Abstract
Objective
To build a prediction model for uveitis in children with JIA for use in current clinical practice.
Methods
Data from the international observational Pharmachild registry were used. Adjusted risk factors as well as predictors for JIA-associated uveitis (JIA-U) were determined using multivariable logistic regression models. The prediction model was selected based on the Akaike information criterion. Bootstrap resampling was used to adjust the final prediction model for optimism.
Results
JIA-U occurred in 1102 of 5529 JIA patients (19.9%). The majority of patients that developed JIA-U were female (74.1%), ANA positive (66.0%) and had oligoarthritis (59.9%). JIA-U was rarely seen in patients with systemic arthritis (0.5%) and RF positive polyarthritis (0.2%). Independent risk factors for JIA-U were ANA positivity [odds ratio (OR): 1.88 (95% CI: 1.54, 2.30)] and HLA-B27 positivity [OR: 1.48 (95% CI: 1.12, 1.95)] while older age at JIA onset was an independent protective factor [OR: 0.84 (9%% CI: 0.81, 0.87)]. On multivariable analysis, the combination of age at JIA onset [OR: 0.84 (95% CI: 0.82, 0.86)], JIA category and ANA positivity [OR: 2.02 (95% CI: 1.73, 2.36)] had the highest discriminative power among the prediction models considered (optimism-adjusted area under the receiver operating characteristic curve = 0.75).
Conclusion
We developed an easy to read model for individual patients with JIA to inform patients/parents on the probability of developing uveitis.
Keywords: uveitis, JIA, prediction model, risk factors, confounders
Rheumatology key messages
We provide for the first time a model for predicting uveitis in juvenile idiopathic arthritis.
Individual risk estimates for uveitis might guide physicians in determining ophthalmological screening frequencies.
Introduction
JIA is a group of diseases characterized by arthritis of unknown origin persisting for >6 weeks before the age of 16 [1, 2]. It is the commonest rheumatic disease in children with a prevalence varying between 3.8 and 400 per 100 000 [3–5]. JIA patients are at an increased risk of developing uveitis, which is an inflammatory condition of the uvea, including the iris, ciliary body and choroid [6]. A systematic review reported a cumulative incidence of JIA-associated uveitis (JIA-U) of 11.4% [7]. Frequency of JIA-U varies geographically and is highest in patients with oligoarthritis while low in patients with systemic and RF positive arthritis [8, 9]. JIA-U occurs more often in girls and known risk factors are younger age at JIA onset and having antinuclear antibodies positivity [6, 10–18]. The estimated prevalence of JIA-U varies up to 30% [6], but the risk of acquiring uveitis for an individual JIA patient is unknown. Chronic anterior uveitis or silent uveitis is the most common form of JIA-U and is usually asymptomatic. On the contrary, acute anterior uveitis presents with apparent symptoms such as eye pain, redness of eyes and headaches [6, 19]. If left un- or undertreated, (silent) uveitis may result in sight-threatening complications including synechiae, cataracts and glaucoma in 25–50% and vision loss in 10–20% of paediatric uveitis cases [20] .
Therefore, early detection and subsequent intensive treatment is the key. Several guidelines exist for the routine screening of JIA patients by ophthalmologists. These include the 1993 American Academy of Pediatrics guidelines, the 2006 British Society for Paediatric and Adolescent Rheumatology (BSPAR) guidelines and the 2019 ACR guidelines, as well as screening recommendations by Heiligenhaus et al. following a 2007 nation-wide study in Germany [6, 11, 20–22]. These guidelines are all based on the risk factors age at JIA onset, ANA status, JIA category and disease duration. Nevertheless, they use different cut-off values for the age at JIA onset, include different JIA categories and recommend different screening frequencies. It can be concluded that while screening for JIA-U is of the utmost importance, there is global consensus neither on the screening frequency, nor on the criteria to identify high-risk patients for uveitis [23]. In fact, the treating physician does not have tools to estimate the real risk of acquiring uveitis for the individual patient.
The objective of this study is to develop a prediction model for JIA-U for use in everyday clinical practice for individual JIA patients rather than arbitrary groups. Individual risk predictions could provide quantitative risk estimates for uveitis to individual patients and guide clinicians in determining screening frequencies.
Methods
Patients
We used data from the international observational Pharmachild registry, an ongoing project that started in 2011 with the primary aim of collecting adverse events in JIA patients undergoing treatment with biologic agents. The scope was later broadened by also including patients on NSAIDs, steroids and synthetic DMARDs. Pharmachild contains information of JIA patients treated in 86 medical centres from 32 countries in Europe, Asia, Africa and South America that belong to the Paediatric Rheumatology International Trials Organisation [24]. Full details of the Pharmachild registry have been published elsewhere [25].
Data were locked on 3 May 2019. For inclusion into the study, patients had to provide informed consent and meet the International League of Associations for Rheumatology (ILAR) criteria for JIA. Exclusion criteria were an age at JIA onset of ≥16 years, development of uveitis prior to JIA, an observation period of <4 years and a diagnosis of systemic JIA with a history of acute anterior uveitis. An observation period of at least 4 years was chosen such that every patient had had enough time to develop uveitis.
