Existing literature suggests that patients with autoimmune diseases might be at increased risk of developing severe COVID-19,1 which emphasises the importance of vaccination against SARS-CoV-2 for this patient group. However, vaccine hesitancy has increased during the past two decades,2 and might have been reinforced by the unavoidable rapid development and approval of multiple COVID-19 vaccines and communication about rare adverse events of thrombosis in women aged younger than 50 years.3 Hesitancy is likely to have increased in patients with autoimmune diseases, since these patients were mostly excluded from COVID-19 vaccine trials. We have previously shown that more than a third of patients with autoimmune diseases remain hesitant about COVID-19 vaccination, with concerns about adverse events and paucity of long-term research identified as the most important reasons.4 At present, data on the safety of COVID-19 vaccines in patients with autoimmune diseases remain scarce, and studies comparing the consequences of different vaccine types between patients and healthy controls are not available. Thus, international COVID-19 vaccination guidelines for patients with autoimmune diseases are based only on expert opinion.5 It is therefore relevant and timely to compare tolerability of different COVID-19 vaccine types between patients with autoimmune diseases and healthy controls, and to assess the effect of COVID-19 vaccination on underlying disease activity.
In this Comment, we describe the results of a questionnaire that assessed adverse events following COVID-19 vaccinations in patients with autoimmune diseases and healthy controls. The questionnaire was sent to patients with systemic autoimmune diseases who were enrolled in two ongoing prospective cohort studies (Netherlands Trial Register, trial ID NL8513 and NCT04498286). Between April 26, 2020 and March 1, 2021, all adult patients with systemic autoimmune diseases from the Amsterdam Rheumatology & Immunology Center (Amsterdam, Netherlands), and all adult patients with multiple sclerosis from the Amsterdam Multiple Sclerosis Center of Amsterdam UMC (Amsterdam, Netherlands) were invited to participate. Patients enrolled in the first study were asked (but not obliged) to recruit their own healthy control participant who was of the same sex and of comparable age (age difference <5 years). Data were collected via online questionnaries distributed via email. Information on demographic data and medication use were collected at baseline, and data on adverse events of COVID-19 vaccinations were collected between April 14 and April 30, 2021.
Participants were asked whether they had received a COVID-19 vaccination. Vaccinated participants were asked to report whether they experienced any local or systemic adverse events in the first 7 days following vaccination, and if so, to report on the severity and duration of the adverse events. Mild adverse events were defined as unpleasant reactions that did not limit daily activities, moderate adverse events as those that limited daily activities, and severe adverse events as those that required medical attention. Serious adverse events were defined as reactions that resulted in hospital admission. Systemic adverse events included fever, chills and shiver, general malaise, nausea, diarrhoea, myalgia, arthralgia, headache, and fatigue. Patients with rheumatic diseases and healthy controls were asked whether they experienced an increased number of joint complaints in the first 2 months after vaccination. All patients with autoimmune diseases were asked whether they experienced a change in their autoimmune disease up to 2 months after vaccination. Additional questions were included to assess whether vaccination would lead to an improved sense of security or quality of life among patients and controls.
All participants who completed the questionnaire at least 4 days after their first COVID-19 vaccination were included in the analyses. We used multivariable logistic regression analyses to compare the occurrence of any adverse event, systemic adverse events, and moderate-to-severe adverse events between patients and controls. Effect modification was investigated for age (stratified to participants aged ≤55 years vs >55 years), sex, and vaccine type. A threshold of p<0·05 was used for interaction terms to identify effect modifiers. Additionally, we did exploratory analyses to compare adverse events between vaccine types, male and female participants, and participants older and younger than 55 years without correction for multiple testing. The research protocols were approved by the medical ethical committee of the VU University medical center (registration number 2020.169 and 2020.370). All participants provided informed consent.
The questionnaire was sent to 2515 patients with autoimmune diseases and 903 healthy controls, of whom 1780 patients and 660 controls completed the questionnaire. 505 patients (including 204 patients with rheumatoid arthritis and 81 patients with multiple sclerosis) and 203 healthy controls completed the questionnaire at least 4 days after receiving their first COVID-19 vaccination and were included in the analyses (appendix p 1). The median number of days between the first vaccination and completing the questionnaire was 15 days (IQR 10–25) for patients and 15 days (10–34) for controls. The mean age of both patients and controls was 64 years (SD 11), and 329 (65%) of 505 patients and 133 (66%) of 203 controls were women (appendix p 2). 348 (69%) of 505 patients (189 [93%] of 204 patients with rheumatoid arthritis and 59 [73%] of 81 patients with multiple sclerosis) received immunosuppressive treatment. Biological agents were prescribed to 147 (29%) patients, predominantly tumour necrosis factor inhibitors in patients with rheumatoid arthritis, and anti-CD20 therapies in patients with multiple sclerosis.
