Supplemental Digital Content is available in the text.
Keywords: adrenergic beta-antagonists, angiotensin receptor antagonists, biomarkers, breast neoplasms, cardiomyopathies, magnetic resonance imaging
Background:
Adjuvant breast cancer therapy containing anthracyclines with or without anti–human epidermal growth factor receptor–2 antibodies and radiotherapy is associated with cancer treatment–related cardiac dysfunction. In the PRADA trial (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy), concomitant treatment with the angiotensin receptor blocker candesartan attenuated the reduction in left ventricular ejection fraction (LVEF) in women receiving treatment for breast cancer, whereas the β-blocker metoprolol attenuated the increase in cardiac troponins. This study aimed to assess the long-term effects of candesartan and metoprolol or their combination to prevent a reduction in cardiac function and myocardial injury.
Methods:
In this 2×2 factorial, randomized, placebo-controlled, double-blind, single-center trial, patients with early breast cancer were assigned to concomitant treatment with candesartan cilexetil, metoprolol succinate, or matching placebos. Target doses were 32 and 100 mg, respectively. Study drugs were discontinued after adjuvant therapy. All 120 validly randomized patients were included in the intention-to-treat analysis. The primary outcome measure was change in LVEF assessed by cardiovascular magnetic resonance imaging from baseline to extended follow-up. Secondary outcome measures included changes in left ventricular volumes, echocardiographic peak global longitudinal strain, and circulating cardiac troponin concentrations.
Results:
A small decline in LVEF but no significant between-group differences were observed from baseline to extended follow-up, at a median of 23 months (interquartile range, 21 to 28 months) after randomization (candesartan, 1.7% [95% CI, 0.5 to 2.8]; no candesartan, 1.8% [95% CI, 0.6 to 3.0]; metoprolol, 1.6% [95% CI, 0.4 to 2.7]; no metoprolol, 1.9% [95% CI, 0.7 to 3.0]). Candesartan treatment during adjuvant therapy was associated with a significant reduction in left ventricular end-diastolic volume compared with the noncandesartan group (P=0.021) and attenuated decline in global longitudinal strain (P=0.046) at 2 years. No between-group differences in change in cardiac troponin I and T concentrations were observed.
Conclusions:
Anthracycline-containing adjuvant therapy for early breast cancer was associated with a decline in LVEF during extended follow-up. Candesartan during adjuvant therapy did not prevent reduction in LVEF at 2 years, but was associated with modest reduction in left ventricular end-diastolic volume and preserved global longitudinal strain. These results suggest that a broadly administered cardioprotective approach may not be required in most patients with early breast cancer without preexisting cardiovascular disease.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01434134.
Clinical Perspective.
What Is New?
In this 2-year follow-up study of the PRADA trial (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy), treatment with candesartan and metoprolol during adjuvant therapy for early breast cancer did not prevent decline in left ventricular ejection fraction.
Candesartan treatment during adjuvant therapy was associated with a small reduction in end-diastolic volume and attenuated decline in global longitudinal strain at 2 years.
What Are the Clinical Implications?
The results do not support broadly administered preventive cardioprotective therapy with β-adrenergic or angiotensin receptor blockade during contemporary adjuvant therapy regimens for early breast cancer.
The focus of future studies should be to identify subgroups at high risk of cardiotoxicity who may benefit from cardioprotective therapy in a precision medicine fashion.
Advances in cancer therapy have led to improved survival, but also to growing concern over adverse cardiovascular effects that may limit the benefit of cancer therapy and reduce quality of life. Cancer survivors have increased risk for cardiovascular disease compared with the general population, and this is most pronounced in patients who have received adjuvant cardiotoxic chemotherapy.1 Adjuvant breast cancer therapy may include several potentially cardiotoxic components, including anthracycline therapy, radiotherapy, and monoclonal antibodies like trastuzumab.2 Together with overlapping risk factors, these multiple hits contribute to breast cancer survivors’ increased cardiovascular risk compared with age-matched controls without cancer.1,3 With an increasing number of long-term early breast cancer survivors, effective strategies to prevent long-term cardiac dysfunction are needed.4
Treatment with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and β-blockers significantly reduce morbidity and mortality in all stages of heart failure,5–7 and previous small-scale studies in heterogeneous patient populations have indicated that neurohormonal blockade may reduce the cardiotoxic effects of cancer therapy.8,9 However, conflicting results from more recent randomized, controlled trials of neurohormonal blockade during adjuvant breast cancer therapy have been reported.10–14
In the PRADA trial (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy), patients with early breast cancer and no serious comorbidities scheduled for adjuvant therapy were randomized to the angiotensin receptor blocker candesartan, the β-adrenoceptor blocker metoprolol, or placebo. The primary outcome measure was change in left ventricular ejection fraction (LVEF) from baseline to end of adjuvant therapy measured by cardiovascular magnetic resonance (CMR) imaging. We demonstrated that adjuvant therapy was associated with a modest overall decline in LVEF that was attenuated by concomitant treatment with candesartan but not by metoprolol.10 We also found that β-blockade may have beneficial effects on myocardial injury during anthracycline therapy, expressed as an attenuated cardiac troponin increase.15 However, the cardiotoxic effects of anthracyclines and radiotherapy may manifest months to years after end of therapy,2 and the long-term effects of the concomitant neurohormonal blockade is unknown. It is also unclear to what extent the initial observations are attributable to direct hemodynamic effects of the neurohormonal blockade or persisting myocardial remodeling.16 We therefore conducted a prespecified follow-up study to test the hypothesis that concomitant therapy with candesartan or metoprolol during adjuvant, anthracycline-containing therapy with or without trastuzumab and radiation for early breast cancer will attenuate the decline in LVEF at extended follow-up. We also assessed the effect of the interventions on several prespecified secondary outcomes.
