Abstract
Background:
More and more scholars have called for the cumulative live birth rate (CLBR) of a complete ovarian stimulation cycle as a key indicator for assisted reproductive technology. This research aims to study the CLBR of the first ovarian hyperstimulation cycles and analyze the related prognosis factors that might affect the CLBR.
Methods:
Our retrospective study included first in vitro fertilization or intracytoplasmic sperm injection (IVF/ICSI) cycles performed between January 2013 to December 2014. A total of 17,978 couples of first ovarian hyperstimulation IVF/ICSI cycles were included. The study was followed up for 4 years to observe the CLBR. The multivariable logistic regression model was used to analyze the prognosis factor, P value of <0.05 was considered statistically significant.
Results:
The cumulative pregnancy rate was 58.14% (10,452/17,978), and the CLBR was 49.66% (8928/17,978). The female age was younger in the live birth group when compared with the non-live birth group (30.81 ± 4.05 vs. 33.09 ± 5.13, P < 0.001). The average duration of infertility was shorter than the non-live birth cohort (4.22 ± 3.11 vs. 5.06 ± 4.08, P < 0.001). The preliminary gonadotropin used and the total number of gonadotropin used were lower in the live birth group when compared with the non-live birth group (both P < 0.001). Meanwhile, the number of oocytes retrieved and transferrable embryos were both significantly higher in the live birth group (15.35 ± 7.98 vs. 11.35 ± 7.60, P < 0.001; 6.66 ± 5.19 vs. 3.62 ± 3.51, P < 0.001, respectively).
Conclusions:
The women's age, body mass index, duration of infertility years, infertility factors, controlled ovarian hyperstimulation protocol, the number of acquired oocytes, and number of transferrable embryos are the prognosis factors that significantly affected the CLBR.
Keywords: Cumulative live birth rates, IVF, ICSI, Controlled ovarian hyperstimulation, In vitro fertilization, Intracytoplasmic sperm injection
Introduction
At present, it is still remaining controversial how to accurately define the success of assisted reproductive technology (ART). More and more scholars have called for the cumulative live birth rate (CLBR) of a complete ovarian stimulation cycle as a key indicator for ART. Combined with domestic and foreign research progress in recent years, a consensus was reached by some experts on the definition, clinical significance, calculation methods, and prognosis factors of CLBR, in order to guide the clinical application of the CLBR all around the world.[1]
Given the encourage of single embryo transfer and the effectiveness of embryo frozen have improved distinctly over the last decades,[2] it has been recommended that the success of ART should be calculated as the live-birth rate per ovarian hyperstimulation in vitro fertilization or intracytoplasmic sperm injection (IVF/ICSI) cycle, including all following fresh and frozen embryo transfers (FET) cycles.
The CLBR at a given cycle was expressed as the probability of a live-birth from all cycles followed up to and including that cycle. This may answer the patient's inquiry that “what is my aggregate chance of a live birth with repeat ovarian stimulation and oocyte retrievals, together with the consequent embryo transfer from every cycle, up to a given cycle number.” However, the patients first come to the reproductive center who may ask “what is my chance of a live-birth with the first ovarian stimulation and oocytes retrieval.” The live-birth rate within the first IVF/ICSI cycle was defined as the probability of a live-birth from the first ovarian stimulation encompassing all following FET from that stimulation.
From this study, the CLBR of the first ovary hyperstimulation IVF/ICSI cycle was analyzed thoroughly. Meanwhile, the prognosis factors which may affect the CLBR are also studied. We hope to optimize the controlled ovarian hyperstimulation (COH) protocol and increase the CLBR. Those data might provide information for the patients during a consultation and assist the clinical doctors in designing the COH protocol.
Methods
Ethical approval
This study was approved by the Ethics Committee of Peking University Third Hospital (PUTH) (No: 2018S2-002).
Study design and participants
This is a retrospective, single-center cohort research. From January 2013 to December 2014, couples who had undergone IVF/ICSI in the Reproductive Center, PUTH, China, were enrolled and the outcomes assessed up to December 2018. The inclusion criteria were: (1) match the indications for IVF/ICSI treatment; (2) all the couples should be the first time undergone ovarian hyperstimulation IVF/ICSI cycles; (3) all cases underwent standard COH protocols. The exclusion criteria were: (1) the couples had previous ovarian hyperstimulation and IVF/ICSI cycles; (2) microstimulation or natural IVF/ICSI cycles; (3) cases for the purpose of sterility preservation or suffer chromosomal abnormality or monogenic diseases for preimplantation genetic testing cycles.
There were 26,826 cases of oocyte retrieval conducted during this period and 17,978 cases were in the first hyperstimulation IVF/ICSI cycles [Figure 1]. All patients underwent a thorough infertility examination for IVF/ICSI treatments. The demographic, clinical, laboratory, and treatment data of patients were extracted from paper and electronic medical records using a standardized electronic data collection form.
Figure 1.
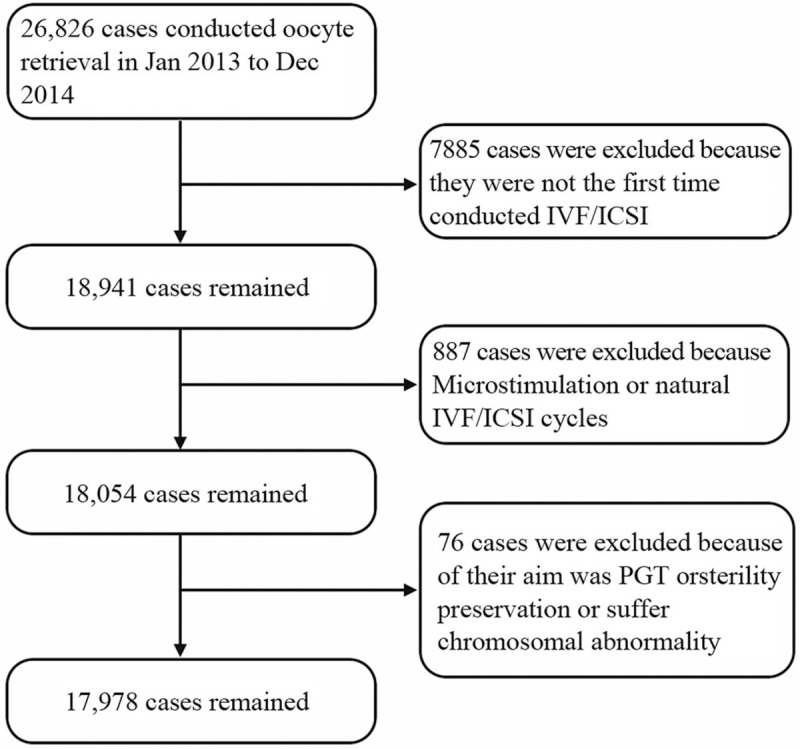
The flow chart of the cases enrolled in the study. There were 26,826 cases of oocyte retrieval conducted during January 2013 to December 2014. A total of 17,978 cases were first hyperstimulation IVF/ICSI cycles and enrolled. IVF/ICSI: In vitro fertilization or intracytoplasmic sperm injection; PGT: Preimplantation genetic testing.
