Abstract
This study was conducted to investigate the effects of the degree of dual-task (DT) interference on gait, dual-task cost (DTC), cognitive ability, balance, and fall efficacy in people with stroke.
In this cross-sectional study, people with chronic stroke (N = 36) performed a DT gait assessment (gait and cognitive task). During the evaluation, DT interference in motor and cognition was evaluated simultaneously. Thus, the group with severe interference in both tasks (mutual interference) was compared with the group with mild interference in either.
The main effects for the degree of motor interference were observed on gait performance, DTC in motor, time up and go, and trail-making test B. In the cognitive interference, the main effects were observed on correct response rate, DTC in cognition, time up and go, and trail-making test B. An interaction effect was observed in the trail-making test B.
The degree of motor interference affected gait, balance ability, and executive function (EF), and the degree of cognitive interference influenced the correct response rate in the DT condition, balance ability, and EF. Furthermore, mutual interference led to a significant reduction in EF in people with stroke.
Keywords: dual-task, gait, rehabilitation, stroke
1. Introduction
The ability to perform two tasks is easily observed in daily activities and is an essential skill for independent living.[1] However, when cognitive and motor tasks are conducted simultaneously, dual-task interference (DTI) occurs, leading to poor performance in one or two tasks during dual-tasking relative to single-tasking.[2] Based on the capacity-sharing theory, when attentional demands elicited during dual-tasking exceeded central processing capacity, it was prominent.[3,4]
Individuals with stroke experienced more DTI than healthy people, and the degree of DTI was high in limited community ambulators (<0.8 m/s, slow gait speed).[5,6] The typical effects of DTI in stroke patients were decreased gait performance, increased postural sway, and declines in cognitive performance such as executive function (EF) due to physical and cognitive impairment.[7] To evaluate this DTI, dual-task cost (DTC) was used to indicate the percentage difference between single-task (ST) and dual-task (DT) performance (single task – dual task/single task ×100).[3,4] In many studies, DTC was used as an indicator of DT ability, the risk of falling, EF, and gait automaticity for people with neurological diseases.[5,8,9] However, many studies only calculated the DTC of motor function (e.g., speed), investigated the effect of DTC of motor function, and did not address the DTC of cognitive tasks applied as a second task.[1,4,10] Although a recent study reported that not only motor DTI (MDTI) but also cognitive DTI (CDTI) was linked to fall risk,[11] Few studies have focused on the DTC of cognition for DT assessment in people with stroke with high DTC. To correctly evaluate DTI, the DTCs of motor and cognition should be calculated simultaneously, which could screen for task prioritization indicative of a strategy that focused on only one task by sacrificing the other and could investigate the overall DT capacity.[4] When assessing both DTIs, the cause of the mutual interference between motor function and cognition was reported to be a decrease in DT capacity and limitation in the flexibility to switch attention, which meant that bilateral severe DTI impaired DT ability more than unilateral DTI.[4,12] We hypothesized there would be a difference in gait, DTC, cognitive ability, balance, and fall efficacy between the group with severe DTIs in motor and cognition and the group with mild DTI in either. Hence, the purpose of our study was to identify the effects of the degree of DTI on gait, DT cost, cognitive ability, balance, and fall efficacy for people with stroke with high DTI. The findings of the present study would provide important information for evaluating DT ability, applying personalized intervention, and identifying potential factors influencing DTI.
2. Methods
2.1. Participants
This study had a cross-sectional design with recruitment and evaluations completed from November 29, 2020, to December 7, 2020. A total of 36 community-dwelling persons with stroke were included. This study was approved by the National Health Insurance Service of Ilsan Hospital Institutional Review Board, and in accordance with the Declaration of Helsinki, all procedures were conducted in public hospital. The study was registered in the Clinical Trial Registry of Korea (https://cris.nih.go.kr; no. KCT0005623, 11/24/2020). All participants provided written informed consent. The inclusion criteria were: 1) patients with a Korean version of the Mini-Mental State Examination (K-MMSE) score of >24, 2) limited community ambulators with gait speeds of less than 0.8 m/s, indicating high DTI,[6,13] 3) > 6 months after the onset of stroke, and 4) independent walking for less than 15 m without using a cane. The exclusion criteria were:
-
1)
neurological disorders other than stroke
-
2)
poor understanding of the assessment process
-
3)
severe aphasia.
2.2. Data analysis
All statistical analyses were conducted using SPSS 18.0 (IBM SPSS, Chicago, IL).