For every patient, information was gathered about the age at JIA onset, observation period, ANA, RF and human leucocyte antigen (HLA) B27 status, use of medication, occurrence of uveitis and JIA ILAR category [2]. A patient was classified as RF positive if two positive RF determinations at least 3 months apart were documented. For ANA positivity, one positive determination was required. The additive value of requiring two positive ANA determinations was also studied. Occurrence of uveitis was determined from three sources: free-text fields and checkmarks indicating a history of uveitis at registration into Pharmachild and adverse events reported during follow-up after registration. All prospective and a number of retrospective uveitis cases were reported using the MedDRA coding system, including a date of onset. Of these, we included the following preferred terms: uveitis, iridocyclitis, autoimmune uveitis and iritis. Since the date of onset was not available for all uveitis cases, ever use of drugs of interest was collected. This was defined as having ever taken the drug during the disease course. Drugs included were NSAIDs, intraarticular steroids, systemic steroids, methotrexate, ciclosporin, anti-TNF, anti-IL-1, anti-IL-6 and other biologics.
Statistical analysis
χ2 and Mann–Whitney U tests at a significance level of 5% were performed to examine differences in characteristics between patients who developed JIA-U and those who did not. Based on the existing literature and consensus of the authors, the following variables were chosen as potential risk factors and confounding variables for JIA-U: age at JIA onset, gender, JIA category, ANA status, RF status and HLA-B27 status. Crude and adjusted odds ratios for the (independent) relationship between these variables and JIA-U were established using logistic regression in a complete-case analysis. A 95% confidence interval for the main effect that did not contain 1 was considered statistically significant. Subsequently, all independent statistically significant risk factors were considered for inclusion into a multivariable logistic regression model, to predict the probability of developing JIA-U. The main effects of this prediction model were selected using a backward procedure based on the Akaike information criterion. For all analyses, oligoarticular JIA was chosen as the reference JIA category. Linearity between continuous predictors and the logit outcome was tested using the Box–Tidwell test. Model performance was assessed based on the area under the receiver operating characteristic curve (AUC) in the training data and 10-fold cross validation. It was also assessed if adding interaction terms for the main effects to the prediction model resulted in improved model performance. The reduced model was internally validated and adjusted for overfitting by bootstrap resampling. A description of the bootstrapping procedure is provided in Supplementary Data S1, available at Rheumatology online. Internal calibration of the final prediction model was assessed by a plot of observed frequencies of JIA-U within deciles of the predicted probabilities vs the mean predicted probabilities. Lastly, a formula for predicting the individual risk of developing uveitis was determined based on the coefficients of the prediction model. All analyses were performed with the stats, car, caret, pROC and rms packages for R version 3.6.3 [26].
Results
Characteristics of study population
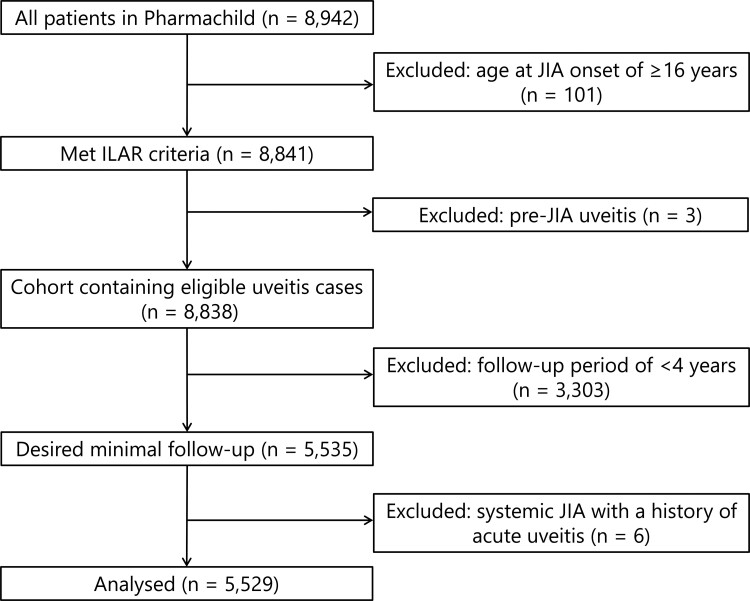
A total of 5529/8942 (62%) patients were included in the analysis (Fig. 1). The majority of excluded patients had a follow-up of <4 years (3303/8838; 37%). Characteristics of patients included and excluded were similar (Supplementary Table S1, available at Rheumatology online).
Fig. 1.
Flowchart of study population
Of the patients analysed, 1102 (19.9%) had ever developed JIA-U (Table 1); 18/91 (20%) cases of uveitis in enthesitis-related arthritis (ERA) patients with available specification were of chronic uveitis type. Children who developed JIA-U, had a longer observation period, younger age at JIA onset and were more often female and HLA-B27 positive compared with patients who did not. The majority of JIA-U patients had oligoarthritis and were ANA positive. RF negative polyarthritis was the second most common JIA category in patients that developed JIA-U. Moreover, RF positivity, systemic arthritis and the ever use of anti-IL-1 and anti-IL-6 were lower in the JIA-U group. On the contrary, the ever use of anti-TNF was more frequent in patients with JIA-U than in those without. We did not find differences among patients receiving systemic steroids.
Table 1.
