Abstract
Objectives
The aetiology of primary chronic pain syndromes (CPS) is highly disputed. We performed a systematic review and meta-analysis aiming to assess differences in circulating cytokine levels in patients with diffuse CPS (fibromyalgia) vs healthy controls (HC).
Methods
Human studies published in English from the PubMed, MEDLINE/Scopus and Cochrane databases were systematically searched from inception up to January 2020. We included full text cross-sectional or longitudinal studies with baseline cytokine measurements, reporting differences in circulating cytokine levels between fibromyalgia patients and HC. Random-effects meta-analysis models were used to report pooled effects and 95% CIs. This study is registered with PROSPERO (CRD42020193774).
Results
Our initial search yielded 324 papers and identified 29 studies (2458 participants) eligible for systematic review and 22 studies (1772 participants) suitable for meta-analysis. The systematic analysis revealed reproducible findings supporting different trends of cytokine levels when fibromyalgia patients were compared with HC, while the chemokine eotaxin, was consistently raised in fibromyalgia. Meta-analysis showed significantly increased TNF-α [standardized mean difference (SMD) = 0.36, 95% CI: 0.12, 0.60, P = 0.0034; I2 = 71%, Q2P = 0.0002], IL-6 (SMD = 0.15, 95% CI: 0.003, 0.29, P = 0.045; I2 = 39%, Q2P = 0.059), IL-8 (SMD = 0.26, 95% CI: 0.05, 0.47, P = 0.01; I2 = 61%, Q2P = 0.005) and IL-10 (SMD = 0.61, 95% CI: 0.34, 0.89, P < 0.001; I2 = 10%, Q2P = 0.34) in fibromyalgia patients compared with HC.
Conclusion
We found evidence of significant differences in the peripheral blood cytokine profiles of fibromyalgia patients compared with HC. However, the distinctive profile associated with fibromyalgia includes both pro-inflammatory (TNF-α, IL-6, IL-8) and anti-inflammatory (IL-10) cytokines in pooled analysis, as well as chemokine (eotaxin) signatures. Further research is required to elucidate the role of cytokines in fibromyalgia.
Keywords: fibromyalgia, cytokines, systematic review, meta-analysis, chronic pain syndromes, chemokine, eotaxin
Rheumatology key messages
A pro-inflammatory cytokine milieu may lead to nociceptive activation in fibromyalgia.
The fibromyalgia signature includes both pro-inflammatory (TNF-α, IL-6, IL-8) and anti-inflammatory (IL-10) cytokines and chemokines (eotaxin).
Better quality studies are needed to draw meaningful conclusions and for potential therapeutic applications.
Research in context
Evidence before this study
We searched PubMed and MEDLINE/Scopus and Cochrane databases from inception to 31 January 2020 for human studies of any design published in English, evaluating circulating (serum, plasma or whole blood) levels of cytokines in patients with chronic pain syndromes (CPS). As very few studies have been identified in patients with localized CPS, we decided to focus exclusively on patients with diffuse CPS (fibromyalgia) without underlying organic pathology.
Previous related systematic reviews and meta-analyses have been focused on specific CPS clinical phenotype, such as fibromyalgia (which also included mainly low-quality studies), or related conditions, such as chronic fatigue syndrome or complex regional pain syndrome. Despite a recent systematic review assessing levels of cytokines in non-specific back pain, a meta-analysis has not been conducted. In addition, the underlying pathology contributing to back pain in the context of increased inflammatory markers found in some patients has been insufficiently explored, limiting the interpretation of findings.
Our literature search did not retrieve any recent systematic review similar in design to this paper, in particular as we focused on a large number of cytokines in fibromyalgia in the absence of underlying organic pathology, and the majority of previous systematic reviews included chronic back pain without confidently excluding associated pathology (such as OA, slipped disc, etc.).
Added value of this study
We conducted a systematic review of 29 studies on fibromyalgia including 2458 participants across 14 countries and four continents; 22 comparative studies, comprising 1772 participants were included in the meta-analysis. Our findings are, to the best of our knowledge, the most comprehensive analysis of the most commonly measured cytokines in fibromyalgia without underlying organic pathology, and therefore provide the best available evidence regarding the unique cytokine profile associated with this condition.
Implications of all available evidence
In view of relatively poor level of evidence for a consistent cytokine signature associated with fibromyalgia, our research highlights the limited number of high-quality studies evaluating fibromyalgia patient groups, and the contradictory evidence provided by many of the studies assessing the same cytokine. Although the meta-analysis of eligible studies showed both increased pro-inflammatory (TNF-α, IL-6, IL-8) and anti-inflammatory (IL-10) cytokine signatures, supporting the potential role for neuro-inflammation in the pathogenesis of fibromyalgia, further research is required to explore the potential role of a systemic cytokine profile in predicting disease severity or response to treatment in fibromyalgia patients. Whilst it is generally accepted that peripheral immune cells traffic to local sites of nerve damage, they are also involved in the development of pathological pain through migration to other tissues relevant for pain processing mechanisms. Harnessing the increased endogenous anti-inflammatory signalling potential associated with chronic pain may offer additional opportunities for pain therapeutic strategies.
Introduction
Pain is an experienced symptom, representing a protective mechanism against a real or potential bodily harm. Pathological pain is defined as an amplified response to normally innocuous stimuli or to acute pain, which is maladaptive and has no biological significance [1, 2]. Pain becomes chronic when it persists for 3 months or longer. Idiopathic chronic pain syndromes (CPS) can be diffuse (e.g. fibromyalgia) or localized (e.g. tension headache, bladder pain syndrome). The prevalence of chronic pain overall ranged between one-third to one-half of the UK population, whilst a pooled analysis found that diffuse CPS affect 11.2–16.5% of the population [3, 4]. Localized CPS are also common—e.g. tension headache, has an estimated prevalence of 38.3%, with 43.3% of people affected reporting significant disability [5]. Despite the significant disease burden of CPS, the pathophysiology is poorly understood.
Idiopathic CPS have no universally accepted anatomical, structural or chemical aetiologies. Due to this, they have been shrouded in controversy, with many believing them to have a significant psychosomatic component. Both, peripheral and central sensitization, are involved in the pathogenesis of chronic pain. Peripheral sensitization is defined by augmented sensitivity to various stimuli after a peripheral nerve insult or lesion due to neurogenic inflammation affecting the low velocity unmyelinated C-fibres and low threshold myelinated Aδ-fibres that transmit pain signals. Increased input from peripheral nociceptors can trigger a prolonged but reversible increase in the excitability and synaptic efficacy of nociceptive pathway neurons [6].
