Abstract
Background
Changes in the management of patients with cancer and delays in treatment delivery during the COVID-19 pandemic may impact the use of hospital resources and cancer mortality.
Patients and methods
Patient flows, patient pathways and use of hospital resources during the pandemic were simulated using a discrete event simulation model and patient-level data from a large French comprehensive cancer centre's discharge database, considering two scenarios of delays: massive return of patients from November 2020 (early-return) or March 2021 (late-return). Expected additional cancer deaths at 5 years and mortality rate were estimated using individual hazard ratios based on literature.
Results
The number of patients requiring hospital care during the simulation period was 13,000. In both scenarios, 6–8% of patients were estimated to present a delay of >2 months. The overall additional cancer deaths at 5 years were estimated at 88 in early-return and 145 in late-return scenario, with increased additional deaths estimated for sarcomas, gynaecological, liver, head and neck, breast cancer and acute leukaemia. This represents a relative additional cancer mortality rate at 5 years of 4.4 and 6.8% for patients expected in year 2020, 0.5 and 1.3% in 2021 and 0.5 and 0.5% in 2022 for each scenario, respectively.
Conclusions
Pandemic-related diagnostic and treatment delays in patients with cancer are expected to impact patient survival. In the perspective of recurrent pandemics or alternative events requiring an intensive use of limited hospital resources, patients should be informed not to postpone care, and medical resources for patients with cancer should be sanctuarised.
Keywords: COVID-19, Delay, Diagnostics, Oncology, Survival, Hospital resources
1. Introduction
The COVID-19 pandemic led to a rapid influx of a large number of patients requiring intensive care. The impact of SARS-coV2 infection specifically in patients with cancer has been previously reported [[1], [2], [3]]. The pandemic has also impacted the management of patients with cancer, including delays in treatment delivery and modifications of standards of care [4,5].
In France, the government implemented a first national lockdown that lasted from 17th March to 10th May 2020, and health authorities enjoined all hospitals, including dedicated cancer centres, to open and extend their intensive care beds to patients with COVID-19 during the peak phase of the epidemic. Non-urgent surgeries and most of those requiring intensive care unit beds in the postoperative period had to be postponed. Several treatment plans deviating from standard practice were considered to minimise the number of hospital visits and hospitalisations as well as to prevent anticancer treatment–induced complications of COVID-19 [[6], [7], [8], [9]]. From the patients' side, a significant portion of those expected for diagnosis, treatment or follow-up during lockdown postponed their visits [[10], [11], [12], [13]]. Moreover, reduction in diagnostic examinations in primary care and cancer screening during lockdown led to a drop in patient referrals to hospitals. In October 2020, previously expected and new patients may have postponed their hospital visits during the second epidemic wave and lockdown. All these changes both in healthcare providers and patients may impact survival of patients with cancer.
Delays in cancer diagnosis and treatment can change the patient's prognosis ([14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], Supplementary Table A). Recent projections in the United Kingdom (UK) and the United States of America reported excess mortality in cancer patients induced by pandemic-related changes in cancer care [[32], [33], [34], [35]]. In a UK study, increase in the number of deaths due to cancer up to 5 years after diagnosis was estimated at 5% for lung, 6% for oesophageal, 8–10% for breast and 15–17% for colorectal cancer [33]. However, little is known about how the pandemic will impact the patient's return to the hospital and hospital resources in the coming months and years.
In this study, our objectives were to assess the impact of delays and changes in cancer care on the use of hospital resources and cancer mortality, using data from the largest comprehensive cancer centre in France.
2. Patients and methods
2.1. Study design and data sources
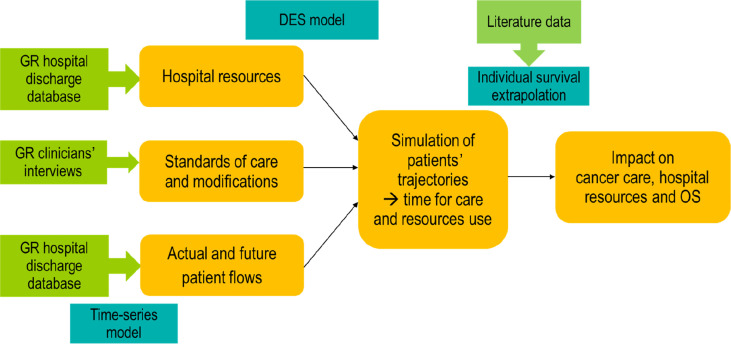
We used a discrete event simulation (DES) model [36] to analyse patient pathways as per different return scenarios (Fig. 1 ). DES models the hospital care pathway as a series of events that occur over time. We used patient-level data from the Gustave Roussy (GR) Cancer Center (Villejuif, France) hospital discharge database (PMSI [programme de médicalisation des systèmes d'information]) from January 2018 to the end of October 2020. We excluded patients in paediatric oncology, neuro-oncology, oncogenetics and early drug development, representing about 11% of patients, as they did not share the same hospital resources with the other specialities. We considered unique patients defined as all patients attending the hospital during this time period for a new episode of care (initial treatment or treatment of recurrences involving surgery, radiotherapy, medical therapies or haematopoietic stem cell transplant), with a 3-month wash-off period. Patients coming for screening or surveillance with no subsequent treatment were not taken into account.
Fig. 1.
Study flow chart. Notes: GR: Gustave Roussy; DES: discrete event simulation; OS: overall survival.
