Abstract
The AIDS Clinical Trials Group (ACTG) A5345 study included an intensively monitored antiretroviral pause (IMAP), during which a cohort of participants temporarily stopped antiretroviral treatment during chronic HIV infection. We surveyed participant perceptions and understanding of A5345 using a cross-sectional sociobehavioral questionnaire. Participants completed the baseline questionnaire either before or after initiating the study's IMAP. Questionnaire responses were linked to existing demographic data. Quantitative responses were analyzed overall and stratified by IMAP status. Open-ended responses were analyzed using conventional content analysis. Thirty-two participants completed the baseline sociobehavioral questionnaire. Half (n = 16) completed it before (i.e., pre-IMAP initiation group) and half (n = 16) after IMAP initiation (i.e., post-IMAP initiation group). Eight pre-IMAP initiation respondents (50%) and 11 post-IMAP respondents (69%) responded “yes” when asked if they perceived any direct benefits from participating in A5345. Perceived societal-level benefits included furthering HIV cure-related research and helping the HIV community. Perceived personal-level benefits included the opportunity to learn about the body's response to IMAP and financial compensation. The majority of respondents—13 from each group (81% of each)—reported risks from participation, for example, viral load becoming detectable. A5345 participants perceived both societal- and personal-level benefits of study participation. While the majority of survey respondents perceived participatory risks, nearly one in five did not. Key messages pertaining to study-related risks and benefits may need to be clarified or reiterated periodically throughout follow-up in HIV cure-related studies with IMAPs.
Clinical Trail Registration Number: NCT03001128.
Keywords: persons living with HIV, HIV cure-related research, intensively monitored antiretroviral pause, analytical treatment interruption, social sciences, behavioral sciences
Introduction
One goal of HIV cure-related research is to identify predictors and interventions associated with sustained suppression of HIV in the absence of antiretroviral therapy (ART).1,2 Intensively monitored antiretroviral pauses (IMAPs) involve stopping ART and then restarting treatment when virus rebounds, whereas analytical treatment interruptions (ATIs) typically have a defined period of treatment interruption to assess viral load set point. IMAPs are used to identify biomarkers associated with viral rebound in persons living with HIV (PLWH).2–5
IMAPs involve the close monitoring of PLWH as they discontinue (and later reinitiate) ART according to prespecified criteria.6 While IMAPs do not offer therapeutic benefit to participants and confer some risks to them and their sexual partners,5,7 the evidence suggests that IMAPs can be relatively safe.6,8–12 A consensus statement developed by a multidisciplinary panel of stakeholders, including HIV research experts, affirmed the necessity of IMAPs, established best practices for their use, and underscored the need for sociobehavioral research embedded within HIV cure-related studies utilizing IMAPs.2
Indeed, given the ethical considerations related to discontinuing ART,2,13,14 the importance of capturing participants' perceptions and experiences throughout their enrollment in HIV cure-related studies is increasingly recognized.2,15–18 However, few studies in the United States have integrated sociobehavioral research into HIV cure-related research.19–21 Assessing the experiences of participants undergoing an IMAP is essential to understanding whether perceived risks and benefits of participation at trial enrollment align with actual experiences, which in turn can inform the development of participant-centered interventions and trials. In this study, we describe the perspectives and experiences of U.S.-based participants entering the AIDS Clinical Trials Group (ACTG) A5345 study.
Methods
ACTG A5345 (A5345)–Cohorts and IMAP definition
A5345 enrolled participants from April 2017 to November 2019 in the United States and Thailand with the intent to identify biomarker predictors of time to viral rebound among PLWH undergoing an IMAP. Clinical research sites participating in the sociobehavioral study included 14 research centers in the United States (including one in Puerto Rico). The A5345 protocol did not deliver an intervention, aside from the IMAP and subsequent ART reinitiation. The study was described to participants during the recruitment and informed consent process as: “The purpose of this study is to collect blood for future studies from participants whose HIV is undetectable by standard tests before, during, and after an intensively monitored antiretroviral pause (or IMAP). The study will also look at how long HIV remains suppressed during IMAP,” as per the study's consent form template.
A5345 included PLWH who had an undetectable viral load (<20 copies/mL) for at least 2 years and CD4+ cell count ≥500 cells/mm3 at screening and who had initiated ART during either chronic HIV infection (Cohort A participants) or acute HIV infection (Cohort B participants). A5345 participants underwent a lead-in period of ≥4 weeks and then proceeded to an IMAP, in which their viral load was monitored at least weekly and ART was reinitiated upon meeting the prespecified criteria (e.g., two consecutive HIV RNA measurements ≥1,000 copies/mL).
Baseline sociobehavioral questionnaire
An amendment to the A5345 protocol in May 2018 added a series of questionnaires to survey participant-centered sociobehavioral outcomes. The amendment received institutional review board (IRB) approval from each participating clinical research site and the UNC Non-Biomedical IRB.
The baseline questionnaire was developed by the A5345 lead social scientist (K.D.) and reviewed extensively with ACTG community representatives (L.B. and D.P.) for clarity and comprehension. Due to time constraints, no formal pilot testing of the questionnaires was done. Sites completed survey administration training before implementation. The baseline questionnaire was designed to be administered at, or closely following, study entry. Since the A5345 clinical study had been initiated some time before the sociobehavioral component was added, the questionnaire could not be administered at the same study time point for all participants. Therefore, we administered the initial sociobehavioral questionnaire as a cross-sectional questionnaire to all participants as soon as it was available. Thus, some participants completed the questionnaire upon entering the study (before IMAP initiation) and others completed the questionnaire after IMAP initiation.
The questionnaire was administered online (using computer or tablet) at the clinic site, in English, using Qualtrics (Provo, UT). Research staff were available on site to help participants complete the sociobehavioral questionnaires. Participants had the option to decline responding to the baseline questionnaire. Those who completed the questionnaire received an additional $25 beyond the compensation received by all participants for their participation in the clinical portion of A5345; all compensation was approved by local IRBs.
The baseline questionnaire assessed participant perceptions and understanding of A5345, including the following domains: history of research participation, motivations to participate/decision-making, expectations about the study, and perceived risks and benefits. Both closed-ended and open-ended questions were included. Open-ended questions elicited free responses and also included strategically placed prompts such as “please explain” and “specify” following selected closed-ended questions.
