INTRODUCTION
Ivermectin is an antiparasitic medication approved by the US Food and Drug Administration (FDA) for use in humans. Ivermectin oral tablets are used worldwide for treatment of certain parasitic infections. An in vitro study has shown that ivermectin inhibits the replication of SARS-CoV-2 in tissue cell cultures.1 Some observational studies and clinical trials have evaluated ivermectin for the treatment and prevention of COVID-19 in humans; however, most had incomplete information and methodological limitations.2 Human data from well-designed and well-conducted clinical trials with robust sample sizes are needed to provide more specific, evidence-based guidance on the role of ivermectin for the treatment or prevention of COVID-19.
METHODS
To understand whether there have been changes in outpatient ivermectin dispensing during the COVID-19 pandemic, CDC tracked outpatient retail prescription data from the IQVIA National Prescription Audit Weekly (NPA Weekly, hereafter abbreviated NPA) for the estimated number of ivermectin prescriptions dispensed from retail pharmacies weekly during March 14, 2020–April 2, 2021, and compared these estimates to a pre-pandemic baseline weekly average during the 52-week period (March 16, 2019–March 13, 2020) before the declaration of a national emergency. Monthly state-level data from the IQVIA Total Patient Tracker (TPT) were then analyzed to assess whether observed changes were due to changes in particular states where the dispensing pharmacies are located. NPA and TPT both collect data from a sample of approximately 48,900 US retail pharmacies, representing 92% of all retail prescription activity nationwide.3 NPA data were used to estimate the total number of ivermectin prescriptions dispensed from retail pharmacies nationally, while TPT data and 2019 intercensal estimates from the US Census Bureau were used to estimate state-specific ivermectin dispensing rates per 100,000 population. These data do not include prescribing indication and ivermectin dispensed by mail order and long-term care pharmacies, prescriptions by veterinarians, and non-oral formulations were not included. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.4
RESULTS
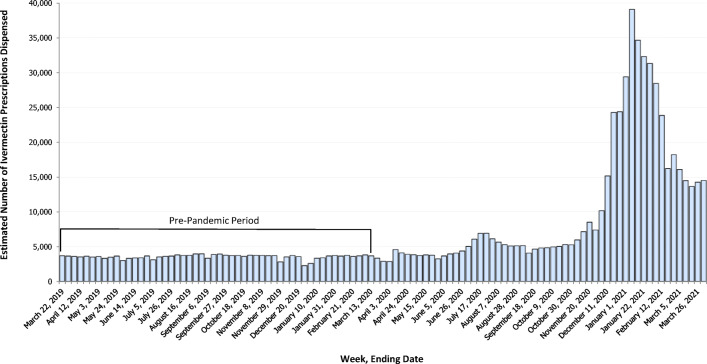
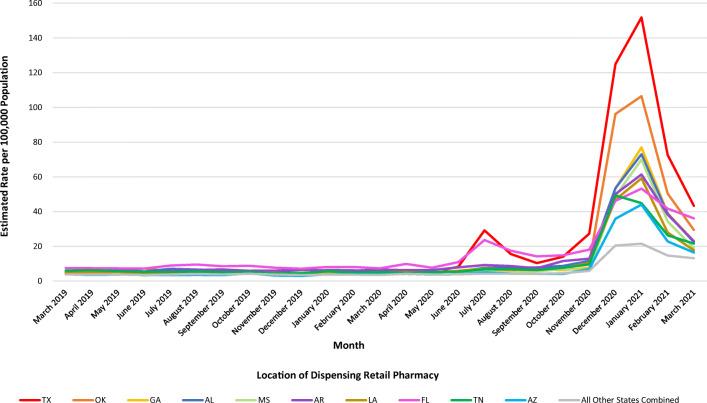
During March 16, 2019–April 2, 2021, national estimates of ivermectin dispensed from outpatient retail pharmacies increased from an average of 3589 prescriptions per week at the pre-pandemic baseline to a peak of 39,102 prescriptions in the week ending on January 8, 2021 (989% relative percent increase) (Fig. 1). Monthly state-based analyses revealed that an initial increase in ivermectin dispensing observed in July 2020 largely involved increased dispensing rates in Texas and Florida (Fig. 2). By January 2021, ivermectin dispensing rates had increased in all states and the three states with the highest estimated rates of dispensing were Texas (151.8 per 100,000 population), Oklahoma (106.5 per 100,000 population), and Georgia (76.9 per 100,000 population). Ivermectin dispensing decreased after January 2021 but remained elevated compared with pre-pandemic levels.
