Abstract
Objective
To investigate the association between neutrophil activation and cardiovascular risk in gout patients. We hypothesize that neutrophil activation mediates inflammation and therefore takes part in atherogenesis in gout patients.
Method
Patient data were collected from 75 consecutive gout patients participating in the Reade gout cohort Amsterdam. Levels of neutrophil extracellular traps (NETs) and neutrophil activation (calprotectin and peroxidase activity) were analysed by ELISA and fluorimetry in plasma and compared with healthy controls. Markers of neutrophil activation were related to clinical markers of cardiovascular risk, including BMI, smoking, blood pressure, lipid profile and 10 year risk of cardiovascular mortality (EU-SCORE).
Results
Increased levels of NETs were found in gout patients, although increased levels were not associated with cardiovascular risk. However, markers of neutrophil activation, including peroxidase activity correlated with waist:hip ratio (β = 0.33, P < 0.001), cholesterol ratio (β = 0.46, P < 0.005) and triglycerides (β = 0.60, P < 0.001) as well as the 10 year risk of cardiovascular mortality (β = 0.44, P = 0.001). Calprotectin levels were elevated in hypertension (P = 0.005) and diabetes (P = 0.02). Finally, gout patients with high levels of both peroxidase and calprotectin (‘neutrophil activation signature’) had a markedly elevated cardiovascular risk score (P = 0.001), with 68% of the patients having high cardiovascular risk (odds ratio 2.9, P = 0.03).
Conclusion
We demonstrated elevated levels of neutrophil activation markers, MPO and calprotectin in gout patients as compared with healthy controls. Of note, neutrophil activation markers were associated with several risk factors for cardiovascular disease, including hyperlipidaemia, hypertension and diabetes. Finally, the presence of a neutrophil activation signature was strongly associated with an increased 10 year risk of cardiovascular mortality. Further studies are needed to determine whether gout-specific factors and/or cardiovascular risk factors contribute to the elevated neutrophil activation observed in these patients.
Keywords: gout, neutrophils, cardiovascular disease, inflammation, atherosclerosis
Rheumatology key messages
Neutrophils play an important role in the pathogenesis of gout.
Patients with gout have elevated levels of NETs in peripheral blood.
Elevated levels of neutrophil activation markers correlate with the 10 year cardiovascular risk in patients with gout.
Introduction
Gout is the most prevalent inflammatory arthritis, characterized initially by acute episodes of joint inflammation alternating with symptom-free periods of variable duration [1]. If not treated adequately, gout evolves into a chronic disease with the presence of tophi and severe, disabling joint damage [2]. Similar to other inflammatory rheumatic diseases, gout also carries an increased risk for cardiovascular disease. This is partly due to an increased prevalence of ‘traditional’ cardiovascular risk factors, but gout itself is an independent risk factor for cardiovascular disease [3]. However, the underlying pathophysiological mechanisms have not yet been fully elucidated. Possible contributing factors such as oxidative stress and chronic inflammation have been suggested but not extensively investigated [4–6] .
The pathogenesis of gout is driven by hyperuricaemia (serum urate levels >360 µmol/l), leading to formation and deposition of MSU crystals in the joints. Upon phagocytosis by monocytes, MSU crystals activate the NLRP3 inflammasome, causing inflammation through production of IL-1β and subsequent neutrophil recruitment and infiltration [7]. The role of neutrophils in the pathogenesis of gout has emerged during the last decade with the intriguing observation of MSU crystal–mediated ‘frustrated phagocytosis’. Incapable of engulfing the large MSU crystals, neutrophils succumb to death through the process of NETosis, extruding chromatin opsonized with cytosolic and granular content in a web-like structure, defined as a neutrophil extracellular trap (NET). Although NETs are present in gout tophi, the role of NETs in systemic inflammation in gout remains unclear [8, 9].
Elevated levels of NETs in the circulation have been reported in several rheumatic diseases, including RA and SLE, and these NETs are thought to take part in disease pathogenesis through different mechanisms, including induction of inflammation and exposure of key autoantigens [10–12]. Furthermore, in murine models of lupus and APS, NETs clearly take part in the development of vascular damage, atherosclerosis and arterial thrombosis, through activation of platelets and endothelial cells [13, 14]. Also in humans, NETs have been detected in atherosclerotic lesions and arterial thrombi [15]. However, whether NETs are present in the peripheral blood of gout patients is unknown. In addition, whether neutrophil activation and NET formation contribute to the elevated risk for cardiovascular disease in patients with gout has not yet been addressed.
