Abstract
Introduction:
Routine viral load (VL) testing is fraught with challenges in resource-limited settings which lead to longer turnaround times for the return of VL results. We assessed the turnaround times for VL testing and factors associated with long turnaround (>30 days) in Marondera, Zimbabwe, between January and September 2018.
Methods:
This was an analytical study of routine program data. Data were extracted from electronic records and paper-based reports at two laboratories and at antiretroviral therapy (ART) facilities. The unit of analysis was the VL sample. Duration (in days) between sample collection and sample testing (pre-test turnaround time), duration between sample testing and receipt of VL result at ART the site (post-test turnaround time), and duration between sample collection and receipt of result at the ART site (overall turnaround time) were calculated. Days on which the VL testing machine was not functional, and workload (number of tests done per month) were used to assess associations. We used binomial log models to assess the factors associated with longer turnaround time.
Results:
A total of 3348 samples were received at the two VL testing laboratories, and 3313 were tested, of these, 1111 were analyzed for overall turnaround time. Pre-test, post-test, and overall turnaround times were 22 days (interquartile range (IQR): 11–41), 51 days (IQR: 30–89), and 67 days (IQR: 46–100), respectively. Laboratory workload (relative risk [RR]: 1.12, 95% confidence interval [CI]: 1.10–1.14) and machine break down (RR: 1.15, 95% CI: 1.14–1.17) were associated with long turnaround time.
Conclusions:
Routine VL turnaround time was long. Decentralizing VL testing and enhancing laboratory capacity may help shorten the turnaround time.
Keywords: Decentralization, operational research, Structured Operational Research and Training Initiative, turnaround time, viral load
INTRODUCTION
Globally, the number of people living with HIV (PLHIV) accessing antiretroviral therapy (ART) increased from 13.2 million to 23.3 million between 2013 and 2018.[1] This was triggered by efforts to achieve the ambitious 90-90-90 targets set by the Joint United Nations Programme on HIV/AIDS (UNAIDS) in 2014.[2] The greatest increase occurred in Sub-Saharan Africa, where the number of PLHIV on ART increased from 8.4 million in 2013 to 13 million in 2018.[1,3]
As HIV programs scale-up ART access, treatment monitoring using viral load (VL) tests is critical. PLHIV who achieve and maintain undetectable levels of VL are less likely to transmit HIV to their sexual partners.[4] Lack of VL monitoring may lead to delays in detecting viral rebound (the earliest indicator of ART failure), which may increase the risk of developing drug resistance and subsequently increase morbidity and mortality in PLHIV.
In 2013, the World Health Organization (WHO) recommended that all PLHIV on ART should receive a VL test 6 months, 12 months after starting ART, and every 12 months thereafter to check that viral suppression is achieved and maintained.[5] Implementation of this WHO recommendation in low- and middle-income high HIV burden countries is challenged by 'unavailability' and 'limited access' to VL testing services.[6] VL testing is a technically complex laboratory process and is typically conducted in a few centralized laboratories in many countries. Although the point of care VL testing is gradually being made available at various facilities, it is not widely available. Other barriers to scaling up VL monitoring include workload, distance, and other characteristics of the ART facility and VL testing laboratory, inefficiencies in the transportation of blood samples from the peripheral health facilities to the VL testing laboratory, and deficiencies in communicating results back from the VL testing laboratories to the ART facility, leading to longer turnaround times.[5] As a consequence, these challenges have led to the inadequate number, and proportion of PLHIV tested for VL, which significantly negate the benefits of VL monitoring.[5,6,7]
Zimbabwe is one of the high HIV burden countries in Southern Africa. The Ministry of Health and Child Care (MoHCC) of Zimbabwe adopted the WHO-recommended VL monitoring guidelines in 2013 starting with a targeted approach where patients who were suspected of clinical or immunological failure were offered VL testing services.[8] The government then made a commitment in 2015 to increase laboratory capacity and rapidly scale-up VL testing services to ensure access to at least 90% PLHIV receiving ART by 2018.[8] Despite such commendable commitments, reports indicate that VL coverage was around 44% in 2018.[9] Factors contributing to the low coverage are unknown. Longer turnaround times in receiving VL test results (if it coexists with low coverage) may lead to a further reduction in the utilization of VL monitoring services by health-care providers. There have been limited published studies examining the turnaround times for VL testing in Zimbabwe. To address this gap in knowledge, we conducted an operational research study to describe the monthly turnaround times for VL testing and factors associated with longer turnaround times (>30 days) in Marondera (urban and rural), Zimbabwe, between January and September 2018.