Figure 1.
Estimated number of outpatient ivermectin prescriptions dispensed from retail pharmacies — US, March 16, 2019–April 2, 2021. Data are from the IQVIA National Prescription Audit Weekly (NPA Weekly) database. NPA Weekly collects data from a sample of approximately 48,900 US retail pharmacies, representing 92% of all retail prescription activity. National estimates of weekly ivermectin prescriptions dispensed indicated by blue bars and bracket indicates the pre-pandemic period from March 16, 2019 to March 13, 2020. Ivermectin dispensed by mail order and long-term care pharmacies, prescriptions by veterinarians, and non-oral formulations were not included.
Figure 2.
Estimated rate per 100,000 population of unique patients receiving ivermectin dispensed from retail pharmacies by state and the District of Columbia — US, March 2019–March 2021. Monthly state-level estimates of outpatient retail prescription data are from the IQVIA Total Patient Tracker (TPT) database and the 2019 intercensal estimates are from the US Census Bureau. TPT collects data from a sample of approximately 48,900 US retail pharmacies, representing 92% of all retail prescription activity. TPT estimates numbers of unique patients receiving ivermectin prescriptions dispensed by the state where the dispensing retail pharmacy is located. The 10 states with the highest estimated rates of ivermectin dispensing from pharmacies located in their state in January 2021 are displayed individually: red line is Texas, orange line is Oklahoma, yellow line is Georgia, dark blue line is Alabama, light green line is Mississippi, purple line is Arkansas, brown line is Louisiana, pink line is Florida, dark green line is Tennessee, light blue line is Arizona; and all other states are combined in the gray line. Ivermectin dispensed by mail order and long-term care pharmacies, prescriptions by veterinarians, and non-oral formulations were not included.
DISCUSSION
Ivermectin dispensed from outpatient retail pharmacies increased substantially in the US during the COVID-19 pandemic. While initial increases largely involved increased dispensing rates in Texas and Florida, by January 2021, ivermectin dispensing rates had increased nationwide. These data are subject to at least three limitations. First, NPA and TPT do not include inpatient prescription data, and thus are not representative of all ivermectin dispensing. Second, without prescribing indication, it is unknown whether the increased ivermectin dispensed was used to treat non-COVID-19 conditions (i.e., parasitic infections). However, the increase in the number of outpatients who received ivermectin from retail pharmacies observed during the pandemic followed publication of the previously mentioned in vitro study1 and increases in COVID-19 cases in the US.5 Third, patients receiving ivermectin may not reside in the same state where the dispensing pharmacy is located.
Clinicians should be aware that ivermectin is not currently authorized or approved by FDA for treatment of COVID-19, and FDA cautions against use for this purpose.6 Furthermore, the National Institutes of Health’s COVID-19 Treatment Guidelines Panel determined that there are currently insufficient data to recommend either for or against the use of ivermectin for treatment of COVID-19.2 As of May 2021, only investigational monoclonal antibody therapies are authorized by FDA for treatment of non-hospitalized high-risk persons with mild-to-moderate COVID-19. It is important for clinicians to consider SARS-CoV-2 infection prevention measures, vaccination, and authorized treatments before prescribing therapies that are not authorized or approved for the treatment or prevention of COVID-19.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/ivermectin. Accessed 11 Feb 2021. [PubMed]
- 3.IQVIA. Prescription Information. Available at: https://www.iqvia.com/locations/united-states/solutions/commercial-operations/essential-information/prescription-information. Accessed 20 Jan 2021.
- 4.United States Government. Federal Regulations and Codes. See e.g., 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
- 5.Centers for Disease Control and Prevention. COVID Data Tracker. Available at: https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases. Accessed 6 May 2021.
- 6.US Food and Drug Administration. Why You Should Not Use Ivermectin to Treat or Prevent COVID-19. Available at: https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19?utm_medium=email&utm_source=govdelivery. Accessed 12 Apr 2021.