 186. Amsterdam: Elsevier; 2010. p. 113–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of tolllike receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 2008;78(3):432–7. 10.1095/biolreprod.107.063545. [DOI] [PubMed] [Google Scholar]
- 286.Riffo-Vasquez Y, Ligeiro de Oliveira AP, Page CP, et al. Role of sex hormones in allergic inflammation in mice. Clin Exp Allergy. 2007;37(3):459–70. 10.1111/j.1365-2222.2007.02670.x. [DOI] [PubMed] [Google Scholar]
- 287.Roomruangwong C, Carvalho AF, Comhaire F, et al. Lowered plasma steady-state levels of progesterone combined with declining progesterone levels during the luteal phase predict peri-menstrual syndrome and its major subdomains. Front Psychol. 2019;10:2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Roy D, Liehr J. Temporary decrease in renal quinone reductase activity induced by chronic administration of estradiol to male Syrian hamsters. Increased superoxide formation by redox cycling of estrogen. J Biol Chem. 1988;263(8):3646–51. [PubMed] [Google Scholar]
- 289.Rubio-Gayosso I, Garcia-Ramirez O, Gutierrez-Serdan R, et al. Testosterone inhibits bradykinin-induced intracellular calcium kinetics in rat aortic endothelial cells in culture. Steroids. 2002;67(5):393–7. [DOI] [PubMed] [Google Scholar]
- 290.Salameh WA, Redor-Goldman MM, Clarke NJ, et al. Validation of a total testosterone assay using high-turbulence liquid chromatography tandem mass spectrometry: total and free testosterone reference ranges. Steroids. 2010;75(2):169–75. 10.1016/j.steroids.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 291.Saldanha PA, Cairrao E, Maia CJ, et al. Longand short-term effects of androgens in human umbilical artery smooth muscle. Clin Exp Pharmacol Physiol. 2013;40(3):181–9. 10.1111/1440-1681.12047. [DOI] [PubMed] [Google Scholar]
- 292.Sathish V, Abcejo AJ, VanOosten SK, et al. Caveolin-1 in cytokine-induced enhancement of intracellular Ca2+ in human airway smooth muscle. Am J Phys Lung Cell Mol Phys. 2011;301(4):L607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Sathish V, Freeman MR, Long E, et al. Cigarette smoke and estrogen signaling in human airway smooth muscle. Cell Physiol Biochem. 2015a;36(3):1101–15. 10.1159/000430282. [DOI] [PubMed] [Google Scholar]
- 294.Sathish V, Martin YN, Prakash YS. Sex steroid signaling: implications for lung diseases. Pharmacol Ther. 2015b;150:94–108. 10.1016/j.pharmthera.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sathish V, Prakash Y. Sex differences in pulmonary anatomy and physiology: implications for health and disease. In: Sex differences in physiology. Elsevier; 2016. p. 89–103. [Google Scholar]
- 296.Sato T, Miyagawa S, Iguchi T. Progesterone. In: Handbook of hormones. Elsevier; 2016. p. 507–594. [Google Scholar]
- 297.Savita Rai U. Sex steroid hormones modulate the activation of murine peritoneal macrophages: receptor mediated modulation. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119(2):199–204. 10.1016/s0742-8413(97)00207-7. [DOI] [PubMed] [Google Scholar]
- 298.Schwartz N, Verma A, Bivens CB, et al. Rapid steroid hormone actions via membrane receptors. Biochimica et Biophysica Acta (BBA) Mol Cell Res. 2016;1863(9):2289–98. [DOI] [PubMed] [Google Scholar]
- 299.See KL, See M, Gluud C. Liver pathology associated with the use of anabolic-androgenic steroids. Liver. 1992;12(2):73–9. [DOI] [PubMed] [Google Scholar]
- 300.Seladi-Schulman J (2020) Everything you need to know about progesterone; https://www.healthline.com/health/progesterone-function#functions. Accessed on May 28, 2020.