COH protocol
All the women who underwent IVF/ICSI cycles received COH protocols as previously described.[3,4] Briefly, in the long protocol, patients received pituitary downregulation by midluteal administration of a gonadotropin-releasing hormone (GnRH) agonist, 0.1 mg triptorelin acetate daily injection or 1.3 mg/1.8 mg triptorelin once injection following hormone test 14 days later. In the short protocol, patients were administered GnRH agonist from the second day of their menstrual cycle onward. COH was given in those patients by recombinant FSH (rFSH) or human menopausal gonadotropin (HMG) in different flexible protocols. In the GnRH agonist ultralong protocol, patients undertook ovarian stimulation that was induced by rFSH protocol initial between day 28 and day 30 of their menstrual cycle following pituitary downregulation by 3.75 mg of triptorelin acetate or leuprorelin acetate on the first day of that cycle. In the antagonist protocol, patients who started with rFSH treatment on the second day of the cycle by once-daily administration. After 5 days of this treatment, the antagonist (ganirelix acetate or cetrorelix acetate) was administered daily. The rFSH dosage was adjusted fitting to individual ovarian response, which was assessed by daily ultrasound examination. The antagonist treatment continued up to, and including the human chorionic gonadotophin (hCG) day. In all treatment procedures, when at least two leading follicles reached 18 mm in size, ovulation was induced by 250 μg recombinant hCG (r-hCG), and ovum collection was performed between 34 and 38 h later.
Oocytes were fertilized by either conventional IVF or ICSI. All embryo collection, thawing, and culture processes were performed under standard protocols as previously published.[5] Briefly, embryos development was evaluated as regular. For day 3 embryos, the consensus scoring system was applied for embryo assessment (“the Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting”). Surplus good quality embryos were cryopreserved using a vitrification protocol as described earlier.[6] FET cycles were conducted throughout natural cycles or hormone replacement therapy cycles. Embryo transfer were accomplished on the 3 or 5 days of the cycle according to the Code of Practice for Assisted Reproductive Technology developed by the Ministry of Health of the People's Republic of China. Embryos were transferred by specified advanced gynecologists with the standard embryo transfer protocol. The luteal phase was supported from the day of embryo transfer (ET) to maintain lutein function through the 10th week of gestation.
Main outcomes
CLBR per cycle is the number of live births that had been achieved from the first cycle divided by the initial number of patients. The live-birth rates were calculated within the first ovarian hyperstimulation IVF/ICSI cycle and the subsequent FET cycles resulting in a live-birth.
Statistical analysis
The demographic and clinical data are recorded as absolute numbers, proportions, or percentages. The database is based on “clinical assisted reproductive technologies management system software (V15.4)” which is managed and conducted quality control by a specialist.
Statistical analyses were performed by SPSS 22.0 (IBM, Armonk, NY, USA). Student's t test was used for continuous variables that satisfy normal distribution and Chi-square test was applied for categorical variables. If the continuous data did not follow a Gaussian distribution, then a Mann-Whitney test was performed. Backward procedure for variables selection was applied for the multivariable logistic regression model. P value of <0.05 was considered statistically significant.
Results
Baseline characteristics
During the period January 2013 to December 2014, a total of 17,978 first IVF/ICSI cycles within 26,826 COH cycles were performed at the reproductive center of PUTH. The final date of follow-up was December 2018. All the information of those patients was collected and there were 107 cases lost to follow up. All the cases lost to follow-up were considered as the worst result that they do not get a live birth. All the information of those patients was collected and there was no missing data. Preliminary characteristics of those patients are showed in Table 1. In the cases with the first IVF/ICSI cycle, 10,452 cycles were getting pregnant and 8928 cycles were giving birth until December 2018. The cumulative pregnancy rate and CLBR were 58.14% (10,452/17,978) and 49.66% (8928/17,978), respectively.
Table 1.
Characteristics of infertility patients who conducted IVF/ICSI.
| Variable | Live birth (n = 8928) | Non-live birth (n = 9050) | Total (n = 17,978) | P |
| Female age (years) | 30.81 ± 4.05 | 33.09 ± 5.13 | 31.96 ± 4.76 | <0.001 |
| Male age (years) | 32.50 ± 5.08 | 34.65 ± 6.03 | 33.58 ± 5.68 | <0.001 |
| BMI of female (kg/m2) | 22.33 ± 3.35 | 22.75 ± 3.61 | 22.54 ± 3.48 | <0.001 |
| Average duration of infertility (years) | 4.22 ± 3.11 | 5.06 ± 4.08 | 4.65 ± 3.66 | <0.001 |
| Classification of infertility | <0.001 | |||
| Tubal factor | 3505 | 3014 | 6519 | |
| Ovulatory dysfunction | 247 | 401 | 648 | |
| Endometriosis | 126 | 115 | 241 | |
| Confounding factor in female | 585 | 747 | 1332 | |
| Other female factor | 82 | 147 | 229 | |
| Cofounding factor in the couple | 1363 | 1839 | 3202 | |
| Male factor only | 2476 | 2175 | 4651 | |
| Unexplained infertility | 544 | 612 | 1156 | |
| Preliminary gonadotropin used | <0.001 | |||
| FSH + HMG | 3397 | 4527 | 7924 | |
| FSH | 5440 | 4282 | 9722 | |
| HMG | 91 | 241 | 332 | |
| Duration of stimulation (days) | 12.07 ± 2.48 | 12.02 ± 2.67 | 12.05 ± 2.58 | 0.267 |
| Number of gonadotropin units used | 2597.86 ± 1141.70 | 3165.42 ± 1456.20 | 2883.44 ± 1339.79 | <0.001 |
| COH protocol | <0.001 | |||
| Long protocol | 4713 | 4177 | 8890 | |
| Ultra-long protocol | 1240 | 1311 | 2551 | |
| Short protocol | 755 | 1252 | 2007 | |
| Antagonist protocol | 2220 | 2310 | 4530 | |
| Number of oocytes retrieved | 15.35 ± 7.98 | 11.35 ± 7.60 | 13.34 ± 8.04 | <0.001 |
| Number of transferrable embryos | 6.66 ± 5.19 | 3.62 ± 3.51 | 10.18 ± 4.35 | <0.001 |
Data are presented as n or mean ± standard deviation. BMI: Body mass index; COH: Controlled ovarian hyperstimulation; FSH: Follicle-stimulating hormone; HMG: Human menopausal gonadotropin; ICSI: Intracytoplasmic sperm injection; IVF: In vitro fertilization.
