Abstract
Background
In order for healthcare systems to prepare for future waves of COVID-19, an in-depth understanding of clinical predictors is essential for efficient triage of hospitalized patients.
Methods
We performed a retrospective cohort study of 259 patients admitted to our hospitals in Rhode Island to examine differences in baseline characteristics (demographics and comorbidities) as well as presenting symptoms, signs, labs, and imaging findings that predicted disease progression and in-hospital mortality.
Results
Patients with severe COVID-19 were more likely to be older (p = 0.02), Black (47.2% vs. 32.0%, p = 0.04), admitted from a nursing facility (33.0% vs. 17.9%, p = 0.006), have diabetes (53.9% vs. 30.4%, p<0.001), or have COPD (15.4% vs. 6.6%, p = 0.02). In multivariate regression, Black race (adjusted odds ratio [aOR] 2.0, 95% confidence interval [CI]: 1.1–3.9) and diabetes (aOR 2.2, 95%CI: 1.3–3.9) were independent predictors of severe disease, while older age (aOR 1.04, 95% CI: 1.01–1.07), admission from a nursing facility (aOR 2.7, 95% CI 1.1–6.7), and hematological co-morbidities predicted mortality (aOR 3.4, 95% CI 1.1–10.0). In the first 24 hours, respiratory symptoms (aOR 7.0, 95% CI: 1.4–34.1), hypoxia (aOR 19.9, 95% CI: 2.6–152.5), and hypotension (aOR 2.7, 95% CI) predicted progression to severe disease, while tachypnea (aOR 8.7, 95% CI: 1.1–71.7) and hypotension (aOR 9.0, 95% CI: 3.1–26.1) were associated with increased in-hospital mortality.
Conclusions
Certain patient characteristics and clinical features can help clinicians with early identification and triage of high-risk patients during subsequent waves of COVID-19.
Introduction
The pandemic due to SARS-CoV-2, a newly described human coronavirus causing the disease known as COVID-19, continues to challenge the U.S. healthcare system. To date, there have been over 30,357,579 cases in the United States (US) and 138,255 cases in Rhode Island (RI), which has one of the highest infection rate per capita (12,910 cases per 100,000) in the country [1, 2]. In the early phase of the pandemic, many studies noted factors associated with severe outcomes [3–5]. Comorbidities like diabetes, obesity, and hypertension were observed to be prevalent in those with severe disease [6, 7]. We aimed to do an in-depth study of such predictors in the state of Rhode Island (RI). Such an understanding is crucial so that healthcare systems can manage the continued influx of patients with COVID-19 [8, 9] by appropriately triaging patients who present to the hospital.
We conducted a retrospective cohort study of persons with COVID-19 who were hospitalized in RI to identify (1) patient demographics and comorbidities associated with severe disease and death, and (2) presenting symptoms and vital signs that predicted progression to severe disease and death.
Methods
Study design and patient selection
We performed a retrospective cohort study of patients hospitalized with COVID-19 at the Lifespan academic hospitals affiliated with Brown University in Providence, RI. Patients of all ages who presented to the hospital with symptoms of COVID-19 and had a positive real time polymerase chain reaction (RT-PCR) result for SARS-CoV-2 were eligible for the study. Patients with asymptomatic infection or those who developed symptoms of COVID-19 after the first 48 hours of hospitalization were excluded.
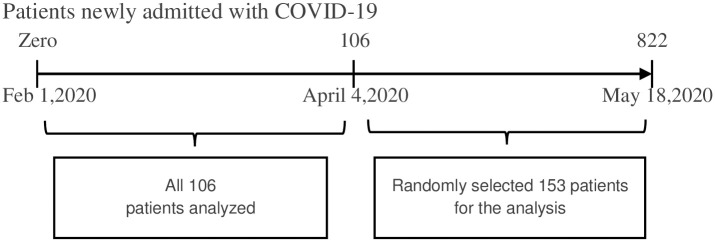
A list of medical record numbers (MRNs) was extracted from the integrated electronic medical record (EMR) for all patients with COVID-19 positive test results hospitalized between February 1, 2020 and May 18, 2020. There were 822 patients admitted during this time period. We included all 106 eligible patients who were hospitalized between February 17 to April 3 and a subset of patients hospitalized between April 4 and May 18, the peak of the pandemic surge in RI. For the latter group, we selected a random sample of 153 from the master patient list (Fig 1). In total, 259 patients, 31.5% of all patients admitted with COVID-19 during the time period, were included in this study. To assess representativeness of our cohort, we compared age, gender, and race among patients selected and not selected for the study. To ensure that we had a representative sample, we compared the weekly case fatality rates between the study sample and all COVID-19 patients admitted to the hospitals during the study period. We did not observe a statistical significance (Wilcoxon test P>0.1) [10].
Fig 1. Participant selection: N = 259.
The Lifespan Academic Medical Center institutional review board approved the study and waived informed consent for participation.
Data collection
Demographic and laboratory data were extracted from the EMR. The following clinical data were collected by manual chart review and entered into a REDCap (Research Electronic Data Capture) database: baseline comorbidities, presenting symptoms and vital signs, microbiology results, imaging results, antimicrobial and other medical treatments (e.g. vasopressors), supplemental oxygen (O2), non-invasive and invasive forms of ventilation, intensive care unit (ICU) admission, renal replacement therapy, prior hospital visits, 15-day follow-up data (including telephone encounters and hospital re-admissions), co-infections, complications, and hospitalization outcome (death or discharge). Study team members were trained to perform chart reviews, and random charts were re-abstracted by the lead author to evaluate interrater agreement and calibrate data collection methods.
