Abstract
Objectives
SSc primary heart involvement (SSc-pHI) is a significant cause of mortality. We aimed to characterize and identify predictors of subclinical SSc-pHI using cardiovascular MRI.
Methods
A total of 83 SSc patients with no history of cardiovascular disease or pulmonary arterial hypertension and 44 healthy controls (HCs) underwent 3 Tesla contrast-enhanced cardiovascular MRI, including T1 mapping and quantitative stress perfusion. High-sensitivity troponin I and N-terminal pro-brain natriuretic peptide were also measured.
Results
Cardiovascular MRI revealed a lower myocardial perfusion reserve in the SSc patients compared with HCs {median (interquartile range (IQR)] 1.9 (1.6–2.4) vs 3 (2–3.6), P < 0.001}. Late gadolinium enhancement, indicating focal fibrosis, was observed in 17/83 patients but in none of the HCs, with significantly higher extracellular volume (ECV), suggestive of diffuse fibrosis, in SSc vs HC [mean (s.d.) 31 (4) vs 25 (2), P < 0.001]. Presence of late gadolinium enhancement and higher ECV was associated with skin score [odds ratio (OR) = 1.115, P = 0.048; R2 = 0.353, P = 0.004], and ECV and myocardial perfusion reserve was associated with the presence of digital ulcers at multivariate analysis (R2 = 0.353, P < 0.001; R2 = 0.238, P = 0.011). High-sensitivity troponin I was significantly higher in patients with late gadolinium enhancement, and N-terminal pro-brain natriuretic peptide was associated with ECV (P < 0.05).
Conclusion
Subclinical SSc-pHI is characterized by myocardial microvasculopathy, diffuse and focal myocardial fibrosis but preserved myocardial contractile function. This subclinical phenotype of SSc-pHI was associated with high-sensitivity troponin I, N-terminal pro-brain natriuretic peptide, SSc disease severity and complicated peripheral vasculopathy. These data provide information regarding the underlying pathophysiological processes and provide a basis for identifying individuals at risk of SSc-pHI.
Keywords: SSc primary heart involvement, cardiovascular magnetic resonance, risk stratification
Rheumatology key messages
Cardiovascular MRI detects subclinical microvasculopathy, myocardial focal and diffuse fibrosis in SSc.
Hs-TnI, NT-proBNP, markers of disease severity and complicated peripheral vasculopathy, predict subclinical SSc-primary heart involvement (SSc-pHI).
This large cardiovascular MRI in SSc study provides a basis for risk stratification in SSc-pHI.
Introduction
SSc is a heterogeneous autoimmune disease characterized by vasculopathy and progressive fibrosis of the skin and internal organs [1, 2]. SSc-primary heart involvement (SSc-pHI) [excluding ischaemic heart disease (IHD) and pulmonary arterial hypertension (PAH)] develops as a direct consequence of the disease and is one of the most common causes of death in SSc, with a clinical prevalence of between 15% and 35% [3]. Its manifestations typically include myocarditis, cardiac failure (systolic or diastolic dysfunction) and arrhythmia [4, 5]. Myocardial fibrosis is the pathological hallmark of SSc-pHI and has been postulated to be the consequence of repeated focal ischaemia due to microvasculopathy [6].
Epidemiological datasets have associated poor prognostic factors of SSc, including diffuse SSc subtype, anti-topoisomerase antibody (Scl-70), male sex and major internal organ involvement with SSc-pHI [7, 8]. However, effective means of risk stratification to guide tailored monitoring and early detection of cardiac involvement in a general, asymptomatic SSc cohort are lacking. Cardiovascular MRI (CMR) can provide a comprehensive assessment of cardiac morphology, function and tissue characterization and can thus detect subclinical SSc-pHI [6, 9–11]; a proportion of patients with subclinical SSc-pHI will develop clinical events associated with SSc-pHI. Late gadolinium enhancement (LGE) CMR can detect focal fibrosis [9, 12, 13], as distinct from IHD, in which the location of infarction on LGE follows the coronary artery distribution. CMR parametric mapping with estimation of myocardial extracellular volume (ECV) and T1 native provides indicators of diffuse extracellular processes, in particular diffuse fibrosis, with histopathological studies confirming findings of myocardial interstitial fibrosis [14–16].
Addition of perfusion CMR with vasodilator stress can assess microvascular dysfunction in SSc [9, 17] in the absence of IHD, although few studies have demonstrated global reduction in myocardial blood flow (MBF) and myocardial perfusion reserve (MPR) [18]. Standard cardiac biomarkers of cardiac injury [troponin I (TnI) and remodelling, N-terminal pro-brain natriuretic peptide (NT-proBNP)] have been shown to be significantly elevated in SSc compared with healthy controls (HCs), and both have been associated with the development of cardiovascular (CV) events in SSc [19–22]. The value of TnI and NT-proBNP in predicting CMR abnormalities in SSc-pHI has however not been explored to date.
The aims of this study were within a general SSc cohort with no known SSc-pHI and no IHD or PAH, first, to characterize subclinical heart involvement using a comprehensive CMR protocol that can evaluate for markers of fibrosis and microvasculopathy. Second, we evaluated for clinical and standard serum cardiac predictors of such a subgroup with subclinical SSc-pHI at risk of future events.
Methods
Participants
Patients recruited to this study fulfilled the 2013 ACR/EULAR criteria for SSc [23] and were classified as lcSSc or dcSSc according to LeRoy classification [24]. Patients were excluded if they had any prior diagnosis of IHD (or clinically overt SSc-pHI), diabetes or more than two traditional CV risk factors [defined as current smoker, hypertension, hypercholesterolaemia/hypertriglyceridaemia and family history of premature CV disease (CVD) and/or any evidence of PAH detected on tissue Doppler echocardiography and confirmed by right heart catheterization]. All patients had echocardiography performed within one year of the study visit, as part of their routine clinical testing. Patients with any other inflammatory musculoskeletal conditions were also excluded. A total of 44 healthy volunteers with no CVD and no CV treatment (frequency matched with the SSc cohort for age and sex) were recruited. The research was carried out according to the Declaration of Helsinki and was approved by the NRES Committee Yorkshire & The Humber–Leeds East Ethics Committee. All participants provided written informed consent.