Characteristics of Pharmachild cohort used for analysis
| Total cohort (n = 5529) |
No uveitis (n = 4427) |
Uveitis (n = 1102) |
P-value | |
|---|---|---|---|---|
| Female gender, n (%) | 3881 (70.2) | 3064 (69.2) | 817 (74.1) | <0.005a |
| Age at JIA onset, median (IQR), years | 4.36 (2.14–8.60) | 5.16 (2.39–9.23) | 2.60 (1.69–4.70) | <0.005a |
| Observation time, median (IQR), years | 7.91 (5.78–11.02) | 7.64 (5.60–10.58) | 9.14 (6.56–12.79) | <0.005a |
| JIA category, n (%) | ||||
| Oligoarthritis | 2182 (39.5) | 1522 (34.4) | 660 (59.9) | <0.005a |
| Persistent oligoarthritis | 1272 (23.0) | 872 (19.7) | 400 (36.3) | <0.005a |
| Extended oligoarthritis | 910 (16.5) | 650 (14.7) | 260 (23.6) | <0.005a |
| Polyarthritis (RF−) | 1504 (27.2) | 1285 (29.0) | 219 (19.9) | <0.005a |
| Polyarthritis (RF+) | 184 (3.3) | 182 (4.1) | 2 (0.2) | <0.005a |
| Psoriatic arthritis | 197 (3.6) | 160 (3.%) | 37 (3.4) | 0.75 |
| ERA | 527 (9.5) | 435 (9.8) | 92 (8.3) | 0.15 |
| Systemic arthritis | 569 (10.3) | 564 (12.7) | 5 (0.5) | <0.005a |
| Undifferentiated arthritis | 366 (6.6) | 279 (6.3) | 87 (7.9) | 0.07 |
| Immunological markers, n (%) | ||||
| 1× ANA positive | 2273 (43.7) (n = 5201) | 1571 (38.0) (n = 4138) | 702 (66.0) (n = 1063) | <0.005a |
| 2× ANA positive | 1372 (34.8) (n = 3946) | 891 (28.9) (n = 3086) | 481 (55.9) (n = 860) | <0.005a |
| RF positive | 190 (3.9) (n = 4877) | 184 (4.7) (n = 3943) | 6 (0.6) (n = 934) | <0.005a |
| HLA-B27 positive | 714 (21.2) (n = 3375) | 555 (20.3) (n = 2729) | 159 (24.6) (n = 646) | 0.02a |
| Anti-inflammatory treatment ever, n (%) | ||||
| NSAIDs | 4635 (83.8) | 3747 (84.6) | 888 (80.6) | <0.005a |
| Intraarticular steroids | 3118 (56.4) | 2389 (54.0) | 729 (66.2) | <0.00a |
| Systemic steroids | 2322 (42.0) | 1838 (41.5) | 484 (43.9) | 0.16 |
| Synthetic DMARDs | 5068 (91.7) | 4012 (90.6) | 1056 (95.8) | <0.005a |
| Methotrexate | 4925 (89.1) | 3883 (87.7) | 1042 (94.6) | <0.005a |
| Ciclosporin | 441 (8.0) | 308 (7.0) | 133 (12.1) | <0.005a |
| Biologic DMARDs | 4157 (75.2) | 3263 (73.7) | 894 (81.1) | <0.005a |
| Anti-TNF | 3801 (68.7) | 2917 (65.9) | 884 (80.2) | <0.005a |
| Anti-IL-1 | 248 (4.5) | 243 (5.5) | 5 (0.5) | <0.005a |
| Anti-IL-6 | 491 (8.9) | 446 (10.1) | 45 (4.1) | <0.005a |
| Other biologics | 530 (9.6) | 425 (9.6) | 105 (9.5) | 0.99 |
Statistically significant difference at α = 0.05. ERA: enthesitis-related arthritis; IQR: interquartile range.
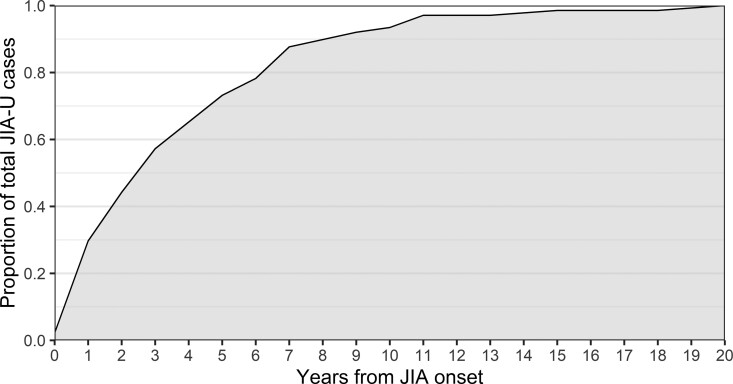
Time between onset of JIA and JIA-U
Of all patients who developed JIA-U, 138 patients had a known date of uveitis onset. We observed that 93/138 patients (67.4%) developed JIA-U within the first 4 years after JIA onset (Fig. 2). Two patients (1.4%) developed JIA-U after 15 years, namely in the 18th and 19th year after JIA onset. Furthermore, the median time interval between JIA onset and onset of JIA-U was 2.4 years.
Fig. 2.
Cumulative juvenile idiopathic arthritis-associated uveitis (JIA-U) onset rate (n = 138)
Adjusted risk factors
Crude odds ratios for JIA-U corresponding to the data presented in Table 1 are presented in Table 2. After correcting for confounding variables, ANA positivity and HLA-B27 turned out to be statistically significant risk factors for JIA-U. Older age at JIA onset (continuous variable), polyarthritis and systemic arthritis were associated with significantly decreased odds for JIA-U (compared with oligoarthritis). While female gender was a risk factor for JIA-U on univariable analysis, this did not hold true after adjusting for confounders. Also, the statistically significant protective effect of psoriatic arthritis, ERA and undifferentiated arthritis for JIA-U compared with oligoarthritis disappeared after confounder adjustment. Patients with a twice positive ANA determination had higher odds for JIA-U than patients with only one positive ANA determination.