In contrast with peripheral sensitization, central sensitization arises from the second-order neurons within the dorsal horn in the spinal cord and brain and is characterized by increased synaptic efficacy and reduction in inhibition, leading to a central amplification of the pain response [7].
Central sensitization is driven by neuroinflammatory processes that affect both the peripheral and central nervous system. Neuroinflammation is characterized by activation of inflammatory cells, such as macrophages (and microglia in the spinal cord), mast cells, platelets, endothelial cells, fibroblasts and other immune cells, and the release of pro-inflammatory molecules, such as cytokines, chemokines and other mediators. Interactions of these mediators with specific nociceptors or spinal cord neurons may lead to various biological processes driving changes in the excitability, conductivity and transmissibility of pain processing pathways, ultimately leading to pain amplification [8]. Central sensitization has been defined as ‘unhelpful neuroplasticity’ leading to development of persistent pain in the absence of tissue inflammation or injury. There is emerging evidence derived from sensory testing and neuro-imaging to support neuroplastic changes associated with CPS [9, 10]. One theory is that chronic pain is caused by, or exacerbated due to, background low-intensity inappropriate systemic inflammation [10].
Cytokines are small molecules released by cells that play an important role in cellular signalling, especially in the context of inflammation and immune responses. Cytokines can be divided into pro-inflammatory (e.g. IL-1, IL-6 and TNF-α) and anti-inflammatory (e.g. IL-4, IL-10 and TGF-β) types, which have opposing mechanisms. Two groups of cytokines, chemokines (cytokines involved in chemotactic functions) and ILs (cytokines involved in lymphocyte-to-lymphocyte signalling) play a central role as mediators of neuro-inflammation associated with chronic pain [11]. Many studies have therefore compared circulating cytokine profiles between patients with CPS and healthy controls (HC), aiming to investigate if CPS are associated with a systemic pro-inflammatory cytokine profile.
The purpose of this systematic review is to quantitatively analyse cross-sectional studies currently in the literature comparing cytokine profiles between CPS patients and HC, to test the hypothesis that CPS are associated with chronic systemic inflammation. As very few studies have been identified in patients with localized CPS, in order to address any potential bias from reporting a pooled data analysis, we decided to focus exclusively on studies in fibromyalgia as these were the most prevalent.
Methods
Search
A full literature search was performed in January 2020 using PubMed, MEDLINE/Scopus and Cochrane databases. The following MeSH terms were used for electronic searches:
(‘chronic pain’ OR ‘diffuse chronic pain’ OR ‘fibromyalgia’) AND (‘cytokine signature’ OR ‘cytokines’ OR ‘inflammatory cytokines’). Following screening of the abstracts of the papers identified using the MeSH terms above, we also repeated the search using the names of individual cytokines that have been reported by the papers identified above, such as TNF-α, TNF-β, IFN-γ, IL-1, IL-1 receptor antagonist (IL-1ra), IL-2, IL-4, IL-6, IL-8, IL-10, IL-17A, IL-18, monocyte chemoattractant protein-1 (MCP-1) and eotaxin, AND Human studies AND English language.
Any reviews identified were screened for relevance, and their references have been manually screened (snowballing) for suitable articles not identified by the computer searches.
Inclusion and exclusion criteria
We included original full text articles describing cross sectional studies comparing serum, plasma or full blood cytokine profiles between fibromyalgia patients and HC. We also included longitudinal studies with a baseline comparison of cytokine profiles between fibromyalgia patients and controls. We defined CPS using the accepted definition as ‘any condition causing pain most of the time for longer than 3 months without any obvious underlying physical or psychiatric cause’ [12]. We excluded articles that considered chronic pain in patients with underlying trauma, and inflammatory/rheumatological, cancer or other conditions without clear evidence that organic pathology has been excluded (e.g. chronic fatigue syndrome, complex regional pain syndrome/chronic back pain), abstracts, reviews, case-series and case-reports, and unpublished studies. We also excluded animal studies and articles not in English. After initial analysis and review of the data, we excluded all papers reporting on localized pain syndromes and only included patients with fibromyalgia to improve homogeneity. For the scope of this systematic review and meta-analysis we used summary estimates of circulating levels of various cytokines. The following cytokines were considered for evaluation in this report, based on the data extracted from papers selected by our automatic and manual search strategy: TNF-α, TNF-β, IFN-γ, IL-1, IL-1ra, IL-2, IL-4, IL-6, IL-8, IL-10, IL-17A, IL-18, MCP-1 and eotaxin.
Data extraction
Two reviewers (L.F.O. and A.S.) independently screened articles for inclusion in the systematic review. First articles were excluded by title, then by abstract and then finally by reading the full text. Reasons for exclusions were recorded. Any disagreements were resolved by a third adjudicator (C.C.), and a Cohen’s κ coefficient was calculated to assess inter-rater reliability. One reviewer (L.F.O.) then collected data for meta-analysis, which was independently verified by a second reviewer (A.S.). We used a predefined and standardized data extraction form to collect information from all the eligible studies. Data collected included: first author, year of publication, diagnostic criteria used for fibromyalgia, number of affected patients, gender ratio of patient group, number of HC, gender ratio of HC, method for measurement of circulating cytokines, modified Newcastle–Ottawa Scale (NOS) score, which cytokines were measured, and how each cytokine level differed between patients and HC. For meta-analysis, further data were collected: mean/median, standard deviation/standard error of the mean/CI/interquartile range/range and distribution of data (normal or non-parametric)—all for each circulating cytokine reported and for both HC and fibromyalgia patients.
Quality assessment
To assess the quality of studies, we used a modified version of the Newcastle–Ottawa Scale for cohort studies [13], adapted for cross sectional studies, shown in Supplementary Table S1, available at Rheumatology online. All included papers were given a score out of 10 for quality. Scores were given by one reviewer (L.F.O.) and independently verified by a second reviewer (A.S.). All articles were then categorized as low quality (0–4/10), medium quality (5–8/10) or high quality (9–10/10).
Data synthesis and analysis
Meta-analysis was performed using R (version 4.0.1) alongside the ‘dmetar’ and ‘meta’ packages. Standardized mean differences (SMD) were calculated by Hedge’s g from means and standard deviations. Interquartile ranges and medians were directly converted to means and standard deviations as described in the Cochrane Handbook [13], whereas medians and ranges were transformed to means and standard deviations as per Hozo et al. [14]. Studies only reporting P-values and means were transformed to Hedge’s g effect sizes and standard error as per Thalheimer and Cook [15]. Any study reporting non-parametric distribution of data [assumed if data were reported as median and interquartile range (IQR)] was labelled for later subgroup analysis.