METHODS
Study design
This was an analytical study of VL testing turnaround times using VL program data from health facilities and VL testing laboratories in Marondera, Zimbabwe.
Setting
General setting
Marondera urban and rural districts are situated in the Mashonaland East Province of Zimbabwe and have a combined population of 178,983.[10] Marondera has 19 health facilities: four (two hospitals and two clinics) in the urban area and 15 (two hospitals and 13 clinics) in the rural areas. The HIV prevalence was estimated to be 15% by 2018.[11] All 19 health facilities offer ART services.
Specific setting
During the study period, individuals diagnosed with HIV were registered for ART with each being given an opportunistic infection ART care booklet and number. In some facilities, the information would then be entered into an electronic patient monitoring system (ePMS) or a paper-based system. Routine clinic follow-up visits were scheduled monthly during the first 6 months of ART initiation and every 2 or 3 months thereafter. VL monitoring was scheduled at 6 months, 12 months, and every 12 months thereafter if the patient had suppressed VL (<1000 copies/ml).[12] Blood samples were collected for VL testing after 3 months when a patient had a previous instance of high VL (defined as >1000 copies/ml) after undergoing three sessions of enhanced adherence counseling.
Information about the VL samples was documented on a VL laboratory requisition form and VL registers. The blood samples collected at peripheral ART sites (urban and rural sites) were transported to the near-by hospital either as a fresh plasma or dry blood spot along with the laboratory requisition form by motorcycle riders. Unlike in urban sites, the collection of VL samples in rural ART sites was done on designated days only upon confirmation of the availability of a motorcycle rider. Samples collected from peripheral ART sites together with samples collected in the urban hospital were transported to the National Microbiological Reference Laboratory or to the Beatrice Road Infectious Disease Hospital in Harare, the two VL testing sites.
Upon receipt of the blood sample at the VL testing laboratory, data from the requisition form were entered into the Laboratory Information Management System (LIMS) and the Viral load Laboratory Information System (VLIS). VL testing was done using the polymerase chain reaction method using Biomerieux (dry blood spot samples) and Roche (plasma samples) machines. The results were entered into the LIMS or VLIS, and a report was printed for delivery back to the referring ART site and patient [Figure 1].
Figure 1.
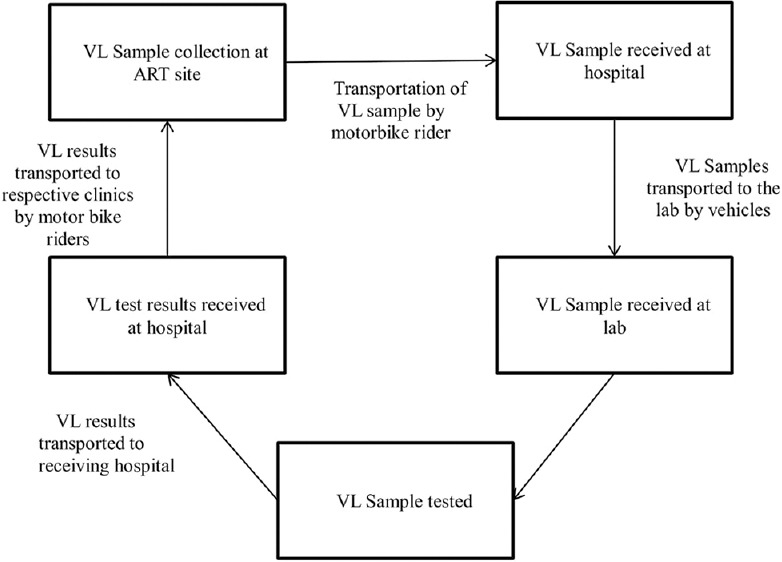
System for viral load blood sample collection, transportation, storage, viral load testing and communication of results in Marondera, Zimbabwe
Study population and data collection
All samples submitted and received at the two VL testing laboratories from January 1 to September 30, 2018, were included in the study. Data were extracted from the LMIS, VLIS, and paper-based VLIS reports at the laboratory and VL registers and the EPMS at the facilities. Data for each VL sample included the name of the ART clinic, type of the facility (government or municipality), location of a facility (urban and rural), date of sample collection, date of sample test, name of the laboratory, date when the VL test result was received at the facility, and number of days the machine was not functional (downtimes). Data from the paper-based system were entered into EpiData entry client (Version 4.4.3.1, EpiData Association, Odense, Denmark) and merged with the electronic data from the LMIS, VLIS, and EPMS.