The values were recorded as the means and standard deviations. The subject's basic characteristics were compared using the independent t test and normal distribution was confirmed by the Shapiro–Wilk test. Two-way analysis of variance was used to analyze the main effect of the degree of MDTI (severe and mild MDTI) and CDTI (severe and mild CDTI), and the interaction effect (degree of MDTI x degree of CDTI). The independent variables were the degrees of DTIs. The significance level was set at 0.05. To identify the association between basic characteristics and DTIs, Pearson correlation analysis was performed. Stepwise multiple linear regression was performed to identify the potential factors influencing DTI. The sample size was confirmed through G∗Power based on the outcome variable of previous research,[14] and a total of 29 subjects was needed. Hence, we included people with stroke (N = 36) in this study indicating that the sample size of this study was set to be larger than that of previous studies addressing the factor related to DTI in people with stroke.[15,16]
2.3. Procedure
All evaluations involved gait analysis and the correct response rate (CRR) under single-task (ST) and DT conditions, DTC in gait parameters and the CRR, balance ability measured by the Berg balance scale (BBS) and time up and go (TUG), cognitive ability by the trail making test B (TMTB), and the fall efficacy scale (FES). Each participant performed all assessments during one day, and only one subject was allowed in the evaluation room while these were conducted. A total of 36 stroke patients completed all tests. First, gait analysis under ST and DT conditions was performed to divide the groups according to the degree of MDTI (severe and mild MDTI) (Fig. 1). To evaluate MDTI, we used speed parameters in the motor DTC formula (ST gait speed – DT gait speed/ST gait speed ×100) because speed was an important and meaningful gait parameter in dual task gait assessment[8,9] In addition, we used 20% DTC of speed as the standard for the degree of MDTI to divide the levels of MDTI in our study based on previous findings.[1,7]
Figure 1.
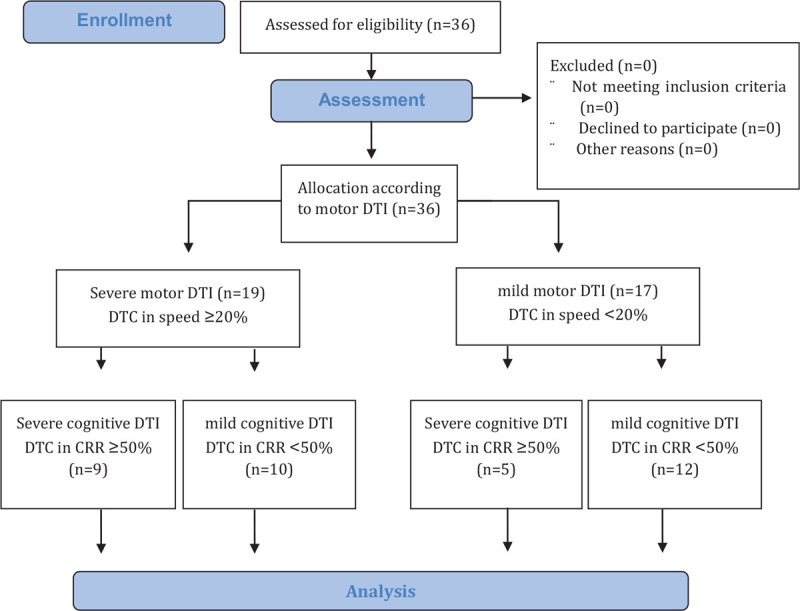
The CONSORT flow diagram.
-
1)
The severe MDTI group was the group with a DTC of speed higher than 20% (DTC of speed ≥ 20%, N = 19).
-
2)
The mild MDTI group was the group with a DTC of speed lower than 20% (DTC of speed < 20%, N = 17).
Together, during the DT gait evaluation the CDTI was also calculated simultaneously. For evaluating the CDTI, the CRR was first calculated (correct response/time ×100) and inserted into the CDTI formula (ST CRR – DT CRR/ST CRR ×100). Here, CRR under DT conditions was the value obtained by dividing the number of correct answers to the cognitive task applied during the DT gait assessment by the time to complete the gait task, and after DT CRR evaluation, the evaluation for ST CRR was performed in which the cognitive task was applied during the time obtained from the previous DT gait in the seated position.[11,17,18] As a result, taking into account the degree of CDTI measured in a previous study the CDTI degree standard was established as 50%,[7] and thus the degree of CDTI was divided into severe and mild levels.
-
1)
The severe CDTI group was the group with a DTC in the CRR higher than 50% (DTC of the CRR ≥ 50%, N = 14).
-
2)
The mild CDTI group was the group with a DTC in the CRR lower than 50% (DTC of the CRR < 50%, N = 22).
The details on the evaluation of MDTI and CDTI are shown in the assessment section.
