Abstract
Objectives
To estimate the incidence and strength of association of extra-articular manifestations [EAMs, here: anterior uveitis (AU), IBD and psoriasis] in patients with AS, undifferentiated SpA (uSpA) and PsA, compared with controls.
Methods
Three mutually exclusive cohorts of patients aged 18–69 years with AS (n = 8517), uSpA (n = 10 245) and PsA (n = 22 667) were identified in the Swedish National Patient Register 2001–2015. Age-, sex- and geography-matched controls were identified from the Swedish Population Register. Follow-up began 1 January 2006, or six months after the first SpA diagnosis, whichever occurred later, and ended at the first date of the EAM under study, death, emigration, 70 years of age, and 31 December 2016. Incidence rates (IRs) and incidence rate ratios were calculated for each EAM, and stratified by sex and age.
Results
Incidence rate ratios for incident AU, IBD and psoriasis were significantly increased in AS (20.2, 6.2, 2.5), uSpA (13.6, 5.7, 3.8) and PsA (2.5, 2.3, n.a) vs controls. Men with AS and uSpA had significantly higher IRs per 1000 person-years at risk for incident AU than women with AS (IR 15.8 vs 11.2) and uSpA (IR 10.1 vs 6.0), whereas no such sex difference was demonstrated in PsA or for the other EAMs.
Conclusions
AU, followed by IBD and psoriasis, is the EAM most strongly associated with AS and uSpA. Among the SpA subtypes, AS and uSpA display a largely similar pattern of EAMs, whereas PsA has a considerably weaker association with AU and IBD.
Keywords: Spondyloarthritis, extra-articular manifestations, anterior uveitis, inflammatory bowel disease, psoriasis
Rheumatology key messages
AS and undifferentiated SpA are strongly associated with future anterior uveitis and IBD.
A weaker but still significant association with anterior uveitis and IBD was observed in PsA.
Men with AS and undifferentiated SpA had higher incidence of anterior uveitis than women.
Introduction
SpA is a cluster of rheumatic diseases comprising AS, PsA, arthritis associated with IBD, reactive arthritis and undifferentiated SpA (uSpA). These diseases are related through overlapping genetic susceptibility, including MHC class I (mainly HLA-B27) and genes involved in the IL-23 pathway, and shared clinical appearance with possible involvement of the spine and sacroiliac joints, peripheral arthritis and, often, enthesitis [1, 2].
Extra-articular manifestations (EAMs) such as anterior uveitis (AU), IBD and psoriasis are common features and included in the majority of classification criteria for SpA; however, not in the modified New York criteria for AS [3–8]. The occurrence of these EAMs is often reported in studies of AS and the broader concept of SpA, but less frequently in patients with PsA. Meta-analyses and systematic literature reviews have been performed to address how these EAMs vary in prevalence between the SpA subtypes [9–11]. Additionally, some studies have explored the risk of incident EAMs in already established AS or PsA [12–18]. However, to our knowledge, no previous study has estimated the risk of future EAMs in the different SpA subtypes in the same setting.
We, therefore, aimed to assess the incidence and strength of association of AU, IBD and psoriasis in the three major clinical subtypes of SpA (AS, uSpA, PsA) [19], in a large and nationwide cohort study based on register data of patients from clinical practice, and to put these rates in relation to the occurrence in the general population.
Methods
Study setting and register sources
This observational, prospective and nationwide cohort study was based on data from a comprehensive linkage of Swedish health care and population registries. The National Patient Register (NPR) records diagnoses from inpatient care according to the International Classification of Diseases (ICD), and has nationwide coverage since 1987. From 2001, the register also includes diagnoses from all specialized outpatient care, but with some incomplete reporting from private caregivers. NPR was used to identify patients with SpA, and EAMs according to pre-specified ICD codes (Supplementary Table S1, available at Rheumatology online).
The Swedish Population Register encompasses demographic data for all Swedish residents and was used to identify matched controls and to ascertain death and emigration for censoring during follow-up.
The Prescribed Drug Register (PDR) was established in July 2005 and holds information on dispensed prescriptions according to the Anatomical Therapeutic Chemical Classification (ATC) system. PDR was used to obtain information on current treatment with DMARDs at start of follow-up. From the Swedish Rheumatology Quality Register, we specifically retrieved data on intravenously administered TNF-inhibitors (infliximab), which are not identified in PDR.
The study was approved by the Regional Ethics Committee, Stockholm, Sweden and has been carried out in compliance with the Helsinki Declaration. Informed consent was not required due to the register-based design.
Cohorts
Patients with AS, uSpA and PsA
Three mutually exclusive cohorts of patients aged 18–69 years with AS (n = 8517), uSpA (n = 10 245) and PsA (n = 22 667) were identified from NPR in 2001 through 2015. Eligible patients had at least one physician visit, within rheumatology or internal medicine outpatient care, reporting an ICD version 10 (ICD-10) code corresponding to an AS, PsA or uSpA diagnosis (Supplementary Table S1, available at Rheumatology online). ICD codes for uSpA comprise both axial and peripheral SpA [20]. To strengthen the case definition, cases with a physician visit reporting an ICD-10 code for RA or SLE during the same time period (2001–2015) were excluded. Further exclusion criteria were emigration or death before start of follow-up.
Patients with ≥2 subtypes of SpA
Patients diagnosed with more than one of the studied subtypes of SpA before start of follow-up (n = 1798) were analysed separately as a mixed SpA cohort. Patients who received a different SpA diagnosis during follow-up left their original cohort at that time-point and entered the mixed SpA cohort (n = 2682). In total, 4480 patients were included in the mixed SpA cohort: 3014 patients (67%) had a mix of AS and uSpA diagnoses, 998 patients (22%) a mix of uSpA and PsA, 399 patients (9%) a mix of AS and PsA and the remaining 69 patients (2%) a mix of all three subtypes.