Statistical analysis
The data were imported into Stata statistical software (version 15.1, StataCorp, College Station Texas, USA) for analysis. The unit of analysis was the individual blood sample. The turnaround time was categorized into two periods: pre-test turnaround time and post-test turnaround time. Pre-test turnaround time was defined as duration (in days) between specimen collection and specimen testing at the laboratory and post-test turnaround time as the duration (in days) between specimen testing and receipt of the result at the referring ART facility for samples with the receipt date. Total turnaround time (in days) was the sum of pre-test and post-test time calculated only for samples with both the dates of collection and receipt of results at the ART facility.
As there were large numbers of samples with missing data on the date of receipt of results at the ART facility, we only examined the association between characteristics of ART facilities and VL testing laboratory with longer pre-test turnaround time. For this, we generated a binary variable for long pre-test turnaround time (≤30 days versus >30 days). We used univariable and multivariable binomial log models (Poison models with robust standard error estimates if the binomial log models failed to converge) to assess the measures of association, risk ratios and adjusted risk ratios. P < 0.05 was considered statistically significant. Since the data did not follow the normal distribution, we decided to summarize the durations using medians and their IQRs (as calculating means and standard deviations would be misleading in this situation).
Ethics approval
The study was approved by the Medical Research Council of Zimbabwe (MRCZ/E/250) and the Union Ethics Advisory Group, Paris, France (EAG number 50/19). The ethics review committees granted consent waiver because this was a retrospective study that used routine ART program data.
RESULTS
Seven out of the eight ART sites selected for the study offered VL testing services during the study period. A total of 3348 samples were received at the two VL testing laboratories from these seven ART sites. Of the samples received, 3313 (99%) were tested and had VL results. Of these samples, we excluded nine from our analysis due to inconsistent dates (i.e., test date was prior to sample collection date), and the remaining 3304 samples were included in our analysis. For these 3304 samples, dates of sample collection and sample testing were available for all of them whereas the date of test report receipt at the ART sites was available for 1111 (34%) samples only [Figure 2].
Figure 2.