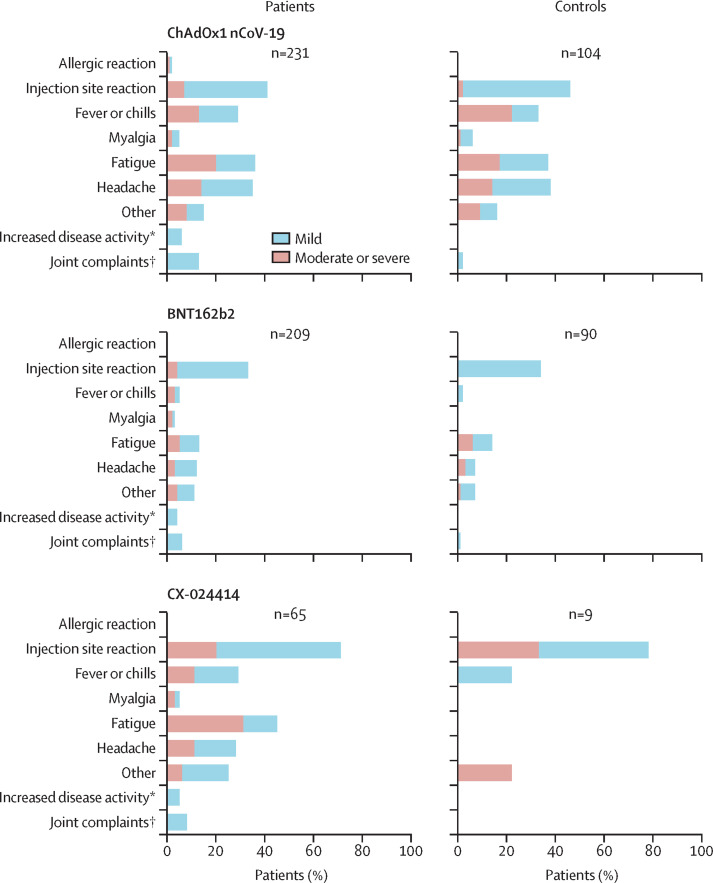
ChAdOx1 nCoV-19 (AstraZeneca) and BNT162b2 (Pfizer/BioNTech) were the most common vaccines in both patients and controls: 231 (46%) of 505 patients and 104 (51%) of 203 controls received the ChAdOx1 nCoV-19 vaccine and 209 (41%) patients and 90 (44%) controls received the BNT162b2 vaccine. 65 (13%) patients and 9 (4%) controls were vaccinated with CX-024414 (Moderna). 258 (51%) patients and 106 (52%) controls had at least one mild adverse event and 105 (21%) patients and 38 (19%) controls reported moderate adverse events. Severe adverse events were rare (six [1%] of 505 patients and none in the control group), and no serious adverse events occurred. Overall, the most commonly reported adverse event in both patients and controls was pain at the injection site (196 [39%] vs 82 [40%]), which was consistent across all vaccines (figure ; appendix p 3). Systemic adverse events occurred in 222 (44%) of 505 patients and 82 (40%) of 203 healthy controls, most frequently fatigue and headache (figure; appendix p 3). Joint complaints were reported more frequently by patients with rheumatic diseases than controls (49 [10%] vs 3 [1%]), but only a small proportion of patients (26 [5%]) reported a deterioration of their autoimmune disease up to 2 months after COVID-19 vaccination. Immunosuppressive treatment was postponed because of COVID-19 vaccination in 26 (6%) of 424 patients with rheumatic diseases. Adverse events stratified by sex are shown in the appendix (p 4).
Figure.
Adverse events following COVID-19 vaccination in patients with autoimmune diseases and healthy controls
*Self-reported increase in disease activity up to 2 months after vaccination; duration and severity of disease flares were not assessed. †Questions about increases in joint complaints up to 2 months after vaccination were only applied to patients with rheumatic diseases and healthy controls, and the duration and severity of joint complaints were not assessed.