Table 2.
ORs for the development of JIA-U per risk factor
| Risk factor | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Female gender | 1.28 (1.10, 1.48)a | 0.90 (0.72, 1.12) |
| Age at JIA onset | 0.83 (0.81, 0.85)a | 0.84 (0.81, 0.87)a |
| Oligoarthritis | 1 | 1 |
| Polyarthritis (RF−) | 0.39 (0.33, 0.47)a | 0.60 (0.47, 0.76)a |
| Polyarthritis (RF+) | 0.03 (0.00, 0.08)a | 0.06 (0.00 -0.48)a |
| Psoriatic arthritis | 0.53 (0.36, 0.76)a | 0.89 (0.55, 1.41) |
| ERA | 0.49 (0.38, 0.62)a | 1.15 (0.77, 1.69) |
| Systemic arthritis | 0.02 (0.01, 0.04)a | 0.07 (0.03, 0.16)a |
| Undifferentiated arthritis | 0.72 (0.55, 0.93)a | 1.30 (0.87, 1.91) |
| 1× ANA positive | 3.18 (2.76, 3.66)a | 1.88 (1.54, 2.30)a |
| 2× ANA positive | 3.13 (2.68, 3.65)a | 2.27 (1.82, 2.85)a |
| RF positive | 0.13 (0.05, 0.27)a | 0.98 (0.22, 3.43) |
| HLA-B27 positive | 1.28 (1.04, 1.56)a | 1.48 (1.12, 1.95)a |
Statistically significant. ERA: enthesitis-related arthritis; JIA-U: juvenile idiopathic arthritis-associated uveitis; OR: odds ratio.
Prediction model
Our best prediction model for estimating the probability of developing JIA-U included the following predictors: age at JIA onset, ANA positivity and JIA category (Table 3). According to this model, the individual risk of developing JIA-U can be calculated using the following formula:
Table 3.
Coefficients table of prediction model for juvenile idiopathic arthritis-associated uveitis (n = 5201)
| Predictor | OR (95% CI) | β | Optimism-adjusted β |
|---|---|---|---|
| (Intercept) | 0.53 (0.44, 0.63)a | −0.63 | −0.65 |
| ANA positive | 2.02 (1.73, 2.36)a | 0.70 | 0.67 |
| Age at JIA onset | 0.84 (0.82, 0.86)a | −0.17 | −0.17 |
| Oligoarthritis | 1 | 0 | 0 |
| Undifferentiated arthritis | 1.11 (0.84, 1.46) | 0.10 | 0.10 |
| Polyarthritis (RF−) | 0.55 (0.46, 0.66)a | −0.60 | −0.58 |
| Polyarthritis (RF+) | 0.06 (0.01, 0.20)a | −2.78 | −2.69 |
| Psoriatic arthritis | 0.85 (0.56, 1.24) | −0.17 | −0.16 |
| ERA | 1.53 (1.13, 2.05)a | 0.42 | 0.41 |
| Systemic arthritis | 0.04 (0.01, 0.08)a | −3.32 | −3.21 |
Optimism-adjusted area under the receiver operating characteristic curve = 0.75. aStatistically significant. ERA: enthesitis-related arthritis; OR: odds ratio.
For this estimation, the age at JIA onset in years, ANA status (1 = positive, 0 = negative) and a JIA category coefficient from Table 3 are needed.
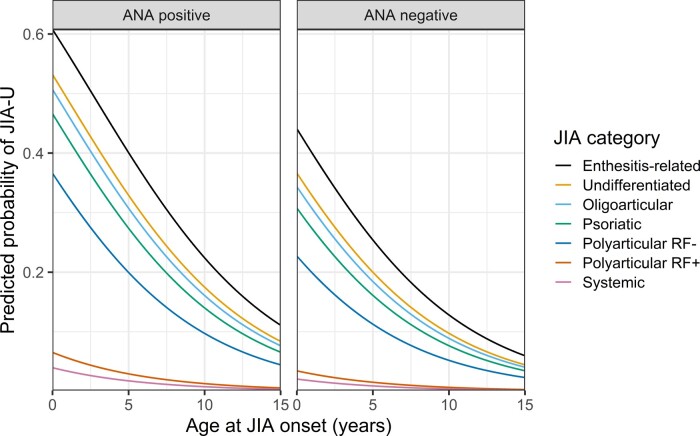
Oligoarticular JIA was chosen as the reference category because this was the largest category and therefore would provide stable odds ratios for the other JIA categories. The analysis eliminated 328/5529 (5.9%) of all patients due to missing ANA determinations, resulting in 5201 patients with 1063 outcome events (Supplementary Table S2, available at Rheumatology online). ANA positivity and a younger age at JIA onset were associated with significantly higher odds for developing uveitis, while systemic arthritis and polyarthritis were associated with significantly decreased odds when compared with oligoarthritis. The prediction model had good discriminative power in the training data [AUC = 0.76 (95% CI: 074, 0.77)]. Ten-fold cross validation revealed similar model performance: the average AUC was 0.75 with a standard deviation of 0.02 (Supplementary Fig. S1, available at Rheumatology online). Internal validation by bootstrap resampling revealed little overfitting, optimism of the AUC estimate was small (0.004) and the shrinkage factor of the model coefficients was close to 1 (0.97). According to the calibration plot of observed vs predicted probabilities of JIA-U (Supplementary Fig. S2, available at Rheumatology online), the optimism-adjusted model fitted the data well. For clinical practice, a diagram is provided from which the predicted individual uveitis risk as a function of the predicting variables can be read (Fig. 3). Individual risks can also be obtained from a risk calculator (Supplementary Data S2, available at Rheumatology online).