2.2. Model development
A DES model was developed to mimic the patient flow and organisation of hospital care, with time-dependent hospital resources corresponding to effective resources available at each time period. The overall study simulation period extended from January 2018 to December 2023. We used patient flows, pathways, treatment changes and hospital resources to populate the model.
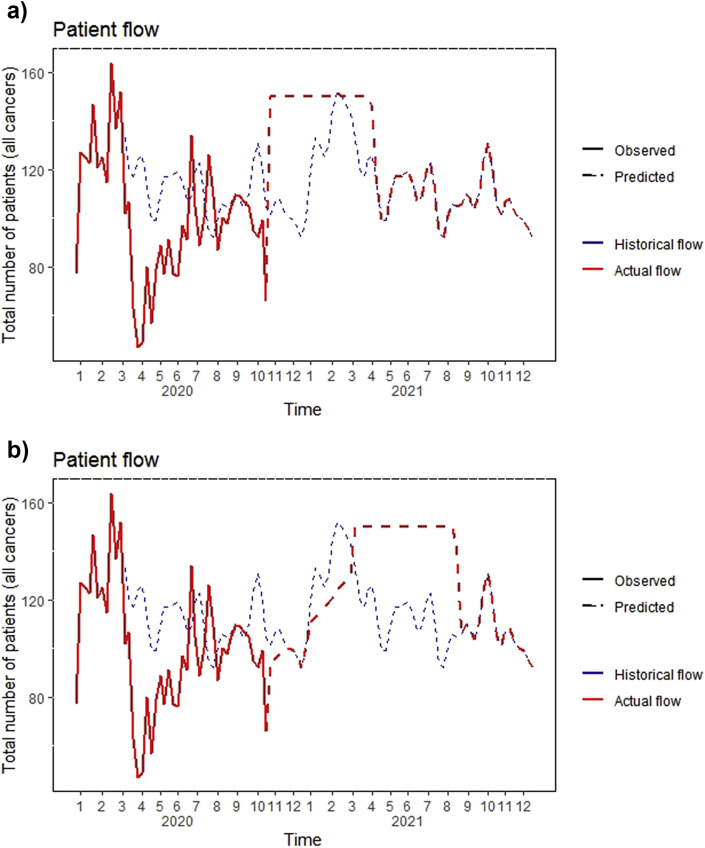
Patient flows were modelled using time-series methods fitted with patient-level data from the GR hospital discharge database (Fig. 2 , see details in Supplementary methods B). At any time point, the number of observed patients is the sum of patients who came to the scheduled appointment (on-time patients) and those who came later than expected (delayed patients), with each component estimated based on different hypotheses (see Simulation scenarios).
Fig. 2.
Observed and predicted patient flows for the simulation period (2019–2022). a) Early-return scenario. b) Late-return scenario. Note: Patients' return is smoothed at 95% of maximum capacity of the centre for ease of visualisation.
Patients' pathways were defined based on the cancer site, stage or histologic type or treatment line, following interviews with expert clinicians based on a standardised questionnaire. Overall, 75 cancer pathways were considered, with each pathway associated with use of specific hospital resources (Supplementary Table C). The distribution of patients in different pathways in 2019 was applied to the simulated patient on-time flow to split patients into the 75 pathways (Table 1—for ease of presentation, pathways were grouped into 21 broader categories as per cancer site). Using the DES model, care and hospital resource use were simulated at the patient level, as well as the individual time needed to receive care, which combined patient-induced delay (delay occurring before the patient's hospital visit) and healthcare-induced delay (delay while waiting for hospital care).
Table 1.
Patient characteristics from the Gustave Roussy hospital discharge database for the year 2019.
| Cancer type | All patientsa (%) | New patients by cancer type (%b) | Metastatic patients by cancer type (%b) |
|---|---|---|---|
| Acute leukaemia | 118 (2.0) | 23 (19.5) | NA |
| Bladder | 64 (1.1) | 5 (7.8) | 36 (56.3) |
| Breast | 1906 (32.0) | 817 (42.9) | 190 (10.0) |
| Cervix | 256 (4.3) | 24 (9.4) | 22 (8.6) |
| Colon | 185 (3.1) | 43 (23.2) | 91 (49.2) |
| Gastroesophageal | 58 (1.0) | 16 (27.6) | 27 (46.6) |
| Germinal seminoma | 61 (1.0) | 2 (3.3) | 33 (54.1) |
| Head and neck | 452 (7.6) | 183 (40.5) | 44 (9.7) |
| Kidney | 46 (0.8) | 8 (17.4) | 46 (100) |
| Liver | 30 (0.5) | 8 (26.7) | 10 (33.3) |
| Lung | 426 (7.2) | 122 (28.6) | 227 (53.3) |
| Lymphoma | 189 (3.2) | 46 (24.3) | NA |
| Myeloma | 87 (1.5) | 38 (43.7) | NA |
| Neuroendocrine tumours | 46 (0.8) | 19 (41.3) | NA |
| Ovary | 143 (2.4) | 52 (36.4) | 87 (60.8) |
| Pancreas | 41 (0.7) | 9 (22.0) | 20 (48.8) |
| Prostate | 390 (6.5) | 63 (16.2) | 85 (21.8) |
| Sarcomas | 268 (4.5) | 74 (27.6) | 62 (23.1) |
| Melanoma | 585 (9.8) | 146 (25.0) | 102 (17.4) |
| Thyroid | 376 (6.3) | 158 (42.0) | 77 (20.5) |
| Endometrium | 237 (4.0) | 33 (13.9) | 45 (19.0) |
| ALL | 5964 (100.0) | 1889 (31.7) | 1204 (20.2) |
NA: not applicable.