Pooled SMD was calculated using a random-effects model, and significance was assessed by z-testing. Heterogeneity was assessed by both I2 and Q2 statistics. Sensitivity analysis was performed for all significant models, and the results were considered robust if SMD remained significant after: omitting any one study; omitting non-parametric or skewed data; and switching to a fixed-effects model. Subgroup analysis was performed using the mixed-effects model. Funnel plots and Egger’s test were used to exclude publication bias.
Results
Study selection
An algorithm detailing the number of studies included and excluded, with reasons for exclusions, is shown in Fig. 1.
Fig. 1.
Study selection algorithm
CSF: cerebrospinal fluid.
The primary literature search yielded 326 papers. After removal of duplicates (n = 0) and exclusion of unsuitable studies on the basis of their abstracts (n = 107) or through examining their full text (n = 166), and additional inclusion of two studies retrieved manually, 29 studies were identified as eligible for inclusion in the systematic review (Fig. 1 and PRISMA chart—see Supplementary Data S1, available at Rheumatology online), containing data on 1322 CPS patients and 1136 controls.
After screening titles and abstracts, 107 papers were excluded. An additional 25 papers were excluded because the samples analysed were not blood, serum or plasma (e.g. cerebrospinal fluid, skin biopsies), while 14 papers were excluded as the cytokines measured did not fall within the scope of this review; 94 papers were excluded as they included CPS patient groups with associated organic disease, three were letters to the editor, and one paper was written in Italian. Sixty-seven full-text articles were reviewed, of which 29 were excluded as they lacked HC, or presented solely longitudinal data. Nineteen reviews were also identified, from which two were deemed relevant for reference manual searches, which identified two additional eligible studies [16, 17]. Finally, 29 articles suitable for systematic review were identified [18–46]. Supplementary Table S1, available at Rheumatology online, summarizes all the studies included. Cohen's κ coefficient for inter-rater agreement was 0.955 (99% agreement).
Out of 29 studies, 22 presented data in the form of means and standard deviation/standard error/CI, or medians and IQR/range, and so were suitable for meta-analysis [18, 19, 23–29, 31–34, 36–42, 45, 46]. Those with medians and IQR were separated to determine whether skewed data would contribute significantly to heterogeneity through later subgroup analysis. Articles were also grouped based on CPS clinical phenotype (e.g. localized or widespread CPS).
Quality of studies
The majority of studies were of medium quality according to the modified NOS score. Only 8 out of 29 studies (28%) were scored as high quality, with the average score 9.4/10. The lowest rated articles had a rating of 5/10. The lowest scores have been attributed to the ‘selection of controls’ item, with 12 out of 29 (41%) studies not reporting where they recruited their HC.
Characteristics of studies
Sample size was highly variable, ranging from 16 to 255 subjects per study. Gender ratios for both control and patient groups were reported in 27 out of 29 (93%) studies. Female participants were predominant in almost all studies, with 15 out of 29 (52%) studies having only female subjects. The overall female: male ratio for studies that reported it was 1227:196 (86% females) and 1485:102 (94% females) for controls and CPS patient groups, respectively. Methods used to measure cytokines varied significantly between studies: 18 (62%) studies used ELISA, seven (24%) used cytometric bead arrays, two (7%) used multiplex immunoassay, one (3%) used a mixture of bead-based methods, and 1 study (3%) had unclear methodology. Kit brands and concentrations of reagents varied significantly even between studies of the same cytokine.
Systematic review and meta-analysis
We evaluated the levels of cytokines across 29 eligible studies as detailed above and identified that some of the most studied cytokines had opposing concentration trends in fibromyalgia patients compared with HC. Opposing findings reaching statistical significance that have been reproduced in at least two independent studies (moderate–high quality), irrespective of the method of analysis have been reported for the following cytokines.
TNF-α levels were significantly increased in fibromyalgia patients compared with HCs in 6 out of 17 studies analysing 722 participants (44%) [23, 26, 35, 39, 40, 44], significantly lower in 2 out of 17 studies including 248 subjects (15%) [22, 29], while 9 out of 17 studies showed no significant difference in circulating TNF-α between the fibromyalgia and HC (n = 678, 41%) [18, 19, 34, 36–38, 41, 42, 46].
IL-1 levels have been found to be lower in fibromyalgia patients compared with HC in 2 out of 10 studies analysing 380 participants (44%) [18, 23]. However, 8 out of 10 studies showed no significant difference in IL-1 levels between the two groups (n = 478, 56%) [27, 28, 30, 34, 37, 38, 40, 46].
IL-6 levels were significantly increased in fibromyalgia in 4/20 studies (n = 477, 27%) [23, 29, 32, 40], while 16 out of 20 studies failed to show any significant difference in fibromyalgia patients vs HC (n = 1316, 73%) [18, 26–28, 30, 31, 34–39, 42, 44–46].
IL-8 levels were significantly increased in patients with fibromyalgia in 9 out of 16 studies (n = 623, 46hx0025;) [18, 20, 21, 27, 28, 30, 32, 43, 44], while 7 out of 16 studies found no significant difference (n = 771, 55%) [23, 36–38, 42, 45, 46].
IL-10 levels were reported as raised in fibromyalgia in 3 out of 9 articles (n = 339, 33%) [18, 36, 46], while 6 out of 9 studies failed to show any significant difference (n = 588, 67%) [23, 35, 37, 38, 42, 44].
For the other cytokines, IL1ra levels were reproducibly increased in fibromyalgia patients compared with controls in 1 out of 4 studies [42], whereas the other 3 out of 4 papers showed no difference [23, 31, 46]. IFN-γ levels were similar in fibromyalgia vs HC in 3 out of 4 studies [23, 42, 46], and IL-2 levels were found to be either high (2 out of 4 studies) [23, 35] or similar (2 out of 4 studies) [42, 46]. IL-4, which is an anti-inflammatory cytokine, was found at similar concentration in both groups in 2 out of 3 studies which measured it [44, 46]. Similarly, there was no difference in IL-17A levels in 2 out of 3 studies [23, 46].
For the chemokines, MCP-1 levels were higher (1 out of 4 studies) [46] or similar (2 out of 4 studies) [23, 33], while eotaxin levels were reproducibly higher in fibromyalgia patients compared with HC in 4 out of 5 studies [22, 23, 25, 46].
