Abstract
Most patients with coronavirus disease 2019 (COVID-19) have mild to moderate illness not requiring hospitalization. However, no study has detailed the evolution of symptoms in the first month of illness.
At our institution, we conducted remote (telephone and video) visits for all adult outpatients diagnosed with COVID-19 within 24 h of a positive nasopharyngeal polymerase chain test for SARS-CoV-2. We repeated regular video visits at 7, 14, and 28 days after the positive test, retrospectively reviewed the prospective data collected in the remote visits, and constructed a week by week profile of clinical illness, through week 4 of illness.
We reviewed the courses of 458 symptomatic patients diagnosed between March 12, 2020, and June 22, 2020, and characterized their weekly courses. Common initial symptoms included fever, headache, cough, and chest pain, which frequently persisted through week 3 or longer. Upper respiratory or gastrointestinal symptoms were much shorter lived, present primarily in week 1. Anosmia/ageusia peaked in weeks 2 to 3. Emergency department visits were frequent, with 128 visits in the 423 patients who were not hospitalized and 48 visits among the 35 outpatients (7.6%) who were eventually hospitalized (2 subsequently died). By the fourth week, 28.9% said their illness had completely resolved. After the 4-week follow up, 20 (4.7%) of the 423 nonhospitalized patients had further medical evaluation and management for subacute or chronic COVID-19 symptoms.
Mild to moderate outpatient COVID-19 is a prolonged illness, with evolving symptoms commonly lasting into the fourth week of illness.
Keywords: adult, coronavirus infections/diagnosis/drug therapy/prevention & control, COVID-19, outpatient, SARS-CoV-2
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), manifests in various ways ranging from asymptomatic infection to severe respiratory failure and multiorgan dysfunction that can result in hospitalization and death. Because little was known about SARS-CoV-2 before 2020, the medical literature has exploded with descriptions of the virus, the illness, and outcomes for a variety of interventions. Among patients with severe illness requiring hospitalization, a syndrome of fever, cough, fatigue, myalgia, and dyspnea is common, often additionally accompanied by gastrointestinal symptoms, such as diarrhea. Chest imaging often shows multifocal ground-glass infiltrates. Symptomatic illness can be prolonged and recovery slow.[1] Mild COVID-19 illness is defined by “mild symptoms without dyspnea, ” whereas moderate illness is defined by the presence of clinical or radiographic evidence of lower respiratory tract infection with oxygen saturations that exceed 94%.[2]
Although approximately 85% of persons with COVID-19 have mild illness,[3] not much has been published to describe the characteristics and courses of adult patients with mild illness in an outpatient setting. Recent studies of outpatients have described similar symptoms at presentation as those of patients who eventually require hospitalisation[4,5] early in the outpatient course. Notably, most outpatients who initially describe symptoms remain symptomatic 1 to 2 weeks after diagnosis.[5,6] With the onset of the pandemic, our infectious disease practice instituted regular telephone or video visits for our outpatients with COVID-19 over the first month of illness. In this study, we aimed to describe the first month of COVID-19 illness among patients with mild to moderate illness.
2. Methods
This study was approved by the Mayo Clinic Institutional Review Board, and written informed consent was waived for those who provided research authorization. The study is reported in keeping with the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline for cohort studies.[7] We retrospectively reviewed electronic health records of all adults aged 18 years and older who had positive results from a polymerase chain reaction (PCR) test for SARS-CoV-2 RNA between March 12, 2020, and June 22, 2020. Only current patients or employees of our institution were tested. All patients had nasopharyngeal swabs collected either in the emergency department (ED) or drive-through testing center, and swabs were analyzed the same day in our institution's laboratory using the RealTime SARS-COV-2 assay and m2000 platform (Abbott Molecular). Patients who were evaluated, tested, and dismissed home from the ED were included, but those who were admitted to the hospital directly from the ED were excluded. We also excluded those whose diagnosis was made during hospitalization.
Test results were reported by telephone to the patient by a medical practitioner or nurse within 24 h, at which time an initial history was taken and counseling conducted. Video (or telephone) visits were then conducted 2 to 3 days after the initial contact and repeated at days 7, 14, and 30 after the positive test date. Because of iterative feedback and patient volumes, we later discontinued the day 2 to 3 visit. All virtual visits were conducted to counsel patients regarding symptom management, to assess risk factors, to advise patients on the need for isolation, to counsel for warning symptoms or signs that suggested a need for further medical evaluation, and/or to coordinate retesting if needed. Patient symptoms, medical history, and epidemiologic risk factors were prospectively collected in a standardized fashion using a templated note in the electronic health record, then retrospectively collected for the purpose of analysis.