In this table, only patients under active treatment using hospital resources (surgery blocks, beds in the postsurgery unit, chemotherapy sessions, radiotherapy sessions, beds for haematopoietic stem cell transplant) considered in the study are taken into account. Percentages are calculated with respect to the total number of patients.
Percentages are calculated with respect to all patients within each cancer type.
Available hospital resources (number of surgery blocks, beds in the postsurgery unit, chemotherapy sessions, radiotherapy sessions, beds for haematopoietic stem cell transplant) per week between March and October 2020 were defined based on the GR hospital discharge database. The term ‘chemotherapy’ refers to medical cancer treatments and includes chemotherapy, targeted treatments, immunotherapies, monoclonal antibodies, etc. Activity in 2019 was considered as maximum capacity (Supplementary Table D).
2.3. Simulation scenarios
We considered two contrasting scenarios. In the earlier return of patients scenario (early-return), usual patient flow was recovered by November 2020. We considered the on-time patient flow from the end of lockdown (week 21, mid-May 2020) to be constant and equal to that of the last lockdown week, until the last observed data date (LODD, week 44, end of October 2020). After the lockdown was lifted, the return of delayed patients was simulated following a first in/first out method: whenever there was a spot available (difference between expected usual patient flow and simulated on-time patient flow), it was given to the patient who had been waiting for the longest time. After the LODD, delayed patients who had not yet returned were added to the expected flow up to the hospital's maximum capacity, until total absorption of delayed patients. In this scenario, we hypothesised that all patients would return after the LODD and that existing delays would only be healthcare-induced delays (the hospital's maximum capacity reached) and not patient-induced delays.
In the later return of patients scenario (late-return), the usual patient flow was recovered by March 2021, taking into account the expected vaccination of people at risk (age, comorbidities) on priority. After the same patient flows as in the first scenario until the LODD, we considered the flow of on-time patients to follow a linear extrapolation from the LODD to reach the level of expected patient flow in the end of March 2021 (week 12). We considered the proportion of on-time patients relative to the observed patients to be constant from the LODD to March 2021. We hypothesised that all delayed patients would return after this date. To assess the robustness of results and give additional insights on alternative scenarios, two main sensitivity analyses on the availability of hospital resources were implemented (Supplementary Methods E).
2.4. Statistical analysis
For cancer mortality, an individual hazard ratio for additional cancer death was derived based on literature data [[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]], in accordance with simulated care and time needed for provision of care (Supplementary Table A). This additional risk was applied to the 5-year net mortality rate for patients with cancer [37], and the additional number of cancer-specific deaths at 5 years after treatment for different types of cancer was calculated based on the assumption that net mortality approximates cancer mortality [38] for patients treated during the simulation period. The percentage of additional cancer deaths was calculated with respect to the usual number of cancer deaths at 5 years, for patients expected to receive care in a calendar year, to provide information on time trends of effects.
The model was programmed in open source R v.4.0.2 software using the Simmer and tsModel packages [39,40].
3. Results
3.1. Patient flows, treatment delays and treatment modifications
Patients undergoing treatment at GR were 5964 in 2019, with 32% of them having breast cancer, 10% melanoma and 8% head and neck cancers (Table 1). Proportions of new patients and metastatic patients/patients with advanced cancer were 31.7% and 20.2%, respectively, both with wide variations across cancer types. Return to usual flows (no more delayed patients) and time for care (no more healthcare-induced delays) is expected to be in mid-May 2022 for the early-return scenario and in the beginning of June 2022 for the late-return scenario (Fig. 2). The number of patients requiring hospital care during the simulation period (from the beginning of lockdown in mid-March 2020 until absorption of all delays) was 13,015 patients for the early-return scenario and 13,328 patients for the late-return scenario. The mean delays were 12 days (standard deviation [SD] = 34, range 0–208) for the early-return scenario and 20 days (SD = 56, range 0–322) for the late-return scenario. In the early-return scenario, 18% of patients had a delay of >1 week, 8% > 1 month and 6% >2 months. In the late-return scenario, 25% of patients had a delay of >1 week, 10% >1 month and 8% >2 months.
Treatment modifications were implemented during the first lockdown only (March–May 2020). Among the 4514 patients expected to receive care in 2020 (from March to December, in the early-return scenario), 360 (8.0%) received modified care adapted to the COVID pandemic context, mainly in breast cancer (n = 208).