Matched controls
For each SpA case, up to five controls were identified from the general population in the Swedish Population Register. The controls were matched for sex, birth-year and county of residence, and were required to be alive and not diagnosed with any of the studied SpA diagnoses at start of follow-up (which was set to the date of start of follow-up for their index patient with SpA).
Follow-up
Follow-up started 1 January 2006, or six months after the first SpA diagnosis in previously undiagnosed cases, whichever occurred later, in order to guarantee at least five years of pre-follow-up data from the specialized outpatient care register and six months data from PDR for each individual. The lag period of six months was used to avoid, as far as possible, inclusion of EAMs already present at the date of SpA diagnosis. Follow-up ended at first occurrence of each respective EAM, death, emigration, 70 years of age or end of study follow-up, 31 December 2016. Patients and their matched controls were censored at the time of a different SpA diagnosis during follow-up and the patients thereafter contributed with person-years at risk and number of EAMs to the mixed SpA cohort after a lag period of six months. Controls were censored if diagnosed with AS, PsA or uSpA during follow-up and consequently eligible to enter the respective SpA cohort.
Extra-articular manifestations
EAMs under study included AU, IBD with subcategories Crohn’s disease (CD) and ulcerative colitis (UC), and psoriasis. AU (flare and chronic) and IBD were identified in NPR according to pre-specified ICD codes. Psoriasis is often managed in primary health care in Sweden and is in those cases not captured by NPR. For that reason, dispensed prescriptions in PDR with an ATC code corresponding to a specific anti-psoriatic drug were also used to identify psoriasis. The pre-specified ICD and ATC codes are described in Supplementary Table S1, available at Rheumatology online.
History of EAM at start of follow-up
Patients with a history of EAMs were identified. For AU and IBD, we further demanded a diagnosis within ophthalmology and internal medicine/gastroenterology/surgery care, respectively. We also identified prior chronic AU (ICD-10 H20.1) within five years before start of follow-up, and for those without chronic AU, also the number of flares within the five-year period before start of follow-up. Flares were defined as a physician visit in ophthalmology care listing an AU diagnosis and with a gap of 90 days or longer from the last visit in ophthalmology care with an AU [21].
Incident EAM during follow-up
Patients (together with their matched controls) and controls with a history of AU, IBD and psoriasis at start of follow-up were excluded from the incident AU, IBD and psoriasis analysis, respectively. Each EAM during follow-up was analysed separately and defined as follows:
First occurrence of AU in specialized ophthalmology care.
First occurrence of IBD, with subcategories CD and UC, in specialized internal medicine, gastroenterology or surgical care.
First occurrence of either psoriasis in NPR or first dispensed prescription of a specific anti-psoriatic drug.
Flares of AU during follow-up
In a further analysis, we investigated flares of AU during follow-up. As previously described, flares were defined as a physician visit in ophthalmology care listing an AU diagnosis (except chronic AU) and with a gap of 90 days or longer from the last visit in ophthalmology care with an AU. Patients (together with their matched controls), and controls with chronic AU in ophthalmology care within five years before start of follow-up were excluded from the analysis. Patients and controls were also censored during follow-up if diagnosed with chronic AU.
Statistics
Descriptive statistics are presented as number (percentage) or mean (standard deviation (s.d.)). For each SpA cohort and their corresponding matched controls, the number of incident EAMs and person-years at risk during follow-up were counted. Incidence rates (IRs) with 95% CI assuming a Poisson distribution were calculated and presented as number of events per 1000 person-years at risk, overall and stratified by sex and age intervals. For flares of AU, robust standard errors without assumption of variance was used to calculate the 95% CI for IRs and incidence rate ratios (IRRs).
Cumulative incidence probability curves were plotted for each EAM, stratified by cohort and sex. For the mixed SpA cohort, the cumulative incidence probability curves for AU were further stratified by the presence of psoriasis at start of follow-up.
For the comparison between SpA cases and matched controls, IRRs with 95% CI were calculated as a measure of the strength of association. Statistical analyses were performed by SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
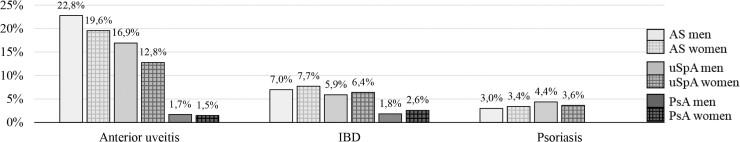
Characteristics of the AS (n = 8517), uSpA (n = 10 245) and PsA (n = 22 667) cohorts and their matched controls are summarized in Table 1 and Fig. 1. A history of AU was registered in 22%, 15% and 2% in the AS, uSpA and PsA cohort, respectively and the majority of diagnoses were made within ophthalmology care. Prior IBD occurred less frequently and was identified in 7%, 6% and 2% of patients with AS, uSpA and PsA. Prior psoriasis was detected in 3% of the patients with AS and in 4% of the patients with uSpA. In the mixed SpA cohort (n = 4480, 53% men, mean age 43(12) years), 20%, 7% and 34% had prior AU, IBD and psoriasis, respectively (Supplementary Table S2, available at Rheumatology online).
Table 1.