2.4. Assessment
2.4.1. Gait
Gait parameters were obtained using the OptoGate (Microgate Srl, Bolzano, Italy), which was composed of 10 bars with sensor, and each bar (1 m) was arranged in both sides (5 m). Hence, when the subject only walked on the structure, the gait pattern was analyzed.[19] This structure was installed in the middle of a 7-m walkway. Each subject was instructed to pass through the structure and return to the starting point (total distance 14 m). Gait analysis was allowed only in the first section passed through the structure, excluding the return section (turning point) where there was no bar with a sensor. Gait analysis in the ST condition was assessed as described above, and DT gait performance was evaluated by concurrently applying serial subtractions of 3 from 2-digit numbers (e.g., 99–91).[12,20] Each subject was not given any instructions about task prioritization for either the motor or cognitive tasks. At the same, the CRR under DT conditions was calculated by reporting the time to complete the gait task and the correct response to the cognitive task (serial subtraction) as mentioned above. After that, a single CRR test was performed 5 minutes after DT evaluation to reduce the learning effect. The 2 evaluation trials were measured and averaged. The order of gait evaluation (ST and DT gait) was randomized, and one practice was allowed for familiarization before 2 test trials.
2.4.2. Balance
The BBS consists of 14 items involving tasks from sitting balance to dynamic standing balance with 4 maximum points for each item. A lower score indicated poor balance performance. TUG was used to evaluate dynamic balance and functional mobility. The time to get up from a chair, walk 3 m, turn around and sit back was measured. These balance tests were conducted according to the regular protocols.[21,22]
2.4.3. Cognitive ability
The TMTB was used to evaluate EF. The subject was instructed to connect the numbers and letters alternately (1, A, 2, B,… etc). A total of 25 circles were connected. The time to complete was reported.[23]
2.4.4. Fall efficacy scale
Fall efficacy was evaluated using the FES translated into Korean, in which high scores revealed a high fear of falling. This tool was comprised of 13 items with a maximum of 10 points each.[24]
3. Results
3.1. Baseline participant characteristics
Table 1 describes the characteristics of the subjects according to the degree of DTIs. There was a difference in gait speed between the groups according to the degree of MDTI (F = 0.252, P = .040). There was no difference between the groups according to the degree of CDTI.
Table 1.
Baseline characteristics of participants.
| baseline characteristics | Severe motor DTI group(N = 19) | Mild motor DTI group(N = 17) | P value | Severe cognitive DTI group(N = 14) | Mild cognitive DTIgroup(N = 22) | P value |
| Age (yr) | 55.68 ± 11.10 | 57.88 ± 7.75 | .50 | 57.29 ± 8.39 | 56.36 ± 10.43 | .78 |
| Sex | ||||||
| Male/Female | 13/6 | 9/8 | .34 | 8/6 | 14/8 | .70 |
| Stroke type | ||||||
| I/H | 8/11 | 6/11 | .68 | 5/9 | 9/13 | .75 |
| Hemiparetic side | ||||||
| Left/Right | 7/12 | 9/8 | .33 | 6/8 | 10/12 | .88 |
| Onset period(month) | 53.00 ± 28.38 | 54.35 ± .26.47 | .88 | 53.86 ± 29.67 | 53.50 ± 26.08 | .97 |
| K-MMSE | 27.00 ± 1.29 | 27.29 ± 1.90 | .60 | 26.50 ± 1.45 | 27.55 ± 1.57 | .06 |
| Gait speed | 0.40 ± 0.13 | 0.51 ± 0.16 | .04∗ | 0.48 ± 0.16 | 0.43 ± 0.14 | .38 |
| FAC | 3.84 ± 0.83 | 4.35 ± 0.86 | .80 | 4.36 ± 0.74 | 3.91 ± 0.92 | .14 |
| Single CRR | 31.73 ± 10.79 | 39.29 ± 15.67 | .99 | 31.93 ± 15.24 | 37.45 ± 12.45 | .24 |
| Use of cane | ||||||
| Yes/ No | 14/5 | 8/9 | .10 | 8/6 | 14/8 | .70 |
3.2. Correlation between DTI and baseline characteristics
Pearson's correlation analysis was performed between DTIs and the different characteristics. A significant association was observed between MDTI and mobility variables such as gait speed (r = −0.331, P = .049) and FAC (r = −0.333, P = .047). CDTI was significantly correlated with FAC (r = 0.395, P = .017). The remaining variables were not significantly correlated with DTI (Table 2).
Table 2.