The 17,978 cycles were then grouped by the number of oocytes retrieved in the fresh period [Figure 2]. The CLBR of the first hyperstimulation cycle was 21.89% (592/2705) when the number of oocytes obtained in the fresh period ≤5, and the CLBR was 43.09% (2062/4785) when the number of oocytes retrieved was 6 to 10, and the cumulative live yield was 56.18% (2549/4537) when the number of oocytes retrieved was 11 to 15. When the number of oocytes retrieved was 16 to 20, the CLBR was 60.60% (1801/2972), and when the number of oocytes retrieved was more than 20, the CLBR was 64.59% (1924/2979). There were statistical differences among all the groups (P < 0.05).
Figure 2.
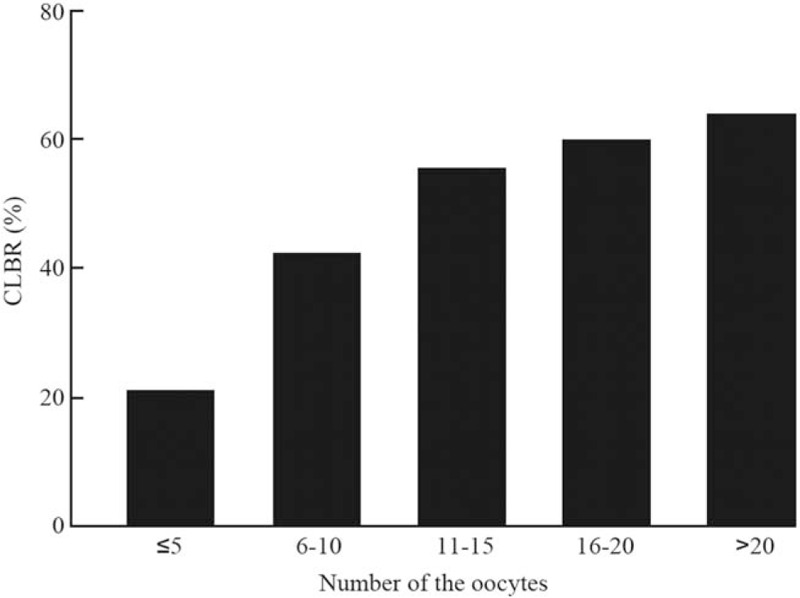
The CLBR of the patients grouped by the number of oocytes obtained. The CLBR of the first hyperstimulation cycle was 21.89% when the number of oocytes obtained in the fresh period ≤5, and the CLBR was 43.09% when the number of oocytes retrieved was 6 to 10, and the cumulative live yield was 56.18% when the number of oocytes retrieved was 11 to 15. When the number of oocytes retrieved was 16 to 20, the CLBR was 60.60%, and when the number of oocytes retrieved was more than 20, the CLBR was 64.59%. There were statistical differences among all the groups (P < 0.05). CLBR: Cumulative live birth rate.
Prognosis factors affected live birth in all participants
The baseline characteristics of the patients were listed in Table 1. Totally, there were 8928 (49.66%) cycles got a live birth while 9050 (50.34%) did not. The average age of women at the beginning of their first complete IVF/ICSI cycle was 30.81 years old and 33.09 years old in the live birth group and non-live birth group, respectively. The average age of women was younger in the live birth group. The body mass index (BMI) of women was also lower in the live birth group (22.33 ± 3.35 kg/m2) than that in the non-live birth group (22.75 ± 3.61 kg/m2). The average duration of infertility was 4.22 years for the live birth group, slightly shorter than the non-live birth group at 5.06 years. The distribution of infertility diagnoses was similar across all groups while the tubal factor was the most frequent diagnosis, followed closely by a male factor [Figure 3A]. Figure 3B and 3C presented treatment characteristics of the couples’ first complete cycle. The preliminary gonadotropin used and the total number of gonadotropin used were less in the live birth group when compared with the non-live birth group. Meanwhile, the number of oocytes retrieved and transferrable embryo were both significantly higher in the live birth group.
Figure 3.
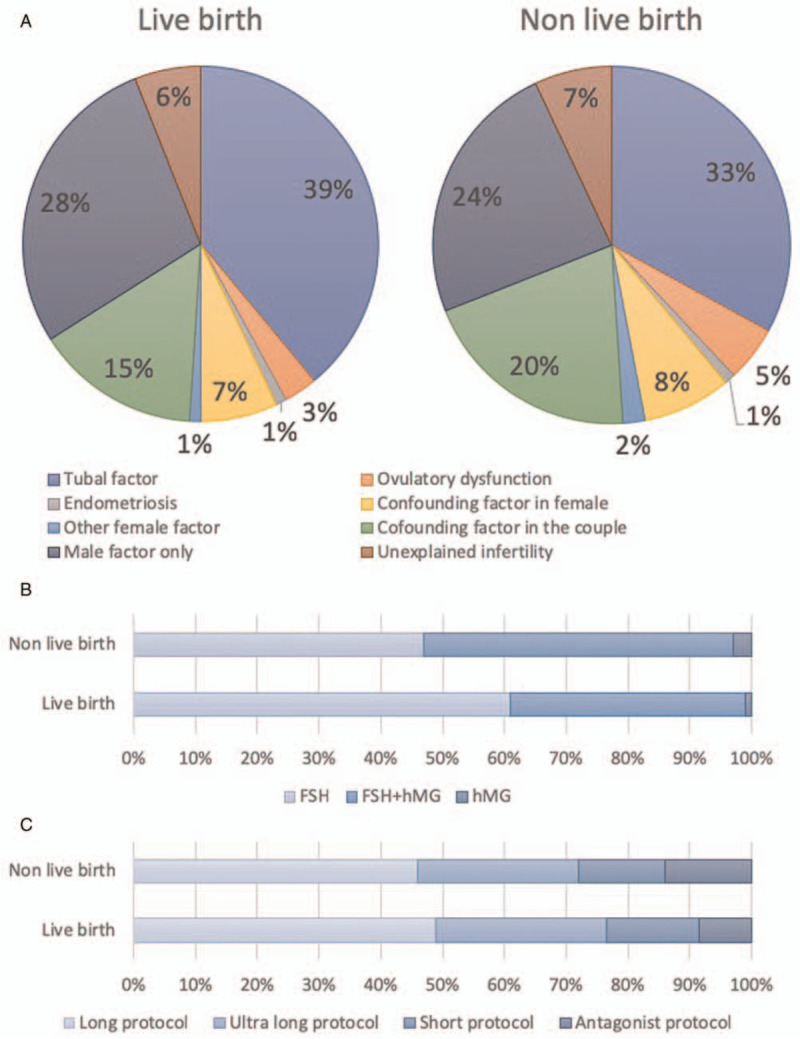
Comparison factors may affect cumulative live birth rate between the live birth group and the non-live birth group. (A) The distribution of infertility diagnoses was similar across all groups while the tubal factor is the most frequent diagnosis, followed closely by male factor. (B) The preliminary gonadotropin used in the live birth group and the non-live birth group. (C) The protocol of controlled ovarian hyperstimulation in the live birth group and the non-live birth group. FSH: Follicle-stimulating hormone; HMG: Human menopausal gonadotropin.