Clinical data
Comprehensive demographic and clinical data were collected, including SSc subtype, duration, serology, organ involvement, treatment and nailfold capillaroscopy findings. Patients also had an electrocardiography performed. All patients had pulmonary function tests that were undertaken as part of their routine clinical assessment, within one year of the CMR visit. A diagnosis of interstitial lung disease was based on HRCT findings.
Serum sample collection
High-sensitivity TnI (Hs-TnI), NT-proBNP and creatine kinase were measured for all participants. Hs-TnI and creatine kinase were tested on a Siemens Advia XPT system (Advia Chemistry XPT and Advia Centaur XPT Immunoassay, respectively) and NT-proBNP on Cobas 6000 (immunochemistry module Cobas e601) using appropriate kits supplied by Roche Diagnostics. All patients had ANA and CRP tested as part of standard care.
CMR imaging
Both SSc and HC cohorts underwent CMR on a 3 Tesla Philips Achieva MRI system as previously described [25, 26], including cine imaging for left ventricle (LV) volume estimation, LGE, native and post-contrast T1 mapping for ECV quantification, and adenosine stress and rest myocardial perfusion, which enabled quantitative assessment of MBF and MPR (a ratio of maximal stress to resting MBF) (full details in Supplementary Data S1, available at Rheumatology online). According to the departmental reference ranges, an ECV >29% and native T1 > 1240 (ms) were classed as abnormal [27, 28].
Statistical analysis
Descriptive summary statistics were provided for all variables. Continuous variables were reported as mean (s.d.) or median [interquartile range (IQR)], and categorical data were reported as percentages. Student’s t test, the Mann–Whitney U test and the χ2 test were used as appropriate to assess for significance of between-group differences. A Bonferroni correction was applied to correct for multiple comparisons when using multiple t tests. Spearman’s rho test was used to assess correlation between CMR indices and clinical and cardiac biomarkers. Linear and logistic regression analyses were used to assess the correlation and predictive value of these biomarkers with CMR measures. For multivariate regression analysis, regressors were eliminated based on the backwards stepwise regression rule. The model with the highest adjusted R square was selected from the range of models generated by backwards regression. For logistic regression, the presence of a CV risk factor was added in the model, as this was considered an important factor for the prediction of CMR fibrosis. Receiver-operating characteristic curves were built to assess the ability of cardiac biomarkers to identify abnormal CMR measures. Statistical analysis was performed using SPSS (IBM SPSS Statistics 22) and Graph Pad Prism 8.
Results
Baseline characteristics
A total of 83 SSc patients were recruited to the study. Details on patient selection, recruitment and feasibility are described in Supplementary Fig. S1, available at Rheumatology online. Participants had a median (IQR) age of 54 (49–54) and disease duration (defined as time from first non-RP) of 7 (2–7) years; 84% were females and 34% had dcSSc. A total of 40% had known interstitial lung disease, and 24% and 25% had a history of digital ulceration (DU) and calcinosis, respectively. A total of 78 (94%) patients were ANA positive, of whom 28 (34%) and 24 (29%) were ACA and Scl-70 positive, respectively (Table 1). A total of 41 (49%) patients were receiving a DMARD at the time of recruitment, the majority receiving MMF (35%). Of those receiving a DMARD, 19 (23%) participants were receiving iloprost vasodilator treatment, 17 (21%) were receiving sildenafil, and 4 (5%) were receiving bosentan (Table 1), (Supplementary Table S1, available at Rheumatology online). A total of 22 (27%) patients had CV risk factors, 17 had one CV risk factor, and the remaining 5 had two CV risk factors. All SSc patients were in sinus rhythm, and none had any signs of IHD on electrocardiography.
Table 1.
Disease characteristics of SSc patients
| Baseline characteristic | SSc cohort, n = 83 |
|---|---|
| Age, median (IQR), years | 54 (49–63) |
| Female, n (%) | 70 (84) |
| Disease subtype, n (%) | |
| lcSSc | 55 (66) |
| dcSSc | 28 (34) |
| Disease duration, median (IQR), years | 7 (2–7) |
| History of, n (%) | |
| Digital ulceration | 20 (24) |
| Calcinosis | 21 (25) |
| Myositis | 3 (4) |
| GORD | 73 (88) |
| Interstitial lung disease | 33 (40) |
| NSIP | 31 (37) |
| UIP | 2 (2) |
| Current use of DMARD, n (%) | 49 (59) |
| Name of current DMARD, n (%) | |
| MMF | 29 (35) |
| MTX | 8 (10) |
| HCQ | 1 (5) |
| SSZ | 1 (1) |
| CYC | 3 (4) |
| Rituximab | 1 (1) |
| Previous use of CYC, n (%) | 23 (28) |
| Current use of prednisolone, n (%) | 18 (22) |
| Current treatment with, n (%) | |
| Iloprost | 19 (23) |
| Sildenafil | 17 (21) |
| Bosentan | 4 (5) |
| ACE inhibitor | 31 (37) |
| Calcium channel blocker | 48 (58) |
| Statin use | 5 (6) |
| Clinical profile | |
| Total modified Rodnan skin score, median (IQR) | 2 (1–6) |
| Presence of, n (%) | |
| Digital pitting scars | 27 (33) |
| Digital ulceration | 14 (17) |
| Tendon friction rubs | 3 (4) |
| Calcinosis | 17 (21) |
| Joint contractures | 13 (16) |
| Serology & acute phase | |
| Antibody positive, n (%) | |
| ANA | 78 (94) |
| ACA | 28 (34) |
| Scl70 | 24 (29) |
| Anti-RNA polymerase III | 3 (4) |
| CRP, median (IQR), mg/l | 5 (5–5.1) |
| Cardiovascular risk profile, n (%) | |
| Dyslipidaemia | 3 (4) |
| Hypertension | 8 (10) |
| Smoking | 7 (8) |
| Family history of CVD | 8 (10) |
| Patients with any CV risk factors, n (%) | 22 (27) |
N % presented unless stated otherwise. ACE: angiotensin-converting enzyme; CV: cardiovascular; CVD: cardiovascular disease; GORD: gastro-oesophageal reflux disease; IQR: interquartile range; NSIP: non-specific interstitial pneumonia; Scl70: anti-topoisomerase antibody; UIP: usual interstitial pneumonia.