Self-reported pain and anxiety were assessed at baseline. The EQ-5D and state anxiety for adults were used, respectively, with license and permission to use. The authors purchased a license for use of the State-Trait Anxiety Inventory for Adults in 100 surveys) for use.22–24 We chose EQ-5D because it is one of the most widely used and efficient scales to assess quality of life in clinical settings and the frequently-used state anxiety inventory because it captures anxiety at the moment of assessment. EQ-5D items for pain and anxiety were analyzed as separate constructs (instead of summing the five EQ-5D items) because three items showed little variance (most participants reported no problems in mobility, self-care, and usual activities). The continuous state anxiety score summed 20 items assessing anxiety frequency in the 7 days before questionnaire completion, with higher scores indicating greater anxiety (possible range 20–80); positively worded items were reverse coded. A score of ≥40 indicated clinically significant anxiety symptoms.25,26
Data analysis
This descriptive analysis includes participants in the United States who initiated ART during chronic HIV infection (A5345 Cohort A). Because A5345 Cohort B (acute cohort) was still enrolling participants at the time of this analysis, we did not include data for cohort B participants in this article. Questionnaire responses were linked to demographic data from the ACTG data management center using coded participant identification numbers.
Responses to closed-ended questions were analyzed overall and stratified by IMAP status at the time of questionnaire (pre-IMAP or post-IMAP initiation) due to the possibility of heterogeneity in experiences by IMAP initiation status at the time of completing the baseline questionnaire. Given small sample sizes, we focused on descriptive summaries and used a two-sided Fisher's exact test to compare categorical responses between the two independent IMAP strata. A 0.05 type I error probability was applied, with no adjustment for multiplicity. Quantitative analyses were conducted using SAS version 9.4 (Cary, NC) and Stata version 15 (College Station, TX). Data visualizations were created in Excel.
Responses to open-ended questions were clustered into themes using conventional content analysis allowing key themes to emerge from the data (e.g., inductive analysis).27A social scientist (K.D. and research assistant) systematically organized the emergent key themes in a Word document without utilizing a preexisting codebook, due to the brevity of the text units. Themes were summarized into an analytic coding tree. In the Results section below, we provide quotations that are broadly representative of the key themes that emerged in the responses.
Results
Demographics and timing of questionnaire completion
A total of 46 participants were enrolled in Cohort A of the A5345 study from September 12, 2017 to July 30, 2019. Of these, 32 (70%) completed the baseline sociobehavioral questionnaire. Half of the 32 respondents (n = 16) completed it before initiating their IMAP (i.e., pre-IMAP initiation group); the remaining (n = 16) completed it after the IMAP initiation (i.e., post-IMAP initiation group). Among the 16 respondents who completed the survey before IMAP initiation, 11 (69%) completed it within 3 days after study entry and the remaining five completed the survey 27–56 days after study entry. Among the 16 respondents who completed the survey following IMAP initiation, survey completion occurred at a median of 110 days after the IMAP was initiated (range: 9–210 days; IQR: 43–150 days). The 14 participants who did not complete the baseline questionnaire had similar demographic characteristics as the 32 who did, except that they were more likely to be Hispanic (5/14 or 35% vs. 3/32 or 9%).
A vast majority of the 32 respondents were men (94%), largely of white, non-Hispanic (75%) or black, non-Hispanic (13%) race/ethnicity, and the median age at entry was 46 (IQR 36–54) years (Table 1). All respondents had an undetectable viral load at study entry; median CD4+ count was 765 (IQR 639–982) cells/mm3 at entry. Overall, 72% of respondents reported no pain/discomfort, while 28% reported moderate pain/discomfort. State anxiety scores ranged from 20 to 56, with a median score of 33.5 (IQR 28.5–45) for pre-IMAP initiation respondents and 38 (IQR 30–48) for post-IMAP initiation respondents, which approaches the threshold of ≥40 for clinically significant anxiety.
Table 1.
Baseline Characteristics Among AIDS Clinical Trials Group A5345 Cohort A Respondents, Stratified by Intensively Monitored Antiretroviral Pause Status, n = 32
| Questionnaire completed before IMAP initiation n = 16 | Questionnaire completed after IMAP initiation n = 16 | Overall n = 32 | ||||
|---|---|---|---|---|---|---|
| Country of residence | ||||||
| United States | 16 | 100% | 16 | 100% | 32 | 100% |
| Sex | ||||||
| Male | 14 | 88% | 16 | 100% | 30 | 94% |
| Female | 2 | 13% | 0 | 0% | 2 | 6% |
| Race/ethnicity | ||||||
| White, non-Hispanic | 12 | 75% | 12 | 75% | 24 | 75% |
| Black, non-Hispanic | 2 | 13% | 2 | 13% | 4 | 13% |
| Hispanic, regardless of race | 1 | 6% | 2 | 13% | 3 | 9% |
| More than one race | 1 | 6% | 0 | 0% | 1 | 3% |
| Age | ||||||
| Median (min–max) | 45 (25–64) | 46 (24–60) | 46 (24–64) | |||
| Interquartile range | 36–56 | 39–52 | 36–54 | |||
| Viral load at study entry | ||||||
| ≤ 20 HIV RNA copies/mL | 16 | 100% | 16 | 100% | 32 | 100% |
| CD4 count at study entry | ||||||
| Median (min–max) | 952 (590–2,372) | 705 (449–1,483) | 765 (449–2,372) | |||
| Interquartile range | 694–1,281 | 606–846 | 639–982 | |||
| Health status at questionnaire completion | ||||||
| No pain or discomfort | 11 | 69% | 12 | 75% | 23 | 72% |
| Moderate pain or discomfort | 5 | 31% | 4 | 25% | 9 | 28% |
| Not anxious or depressed | 10 | 63% | 8 | 50% | 18 | 56% |
| Moderately anxious or depressed | 6 | 38% | 8 | 50% | 14 | 44% |
| No problems in walking about | 13 | 81% | 14 | 88% | 27 | 84% |
| Some problems in walking about | 2 | 13% | 2 | 13% | 4 | 13% |
| Missing | 1 | 6% | 0 | 0% | 1 | 3% |
| No problems with self-care | 13 | 81% | 15 | 94% | 28 | 88% |
| Missing | 3 | 19% | 1 | 6% | 4 | 13% |
| No problem with performing my usual activities | 14 | 88% | 15 | 94% | 29 | 91% |
| Some problems with performing my usual activities | 1 | 6% | 1 | 6% | 2 | 6% |
| Unable to perform my usual activities | 1 | 6% | 0 | 0% | 1 | 3% |
| State anxiety score at questionnaire completion | ||||||
| Median (min–max) | 33.5 (20–53) | 38 (25–56) | 36 (20–56) | |||
| Interquartile range | 28.5–45 | 30–48 | 29–45 | |||
| Elevated anxiety (STAI ≥40) | 6 | 38% | 6 | 43% | 12 | 40% |
| Missing | 0 | 2 | 2 | |||
Data summaries are n and percent (%) or median (min–max) and interquartile ranges.
IMAP, intensively monitored antiretroviral pause; STAI, state anxiety inventory.