We included patients who had at least 1 symptom of COVID-19 at any point during the follow-up time of 4 weeks. We defined mild or moderate severity by the absence of severe symptoms requiring hospitalization. Mild illness was further defined as absence of dyspnea, whereas moderate illness was defined as presence of dyspnea.[2] We also collected information regarding patients’ ability to perform activities of daily living (ADLs). To account for the variation in timing of illness to positive test, we defined “week of illness” by the date of illness onset, not by the date of the first positive PCR test. Where 2 follow-up dates were within the same week, we used the earlier of the 2 dates.
For the patients included in the study cohort, we separately reviewed their electronic health records for continued care for COVID-19–related symptoms after the 4-week follow-up. For the second record review, we defined subacute COVID-19 syndrome by symptoms that persisted for longer than 4 weeks but less than 12 weeks and chronic postacute COVID-19 by symptoms that persisted beyond 12 weeks from the onset of symptoms.[8]
We compiled and managed our data using the REDCap (Research Electronic Data Capture) tool hosted at our institution. We summarized data with number and percentage. The differences in categorical variables were analyzed by the Pearson χ2 test and ordinal variables by the trend test. Continuous variables were compared using the linear model analysis of variance test. A P value of <.05 was considered significant.
3. Results
From March 12, 2020, through June 22, 2020, 550 sequential outpatients had PCR-confirmed SARS-CoV-2 infection diagnosed in the outpatient setting. Of these patients, 92 were excluded: 15 were 17 years of age or younger or were of unknown age, 66 had asymptomatic illness at every time point in follow-up, and 11 had unknown symptoms/severity of illness, yielding a final study cohort of 458 patients. Among the 458 patients, 35 (7.6%) were ultimately hospitalized during the 4 weeks from illness onset, 2 of whom subsequently died of COVID-19. Table 1 summarizes and compares the demographic characteristics, comorbid conditions, and follow-up for the hospitalized and nonhospitalized groups.
Table 1.
Demographic characteristics, medical history, and follow-up of 458 symptomatic adult outpatients with COVID-19.
| No. (%)∗ | |||
| Characteristics | Never hospitalized (n = 423) | Hospitalized during follow-up (n = 35) | P value† |
| Sex | .43 | ||
| Male | 188 (44.4) | 18 (51.4) | |
| Female | 235 (55.6) | 17 (48.6) | |
| Age, median (range), y | 41 (18-93) | 52 (19-76) | .16‡ |
| Race | .82 | ||
| White | 316 (74.7) | 28 (80.0) | |
| Asian | 18 (4.3) | 2 (5.7) | |
| Black or African American | 18 (4.3) | 1 (2.9) | |
| Native American/Alaskan | 4 (1.0) | 0 (0) | |
| Other | 21 (5.0) | 1 (2.9) | |
| Not specified | 36 (8.5) | 3 (8.6) | |
| Hispanic/Latino ethnicity | 41 (9.7) | 4 (11.4) | .96 |
| Body mass index (n = 278) | .42 | ||
| Underweight (<18.5) | 6 (1.4) | 0 (0) | |
| Normal weight (18.5-24.9) | 77 (18.2) | 8 (22.9) | |
| Overweight (25.0–29.9) | 80 (18.9) | 9 (25.7) | |
| Obese (>30.0) | 82 (19.4) | 15 (42.9) | |
| Missing | 178 (42.1) | 3 (8.6) | |
| Comorbid condition§ | |||
| None | 178 (42.1) | 5 (14.3) | .001 |
| Hypertension | 84 (19.9) | 16 (45.7) | <.001 |
| Diabetes | 30 (7.1) | 11 (31.4) | <.001 |
| Pre-diabetes | 18 (4.3) | 2 (5.7) | .69 |
| Hyperlipidemia | 66 (15.6) | 14 (40.0) | <.001 |