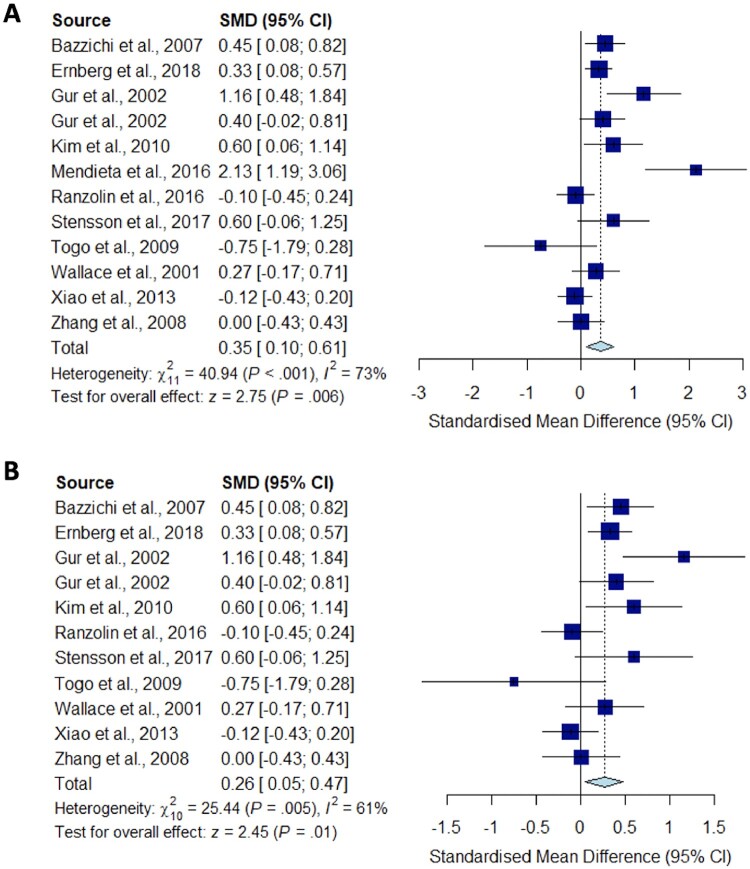
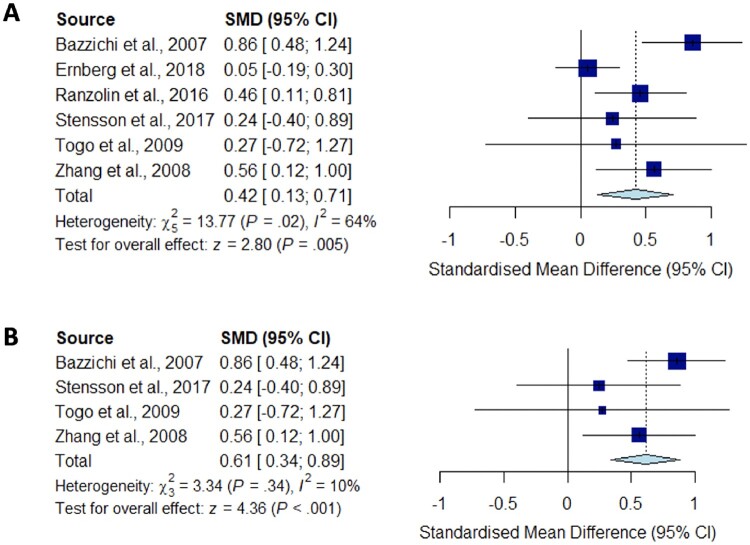
A total of 22 studies reporting on the circulating levels of cytokines of interest were eligible for meta-analysis. We performed meta-analysis and sensitivity analysis to assess the peripheral blood cytokine levels in fibromyalgia compared with HC for TNF-α (Fig. 2), IL-1 (Fig. 3), IL-6 (Fig. 4), IL-8 (Fig. 5) and IL-10 (Fig. 6).
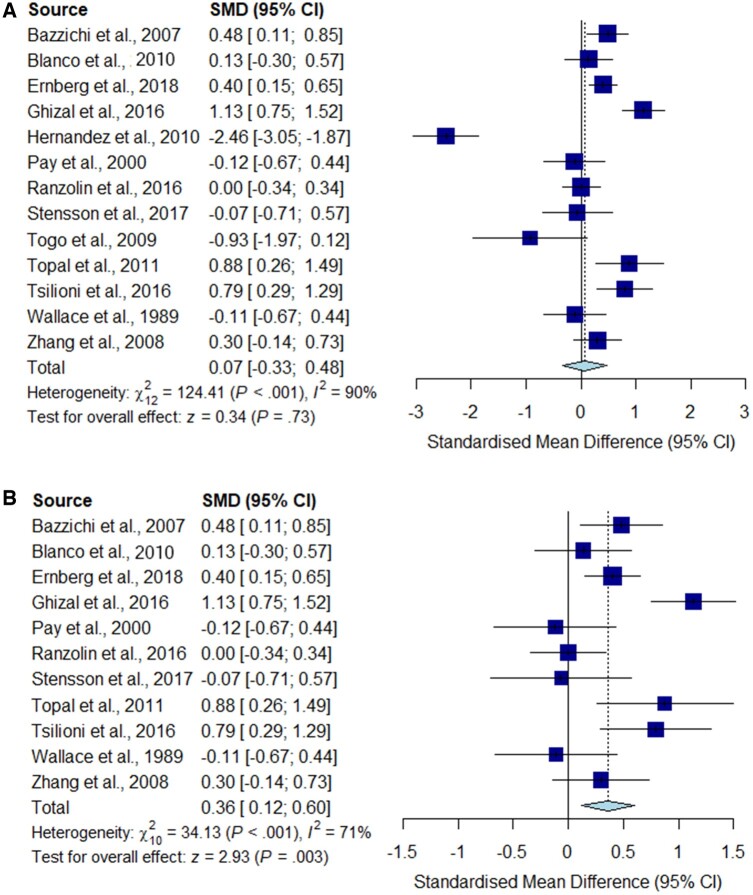
Fig. 2.
Meta-analysis for TNF-α
Forest plots showing the pooled standardized mean difference of circulating TNF-α concentrations between fibromyalgia patients and controls. (A) All studies included;
(B) outliers [Hernandez et al. (2010) and Togo et al. (2009)] excluded.