Motivations to participate and previous experiences
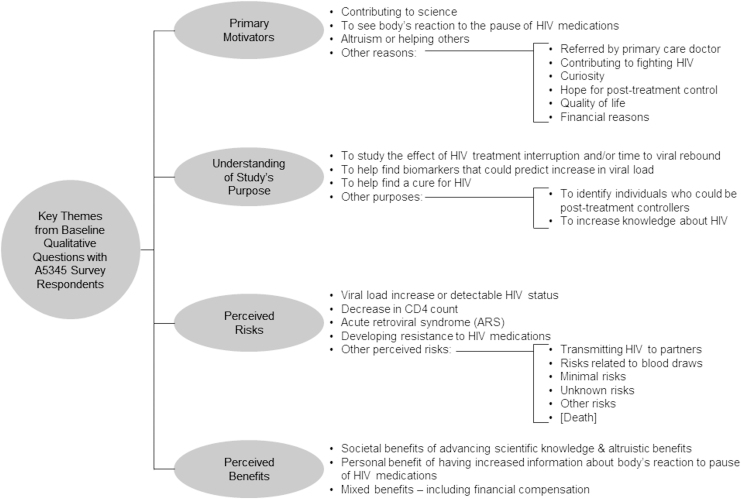
Nearly half of participants (15/32 [47%]) had previously participated in an HIV cure-related research study (e.g., previous ACTG studies, latency-reversing agent studies, or did not remember), and 14/32 (44%) had previously participated in an HIV treatment study (e.g., previous ACTG or industry-sponsored trials). However, A5345 was the first IMAP study for 94% of participants. Respondents were asked to describe the primary reason they decided to participate in a study involving an IMAP using free text. The three most common categories of motivators included: (1) contributing to science (n = 13 responses received—e.g., “I want to help advance HIV research so we can find a cure,” “It will help inform future treatments and may help to develop a cure”); (2) the opportunity to see the body's response to the IMAP (n = 5 responses received—e.g., “[T]o see how my body reacted without being on meds,” “because I want to see how long it would take for my body to regenerate the virus and not suppress it as much”); and (3) altruism or the potential to help others (n = 4 responses received—e.g., “To be part of something that will benefit the overall population of those suffering from HIV/AIDS”) (Fig. 1 and Table 3).
FIG. 1.
Analytic coding tree of emergent themes from select baseline open questions – ACTG A5345. ACTG, AIDS clinical trials group.
Table 3.
Responses from A5345 Survey Respondents Around Primary Motivators, Understanding of Study's Purpose, Perceived Risks, and Perceived Benefits
| Themes | Quotes received Pre-IMAP initiation group | Quotes received from Post-IMAP initiation group |
|---|---|---|
| What is the primary reason that you decided to participate in this study involving a pause in your anti-HIV medications? | ||
| Contributing to science 8 responses pre-IMAP initiation group 5 responses post-IMAP initiation group |
“An interest/willingness to support scientific research in this area. An invitation to participate while being actively enrolled in a different study” “This study is opening a lot of doors and can help with the goal of a cure” “It will help inform future treatments and may help to develop a cure of vaccine” “Aid in the research of HIV cure” “Possibility to stop meds. Help find a cure” “To help find a cure” “To help find a cure” “Research” |
“[S]cience; furthering the cause and information. An opportunity to help provide relevant clinical data to those researching and studying treatment” “I am a health professional and strongly believe in research” “I participate in all the studies I'm qualified to participate in, simply it is the bset way to [g]ive [b]ack to [s]ociety on a broader scale than [sic] donating money” “I want to help advance HIV research so we can find a cure” “[T]o help further the research in finding a cure to HIV” |
| To see body's reaction to the pause of HIV medications 1 response pre-IMAP initiation group 4 responses post-IMAP initiation group |
“I would like to know what would happen if I had to stop taking my [m]y meds for any reason” | “[T]o see how my body reacted without being on meds” “Interested in cure research and to know how quickly my VL [viral load] will come back when stopping meds” “Curiosity about how my body would respond on its own without medication, and curiosity about what the findings would be… plus a desire to help with cure research” “The reason that I have decided to stay in the study involving the stopping of my HIV medications was because I want to see how long it would take for my body to regenerate the virus and not suppress it as much” “I want to contribute. I was also curious how long it would take the virus to come back personally” |
| Altruism or helping others 3 response pre-IMAP initiation group 1 response post-IMAP initiation group |
“[A]ltruism” “To be part of something that will benefit the overall population of those suffering from HIV/AIDS” “I want to be part in helping (…)” |
“To help someone out” |
| Other reasons 3 responses pre-IMAP initiation group 4 responses post-IMAP initiation group |
Contributing to fighting HIV “I want to contribute in the fight against HIV any way I can” Curiosity “Interested in the results of this study” Hope for post-treatment control “In the hope that I may be one of the very few that doesn't need to continue taking medication” |
Referred by primary care doctor “My primary doctor encouraged me to participate because she considered I was a very good candidate” Curiosity “Curiosity” Quality of life “Quality of life, general knowledge and potential for a cure” Financial reasons “Financial” |
| What is the primary purpose of the study? | ||
| To study the effects of HIV treatment interruption and/or time to viral rebound 10 responses pre-IMAP initiation group 5 responses post-IMAP initiation group |
“[T]o understand the effects of going off HIV meds (how quickly does infection rebound in the body)” “[S]ee the reaction of the body after being on med so lon[g] and how my body holds up w out them” “The primary purpose is about seeing how long the HIV lives in the body before your viral load goes down” “To try to find out how fast the virus comes back after stopping the medication” “[T]o see how my body reacts when off meds” “[T]o determine if my viral load will reappear after removing my medication” “[T]o determine the timeline of viral replication” “[T]o discover what happens when HIV me[d]'s are stopped” “To find out how the body reacts to stopping meds” “To see how long it takes for the virus to being multiplying again after being suppressed” |
“To see the time frame it takes an individual to start replicating (detecting) the virus without meds” “Abstain from taking meds to see the time laps before viral load begins to rise” “To track the effects of temporarily stopping HIV medication” “[T]o study the immune response once therapy was withdrawn; to look at my white blood cells, helpers and all, and see if they were impacted by the return of the virus. [T]o see how fast the virus was reactivated. [T]o track or trend how quickly the virus replicated. [T]o track or trend how quickly [I] was able to suppress again” “To identify and document how individuals with HIV will react once they stop taking their medication (…)” |
| 1 response pre-IMAP initiation group 5 responses post-IMAP initiation group |
“To find markers in the blood that may indicate changes in viral loads that may help find or develop a cure. Ultimately it is to find a cure. Also, it may help identify individuals who stay suppressed after stopping ART” | “[T]o see if you can determine identifiers that can help you understand where the virus hides in the body” “Looking at precursors to increased viral load when I stop taking meds” “To gather blood for future research on if there are markers before VL is detected” “To look at the markers of when viral load increases when a person goes off meds” “To identify biomarkers [to] single viral rebound and to be able to find new ways of enhancing those biomarkers or finding strategies to keep those from showing up” |