| Atherosclerotic cardiovascular disease | 18 (4.3) | 8 (22.9) | <.001 |
| COPD | 10 (2.4) | 5 (14.3) | <.001 |
| Asthma | 49 (11.6) | 6 (17.1) | .33 |
| Chronic kidney disease|| | 5 (1.2) | 4 (11.4) | <.001 |
| Immunocompromised state¶ | 2 (0.5) | 3 (8.6) | <.001 |
| No. ED visits during follow-up | <.001# | ||
| 0 | 264 (62.4) | 4 (11.4) | |
| 1 | 89 (21.0) | 21 (60.0) | |
| 2 | 13 (3.1) | 5 (14.3) | |
| 3 or more | 4 (0.9) | 5 (14.3) | |
| Total visits | 128 | 48 | |
| Death from COVID-19 | 0 (0) | 2 (5.7) | |
Table 2 and the Figure 1 summarize the illness characteristics and symptoms of the 423 patients who never required hospitalization by week 4 of illness. Symptoms of upper respiratory illness (rhinitis, sore throat, nasal congestion) and gastrointestinal illness (nausea, vomiting, diarrhea) were common initial symptoms for a few days, but for many patients, headache, fever, and fatigue lasted for weeks. At each time point, cough was the most common symptom and was still present in 15.1% of patients in the fourth week of illness. The presence of dyspnea, distinguishing mild from moderate illness, was seen in a minority of patients and ranged from 14.4% in the first week of illness to 7.8% in the fourth week of illness. Figure 1
Table 2.
Illness characteristics and common symptoms of 423 symptomatic outpatients with mild to moderate COVID-19 during the 4-week follow-up.
| No. (%) | |||||
| Characteristic/symptom | Illness Onset (N = 423) | Week 1 (n = 167) | Week 2 (n = 156) | Week 3 (n = 155) | Week 4 (n = 166) |
| Able to perform all ADLs | 306/389∗ (78.7) | 139 (83.2) | 118 (75.6) | 124 (80.0) | 108 (65.1) |
| Unable to perform all ADLs | 83/389∗ (21.3) | 24 (14.4) | 25 (16.0) | 10 (6.5) | 8 (4.8) |
| Visited the ED | 105 (24.8) | 9 (5.4) | 9 (5.8) | 6 (3.9) | 9 (5.4) |
| Illness resolved | 0 (0) | 2 (1.2) | 12 (7.7) | 21 (13.5) | 48 (28.9) |
| Symptoms | |||||
| Fever | 236 (55.8) | 76 (45.5) | 37 (23.7) | 11 (7.1) | 4 (2.4) |
| Chills or rigors | 22 (5.2) | 42 (25.1) | 11 (7.1) | 8 (5.2) | 2 (1.2) |
| Malaise | 22 (5.2) | 14 (8.4) | 16 (10.3) | 11 (7.1) | 1 (0.6) |
| Arthralgia | 38 (9.0) | 8 (4.8) | 6 (3.8) | 0 (0) | 0 (0) |
| Myalgia | 25 (5.9) | 47 (28.1) | 17 (10.9) | 4 (2.6) | 1 (0.6) |
| Headache | 63 (14.9) | 49 (29.3) | 46 (29.5) | 32 (20.6) | 13 (7.8) |
| Fatigue | 20 (4.7) | 33 (19.8) | 39 (25.0) | 34 (21.9) | 16 (9.6) |
| Anosmia/dysosmia | 84 (19.9) | 33 (19.8) | 45 (28.8) | 28 (18.1) | 19 (11.4) |
| Aguesia/dysguesia | 21 (5.0) | 29 (17.4) | 44 (28.2) | 25 (16.1) | 17 (10.2) |
| Upper respiratory symptoms | |||||
| Sore throat | 92 (21.7) | 14 (8.4) | 7 (4.5) | 0 (0) | 4 (2.4) |
| Rhinorrhea | 49 (11.6) | 1 (0.6) | 3 (1.9) | 2 (1.3) | 0 (0) |
| Nasal/sinus congestion | 88 (20.8) | 12 (7.2) | 16 (10.3) | 6 (3.9) | 5 (3.0) |
| Lower respiratory symptoms | |||||
| Cough | 117 (27.7) | 98 (58.7) | 72 (46.2) | 58 (37.4) | 25 (15.1) |
| Chest pain | 87 (20.6) | 7 (4.2) | 10 (6.4) | 7 (4.5) | 3 (1.8) |
| Dyspnea | 2 (0.5) | 24 (14.4) | 25 (16.0) | 20 (12.9) | 13 (7.8) |
| GI symptoms | |||||
| Anorexia | 127 (30.0) | 11 (6.6) | 15 (9.6) | 8 (5.2) | 2 (1.2) |
| Nausea | 15 (3.5) | 7 (4.2) | 13 (8.3) | 8 (5.2) | 3 (1.8) |
| Vomiting | 58 (13.7) | 1 (0.6) | 6 (3.8) | 1 (0.6) | 0 (0) |
| Diarrhea | 11 (2.6) | 16 (9.6) | 18 (11.5) | 8 (5.2) | 4 (2.4) |
| Palpitations | 165 (39.0) | 2 (1.2) | 1 (0.6) | 0 (0) | 3 (1.8) |
| Light-headedness | 0 (0) | 9 (5.4) | 10 (6.4) | 4 (2.6) | 5 (3.0) |
| Syncope | 11 (2.6) | 0 (0) | 1 (0.6) | 0 (0) | 0 (0) |
Figure 1.