Relationship between baseline characteristics and DTIs.
| Motor DTI | Cognitive DTI | |||||
| Variables | r | P value | 95% CI | r | P value | 95% CI |
| Age | −0.183 | 0.285 | −0.522, 0.213 | −0.133 | 0.438 | −0.408, 0.159 |
| MMSE | −0.059 | 0.733 | −0.392, 0.266 | −0.311 | 0.065 | −0.516, -0.090 |
| Period after stroke | −0.120 | 0.487 | −0.416, 0.205 | 0.014 | 0.937 | −0.346, 0.378 |
| FAC | −0.333 | 0.047∗ | −0.636, 0.004 | 0.395 | 0.017∗ | 0.148, 0.590 |
| Single gait speed | −0.331 | 0.049∗ | −0.601, -0.006 | 0.269 | 0.113 | −0.090, 0.601 |
| Single CRR | −0.287 | 0.089 | −0.560, 0.083 | −0.188 | 0.271 | −0.451, 0.104 |
3.3. Effects of the degree of DTI
Regarding gait performance and the CRR, there was a main effect of the degree of MDTI on speed (F = 5.782, P = .022, mean difference = −0.120, 95% CI = −0.222, −0.018) and stride (F = 4.702, P = .038, mean difference = −8.195, 95% CI = −15.893, −0.497) under ST conditions, and speed (F = 20.315, P < .001, mean difference = −0.187, 95% CI = −0.272, −0.103), stride (F = 11.935, P = .002, mean difference = −10.597, 95% CI = −16.845, −4.349), and cadence (F = 10.563, P = .003, mean difference = −20.874, 95% CI = −33.956, −7.791) under DT conditions. These findings indicated that the group with mild MDTI had better gait performance compared to the severe MDTI group. Additionally, the main effect of the degree of CDTI was observed in the CRR under DT conditions (F = 7.416, P = .010, mean difference = −8.382, 95% CI = −14.651, −2.112), which indicated that the mild CDTI group had better DT CRR compared to the severe CDTI group. In relation to DTC, the main effect of the degree of MDTI was seen in the DTC of speed (F = 99.486, P < .001, mean difference = 19.913, 95% CI = 15.846, 23.979) and cadence (F = 12.208, P = .001, mean difference = 15.382, 95% CI = 6.415, 24,349), and the degree of CDTI in the DTC of the CRR (F = 38.484, P < .001, mean difference = 20.096, 95% CI = 13.498, 26.695). Regarding cognitive ability, the main effects of the degree of MDTI (F = 6.098, P = .019, mean difference = 39.603, 95% CI = 6.935, 72.270) and CDTI (F = 8.695, P = .006, mean difference = 47.291, 95% CI = 14.623, 79.958) were observed on the TMTB, indicating that the groups with severe DTI in motor and cognition showed lower performance in EF compared to those with mild DTI. In addition, the interaction effect on TMTB (F = 5.557, P = .025, mean difference = 85.095, 95% CI = 42.404, 127.787) was confirmed. The main effects of the degree of MDTI (F = 11.933, P = .002, mean difference = 10.662, CI = 4.375, 16.948) and CDTI (F = 6.251, P = .018, mean difference = −7.716, 95% CI = −14.003, −1.430) were observed in the TUG assessment. The group with mild MDTI showed better balance than the severe MDTI group, whereas the group with severe CDTI showed better balance than the mild CDTI group. However, no main or interaction effects were observed for FES and BBS (Table 3).
Table 3.
The effects of the degree of DTI on outcome variables.
| Severe MDTI | Mild MDTI | Main Effects & Interactions | |||||
| Outcome variables | Severe CDTI | Mild CDTI | Severe CDTI | Mild CDTI | MDTI | CDTI | M∗C |
| Single task | |||||||
| Speed, m/s† | 0.43 ± 0.14 | 0.38 ± 0.12 | 0.57 ± 0.16 | 0.48 ± 0.15 | 0.022∗ | 0.164 | 0.602 |
| Stride, cm† | 67.43 ± 9.17 | 63.24 ± 5.81 | 75.27 ± 10.78 | 71.79 ± 14.32 | 0.038∗ | 0.318 | 0.925 |
| Variability, % | 20.18 ± 10.10 | 23.38 ± 9.10 | 16.20 ± 14.19 | 16.03 ± 11.82 | 0.154 | 0.699 | 0.667 |
| Cadence, step/min | 67.54 ± 21.88 | 64.60 ± 16.83 | 84.77 ± 10.22 | 72.25 ± 17.03 | 0.054 | 0.222 | 0.446 |
| CRR, % | 28.27 ± 11.05 | 34.85 ± 10.08 | 38.51 ± 20.65 | 39.62 ± 14.19 | 0.123 | 0.424 | 0.568 |
| Dual-task | |||||||
| Speed, m/s† | 0.30 ± 0.11 | 0.25 ± 0.07 | 0.51 ± 0.14 | 0.42 ± 0.14 | <0.001∗∗∗ | 0.109 | 0.701 |
| Stride, cm† | 63.58 ± 6.23 | 57.72 ± 4.53 | 71.80 ± 12.47 | 70.69 ± 10.95 | 0.002∗∗ | 0.265 | 0.445 |
| Variability, % | 22.41 ± 10.56 | 26.22 ± 7.84 | 17.42 ± 11.29 | 21.03 ± 10.52 | 0.156 | 0.298 | 0.977 |
| Cadence, step/min† | 50.95 ± 22.79 | 48.83 ± 15.39 | 76.59 ± 10.36 | 64.94 ± 18.98 | 0.003∗∗ | 0.29 | 0.46 |
| CRR, % ‡ | 12.92 ± 5.62 | 23.70 ± 7.55 | 18.82 ± 13.55 | 24.79 ± 9.30 | 0.265 | 0.010∗∗ | 0.440 |
| Dual-task cost | |||||||
| Speed, %† | 28.99 ± 6.62 | 33.80 ± 6.88 | 11.43 ± 4.47 | 11.51 ± 3.99 | <0.001∗∗∗ | 0.226 | 0.248 |