Multiple logistic regression analysis on the outcome of live birth showed that the women's age, BMI, duration of infertility years, infertility factors, COH protocol, the number of acquired oocytes, and number of transferrable embryos were the prognosis factors that significantly affected the outcome of the live birth [Table 2]. In the multivariable logistic regression model, female age (odds ratio [OR]: 0.947, 95% confidence interval [CI]: 0.935–0.958), female BMI (OR: 0.983, 95% CI: 0.974–0.993), duration of infertility (OR: 0.990, 95% CI: 0.980–1.000), number of gonadotropin units used (OR: 1, 95% CI: 1.000–1.000) were associated with CLBR (P < 0.05) [Table 2]. The male age, type of infertility, preliminary gonadotropin used were excluded from the model after backward logistic regression (P > 0.05).
Table 2.
The logistic regression result of factors may affect cumulate live birth rate.
| Variable | B | OR | 95% CI | P |
| Female age (years) | –0.055 | 0.947 | 0.935–0.958 | <0.001 |
| Male age (years) | –0.002 | 0.998 | 0.989–1.007 | 0.679 |
| BMI of female (kg/m2) | –0.017 | 0.983 | 0.974–0.993 | 0.001 |
| Average duration of infertility (years) | –0.010 | 0.990 | 0.980–1.000 | 0.048 |
| Classification of infertility | ||||
| Tubal factor | Ref | Ref | – | <0.001 |
| Ovulatory dysfunction | –0.295 | 0.745 | 0.614–0.903 | 0.003 |
| Endometriosis | 0.216 | 1.241 | 0.927–1.663 | 0.147 |
| Confounding factor in female | –0.219 | 0.803 | 0.702–0.920 | 0.002 |
| Other female factor | –0.270 | 0.764 | 0.567–1.029 | 0.076 |
| Cofounding factor in the couple | –0.249 | 0.780 | 0.707–0.860 | <0.001 |
| Male factor only | –0.054 | 0.947 | 0.869–1.032 | 0.218 |
| Unexplained infertility | –0.109 | 0.896 | 0.780–1.029 | 0.121 |
| Duration of stimulation (days) | 0.046 | 1.047 | 1.027–1.068 | <0.001 |
| Number of gonadotropin units used | 0.000 | 1.000 | 1.000–1.000 | <0.001 |
| COH protocol | ||||
| Long protocol | Ref | Ref | – | <0.001 |
| Ultra-long protocol | 0.007 | 1.007 | 0.911–1.114 | 0.888 |
| Short protocol | –0.429 | 0.651 | 0.572–0.741 | <0.001 |
| Antagonist protocol | –0.227 | 0.797 | 0.725–0.876 | <0.001 |
| Number of oocytes retrieved | ||||
| <5 | Ref | Ref | – | <0.001 |
| 5–15 | 0.675 | 1.965 | 1.721–2.243 | <0.001 |
| >15 | 0.343 | 1.410 | 1.209–1.643 | <0.001 |
| Number of transferrable embryos | 0.141 | 1.151 | 1.140–1.162 | <0.001 |
| Constant | 1.262 | 3.533 | – | <0.001 |
BMI: Body mass index; CI: Confidence interval; COH: controlled ovarian hyperstimulation; OR: Odds ratio.
Age and CLBR
Age is a key prognosis factor that may affect the successful rate of ART. So, the 17,978 cycles were then grouped into <35 years old group (n = 12961) and ≥35 years old group (n = 5017) according to the women age of their first COH cycle. The CLBR was 56.37% (7306/12,961) in the women <35 years old group and 32.33% (1622/5017) in the women ≥35 years old group. Interestingly, the highest CLBR were both achieved if the cause of infertility is a pelvic or tubal factor (60.26% and 36.84%, respectively). However, the lowest CLBR in the <35 years group was other female factors (39.16%) while ≥35 years old group was ovulatory dysfunction (23.14%) [Figure 4A]. If further divided the data by the number of oocytes retrieval, in the women <35 years old group, the CLBR was 44.15% (2018/4571) if the obtained oocytes less than ten, which was significantly lower than 63.03% (5288/8390) in the women got more than ten oocytes. The CLBR rose as the number of oocytes retrieved increasing in both cohort [Figure 4B]. Most of the patients in the <35 years old group received FSH only as the preliminary gonadotropin (62.69%) and ≥35 years old group administered with FSH + HMG (64.96%). However, the highest CLBR was archived from FSH as the preliminary gonadotropin in both groups [Figure 4C]. If divided the data by hyper-ovulation protocol, the CLBR was highest in the long protocol (<35 years: 58.56%; ≥35 years: 37.75%) and lowest in the short protocol in both age groups (<35 years: 47.82%; ≥35 years: 20.45%) [Figure 4D].
Figure 4.