- 301.Senn O, Russi EW, Schindler C, et al. Circulating alpha1-antitrypsin in the general population: determinants and association with lung function. Respir Res. 2008;9(1):35. 10.1186/1465-9921-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Seymour BW, Friebertshauser KE, Peake JL, et al. Gender differences in the allergic response of mice neonatally exposed to environmental tobacco smoke. Dev Immunol. 2002;9(1):47–54. 10.1080/1044667021000003989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shah R, Newcomb DC. Sex bias in asthma prevalence and pathogenesis. Front Immunol. 2018;9:2997. 10.3389/fimmu.2018.02997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sochorova R, Mosnarova A, Huzulakova I. The possible influence of testosterone on calcium ion transport (investigated) in Guinea pig uterus. Acta Physiol Hung. 1991;77(1):19–24. [PubMed] [Google Scholar]
- 305.Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Soltanyzadeh M, Ghollasi M, Halabian R, et al. A comparative study of hBM-MSCs’ differentiation toward osteogenic lineage in the presence of progesterone and estrogen hormones separately and concurrently in vitro. Cell Biol Int. 2020; [DOI] [PubMed] [Google Scholar]
- 307.Sørheim I-C, Johannessen A, Gulsvik A, et al. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax. 2010;65(6):480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Speroff L, Fritz MA. Clinical gynecologic endocrinology and infertility. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 309.Spinelli Oliveira E, Hancock JT, Hermes-Lima M, et al. Implications of dealing with airborne substances and reactive oxygen species: what mammalian lungs, animals, and plants have to say? Integr Comp Biol. 2007;47(4):578–91. 10.1093/icb/icm078. [DOI] [PubMed] [Google Scholar]
- 310.Staprans I, Rapp JH, Pan X-M, et al. Testosterone regulates metabolism of plasma chylomicrons in rats. Arteriosclerosis. 1990;10(4):591–6. [DOI] [PubMed] [Google Scholar]
- 311.Stoffel-Wagner B Neurosteroid metabolism in the human brain. Eur J Endocrinol. 2001;145(6):669–80. [DOI] [PubMed] [Google Scholar]
- 312.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–74. [DOI] [PubMed] [Google Scholar]
- 313.Stronge A, Sreenan J, Diskin M, et al. Post-insemination milk progesterone concentration and embryo survival in dairy cows. Theriogenology. 2005;64(5):1212–24. [DOI] [PubMed] [Google Scholar]
- 314.Stumpf W Steroid hormones and the cardiovascular system: direct actions of estradiol, progesterone, testosterone, gluco-and mineralcorticoids, and soltriol [vitamin D] on central nervous regulatory and peripheral tissues. Experientia. 1990;46(1):13–25. [DOI] [PubMed] [Google Scholar]
- 315.Sun Y, Zhang E, Lao T, et al. Progesterone and estradiol synergistically promote the lung metastasis of tuberin-deficient cells in a preclinical model of lymphangioleiomyomatosis. Hormones Cancer. 2014;5(5):284–98. 10.1007/s12672-014-0192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Tam A, Morrish D, Wadsworth S, et al. The role of female hormones on lung function in chronic lung diseases. BMC Womens Health. 2011;11(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Taraborrelli S, Physiologyproduction and action of progesterone. Acta Obstet Gynecol Scand. 2015;94:8–16. [DOI] [PubMed] [Google Scholar]
- 318.Thirumavalavan N, Wilken NA, Ramasamy R. Hypogonadism and renal failure: an update. Indian J Urol. 2015;31(2):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Thomas P, Pang Y. Protective actions of progesterone in the cardiovascular system: potential role of membrane progesterone receptors (mPRs) in mediating rapid effects. Steroids. 2013;78(6):583–8. [DOI] [PubMed] [Google Scholar]
- 320.Tofovic SP. Estrogens and development of pulmonary hypertension – interaction of estradiol metabolism and pulmonary vascular disease. J Cardiovasc Pharmacol. 2010;66:696–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Tofovic SP, Wenzel S, Stewart NA. Role of estradiol metabolism in asthma. Biosci Hypotheses. 2009;2(3):128–34. [Google Scholar]
- 322.Torday J, Nielsen H. The sex difference in fetal lung surfactant production. Exp Lung Res. 1987;12(1):1–19. [DOI] [PubMed] [Google Scholar]