Methods
Study Design and Participants
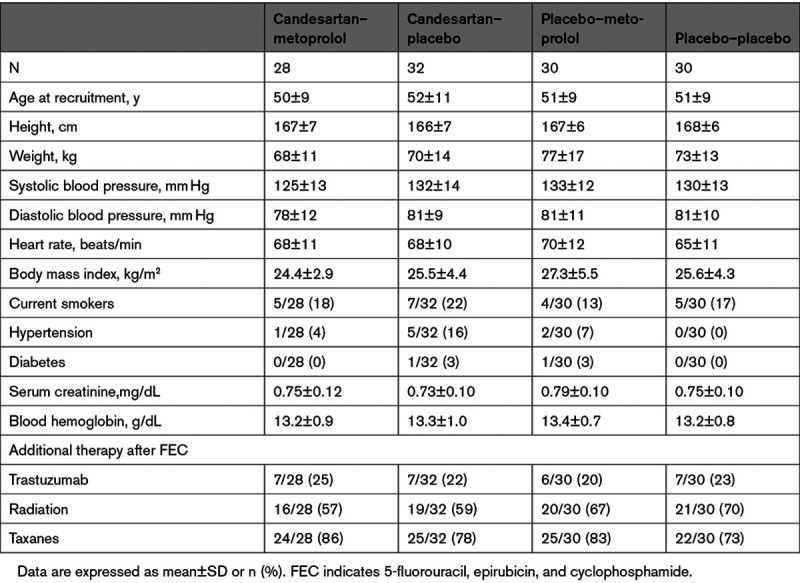
Data from the PRADA trial cannot be publicly shared because of the risk of violating privacy, as regulated by the institutional data protection officer. The PRADA trial was a randomized, 2×2 factorial, placebo-controlled, double-blind clinical trial conducted at Akershus University Hospital in Norway. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the Regional Ethics Committee of South-Eastern Norway (approval number 2010/2890). Study methods and design have been described in detail.10 Eligible patients were adult women between 18 and 70 years of age with LVEF ≥50%, normal kidney function, and no serious comorbidities, who after surgery for early breast cancer were scheduled for adjuvant anthracycline-containing therapy. The main exclusion criteria were previous malignancy requiring treatment with anthracyclines or radiotherapy; clinically significant heart disease; hypotension or hypertension; treatment with or intolerance to angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or β-blockers; and inability to comply with study protocol and procedures. Complete inclusion and exclusion criteria are listed in Table I in the Data Supplement. All participants provided written, informed consent before any study procedures took place.
Randomization and Masking
We randomly assigned participants in a 1:1:1:1 fashion to receive 1 of the following treatment combinations: candesartan cilexetil and metoprolol succinate, candesartan cilexetil and placebo, metoprolol succinate and placebo, or placebo and placebo. A randomization list was created by using a permuted block randomization procedure with block size 4 and 8 in random order, stratified for trastuzumab therapy. The placebos were identical in appearance to the active tablets. The study was double-blind, so both study personnel and participants were masked to group assignments, and all CMR and echocardiography assessments were performed blinded to patient identity, group assignment, and image sequence. Follow-up image analyses were performed blinded to previous imaging.
Procedures
After randomization, patients were instructed to take oral candesartan cilexetil/placebo and metoprolol succinate/placebo tablets (AstraZeneca) with a daily target dose of 32 mg and 100 mg, respectively. Dose modifications according to blood pressure and patient symptoms were allowed. Details on dose titration have been reported previously.10 Patients received study medication throughout adjuvant therapy; the duration depended on the cancer treatment regimen and ranged from 10 to 61 weeks. During adjuvant therapy, patients were serially assessed with CMR, blood samples, physical examinations, and ECGs at baseline, after completion of the first cycle of anthracycline therapy, after completion of the final cycle of anthracycline therapy, and, for those concerned, at completion of trastuzumab or radiation therapy. Echocardiography was performed at the same time points, except for after completion of the first cycle of anthracyclines. Follow-up was performed at a median of 23 months (interquartile range, 21 to 28 months) after baseline examinations, and examination procedures were the same as at baseline.
CMR and Echocardiography
Study procedures have been described in detail previously.10 In short, ventricular volumes, ejection fraction, and left ventricular (LV) mass were assessed in short-axis, steady-state free precession sequences acquired in contiguous, 8-mm slices on a 1.5T magnetic resonance imaging scanner (Achieva; Philips Medical Systems). The radiologist (Dr Heck) who assessed CMRs in the main trial performed follow-up assessments according to the Society for Cardiovascular Magnetic Resonance guidelines17 on dedicated, commercially available software (cmr42, version 5.9.4; Circle Cardiovascular Inc).
Follow-up transthoracic echocardiography recordings were performed by the same cardiologist (Dr Gulati) and with the same Vivid E9 (GE Vingmed) as the initial study. Main study analyses were performed offline by Dr Gulati and extended follow-up by Dr Mecinaj using the same custom software (EchoPAC, version 112; GE Vingmed). LV 2-dimensional peak systolic global longitudinal strain (GLS) was assessed with a semiautomated speckle tracking imaging technique from the 3 standard apical views.