| To help find a cure for HIV 1 response pre-IMAP initiation group 2 responses post-IMAP initiation group |
“To identify and develop a cure for HIV” | “To help find a cure to see the difference of people blood types” “Find early cure based on factors (genetics) in my blood” |
| Other purposes 2 responses pre-IMAP initiation group 1 responses post-IMAP initiation group |
To identify individuals who could be post-treatment controllers “Help science find someone who its body can reproduce its own anti HIV virus” To increase knowledge about HIV “To answer many question[s] about how [HIV] develops and hides in the body” |
To identify individuals who could be post-treatment controllers “[T]o help see what natural immunities certain people may have that could help others” |
| Are there any risks to you from participating in this study? | ||
| Viral load increase or detectable HIV status 6 responses pre-IMAP initiation group 2 responses post-IMAP initiation group |
“My viral load can rise” “I will experience a rise in my viral load while off of HIV meds” “[V]iral load rebound” “Viral load increase and potential drug resistance” “Viral load increase and I may feel ill” “[M]ay become detectable” |
“VL could rebound and then have difficulting [sic] supressing” “Viral rebound and getting sick” |
| Decrease in CD4 count 1 response pre-IMAP initiation group 0 response post-IMAP initiation group |
“Afraid of CD4 going down” | |
| Acute retroviral syndrome 0 response pre-IMAP initiation group 3 responses post-IMAP initiation group |
“Could experience [c]eroconversion [sic] like symptoms” “[A]cute retro-viral syndrome; acute illness” “I could have developed “acute retroviral syndome (…)” |
|
| Developing resistance to HIV medications 2 responses pre-IMAP initiation group 2 responses post-IMAP initiation group |
“[M]y HIV could mutate and I have to use/switch to new meds” “The medication I am currently on will not be effective when I restart treatment” |
“Becoming resistant to medication” “[B]ecoming drug resistant” |
| Other perceived risks 4 responses pre-IMAP initiation group 4 responses post-IMAP initiation group |
Risks related to blood draws “[O]nly with getting my blood drawn” Minimal risks “I would think that stopping my medication even in this controlled atmosphere would still have a minimal risk to my health” Other risks “I might become sick” “[O]pen for infection” |
Transmitting HIV to partners “[B]eing detectable/infecting others” Risks related to blood draws “Blood draws and more nothing serious” Unknown risks “Unknown, a study is never 100% guareenteed [sic]” [Death] “Death” |
| Are there any direct benefits to you from participating in this study? | ||
| Societal benefits of advancing scientific knowledge 3 responses pre-IMAP initiation group 2 responses post-IMAP initiation group |
“Knowledge gained that might potentially result in a cure” “Th[e] potential of a cure” “Finding a cure” |
“Knowledge” “[P]ossible cures (longterm) [sic]” |
| Altruistic benefits 1 response pre-IMAP initiation group 0 response post-IMAP initiation group |
“[A]ltruism” | N/A |
| Increased information about body's reaction to pause of HIV medications 1 response pre-IMAP initiation group 4 responses post-IMAP initiation group |
“[M]ore information on my body's reaction to the meds I am taking” | “Knowing more abuot my body and reactions to meds/not taking meds” “I will become informed about my viral load” “[O]ther than answering a curiosity fact on how [I] will respond to the withdraw and reapplication of meds, no. [T]here is no direct benefit” “For my personal knowledge about the HIV infection and virus behavior” |
| Mixed benefits—including financial compensation 3 responses pre-IMAP initiation group 3 responses post-IMAP initiation group |
“The benefits are finding out what's working to helping people with HIV. Also getting money for doing the study i[s] a good thing” “You keep giving me money and hopefully being able to stay off of any medication” “Monetary and emotional” |
“Knowing that I am heloping mankind, and small financial gain” “Financially and help finding the cure” “Financial, frequent labs & check ups” |
Overall, 75% of participants (24/32) discussed their study enrollment with a family member, partner, HIV clinician, or someone else. Nearly all (30/32, 94%) were satisfied with the informed consent process for A5345 (Table 2). Free-text responses of reasons for being satisfied with the consent process centered around study staff (n = 4 responses received—e.g., “[T]he team is great about going through each of the study criteria and expectations”), thoroughness of information provided (n = 3 responses received—e.g., “The informed consent process was very thorough”), type of information provided (n = 3 responses received—e.g., “The material provided a great understanding of the process and what to expect going forward”), and ease of understanding (n = 3 responses received—e.g., “It was straightforward and well explained”).
Table 2.
Study Expectations and Previous HIV Cure-Related Research Experiences Among AIDS Clinical Trials Group A5345 Cohort A Respondents, by Intensively Monitored Antiretroviral Pause Status, n = 32
| Question Pre-IMAP, n = 16 Post-IMAP, n = 16 | Yes n (%) | No n (%) | Don't know n (%) | Missing n (%) | p valuea |
|---|---|---|---|---|---|
| Are you generally interested in HIV cure research? | |||||
| Pre-IMAP | 16 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
| Post-IMAP | 16 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Have you previously participated in an HIV treatment study? | |||||
| Pre-IMAP | 8 (50%) | 6 (38%) | 2 (13%) | 0 (0%) | .882 |
| Post-IMAP | 6 (38%) | 8 (50%) | 2 (13%) | 0 (0%) | |
| Have you previously participated in an HIV cure study? | |||||
| Pre-IMAP | 5 (31%) | 9 (56%) | 2 (13%) | 0 (0%) | .272 |
| Post-IMAP | 10 (63%) | 5 (31%) | 1 (6%) | 0 (0%) | |
| Have you previously participated in a study that involved pausing your anti-HIV medications? | |||||
| Pre-IMAP | 1 (6%) | 15 (94%) | 0 (0%) | 0 (0%) | 1.000 |
| Post-IMAP | 1 (6%) | 15 (94%) | 0 (0%) | 0 (0%) | |
| Were you satisfied with the informed consent process? | |||||
| Pre-IMAP | 15 (94%) | 0 (0%) | 0 (0%) | 1 (6%) | 1.000 |
| Post-IMAP | 15 (94%) | 1 (6%) | 0 (0%) | 0 (0%) | |
| Are there any risks to you from participating in this study? | |||||
| Pre-IMAP | 13 (81%) | 2 (13%) | 1 (6%) | 0 (0%) | 1.000 |
| Post-IMAP | 13 (81%) | 3 (19%) | 0 (0%) | 0 (0%) | |
| Do you have any concerns about HIV inside your body increasing if you stop your anti-HIV medications? | |||||
| Pre-IMAP | 4 (25%) | 11 (69%) | 1 (6%) | 0 (0%) | .357 |
| Post-IMAP | 8 (50%) | 7 (44%) | 1 (6%) | 0 (0%) | |
| Are there any direct benefits to you from participating in this study? | |||||
| Pre-IMAP | 8 (50%) | 6 (38%) | 2 (13%) | 0 (0%) | .408 |
| Post-IMAP | 11 (69%) | 5 (31%) | 0 (0%) | 0 (0%) | |
| Will you receive a health benefit from being in this study? | |||||
| Pre-IMAP | 6 (38%) | 6 (38%) | 4 (25%) | 0 (0%) | .035 |
| Post-IMAP | 1 (6%) | 13 (81%) | 2 (13%) | 0 (0%) | |
Pre- and post-IMAP groups are independent samples.