Study outcomes
We defined severe COVID-19 as requiring high flow O2 (flow rate of more than 8L/min or use of high flow oxygen cannula), non-invasive ventilation (e.g. BIPAP), or invasive mechanical ventilation at any time point during the hospitalization. We evaluated four combinations of predictors and outcomes. Among all 259 patients, we examined differences in patient demographics and comorbidities in relation to (1) severe disease or (2) death at any time during hospitalization. To examine early clinical predictors of progression to severe disease and death, we excluded patients who met criteria for severe disease or died on the day of admission, and identified presenting symptoms, signs, laboratory results, and imaging findings during the first 24 hours that were associated with (3) developing severe disease or (4) death.
Statistical analysis
Chi-square or Fisher’s exact tests for categorical variables and the Student’s t-test for continuous variables were used to compare demographics, comorbidities, and clinical data between groups. Variables that differed at a significance level of <0.30 were included in stepwise multivariable logistic regression analyses. Variables with a significance level of <0.35 were maintained in the model during stepwise selection. Estimated correlation matrix was used to check multicollinearity between clinically related variables. In multivariate regression, we excluded cases with ≤5 missing values for a given covariate and used the missing indicator method for variables with >5 missing observations. SAS 9.4 (SAS Institute, Cary, U.S.A.) and R 3.6.3 (R Computing, Vienna, Austria) were used for statistical analyses.
Results
The 259 patients included in our cohort did not differ from the 563 patients not selected for the study with respect to age, gender, and race [Supplementary Materials]. Among the participants, median age was 62 years [interquartile range (IQR), 51–73]; 138 (53%) were male; 75 (29%) were Hispanic; and 53 (20.5%) Black [Table 1]. Sixty (23%) participants were admitted from a nursing facility; 52/259 (20%) had additional emergency room (ER) visits in the 15 days before hospitalization. The median length of stay for all patients was 8 days [IQR, 5–15]. ICU admission was required in 74/259 (28%); 42/259 (16%) patients required mechanical ventilation with a median time of 7 days [IQR, 3–12] on a ventilator.
Table 1. Demographics.
| n (%) or median [IQR] | ||||
|---|---|---|---|---|
| All patients | Non-severe disease | Severe disease | p-value | |
| n = 259 (%) | n = 168(%) | n = 91(%) | ||
| Age in years | 62[51–73] | 60[49–72] | 65[57–74] | 0.0162* |
| Gender | 0.6930 | |||
| Male | 138(53.3) | 88(52.4) | 50(55.0) | |
| Female | 121(46.7) | 80(47.6) | 41(45.1) | |
| Ethnicity | 0.1246 | |||
| Hispanic / Latinoa | 75 (29.0) | 54 (32.1) | 21 (23.1) | |
| Non-Hispanic/ Latino | 184 (71.0) | 114 (67.9) | 70 (76.9) | |
| Race | ||||
| Black | 53(20.5) | 28(52.8) | 25(47.2) | 0.0396* |
| Non-Black | 206(79.5) | 140(68) | 66(32.0) | |
| Health care worker | 0.5899 | |||
| Yes | 15(5.8) | 11(6.6) | 4(4.4) | |
| No | 178(68.7) | 117(69.6) | 61(67.0) | |
| Unknown | 66(25.5) | 40(23.8) | 26(28.6) | |
| Skilled nursing facility | 0.0059* | |||
| Yes | 60(23.2) | 30(17.9) | 30(33.0) | |
| No | 199(76.8) | 138(82.1) | 61(67.0) | |
a5 with Black race also identified themselves as Hispanic/Latino.
*p-values of <0.05
Of the 259 participants, 91 (35%) had severe COVID-19, and 38 (15%) died at any time during hospitalization. Two hundred twenty-three participants did not have severe disease or die during the first 24 hours of admission. Among this group, 55 (24.7%) progressed to severe disease, and 24 (10.8%) died. Compared to the 168 participants who did not progress to severe disease, the 55 participants who progressed to severe disease had a higher incidence of arrhythmias (35% versus 5%; p<0.001) and death (40% vs. 1.2%, p<0.001). The differences in incidence of thromboembolic events (11% vs. 4%, p = 0.06) did not reach statistical significance and bleeding was rare (7% vs. 5%; p = 0.6).
Follow-up telehealth visits were completed in 124/221 discharged patients within a median time of 2 days [IQR, 2–4] post-discharge. At the time of follow-up visit, 84/124 (67%) still reported symptoms and 15/124 (12%) still required oxygen. Among all patients who survived, 16/221(7.2%) patients who were discharged required re-admission within 15 days.
We did not find any significant difference in distribution of black patients among remdesivir versus non remdesivir treatment groups (15.9 vs 17.4%, p-value 0.78) in our study.