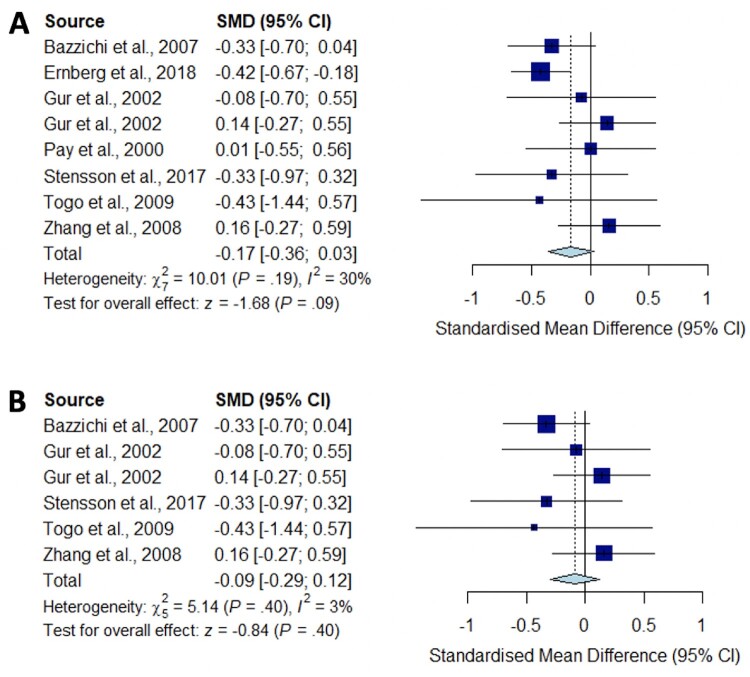
Fig. 3.
Meta-analysis for IL-1
Forest plots showing the pooled standardized mean difference of circulating IL1 concentrations between fibromyalgia patients and controls. (A) All studies included;
(B) studies reporting skewed (non-parametric) data excluded.
Fig. 4.
Meta-analysis for IL-6
Forest plots showing the pooled standardized mean difference of circulating IL-6 concentrations between fibromyalgia patients and controls. (A) All studies included;
(B) outliers [Hernandez et al. (2010)] excluded.
Fig. 5.
Meta-analysis for IL-8
Forest plots showing the pooled standardized mean difference of circulating IL-8 concentrations between fibromyalgia patients and controls. (A) All studies included;
(B) outliers [Mendieta et al. (2016)] excluded.
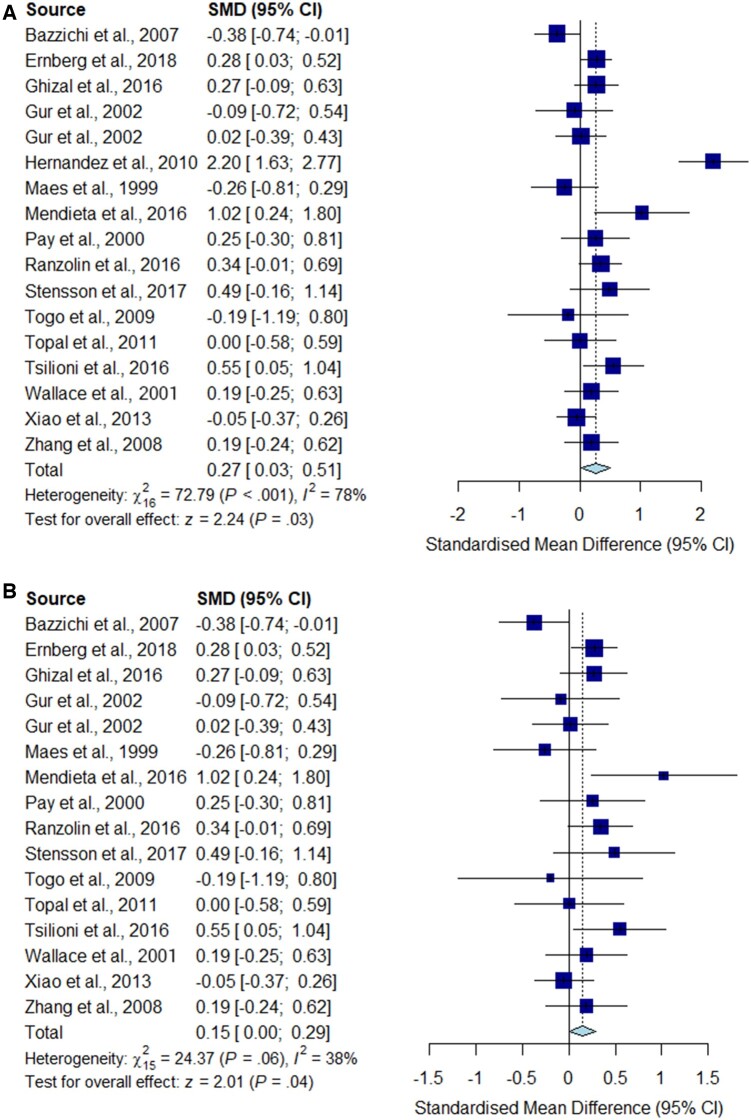
Fig. 6.
Meta-analysis for IL-10
Forest plots showing the pooled standardized mean difference of circulating IL-10 concentrations between fibromyalgia patients and controls. (A) All studies included;
(B) studies reporting skewed (non-parametric) data excluded.
Significantly higher peripheral levels of cytokine concentrations were detected in fibromyalgia patients compared with HC subjects in comparisons of TNF-α, IL-8 and IL-10 (Figs 2B, 5 and 6), which were all robust on sensitivity analysis, while no significant differences were found for IL-1 (Fig. 3). IL-6 concentrations were significantly higher in fibromyalgia patients compared with HC, but not robust on sensitivity analysis (Fig. 4). Results are summarized in Supplementary Table S2, available at Rheumatology online. Results were not significantly different between skewed and normally distributed data (Supplementary Table S3, available at Rheumatology online).
Funnel plot analysis was used for assessment of publication bias. Funnel plots looked symmetrically distributed, excluding some clear outliers (Supplementary Fig. S1, available at Rheumatology online). Egger’s test was performed for all cytokines included in the meta-analysis [TNF-α (P = 0.20), IL-1 (P = 0.37), IL-6 (P = 0.42), IL-8 (P = 0.18) and IL-10 (P = 0.51)] and they were all non-significant, suggesting that overall, publication bias is unlikely.
Discussion
The findings of this systematic review and meta-analysis of circulating cytokine levels in patients with fibromyalgia provides the best available evidence for the existence of a cytokine profile in the peripheral blood associated with diffuse chronic pain in the absence of underlying organic pathology. Our findings suggest that there is an association between fibromyalgia and both a pro-inflammatory cytokine profile (comprising increased TNF-α, IL-6 and IL-8 levels) and an anti-inflammatory signature (characterized by increased IL-10 levels). Both increased and decreased levels of various pro- and anti-inflammatory cytokines and chemokines have been reported in individual studies, and therefore these meta-analysis results strengthen the clinical evidence that fibromyalgia is accompanied by a distinct cytokine profile.