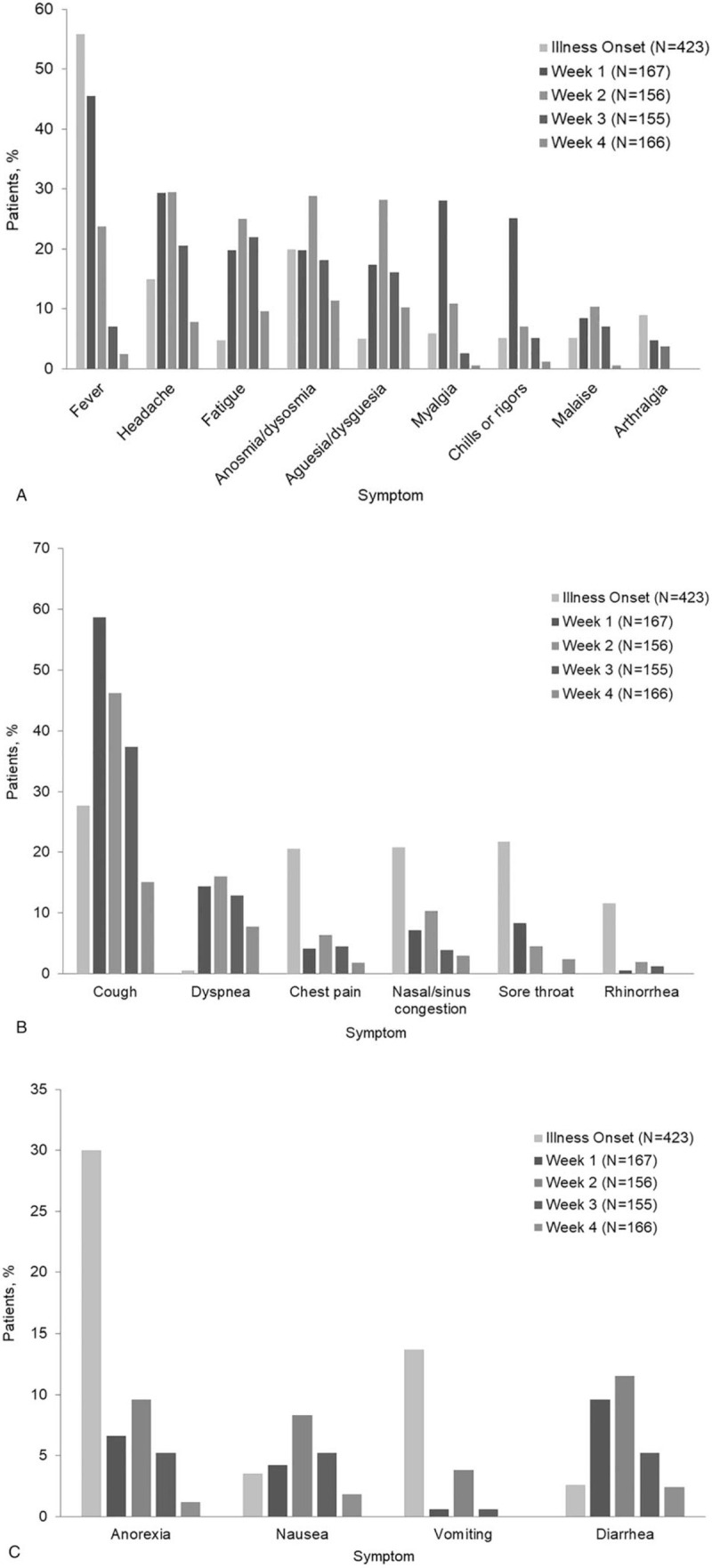
Summary of symptoms by week of illness for 423 patients with mild to moderate COVID-19 who were never hospitalized. (A) General symptoms. (B) Respiratory symptoms. (C) Gastrointestinal symptoms.