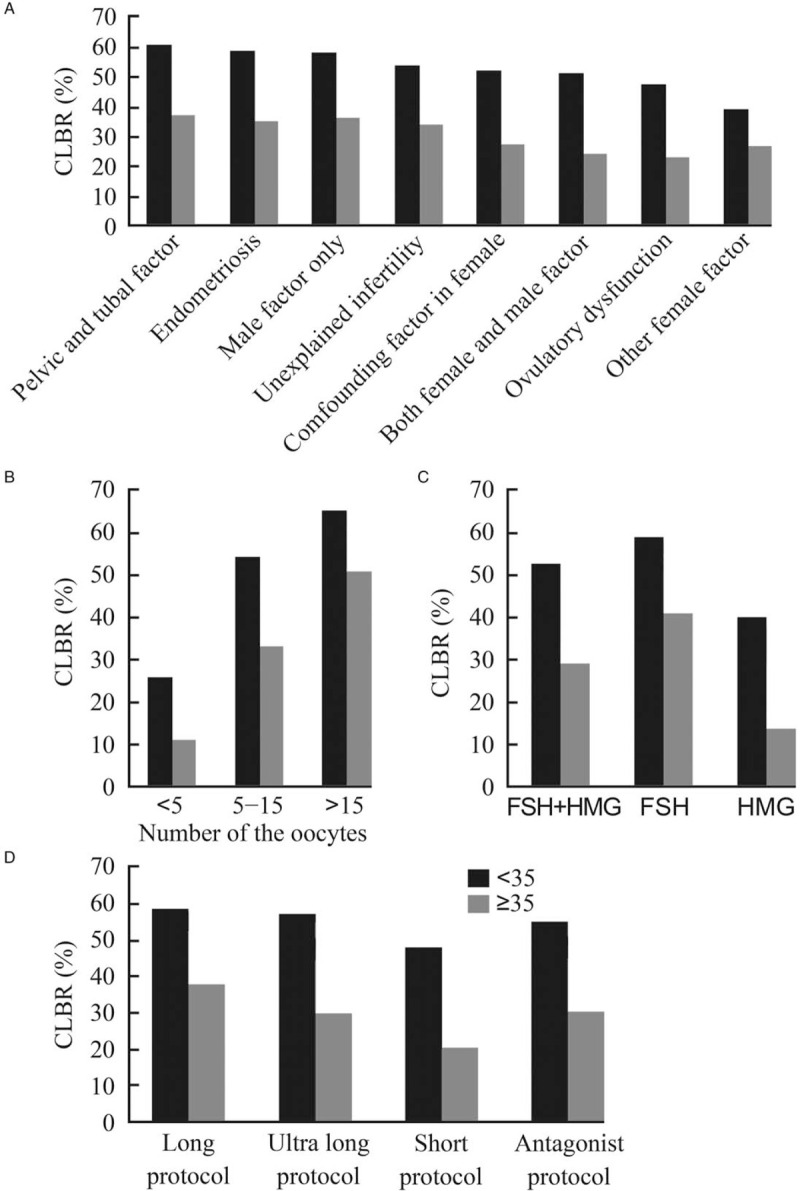
The effect of age on the cumulative live birth rate. (A) The CLBR of patients at different infertility diagnosis. The lowest CLBR in the <35 years group was other female factor (39.16%) while advanced age group was ovulatory dysfunction (23.14%). (B) The CLBR of patients grouped by the number the oocyte obtained. If further divided the data by the number of oocyte retrieval, in the women under 35 years old, the CLBR was 44.15% (2018/4571) if the obtained oocyte less than ten oocytes, which was significantly lower than 63.03% (5288/8390) in the women got more than ten oocytes. The CLBR rises as the number of oocytes retrieved increasing in the both cohort. (C) The CLBR of patients grouped by preliminary gonadotropin used. Most of the patients in the below 35 years group received FSH only as the preliminary gonadotropin (62.69%) and advanced age group administered with FSH + HMG (64.96%). However, the highest CLBR was archived from FSH as the preliminary gonadotropin in both group. (D) The CLBR of patients grouped by the protocol of COH. If divided the data by hyper-ovulation protocol, the CLBR was highest from the long protocol (<35 years: 58.56%; ≥35 years: 37.75%) and lowest from the short protocol in both age group (<35 years: 47.82%; ≥35 years: 20.45%). CLBR: Cumulative live birth rate; COH: Controlled ovarian hyperstimulation; FSH: Follicle-stimulating hormone; HMG: Human menopausal gonadotropin.
The multiple factor analysis showed the BMI of female (OR: 0.983, 95% CI: 0.972–0.994), type of infertility (OR: 1.090, 95% CI: 1.004–1.183), classification of infertility, COH protocol, the number of acquired oocytes, and number of transferrable embryos are prognosis factors significantly affecting CLBR in the women less 35 years old group [Table 3]. In the women over 35 years old group, the CLBR in patients acquired less than five oocytes was 11.15% (116/1040), and the CLBR was 33.4% (984/2946) if the oocytes obtained was between 5–15, and reached 50.63% if the oocytes obtained more than 15. For the logistic regression for women under 35 years old, the age of female, male age, duration of infertility, preliminary gonadotropin used were excluded. The multiple factor regression analysis [Table 4] showed that the age of men (OR: 0.979, 95% CI: 0.963–0.996) and women (OR: 0.837, 95% CI: 0.808–0.868), COH protocol, the number of acquired oocytes, and number of transferrable embryos are the prognosis factors that significantly affected the CLBR which are different when compared with the younger group. For the women age over or equal to 35 years old, BMI of female, average duration of infertility, classification of infertility, preliminary gonadotropin used, and duration of stimulation were not included. The similar and different prognosis factors for different groups were shown in Table 5.
Table 3.
The logistic regression analyses of factors may affect cumulate live birth rate in the female age <35 years group.
| Variable | B | OR | 95% CI | P |
| BMI of female (kg/m2) | –0.017 | 0.983 | 0.972–0.994 | 0.002 |
| Type of infertility | ||||
| Secondary infertility | 0.086 | 1.090 | 1.004–1.183 | 0.040 |
| Classification of infertility | ||||
| Tubal factor | Ref | Ref | – | <0.001 |
| Ovulatory dysfunction | –0.296 | 0.744 | 0.593–0.934 | 0.011 |
| Endometriosis | 0.207 | 1.229 | 0.871–1.734 | 0.239 |
| Confounding factor in female | –0.216 | 0.806 | 0.687–0.945 | 0.008 |
| Other female factor | –0.347 | 0.707 | 0.503–0.993 | 0.045 |
| Cofounding factor in the couple | –0.246 | 0.782 | 0.697–0.877 | <0.001 |
| Male factor only | –0.005 | 0.995 | 0.902–1.097 | 0.918 |
| Unexplained infertility | –0.115 | 0.891 | 0.754–1.053 | 0.176 |
| Duration of stimulation (days) | 0.047 | 1.048 | 1.025–1.072 | <0.001 |
| Number of gonadotropin units used | 0.000 | 1.000 | 1.000–1.000 | <0.001 |
| COH protocol | ||||
| Long protocol | Ref | Ref | – | <0.001 |
| Ultra-long protocol | 0.087 | 1.091 | 0.969–1.228 | 0.149 |
| Short protocol | –0.316 | 0.729 | 0.627–0.848 | <0.001 |
| Antagonist protocol | –0.150 | 0.861 | 0.773–0.958 | 0.006 |
| Number of oocytes retrieved | ||||
| <5 | Ref | Ref | – | <0.001 |
| 5–15 | 0.642 | 1.901 | 1.610–2.244 | <0.001 |
| >15 | 0.316 | 1.372 | 1.140–1.651 | 0.001 |
| Number of transferrable embryo | 0.138 | 1.148 | 1.137–1.160 | <0.001 |
| Constant | –0.212 | 0.809 | – | 0.430 |
BMI: Body mass index; CI: Confidence interval; COH: controlled ovarian hyperstimulation; OR: Odds ratio.