Demographics and disease characteristics at start of follow-up
| AS (n = 8517) |
AS controls (n = 39369) |
uSpA (n = 10245) |
uSpA controls (n = 46903) |
PsA (n = 22667) |
PsA controls (n = 99254) |
|
|---|---|---|---|---|---|---|
| Men | 5771 (67.8) | 26226 (66.6) | 4557 (44.5) | 20767 (44.3) | 10520 (46.4) | 45315 (45.7) |
| Age at start, mean (s.d.) | 47 (13) | 47 (13) | 42 (13) | 42 (13) | 50 (12) | 50 (12) |
| 18–29 years | 990 (11.6) | 4585 (11.6) | 2009 (19.6) | 8947 (19.1) | 1582 (7.0) | 7177 (7.2) |
| 30–39 years | 1649 (19.4) | 7444 (18.9) | 2690 (26.3) | 11879 (25.3) | 3346 (14.8) | 14503 (14.6) |
| 40–49 years | 2019 (23.7) | 9315 (23.7) | 2617 (25.5) | 12069 (25.7) | 5415 (23.9) | 23115 (23.3) |
| 50–59 years | 2161 (25.4) | 10001 (25.4) | 1915 (18.7) | 9111 (19.4) | 6734 (29.7) | 29230 (29.4) |
| 60–69 years | 1698 (19.9) | 8024 (20.4) | 1014 (9.9) | 4897 (10.4) | 5590 (24.7) | 25229 (25.4) |
| Medicationa | ||||||
|
DMARDs csDMARDs TNFi |
2404 (28.2) 1672 (19.6) 1098 (12.9) |
267 (0.7) 257 (0.7) 29 (0.1) |
3224 (31.5) 2701 (26.4) 887 (8.7) |
320 (0.7) 300 (0.6) 35 (0.1) |
10612 (46.8) 10084 (44.5) 1338 (5.9) |
812 (0.8) 771 (0.8) 82 (0.1) |
| NSAIDs | 5021 (59.0) | 3558 (9.0) | 5950 (58.1) | 3986 (8.5) | 11685 (51.6) | 10064 (10.1) |
| Glucocorticoids | 1032 (12.1) | 865 (2.2) | 1760 (17.2) | 1014 (2.2) | 4027 (17.8) | 2732 (2.8) |
| Prior extra-articular manifestations | ||||||
| Anterior uveitis (AU)b | 1852 (21.7) | 215 (0.5) | 1498 (14.6) | 237 (0.5) | 356 (1.6) | 630 (0.6) |
|
AU, ophthalmology care Chronic AU within 5 yearsb AU flare within 5 years One flare Two flares Three flares ≥ Four flares |
1840 (21.6) 151 (1.8) 1488 (17.5) 701 (8.2) 384 (4.5) 209 (2.5) 206 (2.4) |
210 (0.5) 13 (0.0) 131 (0.3) 94 (0.2) 21 (0.1) 10 (0.0) 6 (0.0) |
1491 (14.6) 154 (1.5) 1205 (11.8) 548 (5.3) 322 (3.1) 137 (1.3) 198 (1.9) |
236 (0.5) 19 (0.0) 148 (0.3) 99 (0.2) 28 (0.1) 8 (0.0) 13 (0.0) |
348 (1.5) 37 (0.2) 250 (1.1) 158 (0.7) 45 (0.2) 21 (0.1) 26 (0.1) |
615 (0.6) 50 (0.1) 411 (0.4) 285 (0.3) 63 (0.1) 29 (0.0) 34 (0.0) |
| IBDb | 615 (7.2) | 411 (1.0) | 632 (6.2) | 511 (1.1) | 504 (2.2) | 1166 (1.2) |
| IBD, internal medicine, gastroenterology or surgery care | 592 (7.0) | 391 (1.0) | 595 (5.8) | 496 (1.1) | 466 (2.1) | 1134 (1.1) |
| CD | 230 (2.7) | 102 (0.3) | 212 (2.1) | 162 (0.3) | 170 (0.7) | 358 (0.4) |
| UC | 273 (3.2) | 236 (0.6) | 277 (2.7) | 267 (0.6) | 234 (1.0) | 657 (0.7) |
| Overlap CD and UC | 89 (1.0) | 53 (0.1) | 106 (1.0) | 67 (0.1) | 62 (0.3) | 119 (0.1) |
| Psoriasisb | 264 (3.1) | 589 (1.5) | 405 (4.0) | 610 (1.3) | n.a. | 1740 (1.8) |
| Psoriasis according to NPR | 224 (2.6) | 417 (1.1) | 340 (3.3) | 452 (1.0) | 1218 (1.2) | |
| Psoriasis according to PDR | 100 (1.2) | 322 (0.8) | 152 (1.5) | 313 (0.7) | 1011 (1.0) | |
Data is presented as number (%) if not stated otherwise.
Medication is defined as ≥1 dispensed prescription in Prescribed Drug Register within 6 months before start of follow-up. Intravenous TNFi were identified through the Swedish Rheumatology Quality Register.
Exclusion criteria for the incident AU, AU flares, incident IBD and incident psoriasis analysis, respectively.
CD: Crohn’s disease; csDMARDs: conventional synthetic DMARDs; n.a.: not applicable; NPR: National Patient Register; PDR: Prescribed Drug Register; TNFi: TNF inhibitors; uSpA: undifferentiated SpA; UC: ulcerative colitis.
Fig. 1.