Baseline characteristics associated with severe COVID-19 at any time during hospitalization
Compared to patients with non-severe COVID-19, those with severe COVID-19 were older (65 [IQR 57–74] vs. 60 [IQR 49–72] years, p = 0.02) and more likely to be Black (47.2% vs. 32.0%, p = 0.04), admitted from a nursing facility (33.0% vs. 17.9%, p = 0.006), have diabetes (53.9% vs. 30.4%, p<0.001), or COPD (15.4% vs. 6.6%, p = 0.02) [Table 2]. No significant differences were noted with respect to gender, blood type, or current active immunosuppressive medication. In multivariate regression, only Black race (adjusted odds ratio [aOR] 2.0, 95% confidence interval [CI]: 1.1–3.9) and diabetes (aOR 2.2, 95% CI: 1.3–3.9) were found to be independent predictors of severe disease [Table 3].
Table 2. Medical comorbidities.
| n (%) or median [IQR] | ||||
|---|---|---|---|---|
| All patients | Non-severe disease | Severe disease | p-value | |
| n = 259(%) | n = 168(%) | n = 91(%) | ||
| Smoking history | 93(35.9) | 59(35.1) | 34(37.4) | 0.7194 |
| Obesitya | 114(44) | 67(39.9) | 47(51.7) | 0.0686 |
| Hypertension | 164(63.3) | 101(60.1) | 63(69.2) | 0.1463 |
| Diabetes mellitus | 100(38.6) | 51(30.4) | 49(53.9) | 0.0002* |
| Pre-diabetes | 38(14.7) | 27(16.1) | 11(12.1) | 0.3871 |
| Hyperlipidemia | 134(51.7) | 85(50.6) | 49(53.9) | 0.6172 |
| Coronary artery disease | 38(14.7) | 27(16.1) | 11(12.1) | 0.3871 |
| Cerebrovascular disease | 22(8.5) | 13(7.7) | 9(9.9) | 0.5532 |
| Peripheral vascular disease | 10(3.9) | 5(3.0) | 5(5.5) | 0.3153 |
| COPDb | 25(9.7) | 11(6.6) | 14(15.4) | 0.0215* |
| Asthma | 30(11.6) | 20(12.0) | 10(11.0) | 0.8260 |
| Chronic kidney disease | 45(17.4) | 26(15.5) | 19(20.9) | 0.2733 |
| Congestive heart failure | 37(14.3) | 20(11.9) | 17(18.7) | 0.1368 |
| Chronic liver disease | 16(6.2) | 11(6.6) | 5(5.5) | 0.7368 |
| Neurological diseases | 53(20.5) | 30(17.9) | 23(25.3) | 0.1578 |
| Autoimmune disease | 10(3.9) | 8(4.8) | 2(2.2) | 0.3065 |
| Organ transplant | 10(3.9) | 6(3.6) | 4(4.4) | 0.7424 |
| HIV | 5(1.9) | 1(0.60) | 4(4.4) | 0.0792 |
| Malignancy | 29(11.2) | 18(10.7) | 11(12.1) | 0.7379 |
| Current immunosuppressive medicationc | 25(9.7) | 13(7.7) | 12(13.2) | 0.1563 |
| Blood groupsd | 0.4566 | |||
| Blood group A | 61(23.6) | 39(23.2) | 22(24.2) | |
| Blood group B | 14(5.4) | 6(3.6) | 8(8.8) | |
| Blood group AB | 6(2.3) | 3(1.8) | 3(3.3) | |
| Blood group O | 73(28.2) | 40(23.8) | 33(36.3) | |
aObesity was defined as Body mass index (BMI)> 30kg/m2; BMI was missing in 11 patients
bCOPD: Chronic Obstructive Pulmonary Disease
cImmunosuppressive medications included steroids, calcineurin inhibitors, mTOR inhibitors, antiproliferative agents, active chemotherapy, immunotherapy
bBlood group was missing in 105 patients
*p value<0.05
Table 3. Factors associated with severe disease.
| Adjusted Odds Ratio | 95% Wald Confidence Limits | p-Value | ||
|---|---|---|---|---|
| Patient age at admission | 1.011 | 0.993 | 1.030 | 0.2253 |
| Black race vs. all others | 2.016 | 1.052 | 3.864 | 0.0346* |
| Hospitalized from SNFa | 1.825 | 0.932 | 3.575 | 0.0795 |
| Obesity | 1.444 | 0.820 | 2.544 | 0.2032 |
| Diabetes mellitus | 2.232 | 1.277 | 3.901 | 0.0048* |
| COPD | 2.069 | 0.847 | 5.051 | 0.1104 |
Abbreviations:
aSNF, skilled nursing facility or rehabilitation
*p-value of <0.05, Multivariate stepwise regression for entire cohort (n=259)
Patient characteristics associated with in-hospital mortality
Each one-year increase in patient age (aOR 1.04, 95% CI: 1.01–1.07) and admission from a skilled nursing facility (aOR 2.7, 95% CI 1.1–6.7) were associated with death during hospitalization. While many comorbidities were more common in the deceased group [Supplementary Materials], underlying hematological disorders (chronic anemia, coagulation disorders, hematological malignancies, and sickle cell disease) were the only comorbidity that predicted mortality in multivariate regression (aOR 3.4, 95% CI 1.1–10.0) [Table 4].