Table 4.
The logistic regression analyses of factors may affect cumulate live birth rate in the female age ≥35 years group.
| Variable | B | OR | 95% CI | P |
| Female age (years) | –0.178 | 0.837 | 0.808–0.868 | <0.001 |
| Male age (years) | –0.021 | 0.979 | 0.963–0.996 | 0.016 |
| Number of gonadotropin units used | 0.000 | 1.000 | 1.000–1.000 | <0.001 |
| COH protocol | ||||
| Long protocol | Ref | Ref | – | <0.001 |
| Ultra-long protocol | –0.252 | 0.777 | 0.636–0.950 | 0.014 |
| Short protocol | –0.665 | 0.514 | 0.396–0.667 | <0.001 |
| Antagonist protocol | –0.360 | 0.698 | 0.570–0.855 | <0.001 |
| Number of oocytes retrieved | ||||
| <5 | Ref | Ref | – | <0.001 |
| 5–15 | 0.585 | 1.795 | 1.429–2.256 | <0.001 |
| >15 | 0.258 | 1.294 | 0.962–1.741 | 0.089 |
| Number of transferrable embryo | 0.148 | 1.159 | 1.135–1.184 | <0.001 |
| Constant | 6.529 | 684.703 | – | <0.001 |
CI: Confidence interval; COH: Controlled ovarian hyperstimulation; OR: Odds ratio.
Table 5.
The different prognosis factors of cumulate live birth rate for patients in different groups.
| Variable | All | Female age <35 years group | Female age ≥35 years group |
| Female age | + | − | + |
| Male age | − | − | + |
| BMI of female | + | + | − |
| Average duration of infertility | + | − | − |
| Type of infertility | − | + | − |
| Classification of infertility | + | + | − |
| Preliminary gonadotropin used | − | − | − |
| Duration of stimulation | + | − | − |
| Number of gonadotropin units used | + | + | + |
| COH protocol | + | + | + |
| Number of oocytes retrieved | + | + | + |
| Number of transferrable embryos | + | + | + |
+: P < 0.05; −: P > 0.05. BMI: Body mass index; COH: Controlled ovarian hyperstimulation.
Discussion
CLBR is recommend to be an appropriate indicator to evaluate the success of IVF/ICSI treatment, which revealed results from the fresh embryo transfer and all following FET cycles. In this research, the CLBR in the first IVF/ICSI cycle is 49.66%, which is consistent with some other research (31.2%–52.6%).[6,7] The CLBR of women age below 35 is 56.37% while the CLBR was decreasing to 32.33% in women with advanced age.
We proposing that the CLBR is significantly increased with the number of oocytes retrieved, which was consistent with previous researches.[8,9] These results suggested that a high ovarian response predicted an elevated CLBR, which combined fresh embryo transfer and FET cycles. The number of retrieved oocytes reflected the effectiveness of the COH. Once there was enough oocyte, the more embryos could be obtained which means more chances for embryo transfer and more chances for live birth. In Sunkara's study, no matter in which age group (18–34, 24–37, 38–39, ≥40 years old), the highest CLBR was from the highest number of oocyte retrieval which was similar to our results.[10] Some other studies also indicated that the number of oocytes retrieved positively associated with the chance of accomplishing a live birth,[11–14] hence the number of oocytes retrieved was thought to be an essential predictive factor for CLBR.[11,13] Nonetheless, receiving more oocytes means a higher rate of cycle cancellation to prevent ovarian hyperstimulation syndrome (OHSS).[11] One previous research indicated that the optimal number of oocytes retrieval for a live birth was 6 to 15 in young patients less than 35 years old.[13] Some researchers suggested that the suitable number of oocytes retrieved for the maximum CLBR was between 6 and 15 oocytes in women aged 20 to 34 years when balancing the hazard of OHSS.[13] The study also indicated that the number of oocytes retrieved was positively correlated to the CLBR in women aged 35 to 40 years. While the preferred number of oocytes retrieved in women aged between 35 and 40 years is 10 to 14, receiving a high CLBR while sustaining an acceptable low OHSS rate. This is consistent with our study, while the logistic regression showed it is a protective factor when the number of oocytes retrieved is 5 to 15 in the advanced age group.
The CLBR in the women ≥35 years was 32.33% in our study. Abuzeid found the live birth rate in fresh embryo transfer was 40% and the CLBR was 66% in women aged 35 to 39 years,[15] while Wu et al[16] claimed their live birth rate in fresh ET was 51.8% and the CLBR was 69.8% in women aged 35 to 39 years. Our CLBR was lower than the reported study may be due to only the first cycle was included while others included all the ovarian stimulation cycle. Researches had revealed that the implantation rate remains stable until the age of 35 at when a linear decrease of 2.77% per year is discovered, which maybe because the number of the aneuploid embryos increases linearly after 35 years old.[17,18] Age is a negative factor for live birth initiation from 35 years old, with a rapid decline in live birth rate beyond 40 years old.[19] It is a significant factor in response to COH,[20] as women over 35 years old are at risk of poor ovarian response.[21,22] However, age is an adverse factor for OHSS, as age rises the rate of OHSS declines.[23] Our results also showed that the male age may affect the CLBR especially in the women with advanced age which is also similar to other published research. The relationship of advanced male age alone was not as important as that of advanced female age alone. It may take function based on female age. That might be due to the damage on sperm DNA or changes of epigenetic and following affect the quality of embryo.[24,25]
Age is a negative prognostic reason for live births during IVF/ICSI treatment.[26] It is the main reason affect the fertility of women. With the rise of age, the response and reserve of ovarian are all declined. Meanwhile, the number of retrieved oocytes, quality of oocyte and embryo, implantation rate are all descending noticeably. Poor ovarian function was also related to a parallel decrease in both the oocyte quantity and quality as the abortion rate falling down from 20% to 13% with a growing number of oocytes retrieved.[27] Reports showed that the implantation rate linearly declined after 35 years old.