Blood Sampling and Biochemical Analysis
Serum cardiac troponin I and cardiac troponin T on follow-up were measured by high-sensitivity (hs) assays on an Alinity platform (Abbott Diagnostics) and a cobas 8000 e602 analyzer (Roche Diagnostics), respectively. NT-proBNP (N-terminal pro–B-type natriuretic peptide) in serum was measured by the proBNP II assay on a cobas 8000 e801 analyzer (Roche Diagnostics). Details on the biomarker assays are given in the Data Supplement and in a previous report.15
Outcomes
The primary efficacy end point of this study was change in LVEF assessed by CMR from baseline to extended follow-up. Secondary efficacy end points were incidence of LV systolic dysfunction, defined as LVEF <53% in combination with either an absolute decrease of >10% in LVEF as determined by CMR or clinical heart failure requiring diuretic therapy; incidence of significant decline in LV systolic function, defined as LVEF <53% in combination with an absolute decrease of >5% in LVEF as determined by CMR; change in LV systolic function as determined by GLS via 2-dimensional echocardiography and change in concentrations of hs cardiac troponin I, hs cardiac troponin T, and NT-proBNP; and change in LV end-diastolic and end-systolic volumes as determined by CMR.
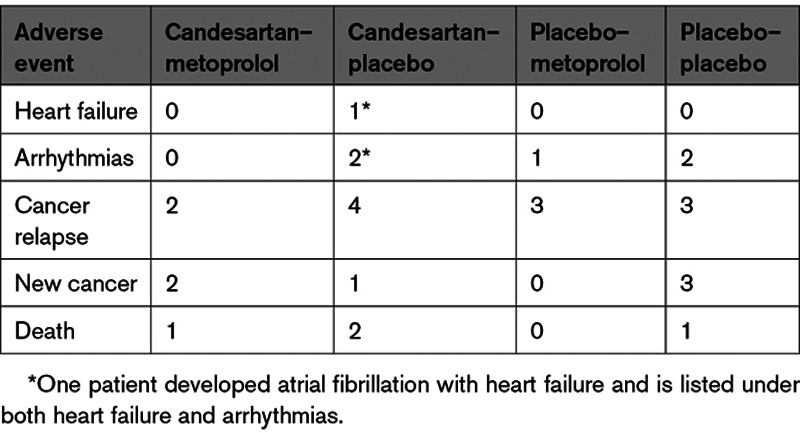
Safety outcomes included development of heart failure, arrhythmia, cancer relapse, new cancer, valvular disease, and death.
Statistical Analysis
Sample size calculations from the original trial showed that we needed a minimum of 120 patients to detect with 95% power an absolute between-group difference in change in LVEF of 5±5 (SD) percentage points.10 The primary efficacy analysis was performed on a modified intention-to-treat sample consisting of all validly randomized patients with, at a minimum, baseline CMR and a per-protocol sample. The per-protocol analysis excluded patients who were not compliant with intervention, withdrew consent, did not complete adjuvant therapy or study medication, or did not undergo follow-up CMR measurements. Secondary efficacy analyses were performed on the intention-to-treat sample.
For each continuous efficacy end point, we fitted a linear mixed model to all available measurements from 5 time points: baseline, after completion of the first cycle of anthracycline therapy, after completion of the final cycle of anthracycline therapy, after completion of trastuzumab or radiation therapy, and at extended follow-up. All models included fixed effects for time, candesartan treatment, metoprolol treatment, candesartan treatment × time interaction, metoprolol treatment × time interaction, age, and left-sided radiation and a random intercept. To investigate possible interactions between the 2 treatments, we fitted additional models that included a candesartan × metoprolol interaction term and applied a likelihood ratio test to the models with and without the treatment interaction term. No statistically significant treatment interactions were observed. On the basis of the fitted models without the treatment interaction term, we estimated baseline, follow-up, and change from baseline to follow-up mean values (with 95% CI). The treatment effects were estimated as the between-group difference in change from baseline to extended follow-up for the comparisons of patients treated with candesartan versus not treated with candesartan and patients treated with metoprolol versus not treated with metoprolol (Figure). All terms in the linear mixed models were prespecified in the statistical analysis plan. A P value of <0.05 was considered statistically significant. The reported P values are 2-sided and not adjusted for multiple comparisons. The statistical analyses were carried out with Stata 16.0 (StataCorp LP).
Figure.