It is well recognized that peripheral immune activation contributes to pathological pain through initial activation of the pattern recognition receptors expressed on immune cells following peripheral nerve injury or inflammation. In addition, peripheral nociceptors involved in pathological pain have been shown to express cytokine and chemokine receptors [47]; this provides supporting evidence for direct immune activation of neuronal activity leading to enhanced excitability to subsequent stimulation, a phenomenon described as peripheral sensitization [48, 49]. Similarly to peripheral neurons, dorsal horn neurons express receptors for many pro-inflammatory cytokines, such as IL-1, TNF-α, IL-6 and IL-17 [50], which when activated can impact the neuronal function (e.g. pro-inflammatory cytokines such as TNF-α and IL-1β enhance excitatory synaptic transmission and suppress inhibitory synaptic transmission in neurons of spinal cord). In contrast, although there is limited evidence for IL-10 receptor expression within the neural tissues [47], it has been postulated that the anti-inflammatory properties of IL-10 could be exploited as a potential therapeutic target for pain modulation [51].
We found evidence that high study heterogeneity influenced significantly our findings related to the TNF-α signature associated with fibromyalgia. Our post hoc analysis showed significantly increased circulating TNF-α in fibromyalgia patients, only after the exclusion of two outlier studies, which was robust on sensitivity analysis. In support of our findings, a recent systematic review of biomarkers associated with chronic non-specific back pain also identified two studies that reported increased levels of circulating TNF-α [52].
Pro-inflammatory cytokines such as IL-1 and IL-6 have also been implicated in pain modulation through specific mechanisms: IL-1β enhances excitatory induced currents within the spinal cord, and IL-1β and IL-6 suppress inhibitory GABA- and glycine-induced currents, leading to a feed-forward cycle of nociceptive signalling leading to central sensitization [53]. Our meta-analysis did not show any significant difference between the concentration of IL-1 in fibromyalgia patients compared with controls, despite a low level of study heterogeneity and opposing trends described in some individual studies. However, we did find significantly increased circulating concentrations of IL-6 in fibromyalgia patients compared with HC. This was not robust on sensitivity analysis, but is consistent with previous systematic reviews [17].
IL‐8 is a member of the CXC chemokine subfamily, which is known to be synthesized by microglial cells and astrocytes with a role in mediating the intercellular communication between glia and neurons by rapidly altering the excitability of neurons, and thereby contributing to pain modulation [54]. Blocking IL-8 receptor signalling in a mouse model of disc degeneration and pain reduced behavioural signs of back pain, suggesting that IL-8 could also be considered as a therapeutic target for chronic pain [55]. Our systematic review and meta-analysis showed increased levels of IL-8 in fibromyalgia patients compared with HC, and the significance was robust on sensitivity analysis. Our findings are similar to the results of a systematic review of 25 studies in fibromyalgia [17], although their meta-analysis did not show increased IL-8 overall.
Our meta-analysis is the first to show increased IL-10 levels in CPS. IL-10 has anti-inflammatory properties that have been shown to reduce nociceptor sensitization [56]. IL-10 levels were decreased in patients with fibromylagia [57]. Despite contradictory findings, our pooled result was robust on sensitivity analysis and displayed no significant study heterogeneity, but was limited by a small pooled sample size.
Eotaxin, a chemokine originally implicated in the selective recruitment of eosinophils into inflammatory sites during allergic reactions, has been shown to be implicated in ageing, neurogenesis and neurodegeneration, and more recently in chronic pain, through increased cellular signalling that may be responsible for changes in neuronal excitability [46]. Our systematic analysis showed reproducible evidence for increased levels in fibromyalgia patients compared with HC, but the lack of suitable data precluded a meta-analysis.
The primary limitation of our analysis is that all cytokine studies were non-randomized. Therefore, we can only show association of various cytokine levels with fibromyalgia, without implying any temporal association or causal relationship. In addition, we found significant study heterogeneity most likely due to highly variable methodology for measuring cytokines and dissimilar sample populations. Many studies did not provide enough data to enable inclusion in meta-analysis, which may introduce bias. Reporting bias was also relevant, as many papers mentioned more cytokines in their methods than they reported in their results, with the explanation that some concentrations were below the manufacturers’ limit of detection. Also, many studies included in our analysis measured a wide range of different cytokines, without appropriate multiple testing correction. It is possible that a few significant results occurred just by accumulated probability of type I error with multiple measurements. We could not analyse differences between circulating cytokines and local cytokine concentrations, although these have been reported [16, 58–60], because their sampling sites were too heterogeneous for meaningful analysis. Finally, most studies could not control for medications taken by patients, which could be a significant confounder, especially in studies comparing them with HC, who were unlikely to be on any medications.
In addition, other molecules implicated in the pathogenesis of pain, which are identified by some authors as cytokines and by other as neurotrophins, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), have not been highlighted by our search strategy and therefore not discussed in any detail in this paper. The majority of the reports identified subsequently have been excluded from this systematic review and meta-analysis based on ineligibility (pre-clinical studies or human studies evaluating cerebrospinal fluid or platelet expression of these neutrophins, or including patients with chronic pain associated with organic pathology, such as cancer, pancreatitis, diabetes and interstitial cystitis).
We would like to highlight two studies that could have been eligible for inclusion if identified by our search strategy, which measured the NGF and BDNF plasma levels in patients with fibromyalgia and primary headaches compared with healthy controls (without assessing any other cytokines) and found no differences [61, 62].
Overall, fibromyalgia seems to be associated with a cytokine profile different from HC based on our systematic appraisal of best available evidence. However, rather than having a purely pro-inflammatory profile, the peripheral cytokine signature associated with fibromyalgia appears to be more complex, involving potential compensatory anti-inflammatory cytokines and chemokines implicated in modulation of neuronal excitability. In addition, it is very difficult to establish a chronological relationship between various levels of cytokines and onset or progression of pain, as well as take into account patient individual characteristics. These aspects may be reflected in biological feed-back mechanisms that could propagate an inflammatory response or, in contrast, trigger effective physiological compensatory mechanisms. The lack of granular data related to the duration, intensity, fluctuation or character of pain, patient medication or use of other therapeutic strategies, levels of fitness, nutritional and BMI status, associated depression, etc. poses significant limitations in interpreting data or generalizing the findings of our analysis.
Further research involving standardized methodology for cytokine measurements, as has previously been suggested [17], is warranted. In particular, higher quality studies focused on hypothesis testing, evaluating the role of both pro- and anti-inflammatory cytokines associated with fibromyalgia are required, which may enable the identification of cytokines as potential therapeutic targets for chronic pain syndromes.
Funding: This work is funded by a Centre of Excellence (Centre for Adolescent Rheumatology Versus Arthritis—21593), as well as grants from NIHR UCLH BRC (BRC 525 III/CC 101350) to C.C.