None of the patients had evidence of PAH on echocardiography.
CMR assessment in SSc vs HCs
Of the 83 patients recruited, complete CMR function/volume assessment were available in all, LGE and native T1 in 80, ECV in 78 and perfusion CMR in 61 patients (Supplementary Fig. S1, available at Rheumatology online). Of the 44 HCs, LV function/volumes, LGE, native T1 and ECV were available in all, and perfusion CMR was available in 36. HCs were well matched to patients, with a median (IQR) age of 55 (37–63), 37 (84%) being females.
Tissue characterization: myocardial inflammation and focal and diffuse fibrosis observed in SSc
A total of 17 (21%) SSc patients (and none of the HCs) had focal LGE fibrosis in a non-ischaemic pattern, with a mean (s.d.) LGE fibrosis mass of 2.08 (1.74). Sixteen additional patients had evidence of right ventricular insertion point LGE, considered a non-specific finding and thus not included in the LGE analysis [29]. ECV, a marker of diffuse fibrosis, was significantly higher in patients with SSc compared with HCs [mean (s.d.) 31% (4) vs 25% (2), P < 0.001] and remained statistically significant after the Bonferroni correction was applied (P < 0.005) (Table 2).
Table 2 .
Cardiovascular MRI parameters in SSc patients vs healthy controls
| CMR variable | HC (n = 44) | SSc patients (n = 83) | P-value | Bonferroni correction |
|---|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | |||
| Fibrosis | ||||
| ECV, % | 25 (2) | 31 (3) | <0.001** | <0.001 |
| Native T1, ms | 1209 (51) | 1241 (76) | 0.008* | NS |
| LGE, n (%) | 0 | 17/80 (21%) | ||
| LGE fibrosis mass, g | 0 | 2.08 (1.74) | ||
| Myocardial perfusion, median (IQR) | ||||
| Stress MBF, ml/g/min | 2.6 (2.2–3.3) | 1.9 (1.4–2.6) | <0.001** | <0.001 |
| Rest MBF, ml/g/min | 0.9 (0.7, 1.2) | 0.9 (0.6–1.2) | 0.734 | NS |
| MPR | 3 (2–3.6) | 1.9 (1.6–2.4) | <0.001** | <0.001 |
| LV function/volume | ||||
| LVEDV/BSA, ml/m2 | 77 (14) | 77 (15) | 0.513 | NS |
| LVESV/BSA, ml/m2 | 30 (9) | 30 (9) | 0.898 | NS |
| LVSV/BSA, ml/m2 | 47 (9) | 48 (7) | 0.553 | NS |
| LVEF, % | 61 (7) | 61 (7) | 0.565 | NS |
| LV mass/BSA, g/m2 | 45 (10) | 43 (10) | 0.415 | NS |
P < 0.05;
P < 0.001.
Mean (s.d.) unless stated otherwise. Bonferroni correction was applied for all t tests (n = 10), with a P < 0.005 indicating statistical significance.
BSA: body surface area; CMR: cardiovascular MRI; ECV: extracellular-volume fraction; EDV: end-diastolic volume; ESV: end-systolic volume; HCs: healthy controls; LGE: late gadolinium enhancement; LV: left ventricular; EF: ejection fraction; MBF: myocardial blood flow; MPR: myocardial perfusion reserve; NS: non-significant; SV: stroke volume.
Native T1 was also higher in SSc compared with HCs but did not reach statistical significance following Bonferroni correction [mean (s.d.) 1241 ms (76) vs 1209 ms (51), P = 0.008, i.e. P > 0.005]. Mean native ECV was above the normal reference value (>29%) and T1 just above (>1240). A total of 51 (61%) and 43 (52%) SSc patients, respectively, had native T1 and ECV above normal values.
LGE pattern and distribution
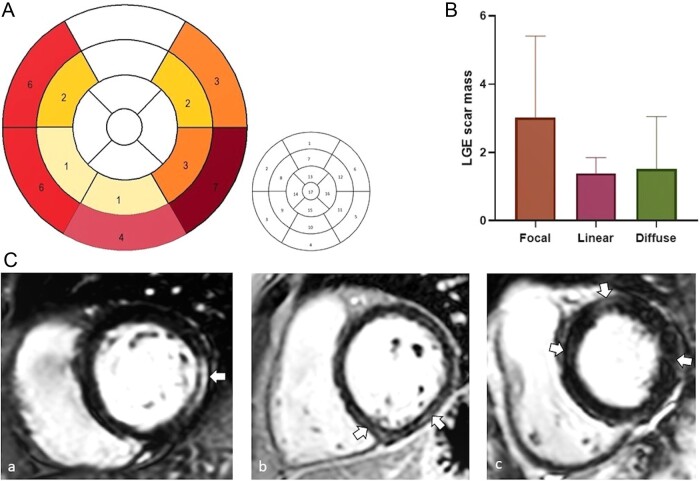
Of the 17 SSc patients with focal fibrosis on LGE CMR, a linear pattern was noted in 9 participants, a focal pattern in 6 and a diffuse pattern in 2 (1 participant had both focal and linear distribution) (Fig. 1C). Higher LGE fibrosis mass was noted in those with a focal LGE pattern compared with those with a linear or diffuse pattern [mean (s.d.) 3.02 (2.3) vs 1.39 (0.45) and 1.5 (1.5), respectively] (Fig. 1B). The distribution of LGE was 7 midwall, 6 subepicardial, 2 midwall-subepicardial and 2 transmural/diffuse. The most commonly affected segment was the basal inferolateral segment (n = 7), followed by the basal anteroseptal and inferoseptal segments. Of 17 patients, 11 had involvement of >1 segment (Fig. 1A).
Fig. 1 .