Table 3 summarizes the illness characteristics and symptoms of the 35 adults who started follow-up as outpatients but were eventually hospitalized within the 4 weeks after symptom onset. Once patients were hospitalized, no further symptoms were tallied for them. The initial characteristics and symptoms of patients eventually hospitalized did not differ from those of patients never hospitalized, although lower respiratory symptoms (chest pain, dyspnea) defining moderate illness were more prevalent at 3 and 4 weeks of follow-up among the group of patients who were eventually hospitalized.
Table 3.
Illness characteristics and symptoms of 35 adult outpatients hospitalized during the 4-week outpatient follow-up for COVID-19∗.
| No. (%) | |||||
| Characteristic/symptom | Illness onset (N = 35) | Week 1 (n = 10) | Week 2 (n = 12) | Week 3 (n = 8) | Week 4 (n = 7) |
| Able to perform all ADLs | 15/33† (45.5) | 4 (40.0) | 5 (41.7) | 5 (62.5) | 6 (85.7) |
| Unable to perform all ADLs | 18/33† (54.5) | 6 (60.0) | 7 (58.3) | 3 (37.5) | 1 (14.3) |
| Visited the ED | 31 (88.6) | 6 (60.0) | 6 (50.0) | 5 (62.5) | 2 (28.6) |
| Illness resolved | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Selected symptoms | |||||
| Fever | 21 (60.0) | 4 (40.0) | 4 (33.3) | 2 (25.0) | 0 (0) |
| Chills or rigors | 3 (8.6) | 2 (20.0) | 4 (33.3) | 1 (12.5) | 1 (14.3) |
| Malaise | 6 (17.1) | 2 (20.0) | 4 (33.3) | 0 (0) | 1 (14.3) |
| Arthralgia | 3 (8.6) | 1 (10.0) | 3 (25.0) | 0 (0) | 0 (0) |
| Myalgia | 2 (5.7) | 1 (10.0) | 2 (16.7) | 0 (0) | 0 (0) |
| Headache | 12 (34.3) | 1 (10.0) | 4 (33.3) | 2 (25.0) | 1 (14.3) |
| Fatigue | 9 (25.7) | 2 (20.0) | 3 (25.0) | 1 (12.5) | 2 (28.6) |
| Anosmia/dysosmia | 4 (11.4) | 1 (10.0) | 2 (16.7) | 0 (0) | 0 (0) |
| Aguesia/dysguesia | 1 (2.9) | 1 (10.0) | 3 (25.0) | 0 (0) | 0 (0) |
| Upper respiratory symptoms | |||||
| Sore throat | 1 (2.9) | 0 (0) | 0 (0) | 1 (12.5) | 0 (0) |
| Rhinorrhea | 2 (5.7) | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) |
| Nasal/sinus congestion | 13 (37.1) | 2 (20.0) | 1 (8.3) | 1 (12.5) | 0 (0) |
| Lower respiratory symptoms | |||||
| Cough | 9 (25.7) | 5 (50.0) | 6 (50.0) | 4 (50.0) | 2 (28.6) |
| Chest pain | 5 (14.3) | 0 (0) | 1 (8.3) | 3 (37.5) | 2 (28.6) |
| Dyspnea | 3 (8.6) | 5 (50.0) | 3 (25.0) | 3 (37.5) | 2 (28.6) |
| GI symptoms | |||||
| Anorexia | 15 (42.9) | 1 (10.0) | 2 (16.7) | 0 (0) | 0 (0) |
| Nausea | 1 (2.9) | 1 (10.0) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 8 (22.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea | 4 (11.4) | 2 (20.0) | 3 (25.0) | 1 (12.5) | 0 (0) |
| Palpitations | 9 (25.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Light-headedness | 2 (5.7) | 0 (0) | 3 (25.0) | 2 (25.0) | 0 (0) |
| Syncope | 1 (2.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
We compared the demographic characteristics, underlying comorbid conditions, and body mass index of those patients who were eventually hospitalized versus those who were not hospitalized. Hospitalized patients were older and more likely to have a host of comorbid conditions such as hypertension, diabetes, hyperlipidemia, atherosclerotic heart disease and arrhythmias, chronic kidney disease, thromboembolic disease such as pulmonary embolus, chronic obstructive pulmonary disease (but not asthma), and others (Table 1). Those who were eventually hospitalized also had more respiratory symptoms, were less likely to have illness resolution, and made more trips to the ED than those who were not hospitalized (P < .001).