Proportion (%) with a prior anterior uveitis, IBD and psoriasis before start, stratified by sex
Incident AU
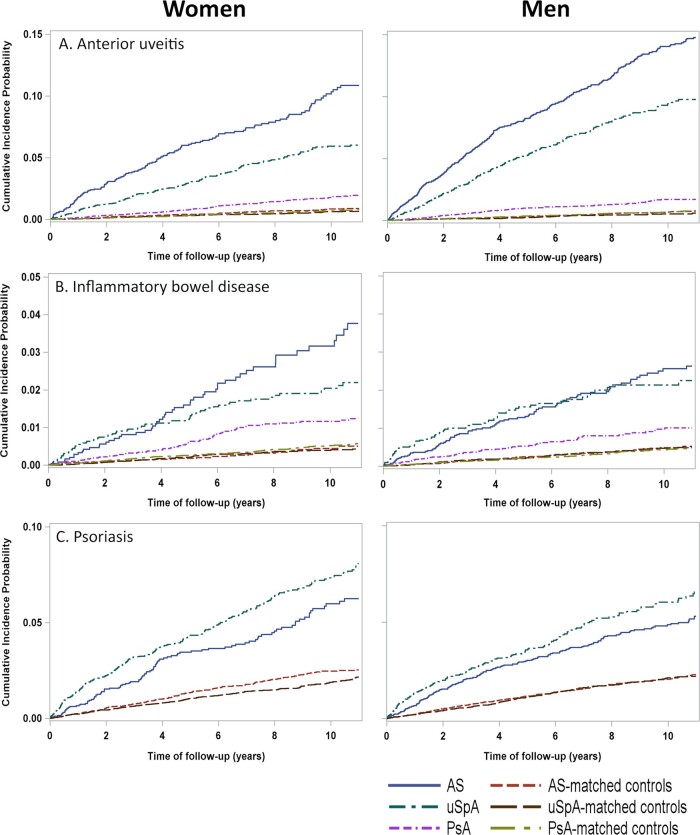
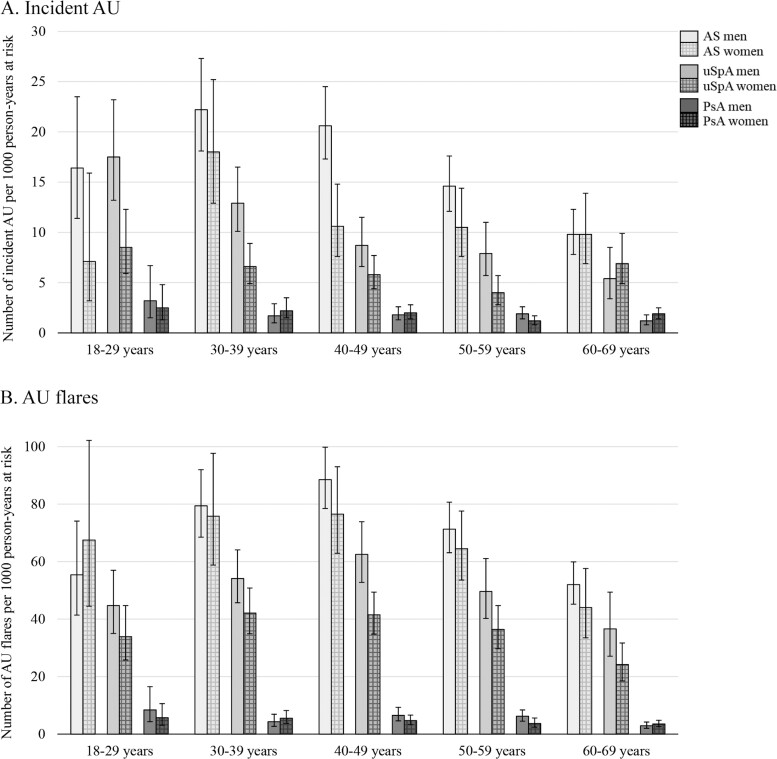
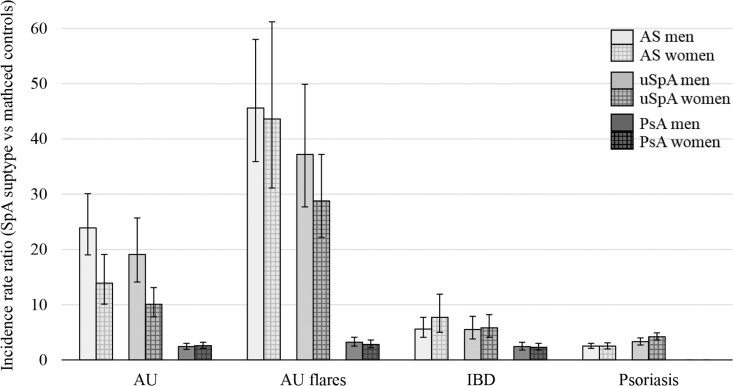
The cumulative incidence plot for first AU is shown in Fig. 2A. The IRs for AU were 14.4, 7.7 and 1.7 events per 1000 person-years at risk in the AS, uSpA and PsA cohort, respectively (Table 2). Women with AS and uSpA had significantly lower IRs than men with AS (IR 11.2 vs 15.8 per 1000 person-years at risk) and uSpA (IR 6.0 vs 10.1 per 1000 person-years at risk), whereas no significant difference between the sexes was found in PsA. The highest IR point estimates were noted in ages 30–39 years in AS and in ages 18–29 years in uSpA and PsA (Fig. 3A). In comparison with matched controls, the highest IRR for incident AU was demonstrated in AS (IRR 20.2), followed by uSpA (IRR 13.6), while the IRR in PsA was considerably lower (IRR 2.5), but yet significantly increased (Table 2, Fig. 4). IRRs were significantly increased in all age intervals with the lowest point estimate for all SpA subtypes in ages 60–69 years (Supplementary Table S3, available at Rheumatology online).
Fig. 2.
Cumulative incidence plots of anterior uveitis, IBD and psoriasis in the SpA cohorts and matched controls
Observe that the y-axis scale differs across the extra-articular manifestations.
Table 2.