| Stride, % | 4.74 ± 12.64 | 7.95 ± 11.84 | 4.53 ± 10.60 | 0.41 ± 8.60 | 0.319 | 0.906 | 0.346 |
| Variability, % | 30.16 ± 51.20 | 16.00 ± 37.12 | 38.22 ± 70.79 | 22.91 ± 43.94 | 0.662 | 0.392 | 0.973 |
| Cadence, %† | 25.55 ± 18.40 | 25.18 ± 9.65 | 8.93 ± 13.39 | 11.04 ± 8.29 | 0.001∗∗ | 0.844 | 0.780 |
| CRR, % ‡ | 54.26 ± 7.46 | 31.61 ± 10.90 | 54.72 ± 11.93 | 37.17 ± 7.58 | 0.359 | <0.001∗∗∗ | 0.438 |
| Balance | |||||||
| BBS, score | 32.11 ± 4.26 | 30.00 ± 4.78 | 35.00 ± 6.93 | 33.08 ± 9.08 | 0.216 | 0.401 | 0.967 |
| TUG, sec †,§ | 27.42 ± 5.33 | 38.58 ± 9.60 | 20.20 ± 7.61 | 24.47 ± 10.35 | 0.002∗∗ | 0.018∗ | 0.273 |
| Cognitive ability | |||||||
| TMTB, sec†,‡,|| | 227.23 ± 38.55 | 142.14 ± 49.55 | 149.83 ± 48.73 | 140.34 ± 45.83 | 0.019∗ | 0.006∗∗ | 0.025∗∗ |
| Fall efficacy | |||||||
| FES, score | 53.67 ± 33.44 | 71.20 ± 24.95 | 51.60 ± 30.44 | 53.17 ± 29.95 | 0.342 | 0.366 | 0.449 |
3.4. Multiple linear regression analysis with stepwise between DTIs and various factors
The dependent variables were selected as MDTI and CDTI and the overall DTI (MDTI + CDTI/ 2).[2,25] MDTI was significantly associated with TUG (β = 0.611, P < .001) and TMTB (β = 0.378, P = .008), whereas CDTI was only significantly associated with TMTB (β = 0.360, P = .031). There was significant association between the overall DTI and TMTB (β = 0.501, P = .001) (Table 4).
Table 4.
Correlation between DTIs and outcome variables.
| Variables | B±SE | Standardized β | T | 95% CI | P value | |
| Motor DTI | TUG | 0.663±0.144 | 0.611 | 4.606 | 0.370, 0.955 | <.001∗∗∗ |
| TMTB | 0.077±0.027 | 0.378 | 2.846 | 0.022, 0.131 | .008∗∗ | |
| Cognitive DTI | TMTB | 0.084±0.037 | 0.360 | 2.250 | 0.008, 0.159 | .031∗ |
| Overall DTI | TMTB | 0.115±0.032 | 0.501 | 3.598 | 0.050, 0.180 | .001∗∗ |
4. Discussion
This study investigated the effects of the degree of DTI on gait, DTC, cognitive ability, balance, and fall efficacy in people with stroke. Our findings showed that the degree of DTI exposure affected gait, DTC, cognitive ability, and balance, and severe DTI in both domains had a great influence on EF. The potential factors influencing DTI were functional mobility (TUG) and EF. Correlation analysis showed that MDTI was negatively correlated with FAC and single gait speed, and CDTI was positively correlated with FAC. These results may indicate that individuals with stroke with high functional mobility showed low MDTI (more attention to motor) and high CDTI (less attention to cognition), which was a natural habit usually observed due to mobility impairment after stroke, indicating excessive motor task prioritization.[26] Similar to this finding, Mori et al reported that patients with stroke were more focused on mobility such as gait and posture control, sacrificing the performance of cognitive tasks due to deficits in sensory and motor function compared to healthy people during dual tasking.[20]
4.1. Effects of the degree of DTI
Gait performance was affected by the degree of MDTI. Depending upon the degree of MDTI, differences were found in speed and stride in ST conditions, and speed, stride, cadence in DT conditions. These results were similar to the findings of Yang et al in which high DTI in gait parameters was observed in the stroke patients with more deficits in gait performance in ST and DT conditions compared to those with fewer deficits in gait performance.[6] The CRR in the DT condition was influenced by the degree of CDTI. However, there were no interaction effects between the degree of DTI on gait performance and the CRR, and only each main effect was found. These findings were based on the capacity-sharing theory in which during dual tasking, the total capacity is divided and processed differently for primary and secondary tasks. However, if the attentional demands excessively exceed the capacity, they could affect each other. Therefore, it seems that the DT test in this study was not considered a challenging task that could affect mutual interference on motor and cognitive task.[3,26] Regarding EF, main effects of the degree of DTI and the interaction effect on TMTB were observed, indicating that severe DTI in both motor and cognition could represent more deficits in EF. A study by Montero-Odasso et al reported that high motor DTC in elderly people with mild cognitive impairment led to more transition to dementia, showing impaired EF compared to those with lower DTC and that measuring the DTC could reveal impaired EF.