Objectives
The objective of this study was to investigate whether markers of neutrophil activation and cell death, i.e. NETs, are present in the peripheral blood of gout patients and are associated with cardiovascular risk. We hypothesize that neutrophil activation, assessed by levels of peroxidase and calprotectin, and NET formation mediate inflammation and therefore take part in atherogenesis in gout patients.
Methods
Patient characteristics
Plasma samples from 75 consecutive gout patients participating in the Reade gout cohort Amsterdam, The Netherlands were analysed and compared with 30 healthy controls. The Reade gout cohort includes patients with a clinical diagnosis of gout diagnosed by a rheumatologist. All patients fulfilled the 2015 gout classification criteria according to the ACR and EULAR collaborative initiative [16]. Clinical data collected included disease activity, demographics, gout history, comorbidities, medication use and cardiovascular risk factor assessments. Measurements included anthropometry, vital parameters and laboratory parameters. Cardiovascular risk was determined using the Dutch Systematic Coronary Risk evaluation (NL-SCORE) and European Heart SCORE (EU-SCORE) [17, 18]. This algorithm includes age, gender, smoking, systolic blood pressure and cholesterol ratio and calculates the 10 year risk of cardiovascular morbidity (NL-SCORE) and mortality (EU-SCORE).
The study was approved by the regional ethics board (METC Slotervaart & Reade Amsterdam) and informed written consent was obtained from all participants in accordance with the Helsinki Declaration.
Neutrophil activation assays
Levels of NETs were analysed using an MPO-DNA ELISA, as described previously [11]. In brief, a 96-well microtitre plate (Corning, Corning, NY, USA) was coated with anti-MPO antibody (4 µg/ml; Bio-Rad Laboratories, Hercules, CA, USA) overnight at 4°C, followed by blocking with 1% bovine serum albumin (BSA) in PBS for 2 h at room temperature. After blocking, plasma samples (10% in PBS-BSA) were added and incubated overnight at 4°C. For detection, dsDNA horseradish peroxidase (HRP) antibody (diluted 1/100; Roche Diagnostic, Rotkreuz, Switzerland) was added for 2 h at room temperature. The reaction was developed with 3,3′,5,5′-tetramethylbenzidine (TMB; BD Biosciences, Franklin Lakes, NJ, USA) and ended by the addition of 2 N sulphuric acid. Absorbance was measured at 450 nm by a plate reader (Synergy; BioTek Instruments, Winooski, VT, USA). NET degradation was assessed using a previously published protocol [19]. Briefly, neutrophils were isolated through density gradient (PolymorphPrep; Axis-Shield, Dundee, Scotland) and seeded at 1 × 106 cells/ml in a poly-l-lysine-coated black 96-well microtitre plate. Neutrophils were induced to undergo NETosis by addition of 20 nM phorbol myristate acetate for 4 h. Upon washing, attached NETs were stained with Sytox green (1:5000; Life Technologies, Waltham, MA, USA) followed by subsequent wash steps. After recording of baseline fluorescence, sera [5%, diluted in nuclease buffer (10 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 2 mM CaCl2, 50 mM NaCl)] were added and incubated for 90 min at 37°C. Micrococcal nuclease was used as a positive control. After the incubation, the wells were washed and residual NETs analysed by a plate reader. NET degradation was calculated as the relative loss of NETs (Sytox green signal) in each well, using the standard curve as a reference value. For DNA degradation, Sytox green–labelled DNA (5 μg/ml) was bound to poly-l-lysine-coated plates and the capacity to degrade the DNA was assessed in the presence of 10% sera in nuclease buffer.