Fig. 3.
Diagram of optimism-adjusted clinical prediction model for juvenile idiopathic arthritis-associated uveitis (JIA-U)
First, distinguish between ANA positive and negative patients (left or right side of the diagram). Then, pick a line corresponding to the JIA category (see legend), and finally, read off the predicted probability for JIA-U (y-axis) as a function of the age at JIA onset (x-axis).
A Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) checklist [27] is provided in Supplementary Data S3 (available at Rheumatology online).
Discussion
In this study, we developed a clinical prediction model for estimating JIA-U in order to be able to inform patients/parents on the probability that they/their child will develop uveitis. For the first time, quantitative risk estimates can easily be obtained for an individual newly diagnosed JIA patient and these estimates could also aid clinicians in determining screening frequencies. As a result, screening frequencies can be tailored towards the individual rather than followed for arbitrary groups. Attributing a percent risk for developing JIA-U is a step forward from only being able to inform patients and parents of a ‘high’, ‘low’ or ‘moderate’ risk as used in existing screening guidelines [20, 21], since these terms are highly subjective and identify an unidentified risk range. In addition, higher risk estimates following our model might encourage clinicians to earlier escalate drug therapy to methotrexate or adalimumab, which is superior to etanercept in the treatment of silent uveitis [6].
The combination of age at JIA onset, ANA status and JIA category had the highest predictive power among the models we considered. HLA-B27 appeared to be statistically significant in predicting JIA-U when added to our model (P < 0.01). However, this addition also decreased the discriminative power of our model (AUC = 0.74). Because there were many missing observations for HLA-B27 and since this is an expensive test that cannot be measured as a point-of-care, we decided not to include HLA-B27 in our model. Including HLA-B27 as a predictor variable in our model could have introduced selection bias and would cause our model to be useless in clinical settings without resources for this determination. Nonetheless, by not including HLA-B27 as a predictor variable, the model might predict acute anterior uveitis slightly worse [6]. We also decided not to incorporate a twice positive ANA determination in the model for similar reasons: a substantial number of patients did not have two ANA determinations while adding this variable to the model did not result in an increase of discriminative power. We furthermore considered adding interaction terms for the main effects to the model to account for possible differences in risk prediction between categories of predictors. Other studies have reported that the age at JIA onset and ANA-associated risk of JIA-U differs between boys and girls [10, 15, 28]. Nonetheless, including interaction terms next to the main effects did not improve the discriminative power of our model. Lastly, we adjusted our model by distinguishing between persistent and extended oligoarthritis within the group of oligoarthritis patients. This was based on a study by Sim et al. that reported that patients with extended oligoarthritis are at higher risk for developing JIA-U and develop JIA-U earlier than patients with persistent oligoarthritis [29]. The adjustment, however, resulted in worse model fit as determined by the calibration plot. We therefore decided to stick with the oligoarthritis group as a whole. Moreover, we wanted our prediction model to predict baseline risks for JIA patients and this is not possible when having to distinguish between persistent and extended oligoarthritis, which might take years to become obvious.
The variables included in our model are identical to the parameters used in current screening guidelines, except for the disease duration. In addition, several studies found that on multivariable analysis, young age at JIA onset, ANA positivity and JIA category were indeed the best predictors based on statistical significance [13, 14, 30, 31]. However, when building prediction models including the JIA categories and cut-off values for age at JIA onset that are used in these guidelines, this resulted in less discriminative power than our individualized model (AUCBSPAR = 0.66 and AUCHeiligenhaus/ACR = 0.71).
Our analyses revealed that the relationship between several factors and JIA-U is confounded by extraneous variables. For the ERA patients in our cohort, the relationship with JIA-U can be explained as due to the predominance of HLA-B27 positivity (data not shown), which is associated with acute anterior uveitis [20]. Similar to ERA, undifferentiated arthritis was no longer associated with decreased odds for JIA-U compared with oligoarthritis after adjusting for confounders. This could be explained by the fact that a large percentage of patients with undifferentiated arthritis in our cohort were ANA and HLA-B27 positive and that a reasonable number of acute anterior uveitis cases were present in this group (data not shown). In addition, male gender, another risk factor for acute anterior uveitis [6, 32], was relatively common in undifferentiated arthritis patients that developed JIA-U in comparison with all other JIA-U patients (data not shown). Although the vast majority of patients that developed JIA-U in our cohort were female, female gender itself was not an independent risk factor for JIA-U after confounder adjustment. Several studies have also reported this bias, explained by the fact that female JIA patients on average have a lower age at JIA onset and are more often ANA positive than male JIA patients [7, 33–35]. In addition, we observed that RF negative polyarthritis was associated with decreased odds for JIA-U compared with oligoarthritis, regardless of adjusting for confounding. In fact, this was the JIA category with the third lowest percentage of JIA-U. This implies that even though it is known that uveitis occurs most frequently in patients with oligoarthritis and RF negative polyarthritis [6, 9], RF negative polyarthritis on its own is not associated with a high risk of developing uveitis. The large cohort study by Heiligenhaus et al. also observed relatively low rates of JIA-U in patients with RF negative polyarthritis [11].