Late gadolinium enhancement fibrosis in SSc patients
(A) Number of patients with LGE fibrosis as per each cardiac segment (left figure). 17-segment model (right figure) 1: basal anterior; 2: basal anteroseptal; 3: basal inferoseptal; 4: basal inferior; 5: basal inferolateral; 6: basal anterolateral; 7: mid anterior; 8: mid anteroseptal; 9: mid inferoseptal; 10: mid inferior; 11: mid inferolateral; 12: mid anterolateral; 13: apical anterior; 14: apical septal; 15: apical inferior; 16: apical lateral; 17: apex. (B) LGE fibrosis mass in those with focal, linear and diffuse pattern. (C) LGE patterns: a-focal; b-linear; c-diffuse. LGE: late gadolinium enhancement.
Lower stress MBF and MPR in SSc
None of the HCs or SSc patients with perfusion CMR data had regional perfusion defects that would be suggestive of coronary artery disease. Quantitative analysis however showed significantly lower global MBF at stress and lower MPR in SSc patients compared with HCs [median (IQR) 1.9 (1.4–2.6) vs 2.6 (2.2–3.3), P < 0.001; median (IQR) 1.9 (1.6–2.4) vs 3 (2–3.6), P < 0.001, respectively] (Table 2). There was no difference in MBF at rest between the two groups.
Normal functional assessment in SSc
LV volumes and function, including LV end-diastolic volume, LV end-systolic volume, LV stroke volume, LV ejection fraction and LV mass were comparable between the HCs and SSc participants, and the means were within normal limits (Table 2). Right ventricular parameters in SSc patients were within normal range (Supplementary Data S2, available at Rheumatology online).
CMR measures of fibrosis and vasculopathy were correlated with SSc disease severity and peripheral vasculopathy
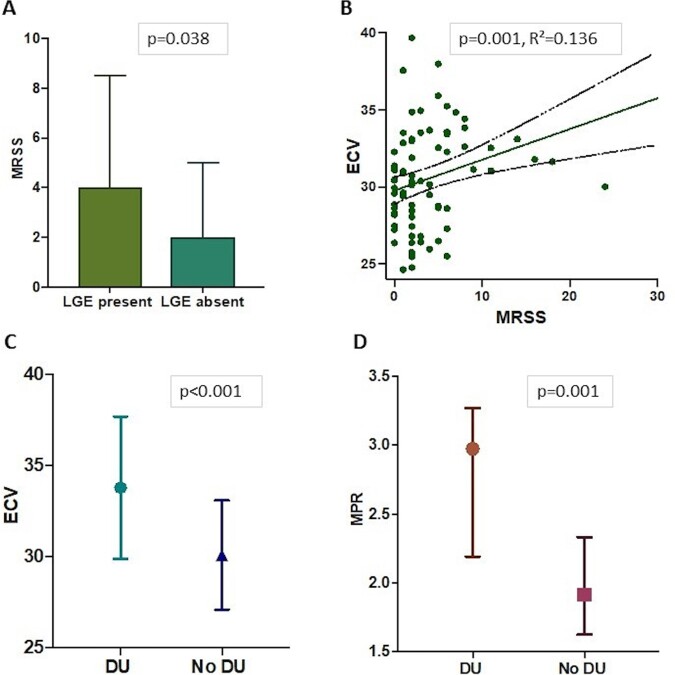
Patients with LGE focal fibrosis had significantly higher modified Rodnan skin score (mRSS) [median (IQR) 4 (2–9) vs 2 (1–5), P = 0.038] (Fig. 2A) and higher, albeit modest, CRP levels [median (IQR) 5 (5–18) vs 5 (5,5), P = 0.038] compared with those without LGE. Logistic regression confirmed an association between mRSS and LGE [odds ratio (OR) = 1.107, P = 0.048], which remained significant after adjusting for the presence of CV risk factors (Table 3). LGE fibrosis mass was also moderately correlated with mRSS (rho = 0.231, P = 0.039).
Fig. 2 .
Disease phenotype and cardiovascular MRI parameters
(A) Presence or absence of LGE fibrosis and median (IQR) mRSS. (B) Association between ECV and mRSS. (C) Presence or absence of DU and ECV. (D) Presence or absence of DUs and MPR. DU: digital ulceration; ECV: extracellular volume; LGE: late gadolinium enhancement; MPR: myocardial perfusion reserve; mRSS: modified Rodnan skin score; IQR: interquartile range.
Table 3.
Logistic and linear regression to predict the relationship between SSc clinical phenotype and CMR variables
| LGE presence (n = 17)/absence (n = 63) |
ECV |
MPR |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Logistic regression— univariate analysis |
Logistic regression— multivariate analysis |
Linear univariate analysis |
Multivariate analysis, R2 = 0.353 |
Linear univariate analysis |
Multivariate analysis, R2 = 0.238 |
||||||
| OR | P-value | OR | P-value | Beta | P-value | Beta | P-value | Beta | P-value | Beta | P-value | |
| Male sex | 2.11 | 0.275 | −0.05 | 0.966 | −0.195 | 0.133 | −0.180 | 0.167 | ||||
| Age | 0.986 | 0.541 | 0.019 | 0.869 | −0.231 | 0.073 | −0.295 | 0.022* | ||||
| Presence of CV risk factors | 1.106 | 0.875 | 1.401 | 0.630 | 0.209 | 0.066 | 0.113 | 0.277 | −0.139 | 0.287 | −0.071 | 0.568 |
| Disease duration | 0.969 | 0.411 | −0.216 | 0.059 | −0.157 | 0.137 | 0.028 | 0.831 | ||||
| Presence of ILD | 1.444 | 0.504 | −0.12 | 0.296 | −0.085 | 0.425 | 0.031 | 0.810 | ||||
| DLCO/VA | 0.893 | 1.003 | −0.238 | 0.040* | −0.112 | 0.356 | ||||||
| mRSS | 1.107 | 0.048* | 1.115 | 0.048* | 0.369 | 0.001* | 0.329 | 0.004* | −0.145 | 0.264 | −0.159 | 0.210 |
| dcSSc | 1.621 | 0.392 | 0.185 | 0.105 | 0.009 | 0.945 | ||||||
| Digital ulcers | 0.222 | 0.154 | 0.395 | <0.001** | 0.388 | <0.001** | 0.299 | 0.013* | 0.319 | 0.011* | ||
| CRP | 1.064 | 0.067 | −0.056 | 0.624 | −0.154 | 0.135 | −0.075 | 0.567 | ||||
| DMARD treatment | 0.402 | 0.148 | 0.151 | 0.186 | −0.003 | 0.984 | ||||||
| ACE inhibitor treatment | 1.428 | 0.447 | −0.087 | 0.451 | −0.04 | 0.978 | ||||||
P < 0.05;
P < 0.001.