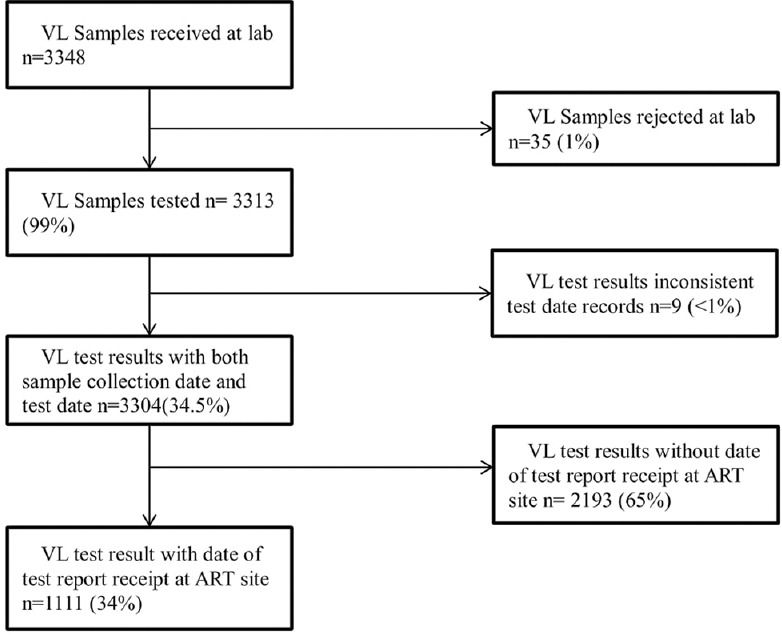
Flow chart of viral load samples tested and result sent to the referring health facility in Marondera, Zimbabwe, between January and September 2018
Pre-test turnaround time and factors associated with >30 days pre-test turnaround time
The median pre-test turnaround time for the 3304 samples was 22 days (interquartile range (IQR): 11–41), with 1175 (36%) samples having a pre-test turnaround time >30 days (i.e., longer pre-test turnaround time). The factors associated with longer pre-test turnaround time are given in Table 1. Longer turnaround time was observed when the VL testing machines were not functional (due to breakdown) with the risk of longer turnaround time increasing by 1.2 times for every day the machine was not functional during the month. Figure 3 shows the relationship between median pre-test turnaround time and the number of days of VL testing machine break down per month. An increase in the median pre-test turnaround time paralleled an increased number of days the VL testing machine was non-functional during the month. Monthly workload (i.e., number of tests per month) at the VL testing sites was another factor associated with the risk of longer turnaround time with the pre-test turnaround time increasing by 1.1 times for every 500-sample increase in the monthly workload at the laboratory. Pre-test turnaround times were not associated with ART site characteristics [Figure 3].
Table 1.
Factors associated with the longer viral load turnaround time (>30 days) for people living with HIV on antiretroviral therapy in Marondera between January and September 2018 (n=3304)
| Characteristics | n | Pretest turnaround time >30 days), n (%) | Adjusted risk ratio (95% CI) |
|---|---|---|---|
| Laboratory | |||
| Lab name | |||
| Lab 1 | 166 | 19 (19) | 1.74 (0.95- 3.21) |
| Lab 2 | 3138 | 1157 (37) | Reference |
| Tests per month (baseline ≤500 tests per month) | - | - | 1.12 (1.10- 1.14)* |
| Days machine not functional/per month (baseline 0 days) | - | - | 1.15 (1.14- 1.17)* |
| ART site | |||
| Location | |||
| Urban | 1034 | 323 (31) | 1.00 (0.91- 1.09) |
| Rural | 2270 | 853 (38) | Reference |
| Ownership | |||
| Government | 2583 | 901 (35) | 1.09 (0.99- 1.19) |
| Municipality | 721 | 275 (38) | Reference |
*Statistically significant with P<0.05. PLHIV: People living with HIV, ART: Antiretroviral therapy, CI: Confidence interval
Figure 3.
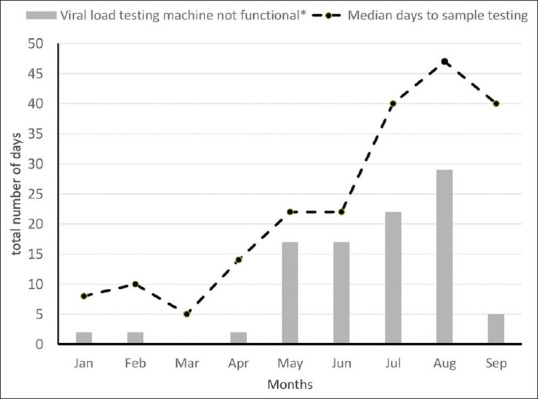
Trends in median pre-test turnaround time (in days) and viral load machine was not functional for the Viral load testing machines (in days) in Marondera, Zimbabwe between January and September 2018* (n = 3304 samples)
Post-test turnaround time and total turnaround time
We were able to calculate the post-test turnaround for 34% of the samples that had the date when the sample was received at the referring facility. Of these samples, the median post-test turnaround time for the 1111 samples was 51 days (IQR: 30-89), with 826 (74%) having a post-test turnaround time >30 days [Figure 4]. The overall turnaround time was estimated for the 1111 samples that had both the sample collection date and the result receipt date at the facility. The median turnaround time was 67 days (IQR: 46-100). VL samples that had both sample collection date and result receipt at the facility date had a shorter median pre-test turnaround time compared to VL samples without receipt date (13 vs. 28 days) (P < 0.001) [Figure 4].