Peroxidase activity was analysed as previously described. Briefly, plasma samples (10%) were incubated with TMB (BD Biosciences) at a final volume of 100 µL for 30 min at room temperature. The reaction was ended by the addition of 2 N sulphuric acid. The absorbance was analysed by a plate reader at 450 nm. Values are reported as mU/ml using HRP (Sigma-Aldrich, St. Louis, MO, USA) as a standard curve. Levels of calprotectin (S100A8/A9) were analysed in plasma using a commercial ELISA kit according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). All of the analysed markers were compared between gout patients and healthy individuals. In the gout population, markers of neutrophil activation and NETosis were related to clinical markers of cardiovascular risk, including BMI, smoking, blood pressure, lipid profile and 10 year risk of cardiovascular mortality (EU-SCORE).
Statistics
Patient characteristics were expressed as number (percentage) or mean (s.d.) when normally distributed or median [interquartile range (IQR)] when skewed. Distribution was explored using visualization techniques as histograms and QQ plots and when not conclusive, the Shapiro–Wilk test was used (P < 0.05). Linear regression analysis was performed in normally distributed continuous variables and skewed variables if log transformation was successful. For sample sets with a non-Gaussian distribution, Mann–Whitney U test was used. Logistic regression was used for dichotomous variables. Adjustments for possible confounding by age, gender, diabetes mellitus and gout disease load were performed afterwards. Gout disease load was defined as the number of gout attacks since diagnosis as reported by the patient. The 90th percentile of the healthy controls was used to define positive samples. All analyses were considered statistically significant at P < 0.05.
Results
Patient and control characteristics
Data from the first 75 patients included in the Reade gout cohort and 30 healthy controls were analysed. The gout patients were included between April 2015 and April 2019. Samples were obtained during their baseline visit. The healthy controls gave informed consent between January 2013 and December 2018 and samples were obtained directly afterwards. In more than half of the gout patients, diagnosis was confirmed by MSU crystal detection in synovial fluid. In 22 patients (29%) the disease was limited to podagra, while 53 patients (71%) had extended gout with involvement of more than one joint in the past. Subcutaneous tophaceous gout was seen in 29% of the patients and the median disease activity according the visual analogue scale for disease activity was 18.5 mm (IQR 1–48.5). The mean duration of gout flares was 9 days. Sixty-nine percent of the patients used colchicine during the last flare, 55% used NSAIDs and 43% used corticosteroids. Sixty-six percent of the patients received urate-lowering therapy, predominantly allopurinol (96%), otherwise benzbromarone (2%) or febuxostat (2%). The mean serum urate level at inclusion was 0.42 mmol/l. More patient characteristics from both groups are depicted in Table 1.
Table 1.
Patient characteristics of the gout cohort
| Characteristics | Gout cohort (N = 75) | Control group (N = 30) |
|---|---|---|
| Sex (male), n (%) | 68 (89.5) | 14 (46.7) |
| Age, mean (s.d.), years | 58 (11.7) | 49 (15.2) |
| Disease duration, median (IQR), years | 4 (2–10) | N.A. |
| Mono-articular gout, n (%) | 22 (29) | N.A. |
| Polyarticular gout, n (%) | 53 (71) | N.A. |
| Subcutaneous tophaceous gout, n (%) | 22 (29) | N.A. |
| VAS pain, median (IQR) | 11 (1–45) | N.A. |
| VAS disease activity, median (IQR) | 19 (1–48.5) | N.A. |
| Serum urate, mean (s.d.), mmol/l | 0.42 (0.09) | N.D. |
| Systolic blood pressure, mean (s.d.), mmHg | 142 (17.1) | 125 (16.6) |
| Smoking, yes, n (%) | 13 (17.1) | 5 (17) |
| BMI, mean (s.d.), kg/m2 | 30.4 (4.9) | 24.1 (3.2) |
| Total cholesterol, mean (s.d.), mmol/l | 5.1 (1.2) | 5.5 (1.1) |
| Triglycerides, mean (s.d.), mmol/l | 2.4 (1.8) | N.D. |
| LDL cholesterol, mean (s.d.), mmol/l | 3.3 (1.0) | 3.2 (0.9) |
| HDL cholesterol, mean (s.d.), mmol/l | 1.3 (0.5) | 1.9 (0.5) |
| Cholesterol ratio (total:HDL), mean (s.d.) | 4.4 (1.5) | 3.0 (1.0) |
N.A., not applicable; N.D., not determined; VAS, visual analogue scale.