Our study supports well-established epidemiological features of JIA-U. The prevalence of JIA-U in our cohort used for analysis was 19.9%, which is in line with a review by Clarke et al. [6]. The (independent) association with a younger age at JIA onset, ANA positivity and oligoarthritis is also frequently described [13, 14, 30, 36, 37]. Furthermore, we observed that JIA-U is extremely rare in systemic arthritis and RF positive polyarthritis patients, which is in concordance with other studies [8, 11–13, 30, 36]. Occurrence of uveitis in systemic JIA patients might therefore be a good moment for reconsidering the initial diagnosis. Nevertheless, because of diagnosis uncertainty and overlapping symptoms, the guidelines indicate screening in this group of patients and thus recognize a small risk of uveitis in patients initially labelled as systemic JIA [6, 38, 39].
We excluded patients with an observation period of <4 years since the risk of developing uveitis after 4 years of JIA is markedly reduced [21, 40] and we only wanted to analyse patients who had had enough time to develop uveitis. Extending this period would lead to a decreased sample size for analyses. We observed that 67% of JIA-U cases occurred within the first 4 years since JIA onset and other studies have reported numbers between 63% and 91% [11, 12, 15, 34, 41]. The cut-off value of 4 years has also been used in other studies on JIA-U on the basis of the aforementioned reasons [7, 30, 42].
The study has some limitations given it lacks an association with relevant medication prior to uveitis onset as well as the disease duration. Because of the latter, our prediction model should not directly replace current screening guidelines. Nevertheless, we believe it is very useful for clinicians, parents and patients to estimate an individual ‘starting risk’ of developing uveitis. A great strength of our model is its large sample size with data of patients from 32 countries around the world, making its risk predictions well generalizable. However, it should be mentioned that our cohort is subject to a certain amount of referral bias since in the Pharmachild registry many of the contributing centres are academic hospitals, which might lead to an under-representation of JIA patients with low disease activity and not in need of DMARDs. Therefore, our prediction model might perform worse and might need to be recalibrated especially for non-academic centres with a higher proportion of JIA patients that do well on NSAIDs and intra-articular injections only.
For the future, a dynamic model for predicting JIA-U that incorporates medication and disease duration would certainly be ideal. A further step in modelling JIA-U would be to include additional information on relevant biomarkers, including HLA-B27. Studies have already indicated HLA type DRB1*11, anti-histone antibodies, an elevated erythrocyte sedimentation rate and calcium-binding protein S100A12 as predictive factors for JIA-U [14, 15, 28, 41–45]. Furthermore, some studies have identified particular T cell subsets and monocyte phenotypes as potential biomarkers for JIA-U [46, 47].
In conclusion, here, we provide a clinical tool for predicting JIA-U based on data from the largest registry of JIA patients. For every individual with JIA, this model informs patients/parents on the probability of developing uveitis. Known risk factors of JIA-U have been confirmed. In our model, ANA-positive patients with early-onset JIA are at highest risk for JIA-U contrary to systemic and RF positive polyarticular JIA patients.
Supplementary Material
Acknowledgements
We thank all participating PRINTO centres for the data collection. We also thank all patients and their families for their consent to this study.
Ethics statement: All participating centres obtained approval from their respective ethics committees. All subjects provided written informed consent/assent based on existing national regulations.
Funding: This work was supported by a research grant from FOREUM Foundation for Research in Rheumatology. Pharmachild has been supported by a grant from the European Union (grant 260353) and by funding from the Italian public hospital IRCCS Istituto Giannina Gaslini.
Disclosure statement: N.R. has received honoraria for consultancies or speaker bureaus (<10 000 USD each) from the following pharmaceutical companies in the past 3 years: Ablynx, Astrazeneca-Medimmune, Aurinia, Biogen, Boehringer, Bristol Myers Squibb, Cambridge Healthcare Research (CHR), Centrical Global, Domain Therapeutics, Eli-Lilly, EMD Serono, GlaxoSmithKline, Hoffmann-La Roche, Idorsia, Janssen, Merck, MSD, Novartis, Pfizer, R-Pharma, Sanofi, Servier, Sinergie, Sobi and UCB.
The IRCCS Istituto Giannina Gaslini (IGG), where N.R. works as full-time public employee has received contributions (>10 000 USD each) from the following industries in the past 3 years: BMS, Eli-Lilly, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Sobi. This funding has been reinvested for the research activities of the hospital in a fully independent manner, without any commitment with third parties. J.S. has received a symposium grant from Sobi in 2018 (not exceeding EUR 1500). C.L. has received sponsorship for a congress from AbbVie in 2016 and a speaker fee for a conference from Roche in 2013. G.F. has received consultancy fees from Sobi. All other authors declare no conflicts of interest.
Data availability statement
All relevant data are reported in the article. Additional details can be provided by the corresponding author upon reasonable request. The Pharmachild registry is registered at Clinicaltrials.gov (NCT01399281) and at the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP; http://www.encepp.eu/encepp/viewResource.htm? id=19362).