Figure 4.
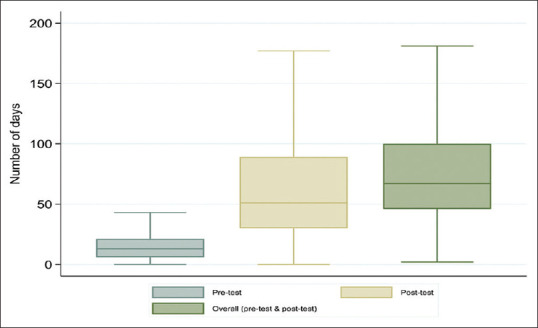
Median pre-test, post-test and overall turnaround time (in days) for returning viral load test results in Marondera, Zimbabwe, between January and September 2018 (n = 1111 samples)
DISCUSSION
This is the first study in Zimbabwe to assess the pre-test, post-test, and overall turnaround times and the factors associated with longer pre-test turnaround times for VL testing in routine ART programs. The median pre-test turnaround time was 22 days, and the median post-test turnaround time was 51 days and the median total turnaround time 67 days (9 weeks). The overall turnaround is higher than the recommended Zimbabwe MOHCC standard total turnaround time of (30 days). The number of days the VL testing machine was down and the monthly VL testing workload contributed to longer turnaround time.
The strengths of our study were as follows: first, we used routine programmatic data of VL samples that were received for VL testing at the two laboratories. Therefore, we strongly believe that our findings reflect the situation on the ground. Second, apart from providing information about turnaround times, the study also attempts to provide information on a few causes of longer turnaround time. This can help in improving turn-around times not only in Marondera but in other parts of the country as the process of VL testing (collection of blood from peripheral ART sites, transporting them to centralized laboratory and carrying back the test results from VL testing labs to peripheral ART sites) is similar in most parts of Zimbabwe.
The study has some limitations. First, nearly 66% of the samples did not have a date when the VL results were received at the referring ART site. Comparisons of samples with and without date of VL test result receipt on some measured variables indicated that the two groups of samples were not similar. Therefore, our estimates of the post-test and overall turnaround time may not be generalizable. Second, dates, when samples were received at the VL testing laboratories, were not available. Lack of these dates prevented us from estimating the time taken between receipt of the sample at the laboratory to VL testing. Third, we were able to include only a few characteristics for ART facilities and VL testing laboratory to assess the factors associated with longer turnaround time. There could be several other factors such as availability/absence of bike riders to transport the samples and carry the results to the ART facilities, patient factors, and health system factors that may be associated with longer turnaround times. Since this information was not systematically documented in the ART program records that we reviewed for our study, we could not include them in our data analysis. Fourth, our study included only samples received at the VL testing laboratories. There may be blood samples that were collected at the peripheral ART centers that were not transported to the VL testing laboratories. Our study does not provide information on these losses. Assessing this could be an area for future research.
Notwithstanding these limitations, the study findings have the following three implications for policy and practice. First, longer turnaround time puts PLHIV at risk of poor ART outcomes as it delays necessary interventions when there is ART failure. Trends in pre-test turnaround times over the study period showed that the turnaround time increased as the year progressed from a median of 10 days in February to 47 days in August. Our study also shows an association between long turnaround times with machine breakdowns and increased workload at the laboratory. Although reasons for the breakdown were not explored in this study, ensuring the timely repair of the machines and adequate training for the laboratory technicians may reduce the turnaround time. Laboratories should also ensure consistent placement of enough workforce in the VL testing laboratory to provide better services.