Multivariable logistic regression analyses showed similar odds for any adverse event, systemic adverse events, or moderate or severe adverse events between patients and controls, which was consistent when patients with rheumatoid arthritis or multiple sclerosis were compared with healthy controls (appendix p 5). Age was a significant effect modifier in the association between autoimmune status and the risk of moderate or severe adverse events, but additional age-stratified multivariable regression analyses did not reach statistical significance in both strata (data not shown). Vaccination with ChAdOx1 nCoV-19, female sex, and younger age (≤55 years) were independently associated with an increased likelihood of reporting any adverse event, systemic adverse events, and moderate or severe adverse events after a COVID-19 vaccination (appendix p 5). All results remained similar when participants who completed the questionnaire within 7 days after the first COVID-19 vaccination were excluded from the analyses.
Most participants (217 [75%] of 289 patients and 91 [76%] of 120 controls) indicated that they felt safer from 2 weeks after their first COVID-19 vaccination (appendix p 5). Additionally, 58 (20%) of 288 patients and 17 (14%) of 120 controls also reported to perceive an overall improvement in their quality of life.
Analysis of the results of our questionnaire demonstrate that adverse events of COVID-19 vaccinations in patients with autoimmune diseases are comparable with controls, independent of the type of vaccine. The observed adverse events consisted of expected transient local or systemic reactions that were mostly self-limiting. The frequency of participants who reported adverse events was lower than that reported in clinical trials,6 but similar to a nationwide observational study on adverse events of COVID-19 vaccinations in the general population done in the UK.7 Our data are consistent with previous studies that reported higher frequencies of adverse events in women and younger people.6, 7 We did not observe any serious adverse events, but the number of participants included in our study was too low to draw conclusions about rare serious events. Additionally, our data suggest that COVID-19 vaccinations do not seem to trigger autoimmune disease flares, which is in accordance with data from previous small studies that assessed consequences of mRNA vaccines in patients with autoimmune diseases.8, 9 However, a limitation of our study is that data on disease activity were based on self-report rather than validated disease activity measures, and we did not assess the duration and severity of disease flares. Known pathophysiological effects of mRNA on the innate immune system could be both immunostimulatory and immunosuppressive,10, 11 thus as COVID-19 vaccines are the first mRNA vaccines to be widely applied, prospective controlled studies with adequate follow-up are required to draw robust conclusions about long-term effects of COVID-19 vaccines on autoimmune disease activity. Another limitation of our study was that control participants were not a random sample of the general population, nor were they an exact match for patients with autoimmune diseases with regard to age and sex distribution. However, we adjusted for these variables as possible confounders in our analysis. We found that vaccination had favourable effects on psychological wellbeing; the majority of participants indicated that they felt safer after receiving a COVID-19 vaccination, and 20% of patients with autoimmune diseases reported a perceived overall increase of their quality of life. Overall, our data indicated that COVID-19 vaccines are well tolerated by patients with autoimmune diseases. Such findings might reassure patients who remain hesitant about COVID-19 vaccinations, and help physicians in guiding their patients towards accepting such vaccines.
Acknowledgments
JK reports grants from Biogen Idec, Novatis, TEVA, Bayer Schering Pharma, GlaxoSmith Kline, Merck Serono, Genzyme, and Roche, outside the submitted work. TR reports grants from ZonMw during the conduct of the study. GW reports granst from ZonMw, Reade foundation, and the T2B!-consortium during the conduct of the study. All other authors declare no competing interests.
Supplementary Material
References
- 1.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubé E, Vivion M, MacDonald NE. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: influence, impact and implications. Expert Rev Vaccines. 2015;14:99–117. doi: 10.1586/14760584.2015.964212. [DOI] [PubMed] [Google Scholar]
- 3.Trogen B, Oshinsky D, Caplan A. Adverse consequences of rushing a SARS-CoV-2 vaccine: implications for public trust. JAMA. 2020;323:2460–2461. doi: 10.1001/jama.2020.8917. [DOI] [PubMed] [Google Scholar]
- 4.Boekel L, Hooijberg F, van Kempen ZLE, et al. Perspective of patients with autoimmune diseases on COVID-19 vaccination. Lancet Rheumatol. 2021;3:e241–e243. doi: 10.1016/S2665-9913(21)00037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American College of Rheumatology COVID-19 vaccine clinical guidance summary for patients with rheumatic and musculoskeletal diseases. https://www.rheumatology.org/Portals/0/Files/COVID-19-Vaccine-Clinical-Guidance-Rheumatic-Diseases-Summary.pdf
- 6.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00224-3. published April 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connolly CM, Ruddy JA, Boyarsky BJ, et al. Safety of the first dose of mRNA SARS-CoV-2 vaccines in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220231. published online March 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220272. published online March 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult Scler. 2021;27:864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velikova T, Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int. 2021;41:509–518. doi: 10.1007/s00296-021-04792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.