3.2. Hospital resources
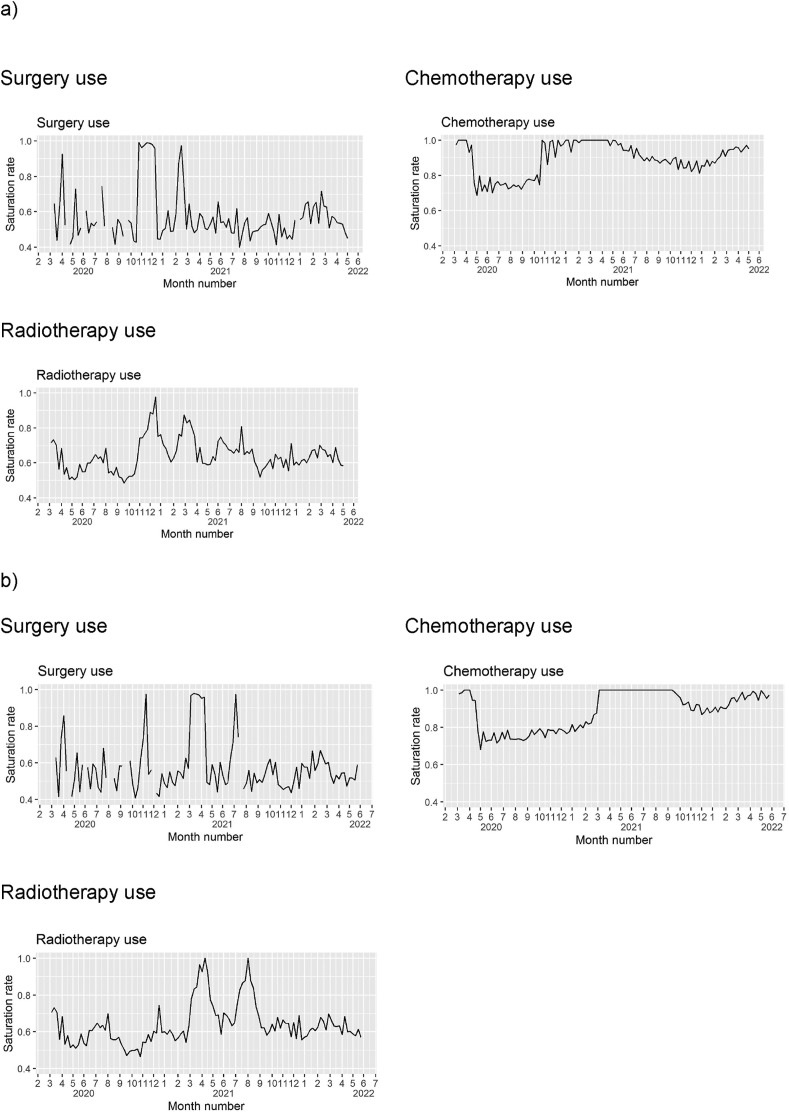
The last resource creating healthcare-induced delay is estimated to be chemotherapy in both scenarios, with delays existing until 2022. Surgery is expected to present overload during about 60 days over the study period, for chemotherapy sessions, 200 days and for radiotherapy, between 3 and 10 days, as per simulation scenarios (Fig. 3 ).
Fig. 3.
Hospital resources. a) Early-return scenario. b) Late-return scenario. Notes: Overload is defined by saturation rate equals to 1. Mean weekly saturation rates are represented.
3.3. Cancer outcomes
Impact on cancer mortality as per cancer type for each scenario is shown in Table 2 . In the early-return scenario, the expected number of cancer deaths at 5 years under standard conditions is 4639 for the 13,015 patients in the simulation period. The overall additional cancer deaths at 5 years due to treatment delays were estimated at 88. This excess risk is mainly present for patients who should have received care in 2020, with a cancer mortality increase of 4.4%, whereas it represents 0.5% in both 2021 and 2022. For the late-return scenario, the expected number of cancer deaths at 5 years under standard conditions is 4769 for 13,328 patients, and additional deaths were estimated at 145, which represents a 6.8% increase for patients who should have received care in 2020, 1.3% in 2021 and 0.5% in 2022. The strongest increase in cancer deaths for patients to be treated in 2020, considering the proportion of additional mortality, was found for the following cancer types for the two scenarios: sarcomas (21 and 29%), cervix (16 and 21%), liver (9 and 8%), endometrium (8 and 12%), acute leukaemia (8 and 13%), head and neck (8 and 15%) and breast (7 and 11%). Sensitivity analyses supported findings from the main scenarios (Supplementary Data F).
Table 2.
Additional number of deaths at 5 years as per cancer type.
| a) Early-return scenario | |||||||
|---|---|---|---|---|---|---|---|
| Cancer type | Patients during the simulation perioda | Metastatic patients (%) | Expected number of cancer-specific deaths at 5 years | Additional number of cancer-specific deaths at 5 years | Additional cancer mortality rate in year 2020b (%) | Additional cancer mortality rate in year 2021b (%) | Additional cancer mortality rate in year 2022b (%) |
| Sarcomas | 536 | 138 | 235 | 19 | 20.8 | 1.4 | 1.3 |
| Cervix | 584 | 50 | 177 | 11 | 15.5 | 1.3 | 1.5 |
| Liver | 76 | 24 | 63 | 3 | 8.7 | 2.2 | 1.9 |
| Endometrium | 558 | 107 | 135 | 4 | 8.1 | 0.3 | 0.3 |
| Acute leukaemia | 355 | NA | 171 | 5 | 7.9 | 0.2 | 0.2 |
| Head and neck | 960 | 98 | 371 | 12 | 7.8 | 0.8 | 0.8 |
| Breast | 3922 | 401 | 728 | 21 | 6.7 | 0.9 | 0.8 |
| Bladder | 140 | 71 | 80 | 1 | 3.2 | 0.1 | 0.1 |
| Colon | 476 | 245 | 249 | 7 | 2.6 | 2.7 | 2.7 |
| Lung | 915 | 479 | 749 | 4 | 1.5 | 0.1 | 0.1 |
| Melanoma | 1188 | 195 | 643 | 1 | 0.5 | 0 | 0 |
| Germinal seminoma | 147 | 79 | 7 | 0 | 0 | 0 | 0 |
| Lymphoma | 419 | NA | 127 | 0 | 0 | 0 | 0 |
| Myeloma | 207 | NA | 130 | 0 | 0 | 0 | 0 |
| Neuroendocrine tumours | 128 | NA | 31 | 0 | 0 | 0 | 0 |
| Gastroesophageal | 168 | 80 | 126 | 0 | 0 | 0 | 0 |
| Ovary | 342 | 214 | 176 | 0 | 0 | 0 | 0 |
| Pancreas | 96 | 60 | 88 | 0 | 0 | 0 | 0 |
| Prostate | 893 | 197 | 143 | 0 | 0 | 0 | 0 |
| Thyroid | 760 | 126 | 133 | 0 | 0 | 0 | 0 |
| Kidney | 95 | 95 | 77 | 0 | 0 | 0 | 0 |
| All | 13,015 | 2659 | 4639 | 88 | 4.4 | 0.5 | 0.5 |
| b) Late-return scenario | |||||||
|---|---|---|---|---|---|---|---|
| Cancer type | Patients during the simulation perioda | Metastatic patients (%) | Expected number of cancer-specific deaths at 5 years | Additional number of cancer-specific deaths at 5 years | Additional cancer mortality rate in year 2020b (%) | Additional cancer mortality rate in year 2021b (%) | Additional cancer mortality rate in year 2022b (%) |