Previous reports showed that the targeted demand for VL testing countrywide was 541,857-850,199 samples between 2017 and 2018 with a lab capacity gap of 158,555 samples by 2018 because of the adoption of the National VL Scale-Up Plan of 2015 and 'Treat All' Guidelines of 2016.[13,14] This increase in VL demand has not been complemented by an increase in the number of VL testing laboratories and/or an increase in the capacity of the existing laboratories to handle the additional workload through the provision of high throughput machines and better blood collection and transportation systems. With the increased volume of VL samples at national level, longer turnaround times may decrease demand for VL load testing among ART providers and strategies to shorten turnaround time such as decentralization of VL testing services and/or speeding up the adoption of point of care technology may improve quality of VL testing services across the VL monitoring spectrum. As of September 2018, there were 58 point-of-care VL testing machines benefiting 257 out of the 1848 hospitals and primary health care facilities in Zimbabwe.[15]
Second, the median post-test turnaround time of 51 days is high and the exact reasons for such a long turnaround time to receive the results at the ART sites are unknown. According to Zimbabwe laboratory standards, VL samples should be tested and released within 14 days. Anecdotal evidence suggests that there are delays in printing VL results at the laboratories and delays in documenting the arrival of the VL test results/reports at the ART sites, especially when the VL test results show that the VLs are suppressed (<1000 copies per ml).
Third, previous assessments of national VL monitoring scale-up in seven Sub-Saharan African (SSA) countries found that the total turnaround time ranged from three and 4 days in South Africa and Namibia to 42 and 50 days in Malawi and Cote d'Ivoire.[7] South Africa and Namibia adopted strategies that reduce or eliminate hidden costs and incentivize maximum instrument use to achieve an all-inclusive price per patient VL result.[8] It is useful to draw from the South African and Namibian experiences on what steps can be taken to ensure that turnaround times are shortened. Population-based HIV Impact Assessment surveys recruited dedicated staff that worked directly with laboratory staff at the central reference laboratory to implement all steps in the VL testing process to shorten the turnaround time.[16]
Finally, a substantial proportion of the samples did not have dates when the samples were received at the laboratory, dates for VL testing, or when the samples were received at the facility, suggesting the need for improvement in data quality. Optimizing the use of LMIS, VLIS, or ePMS in capturing all necessary dates across the VL monitoring spectrum will ensure timely and accurate information about the gaps in the VL testing process, which may inform the implementation of corrective measures that reduce VL turnaround times.
CONCLUSIONS
The total turnaround times for VL tests were more than 2 months. High workload and higher laboratory equipment breakdown time are associated with longer pre-test turnaround time. Decentralizing VL testing services, increasing the laboratory workforce, and ensuring uninterrupted availability of VL testing services should be considered essential in VL monitoring and achieving 90-90-90 UNAIDS targets.
Research quality and ethics statement
This study was approved by the Institutional Review Board / Ethics Committee approval number (MRCZ/E/250) for the Medical Research Council of Zimbabwe and 49/19 for Union Ethics Advisory Group, Paris, France (50/19). The authors followed applicable EQUATOR Network (http://www.equator-network.org/) guidelines during the conduct of this research project.
Financial support and sponsorship
The training course under which this study was conducted was funded by: the United Kingdom's Department for International Development (DFID); and the WHO Zimbabwe Country Office. The open-access publications costs were funded by the Department for International Development (DFID), UK and La Fondation Veuve Emile Metz-Tesch (Luxembourg). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the WHO (WHO/TDR). The training model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union) and Medécins sans Frontières (MSF). The specific SORT IT program which resulted in this publication was implemented by the Centre for Operational Research, The Union, Paris, France. Mentorship and the coordination/facilitation of this particular SORT IT workshop was provided through the Centre for Operational Research, The Union, Paris, France; the Department of Tuberculosis and HIV, The Union, Paris, France; The Union, Zimbabwe Office; The Union, South East Asia Office; and AIDS and TB Department, MoHCC, Harare, Zimbabwe.