Table 4. Factors associated with in-hospital mortality.
| Adjusted Odds Ratio | 95% Wald Confidence Limits | p-value | ||
|---|---|---|---|---|
| Patient age at admission | 1.035 | 1.005 | 1.067 | 0.0242* |
| Hospitalized from SNF | 2.742 | 1.130 | 6.654 | 0.0258* |
| Hypertension | 1.859 | 0.662 | 5.220 | 0.2392 |
| Diabetes mellitus | 2.291 | 0.962 | 5.455 | 0.0611 |
| Hyperlipidemia | 0.520 | 0.203 | 1.333 | 0.1732 |
| Peripheral Vascular Disease | 3.051 | 0.674 | 13.823 | 0.1478 |
| Asthma | 0.121 | 0.013 | 1.135 | 0.0644 |
| Hematological Disorders | 3.373 | 1.134 | 10.036 | 0.0288* |
*p-value of <0.05
Multivariate stepwise regression for entire cohort (n=259)
Progression to severe disease after the first 24 hours of hospitalization
Among the 223 participants in this analysis, respiratory symptoms (94.6% vs. 83.3%, p = 0.04), tachypnea (85.5% vs. 64.9%, p = 0.003), and hypoxia (96.4% vs. 67.9%, p<0.0001) in the first 24 hours were associated with progressing to severe COVID-19 [Table 5]. For laboratory values, only estimated glomerular filtration rate (eGFR) <60 mL/min (48% versus 31%, p = 0.02), hypoalbuminemia (<3.5g/dl) (38% versus 17%, p = 0.01), and elevated D-dimer (>300ng/ml) (71% versus 44%, p = 0.02) were found to be significantly more common during the first 24 hours of admission among those who progressed to severe disease compared to those who did not [Table 6]. A higher proportion of patients who progressed to severe disease had abnormal findings on chest imaging, including bilateral disease on chest x-ray (44% versus 29%, p = 0.04) or chest CT (27% versus 15%, p = 0.03), and ground glass opacities on chest CT (35% versus 14%; p<0.001); a higher proportion of patients with non-severe disease had normal chest x-rays (23% versus 9%; p = 0.02) [Table 7]. In multivariate logistic regression, progression to severe disease was associated with respiratory symptoms (aOR 7.0, 95% CI: 1.4–34.1), hypoxia (aOR 19.9, 95% CI: 2.6–152.5), and hypotension (aOR 2.7, 95% CI) during first 24 hours [Table 8].
Table 5. Presenting symptoms and signs during the first 24 hours of admission.
| n (%) or median [IQR] | ||||
|---|---|---|---|---|
| All patients | Non-severe disease | Severe disease | p-value | |
| n = 223(%) | n = 168(%) | n = 55(%) | ||
| Days from symptom onset to presentation, median | 5[8–3] | 5[3–8] | 7[2–9] | 0.9277 |
| Subjective | ||||
| Respiratory symptomsa | 192(86.1) | 140(83.3) | 52(94.6) | 0.0370* |
| GI symptomsb | 118(52.9) | 88(52.4) | 30(54.6) | 0.7801 |
| Systemic symptomsc | 178(79.8) | 136(81.0) | 42(76.4) | 0.4617 |
| Chest pain | 52(23.3) | 46(27.4) | 6(10.9) | 0.0015 |
| Objective | ||||
| Feverd | 133(59.6) | 96(57.1) | 37(67.3) | 0.1838 |
| Hypothermiae | 21(9.4) | 15(8.9) | 6(10.9) | 0.6625 |
| Tachycardiaf | 118(52.9) | 84(50.0) | 34(61.8) | 0.1275 |
| Tachypneag | 156(70) | 109(64.9) | 47(85.5) | 0.0039* |
| Hypoxiah | 167(74.9) | 114(67.9) | 53(96.4) | < .0001* |
| Hypotensioni | 27(12.1) | 14(8.3) | 13(23.6) | 0.0025* |
aSymptoms of cough, shortness of breath, chest pain, sore throat, and congestion were grouped as respiratory;
bGI symptoms were nausea, vomiting, diarrhea, abdominal pain;
csystemic symptoms were fever, myalgias, rash, encephalopathy, dizziness.
Abbreviations: bGI, gastrointestinal
dFever was defined as the highest temp of >38C;
ehypothermia as the lowest temp of <36C;
ftachycardia was defined as having a heart rate of >100 beats per minute;
gtachypnea was defined as having a respiratory rate of >20 breaths per minute;
hhypoxia was defined as having an O2 saturation of <95% on room air;
ihypotension was having a systolic blood pressure of <90mm Hg.
*p-values of <0.05
Table 6. Laboratory values during the first 24 hours of admission.