Fisher's exact test p-value (missing excluded).
IMAP, intensively monitored antiretroviral pause; N/A, not applicable.
Understanding of the A5345 study
Participants were asked to report the primary purpose of A5345 using free text (Fig. 1 and Table 3). Responses focused on the effect of HIV treatment interruption and/or time to viral rebound. In addition, several participants demonstrated a detailed understanding of the study, accurately identifying biomarker prediction of viral load/rebound as the purpose: “To identify biomarkers [to] single viral rebound and to be able to find new ways of enhancing those biomarkers or finding strategies to keep those from showing up” and “[T]o see if you can determine identifiers that can help you understand where the virus hides in the body.”
Comprehension of the study was also assessed through a series of true or false items (Supplementary Fig. S1). All 32 participants correctly understood that one of the main goals of the study was to “develop knowledge that may be used one day to develop new therapies to cure or control HIV.” All but one participant understood that pausing antiretroviral medication might result in an increase in viral load. In a series of 9 true or false questions about the study's purpose, 56% of pre-IMAP and 50% of post-IMAP initiation respondents got either 8 or all 9 correct (Supplementary Fig. S1). In response to the statement “One of the main goals of this study is to benefit my personal health,” 5/16 pre-IMAP initiation respondents (31%) and 7/16 post-IMAP initiation respondents (44%) believed this to be true (p value = .7).
Perceived benefits and expectations from study participation
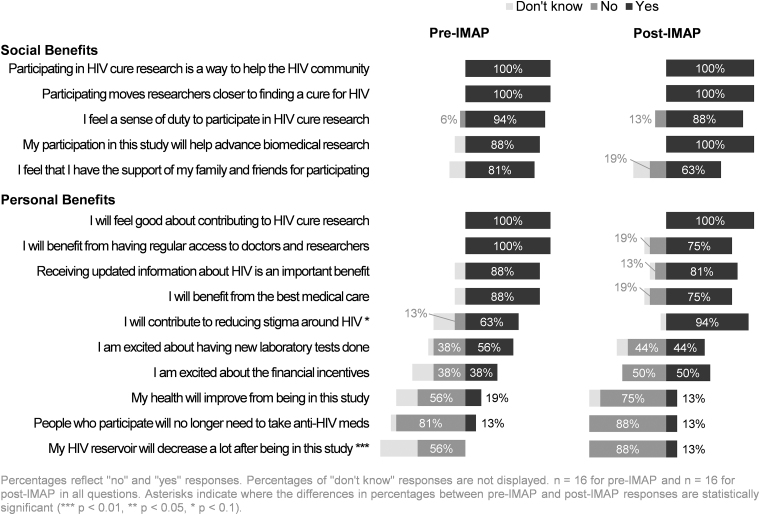
When participants were asked if there were any direct benefits from participating in A5345 (“Are there any direct benefits to you from participating in this study?”), 8 pre-IMAP initiation respondents (50%) and 11 post-IMAP respondents (69%) responded yes (p value = .4) (Table 2). Among those who indicated that there were direct benefits to them and elaborated in the open-ended field, the responses could be grouped into three main categories: (1) benefits of advancing scientific knowledge/altruistic benefits (6 responses received); (2) financial compensation (6 responses received); and (3) increased information about one's own body response to the IMAP (5 responses received) (Table 3). Participants were asked to indicate which potential benefits they perceived as societal benefits of participation (Fig. 2). All 32 participants perceived “a way to help the HIV community” and “moving researchers closer to finding an HIV cure” as societal benefits. In addition, 14 of 16 pre-IMAP and all 16 post-IMAP initiation participants identified “helping advance biomedical research” as a societal benefit (88% vs. 100%, p value = .5) (Fig. 2).
FIG. 2.
Acknowledgment of potential personal and social benefits among ACTG A5345 Cohort A respondents, stratified by IMAP status (n = 32). IMAP, intensively monitored antiretroviral pause.
Participant expectations of the clinical research team during the IMAP were categorized into three main themes: (1) adequate information and communication received (5 responses received); (2) honesty, compassion, and other positive personality traits (5 responses received); and (3) expertise and professionalism (4 responses received). When asked what participants needed from the research team to feel supported to participate in the IMAP, participant responses related to flexibility around scheduling (7 responses received), transparency of information and communication (7 responses received), and moral support (6 responses received). Five respondents indicated that they did not need anything in particular.
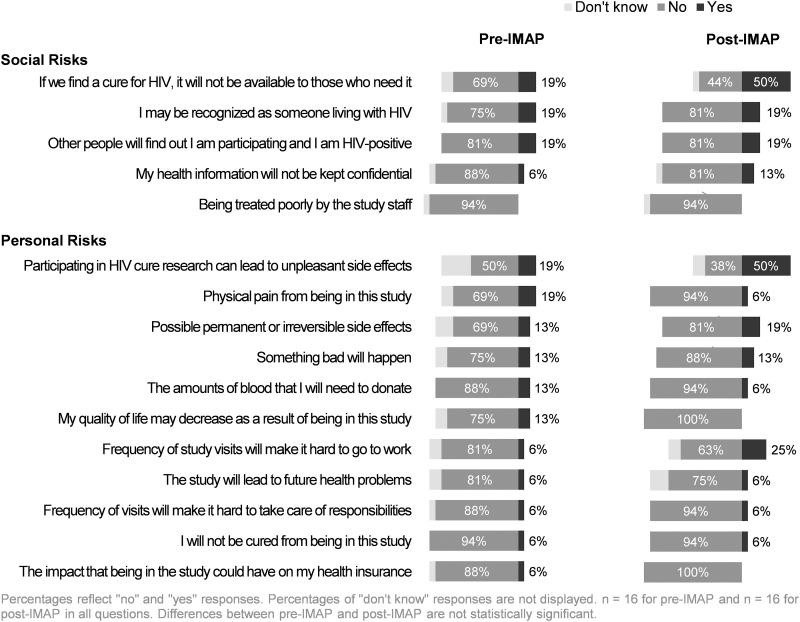
Perceived risks and concerns
Participants were asked to answer questions around perceived risks of A5345. Among the respondents, 13/16 pre-IMAP initiation participants (81%) and 13/16 post-IMAP initiation participants (81%) reported that there were risks to participation (Table 2). When provided with a structured list of potential personal and social risks of participation (Fig. 3), the two most prevalent perceived personal risks were unpleasant side effects (3/16, 19%) and physical pain from being in the study (3/16, 19%) for pre-IMAP initiation respondents. For post-IMAP initiation participants, perceived risks included unpleasant side effects (8/16, 50%) and difficulty going to work because of the frequency of study visits (4/16, 25%) (Fig. 3). The three most prevalent perceived social risks were similar for pre-IMAP and post-IMAP initiation groups: (1) if an HIV cure was found, it will not be available to those who need it (reported by 19% of pre-IMAP respondents vs. 50% of post-IMAP respondents); (2) other people finding out about the individual's study participation and their HIV status (19% of each IMAP group); and (3) being recognized as someone living with HIV (19% of each IMAP group).