REFERENCES
- 1.UNAIDS Data 2019 UNAIDS. [Last accessed on 2019 Nov 13]. Available from: https://www.unaids.org/en/resources/documents/2019/2019-UNAIDS-data .
- 2.90-90-90: A Transformative Agenda to Leave no One Behind UNAIDS. [Last accessed on 2019 Nov 13]. Available from: https://www.unaids.org/en/speeches/2014/20141025_SP_EXD_Vietnam_launch_of_909090_en.pdf .
- 3.UNAIDS. UNAIDS Data 2018. 2018:1–376. [Google Scholar]
- 4.WHO Consolidated Guidelines on the use of Antiretroviral Drugs for Treating and Preventing HIV Infection. [Last accessed on 2019 Nov 13]. Available from: https://www.who.int/hiv/pub/arv/arv-2016/en/
- 5.Minchella PA, Chipungu G, Kim AA, Sarr A, Ali H, Mwenda R, et al. Charpentier C, editor. Specimen origin, type and testing laboratory are linked to longer turn-around times for HIV viral load testing in Malawi. PLoS One. 2017;12:e0173009. doi: 10.1371/journal.pone.0173009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mwau M, Syeunda CA, Adhiambo M, Bwana P, Kithinji L, Mwende J, et al. Scale-up of Kenya's national HIV viral load program: Findings and lessons learned. PLoS One. 2018;13:e0190659. doi: 10.1371/journal.pone.0190659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lecher S, Williams J, Fonjungo PN, Kim AA, Ellenberger D, Zhang G, et al. Progress with scale-Up of HIV viral load monitoring Seven sub-Saharan African countries, January 2015–June 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1332–5. doi: 10.15585/mmwr.mm6547a2. [DOI] [PubMed] [Google Scholar]
- 8.Viral IV, Plan LS. Zimbabwe h iv viral Load Scale-up Plan 2015-2018. 2018 [Google Scholar]
- 9.Activity Report , Global Fund Supported Funding Model HIV/AIDS Grant Number GA 1393. 2019:1–27. [Google Scholar]
- 10.Zimstat. Provincial Report Mashonaland East Zimbabwe Population. 2012 [Google Scholar]
- 11.Zimbabwe Ministry of Health and Child Care. Zimbabwe National and Sub-National HIV Estimates Report 2017. 2018. [Last accessed on 2019 Nov 06]. Available from: http://nac.org.zw/wp.content/uploads/2019/01/Zimbabwe-HIV-Estimates-Report-2018.pdf .
- 12.Care C. AIDS TB Programme for the Prevention, Care and Treatment of HIV in Zimbabwe Operational and Service Delivery Manual Operational and Service Delivery Manual for the Prevention, Care and Treatment of HIV in Zimbabwe Content Abbreviations 2 Background. 2017. [Last accessed on 2019 Nov 17]. Available from: http://www.differentiatedcare.org/Portals/0/adam/Content/m2an155byU6RIoHeF4e4FQ/File/Zimbabwe_OSDM_2017.pdf .
- 13.Ministry of Health and Child Care (MOHCC). Zimbabwe HIV Viral Load Scale-up Plan (2015-2018) 2015:1–48. [Google Scholar]
- 14.Ministry of Health and Child Care. Guidelines for Antiretroviral Therapy for the Prevention and Treatment of HIV in Zimbabwe. 2016. [Last accessed on 2019 Nov 20]. p. 23. Available from: https://aidsfree.usaid.gov/sites/default/files/zw_arv_therapy_prevention.pdf .
- 15.Ministry Of Health and Child Care Global Fund Final Quarter 3 Report [Google Scholar]
- 16.El-Sadr W, Rabkin M, Birx D, Nkengasong J. Reaching the third 90: Taking routine viral load monitoring to scale. JIA2_v20_s7_cover.indd 1 JIA2_v20_s7_cover. INDD. 2017;20:57. [Google Scholar]