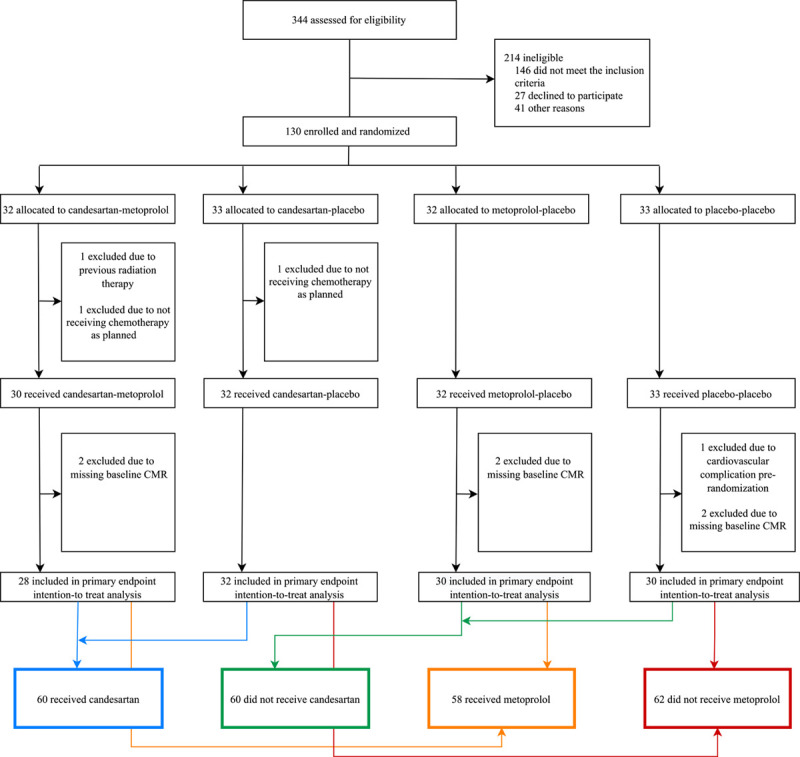
Consolidated Standards of Reporting Trials diagram. PRADA trial (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) screening and randomization. The primary efficacy analysis was performed on a modified intention-to-treat sample consisting of all validly randomized patients with, at a minimum, baseline cardiovascular magnetic resonance (CMR) imaging.
Before study initiation, a data safety and monitoring board consisting of a cardiologist, an oncologist, and a statistician was constituted to monitor adverse events and the trial was registered in the ClinicalTrials.gov registry (URL: https://www.clinicaltrials.gov; Unique identifier: NCT01434134).
Results
Between September 15, 2011, and September 11, 2014, 344 patients with early breast cancer who after surgery were scheduled for adjuvant therapy involving the anthracycline epirubicin at the Department of Oncology, Akershus University Hospital, were screened for eligibility. A Consolidated Standards of Reporting Trials diagram (Figure) summarizes patient screening and randomization. Similar details for the per-protocol cohort are provided in Figure I in the Data Supplement. Of these patients, 120 were eligible and validly randomized to 1 of 4 treatment groups, with well-balanced baseline characteristics and intended adjuvant anticancer therapy (Table 1). Participants received adjuvant therapy according to the recommendations of the Norwegian Breast Cancer Group at the time of diagnosis. All patients received 5-fluorouracil, epirubicin, and cyclophosphamide, and if indicated, taxanes (80%), trastuzumab (23%), and radiotherapy (63%). The median epirubicin dose was 240 (range 240 to 400) mg/m2. Details of the cancer characteristics and therapy are listed in Table II in the Data Supplement. No patient fulfilled the predefined criteria for unblinding of the randomized treatment assignment during the study intervention period. Extended follow-up examinations were performed between April 25, 2013, and June 30, 2016, after a median follow-up of 23 months (interquartile range, 21 to 28 months) from randomization. Of the 120 participants, 98 were assessed by CMR at extended follow-up.
Table 1.
Baseline Characteristics of the Study Population
There was no statistical interaction between candesartan and metoprolol treatment on the primary end point or on any of the secondary end points, allowing the patients in the 2 groups receiving candesartan to be compared with patients receiving placebo–placebo or metoprolol–placebo and the 2 groups receiving metoprolol to be compared with patients receiving placebo–placebo or candesartan–placebo. There were no between-group differences in the primary outcome measure (Table 2). From baseline to extended follow-up, the overall decline in LVEF in patients in the candesartan group was 1.7 (95% CI, 0.5 to 2.8) percentage points and in patients not receiving candesartan 1.8 (95% CI, 0.6 to 3.0) percentage points in the intention-to-treat analysis (P=0.91 for between-group difference in linear mixed model analysis). For patients in the metoprolol group, the decline in LVEF was 1.6 (95% CI, 0.4 to 2.7) percentage points and for patients not receiving metoprolol the decline was 1.9 (95% CI, 0.7 to 3.0; P=0.73) percentage points. Per-protocol analysis did not materially change these results, nor was there a significant difference in effect of the interventions between patients receiving low versus higher anthracycline doses (240 mg/m2 epirubicin versus higher doses).
Table 2.