FIG. 3.
Acknowledgment of potential personal and social risks among ACTG A5345 Cohort A respondents, stratified by IMAP status (n = 32).
Participants reported IMAP-specific concerns as well. For example, when asked if they had any concerns about HIV increasing inside their body if they stopped their anti-HIV medications, 4 of 16 pre-IMAP and 8 of 16 post-IMAP initiation respondents said “yes” (25% vs. 50%, p value = .4) (Table 2). When provided with a list of IMAP-specific concerns and asked to respond “yes” or “no” to whether the concern was a potential risk of the IMAP, the three most common concerns among both pre- and post-IMAP initiation respondents were: (1) CD4+ cell count decreasing (94% of pre-IMAP, 100% of post-IMAP; p value >.9); (2) becoming detectable for HIV (63% of pre-IMAP, 81% of post-IMAP; p value = .2); and (3) developing acute retroviral syndrome (63% of pre-IMAP, 81% of post-IMAP; p value = .5) (data not shown). Similarly, among respondents who reported risks associated with participation and who completed the free-text responses, the most frequently reported concern was the potential for an increasing and detectable viral load (Table 3). Other concerns noted were the possibility of CD4+ cell count decreasing, developing acute retroviral syndrome, or resistance to anti-HIV medications (Table 3).
Discussion
Our study used convergent closed-ended and open-ended questions to provide valuable insights relating to perceived risks and benefits of study participation, motivations to participate, and comprehension of the clinical trial protocol among PLWH entering an IMAP study. A variety of motivations for participation were identified, and we found that the majority of participants perceived both benefits and risks associated with study participation, regardless of IMAP initiation status at time of questionnaire completion. Overall, participants understood the purpose of the A5345 study and its ultimate goal of advancing research toward an HIV cure. This work extends previous sociobehavioral analyses of participant experiences in HIV cure-related trials in the United States to the setting in which the trial's sole intervention is an IMAP.
Several participants perceived personal benefits resulting from A5345 study participation. This finding is consistent with a previous study of U.S. women participating in an HIV cure-related trial, which found that over half believed the study would benefit them.19 However, the A5345 informed consent template explicitly states: “no direct benefits should be expected from participating in this study.” Our results indicate that participants may view certain broader aspects of study participation as benefits, even if informed consent processes state otherwise.28 Specifically, the majority of A5345 participants perceived that receiving updated information about HIV, having regular access to doctors and researchers, and the opportunity to learn about the body's response to the IMAP were beneficial (Fig. 2). These benefits may be categorized as indirect or collateral benefits.29,30
Participants reported diverse perceived personal and societal-level benefits that overlapped with their motivations for participation. The opportunities to contribute to science and participate in furthering HIV research toward a cure were the most prevalent motivators. Altruism was cited as a source of motivation and has previously been identified as a motivator in analyses of social science data collected from HIV cure-related trial participants (or potential participants).19–21,31–34 Specifically, previous sociobehavioral sciences research conducted in Europe and the United States have identified scientific altruism35–38—akin to activism39—as well as self-confidence39 being a PLWH as strong motivators to being willing to participate in these types of studies.
Some participants also considered monetary compensation as a benefit of study participation. This is ethically relevant because, even though participants may view compensation as a benefit, reimbursements are not formally recognized by many IRBs as a benefit.19,40–42 A similar finding was noted in the ACTG A5366 HIV cure-related trial43 where at least one third of participants viewed compensation as a benefit. For some people living with HIV, compensation may help overcome barriers to research participation, particularly for intensive studies requiring frequent visits. However, Arnold and colleagues found that theoretical willingness to participate in an HIV treatment interruption was not associated with the importance of compensation to participate in research.35
Interestingly, learning about the body's response to the IMAP was a frequently reported motivator and perceived benefit. Similarly, Henderson et al. identified curiosity about the immune system's response to a pause in ART as a motivator among acutely-diagnosed PLWH who were recruited to participate in an ATI study in Thailand.20 Anecdotally, one A5345 participant was going to stop ART on his own, but enrolled in the study because he viewed participation as an opportunity to safely undergo a treatment interruption due to the additional safety monitoring of the IMAP. One could see how this participant would perceive a direct benefit of being in A5345. One may suspect that satisfaction of curiosity—including seeing how quickly HIV can rebound following ART discontinuation—could also improve ART adherence in the long run. Researchers should be aware that participants may be curious about their own body's response to IMAPs and other HIV cure-related interventions. Our results underscore the importance of considering perceived benefits of participation (both physical and psychological) from the participant's perspective.
One quarter of post-IMAP initiation respondents (n = 4) were concerned that the twice-weekly study visit frequency could make going to work difficult. This concern highlights the difficulty of recruiting PLWH who are otherwise healthy and often have work commitments or other obligations.43 Frequent monitoring of participants is essential for safely implementing IMAPs, and flexibility in scheduling visits may help offset the burden of frequent visits. This finding is consistent with Protière and colleagues' study which found that the perceived burden of participation remains an important factor in decisions to participate.38 This result also suggests that future home-based viral load testing methods may be desirable in the setting of HIV cure-related research.2,44
The majority of participants correctly perceived general and IMAP-specific risks. However, nearly one fifth of all respondents (n = 6) reported no risks to participation—a proportion similar to that found among women in the A5366 HIV cure-related trial.19 Most participants perceived risks pertaining to the HIV treatment interruption (e.g., viral load increase, CD4+ cell count decline, acute retroviral syndrome).45 Some participants also mentioned physical pain as a perceived side effect of the study, which is interesting for a study that did not include an experimental intervention. HIV treatment interruption studies also carry risks to sexual partners of study participants.5,7,46 Participants were counseled on the possible risk of HIV transmission to sexual partners during the IMAP during the informed consent process. The fear of transmitting HIV to sexual partners during the brief IMAP did not emerge as a prominent concern in our study; however, this may represent a significant worry for participants in future extended ATI studies.46 Furthermore, our results indicate that key messages pertaining to risks may need to be simplified and reiterated periodically throughout study follow-up. The message may also need to be adapted contingent upon the IMAP phase.