Cardiovascular comorbidity
Sixty percent of the patients were diagnosed with hypertension; of these, 44% were treated with antihypertensive medication. Medical history revealed that 41% of the patients had hypercholesterolaemia and in 25% of all patients a statin was used. A cholesterol ratio >5.0 was found in 23 patients (31%), a low-density lipoprotein (LDL) level >2.5 mmol/l in 59 patients (79%) and a high-density lipoprotein (HDL) level <1.0 mmol/l in 15 patients (20%). Ten patients (13%) were treated for type 2 diabetes mellitus and eight patients (11%) had a history of cardiovascular disease (acute coronary syndrome, stroke or aneurysm/dissection). The 10 year risk of cardiovascular morbidity was 17.4%, whereas the mean 10 year mortality was 3%.
Neutrophil activation
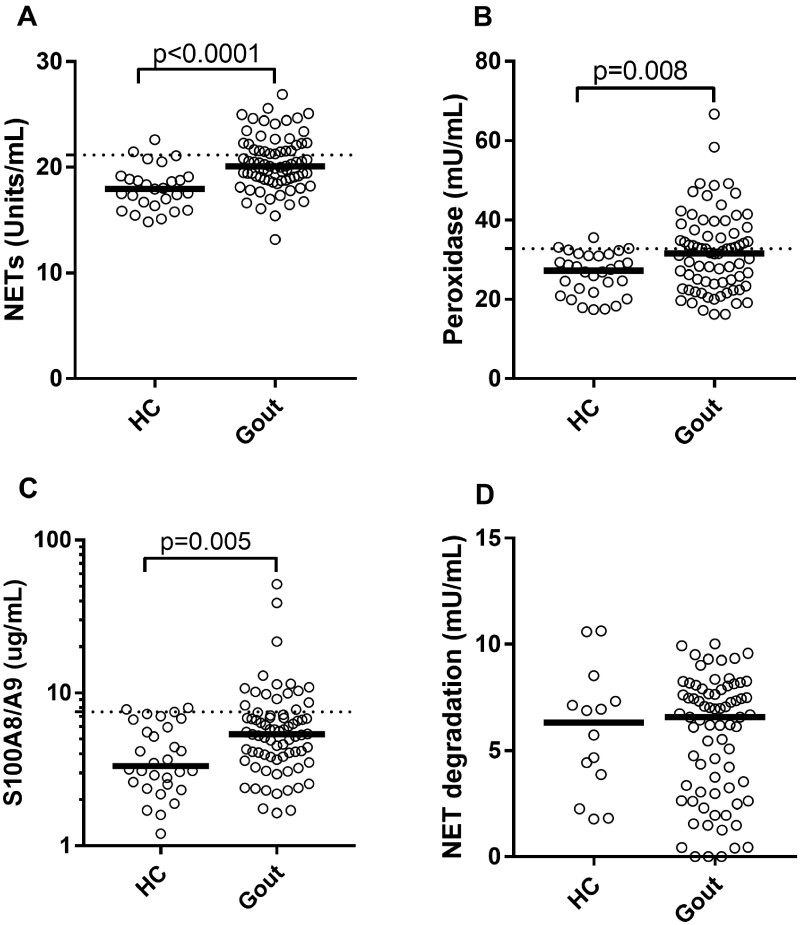
Levels of NETs, as well as other neutrophil biomarkers, were significantly increased in gout patients as compared with healthy subjects (P < 0.01; Fig. 1A–C). NET degrading capacity was not impaired in gout sera (Fig. 1D). NET formation was not influenced by age (β = 0.09, P = 0.40) or gender (P = 0.59). There was no association between NETs and BMI in the gout cohort (β = 0.02, P = 0.89) or healthy control group (β = 0.16, P = 0.42). Within the healthy control group, the amount of NETs was not associated with any of the cardiovascular risk factors [total cholesterol (β = 0.02), LDL cholesterol (β = 0.04), HDL cholesterol (β = 0.01) or systolic blood pressure (β = 0.22, P = 0.26)].
Fig. 1.
Neutrophil activation in gout
(A) Levels of circulating NETs were analysed in gout patients (n = 75) and healthy controls (HCs, n = 30). Levels of neutrophil activation marker (B) peroxidase activity and (C) calprotectin (S100A8/A9) were analysed using enzymatic assay and ELISA, respectively. (D) NET degradation was assessed in gout patients and HCs. Statistical analyses were done using Mann–Whitney U test with P < 0.05.