ACE: angiotensin-converting enzyme; CMR: cardiovascular MRI; CV: cardiovascular; DLCO/VA: DLCO adjusted for volume; ECV: extracellular volume; ILD: interstitial lung disease; LGE: late gadolinium enhancement; MPR: myocardial perfusion reserve; mRSS: modified Rodnan skin score; OR: odds ratio.
ECV was significantly higher in patients with DU compared with those without [mean (s.d.) 34 (4) vs 29 (3), respectively, P < 0.001] (Fig. 2C). Univariate analysis indicated associations of ECV with DU (P < 0.001), mRSS (P = 0.001) (Fig. 2B) and DLCO/VA (P = 0.040). Multivariate analysis confirmed ECV was associated with mRSS and the presence of DU (R2 = 0.353, P = 0.004, P < 0.001, respectively) (Table 3).
Significantly higher MPR values were noted in SSc patients with DU compared with those without [median (IQR) 3 (2–3) vs 2 (2,2), P = 0.001] (Fig. 2D). The presence of DU was associated with MPR in both univariate (P = 0.013) and multivariate analysis (R2 = 0.238, P = 0.011) (Table 3). Age was negatively associated with MPR at multivariate analysis (R2 = 0.238, P = 0.022).
Neither DMARD nor angiotensin-converting enzyme inhibitor treatment were associated with the CMR measures of fibrosis and vasculopathy (P > 0.05).
The association between native T1 and clinical phenotype is detailed in Supplementary Table S2 and Supplementary Data S2, available at Rheumatology online.
CMR measures of fibrosis and vasculopathy were associated with serum cardiac biomarkers
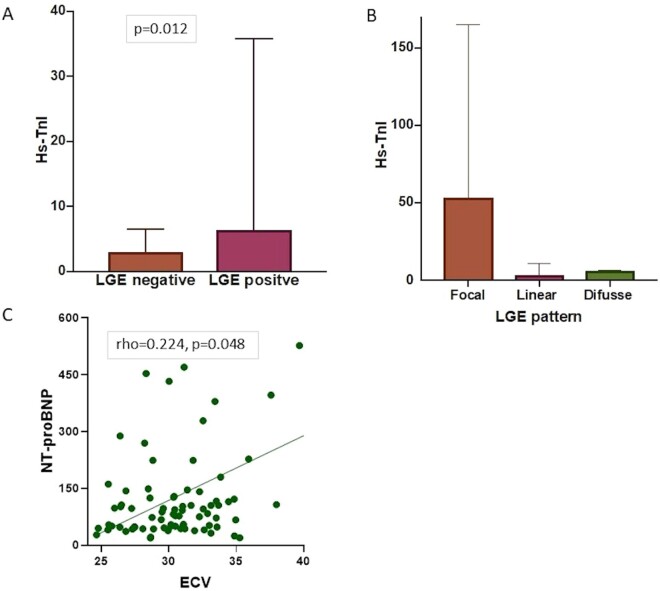
Hs-TnI was significantly higher in patients with LGE focal fibrosis compared with those without [median (IQR) 6.4 (4–36) vs 2.9 (3–7), P = 0.012] (Fig. 3A) and was correlated with LGE fibrosis mass (rho = 0.283; P = 0.014). Patients with a focal LGE pattern (also with greater fibrosis mass) had higher hs-TnI levels compared with those with a linear or diffuse pattern [median (IQR) 53 (6–165) vs 4 (3–11) vs 6 (5–6), respectively] (Fig. 3B).
Fig. 3 .
Association between cardiac biomarkers and cardiovascular MRI variables
(A) Presence or absence of LGE and hs-TnI. (B) Hs-TnI in focal, linear or diffuse LGE pattern. (C) Association between ECV and NT-proBNP. ECV: extracellular volume; hs-TnI: high-sensitivity troponin I; LGE: late gadolinium enhancement; NT-proBNP: N-terminal pro-brain natriuretic peptide.
A positive correlation was observed between NT-proBNP and ECV (rho = 224; P = 0.048) (Fig. 3C) and higher levels of NT-proBNP were associated with the presence of LGE fibrosis, although this did not reach statistical significance [median (IQR) 105 (49–377) vs 77 (46–122), P = 0.083]. Receiver-operating characteristic curves were plotted to assess the ability of serum cardiac biomarkers to identify focal or diffuse myocardial fibrosis. AUC (95% CI) of Hs-TnI for identifying LGE was significant at 0.695 (0.55–0.837), P = 0.015. A hs-TnI ≥ 5.5 ng/l had a sensitivity of 65% and a specificity of 70% to predict the presence of focal LGE. AUC (95% CI) of NT-proBNP for identifying ECV (ECV < 29%; ECV ≥ 29%) was 0.586 (0.447–0.726), P = 0.213 (Supplementary Fig. S2, available at Rheumatology online). There was no significant correlation between MPR and cardiac biomarkers (P > 0.05) or between creatine kinase and CMR measures.
Discussion
We report on the largest study to date to phenotype subclinical SSc-pHI using CMR markers of myocardial fibrosis and perfusion, and to identify clinical and serum predictors of subclinical SSc-pHI. Myocardial microvasculopathy, as well as focal and diffuse myocardial fibrosis, are suggestive of SSc-pHI. In this study, presence of fibrosis was associated with raised hs-TnI and NT-proBNP, and both myocardial perfusion impairment and fibrosis were associated with SSc disease severity and peripheral vasculopathy. These data provide information regarding the underpinning processes of SSc-pHI and provide a basis for risk stratifying a SSc cohort without overt cardiac involvement for CMR-detected subclinical SSc-pHI who would benefit from more focused monitoring.