[1] In addition, the findings of Etemadi reported that a high DTC of the CRR was associated with falls indicative of EF deficits in multiple sclerosis patients.[11] Eventually, mutual interference could indicate more impaired EF. In brain-imaging studies, more activation in the prefrontal cortex was observed in healthy people and stroke patients during dual tasking.[27,28] This area was primarily responsible for EF with key functions of planning, execution, monitoring, inhibition, and attention-shifting. Thus, EF plays the most important role in dual tasking.[29] More impairment in EF could lead to poor DT performance and high DTI. Regarding balance, there were main effects of the degree of DTIs on TUG. Interestingly, the group with mild MDTI completed TUG faster than the group with severe MDTI, and the group with severe CDTI completed TUG faster than the mild CDTI group, indicating that the group who paid more attention to motor tasks, reducing attention to cognitive tasks, showed better performance in TUG. These results were similar to the correlation analysis findings shown above (FAC). We speculated that the reasons for these findings were the natural task prioritization of stroke patients and the difficult environment for the subjects with slow speed (< 0.8 m/s), which could sufficiently affect DTI.[12,13,20] Unlike healthy people who could focus on a secondary task during DTs because there was no need to pay more attention to posture control in ST and DT environments, people with stroke might tend to pay more attention to postural control (motor) over cognition in ST conditions as well as DT conditions due to limited capacity and motor deficits, indicating excessive posture-first.[3,26] In addition, because TUG was not a simple task but a more complex task including standing up, walking, turning, and sitting, which required more attention similar to that of a DT, the groups with higher motor prioritization would have better performance in difficult environments such as TUG. However, there were no effects on BBS and, it was assumed that was because the BBS was a less dynamic task than TUG and composed of simple tasks that did not require more attention, unlike DTs and TUG, so the degree of DTI would not have affected the BBS. Fall efficacy was not affected by the degree of DTI. We speculated that the subjects had relatively low fall efficacy (less concern of falling), which could not influence DTI. Similar to our results, the findings of Reelick et al showed that fear of falling did not affect DT gait and balance.[30] In the multiple regression analysis results, there was association between overall DTI and EF. In particular, TUG and EF were regarded as the factors most influencing MDTI, and CDTI was greatly influenced by EF. These results were consistent with previous findings that DTI differed depending on the degree of postural and cognitive reserves and was associated with impairments in functional mobility and EF for older adults and stroke patients.[14,23] This study had some limitations.
-
1)
It is difficult to generalize these results for all people with stroke due to the small sample size and inclusion of only stroke patients with lower speed.
-
2)
The methodological design did not address the location of lesions related to dual-tasking.
-
3)
There was only one type of cognitive task applied in the DT assessment (subtraction).
Considering these points, future research is warranted. People with stroke have poor DT performance and high DTI during dual-tasking. To evaluate DTI in those people, simultaneously measuring DTI in motor function and cognition must be done. Hence, it is important to identify the effects of the degree of DTI on gait, balance, DTC, cognitive ability, balance, and fall efficacy in people with stroke and identify the factors related to DTI. Our findings indicated the degree of MDTI affected gait, balance, and EF, and the degree of CDTI affected the CRR in DT conditions, balance, and EF. Furthermore, mutual interference showed a significant reduction in EF. Therefore, our study provided information for designing interventions and evaluating DT ability in the clinic.