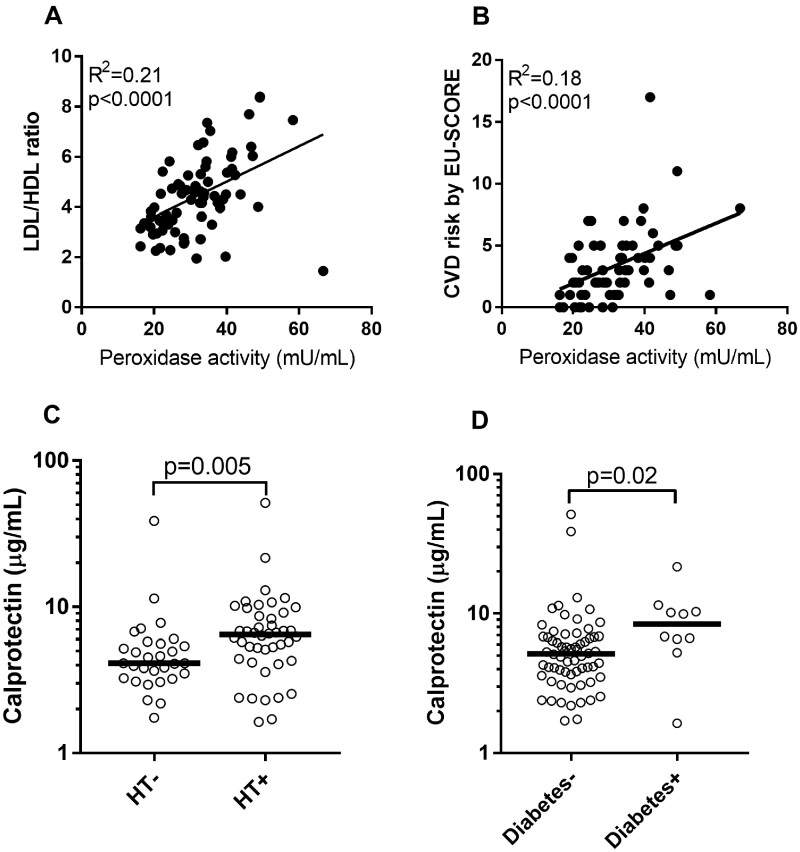
Both of the neutrophil activation markers (peroxidase and calprotectin) were associated with cardiovascular risk in the gout cohort. In the adjusted analysis, peroxidase activity correlated with waist:hip ratio (β = 0.33, P < 0.001), cholesterol ratio (β = 0.46, P = 0.005; Fig. 2A) and triglycerides (β = 0.60, P < 0.001). The associations were even stronger in patients with chronic polyarticular gout [cholesterol ratio (β = 0.66, P < 0.001), triglycerides (β = 0.67, P < 0.001)]. Peroxidase activity was also associated with the 10 year risk of cardiovascular mortality (β = 0.44, P = 0.001; Fig. 2B). Calprotectin levels were elevated in patients with hypertension (P = 0.005; Fig. 2C) and diabetes (P = 0.02; Fig. 2D), with calprotectin levels associating with diabetes independent of BMI [odds ratio (OR) 6.2, P = 0.04). Peroxidase and calprotectin were not associated with these risk factors in the healthy control group.
Fig. 2.
Neutrophil activation and cardiovascular disease
(A) Levels of peroxidase were correlated with cholesterol ratio in gout patients. (B) Peroxidase activity was correlated with the 10 year risk EU-SCORE in gout patients. Calprotectin levels were associated with (C) hypertension and (D) diabetes in gout patients. Statistical analyses were done using Mann–Whitney U test and linear regression analysis with P < 0.05.