Primary and Secondary End Points: Estimated Values From Linear Mixed Models
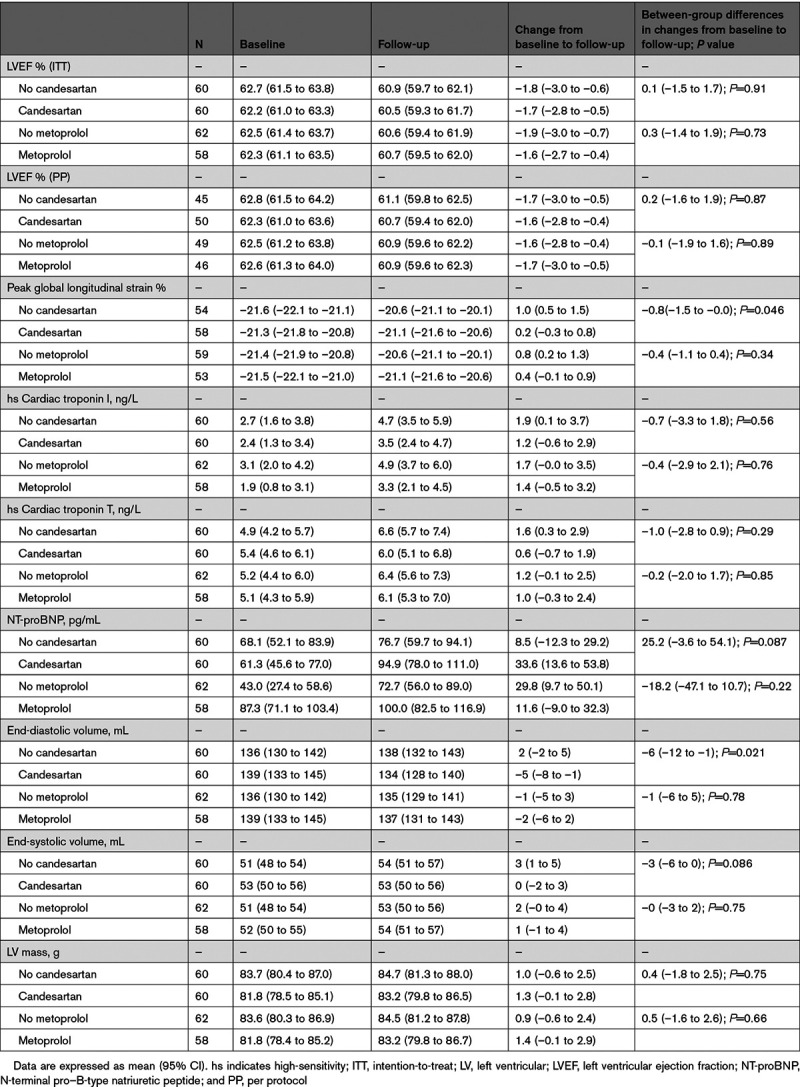
Candesartan treatment during adjuvant therapy was associated with reduction in the secondary outcome measure end-diastolic volume of 5 (95% CI, 1 to 8) mL compared with an increase of 2 (95% CI, –2 to 5) mL in the no candesartan group (between-group difference, 6 [95% CI, 1 to 12] mL; P=0.021). Patients not receiving candesartan experienced increase in end-systolic volumes (3 [95% CI, 1 to 5] mL) whereas patients receiving candesartan did not (0 [95% CI, –2 to 3] mL; P=0.086). There was no effect of the intervention on change in cardiac troponins. Changes in hs cardiac troponin I and hs cardiac troponin T were 1.2 (95% CI, –0.6 to 2.9) and 0.6 (95% CI, –0.7 to 1.9) ng/L in the candesartan group, 1.9 (95% CI, 0.1 to 3.7) and 1.6 (95% CI, 0.3 to 2.9) ng/L in the no candesartan group, 1.4 (95% CI, –0.5 to 3.2) and 1.0 (95% CI, –0.3 to 2.4) ng/L in the metoprolol group, and 1.7 (95% CI, –0.0 to 3.5) and 1.2 (95% CI, –0.1 to 2.5) ng/L in the no metoprolol group, respectively. There was a small decline in GLS in absolute values, which was attenuated by candesartan, but not metoprolol. The decline was 0.2 (95% CI, –0.3 to 0.8) percentage points in patients receiving candesartan and 1.0 (95% CI, 0.5 to 1.5) percentage points in the no candesartan group in the intention-to-treat analysis (P=0.046 for between-group difference in linear mixed model analysis). During extended follow-up, 1 participant treated with candesartan developed symptomatic heart failure. There were no significant between-group differences in incidence of heart failure or significant decline in LVEF. No effect of candesartan or metoprolol treatment was observed on any of the other secondary outcome measures (Table 2). Four patients died between baseline examinations and December 2020, all from cancer. Median time to death from baseline was 63 months (range, 31 to 78 months). Serious adverse events are summarized in Table 3.
Table 3.
Incidence of Serious Adverse Events From End of Study to December 2020
Discussion
In this follow-up study of the PRADA trial, we made 4 important observations. First, we found that adjuvant, epirubicin-containing therapy for early breast cancer in women was associated with a modest but persistent decline in LV systolic function. Second, concomitant angiotensin and β-adrenergic receptor blockade during adjuvant breast cancer therapy did not affect the decline in LVEF at 2 years as compared with placebo. Third, treatment with candesartan was associated with attenuated decline in GLS as well as decline in LV end-diastolic volumes, whereas end-systolic volumes increased in patients not treated with candesartan. Last, no significant effect of metoprolol on GLS or LV volumes was observed, nor did the early interventions significantly affect the levels of cardiac troponin I and cardiac troponin T or NT-proBNP. Our findings provide insight in the long-term effect of β-adrenergic and angiotensin receptor blockade during adjuvant chemotherapy for early breast cancer and will be important when defining its role in cardioprotective primary prevention.