- 323.Townsend EA, Meuchel LW, Thompson MA, et al. Estrogen increases nitric-oxide production in human bronchial epithelium. J Pharmacol Exp Ther. 2011;339(3):815–24. 10.1124/jpet.111.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Townsend EA, Miller VM, Prakash Y. Sex differences and sex steroids in lung health and disease. Endocr Rev. 2012a;33(1):1–47. 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Townsend EA, Sathish V, Thompson MA, et al. Estrogen effects on human airway smooth muscle involve cAMP and protein kinase A. Am J Physiol Lung Cell Mol Physiol. 2012b;303(10):L923–8. 10.1152/ajplung.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Townsend EA, Thompson MA, Pabelick CM, et al. Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. Am J Physiol. 2010;298(4):L521–30. 10.1152/ajplung.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Tranguch S, Wang H, Daikoku T, et al. FKBP52 deficiency–conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest. 2007;117(7):1824–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Triebner K, Accordini S, Calciano L, et al. Exogenous female sex steroids may reduce lung ageing after menopause: a 20-year follow-up study of a general population sample (ECRHS). Maturitas. 2019;120:29–34. [DOI] [PubMed] [Google Scholar]
- 329.Tyagi V, Scordo M, Yoon RS, et al. Revisiting the role of testosterone: are we missing something? Reviews Urol. 2017;19(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Tzouvelekis A, Bouros D. Estrogen signaling and microRNAs in lung fibrosis. Sex, hormones, and rock scars. Am J Respir Crit Care Med. 2019;200(10):1199–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Vermeulen A Androgen replacement therapy in the aging male – a critical evaluation. J Clin Endocrinol Metabol. 2001;86(6):2380–90. [DOI] [PubMed] [Google Scholar]
- 332.Vermeulen A, Kaufman J, Goemaere S, et al. Estradiol in elderly men. Aging Male. 2002;5(2):98–102. [PubMed] [Google Scholar]
- 333.Vermeulen A, Rubens R, Verdonck L. Testosterone secretion and metabolism in male senescence. J Clin Endocrinol Metabol. 1972;34(4):730–5. [DOI] [PubMed] [Google Scholar]
- 334.Villa A, Rizzi N, Vegeto E, et al. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep. 2015;5(1):15224. 10.1038/srep15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Voltz JW, Card JW, Carey MA, et al. Male sex hormones exacerbate lung function impairment after bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Wang S-F, Chen X-H, He B, et al. Acute restraint stress triggers progesterone withdrawal and endometrial breakdown and shedding through corticosterone stimulation in mouse menstrual-like model. Reproduction. 2019;157(2):149–61. [DOI] [PubMed] [Google Scholar]
- 337.Wang SY, Freeman MR, Sathish V, et al. Sex steroids influence brain-derived neurotropic factor secretion from human airway smooth muscle cells. J Cell Physiol. 2016;231(7):1586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones. J Clin Endocrinol Metabol. 2011;96(4):885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Weetman AP, McGregor AM, Smith BR, et al. Sex hormones enhance immunoglobulin synthesis by human peripheral blood lymphocytes. Immunol Lett. 1981;3(6):343–6. 10.1016/0165-2478(81)90064-x. [DOI] [PubMed] [Google Scholar]
- 340.Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106(1):306–16. [DOI] [PubMed] [Google Scholar]
- 341.Wierman ME. Sex steroid effects at target tissues: mechanisms of action. Adv Physiol Educ. 2007;31(1):26–33. [DOI] [PubMed] [Google Scholar]
- 342.Williams DG. Gender, masculinity-femininity, and emotional intimacy in same-sex friendship. Sex Roles. 1985;12(5–6):587–600. [Google Scholar]
- 343.Wise RA. Changing smoking patterns and mortality from chronic obstructive pulmonary disease. Prev Med. 1997;26(4):418–21. [DOI] [PubMed] [Google Scholar]
- 344.Wulfsohn N, Politzer W, Henrico J. Testosterone therapy in bronchial asthma. Afr J Health Prof Educ. 1964;38(9):170–2. [PubMed] [Google Scholar]
- 345.Yamaguchi M, Hosoda Y, Sasaki R, et al. Epidemiological study on sarcoidosis in Japan. Recent trends in incidence and prevalence rates and changes in epidemiological features. Sarcoidosis. 1989;6(2):138–46. [PubMed] [Google Scholar]
- 346.Yap HM, Israf DA, Harith HS, et al. Crosstalk between signaling pathways involved in the regulation of airway smooth muscle cell hyperplasia. Front Pharmacol. 2019;10:1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Yarova PL, Stewart AL, Sathish V, et al. Calcium-sensing receptor antagonists abrogate airway hyperresponsiveness and inflammation in allergic asthma. Sci Transl Med. 2015;7(284):284ra260. 10.1126/scitranslmed.aaa0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Yovel G, Shakhar K, Ben-Eliyahu S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol. 2001;81(2):254–62. 10.1006/gyno.2001.6153. [DOI] [PubMed] [Google Scholar]
- 349.Yu CK, Liu YH, Chen CL. Dehydroepiandrosterone attenuates allergic airway inflammation in Dermatophagoides farinae-sensitized mice. J Microbiol Immunol Infect = Wei mian yu gan ran za zhi. 2002;35(3):199–202. [PubMed] [Google Scholar]
- 350.Zhang X, Wang L, Zhang H, et al. Estrogen inhibits lipopolysaccharide-induced tumor necrosis factor-alpha release from murine macrophages. Methods Find Exp Clin Pharmacol. 2001;23(4):169–73. 10.1358/mf.2001.23.4.634640. [DOI] [PubMed] [Google Scholar]