Incidence rates and IRRs of the extra-articular manifestations in SpA subtypes vs matched controls
| AS |
Undifferentiated SpA |
PsA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Event | IR (95% CI) | IRR (95% CI) | Event | IR (95% CI) | IRR (95% CI) | Event | IR (95% CI) | IRR (95% CI) | |
| Incident AU | |||||||||
| All | 581 | 14.4 (13.2, 15.6) | 20.2 (16.8, 24.3) | 397 | 7.7 (7.0, 8.5) | 13.6 (11.2, 16.5) | 253 | 1.7 (1.5, 2.0) | 2.5 (2.1, 2.9) |
| Men | 435 | 15.8 (14.4, 17.4) | 23.9 (19.0, 30.1) | 217 | 10.1 (8.8, 11.6) | 19.1 (14.1, 25.7) | 114 | 1.7 (1.4, 2.0) | 2.4 (1.9, 3.0) |
| Women | 146 | 11.2 (9.6, 13.2) | 13.9 (10.1, 19.1) | 180 | 6.0 (5.2, 6.9) | 10.1 (7.8, 13.1) | 139 | 1.8 (1.5, 2.1) | 2.6 (2.1, 3.2) |
| AU flares | |||||||||
| All | 3714 | 68.5 (64.3, 72.9) | 45.1 (37.0, 54.8) | 2625 | 43.3 (40.2, 46.7) | 32.6 (26.8, 39.5) | 688 | 4.6 (4.0, 5.3) | 3.0 (2.5, 3.6) |
| Men | 2636 | 70.3 (65.4, 75.6) | 45.6 (35.9, 58.0) | 1352 | 51.6 (46.6, 57.3) | 37.2 (27.7, 49.9) | 355 | 5.1 (4.2, 6.3) | 3.2 (2.5, 4.1) |
| Women | 1078 | 64.3 (57.1, 72.4) | 43.6 (31.1, 61.2) | 1273 | 37.0 (33.2, 41.3) | 28.8 (22.2, 37.2) | 333 | 4.1 (3.4, 5.0) | 2.8 (2.2, 3.6) |
| IBD | |||||||||
| All | 143 | 2.8 (2.4, 3.3) | 6.2 (4.8, 8.0) | 142 | 2.5 (2.1, 2.9) | 5.7 (4.4, 7.3) | 162 | 1.1 (1.0, 1.3) | 2.3 (1.9, 2.8) |
| Men | 90 | 2.6 (2.1, 3.1) | 5.6 (4.1, 7.7) | 65 | 2.6 (2.0, 3.3) | 5.5 (3.8, 7.9) | 68 | 1.0 (0.8, 1.3) | 2.4 (1.8, 3.2) |
| Women | 53 | 3.4 (2.6, 4.4) | 7.7 (5.0, 11.9) | 77 | 2.4 (1.9, 3.0) | 5.8 (4.1, 8.2) | 94 | 1.2 (1.0, 1.5) | 2.3 (1.8, 3.0) |
| CD | |||||||||
| All | 73 | 1.4 (1.1, 1.8) | 9.8 (6.5, 14.8) | 70 | 1.2 (1.0, 1.5) | 7.0 (4.8, 10.2) | 72 | 0.5 (0.4, 0.6) | 2.9 (2.1, 3.9) |
| Men | 44 | 1.2 (0.9, 1.7) | 9.4 (5.6, 15.8) | 29 | 1.2 (0.8, 1.7) | 5.7 (3.3, 9.7) | 31 | 0.5 (0.3, 0.6) | 3.1 (1.9, 4.9) |
| Women | 29 | 1.9 (1.3, 2.7) | 10.7 (5.6, 20.7) | 41 | 1.3 (0.9, 1.7) | 8.4 (5.0, 14.2) | 41 | 0.5 (0.4, 0.7) | 2.7 (1.8, 4.0) |
| UC | |||||||||
| All | 70 | 1.4 (1.1, 1.7) | 4.5 (3.2, 6.3) | 72 | 1.2 (1.0, 1.6) | 4.8 (3.4, 6.7) | 92 | 0.6 (0.5, 0.8) | 2.1 (1.6, 2.6) |
| Men | 46 | 1.3 (1.0, 1.7) | 4.0 (2.7, 6.0) | 36 | 1.4 (1.0, 2.0) | 5.4 (3.3, 8.8) | 37 | 0.5 (0.4, 0.8) | 2.0 (1.3, 2.9) |
| Women | 24 | 1.5 (1.0, 2.3) | 5.8 (3.2, 10.5) | 36 | 1.1 (0.8, 1.5) | 4.3 (2.7, 6.8) | 55 | 0.7 (0.5, 0.9) | 2.1 (1.5, 2.9) |
| Psoriasis | |||||||||
| All | 302 | 5.6 (5.0, 6.3) | 2.5 (2.2, 2.9) | 458 | 7.7 (7.0, 8.5) | 3.8 (3.3, 4.3) | |||
| Men | 200 | 5.4 (4.7, 6.2) | 2.5 (2.1, 3.0) | 177 | 6.9 (6.0, 8.1) | 3.3 (2.7, 4.0) | |||
| Women | 102 | 6.1 (5.0, 7.5) | 2.5 (2.0, 3.1) | 281 | 8.3 (7.4, 9.4) | 4.2 (3.6, 4.9) | |||
IRs are presented as number of events per 1000 person-years at risk with 95% CI. IRRs with estimated 95% CI are the ratios between the IRs in the AS, uSpA and PsA cohorts and their respective matched controls.
AU: anterior uveitis; CD: Crohn’s disease; IR: incidence rate; IRR: incidence rate ratios; UC: ulcerative colitis.
Fig. 3.
Incidence rates for (A) incident AU and (B) AU flares, stratified sex and age interval
anterior uveitis
Fig. 4.
Incidence rate ratios (SpA subtype vs matched controls) with 95% CI for each extra-articular manifestation
Flares of AU during follow-up
During follow-up, 1494 (18%) patients with AS, 1094 (11%) patients with uSpA and 358 (2%) patients with PsA had at least one flare of AU. Of the patients with a flare during follow-up, 58%, 61% and 29% in the AS, uSpA and PsA cohort, respectively, had a prior AU flare within five years before start of follow-up. In contrast to incident AU, there was no significant difference in IRs between men and women with AS (Table 2). The highest IR point estimates were noted in ages 40–49 years in AS and uSpA, and in ages 18–29 years in PsA (Fig. 3B). The IRRs for AU flares were 45.1 in AS vs controls, 32.6 in uSpA vs controls, and 3.0 in PsA vs controls (Table 2, Fig. 4).