| Sarcomas | 609 | 152 | 248 | 30 | 29.1 | 4.7 | 1.5 |
| Cervix | 620 | 62 | 194 | 15 | 20.7 | 2.0 | 1.3 |
| Head and neck | 970 | 94 | 375 | 23 | 14.8 | 2.0 | 0.8 |
| Acute leukaemia | 365 | NA | 176 | 9 | 13.4 | 0.8 | 0.2 |
| Endometrium | 573 | 111 | 140 | 6 | 12.2 | 1.0 | 0.2 |
| Breast | 4021 | 404 | 740 | 40 | 10.9 | 3.3 | 0.8 |
| Liver | 81 | 26 | 67 | 4 | 8.0 | 5.4 | 2.2 |
| Bladder | 150 | 79 | 87 | 2 | 5.7 | 0.8 | 0.1 |
| Colon | 485 | 246 | 251 | 7 | 2.6 | 2.8 | 2.6 |
| Lung | 931 | 503 | 766 | 7 | 2.1 | 0.3 | 0.1 |
| Melanoma | 1214 | 204 | 656 | 2 | 0.8 | 0.1 | 0 |
| Germinal seminoma | 141 | 80 | 7 | 0 | 0 | 0 | 0 |
| Lymphoma | 430 | 0 | 130 | 0 | 0 | 0 | 0 |
| Myeloma | 209 | 0 | 131 | 0 | 0 | 0 | 0 |
| Neuroendocrine tumours | 122 | 0 | 29 | 0 | 0 | 0 | 0 |
| Gastroesophageal | 176 | 81 | 132 | 0 | 0 | 0 | 0 |
| Ovary | 342 | 219 | 179 | 0 | 0 | 0 | 0 |
| Pancreas | 108 | 64 | 98 | 0 | 0 | 0 | 0 |
| Prostate | 901 | 199 | 144 | 0 | 0 | 0 | 0 |
| Thyroid | 780 | 130 | 138 | 0 | 0 | 0 | 0 |
| Kidney | 100 | 100 | 81 | 0 | 0 | 0 | 0 |
| All | 13,328 | 2754 | 4769 | 145 | 6.8 | 1.3 | 0.5 |
Categories are listed by decreasing order of additional cancer mortality rate in year 2020.
Simulation period = from March 2020 until return to normal (mid-May 2022 and June 2022 in Early-Return and Late-Return scenarios respectively).
The mortality rate was calculated considering the number of additional deaths occurring in patients who should have received care in the respective year over the theoretical number of deaths in the same population.
4. Discussion
This study assessed the impact of COVID-19 disease on patients with cancer and without COVID in France. France has dedicated centres for patients with cancer (Centres de Lutte Contre le Cancer), which represent a protected resource. Although not all patients with cancer are cared for using this pathway, we believe this study to be informative for other hospital settings and countries with similar organisations.
The impact we found on sarcomas, gynaecological, liver, head and neck, breast cancer and acute leukaemia can be due to the existence of risk associated with diagnosis and treatment delay, overload in hospital resources needed for patients and/or volume of patients in these cancer types. Absorption of all delays could only be expected in May or June 2022 in the two scenarios, with delays existing until 2022 for chemotherapy. Sensitivity analyses show the sensible impact of punctual disruptions in the care pathways. These results advocate for a refined programming of activity in the coming months, to ensure appropriate allocation and protection of resources, including healthcare professionals.
The strengths of our simulation study should be underlined. First, we used robust individual data from the GR hospital discharge database. The DES model allowed us to make precise predictions on patient delays, rather than relying on strong and impacting hypotheses of generalised delays, and to take into account and adapt to multiple environments. In addition, we used published literature for hazard ratios on mortality. The information relying on best evidence has been used, even if heterogeneity of some cancers (e.g. sarcomas) can limit the validity of data. Two contrasting scenarios based on different dynamics for patients’ return were considered, with supportive sensitivity analyses. The use of this additional risk for cancer mortality associated with treatment delay allowed us to implicitly take into account disease progression. Finally, the observation period was long, from March to the end of October 2020, including periods of lockdown, curfew and other restrictive measures.