Cardiac involvement is a significant cause of morbidity and mortality in SSc, but we have limited understanding of the pathophysiological basis and the patients most at risk. In asymptomatic SSc individuals, we demonstrated impaired microvascular perfusion and tissue changes suggestive of focal and diffuse fibrosis, with preservation of ventricular dimensions and function. One-quarter of the SSc patients (and no HCs) had evidence of focal myocardial fibrosis in a non-coronary LGE pattern. The study did not include patients with IHD, PAH, diabetes, other inflammatory musculoskeletal diseases, and allowed a minimum presence of traditional CV risk factors, thus reducing the risk of including cardiac fibrosis associated with IHD or other conditions. Moreover, the study excluded focal LGE localized at the inferior right ventricular insertion point that is considered non-specific and may represent a normal variant [29]. Three distinctive patterns of focal fibrosis were observed; linear, diffuse and focal, the latter being associated with higher LGE fibrosis mass, although lower compared with fibrosis mass reported in IHD [30, 31]. LGE was localized predominantly at the basal anteroseptal and inferoseptal segments and had a midwall or subepicardial distribution, similar to previously reported studies [32, 33]. A lateral subepicardial LGE distribution which is more commonly described in myocarditis [34], was present in nearly half of the SSc patients. This implies that at least in a subgroup of SSc patients, a silent inflammatory process could be the substrate for the development of myocardial fibrosis. The relevance and mechanism for these different LGE patterns needs to be further explored.
By applying T1 mapping, we also demonstrated higher ECV, suggestive of diffuse myocardial fibrosis in SSc compared with HCs [15, 35]. In non-ischaemic cardiomyopathies, ECV is a poor prognostic marker for CV outcomes [36], with pathophysiological studies suggesting that interstitial fibrosis can progress to replacement fibrosis (seen as LGE on CMR) [37, 38]. We recently reported an association between ECV and implantable loop recorder–detected arrhythmia in asymptomatic SSc patients [26], underscoring the potential clinical importance of raised ECV in SSc. Further studies are needed to fully establish the prognostic relevance of this finding.
The study also demonstrated significantly lower MPR in SSc patients compared with HCs with no regional perfusion defects, indicating myocardial microvascular disease. One previous small study (n = 19) also performed quantitative perfusion CMR and demonstrated lower stress MBF in SSc patients compared with HCs [18]. In our study, microvascular impairment occurred in SSc patients with normal left ventricular dimensions and function, with and without myocardial fibrosis, suggesting microvascular disease may be the earliest manifestation of heart involvement in SSc. Longitudinal studies are needed to understand whether and how these pathophysiological processes are interrelated and develop over time, and ultimately lead to the clinical manifestations of SSc-pHI.
Of particular value are the analyses identifying possible predictors of subclinical SSc-pHI. Several smaller studies reported an association of ECV with mRSS [15, 39] and longer history of RP in those with focal fibrosis [12], with another study failing to reveal an association between the CMR measures of fibrosis and SSc disease phenotype [33]. Our larger study showed that both LGE and ECV were correlated with mRSS in a predictive model. Patients with LGE also had higher CRP levels. Collectively, these findings support the association of myocardial fibrosis with disease severity and suggest likely concurrent fibrotic process affecting both the skin and myocardium.
Peripheral vasculopathy is considered an early inciting event in the pathogenesis of SSc. In our study, ECV correlated with the presence of DU, indicating co-existing peripheral and myocardial processes. The observed increase in MPR in the presence of DU however appears at first counterintuitive. Patients with DU are commonly treated with vasodilators (80% in our cohort). An increase in resting MBF would have been expected along with a reduction in stress perfusion in the presence of microvascular disease, with consequent reduction, rather than increase, in MPR. However, the coronary microvasculature of SSc patients, and responsiveness to vasodilator treatment compared with a general population may be altered and has not been extensively evaluated. Other studies have also reported an increased myocardial perfusion index following vasodilatory therapies [40, 41], while one recent study showed a protective role of vasodilator treatment in SSc-pHI [42]. Further mechanistic investigation is required to explain these findings and the role of vasodilator therapy on CMR-detected SSc-pHI. The results also confirmed a negative association between age and MPR, suggesting a reduction of MPR with age, as documented in the general population [43, 44].
The relevance of modest hs-TnI elevation in the general population is not clear, with renal pathology, inflammation and/or infection being possible contributors [45]. Our results showed that the moderate hs-TnI levels observed in SSc were correlated with the presence of LGE and LGE fibrosis mass, indicating that this biomarker is sensitive in detecting myocardial injury in SSc. Moreover, hs-TnI was higher in patients demonstrating a focal pattern of fibrosis, implying a direct relationship between myocardial fibrosis mass (that we observed is increased in focal pattern of fibrosis) and hs-TnI. This is of particular interest, as the extent of LGE has been associated with worse CV outcomes in both ischaemic and non-ischaemic cardiomyopathies [46, 47]. NT-proBNP, released by ventricular myocytes in response to increased wall tension was associated with ECV, which might be explained by interstitial expansion and remodelling of the myocardium. Higher levels of NT-proBNP were also associated with LGE, although the results did not reach statistical significance. Both hs-TnI and NT-proBNP thus appear to be sensitive tools for detecting (focal and diffuse) fibrosis. While receiver-operating characteristic curve analysis showed a moderate diagnostic performance of hs-TnI for identifying LGE, the diagnostic performance of NT-proBNP for identifying ECV was poor. This was likely due to a relatively modest sample size, despite this study being the largest CMR study in SSc to look at the association between cardiac biomarkers and CMR measures.
There are some limitations to this study. Time of occurrence of SSc-pHI and whether treatment can alter the course of SSc-pHI are important clinical questions that would ideally require recruitment of an inception cohort. Such studies in a rare disease are challenging. Atherosclerotic disease is also difficult to exclude fully; however, we minimized this risk by excluding patients with CVD, diabetes and more than two CV risk factors. None of the patients had evidence of IHD on electrocardiography or myocardial perfusion defects indicative of flow-limiting IHD, or evidence of ischaemic fibrosis on CMR, making inadvertent inclusion of secondary IHD in our cohort unlikely. While myocardial stress perfusion imaging provides important information on the pathophysiological process of SSc-pHI, there is not enough evidence currently to support its use in routine clinical practice. T2-weighted imaging was not included in the CMR protocol; however, CMR mapping techniques (which have shown superior accuracy for detecting myocardial oedema and fibrosis compared with T2-weighted imaging) were instead used [48, 49]. The use of a more specific investigative tool for assessing peripheral vasculopathy would have provided more insights into the association between peripheral and myocardial vascular involvement in SSc.