Of the 423 nonhospitalized patients included in the study, 20 (4.7%) had continued medical evaluation and management for known or suspected persistent COVID-19–related symptoms beyond the first 4 weeks of their COVID-19 illness and met the definition for subacute or chronic postacute COVID-19 syndrome. Among the 20 patients, 7 (35%) had persistent COVID-19 symptoms that were present in their acute illness. There were no demographic characteristics of those with postacute COVID-19 symptoms that predicted persistent symptoms (calculations not shown).
4. Discussion
Relatively little has been published regarding the course and outcomes for outpatients with mild or moderate symptoms of COVID-19. This study adds to the existing knowledge of COVID-19 by providing a detailed weekly summary of follow-up for patients with illness mild enough for outpatient treatment. At the time of our study, no outpatient treatment trials or new interventions were available for patients, other than standard treatments for symptoms. A distinguishing characteristic of our study is that the cohort had real-time follow-up at days 7, 14, and 28 after their positive test results, which gave us a unique opportunity to assess the duration and timing of symptom resolution. By retiming those follow-up visits to the day of illness onset, rather than the day of test positivity, we were able to construct a weekly profile of symptoms for the first 4 weeks of infection.
Our results show the wide range of COVID-19 symptoms and their evolution over the 4-week follow-up. Because of the difficulty of accessing outpatient testing in the community, a few patients came to our institution late into their illness for testing, and although we recorded their initial symptoms, we were unable to collect a tally of symptoms by week. However, most patients sought testing 1 to 2 days after illness onset, and few were found to be positive before the onset of symptoms. Like the findings of other studies,[2,6,9] our results showed that patients had a host of common symptoms and occasionally atypical symptoms.[10] Common initial symptoms included fever, headache, malaise, cough, and chest pain, which frequently persisted through week 3 (or longer), and upper respiratory (except for anosmia/ageusia) or gastrointestinal symptoms, which were present primarily in the first week. Anosmia and ageusia were most common in the second and third weeks of illness. Inability to perform ADLs was more prevalent in the first and second weeks of illness, and most patients had recovered sufficiently to perform most ADLs by the fourth week of illness. Visits to the ED occurred primarily in the first week of illness, although ED visits continued for some patients throughout the entire 4-week observation period. Only 28.9% of the 423 patients who remained outpatients for the entire follow-up reported that their illness resolved completely by the end of week 4.
We frequently observed in the video visits that symptoms were not consistent over time. They could be better, worse, or even resolve, only to return again—making it difficult to determine the true date the illness resolved. For some patients, symptoms such as fatigue or sense of “not quite back to normal” lasted longer than the follow-up time of this study, which could have contributed to the reasons for the low (28.9%) percentage of patients endorsing “resolved illness” by week 4.
A few studies have described mild or moderate COVID-19 illness.[6,9,11] Kim et al[9] summarized symptoms of 172 patients who were in community isolation facilities in South Korea for mild illness; the most common symptom was cough in 40.2%, and fever was noted in only 11.6%. By contrast, we noted fever to be the most common initial symptom (reported by 55.8%) and cough a predominant symptom initially, increasing in the first and second weeks before decreasing to 37.4% and 15.1% in the third and fourth weeks of illness, respectively; these differences may reflect differences in the approach to referring for testing. Another study described 282 patients with mild to moderate COVID-19 illness in patients hospitalized in China and described the duration (mean, 9 days; range, 1–37) of symptoms or recovery.[9,11] A recent study of 274 outpatients with confirmed, symptomatic, mild COVID-19 infection also described symptoms and outcomes, but from a single telephone interview at a median (range) of 16 (14–21) days after a positive test.[6] At the time of the call, at least 94% of patients still had at least 1 symptom. In addition, persons in progressively older age groups were less likely than younger adults to have returned to normal health, and persons with more concomitant risk and comorbid conditions were less likely than those with no comorbid conditions to have returned to normal health. The most common symptoms in that study included fatigue, cough, dyspnea, congestion, and anosmia/ageusia.[6] Our findings from the current study are congruent with prior studies but provide further detail regarding symptoms in the third and fourth weeks after illness onset, details that were lacking in prior studies.