Several limitations must be acknowledged. First, this study was based on patient flows of a single hospital, and even though it is one of the largest comprehensive cancer centres in Europe (6000 patients treated for cancer every year), the impact on cancer mortality may not be representative of all French patients with cancer. A substantial and heterogeneous decrease in national cancer care activity has been reported, affecting mostly general hospitals [9]. The hospital casemix is not fully representative of the general cancer population, GR being a reference centre for some cancer types (e.g. sarcomas) and a notable proportion of patients being included in clinical trials. However, the model is applicable to other settings, and external data could be integrated in future studies. Second, we had to simplify some of the model inputs. Patients' individual risk factors were not considered, and delays were applied homogeneously to all patients, whatever the cancer type. Patients with no subsequent treatment for cancer were not taken into account because of their low use of limited medical resources, which could lead to an underestimation of delays for care. We assumed that no prioritisation was made between patients, whereas it was actually implemented within each tumour group. However, haematopoietic stem cell transplant beds were considered as not limiting to take into account potential faster return and care for haematological patients. Patient-induced delays can in fact reflect a variety of delays, which may not be due to the patient's choice to postpone. Delays can present differences between patients included or not in clinical trials. Furthermore, additional number of deaths is not as exhaustive as the number of life years lost. Finally, although we chose two contrasting scenarios to address the impact on cancer mortality, the impact of the pandemic appears to be long-lasting, and our scenarios are likely optimistic and the impact on cancer mortality underestimated. Estimates will be updated as more recent discharge data become available.
This work has shown the existence of long-term impacts of COVID-19 pandemic, regarding patients' demand for care, provision of care and cancer outcomes. Further investigations will be implemented from the present work, including patient perspectives and behaviours. It seems important that cancer care providers fully maintain their activities even during lockdown periods and that patients are informed not to postpone their diagnosis and treatment.
Funding
This study was partially supported by la Ligue contre le Cancer.
Role of the funding source
La Ligue contre le Cancer provided financial support to the Oncostat U1018, Inserm team. It has not been involved in this study design, analysis or interpretation.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: No author reported potential conflict of interest in link with the submitted work. A.B. reports personal fees from Roche SAS. I.B. reports personal fees from Roche, Novartis, CSL Behring and Takeda. M.F. reports fees from HRA Pharma (participation to board) and travel grants from Ipsen, Pfizer and Novartis. S.D. reports grants and non-financial support from Pfizer, grants from Novartis, grants and non-financial support from AstraZeneca, grants and non-financial support from Roche Genentech, grants from Lilly, grants from Puma, grants from Myriad, grants from Orion, grants from Amgen, grants from Sanofi, grants from Genomic Health, grants from GE, grants from Servier, grants from MSD, grants from BMS, grants from Pierre Fabre, grants from Seagen and grants from Exact Sciences. L.A. reports a consulting/advisory role for Novartis (Institution), Amgen (Institution), Bristol Myers Squibb (Institution), Ipsen (Institution), Roche (Institution), Pfizer (Institution), Astellas Pharma (Institution), Merck (Institution), MSD (Institution), AstraZeneca (Institution), Exelixis (Institution), Corvus Pharmaceuticals (Institution) and Peloton Therapeutics (Institution) and research Funding from Bristol Myers Squibb (Institution). D.P. reports a consulting, advisory role or lectures for AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche and Samsung; honoraria from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche and Samsung; institutional financial interests (clinical trials research as the principal or co-investigator) from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Pfizer, Roche, MedImmune, Sanofi-Aventis, Taiho Pharma, Novocure and Daiichi Sankyo and travel and accommodation expenses from AstraZeneca, Roche, Novartis, prIME Oncology and Pfizer. M.D. reports honoraria for advisory boards or as a presenter in symposium from Merck Serono, MSD, AMGEN, Roche, Bayer, Ipsen, Pfizer, Servier, Pierre Fabre, HalioDx, Lilly and Sanofi. E.C. reports consulting advisory board activity for BMS, Ipsen, Sanofi, Pfizer, GSK and Merck. C.R. reports a consultant/advisory role for Bristol Myers Squibb, MSD, Roche, Novartis, Amgen, Sanofi and Pierre Fabre. C.M. reports consultant/advisory fees from Amgen, Astellas, AstraZeneca, Bayer, BeiGene, BMS, Celgene, Debiopharm, Genentech, Ipsen, Janssen, Lilly, MedImmune, MSD, Novartis, Pfizer, Roche, Sanofi and Orion and as acted as a principal/sub-investigator of clinical trials for AbbVie, Aduro, Agios, Amgen, Argen-x, Astex, AstraZeneca, Aveo pharmaceuticals, Bayer, BeiGene, Blueprint, BMS, Boehringer Ingelheim, Celgene, Chugai, Clovis, Daiichi Sankyo, Debiopharm, Eisai, Eos, Exelixis, Forma, GamaMabs, Genentech, Gortec, GSK, H3 Biomedecine, Incyte, Innate Pharma, Janssen, Kura Oncology, Kyowa, Lilly, Loxo, Lysarc, Lytix Biopharma, MedImmune, Menarini, Merus, MSD, Nanobiotix, Nektar Therapeutics, Novartis, Octimet, Oncoethix, Oncopeptides AB, Orion, Pfizer, PharmaMar, Pierre Fabre, Roche, Sanofi, Servier, Sierra Oncology, Taiho, Takeda, Tesaro and Xencor. J.B.M. reports personal fees from AbbVie, Jazz PHARMA and Astellas. S.M. reports personal fees from statistical advice to IDDI, Janssen Cilag and Amaris and from data and safety monitoring membership of clinical trials (Hexal, Steba, IQVIA, Roche, Sensorion, Biophytis, Servier and Yuhan). A.A. reports grant support, paid to her institution, from F. Hoffmann-La Roche. F.B. reports personal financial interests with AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly Oncology, F. Hoffmann–La Roche Ltd, Novartis, Merck, Mirati, MSD, Pierre Fabre, Pfizer, Seattle Genetics and Takeda; institutional financial interests with AbbVie, ACEA, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Eisai, Eli Lilly Oncology, F. Hoffmann–La Roche Ltd, Genentech, Ipsen, Ignyta, Innate Pharma, Loxo, Novartis, MedImmune, Merck, MSD, Pierre Fabre, Pfizer, Sanofi-Aventis and Takeda and non-financial interests (as a principal investigator) for AstraZeneca, BMS, Merck, Pierre Fabre and F. Hoffmann-La Roche, Ltd–sponsored trials (or ISR). J.B. reports honoraria from Bristol Myers Squibb; a consulting or advisory role for Bristol Myers Squibb, MSD, PharmaMar (Inst), Bristol Myers Squibb (Inst) and Merck Serono (Inst) and travel and accommodation expenses from Bristol Myers Squibb. Other authors report no conflict of interest. Y. Takahashi declares no conflict of interest. Her editorial work for the present article has been funded by Inserm.