In conclusion, we report on the pathophysiological basis of subclinical SSc-pHI in the largest CMR study in SSc to employ quantitative myocardial perfusion, quantitative fibrosis mass and T1 mapping. In asymptomatic SSc patients, microvasculopathy and myocardial focal and diffuse fibrosis but preserved myocardial contractile function characterise subclinical SSc-pHI. Hs-TnI, NT-proBNP, markers of SSc disease severity and complicated peripheral vasculopathy may predict subclinical SSc-pHI. Collectively, these data provide an initial basis for risk stratifying for subclinical SSc-pHI and the opportunity for more tailored monitoring and intervention of those liable to develop clinically overt SSc-pHI.
Supplementary Material
Acknowledgements
We wish to thank all patients participating in the study. We also thank Petra Bijsterveld for helping with the coordination of the CMR visit, Margaret Saysell, Lisa Lewis and Gavin Bainbridge, radiographers who performed the CMR studies, and Dr Arka Das, who helped with the CMR image preparation. The research was supported by the National Institute for Health Research (NIHR) infrastructure at Leeds. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Funding: The study was partly supported by Scleroderma and Raynaud’s UK. R.B.D. and L.-A.B. have been funded by the ACORN charity. R.B.D. has been also funded by the Charitable Foundation Fellowship, Leeds Teaching Hospital. J.B. is funded by a National Institute of Health Research (NIHR) Clinical Lectureship (ICA-CL-2016–02-017). G.F. was funded by an NIHR grant (number: 11/117/27). S.P. is funded by a British Heart Foundation Personal Chair (CH/16/2/32089).
Disclosure statement: The authors have no competing interests to declare.
Data availability statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Ferri C, Valentini G, Cozzi F. et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine 2002;81:139–53. [DOI] [PubMed] [Google Scholar]
- 2. Deswal A, Follansbee WP.. Cardiac involvement in scleroderma. Rheum Dis Clin North Am 1996;22:841–60. [DOI] [PubMed] [Google Scholar]
- 3. Tyndall AJ, Bannert B, Vonk M. et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809–15. [DOI] [PubMed] [Google Scholar]
- 4. Lambova S. Cardiac manifestations in systemic sclerosis. World J Cardiol 2014;6:993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medsger TA Jr, Masi AT.. Survival with scleroderma. II. A life-table analysis of clinical and demographic factors in 358 male U.S. veteran patients. J Chron Dis 1973;26:647–60. [DOI] [PubMed] [Google Scholar]
- 6. Follansbee WP, Miller TR, Curtiss EI. et al. A controlled clinicopathologic study of myocardial fibrosis in systemic sclerosis (scleroderma). J Rheumatol 1990;17:656–62. [PubMed] [Google Scholar]
- 7. Steen VD, Medsger TA Jr.. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000;43:2437–44. [DOI] [PubMed] [Google Scholar]
- 8. Allanore Y, Meune C, Vonk MC. et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010;69:218–21. [DOI] [PubMed] [Google Scholar]
- 9. Rodriguez-Reyna TS, Morelos-Guzman M, Hernandez-Reyes P. et al. Assessment of myocardial fibrosis and microvascular damage in systemic sclerosis by magnetic resonance imaging and coronary angiotomography. Rheumatology (Oxford) 2015;54:647–54. [DOI] [PubMed] [Google Scholar]
- 10. Mueller KA, Mueller II, Eppler D. et al. Clinical and histopathological features of patients with systemic sclerosis undergoing endomyocardial biopsy. PLoS One 2015;10:e0126707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mavrogeni SI, Bratis K, Karabela G. et al. Cardiovascular Magnetic Resonance Imaging clarifies cardiac pathophysiology in early, asymptomatic diffuse systemic sclerosis. Inflamm Allergy Drug Targets 2015;14:29–36. [DOI] [PubMed] [Google Scholar]
- 12. Tzelepis GE, Kelekis NL, Plastiras SC. et al. Pattern and distribution of myocardial fibrosis in systemic sclerosis: a delayed enhanced magnetic resonance imaging study. Arthritis Rheum 2007;56:3827–36. [DOI] [PubMed] [Google Scholar]
- 13. Hachulla AL, Launay D, Gaxotte V. et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009;68:1878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barison A, Gargani L, De Marchi D. et al. Early myocardial and skeletal muscle interstitial remodelling in systemic sclerosis: insights from extracellular volume quantification using cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging 2015;16:74–80. [DOI] [PubMed] [Google Scholar]
- 15. Ntusi NA, Piechnik SK, Francis JM. et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis—a clinical study using myocardial T1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson 2014;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diao K-Y, Yang Z-G, Xu H-y. et al. Histologic validation of myocardial fibrosis measured by T1 mapping: a systematic review and meta-analysis. J Cardiovasc Magn Reson 2016;18:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schicchi N, Valeri G,, Moroncini G. et al. Myocardial perfusion defects in scleroderma detected by contrast-enhanced cardiovascular magnetic resonance. Radiol Med 2014;119:885–94. [DOI] [PubMed] [Google Scholar]
- 18. Gyllenhammar T, Kanski M, Engblom H. et al. Decreased global myocardial perfusion at adenosine stress as a potential new biomarker for microvascular disease in systemic sclerosis: a magnetic resonance study. BMC Cardiovasc Disord 2018;18:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bosello S, De Luca G, Berardi G. et al. Cardiac troponin T and NT-proBNP as diagnostic and prognostic biomarkers of primary cardiac involvement and disease severity in systemic sclerosis: A prospective study. Eur J Intern Med 2019;60:46–53. [DOI] [PubMed] [Google Scholar]
- 20. Avouac J, Meune C, Chenevier-Gobeaux C. et al. Cardiac biomarkers in systemic sclerosis: contribution of high-sensitivity cardiac troponin in addition to N-terminal pro-brain natriuretic peptide. Arthritis Care Res 2015;67:1022–30. [DOI] [PubMed] [Google Scholar]
- 21. Allanore Y, Wahbi K, Borderie D. et al. N-terminal pro-brain natriuretic peptide in systemic sclerosis: a new cornerstone of cardiovascular assessment? Ann Rheum Dis 2009;68:1885–9. [DOI] [PubMed] [Google Scholar]