Acknowledgments
We thank Harrisco (https://en.harrisco.net) for editing and reviewing this manuscript for English language
Author contributions
Conceptualization: Chang Yoon Baek.
Data curation: Hyun Sik Yoon, Kyoung Yee Kang.
Formal analysis: Chang Yoon Baek, Hyeong Dong Kim.
Investigation: Hyun Sik Yoon, Kyoung Yee Kang.
Methodology: Chang Yoon Baek, Hyun Sik Yoon.
Project administration: Chang Yoon Baek, Hyun Sik Yoon.
Resources: Kyoung Yee Kang.
Supervision: Chang Yoon Baek, Hyeong Dong Kim.
Validation: Chang Yoon Baek.
Visualization: Hyun Sik Yoon, Kyoung Yee Kang.
Writing – original draft: Chang Yoon Baek.
Writing – review & editing: Chang Yoon Baek.
Footnotes
Abbreviations: BBS = berg balance scale, CDTI = cognitive dual-task interference, CRR = correct response rate, DT = dual-task, DTC = dual-task cost, DTI = dual-task interference, FES = fall efficacy scale, K-MMSE = Korean version of the Mini-Mental State Examination, MDTI = motor dual-task interference, ST = single-task, TMTB = trail making test B, TUG = time up and go.
How to cite this article: Baek CY, Yoon HS, Kim HD, Kang KY. The effect of the degree of dual-task interference on gait, dual-task cost, cognitive ability, balance, and fall efficacy in people with stroke: a cross-sectional study. Medicine. 2021;100:24(e26275).
This study was registered at the Clinical Trial Registry of Korea (https://cris.nih.go.kr. No. KCT0005623; 11/24/2020).
The authors have no funding and conflicts of interest to disclose. Data are available by requesting the corresponding author.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data are reported as mean ± standard deviation (SD), CRR = correct response rate, DTI = dual-task interference, FAC = functional ambulation classification, H = hemorrhage, I = infarction, K-MMSE = Korean version of Mini-Mental State Examination.
P < .05 by independent t test.
CRR = correct response rate, DTI = dual-task interference, FAC = functional ambulation classification, MMSE = mini-mental state examination.
P < .05 by Pearson correlation analysis.
Data are reported as mean ± standard deviation (SD), BBS = berg balance scale, CDTI = cognitive DTI, CRR = correct response rate, DTI = dual-task interference, FES = fall efficacy scale, MDTI = motor DTI, TMTB = trail making test B, TUG = time up and go.
depending on the degree of MDTI difference in performance (mild MDTI > severe MDTI)
depending on the degree of CDTI difference in performance (mild CDTI > severe CDTI)
depending on the degree of CDTI difference in performance (mild CDTI < severe CDTI)
difference between group with severe MDTI and CDTI and group with severe MDTI and mild CDTI.
P < .05.
P < .01.
P < .001 by two-way analysis of variance.
DTI = dual-task interference, TMTB = trail making test B, TUG = time up and go.
P < .05.
P < .01.
P < .001 by stepwise multiple linear regression.
References
- [1].Montero-Odasso MM, Sarquis-Adamson Y, Speechley M, et al. Association of dual-task gait with incident dementia in mild cognitive impairment: results from the gait and brain study. JAMA Neurol 2017;74:857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brustio PR, Magistro D, Zecca M, Rabaglietti E, Liubicich ME, et al. Age-related decrements in dual-task performance: Comparison of different mobility and cognitive tasks. A cross sectional study. PLoS One 2017;12:e0181698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bayot M, Dujardin K, Tard C, et al. The interaction between cognition and motor control: A theoretical framework for dual-task interference effects on posture, gait initiation, gait and turning. Neurophysiol Clin 2018;48:361–75. [DOI] [PubMed] [Google Scholar]
- [4].Plummer P, Eskes G. Measuring treatment effects on dual-task performance: a framework for research and clinical practice. Front Hum Neurosci 2015;9:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee KB, Kim JH, Lee KS. The relationship between motor recovery and gait velocity during dual tasks in patients with chronic stroke. J Phys Ther Sci 2015;27:1173–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yang Y-R, Chen Y-C, Lee C-S, Cheng SJ, Wang RY, et al. Dual-task-related gait changes in individuals with stroke. Gait Posture 2007;25:185–90. [DOI] [PubMed] [Google Scholar]
- [7].Patel P, Bhatt T. Task matters: influence of different cognitive tasks on cognitive-motor interference during dual-task walking in chronic stroke survivors. Top Stroke Rehabil 2014;21:347–57. [DOI] [PubMed] [Google Scholar]