Disclosure statement: P.M. is an MRC-GSK EMINENT clinical training fellow with project funding outside the submitted work. P.M. receives co-funding by the NIHR University College London Hospitals Biomedical Research Centre (UCLH BRC). P.M. has served on an advisory board for SOBI, outside the submitted work. C.C. has received consultancy fees from Roche and ModernBiosciences, and educational financial support from Novartis, Lilly, Chugai outside the submitted work.
Data availability statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Calzá L. Neurochemistry of pain circuits: physiological versus pathological pain. In: Tiengo MA, ed. Neuroscience: Focus on Acute and Chronic Pain. Milan: Springer-Verlag Italy, 2001, 9.– . [Google Scholar]
- 2. Cheng J. Mechanisms of pathologic pain. In: Cheng J, Rosenquist RW, eds. Fundamentals of Pain Medicine. Cham: Springer International Publishing, 2018, 21–5. [Google Scholar]
- 3. Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT.. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open 2016;6:e010364. doi:10.1136/bmjopen-2015-010364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wells G, Shea B, O’Connell D et al. Newcastle-Ottawa quality assessment scale cohort studies 2014.
- 5. Schwartz BS, Stewart WF, Simon D, Lipton RB.. Epidemiology of tension-type headache. JAMA 1998;279:381–3. [DOI] [PubMed] [Google Scholar]
- 6. Julius D, Basbaum AI.. Molecular mechanisms of nociception. Nature 2001;413:203–10. [DOI] [PubMed] [Google Scholar]
- 7. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152:S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grace PM, Hutchinson MR, Maier SF, Watkins LR.. Pathological pain and the neuroimmune interface. Nat Rev Immunol 2014;14:217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pomares FB, Funck T, Feier NA. et al. Histological underpinnings of grey matter changes in fibromyalgia investigated using multimodal brain imaging. J Neurosci 2017;37:1090–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sluka KA, Clauw DJ.. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016;338:114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang J-M, An J.. Cytokines, inflammation and pain. Int Anesthesiol Clin 2007;45:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jellinger K. Headache and chronic pain syndromes: the case‐based guide to targeted assessment and treatment. Eur J Neurol 2007;14:e25. [Google Scholar]
- 13. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1. 7.7.3.5 Medians and interquartile ranges. London: Cochrane. https://handbook-5-1.cochrane.org/chapter_7/7_7_3_5_mediansand_interquartile_ranges.htm (January 2020, date last accessed). [Google Scholar]
- 14. Hozo SP, Djulbegovic B, Hozo I.. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res 2005;5. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thalheimer W., Cook S.. How to Calculate Effect Sizes from Published Research Articles: A Simplified Methodology. Work-Learning Research 2002;http://www.work-learning.com/. [Google Scholar]
- 16. Kadetoff D, Lampa J, Westman M, Andersson M, Kosek E.. Evidence of central inflammation in fibromyalgia—increased cerebrospinal fluid interleukin-8 levels. J Neuroimmunol 2012;242:33–8. [DOI] [PubMed] [Google Scholar]
- 17. Üçeyler N, Häuser W, Sommer C.. Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC Musculoskelet Disord 2011;12:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bazzichi L, Rossi A, Massimetti G. et al. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin Exp Rheumatol 2007;25:225–30. [PubMed] [Google Scholar]
- 19. Blanco I, Janciauskiene S, Nita I. et al. Low plasma levels of monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-alpha (TNFα), and vascular endothelial growth factor (VEGF) in patients with alpha1-antitrypsin deficiency-related fibromyalgia. Clin Rheumatol 2010;29:189–97. [DOI] [PubMed] [Google Scholar]
- 20. Bote ME, García JJ, Hinchado MD, Ortega E.. Inflammatory/stress feedback dysregulation in women with fibromyalgia. Neuroimmunomodulation 2012;19:343–51. [DOI] [PubMed] [Google Scholar]
- 21. Bote ME, Garcia JJ, Hinchado MD, Ortega E.. Fibromyalgia: anti-inflammatory and stress responses after acute moderate exercise. PLoS One 2013;8:e74524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clos-Garcia M, Andrés-Marin N, Fernández-Eulate G. et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine 2019;46:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ernberg M, Christidis N, Ghafouri B. et al. Plasma cytokine levels in fibromyalgia and their response to 15 weeks of progressive resistance exercise or relaxation therapy. Mediators Inflamm 2018;2018:3985154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Furer V, Hazan E, Mor A. et al. Elevated levels of eotaxin-2 in serum of fibromyalgia patients. Pain Res Manag 2018;2018:7257681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. García JJ, Cidoncha A, Bote ME, Hinchado MD, Ortega E.. Altered profile of chemokines in fibromyalgia patients. Ann Clin Biochem 2014;51:576–81. [DOI] [PubMed] [Google Scholar]
- 26. Ghizal F, Das SK, Verma N, Mahdi AA.. Evaluating relationship in cytokines level, fibromyalgia impact questionnaire and body mass index in women with fibromyalgia syndrome. J Back Musculoskelet Rehabil 2016;29:145–9. [DOI] [PubMed] [Google Scholar]
- 27. Gur A,, Karakoc M,, Erdogan S. et al. Regional cerebral blood flow and cytokines in young females with fibromyalgia. Clin Exp Rheumatol 2002;20:753–60. [PubMed] [Google Scholar]
- 28. Gür A,, Karakoç M,, Nas K. et al. Cytokines and depression in cases with fibromyalgia. J Rheumatol 2002;29:358–61. [PubMed] [Google Scholar]
- 29. Hernandez ME, Becerril E, Perez M. et al. Proinflammatory cytokine levels in fibromyalgia patients are independent of body mass index. BMC Res Notes 2010;3:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim SK, Kim KS, Lee YS, Park SH, Choe JY.. Arterial stiffness and proinflammatory cytokines in fibromyalgia syndrome. Clin Exp Rheumatol 2010;28:S71–77. [PubMed] [Google Scholar]
- 31. Maes M, Libbrecht I, Van Hunsel F. et al. The immune-inflammatory pathophysiology of fibromyalgia: increased serum soluble gp130, the common signal transducer protein of various neurotrophic cytokines. Psychoneuroendocrinology 1999;24:371–83. [DOI] [PubMed] [Google Scholar]
- 32. Mendieta D, la Cruz-Aguilera DLD, Barrera-Villalpando MI. et al. IL-8 and IL-6 primarily mediate the inflammatory response in fibromyalgia patients. J Neuroimmunol 2016;290:22–5. [DOI] [PubMed] [Google Scholar]