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Prakken B, Albani S, Martini A.. Juvenile idiopathic arthritis. Lancet 2011;377:2138–49. [DOI] [PubMed] [Google Scholar]
- 2. Petty RE, Southwood TR, Manners P. et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 3. Ravelli A, Martini A.. Juvenile idiopathic arthritis. Lancet 2007;369:767–78. [DOI] [PubMed] [Google Scholar]
- 4. Thierry S, Fautrel B, Lemelle I, Guillemin F.. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Jt Bone Spine 2014;81:112–7. [DOI] [PubMed] [Google Scholar]
- 5. Palman J, Shoop-Worrall S, Hyrich K, McDonagh JE.. Update on the epidemiology, risk factors and disease outcomes of Juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol 2018;32:206–22. [DOI] [PubMed] [Google Scholar]
- 6. Clarke SLN, Sen ES, Ramanan AV.. Juvenile idiopathic arthritis-associated uveitis. Pediatr Rheumatol 2016;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carvounis PE, Herman DC, Cha S, Burke JP.. Incidence and outcomes of uveitis in juvenile rheumatoid arthritis, a synthesis of the literature. Graefes Arch Clin Exp Ophthalmol 2006;244:281–90. [DOI] [PubMed] [Google Scholar]
- 8. Hayworth JL, Turk MA, Nevskaya T, Pope JE.. The frequency of uveitis in patients with juvenile inflammatory rheumatic diseases. Jt Bone Spine 2019;86:685–90. [DOI] [PubMed] [Google Scholar]
- 9. Heiligenhaus A, Heinz C, Edelsten C, Kotaniemi K, Minden K.. Review for disease of the year: epidemiology of juvenile idiopathic arthritis and its associated uveitis: the probable risk factors. Ocul Immunol Inflamm 2013;21:180–91. [DOI] [PubMed] [Google Scholar]
- 10. Saurenmann RK, Levin AV, Feldman BM. et al. Risk factors for development of uveitis differ between girls and boys with juvenile idiopathic arthritis. Arthritis Rheum 2010;62:1824–8. [DOI] [PubMed] [Google Scholar]
- 11. Heiligenhaus A, Niewerth M, Ganser G, Heinz C, Minden K.. Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population-based nation-wide study in Germany: suggested modification of the current screening guidelines. Rheumatology 2007;46:1015–9. [DOI] [PubMed] [Google Scholar]
- 12. Yasumura J, Yashiro M, Okamoto N. et al. Clinical features and characteristics of uveitis associated with juvenile idiopathic arthritis in Japan: first report of the pediatric rheumatology association of Japan (PRAJ). Pediatr Rheumatol Online J 2019;17:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee JJY, Duffy CM, Guzman J. et al. Prospective determination of the incidence and risk factors of new‐onset uveitis in juvenile idiopathic arthritis: the research in arthritis in Canadian children emphasizing outcomes cohort. Arthritis Care Res 2019;71:1436–43. [DOI] [PubMed] [Google Scholar]
- 14. Tappeiner C, Klotsche J, Sengler C. et al. Risk factors and biomarkers for the occurrence of uveitis in juvenile idiopathic arthritis: data from the inception cohort of newly diagnosed patients with juvenile idiopathic arthritis study. Arthritis Rheumatol (Hoboken, NJ) 2018;70:1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nordal E, Rypdal V, Christoffersen T. et al. Incidence and predictors of Uveitis in juvenile idiopathic arthritis in a Nordic long-term cohort study. Pediatr Rheumatol Online J 2017;15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cosickic A, Halilbasic M, Selimovic A, Avdagic H.. Uveitis associated with juvenile idiopathic arthritis, our observations. Med Arch 2017;71:52–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanski JJ. Screening for uveitis in juvenile chronic arthritis. Br J Ophthalmol 1989;73:225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kodsi S, Rubin S, Milojevic D, Ilowite N, Gottlieb B.. Time of onset of uveitis in children with juvenile rheumatoid arthritis. J AAPOS 2002;6:373–6. [DOI] [PubMed] [Google Scholar]
- 19. Holland GN, Denove CS, Yu F.. Chronic anterior uveitis in children: clinical characteristics and complications. Am J Ophthalmol 2009;147:667–78.e5. [DOI] [PubMed] [Google Scholar]
- 20. Angeles‐Han ST, Ringold S, Beukelman T. et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis–associated uveitis. Arthritis Care Res (Hoboken) 2019;71:703–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics. Guidelines for ophthalmologic examinations in children with juvenile rheumatoid arthritis (RE9320). Pediatrics 1993;92:9–11. [PubMed] [Google Scholar]
- 22. Cassidy J, Kivlin J, Lindsley C, Nocton J; Section on Rheumatology; Section on Ophthalmology. Ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics 2006;117:1843–5. [DOI] [PubMed] [Google Scholar]
- 23. Constantin T, Foeldvari I, Anton J. et al. Consensus-based recommendations for the management of uveitis associated with juvenile idiopathic arthritis: the SHARE initiative. Ann Rheum Dis 2018;77:1107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruperto N, Martini A.. Networking in paediatrics: the example of the Paediatric Rheumatology International Trials Organisation (PRINTO). Arch Dis Child 2011;96:596–601. [DOI] [PubMed] [Google Scholar]