Acknowledgements
The authors sincerely thank Yuki Takahashi for medical writing and editorial assistance.
The authors thank Fanny Pommeret, medical oncologist, head of the COVID unit in Gustave Roussy.
The authors thank the anonymous reviewer for precious comments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2021.05.012.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Albiges L., Foulon S., Bayle A., et al. Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: results from the Gustave Roussy cohort. Nat Canc. 2020;1:965–975. doi: 10.1038/s43018-020-00120-5. [DOI] [PubMed] [Google Scholar]
- 2.Lièvre A., Turpin A., Ray-Coquard I., et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study(GCO-002 CACOVID-19) Eur J Canc. 2020;141:62–81. doi: 10.1016/j.ejca.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Joode K., Dumoulin D.W., Tol J., et al. Dutch Oncology COVID-19 consortium: outcome of COVID-19 in patients with cancer in a nationwide cohort study. Eur J Canc. 2020;141:171–184. doi: 10.1016/j.ejca.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Haar J., Hoes L.R., Coles C.E., et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26(5):665–671. doi: 10.1038/s41591-020-0874-8. [DOI] [PubMed] [Google Scholar]
- 5.Spicer J., Chamberlain C., Papa S. Provision of cancer care during the COVID-19 pandemic. Nat Rev Clin Oncol. 2020;17(6):329–331. doi: 10.1038/s41571-020-0370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gligorov J., Bachelot T., Pierga J.Y., et al. COVID-19 et personnes suivies pour un cancer du sein: recommandations françaises pour la pratique clinique de Nice-St Paul de Vence, en collaboration avec le Collège Nationale des Gynécologues et Obstétriciens Français (CNGOF), la Société d'Imagerie de la Femme (SIFEM), la Société Française de Chirurgie Oncologique (SFCO), la Société Française de Sénologie et Pathologie Mammaire (SFSPM) et le French Breast Cancer Intergroup-UNICANCER (UCBG) [COVID-19 and people followed for breast cancer: French guidelines for clinical practice of Nice-St Paul de Vence, in collaboration with the Collège Nationale des Gynécologues et Obstétriciens Français (CNGOF), the Société d'Imagerie de la Femme (SIFEM), the Société Française de Chirurgie Oncologique (SFCO), the Société Française de Sénologie et Pathologie Mammaire (SFSPM) and the French Breast Cancer Intergroup-UNICANCER (UCBG)] Bull Canc. 2020;107(5):528–537. doi: 10.1016/j.bulcan.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radiothérapie de la sphère ORL en période de pandémie COVID-19, Propositions d'adaptations pratiques validées par le bureau du GORTEC au 28/3/2020. https://gortec.net/index.php/fr/actualites/476-covid19-radiotherapie-orl-recommandations-gortec.
- 8.Groupe d'Etude des Tumeurs Uro-Génitales (GETUG) Options thérapeutiques en cancérologie génito-urinaire en période épidémique de COVID19. Bull Cancer. 2020;107(4):395–397. doi: 10.1016/j.bulcan.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prise en charge des cancers digestifs en fonction de la situation épidémique COVID-19. https://www.snfge.org/content/21-prise-en-charge-des-cancers-digestifs-en-fonction-de-la-situation-epidemique-covid-19.