Besides age, many factors may also affect the CLBR. A large retrospective study suggests that the age of women, number of oocytes retrieved and embryo fertilization rate are the main factors contribute to the CLBR.[12] Another research also indicated the number of oocytes retrieved and BMI could affect the CLBR[11] which are consistent with our results. The application of the initial period of FSH was higher than the initial period of the simple HMG. Shorter period of infertility in patients reached better outcomes and also needs fewer gonadotrophin. Our results indicated that the CLBR of the single COH cycle increased significantly with the increase of the number of oocyte retrieval and the number of transferable, and it was significantly associated with the age of women and ovarian hyperstimulation protocol. In view of the CLBR when FET is involved, the day of ET does not affect the chances to get a live-birth baby.[28] CLBR from the first oocyte retrieval including fresh and all subsequent FET cycles were comparable between the GnRH-agonist and GnRH-antagonist groups, with 31.2% (161/516) and 34.1% (182/534) received at least one live birth.[7] A previous study demonstrated that the days of embryo transfer do not affect the CLBR. It was similar between the cleavage-stage embryo and blastocyst-stage (48.5% vs. 48.0%).[29]
How to compare the successful rate of IVF/ICSI among different center is still remains controversial. The successful rate for each transfer cycle only reflects the outcome of a single transfer cycle, which may neglect the ovarian hyper-stimulation protocol and the number of oocytes retrieved. Thus, it might be more proper to evaluate the cumulative success rate per woman in one complete cycle.[30,31] By means of one complete IVF/ICSI cycle, the total reproductive potential of one oocyte retrieval offers a comprehensive success rate that is related and important.[32,33] CLBR per oocyte retrieval is an appropriate indicator of the quality and success in an IVF/ICSI treatment, as FET has become an essential part of one complete cycle of ART treatment. Reporting the CLBR will be more applicable while comparing the IVF/ICSI treatment outcome between different centers and making economic and political decisions regarding treatment efficacy and cost effectiveness, instead of reporting live birth rate based on fresh ET or FET. Maheshwari et al[33] suggested a triple outcome approach for reporting the short-term, medium-term, and long-term CLBR.
The application of CLBR could encourage the single embryo transfer which may achieve a higher rate of live birth and singleton pregnancy. It may also reflect the utilization of oocyte and embryo per IVF/ICSI cycle. Moreover, it may increase the chance of embryo transfer in nature conditions which may avoid the side effect of higher estrogen. On the other hand, the application of CLBR may make it easier and more objective when compare the various protocols of hyperstimulation, and guide the individualized treatment. When connecting with infertile patients, CLBR is an appropriate representation of success rates over a complete journey of IVF/ICSI and so our analysis of success rates would assist informed decision-making and aid tailor expectations for these patients.
Our outcomes are valuable both for doctors and infertile couples who will carry out the first IVF/ICSI cycle, especially when the financially and emotionally burdened decisions for the continued treatment are taken into consideration. Furthermore, this large retrospective study in Chinese population may provide information for the CLBR in Chinese race.
The CLBR of the single hyperstimulation cycle increased significantly with the increase of the number of oocyte retrieval, and it was significantly related to the age of women and the number of transferrable embryos. The age, ovarian reserve function, duration of infertility, and the cause of infertility could not be altered or controlled by the doctor. However, our data indicated that the protocol of ovarian stimulation and the preliminary gonadotropin are related to the CLBR which means the doctors could optimize those to increase CLBR.
Acknowledgements
The authors thank all the staff in the reproductive center and Obstetrics and Gynecology in PUTH for their work for this study.
Funding
This work was supported by grants from the National Key Research and Development Program of China (No. 2018YFC1002106) and the National Science Foundation for Young Scientists of China (No. 81801447).
Conflicts of interest
None.
Footnotes
How to cite this article: Yang R, Niu ZR, Chen LX, Liu P, Li R, Qiao J. Analysis of related factors affecting cumulative live birth rates of the first ovarian hyperstimulation in vitro fertilization or intracytoplasmic sperm injection cycle: a population-based study from 17,978 women in China. Chin Med J 2021;134:1405–1415. doi: 10.1097/CM9.0000000000001586
Rui Yang and Zi-Ru Niu contributed equally to this work.
References
- 1.Professional Committee on Reproductive Medicine CMDA. Cumulative delivery/live birth rate of complete ovarian stimulation cycle consensus. Chin J Reprod Contracep 2018; 38:963–968. doi: 10.3760/cma.j.issn.2096-2916.2018.12.001. [Google Scholar]
- 2.Kupka MS, Ferraretti AP, de Mouzon J, Erb K, D’Hooghe T, Castilla JA, et al. Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHRE. Hum Reprod 2014; 29:2099–2113. doi: 10.1093/humrep/deu175. [DOI] [PubMed] [Google Scholar]
- 3.Chi H, Qiao J, Li H, Liu P, Ma C. Double measurements of serum HCG concentration and its ratio may predict IVF outcome. Reprod Biomed Online 2010; 20:504–509. doi: 10.1016/j.rbmo.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Gao H, Chi H, Zeng L, Xiao W, Wang Y, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA 2017; 318:2190–2198. doi: 10.1001/jama.2017.18249. [DOI] [PubMed] [Google Scholar]
- 5.Zhu P, Guo H, Ren Y, Hou Y, Dong J, Li R, et al. Single-cell DNA methylome sequencing of human preimplantation embryos. Nat Genet 2018; 50:12–19. doi: 10.1038/s41588-017-0007-6. [DOI] [PubMed] [Google Scholar]
- 6.Van Landuyt L, Verpoest W, Verheyen G, De Vos A, Van de Velde H, Liebaers I, et al. Closed blastocyst vitrification of biopsied embryos: evaluation of 100 consecutive warming cycles. Hum Reprod 2011; 26:316–322. doi: 10.1093/humrep/deq338. [DOI] [PubMed] [Google Scholar]
- 7.Toftager M, Bogstad J, Lossl K, Praetorius L, Zedeler A, Bryndorf T, et al. Cumulative live birth rates after one ART cycle including all subsequent frozen-thaw cycles in 1050 women: secondary outcome of an RCT comparing GnRH-antagonist and GnRH-agonist protocols. Hum Reprod 2017; 32:556–567. doi: 10.1093/humrep/dew358. [DOI] [PubMed] [Google Scholar]