In contrast to the primary results of the PRADA trial, the attenuating effect of candesartan on decline in LVEF observed during adjuvant therapy for early breast cancer did not persist 2 years after randomization. There may be several reasons for this. First, the observed attenuation may have been attributable to direct hemodynamic and neurohormonal effects of angiotensin receptor blockade that ceased on discontinuation.18 Second, ejection fraction is a product of end-diastolic and end-systolic volumes, and physiologic and pathophysiologic processes may affect these volumes differentially. Decline in ejection fraction after cardiotoxic therapy may occur through increased end-systolic volumes related to myocellular dysfunction, but may also be caused by decreased end-diastolic volumes from cancer therapy–related nausea and volume depletion.19,20 At follow-up, patients treated with candesartan had a decline in end-diastolic volumes but no change in end-systolic volumes, whereas participants who did not receive candesartan experienced increasing end-systolic volumes, but no significant change in end-diastolic volumes. It seems unlikely that the decline in end-diastolic volume is caused by volume depletion >1 year after end of cancer therapy. Our findings suggest that different mechanisms may be at play: the decline in ejection fraction in patients treated with candesartan may be attributable to long-term reverse myocardial remodeling effects of candesartan with reduced end-diastolic volumes whereas in patients not treated with candesartan it may be an expression of decline in myocardial contractility and increased end-systolic volumes.19,21
GLS is considered a more sensitive marker of subclinical myocardial dysfunction than echocardiographic LVEF.2,22 A relative drop of >15% or a value below the normal lower limit may be associated with cardiotoxicity during cancer treatment and may predict later decline in LVEF. Several studies have shown subclinical reductions in GLS years after anthracycline therapy.2,23,24 In PRADA, we found that candesartan attenuated a numerically small decline in GLS. However, in a study of 2625 patients followed for 4 years after anthracycline therapy, 98% of cardiotoxicity cases occurred within the first year after therapy.25 This could imply that further significant deterioration of cardiac function in our cohort is unlikely, and whether candesartan’s effect on ventricular volumes and GLS 2 years after randomization will translate into future clinically relevant benefit is uncertain.
We have previously shown that anthracycline therapy was associated with an increase in circulating troponins that was attenuated by β-blockade.15 These findings suggested a beneficial effect on the acute cardiotoxic effects of anthracyclines and were confirmed by the CECCY trial (Carvedilol Effect in Preventing Chemotherapy-Induced Cardiotoxicity).13 However, at the current follow-up, we found no difference between patients treated with metoprolol and placebo in any of the outcome measures, indicating that the initial effect does not provide long-term clinical benefit in our patient cohort.
The overall decline in LV ejection fraction was <2 percentage points, which is less than anticipated when compared with early reports on the cardiotoxic effects of anthracyclines, trastuzumab, and radiotherapy.26,27 A meta-analysis from 2013 including 22 815 patients treated with anthracyclines identified cumulative anthracycline dose, extremes of age, serious comorbidities, and other cardiovascular risk factors as important predictors of cardiotoxicity.28 Contemporary adjuvant regimens with dose limitations, less cardiotoxic anthracycline analogues, and sequential administration of anthracyclines and trastuzumab have reduced the risk of cardiotoxicity.29 The PRADA cohort was relatively young, without serious comorbidities, and treated with low to moderate anthracycline doses, and therefore is at low risk for cardiac dysfunction. Our findings indicate that contemporary adjuvant therapy for early breast cancer is safe in these patients.
Our findings 2 years after randomization add important knowledge about the effect of neurohormonal blockade during adjuvant therapy for breast cancer, where results from recent trials have been inconsistent. The CECCY trial found no beneficial effect of β-adrenergic blockade during anthracycline therapy on the primary end point cardiotoxic events or change in LVEF.13 Three trials assessing the effect of neurohormonal interventions during trastuzumab therapy failed to demonstrate any effect on the primary end points: change in end-diastolic volume (MANTICORE [Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research])11 and cardiotoxic events.12,14 MANTICORE reported cardioprotective effects of angiotensin-converting enzyme inhibition and β-adrenergic blockade on the secondary end point change in LVEF, and Guglin et al.12 found that angiotensin-converting enzyme inhibition and β-adrenergic blockade significantly reduced both cardiotoxic events and decline in ejection fraction in the subgroup of patients who had previously received anthracyclines. Reasons for these discrepancies may be differences in cancer therapy regimens, risk factors, cardioprotective medication, choice of primary end point, and measurement method, as well as varying and relatively short follow-up time. Overall, there is limited evidence that general, primary preventive neurohormonal blockade provides significant long-term clinical benefit. Future trials should focus on whether preventive strategies such as neurohormonal blockade are more efficient in patients at higher risk of developing cardiac dysfunction. Possible target populations are patients with cardiovascular risk factors or established disease, patients genetically disposed for cardiotoxicity, or patients receiving higher cumulative anthracycline doses.30 Other strategies such as exercise programs, strict risk factor control, or prolonged use of cardioprotective agents should also be investigated to identify the optimal cardioprotective strategy to mitigate cancer-related cardiovascular toxicity.
Strengths and Limitations
Strengths of our study are the extended follow-up; the 2×2 factorial design that simultaneously tests 2 interventions; the study population, representative of a large group of patients with early breast cancer and no serious comorbidities; and the longitudinal use of sensitive CMR and echocardiographic measures of cardiac function. The main limitation is that the decline in the primary outcome measure LVEF was less than anticipated in this low-risk cohort. The study may therefore have been underpowered to detect small between-group differences and lacks power for subgroup analyses. The study was also underpowered to detect an interaction between candesartan and metoprolol. Not all patients returned for the final follow-up examinations, but this was partly accounted for in the statistical model in the intention-to-treat analyses. Given that cardiovascular events may occur several years after administration of potentially cardiotoxic cancer therapy, we cannot rule out a beneficial effect of broadly administered preventive therapy if the follow-up period had been longer than 23 months. New versions of the biomarker assays were used at follow-up, and using different assays during adjuvant therapy and at follow-up may have affected the assessment of longitudinal biomarker changes, but should not affect between-group differences.