Incident IBD
The cumulative incidence plot for IBD is shown in Fig. 2B. The IRs were 2.8, 2.5 and 1.1 IBD per 1000 person-years at risk in the AS, uSpA and PsA cohort, respectively (Table 2). There were no significant differences across the sexes in any of the cohorts. The IRRs for incident IBD were similar in AS (IRR 6.2) and uSpA (IRR 5.7), while PsA had a lower association (IRR 2.3). In the sub-analyses of CD and UC, patients with AS had a significantly stronger association with CD than UC (IRR 9.8 vs IRR 4.5).
Incident psoriasis
The cumulative incidence plot for psoriasis is shown in Fig. 2C. The IRs were 5.6 and 7.7 psoriasis per 1000 person-years at risk in the AS and uSpA cohort, respectively (Table 2). Patients with uSpA had a slightly stronger association with psoriasis (IRR 3.8) than patients with AS (IRR 2.5).
Results for the mixed SpA cohort
The IRs and IRRs for the studied EAMs in the mixed SpA cohort vs matched controls are summarized in Supplementary Table S4, available at Rheumatology online. Overall, the IRRs were 18.6, 38.4, 5.0 and 4.4 for incident AU, flares of AU, incident IBD and psoriasis. Patients with psoriasis at baseline had significantly lower IRR for incident AU vs matched controls than patients without psoriasis at baseline (IRR 6.8 vs 27.4) (Supplementary Fig. S1, available at Rheumatology online).
Sensitivity analyses
In a sensitivity analysis, the patients with mixed SpA were not excluded from their original cohorts and baseline characteristics regarding prior EAMs were re-calculated. This resulted in a higher prevalence of psoriasis at baseline for AS [6.5%, difference between sensitivity and main analyses (Δ) 3.4%] and uSpA (9.4%, Δ5.4%), whereas the prevalence of AU in ophthalmology care (AS 22.0%, Δ0.4%; uSpA 15.6%, Δ1.0%) and IBD in internal medicine/gastroenterology/surgery care (AS 6.9%, Δ-0.1%; uSpA 6.1%, Δ0.3%) were similar. For PsA, the prevalence of AU (2.0%, Δ0.5%) and IBD (2.3%, Δ0.2%) were slightly changed.
Supplementary materials not described elsewhere
For all studied EAMs, the number of events, person-years at risk, IRs and IRRs (per age intervals) are presented in Supplementary Tables S3, S5 and S6, available at Rheumatology online.
Discussion
In this register-based cohort study investigating the incidence and strength of association of different EAMs in the SpA subtypes, we demonstrate a similarly increased association of AU, IBD and psoriasis in patients with AS and uSpA but a clear difference compared with PsA, where the associations of AU and IBD were much weaker, yet still significantly increased compared with the general population.
A few previous studies have reported the incidence of EAMs in established AS and PsA vs controls from the general population. However, none of the studies have analysed AS and PsA in the same setting, and for uSpA, data is lacking. Reassuringly, the relative risks found in the present study for incident AU, IBD and psoriasis in AS vs controls and for incident AU in PsA are in the same range as previously demonstrated for AS and PsA separately [12–14, 16]. Furthermore, a Danish register-based study observed an increased risk of incident CD and UC in PsA compared with the general population, also with risk levels in accordance with the present study [15]. Two other studies, though, described a significantly increased risk of CD but not of UC in patients with PsA [12, 17]. However, the results in these studies may have been hampered by a low number of incident IBD in the PsA cohort. Nevertheless, in the AS cohort (vs matched controls) we also observed a significantly stronger association with CD than with UC.
With regard to the prevalence of EAMs in SpA, prior meta-analyses of patients with AS and non-radiographic axial SpA (nr-axSpA) have reported prevalence of AU and IBD in accordance with our baseline results in AS and uSpA, respectively [9, 11]. The prevalence of psoriasis was higher in the meta-analyses than the detected prevalence of psoriasis in AS and uSpA in our study. This is probably in part explained by the exclusion of patients with mixed diagnoses, because the sensitivity analyses demonstrated increased prevalence of psoriasis in both AS and uSpA when the patients with mixed SpA were kept in their original cohorts. Also, the register-based design has most likely resulted in some underestimation of the prevalence of psoriasis. In contrast to our results, a systematic literature review of uveitis in SpA reported a history of uveitis in 25.1% of patients with PsA, whereas a register-based study reported (similarly with our results) prior uveitis in only 1.5% of the patients with PsA [10, 12]. However, the prevalence reported by the systematic literature review was based on 1341 patients in total, which may have been highly selected and not representative of the whole heterogeneous PsA spectrum. Our estimates in AS and uSpA are consistent with other studies that further support the reliability of our results for PsA.
The uSpA cohort comprises nr-axSpA as well as peripheral SpA and the associations of EAMs should preferably have been related to these clinical phenotypes, but cannot be discriminated by the ICD codes. In a Swedish validation study of uSpA, 44% fulfilled the ASAS criteria for axial SpA and, additionally, 29% the ASAS criteria for peripheral SpA [20]. Due to their similar clinical appearance, nr-axSpA and AS are often grouped together into one entity and it is well recognized that nr-axSpA can evolve into AS over time [22, 23]. In accordance with this, the greatest overlap of diagnoses in the present study was between AS and uSpA. Thus, the similarities of the associations of EAMs in AS and uSpA are not unexpected.