Another early study described the symptoms of outpatients with confirmed, probable, and possible SARS-CoV-2 infection enrolled in a phase III randomized trial comparing hydroxychloroquine with placebo.[5] In this study, early infection was defined as 0 to 2 days of symptoms; mid infection, 3 to 5 days; and late infection, 5 to 30 days since symptom development, although the longest duration of symptoms reported was 11 days. Cough (82%), fever (67%), fatigue (62%), headache (60%), and dyspnea (46%) were the most common symptoms regardless of time from symptom onset, and there was no clear pattern of resolution, perhaps because of the short timeframe of symptoms.[5] Other studies reported similar types of symptoms at a patient's first contact with a health care practitioner,[4,12] but none of these studies described the study patients’ clinical course to complete recovery.[4,5,12]
Anosmia and ageusia are unusual symptoms among persons with other respiratory viruses, although they are markers of SARS-CoV-2 infection and have been suggested as possible prognostic factors for less severe COVID-19 illness.[13] In our study, a substantial proportion of patients had anosmia or ageusia (20% initially and up to 29% during follow-up), with onset in the first week, which improved but was not gone by week 4. Although this finding has been thought to be a prognostic factor for less severe COVID-19 illness, we found anosmia present in up to 16.7% of those who were ultimately hospitalized.
Patients in both cohorts (never hospitalized and eventually hospitalized) frequently went to the ED (never hospitalized, 105/423 [24.8%]; eventually hospitalized, 31/35 [88.6%]). Although visits to the ED occurred with decreasing frequency during follow-up, some patients were seen up to 5 times during their outpatient course, and the number of ED visits was predictive of hospitalization (P < .001). Race, sex, and body mass index were not predictive of hospitalization, although older age and comorbid conditions significantly predicted eventual hospitalization.
Because this cohort exhibited symptoms into the third and fourth weeks following the onset of COVID-19 illness, we conducted a follow-up record review looking for evidence of persistent symptoms beyond the first 4 weeks. From this review, we identified 20 of the 423 outpatients (4.7%) who sought medical evaluation for subacute or chronic symptoms attributed to their COVID-19 illness. Thirteen of the 20 patients (65%) had different COVID-19–attributed symptoms in their extended follow-up than the symptoms they reported in the third and fourth week of video visits, and at their last video visit, they reported that their COVID-19–related symptoms had resolved (an example of this situation would be a patient who sought medical evaluation for COVID-19–related depression after an illness with fever, respiratory symptoms, and fatigue). Therefore, most symptoms expressed in the third- and fourth-week video visits likely represented the tail end of the spectrum of acute COVID-19 symptoms rather than the beginning of postacute COVID-19 syndrome.
A strength of this study was the real-time collection of data at routinely scheduled video visits, thereby reducing the likelihood of recall bias. However, the study also has several limitations. We tested only patients and employees of our institution. Because our institution has primary and tertiary care practices, patients with underlying good health as well as complex medical illnesses were included, with a high proportion of our patients requiring complex medical care. Thus, our study patients may not reflect the general population. Patients were limited to those who either self-referred or were referred by physicians of our local practice. Most were White, also limiting the generalizability of the findings. Although we prospectively collected data, when our region experienced a surge of COVID-19 cases, we recruited a number of medical practitioners from our general and specialty medical practices to assist us in completing our video visits and data collection, potentially inadvertently inserting practice bias.
In summary, most symptomatic adult outpatients with COVID-19 symptoms did not require hospitalization in the 4-week follow-up period; however, a substantial proportion had illness that precluded participation in ADLs, and their symptoms persisted and commonly evolved throughout the follow-up period. These patients required numerous ED visits, and 7.6% required hospitalization. The outpatient course was protracted, and by week 4, illness had completely resolved in less than one-third of patients.