| n (%) or median [IQR] | ||||
|---|---|---|---|---|
| All patients | Non-severe disease | Severe disease | p-value | |
| n = 223 | n = 168(%) | n = 55(%) | ||
| Leukocytosisa | 28(12.6) | 20(12.1) | 8(14.8) | 0.4967 |
| Leucopeniab | 21(9.4) | 14(8.4) | 7(13.0) | |
| Normal WBC | 171(76.7) | 132(79.5) | 39(72.2) | |
| Lymphopeniac | 136(61) | 98(59.4) | 38(70.4) | 0.2991 |
| Thrombocytopeniad | 48(21.5) | 38(22.9) | 10(18.5) | 0.6189 |
| ALT> 45 IU/Le | 40(17.9) | 29(21.2) | 11(24.4) | 0.6451 |
| AST> 42 IU/Le | 53(23.8) | 35(25.6) | 18(40.0) | 0.0641 |
| Elevated BUNf | 57(25.6) | 38(22.9) | 19(35.2) | 0.0733 |
| Elevated creatinineg | 54(24.2) | 36(21.7) | 18(33.3) | 0.0841 |
| eGFR<60h | 76(34.1) | 50(30.5) | 26(48.2) | 0.0182* |
| Hyponatremiai | 97(43.5) | 70(41.7) | 27(49.0) | 0.3350 |
| Hypokalemiaj | 61(27.4) | 50(30.1) | 11(20.4) | 0.3787 |
| Hypocalcemiak | 54(24.2) | 38(22.9) | 16(29.6) | 0.4558 |
| Elevated troponinl | 32(14.3) | 21(16.7) | 11(25) | 0.2234 |
| Hypoalbuminemiam | 40(17.9) | 23(16.8) | 17(37.8) | 0.0116* |
| Elevated CRPn | 112(50.2) | 86(90.5) | 26(100) | 0.1028 |
| Elevated D-dimero | 43(19.3) | 28(43.8) | 15(71.4) | 0.0277* |
| Elevated Ferritinp | 74(33.2) | 51(82.3) | 23(95.8) | 0.1032 |
| Elevated LDHq | 73(32.7) | 54(68.4) | 19(86.4) | 0.0951 |
Abbreviations: heGFR, estimated glomerular filtration rate; gAKI/ CKD, acute kidney injury/ chronic kidney injury; fBUN, blood urea nitrogen
aLeukocytosis is defined as WBC> 11x10exp9/L;
bleucopenia is defined as WBC< 3.5x10exp9/L;
clymphopenia is defined as <1x10exp9/L;
dthrombocytopenia is defined as platelet count below 150x10exp9/L;
felevated BUN defined as >24 mg/dl;
gElevated creatinine defined as serum creatinine>1.27 mg/dl;
ihyponatremia is defined as serum sodium<135mEq/L;
jhypokalemia is defined as serum potassium < 3.5mEq/L;
khypocalcemia is defined as serum calcium< 8.5 mEq/L;
lelevated troponin is defined as troponin of >0.06ng/ml;
mhypoalbuminemia is defined as serum albumin of <3.5 g/dl;
nelevated CRP (C-reactive protein) is defined as >10mg/L;
oelevated D-dimer is defined as >300ng/mL;
pelevated ferritin is defined as >120 ng/ml;
qelevated LDH defined as >220 IU/L
oD-dimer was missing in 138 patients; pferritin was missing in 137 patients; qLDH was missing in 122 patients; nCRP was missing in 102 patients; ltroponin was missing in 53 patients; eALT, eAST, malbumin was missing in 41 patients;
heGFR was missing in 5 patients;
clymphocyte count was missing in 4 patients; WBC count, dplatelet count, ecreatinine, fBUN, kcalcium, and jpotassium was missing in 3 patients
*p-values of <0.05
Table 7. Radiology imaging results during the first 24 hours of admission.
| All patients | Non- severe disease | Severe disease | p-value | |
|---|---|---|---|---|
| n = 223(%) | n = 168(%) | n = 55(%) | ||
| CXRa | 218(97.8) | |||
| Normal | 44(19.7) | 39(23.2) | 5(9.1) | 0.0223* |
| Unilateral abnormalitiesb | 9(4) | 6(3.6) | 3(5.5) | 0.5379 |
| Bilateral abnormalitiesc | 73(32.7) | 49(29.2) | 24(43.6) | 0.0472* |
| Multifocal abnormalitiesd | 58(26) | 40(23.8) | 18(32.7) | 0.1907 |
| Airspace diseasee | 145(65) | 104(61.9) | 41(74.6) | 0.0880 |
| Consolidatione | 8(3.6) | 6(3.6) | 2(3.6) | 0.9821 |
| Ground glasse | 9(4) | 6(3.6) | 3(5.4) | 0.5379 |
| Interstitiale | 30(13.5) | 24(14.3) | 6(10.9) | 0.5241 |
| Nodulare | 0(0) | 0(0) | 0(0) | NAg |
| Peripherale | 14(6.3) | 10(6.0) | 4(7.3) | 0.7261 |
| Pleural effusione | 9(4) | 6(3.6) | 3(5.5) | 0.5379 |
| CT scanf | 70(31.4) | |||
| Normal | 6(2.7) | 5(3.0) | 1(1.8) | 0.6450 |
| Unilateral abnormalitiesb | 0(0) | 0(0.0) | 0(0.0) | NAg |
| Bilateral abnormalitiesc | 40(17.9) | 25(14.9) | 15(27.3) | 0.0376* |
| Multifocal abnormalitiesd | 25(11.2) | 15(8.9) | 10(18.2) | 0.0590 |
| Airspace diseasee | 28(12.6) | 21(12.5) | 7(12.7) | 0.9648 |
| Consolidatione | 6(2.7) | 3(1.8) | 3(5.5) | 0.1444 |
| Ground glasse | 43(19.3) | 24(14.3) | 19(34.6) | 0.0009* |
| Interstitiale | 1(0.4) | 1(0.6) | 0(0) | 0.5663 |
| Nodulare | 5(2.2) | 5(3.0) | 0(0) | 0.1957 |
| Peripherale | 23(10.3) | 17(10.1) | 6(10.9) | 0.8672 |
| Pleural effusione | 8(3.6) | 4(2.4) | 4(7.3) | 0.0904 |
aFor chest x ray, laterality was not reported in 34 patients. Chest x ray was not done in 5 patients.
bUnilateral was the presence of abnormalities in one lung;
cbilateral was presence of abnormalities in both lungs;
dmultifocal was abnormalities in multiple foci in same or both lungs. They are mutually exclusive.
eDescriptive variables, mutually exclusive
fCT scan was only done in 70 patients and laterality with or without multifocality was reported in all of them.