Despite the overall similar pattern of EAMs in AS and uSpA, patients with AS had a stronger association with AU than patients with uSpA (IRR 20.2 vs 13.6). In the present study, the AS cohort had a higher mean age and probably a longer symptom duration than the uSpA cohort. Symptom duration has been positively associated with AU and could at least partly account for the higher prevalence of prior AU in AS compared with uSpA [9, 24, 25]. A presumably higher proportion of HLA-B27 in AS than in uSpA could further explain the difference found [20, 26]. The typical HLA-B27-associated AU has an acute onset, is unilateral, often reoccurs and, in line with our results in AS and uSpA, has a male preponderance [27]. We also demonstrated a stronger association of AU flares (vs incident AU) in patients with AS and uSpA, which likely mirrors the higher tendency for reoccurrence in these two subtypes both in comparison to the general population and PsA. Interestingly, the difference between men and women with AS was less pronounced for AU flares than for incident AU. One possible hypothesis, which needs to be further explored, is that women with AS have a lower risk than men of incident AU but a more similar risk of reoccurrence.
Some limitations with the study need to be recognized. First, due to the register-based study design, we cannot exclude misclassifications. Previous studies have validated the ICD codes in NPR for the SpA subtypes, psoriasis and IBD. For patients with available imaging and/or HLA-B27, the positive predictive values (PPVs) for fulfilling the modified New York criteria and any set of SpA criteria were 80% and 97%, respectively, in AS and corresponding PPVs in uSpA were 26% and 89%, respectively [20]. Importantly, the ICD codes for uSpA cannot discriminate between axial and peripheral SpA. Another Swedish validation study, also including primary care, demonstrated PPVs within the range of 81% to 100% for psoriasis and 63% to 92% for PsA [28]. At least one registered diagnosis with IBD in NPR corresponded to a PPV of 88% for any IBD, whereas lower PPVs were noted for each of CD and UC [29]. Second, despite the separate analyses for patients with more than one SpA subtype, some remaining overlap between the diagnoses are unavoidable. Third, patients and EAMs managed in primary care are not captured by the NPR. Thus, patients with a probable milder SpA might be missed out and may limit the generalizability of the present results. Regarding the outcomes, psoriasis especially may be underestimated due to possible management exclusively in primary care. Moreover, the ATC codes used, in addition to ICD codes, to identify psoriasis were not available before July 2005 and do not capture non-specific treatment such as topical steroids. Fourth, outcomes managed in outpatient specialized care before 2001 are not identified, which might have underestimated (in all cohorts) the proportion with a prior outcome, particularly for non-chronic conditions such as AU flares. Consequently, some truly prevalent outcomes might have been identified as incident. Fifth, there is a risk of detection bias due to the well-known association of EAMs in SpA, which could underestimate the prevalence and incidence of in particular psoriasis in the matched controls. However, the prevalence of psoriasis in controls (1.3–1.8%) was only somewhat lower than the expected prevalence, around 2%, for the total population [30]. Last, we lack HLA-B27, which had been of interest especially in relation to AU, and clinical data of axial and peripheral involvement, which precludes exploration of the results in relation to clinical phenotype.
The major strength of the study is the large sample of patients with different SpA diagnoses derived from everyday clinical practice and analysed in the same setting. In addition, by using data from a comprehensive register-linkage, the loss of follow-up is minimized.
Conclusion
AU, followed by IBD and psoriasis, is the EAM most strongly associated with AS and uSpA. Among the SpA subtypes, AS and uSpA display a largely similar pattern of EAMs, whereas PsA is considerably less associated with AU and IBD. Men with AS and uSpA had higher incidence of AU than women with AS and uSpA, whereas no such sex difference was demonstrated in PsA or for the other EAMs.
K.B. contributed to the design of the study, data acquisition, analysis and interpretation of data and drafting the first version of the manuscript. H.F.-dE., A.D., E.K., M.D., S.E., U.L., J.A. and L.T.H.J. contributed to the design of the study and interpretation of data. All authors have revised the first version of the manuscript critically and participated in the editing until its final version. All authors agreed to be accountable for all aspects of the work and have read and approved the final manuscript. Preliminary results have been presented as an abstract with poster presentation at EULAR in Madrid 2019 and at the Swedish Rheumatology Meeting in Falun 2019.
Supplementary Material
Acknowledgements
We thank Jonas Söderling for helping us with the initial extraction of the register-linked data and statistician Tatiana Zverkova Sandström for preparation of the extracted data.
Funding: This study was funded by the Swedish state under the agreement between the Swedish government and the county councils (the ALF agreement) (ALFGBG-141111, ALFGBG-430851, ALFGBG-678731), Göteborg’s Medical Society, Swedish Rheumatism Association (R-750931, R-854131), the Swedish Research Council (2016–02035), the Swedish Heart-Lung Foundation, the Swedish Cancer Society (190336 Pj), the Nordic Research Council (75815) and Vinnova (2019–01188).
Disclosure statement: L.T.H.J. has received advisory board fees from Eli Lilly, Pfizer and Novartis. H.F.-dE. has received advisory board fees from Sandoz, Abbvie and Novartis and an unrestricted grant from Novartis. E.K. has received Advisory Board Fees from Novartis, lecturing fees from Lilly and an unrestricted grant from Roche. J.A. acts or has acted PI in agreements between Karolinska Institutet and the following entities, mainly for the nationwide safety monitoring of biologics in rheumatology: Abbvie, BMS, Eli Lilly, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi and UCB. The other authors have declared no conflicts of interest.