- [8].Baetens T, De Kegel A, Palmans T, et al. Gait analysis with cognitive-motor dual tasks to distinguish fallers from nonfallers among rehabilitating stroke patients. Arch Phys Med Rehabil 2013;94:680–6. [DOI] [PubMed] [Google Scholar]
- [9].Wajda DA, Motl RW, Sosnoff JJ. Dual task cost of walking is related to fall risk in persons with multiple sclerosis. J Neurol Sci 2013;335:160–3. [DOI] [PubMed] [Google Scholar]
- [10].Demirdel S, Erbahçeci F. Investigation of the Effects of dual-task balance training on gait and balance in transfemoral amputees: a randomized controlled trial. Arch Phys Med Rehabil 2020;101:1675–82. [DOI] [PubMed] [Google Scholar]
- [11].Etemadi Y. Dual task cost of cognition is related to fall risk in patients with multiple sclerosis: a prospective study. Clin Rehabil 2017;31:278–84. [DOI] [PubMed] [Google Scholar]
- [12].Yang L, Lam FM, Huang M, et al. Dual-task mobility among individuals with chronic stroke: changes in cognitive-motor interference patterns and relationship to difficulty level of mobility and cognitive tasks. Eur J Phys Rehabil Med 2017;54:526–35. [DOI] [PubMed] [Google Scholar]
- [13].Schmid A, Duncan PW, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke 2007;38:2096–100. [DOI] [PubMed] [Google Scholar]
- [14].Muci B, Keser I, Meric A, et al. What are the factors affecting dual-task gait performance in people after stroke? Physiother Theory Pract 2020;01–8. [DOI] [PubMed] [Google Scholar]
- [15].Kim J-S, Jeon H-S, Jeong Y-G. Relationships between cognitive function and gait-related dual-task interference after stroke. Phys Ther Korea 2014;21:80–8. [Google Scholar]
- [16].Plummer-D’Amato P, Altmann LJ. Relationships between motor function and gait-related dual-task interference after stroke: a pilot study. Gait Posture 2012;35:170–2. [DOI] [PubMed] [Google Scholar]
- [17].Patel P, Lamar M, Bhatt T. Effect of type of cognitive task and walking speed on cognitive-motor interference during dual-task walking. Neuroscience 2014;260:140–8. [DOI] [PubMed] [Google Scholar]
- [18].Tsang CSL, Pang MYC. Association of subsequent falls with evidence of dual-task interference while walking in community-dwelling individuals after stroke. Clin Rehabil 2020;34:971–80. [DOI] [PubMed] [Google Scholar]
- [19].Lienhard K, Schneider D, Maffiuletti NA. Validity of the Optogait photoelectric system for the assessment of spatiotemporal gait parameters. Med Eng Phys 2013;35:500–4. [DOI] [PubMed] [Google Scholar]
- [20].Mori T, Takeuchi N, Izumi S-I. Prefrontal cortex activation during a dual task in patients with stroke. Gait Posture 2018;59:193–8. [DOI] [PubMed] [Google Scholar]
- [21].Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther 2008;88:559–66. [DOI] [PubMed] [Google Scholar]
- [22].Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. [DOI] [PubMed] [Google Scholar]
- [23].Parikh H, Shah C. Relationship between executive function and dual task physical performance among older adults-a cross sectional study. Int J Phys Med Rehabil 2017;5:4172. [Google Scholar]
- [24].Hellström K, Lindmark B, Fugl-Meyer A, et al. Swedish version: does it reflect clinically meaningful changes after stroke? Disabil Rehabil 2002;24:471–81. [DOI] [PubMed] [Google Scholar]
- [25].Schwenk M, Zieschang T, Oster P, et al. Dual-task performances can be improved in patients with dementia: a randomized controlled trial. Neurology 2010;74:1961–8. [DOI] [PubMed] [Google Scholar]
- [26].Timmermans C, Roerdink M, Janssen TW, et al. Dual-task walking in challenging environments in people with stroke: cognitive-motor interference and task prioritization. Stroke Res Treat 2018;2018:01–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lu C-F, Liu Y-C, Yang Y-R, et al. Maintaining gait performance by cortical activation during dual-task interference: a functional near-infrared spectroscopy study. PLoS One 2015;10:e0129390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Al-Yahya E, Johansen-Berg H, Kischka U, et al. Prefrontal cortex activation while walking under dual-task conditions in stroke: a multimodal imaging study. Neurorehabil Neural Repair 2016;30:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Belghali M, Chastan N, Davenne D, et al. Improving dual-task walking paradigms to detect prodromal Parkinson's and Alzheimer's diseases. Front Neurol 2017;8:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Reelick MF, van Iersel MB, Kessels RP, et al. The influence of fear of falling on gait and balance in older people. Age Ageing 2009;38:435–40. [DOI] [PubMed] [Google Scholar]