*p-value of <0.05
gAbbreviations: NA, non-applicable.
Table 8. Symptoms, signs, and findings associated with progression to severe disease.
Multivariate stepwise regression for patients who did not have severe disease or die in the first day of hospitalization (n=223).a
| Effect | Odds Ratio | 95% Wald Confidence Limits | P-Values | |
|---|---|---|---|---|
| Respiratory symptoms | 7.008 | 1.442 | 34.070 | 0.0158* |
| Tachypnea | 2.211 | 0.911 | 5.368 | 0.0796 |
| Hypoxia | 19.946 | 2.609 | 152.490 | 0.0039* |
| Hypotension | 2.677 | 1.036 | 6.914 | 0.0420* |
| eGFR<60 | 1.974 | 0.937 | 4.162 | 0.0737 |
| Elevated D-Dimer (Elevated vs Normal) | 0.413 | 0.128 | 1.333 | 0.1392 |
| Elevated D-Dimer (Missing vs Normal) | 1.064 | 0.457 | 2.476 | 0.8861 |
aN = 223, Used in Regression = 218, Severe = 54, Non-Severe = 164; 5 observations were not used because of missing x-ray;
*p-value of <0.05
Progression to death after the first 24 hours of hospitalization
On admission, chest pain, fever, elevated troponin, elevated BUN, elevated creatinine, and eGFR<60 were more common in patients who died during hospitalization, compared to those who were discharged [Supplementary Materials]. In the adjusted model, only the presence of tachypnea (aOR 8.7, 95% CI: 1.1–71.7) or hypotension (aOR 9.0, 95% CI: 3.1–26.1) during the first 24 hours were independently associated with in-hospital mortality [Table 9].
Table 9. Symptoms, signs, and findings associated with in-hospital mortality.
Multivariate stepwise regression for patients who did not have severe disease or die in the first day of hospitalization (n=223).a
| Effect | Adjusted Odds Ratio | 95% Wald Confidence Limits | P-Values | |
|---|---|---|---|---|
| Tachypnea | 8.699 | 1.056 | 71.682 | 0.0444* |
| Hypoxia | 3.353 | 0.409 | 27.468 | 0.2595 |
| Hypotension | 9.022 | 3.118 | 26.102 | < .0001* |
| eGFR<60 | 2.559 | 0.949 | 6.902 | 0.0635 |
aN = 223, Used in Regression = 218, Severe = 54, Non-Severe = 164; 5 observations were not used because of missing x- ray;
*p-value of <0.05
Discussion
In this study, we identified baseline patient characteristics that were associated with severe COVID-19 or death; and presenting signs and symptoms that were associated with progressing to severe COVID-19 or death after the first 24 hours of admission. These findings will help providers on the front lines of the pandemic triage patients and prioritize hospital resources.
Patients who developed severe disease were older, and age was an independent predictor of mortality, similar to findings from other studies [11–14]. We did not observe any significant effects of gender on outcomes. Multivariate regression revealed Black race to be an independent predictor of severe disease. Our findings are aligned with emerging literature on racial and socioeconomic disparities affecting COVID-19 outcomes [15–18]. Almost half of Black persons in our study developed severe disease, but Black race was not independently associated with increased mortality. Rather, advanced age or admission from a nursing home/rehabilitation center was associated with mortality, reflecting that the number and severity of comorbidities is an important driver behind risk of death during hospitalization. This finding underscores the importance of adjusting for age as well as comorbidities when interpreting the impact of race on mortality. Other studies from the U.S. also found that when adjusted for other covariates including age and comorbidities, Black race was not an independent predictor of death [15, 19]. While data from the United Kingdom (UK) [20] has also suggested increased severity of COVID-19 among those with Black race, provisional nationwide analysis from the UK points towards increased mortality even after adjusting for age and comorbidities [21]. Additional research is needed to determine the extent of racial disparities among persons who die from COVID-19.
Comorbidities like diabetes, hypertension, obesity, etc. have been associated with poor COVID-19 outcomes [13, 22–24]. In our study, comorbidities were also found to be associated with severe disease. Notably, while the prevalence of diabetes, obesity, and COPD was higher in those with severe disease, on adjusted regression analysis only diabetes was found to be an independent predictor of severe disease. Diabetes creates a hyperinflammatory state and impairs innate and cell-mediated immunity, which may predispose patients to the cytokine storm known to occur in severe COVID-19 [25, 26]. Furthermore, increased release of cytokines like interleukin-6 in patients with diabetes and COVID-19, in the face of possible blunted antiviral interferon responses and the delayed activation of Th1/Th17, may contribute to worse outcomes [27–29]. However, causality remains to be proven and severe manifestations could be reflective of other factors such as high viral burden, therefore preemptive use of immunosuppressive agents or IL-6 inhibitors remains controversial [30].