- 25. Swart J, Giancane G, Horneff G. et al. Pharmacovigilance in juvenile idiopathic arthritis patients treated with biologic or synthetic drugs: combined data of more than 15,000 patients from Pharmachild and national registries. Arthritis Res Ther 2018;20:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- 27. Collins GS, Reitsma JB, Altman DG, Moons KGM.. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD Statement. Ann Intern Med 2015;162:55. [DOI] [PubMed] [Google Scholar]
- 28. Haasnoot A-M, Kuiper JJW, Boer J. D.. Predicting uveitis in juvenile idiopathic arthritis: from biomarkers to clinical practice. Expert Rev Clin Immunol 2019;15:657–66. [DOI] [PubMed] [Google Scholar]
- 29. Sim KT, Venning HE, Barrett S, Gregson RM, Amoaku WM.. Extended oligoarthritis and other risk factors for developing JIA-associated uveitis under ILAR classification and its implication for current screening guideline. Ocul Immunol Inflamm 2006;14:353–7. [DOI] [PubMed] [Google Scholar]
- 30. Angeles-Han ST, McCracken C, Yeh S. et al. Characteristics of a cohort of children with Juvenile Idiopathic Arthritis and JIA-associated Uveitis. Pediatr Rheumatol 2015;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Papadopoulou M, Zetterberg M, Oskarsdottir S, Andersson Grönlund M.. Assessment of the outcome of ophthalmological screening for uveitis in a cohort of Swedish children with juvenile idiopathic arthritis. Acta Ophthalmol 2017;95:741–7. [DOI] [PubMed] [Google Scholar]
- 32. Moradi A, Amin RM, Thorne JE.. The role of gender in juvenile idiopathic arthritis-associated uveitis. J Ophthalmol 2014;2014:461078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saurenmann RK, Rose JB, Tyrrell P. et al. Epidemiology of juvenile idiopathic arthritis in a multiethnic cohort: ethnicity as a risk factor. Arthritis Rheum 2007;56:1974–84. [DOI] [PubMed] [Google Scholar]
- 34. Kotaniemi K, Kautiainen H, Karma A, Aho K.. Occurrence of uveitis in recently diagnosed juvenile chronic arthritis: a prospective study. Ophthalmology 2001;108:2071–5. [DOI] [PubMed] [Google Scholar]
- 35. Cattalini M, Soliani M, Caparello MC, Cimaz R.. Sex differences in pediatric rheumatology. Clin Rev Allergy Immunol 2019;56:293–307. [DOI] [PubMed] [Google Scholar]
- 36. Ravelli A, Felici E, Magni-Manzoni S. et al. Patients with antinuclear antibody-positive juvenile idiopathic arthritis constitute a homogeneous subgroup irrespective of the course of joint disease. Arthritis Rheum 2005;52:826–32. [DOI] [PubMed] [Google Scholar]
- 37. Campanilho-Marques R, Bogas M, Ramos F, Santos MJ, Fonseca JE.. Prognostic value of antinuclear antibodies in juvenile idiopathic arthritis and anterior uveitis. Results from a systematic literature review. Acta Reumatol Port 2014;39:116–22. [PubMed] [Google Scholar]
- 38. Sen ES, Dick AD, Ramanan AV.. Uveitis associated with juvenile idiopathic arthritis. Nat Rev Rheumatol 2015;11:338–48. [DOI] [PubMed] [Google Scholar]
- 39. Petty RE, Zheng Q.. Uveitis in juvenile idiopathic arthritis. World J Pediatr 2020;16:562–5. [DOI] [PubMed] [Google Scholar]
- 40. Calandra S, Gallo MC, Consolaro A. et al. Female sex and oligoarthritis category are not risk factors for uveitis in Italian children with juvenile idiopathic arthritis. J Rheumatol 2014;41:1416–25. [DOI] [PubMed] [Google Scholar]
- 41. Grassi A, Corona F, Casellato A, Carnelli V, Bardare M.. Prevalence and outcome of juvenile idiopathic arthritis-associated uveitis and relation to articular disease. J Rheumatol 2007;34:1139–45. [PubMed] [Google Scholar]
- 42. Haasnoot AJW, Tent-Hoeve M V, Wulffraat NM. et al. Erythrocyte sedimentation rate as baseline predictor for the development of uveitis in children with juvenile idiopathic arthritis. Am J Ophthalmol 2015;159:372–7.e1. [DOI] [PubMed] [Google Scholar]
- 43. Angeles-Han ST, McCracken C, Yeh S. et al. HLA associations in a cohort of children with juvenile idiopathic arthritis with and without uveitis. Invest Ophthalmol Vis Sci 2015;56:6043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nordal EB, Songstad NT, Berntson L. et al. Biomarkers of chronic uveitis in juvenile idiopathic arthritis: predictive value of antihistone antibodies and antinuclear antibodies. J Rheumatol 2009;36:1737–43. [DOI] [PubMed] [Google Scholar]
- 45. Haasnoot A-M, Schilham MW, Kamphuis S. et al. Identification of an amino acid motif in HLA-DRβ1 that distinguishes uveitis in patients with juvenile idiopathic arthritis. Arthritis Rheumatol (Hoboken, NJ) 2018;70:1155–65. [DOI] [PubMed] [Google Scholar]
- 46. Walscheid K, Neekamp L, Heiligenhaus A. et al. Increased circulating proinflammatory T lymphocytes in children with different forms of anterior uveitis: results from a pilot study. Ocul Immunol Inflamm 2019;27:788–97. [DOI] [PubMed] [Google Scholar]
- 47. Walscheid K, Neekamp L, Heiligenhaus A. et al. Peripheral blood monocytes reveal an activated phenotype in pediatric uveitis. Clin Immunol 2018;190:84–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are reported in the article. Additional details can be provided by the corresponding author upon reasonable request. The Pharmachild registry is registered at Clinicaltrials.gov (NCT01399281) and at the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP; http://www.encepp.eu/encepp/viewResource.htm? id=19362).