- 8.Tannus S, Turki R, Cohen Y, Son WY, Shavit T, Dahan MH. Reproductive outcomes after a single dose of gonadotropin-releasing hormone agonist compared with human chorionic gonadotropin for the induction of final oocyte maturation in hyper-responder women aged 35-40 years. Fertil Steril 2017; 107:1323–1328.e2. doi: 10.1016/j.fertnstert.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Vlaisavljevic V, Kovacic B, Knez J. Cumulative live birth rate after GnRH agonist trigger and elective cryopreservation of all embryos in high responders. Reprod Biomed Online 2017; 35:42–48. doi: 10.1016/j.rbmo.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Sunkara SK, Khalaf Y, Maheshwari A, Seed P, Coomarasamy A. Association between response to ovarian stimulation and miscarriage following IVF: an analysis of 124 351 IVF pregnancies. Hum Reprod 2014; 29:1218–1224. doi: 10.1093/humrep/deu053. [DOI] [PubMed] [Google Scholar]
- 11.Chen YH, Xu XH, Wang Q, Zhang SD, Jiang LL, Zhang CL, et al. Optimum oocyte retrieved and transfer strategy in young women with normal ovarian reserve undergoing a long treatment protocol: a retrospective cohort study. J Assist Reprod Genet 2015; 32:1459–1467. doi: 10.1007/s10815-015-0571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H, et al. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod 2016; 31:370–376. doi: 10.1093/humrep/dev316. [DOI] [PubMed] [Google Scholar]
- 13.Ji J, Liu Y, Tong XH, Luo L, Ma J, Chen Z. The optimum number of oocytes in IVF treatment: an analysis of 2455 cycles in China. Hum Reprod 2013; 28:2728–2734. doi: 10.1093/humrep/det303. [DOI] [PubMed] [Google Scholar]
- 14.van der Gaast MH, Eijkemans MJ, van der Net JB, de Boer EJ, Burger CW, van Leeuwen FE, et al. Optimum number of oocytes for a successful first IVF treatment cycle. Reprod Biomed Online 2006; 13:476–480. doi: 10.1016/s1472-6483(10)60633-5. [DOI] [PubMed] [Google Scholar]
- 15.Abuzeid MI, Bolonduro O, La Chance J, Abozaid T, Urich M, Ullah K, et al. Cumulative live birth rate and assisted reproduction: impact of female age and transfer day. Facts Views Vis Obgyn 2014; 6:145–149. [PMC free article] [PubMed] [Google Scholar]
- 16.Wu CH, Lee TH, Chen HH, Chen CI, Huang CC, Lee MS. The influence of female age on the cumulative live-birth rate of fresh cycles and subsequent frozen cycles using vitrified blastocysts in hyper-responders. Taiwan J Obstet Gynecol 2015; 54:567–571. doi: 10.1016/j.tjog.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Demko ZP, Simon AL, McCoy RC, Petrov DA, Rabinowitz M. Effects of maternal age on euploidy rates in a large cohort of embryos analyzed with 24-chromosome single-nucleotide polymorphism-based preimplantation genetic screening. Fertil Steril 2016; 105:1307–1313. doi: 10.1016/j.fertnstert.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Spandorfer SD, Chung PH, Kligman I, Liu HC, Davis OK, Rosenwaks Z. An analysis of the effect of age on implantation rates. J Assist Reprod Genet 2000; 17:303–306. doi: 10.1023/a:1009422725434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrido N, Bellver J, Remohi J, Simon C, Pellicer A. Cumulative live-birth rates per total number of embryos needed to reach newborn in consecutive in vitro fertilization (IVF) cycles: a new approach to measuring the likelihood of IVF success. Fertil Steril 2011; 96:40–46. doi: 10.1016/j.fertnstert.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Templeton A, Morris JK, Parslow W. Factors that affect outcome of in-vitro fertilisation treatment. Lancet 1996; 348:1402–1406. doi: 10.1016/S0140-6736(96)05291-9. [DOI] [PubMed] [Google Scholar]
- 21.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 2011; 26:1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 22.Vuong TN, Phung HT, Ho MT. Recombinant follicle-stimulating hormone and recombinant luteinizing hormone versus recombinant follicle-stimulating hormone alone during GnRH antagonist ovarian stimulation in patients aged >/=35 years: a randomized controlled trial. Hum Reprod 2015; 30:1188–1195. doi: 10.1093/humrep/dev038. [DOI] [PubMed] [Google Scholar]
- 23.Ashrafi M, Bahmanabadi A, Akhond MR, Arabipoor A. Predictive factors of early moderate/severe ovarian hyperstimulation syndrome in non-polycystic ovarian syndrome patients: a statistical model. Arch Gynecol Obstet 2015; 292:1145–1152. doi: 10.1007/s00404-015-3723-0. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins TG, Aston KI, Pflueger C, Cairns BR, Carrell DT. Age-associated sperm DNA methylation alterations: possible implications in offspring disease susceptibility. PLoS Genet 2014; 10:e1004458.doi: 10.1371/journal.pgen.1004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vagnini L, Baruffi RL, Mauri AL, Petersen CG, Massaro FC, Pontes A, et al. The effects of male age on sperm DNA damage in an infertile population. Reprod Biomed Online 2007; 15:514–519. doi: 10.1016/s1472-6483(10)60382-3. [DOI] [PubMed] [Google Scholar]
- 26.Luke B, Brown MB, Missmer SA, Spector LG, Leach RE, Williams M, et al. Assisted reproductive technology use and outcomes among women with a history of cancer. Hum Reprod 2016; 31:183–189. doi: 10.1093/humrep/dev288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod 2011; 26:1768–1774. doi: 10.1093/humrep/der106. [DOI] [PubMed] [Google Scholar]
- 28.De Vos M, Pareyn S, Drakopoulos P, Raimundo JM, Anckaert E, Santos-Ribeiro S, et al. Cumulative live birth rates after IVF in patients with polycystic ovaries: phenotype matters. Reprod Biomed Online 2018; 37:163–171. doi: 10.1016/j.rbmo.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 29.De Vos A, Santos-Ribeiro S, Van Landuyt L, Van de Velde H, Tournaye H, Verheyen G. Birthweight of singletons born after cleavage-stage or blastocyst transfer in fresh and warming cycles. Hum Reprod 2018; 33:196–201. doi: 10.1093/humrep/dex361. [DOI] [PubMed] [Google Scholar]
- 30.McLernon DJ, Maheshwari A, Lee AJ, Bhattacharya S. Cumulative live birth rates after one or more complete cycles of IVF: a population-based study of linked cycle data from 178,898 women. Hum Reprod 2016; 31:572–581. doi: 10.1093/humrep/dev336. [DOI] [PubMed] [Google Scholar]
- 31.Moragianni VA, Penzias AS. Cumulative live-birth rates after assisted reproductive technology. Curr Opin Obstet Gynecol 2010; 22:189–192. doi: 10.1097/GCO.0b013e328338493f. [DOI] [PubMed] [Google Scholar]
- 32.Jones HW, Jr, Jones D, Kolm P. Cryopreservation: a simplified method of evaluation. Hum Reprod 1997; 12:548–553. doi: 10.1093/humrep/12.3.548. [DOI] [PubMed] [Google Scholar]
- 33.Maheshwari A, McLernon D, Bhattacharya S. Cumulative live birth rate: time for a consensus? Hum Reprod 2015; 30:2703–2707. doi: 10.1093/humrep/dev263. [DOI] [PubMed] [Google Scholar]