- 22. Nordin A, Svenungsson E, Bjornadal L. et al. Troponin I and echocardiography in patients with systemic sclerosis and matched population controls. Scand J Rheumatol 2017;46:226–35. [DOI] [PubMed] [Google Scholar]
- 23. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. [DOI] [PubMed] [Google Scholar]
- 24. LeRoy EC, Black C, Fleischmajer R. et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15:202–5. [PubMed] [Google Scholar]
- 25. Erhayiem B, Pavitt S, Baxter P. et al. Coronary Artery Disease Evaluation in Rheumatoid Arthritis (CADERA): study protocol for a randomized controlled trial. Trials 2014;15:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bissell LA, Dumitru RB, Erhayiem B. et al. Incidental significant arrhythmia in scleroderma associates with cardiac magnetic resonance measure of fibrosis and hs-TnI and NT-proBNP. Rheumatology (Oxford) 2019;58:1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dabir D, Child N, Kalra A. et al. Reference values for healthy human myocardium using a T1 mapping methodology: results from the International T1 Multicenter cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2014;16:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McDiarmid AK, Swoboda PP, Erhayiem B. et al. Athletic cardiac adaptation in males is a consequence of elevated myocyte mass. Circ Cardiovasc Imaging 2016;9:e003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yi JE, Park J, Lee HJ. et al. Prognostic implications of late gadolinium enhancement at the right ventricular insertion point in patients with non-ischemic dilated cardiomyopathy: a multicenter retrospective cohort study. PLoS One 2018;13:e0208100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Plein S, Younger JF, Sparrow P. et al. Cardiovascular magnetic resonance of scar and ischemia burden early after acute ST elevation and non-ST elevation myocardial infarction. J Cardiovasc Magn Reson 2008;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alexandre J, Saloux E, Dugué AE. et al. Scar extent evaluated by late gadolinium enhancement CMR: a powerful predictor of long term appropriate ICD therapy in patients with coronary artery disease. J Cardiovasc Magn Reson 2013;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krumm P, Mueller KA, Klingel K. et al. Cardiovascular magnetic resonance patterns of biopsy proven cardiac involvement in systemic sclerosis. J Cardiovasc Magn Reson 2017;18:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hachulla AL, Launay D, Gaxotte V. et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009;68:1878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Friedrich MG, Sechtem U, Schulz-Menger J. et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 2009;53:1475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thuny F, Lovric D, Schnell F. et al. Quantification of myocardial extracellular volume fraction with cardiac MR imaging for early detection of left ventricle involvement in systemic sclerosis. Radiology 2014;271:373–80. [DOI] [PubMed] [Google Scholar]
- 36. Wong TC, Piehler K, Meier CG. et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation 2012;126:1206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martos R, Baugh J, Ledwidge M. et al. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation 2007;115:888–95. [DOI] [PubMed] [Google Scholar]
- 38. Weber KT, Brilla CG.. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin–angiotensin–aldosterone system. Circulation 1991;83:1849–65. [DOI] [PubMed] [Google Scholar]
- 39. Lee DC, Hinchcliff ME, Sarnari R. et al. Diffuse cardiac fibrosis quantification in early systemic sclerosis by magnetic resonance imaging and correlation with skin fibrosis. J Scleroderma Relat Disord 2018;3:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vignaux O, Allanore Y, Meune C. et al. Evaluation of the effect of nifedipine upon myocardial perfusion and contractility using cardiac magnetic resonance imaging and tissue Doppler echocardiography in systemic sclerosis. Ann Rheum Dis 2005;64:1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Allanore Y, Meune C, Vignaux O. et al. Bosentan increases myocardial perfusion and function in systemic sclerosis: a magnetic resonance imaging and Tissue-Doppler echography study. J Rheumatol 2006;33:2464–9. [PubMed] [Google Scholar]
- 42. Valentini G, Huscher D, Riccardi A. et al. Vasodilators and low-dose acetylsalicylic acid are associated with a lower incidence of distinct primary myocardial disease manifestations in systemic sclerosis: results of the DeSScipher inception cohort study. Ann Rheum Dis 2019;78:1576–82. [DOI] [PubMed] [Google Scholar]
- 43. Uren NG, Camici PG, Melin JA. et al. Effect of aging on myocardial perfusion reserve. J Nucl Med 1995;36:2032–6. [PubMed] [Google Scholar]
- 44. Dandekar VK, Bauml MA, Ertel AW. et al. Assessment of global myocardial perfusion reserve using cardiovascular magnetic resonance of coronary sinus flow at 3 Tesla. J Cardiovasc Magn Reson 2014;16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tanindi A, Cemri M.. Troponin elevation in conditions other than acute coronary syndromes. Vasc Health Risk Manag 2011;7:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neilan TG, Farhad H, Mayrhofer T. et al. Late gadolinium enhancement among survivors of sudden cardiac arrest. JACC Cardiovasc Imaging 2015;8:414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alexandre J, Saloux E, Dugue AE. et al. Scar extent evaluated by late gadolinium enhancement CMR: a powerful predictor of long term appropriate ICD therapy in patients with coronary artery disease. J Cardiovasc Magn Reson 2013;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferreira VM, Piechnik SK, Dall’Armellina E. et al. T(1) mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging 2013;6:1048–58. [DOI] [PubMed] [Google Scholar]
- 49. Nazir SA, Shetye A, Khan JN. et al. Comparison of T1-mapping and T2-weighted imaging for diagnostic oedema assessment in ST-segment elevation myocardial infarction. J Cardiovasc Magn R 2016;18(Supp.1):Q9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.