Data availability statement
The data sets generated and analysed during the present study are not publicly available due to the General Data Protection Regulation. For data access requests, please contact the corresponding author. All data relevant to the study are included in the article.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Dougados M, Baeten D.. Spondyloarthritis. Lancet 2011;377:2127–37. [DOI] [PubMed] [Google Scholar]
- 2. Reveille JD. Genetics of spondyloarthritis–beyond the MHC. Nat Rev Rheumatol 2012;8:296–304. [DOI] [PubMed] [Google Scholar]
- 3. Taylor W, Gladman D, Helliwell P. et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 4. Rudwaleit M, van der Heijde D, Landewe R. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 5. Rudwaleit M, van der Heijde D, Landewe R. et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25–31. [DOI] [PubMed] [Google Scholar]
- 6. Dougados M, van der Linden S, Juhlin R. et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991;34:1218–27. [DOI] [PubMed] [Google Scholar]
- 7. Amor B, Dougados M, Mijiyawa M.. [Criteria of the classification of spondylarthropathies]. Rev Rhum Mal Osteoartic 1990;57:85–9. [PubMed] [Google Scholar]
- 8. van der Linden S, Valkenburg HA, Cats A.. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 9. Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A.. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:65–73. [DOI] [PubMed] [Google Scholar]
- 10. Zeboulon N, Dougados M, Gossec L.. Prevalence and characteristics of uveitis in the spondyloarthropathies: a systematic literature review. Ann Rheum Dis 2008;67:955–9. [DOI] [PubMed] [Google Scholar]
- 11. de Winter JJ, van Mens LJ, van der Heijde D, Landewé R, Baeten DL.. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther 2016;18:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Charlton R, Green A, Shaddick G. et al. Risk of uveitis and inflammatory bowel disease in people with psoriatic arthritis: a population-based cohort study. Ann Rheum Dis 2018;77:277–80. [DOI] [PubMed] [Google Scholar]
- 13. Stolwijk C, Essers I, van Tubergen A. et al. The epidemiology of extra-articular manifestations in ankylosing spondylitis: a population-based matched cohort study. Ann Rheum Dis 2015;74:1373–8. [DOI] [PubMed] [Google Scholar]
- 14. Egeberg A, Khalid U, Gislason GH. et al. Association of psoriatic disease with uveitis: a Danish Nationwide Cohort Study. JAMA Dermatol 2015;151:1200–5. [DOI] [PubMed] [Google Scholar]
- 15. Egeberg A, Mallbris L, Warren RB. et al. Association between psoriasis and inflammatory bowel disease: a Danish nationwide cohort study. Br J Dermatol 2016;175:487–92. [DOI] [PubMed] [Google Scholar]
- 16. Chi CC, Tung TH, Wang J. et al. Risk of uveitis among people with psoriasis: a Nationwide Cohort Study. JAMA Ophthalmol 2017;135:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li WQ, Han JL, Chan AT, Qureshi AA.. Psoriasis, psoriatic arthritis and increased risk of incident Crohn’s disease in US women. Ann Rheum Dis 2013;72:1200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walsh JA, Song X, Kim G, Park Y.. Evaluation of the comorbidity burden in patients with ankylosing spondylitis using a large US administrative claims data set. Clin Rheumatol 2018;37:1869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haglund E, Bremander AB, Petersson IF. et al. Prevalence of spondyloarthritis and its subtypes in southern Sweden. Ann Rheum Dis 2011;70:943–8. [DOI] [PubMed] [Google Scholar]
- 20. Lindstrom U, Exarchou S, Sigurdardottir V. et al. Validity of ankylosing spondylitis and undifferentiated spondyloarthritis diagnoses in the Swedish National Patient Register. Scand J Rheumatol 2015;44:369–76. [DOI] [PubMed] [Google Scholar]
- 21. Lie E, Lindstrom U, Zverkova-Sandstrom T. et al. Tumour necrosis factor inhibitor treatment and occurrence of anterior uveitis in ankylosing spondylitis: results from the Swedish biologics register. Ann Rheum Dis 2017;76:1515–21. [DOI] [PubMed] [Google Scholar]
- 22. Protopopov M, Poddubnyy D.. Radiographic progression in non-radiographic axial spondyloarthritis. Expert Rev Clin Immunol 2018;14:525–33. [DOI] [PubMed] [Google Scholar]
- 23. Sieper J, Poddubnyy D.. Axial spondyloarthritis. Lancet 2017;390:73–84. [DOI] [PubMed] [Google Scholar]
- 24. Essers I, Ramiro S, Stolwijk C. et al. Characteristics associated with the presence and development of extra-articular manifestations in ankylosing spondylitis: 12-year results from OASIS. Rheumatology 2015;54:633–40. [DOI] [PubMed] [Google Scholar]
- 25. Rojas-Vargas M, Munoz-Gomariz E, Escudero A. et al. First signs and symptoms of spondyloarthritis–data from an inception cohort with a disease course of two years or less (REGISPONSER-Early. Rheumatology 2009;48:404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khan MA. Update on spondyloarthropathies. Ann Intern Med 2002;136:896–907. [DOI] [PubMed] [Google Scholar]
- 27. Chang JH, McCluskey PJ, Wakefield D.. Acute anterior uveitis and HLA-B27. Surv Ophthalmol 2005;50:364–88. [DOI] [PubMed] [Google Scholar]
- 28. Lofvendahl S, Theander E, Svensson A. et al. Validity of diagnostic codes and prevalence of physician-diagnosed psoriasis and psoriatic arthritis in southern Sweden–a population-based register study. PLoS One 2014;9:e98024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jakobsson GL, Sternegard E, Olen O. et al. Validating inflammatory bowel disease (IBD) in the Swedish National Patient Register and the Swedish Quality Register for IBD (SWIBREG). Scand J Gastroenterol 2017;52:216–21. [DOI] [PubMed] [Google Scholar]
- 30. Boehncke WH, Schon MP.. Psoriasis. Lancet 2015;386:983–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and analysed during the present study are not publicly available due to the General Data Protection Regulation. For data access requests, please contact the corresponding author. All data relevant to the study are included in the article.