- 10.Kempf E., Lamé G., Layese R., et al. New cancer cases at the time of SARS-Cov2 pandemic and related public health policies: a persistent and concerning decrease long after the end of national lockdown. Eur J Canc. 2021 doi: 10.1016/j.ejca.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brugel M., Carlier C., Essner C., et al. Dramatic changes in oncology care pathways during the COVID-19 pandemic: the French ONCOCARE-COV study. The Oncologist. 2020;25:1–4. doi: 10.1002/onco.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar D., Dey T. Treatment delays in oncology patients during COVID-19 pandemic: a perspective. J Glob Health. 2020;10(1) doi: 10.7189/jogh.10.010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lou E., Teoh D., Brown K., et al. Perspectives of cancer patients and their health during the COVID-19 pandemic. PloS One. 2020;15(10) doi: 10.1371/journal.pone.0241741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekeres M.A., Elson P., Kalaycio M.E., et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28–36. doi: 10.1182/blood-2008-05-157065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanna T.P., King W.D., Thibodeau S., et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards M.A., Westcombe A.M., Love S.B., et al. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 17.Perri T., Issakov G., Ben-Baruch G., et al. Effect of treatment delay on survival in patients with cervical cancer: a historical cohort study. Int J Gynecol Canc. 2014;24(7):1326–1332. doi: 10.1097/IGC.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 18.Hangaard Hansen C., Gögenur M., Tvilling Madsen M., et al. The effect of time from diagnosis to surgery on oncological outcomes in patients undergoing surgery for colon cancer: a systematic review. Eur J Surg Oncol. 2018;44(10):1479–1485. doi: 10.1016/j.ejso.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Brenkman H.J.F., Visser E., van Rossum P.S.N., et al. Association between waiting time from diagnosis to treatment and survival in patients with curable gastric cancer: a population-based study in The Netherlands. Ann Surg Oncol. 2017;24(7):1761–1769. doi: 10.1245/s10434-017-5820-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huyghe E., Muller A., Mieusset R., et al. Impact of diagnostic delay in testis cancer: results of a large population-based study. Eur Urol. 2007;52(6):1710–1716. doi: 10.1016/j.eururo.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Graboyes E.M., Kompelli A.R., Neskey D.M., et al. Association of treatment delays with survival for patients with head and neck cancer: a systematic review. JAMA Otolaryngol Head Neck Surg. 2019;145(2):166–177. doi: 10.1001/jamaoto.2018.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singal A.G., Waljee A.K., Patel N., et al. Therapeutic delays lead to worse survival among patients with hepatocellular carcinoma. J Natl Compr Canc Netw. 2013;11(9):1101–1108. doi: 10.6004/jnccn.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samson P., Patel A., Garrett T., et al. Effects of delayed surgical resection on short-term and long-term outcomes in clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2015;99(6):1906–1912. doi: 10.1016/j.athoracsur.2015.02.022. discussion 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kariyawasan C.C., Hughes D.A., Jayatillake M.M., et al. Multiple myeloma: causes and consequences of delay in diagnosis. QJM. 2007;100(10):635–640. doi: 10.1093/qjmed/hcm077. [DOI] [PubMed] [Google Scholar]
- 25.Jooste V., Dejardin O., Bouvier V., et al. Pancreatic cancer: wait times from presentation to treatment and survival in a population-based study. Int J Canc. 2016;139(5):1073–1080. doi: 10.1002/ijc.30166. [DOI] [PubMed] [Google Scholar]
- 26.Comandone A., Berno E., Boglione A., et al. Delay in diagnosis and treatment of soft tissue sarcomas (STS): causes of late intervention and their role in prognosis - a prospective, multidisciplinary group study. J Clin Oncol. 2012;30(15):suppl–10055. [Google Scholar]
- 27.Featherall J., Curtis G.L., Lawrenz J.M., et al. Time to treatment initiation and survival in adult localized, high-grade soft tissue sarcoma. J Surg Oncol. 2019;120(7):1241–1251. doi: 10.1002/jso.25719. [DOI] [PubMed] [Google Scholar]
- 28.Conic R.Z., Cabrera C.I., Khorana A.A., et al. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78(1) doi: 10.1016/j.jaad.2017.08.039. 40–46.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amit M., Rudnicki Y., Binenbaum Y., et al. Defining the outcome of patients with delayed diagnosis of differentiated thyroid cancer. Laryngoscope. 2014;124(12):2837–2840. doi: 10.1002/lary.24744. [DOI] [PubMed] [Google Scholar]
- 30.Dolly D., Mihai A., Rimel B.J., et al. A delay from diagnosis to treatment is associated with a decreased overall survival for patients with endometrial cancer. Front Oncol. 2016;6:31. doi: 10.3389/fonc.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartman H.E., Sun Y., Devasia T.P., et al. Integrated survival estimates for cancer treatment delay among adults with cancer during the COVID-19 pandemic. JAMA Oncol. 2020;6(12):1881–1889. doi: 10.1001/jamaoncol.2020.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sud A., Torr B., Jones M.E., et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21(8):1035–1044. doi: 10.1016/S1470-2045(20)30392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sud A., Jones M.E., Broggio J., et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020;31(8):1065–1074. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai A., Pasea L., Banerjee A., et al. Estimating excess mortality in people with cancer and multimorbidity in the COVID-19 emergency. 2020. Preprint at. [DOI]
- 36.Cudney E.A., Baru R.A., Guardiola I., et al. A decision support simulation model for bed management in healthcare. Int J Health Care Qual Assur. 2019;32(2):499–515. doi: 10.1108/IJHCQA-10-2017-0186. [DOI] [PubMed] [Google Scholar]
- 37.Mazeau-Woynar V., Cerf N. Institut National du Cancer (INCA); 2010. Survie attendue des patients atteints de cancers en France: état des lieux. [Google Scholar]
- 38.Dambaard Skyrud K., Bray F., Møller B. A comparison of relative and cause-specific survival by cancer site, age and time since diagnosis. Int J Canc. 2014;135(1):196–203. doi: 10.1002/ijc.28645. [DOI] [PubMed] [Google Scholar]
- 39.Ucar I., Smeets B., Azcorra A. Simmer: discrete-event simulation for R. J Stat Software. 2019;90(2):1–30. [Google Scholar]
- 40.https://cran.r-project.org/web/packages/tsModel/tsModel.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.