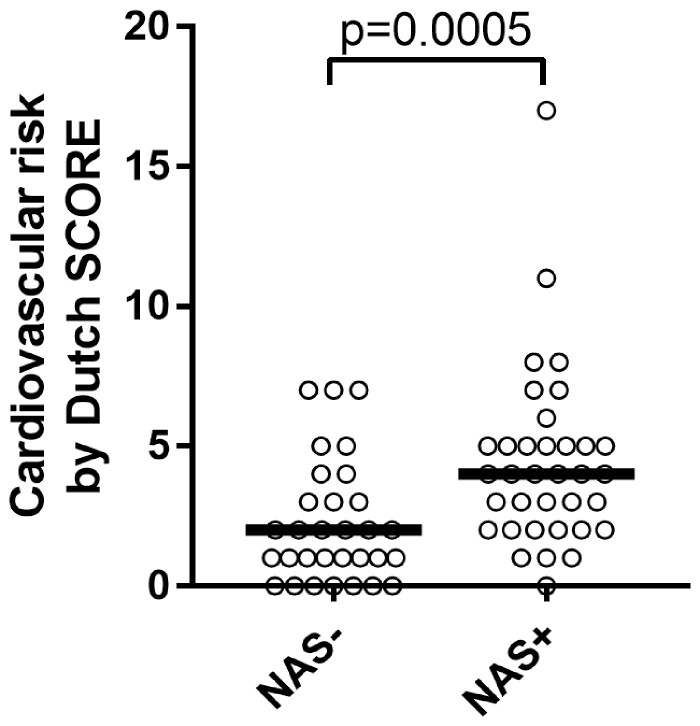
To look further into the association of neutrophil activation and cardiovascular risk in the gout cohort, we combined peroxidase activity and calprotectin into a ‘neutrophil activation signature’ that was positive for patients with elevated levels of peroxidase or calprotectin. Patients with a positive neutrophil activation signature had a markedly elevated EU-SCORE compared with patients without a positive neutrophil activation signature (P = 0.001) and 68% had a high cardiovascular risk according to the NL-SCORE compared with 32% of the patients without a neutrophil activation signature (OR 2.9, P = 0.03; Fig. 3).
Fig. 3.
Neutrophil activation signature and cardiovascular disease
Patients with a positive NAS more often had a high cardiovascular risk according the NL-SCORE compared with patients without a NAS. NAS: neutrophil activation signature.
Discussion
This is the first study investigating neutrophil activation and NETosis in gout patients in relation to cardiovascular risk. Consistent with prior work in RA and SLE, conditions with a substantial cardiovascular risk, we found elevated levels of NETs in gout patients compared with healthy controls. In contrast to RA and SLE, we did not find signs of impaired NET degradation in gout. The contrasting results are most likely due to the presence of anti-NET antibodies in RA and SLE, but not in gout, which act to prevent DNase-mediated NET degradation. As such, our results strengthen the concept that elevated levels of NETs in gout are not due to lack of degradation, but rather to enhanced induction of NETs.
The elevated levels of NETs found in our gout patients could be due to systemic inflammation with exaggerated neutrophil activation and subsequent release of NETs during their process of cell death, a phenomenon that has also been described in studies in SLE and ANCA vasculitis [11, 20]. An alternative explanation for the presence of elevated levels of NETs in the peripheral blood could be the presence of circulating MSU crystals in peripheral blood. Pieterse et al. [21] showed ex vivo that larger, needle-shaped MSU crystals engage neutrophils to undergo frustrated phagocytosis and subsequent cell death with the release of NETs in peripheral blood. This is in contrast to urate microaggregates in asymptomatic hyperuricaemia, which are readily cleared by phagocytes without formation of NETs. The fact that MSU crystals have also been detected in the vessel wall with dual-energy CT scan supports this hypothesis [22].
Although NETs have been proposed to contribute to cardiovascular disease in several disease models through interactions with platelets and endothelium, we did not find any correlations between NETs and cardiovascular risk in gout patients. The absence of this relation could be explained by suboptimal timing of our sampling (different levels of current gout disease activity) or localization of the NETs (circulation vs synovial fluid and/or tophi). Furthermore, the lack of association may also indicate that other mechanisms not related to NETosis contribute to cardiovascular disease in gout, for example, excessive activity of the enzyme xanthine oxidase with high production of reactive oxygen species resulting in high levels of oxidative stress.