Conclusions
Concomitant therapy with candesartan and metoprolol during adjuvant therapy for early breast cancer did not protect against a small decline in LVEF as compared with placebo 2 years after randomization. Candesartan treatment protected against a small decline in GLS and was associated with decline in end-diastolic volumes. In contrast, in patients not treated with candesartan, we observed a decrease in myocardial contractility expressed as increased end-systolic volumes. However, the effect size of these secondary end points was small, and unlikely to confer long-term clinically relevant benefits. Broadly administered, general cardioprotective therapy in this patient group seems unwarranted. Future studies should aim to identify subgroups who may benefit from cardioprotective strategies.
Acknowledgments
The authors thank the Data and Safety Monitoring Board; the staff of the Clinical Research Unit, Division of Medicine; and the radiographers at the Cardiac Magnetic Resonance Unit of the Department of Diagnostic Imaging, Akershus University Hospital, for assistance with all aspects of trial execution. Dr Omland, Dr Ree, and Dr Geisler conceived and designed the research. Dr Gulati, Dr Heck, Dr Mecinaj, Dr Gravdehaug, and Dr Røsjø acquired the data. Dr Omland, Dr Geisler, Dr Steine, Dr Hoffmann, and Dr Schulz-Menger handled funding and supervision. Dr Fagerland performed statistical analysis. Dr Heck, Dr Mecinaj, Dr Gulati, and Dr Omland drafted the manuscript. Dr Ree, Dr Hoffmann, Dr Schulz-Menger, Dr Fagerland, Dr Gravdehaug, Dr Røsjø, Dr Steine, and Dr Geisler made critical revisions to the manuscript.
Sources of Funding
This work was supported by The South-Eastern Norway Regional Health Authority, The University of Oslo, The Extra Foundation for Health and Rehabilitation, The Norwegian Cancer Society, and Akershus University Hospital. Study medications and matching placebos were provided free of charge by AstraZeneca. Reagents for the analysis of high-sensitivity cardiac troponin I were provided by Abbott Diagnostics.
Disclosures
Dr Gulati has received speaker honoraria from Novartis, AstraZeneca, Roche, and Bristol Myers Squibb. Dr Omland has served on advisory boards for Abbott Diagnostics, Roche Diagnostics, and Bayer and has received research support from Abbott Diagnostics, AstraZeneca, Novartis, Roche Diagnostics, Singulex, and SomaLogic via Akershus University Hospital and speaker or consulting honoraria from Roche Diagnostics, Siemens Healthineers, and CardiNor. Dr Røsjø has received speaker honoraria from Novartis and personal fees from CardiNor and SpinChip Diagnostics. The other authors report no conflicts.
Supplemental Materials
Blood Sampling and Biochemical Analysis
Data Supplement Figure I
Data Supplement Tables I and II
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ACEI
- angiotensin converting enzyme inhibitor
- ARB
- angiotensin receptor blocker
- CECCY
- Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity
- CMR
- Cardiovascular magnetic resonance
- cTn
- cardiac troponin
- CV
- cardiovascular
- GLS
- global longitudinal strain
- LVEF
- left ventricular ejection fraction
- MANTICORE
- Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research
- NT-proBNP
- N-terminal pro B-type natriuretic peptide
- PRADA
- Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy
S.L. Heck and A. Mecinaj contributed equally.
G. Gulati and T. Omland contributed equally.
This manuscript was sent to Prof Steven Lloyd, Guest Editor, for review by expert referees, editorial decision, and final disposition.
This work was presented as an abstract at the American College of Cardiology Scientific Session, May 15–17, 2021.
The Data Supplement, podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.121.054698.
For Sources of Funding and Disclosures, see page 2439.