- 33. Paiva ES, Andretta A, Batista ED. et al. Serum levels of leptin and adiponectin and clinical parameters in women with fibromyalgia and overweight/obesity. Arch Endocrinol Metab 2017;61:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pay S,, Calgüneri M,, Calişkaner Z. et al. Evaluation of vascular injury with proinflammatory cytokines, thrombomodulin and fibronectin in patients with primary fibromyalgia. Nagoya J Med Sci 2000;63:115–22. [PubMed] [Google Scholar]
- 35. Pernambuco AP, Schetino LPL, Alvim CC. et al. Increased levels of IL-17A in patients with fibromyalgia. Clin Exp Rheumatol 2013;31:S60–63. [PubMed] [Google Scholar]
- 36. Ranzolin A, Duarte ALBP, Bredemeier M. et al. Evaluation of cytokines, oxidative stress markers and brain-derived neurotrophic factor in patients with fibromyalgia – A controlled cross-sectional study. Cytokine 2016;84:25–8 [DOI] [PubMed] [Google Scholar]
- 37. Stensson N, Ghafouri B, Gerdle B, Ghafouri N.. Alterations of anti-inflammatory lipids in plasma from women with chronic widespread pain – a case control study. Lipids Health Dis 2017;16:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Togo F, Natelson BH, Adler GK. et al. Plasma cytokine fluctuations over time in healthy controls and patients with fibromyalgia. Exp Biol Med (Maywood) 2009;234:232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Topal G, Donmez A, Doğan BSU. et al. Asymmetric dimethylarginine (ADMA) levels are increased in patients with fibromyalgia: correlation with tumor necrosis factor-α (TNF-α) and 8-iso-prostaglandin F2α (8-iso-PGF2α). Clin Biochem 2011;44:364–7. [DOI] [PubMed] [Google Scholar]
- 40. Tsilioni I, Russell IJ, Stewart JM, Gleason RM, Theoharides TC.. Neuropeptides CRH, SP, HK-1, and inflammatory cytokines IL-6 and TNF are increased in serum of patients with fibromyalgia syndrome, implicating mast cells. J Pharmacol Exp Ther 2016;356:664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wallace DJ, Bowman RL, Wormsley SB, Peter JB.. Cytokines and immune regulation in patients with fibrositis. Arthritis Rheum 1989;32:1334–5. [DOI] [PubMed] [Google Scholar]
- 42. Wallace DJ, Linker‐Israeli M, Hallegua D. et al. Cytokines play an aetiopathogenetic role in fibromyalgia: a hypothesis and pilot study. Rheumatology (Oxford) 2001;40:743–9. [DOI] [PubMed] [Google Scholar]
- 43. Wang H, Buchner M, Moser MT, Daniel V, Schiltenwolf M.. The role of IL-8 in patients with fibromyalgia: a prospective longitudinal study of 6 months. Clin J Pain 2009;25:1–4. [DOI] [PubMed] [Google Scholar]
- 44. Wang H, Moser M, Schiltenwolf M, Buchner M.. Circulating cytokine levels compared to pain in patients with fibromyalgia – a prospective longitudinal study over 6 months. J Rheumatol 2008;35:1366–70. [PubMed] [Google Scholar]
- 45. Xiao Y, Haynes WL, Michalek JE, Russell IJ.. Elevated serum high-sensitivity C-reactive protein levels in fibromyalgia syndrome patients correlate with body mass index, interleukin-6, interleukin-8, erythrocyte sedimentation rate. Rheumatol Int 2013;33:1259–64. [DOI] [PubMed] [Google Scholar]
- 46. Zhang Z, Cherryholmes G, Mao A. et al. High plasma levels of MCP-1 and eotaxin provide evidence for an immunological basis of fibromyalgia. Exp Biol Med (Maywood) 2008;233:1171–80. [DOI] [PubMed] [Google Scholar]
- 47. Crosson T, Roversi K, Balood M. et al. Profiling of how nociceptor neurons detect danger—new and old foes. J Int Med 2019;286:268–89. [DOI] [PubMed] [Google Scholar]
- 48. Basbaum AI, Bautista DM, Scherrer G, Julius D.. Cellular and molecular mechanisms of pain. Cell 2009;139:267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chavan SS, Pavlov VA, Tracey KJ.. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 2017;46:927–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scholz J, Woolf CJ.. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007;10:1361–8. [DOI] [PubMed] [Google Scholar]
- 51. Milligan ED, Penzkover KR, Soderquist RG, Mahoney MJ.. Spinal interleukin‐10 therapy to treat peripheral neuropathic pain. Neuromodulation 2012;15:520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morris P, Ali K, Merritt M, Pelletier J, Macedo LG.. A systematic review of the role of inflammatory biomarkers in acute, subacute and chronic non-specific low back pain. BMC Musculoskelet Disord 2020;21:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kawasaki Y, Zhang L, Cheng J-K, Ji R-R.. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 2008;28:5189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Puma C, Danik M, Quirion R, Ramon F, Williams S.. The chemokine interleukin‐8 acutely reduces Ca2+ currents in identified cholinergic septal neurons expressing CXCR1 and CXCR2 receptor mRNAs. J Neurochem 2001;78:960–71. [DOI] [PubMed] [Google Scholar]
- 55. Krock E, Millecamps M, Anderson KM. et al. Interleukin-8 as a therapeutic target for chronic low back pain: upregulation in human cerebrospinal fluid and pre-clinical validation with chronic reparixin in the SPARC-null mouse model. EBioMedicine 2019;43:487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shao Q, Li Y, Wang Q, Zhao J.. IL-10 and IL-1β mediate neuropathic-pain like behavior in the ventrolateral orbital cortex. Neurochem Res 2015;40:733–9. [DOI] [PubMed] [Google Scholar]
- 57. Uçeyler N et al. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum 2656;54:2664. [DOI] [PubMed] [Google Scholar]
- 58. Rosendal L, Kristiansen J, Gerdle B. et al. Increased levels of interstitial potassium but normal levels of muscle IL-6 and LDH in patients with trapezius myalgia. Pain 2005;119:201–9. [DOI] [PubMed] [Google Scholar]
- 59. Salemi S, Rethage J, Wollina U. et al. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol 2003;30:146–50. [PubMed] [Google Scholar]
- 60. Üçeyler N, Kewenig S, Kafke W, Kittel-Schneider S, Sommer C.. Skin cytokine expression in patients with fibromyalgia syndrome is not different from controls. BMC Neurol 2014;14:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baumeister D, Eich W, Saft S. et al. No evidence for altered plasma NGF and BDNF levels in fibromyalgia patients. Sci Rep 2019;9:13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Blandini F, Rinaldi L, Tassorelli C. et al. Peripheral levels of BDNF and NGF in primary headaches. Cephalalgia 2006;26:136–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.