Although this study was not developed to establish causal relations, our results suggest a role for systemic inflammation, through neutrophil activation, in the link between gout and cardiovascular disease. Even though neutrophils are thought to be the main producers of calprotectin, other cells, including monocytes and platelets, should also to be considered given their capacity to produce, albeit lesser amounts of, calprotectin [23, 24]. Of note, previous work from our group demonstrated that platelet-derived calprotectin is associated with cardiovascular disease in SLE. Further studies are needed to investigate the source of calprotectin in gout and the role of platelet activation in gout-associated cardiovascular disease.
Systemic inflammation has also been linked in other inflammatory disease to the initiation of cardiovascular disease by activation of endothelial cells followed by increased permeability of the vascular wall for immune cells and oxidized LDL, both contributing to the process of atherosclerosis. Moreover, systemic inflammation has been linked to activation of the coagulation system increasing the risk of both arterial and venous thrombosis [25]. Altogether, our findings demonstrate a clear association between neutrophil activation markers and cardiovascular risk in gout. This is consistent with prior work from our group in juvenile dermatomyositis and SLE, where neutrophil activation, including NET formation, is associated with dyslipidaemia, endothelial activation and thrombus development [26–28]. However, it remains unclear if neutrophil activation is caused by gout itself or other possible stimuli, for example, cholesterol crystals, as earlier studies have shown that crystallization of cholesterol in the circulation can trigger the release of IL-1β and activation of IL-1 receptors on the surface of neutrophils [29]. Unfortunately we were unable to compare levels of neutrophil activation and cardiovascular risk between groups because of a lack of cardiovascular risk factor data in the control group. In addition, individuals in the healthy control group were also significantly younger and had a lower mean BMI. It is possible that the prevalence of traditional risk factors in this group is lower and this could contribute to differences in the amount of NETs and neutrophil activation between groups. Therefore it is necessary to validate our data in other cohorts, preferably with a control group matched for age, gender and traditional cardiovascular risk factors. Furthermore, we did not assess the contribution of other (not cardiovascular) comorbidities and medication use, since, to date, the role of NETosis in many other diseases and the influence of medication is unknown.
In conclusion, we have demonstrated elevated levels of neutrophil activation markers, MPO and calprotectin in gout patients compared with healthy controls. In addition, for the first time we found elevated levels of NETs in the peripheral blood of gout patients. Neutrophil activation markers are associated with several risk factors for cardiovascular disease, including hyperlipidaemia, hypertension and diabetes. Furthermore, the presence of a neutrophil activation signature was strongly associated with an increased 10 year risk of cardiovascular mortality.
Acknowledgements
We are grateful to all the patients participating in the Reade gout cohort and all rheumatologists from Reade Amsterdam for the recruitment. We want to thank Grunenthal and Horizon Pharma for financial support of this cohort. This work has been previously presented at the 2019 EULAR congress in Madrid (abstract OP0300; https://ard.bmj.com/content/78/Suppl_2/231.2) and 2019 ACR congress in Atlanta. All authors fulfil the four International Committee of Medical Journal Editors criteria and contributed to this manuscript and approved the final version. This study was approved by the local ethics committee METC Slotervaart Amsterdam, The Netherlands.