References
- 1.Strongman H, Gadd S, Matthews A, Mansfield KE, Stanway S, Lyon AR, Dos-Santos-Silva I, Smeeth L, Bhaskaran K. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394:1041–1054. doi: 10.1016/S0140-6736(19)31674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, et al. ; ESC Scientific Document Group. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211 [DOI] [PubMed] [Google Scholar]
- 3.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. doi: 10.1016/j.jacc.2007.06.037 [DOI] [PubMed] [Google Scholar]
- 4.Lenihan DJ, Fradley MG, Dent S, Brezden-Masley C, Carver J, Filho RK, Neilan TG, Blaes A, Melloni C, Herrmann J, et al. Proceedings from the Global Cardio-Oncology Summit: the top 10 priorities to actualize for cardiooncology. JACC CardioOncol. 2019;1:256–272. doi: 10.1016/j.jaccao.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501 [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Pitt B, Davis CE, Hood WB, Jr, Cohn JN. SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003 [DOI] [PubMed] [Google Scholar]
- 7.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 8.Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Martinelli G, Veglia F, Fiorentini C, Cipolla CM. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144 [DOI] [PubMed] [Google Scholar]
- 9.Bosch X, Rovira M, Sitges M, Domènech A, Ortiz-Pérez JT, de Caralt TM, Morales-Ruiz M, Perea RJ, Monzó M, Esteve J. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (Prevention of Left Ventricular Dysfunction With Enalapril and Carvedilol in Patients Submitted to Intensive Chemotherapy for the Treatment of Malignant Hemopathies). J Am Coll Cardiol. 2013;61:2355–2362. doi: 10.1016/j.jacc.2013.02.072 [DOI] [PubMed] [Google Scholar]
- 10.Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff-Brenkenhoff F, Bratland Å, Storås TH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–1680. doi: 10.1093/eurheartj/ehw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, Pagano JJ, Chow K, Thompson RB, Vos LJ, et al. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J Clin Oncol. 2017;35:870–877. doi: 10.1200/JCO.2016.68.7830 [DOI] [PubMed] [Google Scholar]
- 12.Guglin M, Krischer J, Tamura R, Fink A, Bello-Matricaria L, McCaskill-Stevens W, Munster PN. Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol. 2019;73:2859–2868. doi: 10.1016/j.jacc.2019.03.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR, Jr, das Dores Cruz F, Gonçalves Brandão SM, Rigaud VOC, Higuchi-Dos-Santos MH, Hajjar LA, Kalil Filho R, Hoff PM, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018;71:2281–2290. doi: 10.1016/j.jacc.2018.02.049 [DOI] [PubMed] [Google Scholar]
- 14.Boekhout AH, Gietema JA, Milojkovic Kerklaan B, van Werkhoven ED, Altena R, Honkoop A, Los M, Smit WM, Nieboer P, Smorenburg CH, et al. Angiotensin II-receptor inhibition with candesartan to prevent trastuzumab-related cardiotoxic effects in patients with early breast cancer: a randomized clinical trial. JAMA Oncol. 2016;2:1030–1037. doi: 10.1001/jamaoncol.2016.1726 [DOI] [PubMed] [Google Scholar]
- 15.Gulati G, Heck SL, Røsjø H, Ree AH, Hoffmann P, Hagve TA, Norseth J, Gravdehaug B, Steine K, Geisler J, et al. Neurohormonal blockade and circulating cardiovascular biomarkers during anthracycline therapy in breast cancer patients: results from the PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) study. J Am Heart Assoc. 2017;6:e006513. doi: 10.1161/JAHA.117.006513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Meer P, Gietema JA, Suter TM, van Veldhuisen DJ. Cardiotoxicity of breast cancer treatment: no easy solution for an important long-term problem. Eur Heart J. 2016;37:1681–1683. doi: 10.1093/eurheartj/ehw133 [DOI] [PubMed] [Google Scholar]
- 17.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35. doi: 10.1186/1532-429X-15-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaiswal A, Nguyen VQ, Carry BJ, le Jemtel TH. Pharmacologic and endovascular reversal of left ventricular remodeling. J Card Fail. 2016;22:829–839. doi: 10.1016/j.cardfail.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 19.Suerken CK, D’Agostino RB, Jr, Jordan JH, Meléndez GC, Vasu S, Lamar ZS, Hundley WG. Simultaneous left ventricular volume and strain changes during chemotherapy associate with 2-year postchemotherapy measures of left ventricular ejection fraction. J Am Heart Assoc. 2020;9:e015400. doi: 10.1161/JAHA.119.015400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meléndez GC, Sukpraphrute B, D’Agostino RB, Jr, Jordan JH, Klepin HD, Ellis L, Lamar Z, Vasu S, Lesser G, Burke GL, et al. Frequency of left ventricular end-diastolic volume-mediated declines in ejection fraction in patients receiving potentially cardiotoxic cancer treatment. Am J Cardiol. 2017;119:1637–1642. doi: 10.1016/j.amjcard.2017.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 22.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073 [DOI] [PubMed] [Google Scholar]
- 23.Maestrini V, Cheang MH, Kotwinski P, Rosmini S, Lloyd G, Kellman P, Pennell DJ, Montgomery H, Moon JC, Manisty C. Late anthracycline-related cardiotoxicity in low-risk breast cancer patients. J Am Coll Cardiol. 2017;69:2573–2575. doi: 10.1016/j.jacc.2017.03.560 [DOI] [PubMed] [Google Scholar]
- 24.Zhang KW, Finkelman BS, Gulati G, Narayan HK, Upshaw J, Narayan V, Plappert T, Englefield V, Smith AM, Zhang C, et al. Abnormalities in 3-dimensional left ventricular mechanics with anthracycline chemotherapy are associated with systolic and diastolic dysfunction. JACC Cardiovasc Imaging. 2018;11:1059–1068. doi: 10.1016/j.jcmg.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777 [DOI] [PubMed] [Google Scholar]
- 26.Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008;14:14–24. doi: 10.1158/1078-0432.CCR-07-1033 [DOI] [PubMed] [Google Scholar]
- 27.Ewer SM, Ewer MS. Cardiotoxicity profile of trastuzumab. Drug Saf. 2008;31:459–467. doi: 10.2165/00002018-200831060-00002 [DOI] [PubMed] [Google Scholar]
- 28.Lotrionte M, Biondi-Zoccai G, Abbate A, Lanzetta G, D’Ascenzo F, Malavasi V, Peruzzi M, Frati G, Palazzoni G. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. 2013;112:1980–1984. doi: 10.1016/j.amjcard.2013.08.026 [DOI] [PubMed] [Google Scholar]
- 29.McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31:63–75. doi: 10.1007/s10557-016-6711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott-Roe C, Ky B. Genetics of anthracycline-mediated cardiotoxicity: current status and challenges. Curr Cardiovasc Risk Rep. 2020;14:14. doi: 10.1007/s12170-020-00647-3 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