In addition to diabetes, the presence of hematological disorders was independently associated with mortality. Chronic anemia was the most common in this subgroup, followed by coagulation disorders, hematological malignancies, and sickle cell disease. This finding may be indicative of underlying chronic inflammation or baseline dysregulation of the coagulation/endothelial dysfunction interplay, which is another driver of severity in COVID-19 [26, 31, 32].
In our cohort, hypoxia in the first 24 hours was an independent predictor of progression to severe disease. Likewise, presence of tachypnea was also an early indicator of subsequent worsening. Hypotension predicted mortality in addition to clinical worsening. These findings underscore the importance of early frequent monitoring of vital signs, which could provide early clues of impending decompensation or death. Patients with these vital sign abnormalities merit close monitoring.
Higher prevalence of hypoalbuminemia in patients with severe COVID-19 likely reflects a catabolic state and critical illness [33]. We also noted eGFR<60 to be common in the severe group. The kidney damage could be due to direct cytopathic effects of the virus from ACE receptor mediated entry [34] or from hypotension. Elevations in D-dimer reflect thrombosis and abnormal coagulation cascade that is common in COVID-19 [35]. Chest x-rays were normal in many patients with non-severe disease. This could be due to lower sensitivity of chest x-ray earlier in the disease [36, 37]. Presence of bilateral infiltrates and ground glass opacities were also associated with disease progression. However, on multivariate regression analysis none of these lab makers or imaging findings independently predicted outcomes.
This study had limitations. First, we analyzed a subset of all patients admitted to our hospitals during the time period of interest, and thus may have introduced selection bias. However, selected patients did not differ from unselected patients with respect to demographics. Second, due to missing D-dimer, ferritin, LDH, and CRP in a subset of patients, we may not have captured associations between these laboratory values and outcomes. Third, imaging findings were compiled from radiology reports. Therefore, subjectivity in reporting style may affect whether or not our descriptive variables were used by the reporting radiologist. Finally, the evaluation of treatment was beyond the scope of this study, and the statistical models did not adjust for treatment. However, remdesivir and steroids, the treatments that have been shown to improve outcomes in patients with COVID-19 [38–43], were administered to a small percentage of patients with non-severe disease [Supplementary materials]. Therefore, treatment would not be expected to significantly alter our findings of factors associated with severe COVID-19, but we cannot confirm this hypothesis. Although racial disparities could affect treatment outcomes [44–46] we did not find any significant difference in distribution of black patients among remdesivir versus non remdesivir treatment groups in our study.
Conclusions
In this cohort of hospitalized patients with COVID-19 in RI, Black race and diabetes were found to be independent predictors of severe disease. Older age, admission from nursing home or rehabilitation facilities, and presence of hematological disorders predicted mortality. Tachypnea, hypoxia, and hypotension in the first 24 hours predicted progression to severe disease or death later during the hospital stay. These findings can help clinicians with early identification and triage of high-risk patients in order to optimize the allocation of hospital resources.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to acknowledge Dr. Erika D’ Agata for her help in preparation of this manuscript.
Data Availability
All relevant data are within the manuscript and S1–S7 Tables. Due to institution IRB restrictions, unable to provide access to dataset beyond tables in manuscript and supportive information file.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.CDC. Cases in the U.S. 2020 [cited 28 Jul 2020]. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- 2.CDC COVID Data Tracker. [cited 28 Jul 2020]. https://www.cdc.gov/covid-data-tracker/index.html#cases
- 3.Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of Hospitalized Adults With COVID-19 in an Integrated Health Care System in California. JAMA. 2020. doi: 10.1001/jama.2020.7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382: 2372–2374. doi: 10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369: m1966. doi: 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, et al. Coronavirus Disease 2019 Case Surveillance—United States, January 22–May 30, 2020. MMWR. Morbidity and Mortality Weekly Report. 2020. pp. 759–765. doi: 10.15585/mmwr.mm6924e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen D. Roadmap to pandemic resilience. 2020. https://ethics.harvard.edu/files/center-for-ethics/files/roadmaptopandemicresilience_updated_4.20.20_1.pdf
- 9.Madhuripan N, Cheung HMC, Alicia Cheong LH, Jawahar A, Willis MH, Larson DB. Variables Influencing Radiology Volume Recovery During the Next Phase of the Coronavirus Disease 2019 (COVID-19) Pandemic. J Am Coll Radiol. 2020;17: 855–864. doi: 10.1016/j.jacr.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y, Pandita A, Hardesty A, McCarthy M, Aridi J, Weiss ZF, et al. Validation of pneumonia prognostic scores in a statewide cohort of hospitalised patients with COVID-19. International Journal of Clinical Practice. 2021. doi: 10.1111/ijcp.13926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395: 1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Internal Medicine. 2020. p. 934. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu L, Chen S, Fu Y, Gao Z, Long H, Wang J-M, et al. Risk Factors Associated with Clinical Outcomes in 323 COVID-19 Hospitalized Patients in Wuhan, China. Clinical Infectious Diseases. 2020. doi: 10.1093/cid/ciaa539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imam Z, Odish F, Armstrong J, Elassar H, Dokter J, Langnas E, et al. Independent Correlates of Hospitalization in 2040 Patients with COVID-19 at a Large Hospital System in Michigan, United States. Journal of General Internal Medicine. 2020. doi: 10.1007/s11606-020-05937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N Engl J Med. 2020;382: 2534–2543. doi: 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan D, Sze S, Minhas JS, Bangash MN, Pareek N, Divall P, et al. The impact of ethnicity on clinical outcomes in COVID-19: A systematic review. EClinicalMedicine. 2020. p. 100404. doi: 10.1016/j.eclinm.2020.100404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Website. [cited 30 Jul 2020]. Available: The COVID Racial Data Tracker. https://covidtracking.com/race. Accessed 28 July 2020.