This study has limitations. First, due to timing of the sociobehavioral questionnaire being added to the study, some participants had already initiated the IMAP when the first questionnaire was administered. To help ensure successful and complete data collection in future studies, sociobehavioral measures should be included in the early design phase of clinical trials. Second, the sample was small and necessarily stratified by IMAP status; thus, statistical power is limited. We did not adjust for multiple testing due to the exploratory nature of the analysis. Third, while our questionnaire potentially captured more nuance than a solely quantitative, closed-ended survey approach, we may still have missed important heterogeneity in participants' perceptions and experiences. The language used in the survey may have left some responses open to interpretation. Since we needed to be parsimonious with our data collection and limit the length of the questionnaire, we did not obtain socio-professional data from the participants (e.g., financial situation, professional activity, and so on). Importantly, our sample was primarily composed of White men. It is unclear if our findings would generalize to other populations of PLWH entering an IMAP study in the United States, in particular Black women and Latinx populations.47 Our observation that roughly one-third of A5345 participants who did not take the baseline questionnaire were Hispanic underscores the importance of making social science questionnaires available in both English and Spanish in the U.S. context. Finally, we did not survey individuals who declined participation in A5345. Future studies should assess perceived risks and benefits of PLWH who decline participation in IMAP-containing clinical trials.
A5345 participants perceived both societal and personal health benefits of study participation. While the majority of survey respondents perceived risks to being in the study, nearly one in five participants did not. Future IMAP studies should explore the use of clarified and/or periodic informed consent at critical time points, such as immediately before treatment interruption. Moving forward, it will also be important for multidisciplinary research teams to consider participant perceptions of risks and benefits—both physical and psychological—in greater detail.
Supplementary Material
Acknowledgments
Their study team is indebted to all A5345 participants who volunteered their time to participate in the A5345 biomarker study, as well as complete the social science questionnaires. The authors thank the A5345 study team, the ACTG data management center, the ACTG Clinical Research Sites—in particular, A5345 Study Coordinators, and Social & Scientific Systems, a DLH Company, for their assistance with the study. The authors are most grateful to the ACTG Cure Transformative Science Group (TSG), the ACTG Community Scientific Sub-Committee, and the ACTG leadership for supporting this study. The authors thank Dr. David A. Wohl for early guidance with this research. The authors thank Shadi Eskaf for assistance with the data analysis and visualization. The authors thank Kelly E. Perry for assistance with Figure 1 (analytic coding tree). The authors are grateful to Jo Gerrard of the University of California, Riverside School of Medicine, who provided editorial assistance for this article.
Author Disclosure Statement
Jeremy Sugarman is a member of Merck KGaA's Bioethics Advisory Panel and Stem Cell Research Oversight Committee; a member of IQVIA's Ethics Advisory Panel; a member of Aspen Neurosciences Scientific Advisory Board; a consultant to Biogen; and a consultant to Portola Pharmaceuticals, Inc. None of these activities is related to the material discussed in this article. No other authors have outside interests to declare.
Funding Information
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers: UM1 AI068634, UM1 AI068636, UM1 AI069412, and UM1 AI106701. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The ACTG A5345 study corresponds to NCT03001128.
This work was supported by a grant from the National Institute of Mental Health (NIMH) to K.D. (R21MH118120). K.D. is also grateful for support received from the amfAR Institute for HIV Cure Research (amfAR 109301), UM1AI126620 (BEAT-HIV Collaboratory) cofunded by NIAID, NIMH, NINDS and NIDA, and AI131385 (P01 Smith–Revealing Reservoirs during Rebound (R3)–Last Gift). K.R.M. was supported by P30AI050410 (UNC Center for AIDS Research). J.M.S. was supported by P30 AI027757 (University of Washington/Fred Hutch Center for AIDS Research).
Supplementary Material
References
- 1. Fauci AS, Folkers GK, Dieffenbach CW: HIV-AIDS: Much accomplished, much to do. Nat Immunol 2013;14:1104–1107 [DOI] [PubMed] [Google Scholar]
- 2. Julg B, Dee L, Ananworanich J, et al. : Recommendations for analytical antiretroviral treatment interruptions in HIV research trials—report of a consensus meeting. Lancet HIV 2019;6:e259–e268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deeks SG, Lewin SR, Ross AL, et al. : Towards an HIV Cure: A global scientific strategy. Nat Rev Immunol 2012;12:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dubé K, Henderson GE, Margolis DM: Framing expectations in early HIV cure research. Trends Microbiol 2014;22:547–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Margolis DM, Deeks SG: How unavoidable are analytical treatment interruptions in HIV cure–related studies? J Infect Dis 2019;220:S24-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lau JSY, Smith MZ, Lewin SR, McMahon JH: Clinical Trials of Antiretroviral Treatment Interruption in HIV-Infected individuals. AIDS 2019;33:773–791 [DOI] [PubMed] [Google Scholar]
- 7. Lelièvre JD, Hocqueloux L: Unintended HIV-1 transmission to a sex partner in a study of a therapeutic vaccine candidate. J Infect Dis 2019;220(Suppl 1):S5–S6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Scheerder M.-A, Van Hecke C, Zetterberg H, et al. : Evaluating Predictive Markers for Viral Rebound and Safety Assessment in Blood and Lumbar Fluid during HIV-1 Treatment Interruption. J Antimicrob Chemother 2020;75:1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pannus P, Rutsaert S, De Wit S, et al. : Rapid viral rebound after analytical treatment interruption in patients with very small HIV reservoir and minimal on-going viral transcription. J Int AIDS Soc 2020;23:e25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clarridge KE, Blazkova J, Einkauf K, et al. : Effect of Analytical Treatment Tnterruption and Reinitiation of Antiretroviral Therapy on HIV Reservoirs and Immunologic Parameters in Infected Individuals. PLoS Pathog 2018;14:e1006792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stecher M, Claßen A, Klein F, et al. : Systematic review and meta-analysis of treatment interruptions in human immunodeficiency virus (HIV) Type 1-infected Patients Receiving Antiretroviral Therapy: Implications for Future HIV Cure Trials. Clin Infect Dis 2020;70:1406–1417 [DOI] [PubMed] [Google Scholar]
- 12. Hardy DW: Analytical Treatment Interruptions and Human Immunodeficiency Virus Cure Research: Seizing the opportunity while maintaining safety and respect. Clin Infect Dis 2019;70:1418–1420 [DOI] [PubMed] [Google Scholar]