Funding: The Reade gout cohort received funding from Grunenthal and Horizon Pharma.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
References
- 1. Kuo CF, Grainge MJ, Zhang W, Doherty M.. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol 2015;11:649–62. [DOI] [PubMed] [Google Scholar]
- 2. Dalbeth N, Merriman TR, Stamp LK.. Gout. Lancet 2016;388:2039–52. [DOI] [PubMed] [Google Scholar]
- 3. Lottmann K, Chen X, Schadlich PK.. Association between gout and all-cause as well as cardiovascular mortality: a systematic review. Curr Rheumatol Rep 2012;14:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dai Y, Cao Y, Zhang Z, Vallurupalli S, Mehta JL.. Xanthine oxidase induces foam cell formation through LOX-1 and NLRP3 activation. Cardiovasc Drugs Ther 2017;31:19–27. [DOI] [PubMed] [Google Scholar]
- 5. George J, Struthers A.. The role of urate and xanthine oxidase in vascular oxidative stress: future directions. Ther Clin Risk Manag 2009;5:799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zamudio-Cuevas Y, Martinez-Flores K, Fernandez-Torres J. et al. Monosodium urate crystals induce oxidative stress in human synoviocytes. Arthritis Res Ther 2016;18:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. So AK, Martinon F.. Inflammation in gout: mechanisms and therapeutic targets. Nat Rev Rheumatol 2017;13:639–47. [DOI] [PubMed] [Google Scholar]
- 8. Chatfield SM, Grebe K, Whitehead LW. et al. Monosodium urate crystals generate nuclease-resistant neutrophil extracellular traps via a distinct molecular pathway. J Immunol 2018;200:1802–16. [DOI] [PubMed] [Google Scholar]
- 9. Schett G, Schauer C, Hoffmann M, Herrmann M.. Why does the gout attack stop? A roadmap for the immune pathogenesis of gout. RMD Open 2015;1:e000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A. et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 2013;5:178ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lood C, Blanco LP, Purmalek MM. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med 2016;22:146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villanueva E, Yalavarthi S, Berthier CC. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 2011;187:538–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ali RA, Gandhi AA, Meng H. et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat Commun 2019;10: 1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meng H, Yalavarthi S, Kanthi Y. et al. In vivo role of neutrophil extracellular traps in antiphospholipid antibody-mediated venous thrombosis. Arthritis Rheumatol 2017;69:655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doring Y, Soehnlein O, Weber C.. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res 2017;120:736–43. [DOI] [PubMed] [Google Scholar]
- 16. Neogi T, Jansen TL, Dalbeth N. et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2015;74: 1789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Association of Preventive Cardiology. Heartscore 2012. https://heartscore.escardio.org (17 March 2020, date last accessed).
- 18.Caresharing. Scoremeter (NHG standard CVRM 2019). https://www.scoremeter.nl (17 March 2020, date last accessed).
- 19. Bach M, Moon J, Moore R. et al. A neutrophil activation biomarker panel in prognosis and monitoring of patients with rheumatoid arthritis. Arthritis Rheumatol 2020;72:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kraaij T, Kamerling SWA, van Dam LS. et al. Excessive neutrophil extracellular trap formation in ANCA-associated vasculitis is independent of ANCA. Kidney Int 2018;94:139–49. [DOI] [PubMed] [Google Scholar]
- 21. Pieterse E, Jeremic I, Czegley C. et al. Blood-borne phagocytes internalize urate microaggregates and prevent intravascular NETosis by urate crystals. Sci Rep 2016;6:38229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barazani SC, Pyzik R, Jacobi A. et al. Detection of uric acid crystals in the vasculature of patients with gout using dual-energy computed tomography. Arthritis Rheumatol 2018;70(Suppl 10):abstract 2964. [Google Scholar]
- 23. Larsen SB, Grove EL, Pareek M, Kristensen SD, Hvas AM.. Calprotectin and platelet aggregation in patients with stable coronary artery disease. PLoS One 2015;10:e0125992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lood C, Tyden H, Gullstrand B. et al. Platelet-derived S100A8/A9 and cardiovascular disease in systemic lupus erythematosus. Arthritis Rheumatol 2016;68:1970–80. [DOI] [PubMed] [Google Scholar]
- 25. Qi H, Yang S, Zhang L.. Neutrophil extracellular traps and endothelial dysfunction in atherosclerosis and thrombosis. Front Immunol 2017;8:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moore S, Juo HH, Nielsen CT. et al. Neutrophil extracellular traps identify patients at risk of increased disease activity and cardiovascular comorbidity in systemic lupus erythematosus. J Rheumatol 2019; doi: 10.3899/jrheum.190875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duvvuri B, Pachman LM, Morgan G. et al. Neutrophil extracellular traps in tissue and periphery in juvenile dermatomyositis. Arthritis Rheumatol 2020;72:348–58. [DOI] [PubMed] [Google Scholar]
- 28. Tyden H, Lood C, Gullstrand B. et al. Increased serum levels of S100A8/A9 and S100A12 are associated with cardiovascular disease in patients with inactive systemic lupus erythematosus. Rheumatology (Oxford) 2013;52:2048–55. [DOI] [PubMed] [Google Scholar]
- 29. Westerterp M, Fotakis P, Ouimet M. et al. Cholesterol efflux pathways suppress inflammasome activation, NETosis, and atherogenesis. Circulation 2018;138:898–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.