- 18.Holmes L, Enwere M, Williams J, Ogundele B, Chavan P, Piccoli T, et al. Black–White Risk Differentials in COVID-19 (SARS-COV2) Transmission, Mortality and Case Fatality in the United States: Translational Epidemiologic Perspective and Challenges. International Journal of Environmental Research and Public Health. 2020. p. 4322. doi: 10.3390/ijerph17124322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold JAW, Wong KK, Szablewski CM, Patel PR, Rossow J, da Silva J, et al. Characteristics and Clinical Outcomes of Adult Patients Hospitalized with COVID-19—Georgia, March 2020. MMWR. Morbidity and Mortality Weekly Report. 2020. pp. 545–550. doi: 10.15585/mmwr.mm6918e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lassale C, Gaye B, Hamer M, Gale CR, Batty GD. Ethnic Disparities in Hospitalization for COVID-19: a Community-Based Cohort Study in the UK. medRxiv. 2020. doi: 10.1101/2020.05.19.20106344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White C, Nafilyan V. Coronavirus (COVID-19) related deaths by ethnic group, England and Wales—Office for National Statistics. In: Office for National Statistics [Internet]. 6 May 2020 [cited 28 Jul 2020]. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/coronavirusrelateddeathsbyethnicgroupenglandandwales/2march2020to10april2020?hootPostID=b229db5cd884a4f73d5bd4fadcd8959b
- 22.Zhang Y, Li H, Zhang J, Cao Y, Zhao X, Yu N, et al. The clinical characteristics and outcomes of patients with diabetes and secondary hyperglycaemia with coronavirus disease 2019: A single-centre, retrospective, observational study in Wuhan. Diabetes, Obesity and Metabolism. 2020. pp. 1443–1454. doi: 10.1111/dom.14086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L, She Z-G, Cheng X, Qin J-J, Zhang X-J, Cai J, et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31: 1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji D, Zhang D, Xu J, Chen Z, Yang T, Zhao P, et al. Prediction for Progression Risk in Patients with COVID-19 Pneumonia: the CALL Score. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020. pp. 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020; e3319. doi: 10.1002/dmrr.3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan Y, Yang Y, Wang F, Ren H, Zhang S, Shi X, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8. doi: 10.1136/bmjdrc-2020-001343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2015. pp. 171–185. doi: 10.1111/imm.12394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318: E736–E741. doi: 10.1152/ajpendo.00124.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? The Lancet. 2020. p. 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. Journal of Leukocyte Biology. 2020. pp. 17–41. doi: 10.1002/JLB.3COVR0520-272R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao W, Li T. COVID-19: towards understanding of pathogenesis. Cell Research. 2020. pp. 367–369. doi: 10.1038/s41422-020-0327-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aziz M, Fatima R, Lee-Smith W, Assaly R. The association of low serum albumin level with severe COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24: 255. doi: 10.1186/s13054-020-02995-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney International. 2020. pp. 829–838. doi: 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395: 1763–1770. doi: 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, et al. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology. 2020;296: E15–E25. doi: 10.1148/radiol.2020200490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong HYF, Lam HYS, Fong AH-T, Leung ST, Chin TW-Y, Lo CSY, et al. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology. 2020;296: E72–E78. doi: 10.1148/radiol.2020201160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19—Final Report. N Engl J Med. 2020;383: 1813–1826. doi: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19 –Preliminary Report. doi: 10.1101/2020.06.22.20137273 32690491 [DOI] [Google Scholar]
- 40.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395: 1569–1578. doi: 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2020;324: 1048–1057. doi: 10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020;383: 1827–1837. doi: 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalligeros M, Tashima KT, Mylona EK, Rybak N, Flanigan TP, Farmakiotis D, et al. Remdesivir Use Compared With Supportive Care in Hospitalized Patients With Severe COVID-19: A Single-Center Experience. Open Forum Infect Dis. 2020;7: ofaa319. doi: 10.1093/ofid/ofaa319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandita A, Gil RM, Farmakiotis D. Call for Action: Racial Disparities in Clinical Research. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa1349 [DOI] [PubMed] [Google Scholar]
- 45.Olender SA, Perez KK, Go AS, Balani B, Price-Haywood EG, Shah NS, et al. Remdesivir for Severe COVID-19 versus a Cohort Receiving Standard of Care. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivera DR, Peters S, Panagiotou OA, Shah DP, Kuderer NM, Hsu C-Y, et al. Utilization of COVID-19 Treatments and Clinical Outcomes among Patients with Cancer: A COVID-19 and Cancer Consortium (CCC19) Cohort Study. Cancer Discovery. 2020. pp. 1514–1527. doi: 10.1158/2159-8290.CD-20-0941 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and S1–S7 Tables. Due to institution IRB restrictions, unable to provide access to dataset beyond tables in manuscript and supportive information file.