- 13. Garner SA, Rennie S, Ananworanich J, et al. : Interrupting Antiretroviral Treatment in HIV Cure Research: Scientific and ethical considerations. J. Virus Erad 2017;3:82–84 [PMC free article] [PubMed] [Google Scholar]
- 14. Eyal N, Holtzman LG, Deeks SG: Ethical Issues in HIV Remission Trials. Curr Opin HIV AIDS 2018;13:422–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dubé K, Barr L, Palm D, Brown B, Taylor J: Putting participants at the centre of HIV cure research. Lancet HIV 2019;6:e147–e149 [DOI] [PubMed] [Google Scholar]
- 16. Dubé K, Auerbach JD, Stirratt MJ, Gaist P: Applying the behavioural and social sciences research (BSSR) functional framework to HIV cure research. J Int AIDS Soc 2019;22:e25404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henderson GE.Peay H. L., Kroon E, et al. : Ethics of Treatment Interruption Trials in HIV Cure Research: Addressing the conundrum of risk/benefit assessment. J Med Ethics 2018;44:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grossman CI, Ross AL, Auerbach JD, et al. : Towards multidisciplinary HIV-cure research: Integrating social science with biomedical research. Trends Microbiol 2016;24:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dubé K, Hosey L, Starr K, et al. : Participant Perspectives in an HIV Cure-Related Trial Conducted Exclusively in Women in the United States: Results from AIDS Clinical Trials Group (ACTG) 5366. AIDS Res Hum Retroviruses 2020;36:268–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henderson GE, Waltz M, Meagher M, et al. : Going Off Antiretroviral Treatment in a Closely Monitored HIV ‘Cure’ Trial: Longitudinal assessments of acutely diagnosed trial participants and decliners. J Int 2019;22:e25260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gilbertson A, Kelly EP, Rennie R, et al. : Indirect benefits in HIV cure clinical research: A qualitative analysis. AIDS Res Hum Retroviruses 2019;35:100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tran BX, Ohinmaa A, Nguyen LT: Quality of Life Profile and Psychometric Properties of the EQ-5D-5L in HIV/AIDS Patients. Health Qual Life Outcomes 2012;10:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. EQ-5D User Guides – EQ-5D. Available at: https://euroqol.org/publications/user-guides/
- 24. Spielberger CD: State-Trait Anxiety Inventory for Adults - Manual, Instrument and Scoring Guide. Consulting Psychologists Press, Inc., 1983 [Google Scholar]
- 25. Knight RG, Waal Manning HJ, Spears GF: Some norms and reliability data for the State-Trait Anxiety Inventory and the Zung Self-Rating Depression scale. Br J Clin Psychol 1983;22(Pt 4):245–249 [DOI] [PubMed] [Google Scholar]
- 26. Julian LJ: Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res 2011;63(Suppl 11):S467–S472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hsieh HF, Shannon SE: Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–1288 [DOI] [PubMed] [Google Scholar]
- 28. Henderson GE: The Ethics of HIV ‘Cure’ Research: What can we learn from consent forms? AIDS Res Hum Retroviruses 2015;31:56–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rennie S, Day S, Mathews A, et al. : The Role of Inclusion Benefits in Ethics Committee Assessment of Research Studies. Ethics Hum Res 2019;41:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lantos J: The “Inclusion Benefit” in clinical trials. J Pediatr 1999;130–131 [DOI] [PubMed] [Google Scholar]
- 31. Balfour L, Corace K, Tasca GA, et al. : Altruism Motivates Participation in a Therapeutic HIV Vaccine Trial (CTN 173). AIDS Care 2010;22:1403–1409 [DOI] [PubMed] [Google Scholar]
- 32. Dubé K, Taylor J, Sylla L, et al. : ‘Well, It's the Risk of the Unknown. .. Right?’: A Qualitative Study of Perceived Risks and Benefits of HIV Cure Research in the United States. PLoS One 2017;12:1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Protière C, Fiorentino M, Sow A, et al. : Who are the PLWH who might refuse to participate in HIV cure-related clinical trials with treatment interruption? AIDS 2020;34:1095–1099 [DOI] [PubMed] [Google Scholar]
- 34. Saberi P, Eskaf S, Sauceda JA, Dubé K: Perceptions of HIV Virologic Control Strategies among Younger and Older Age Groups of People Living with HIV in the United States: A Cross-Sectional Survey. AIDS Res Hum Retroviruses 2020;36:606–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arnold M, Evans D, Vergel N: Recruitment and ethical considerations in HIV cure trials requiring treatment interruption. J Virus Erad 2015;1:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Protière C, Spire B, Mora M, et al. : Patterns of Patient and Healthcare Provider Viewpoints Regarding Participation in HIV Cure-Related Clinical Trials. Findings from a Multicentre French Survey Using Q Methodology (ANRS-ASPECT). PLoS One 2017;12:e0187489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Préau M, Doumergue M, Protière C, et al. : Acceptability of HIV cure-related trials: The challenges for physicians and people living with HIV. AIDS Care 2018;30:914–920 [DOI] [PubMed] [Google Scholar]
- 38. Protière C, Arnold M, Fiorentino M, et al. : Differences in HIV cure clinical trial preferences of french people living with HIV and physicians in the ANRS-APSEC Study: A Discrete Choice Experiment. J Int AIDS Soc 2020;23:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fiorentino M, Protière C, Sagaon-Teyssier L, et al. : What is the Effect of Self-Identified HIV Activism in Willingness to Participate in HIV Cure-Related Clinical Trials? Results from the ANRS-APSEC Study. J Virus Erad 2019;5:152–162 [PMC free article] [PubMed] [Google Scholar]
- 40. Brown B, Galea JT, Dubé K, et al. : The need to track payment incentives to participate in HIV research. IRB 2018;40:8–12 [PubMed] [Google Scholar]
- 41. Brown B, Galea JT, Davidson P, Khoshnood K: Transparency of Participant Incentives in HIV Research. Lancet HIV 2016;3:e456–e457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gelinas L, Largent E, Cohen G, et al. : A Framework for Ethical Payment to Research Participants. N Engl J Med 2018;378:766–771 [DOI] [PubMed] [Google Scholar]
- 43. Dubé K, Dee L, Evans D, et al. : Perceptions of Equipoise, Risk–Benefit Ratios, and “Otherwise Healthy Volunteers” in the Context of Early-Phase HIV Cure Research in the United States: A Qualitative Inquiry. J Empir Res Hum Res Ethics 2018;13:3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dubé K, Evans D, Dee L, et al. : ‘We Need to Deploy Them Very Thoughtfully and Carefully’: Perceptions of analytical treatment interruptions in HIV cure. AIDS Res Hum Retroviruses 2018;34:67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wen Y, Bar KJ, Li JZ: Lessons Learned from HIV Antiretroviral Treatment Interruption Trials. Curr Opin HIV AIDS 2018;13:416–421 [DOI] [PubMed] [Google Scholar]
- 46. Peluso MJ, Dee L, Campbell D, et al. : A collaborative, multidisciplinary approach to HIV transmission risk mitigation during analytic treatment interruption. J Virus Erad 2020;6:34–37 [PMC free article] [PubMed] [Google Scholar]
- 47. Lesko CR, Buchanan AL, Westreich D, et al. : Generalizing Study Results: A potential outcomes perspective. Epidemiology 2017;28:553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.