Author contributions
Conceptualization: Janis Blair.
Data curation: Ashwini Gotimukul, Fangfang Wang, Syeda A. Mina, Helen C. Bartels, Mark W. Burns, Amy E. Kole, Avinash Vikram.
Formal analysis: Janis Blair, Skye A. Buckner Petty.
Project administration: Janis Blair.
Supervision: Janis Blair, Ashwini Gotimukul.
Writing – original draft: Janis Blair.
Correction
The affiliations for three authors have been updated. Holenarasipur R. Vikram has been changed from a,d to a. Avinash Vikram has been changed from a,e to a,d. Ashwini Gotimukul has been changed from a to a,e.
Footnotes
Abbreviations: ADLs = activities of daily living, COVID-19 = coronavirus disease 2019, ED = emergency department, PCR = polymerase chain reaction, REDCap = Research Electronic Data Capture, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
How to cite this article: Blair JE, Gotimukul A, Wang F, Mina SA, Bartels HC, Burns MW, Kole AE, Vikram HR, Gea-Banacloche JC, Seville MT, Petty SA, Vikram A, Orenstein R. Mild to moderate COVID-19 illness in adult outpatients: Characteristics, symptoms, and outcomes in the first 4 weeks of illness. Medicine. 2021;100:24(e26371).
The authors have no conflicts of interest to disclose.
Data Availability Statement: Data for the study are available on request from the author.
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease 2019; ED = emergency department.
Unless indicated otherwise.
Pearson χ2 test, unless noted otherwise.
Linear model analysis of variance.
Other medical conditions more likely to be present in hospitalized patients: pulmonary embolism (but not deep venous thrombosis), cardiac arrhythmias, gastroesophageal reflux disease, hypothyroidism, obstructive sleep apnea.
Chronic kidney disease grade II or greater, or estimated glomerular filtration rate less than 50.
Immunocompromised state was defined as the presence of organ transplantation, hematologic malignancy, infection with human immunodeficiency virus with <200 CD4 cells, or immunosuppressant medications for autoimmune or inflammatory conditions. In the present cohort, the immunocompromised group consisted solely of patients with hematologic malignancy (n = 4) or kidney transplantation (n = 1).
Trend test for ordinal variables.
ADLs = activities of daily living; COVID-19 = coronavirus disease 2019; ED = emergency department; GI = gastrointestinal.
Thirty-four missing assessments of ADLs.
ADLs = activities of daily living; COVID-19 = coronavirus disease 2019; ED = emergency department; GI = gastrointestinal.
Tallied symptoms include only those recorded before hospitalization.
Two missing assessments of ADLs.
References
- [1].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med 2020;383:1757–66. [DOI] [PubMed] [Google Scholar]
- [3].World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. World Health Organization. Available at: https://apps.who.int/iris/handle/10665/330893. Accessed January 11, 2021 (English version). [Google Scholar]
- [4].Lapostolle F, Schneider E, Vianu I, et al. Clinical features of 1487 COVID-19 patients with outpatient management in the Greater Paris: the COVID-call study. Intern Emerg Med 2020;15:813–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pullen MF, Skipper CP, Hullsiek KH, et al. Symptoms of COVID-19 outpatients in the United States. Open Forum Infect Dis 2020;7:ofaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network: United States, March–June 2020. MMWR Morb Mortal Wkly Rep 2020;69:993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–7. [DOI] [PubMed] [Google Scholar]
- [8].Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021;27:601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim GU, Kim MJ, Ra SH, et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect 2020;26:948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Singhania N, Bansal S, Singhania G. An atypical presentation of novel coronavirus disease 2019 (COVID-19). Am J Med 2020;133:e365–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xia L, Chen J, Friedemann T, et al. The course of mild and moderate COVID-19 infections: the unexpected long-lasting challenge. Open Forum Infect Dis 2020;7:ofaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lam PW, Sehgal P, Andany N, et al. A virtual care program for outpatients diagnosed with COVID-19: a feasibility study. CMAJ Open 2020;8:E407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Foster KJ, Jauregui E, Tajudeen B, Bishehsari F, Mahdavinia M. Smell loss is a prognostic factor for lower severity of coronavirus disease 2019. Ann Allergy Asthma Immunol Oct 2020;125:481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]


