Abstract
Uveal melanoma (UM) is a rare cancer in adults but its treatment is one of the clinical unmet needs in the melanoma field. Metastatic disease develops in approximately 50% of patients and is associated with poor survival due to the lack of effective treatment options. It provides a paradigm for cancers that show evidence of aberrant G-protein coupled receptor signaling, tumor dormancy, liver-selective metastatic tropism and are associated with the loss of the BAP1 tumor suppressor. At the Melanoma Research Foundation (MRF) CURE OM Science Meeting at the Society for Melanoma Research (SMR) Meeting held in Utah, on November 20, 2019, clinicians and researchers presented findings from their studies according to three themes within UM: 1) ongoing clinical trials; 2) molecular determinants; and 3) novel targets that could be translated into clinical trials. This meeting underscored the high interest in the UM research field and the unmet need for effective treatment strategies for late-stage disease. Findings from ongoing clinical trials are promising and multiple studies show how novel combinatorial strategies increase response rates. Novel targets and tumor vulnerabilities identified bioinformatically or through high-throughput screens also reveal new opportunities to target UM. The future directions pursued by the UM research field will likely have impact of other cancer types, which harbor similar genetic alterations and/or show similar biological properties.
Keywords: Uveal melanoma, liver-directed therapy, clinical trials, novel targets
Introduction
UM is the most common intraocular malignancy in adults. It represents about 5% of all melanoma diagnoses in the United States and has an incidence of approximately 2,000 cases per year (1). Primary disease can be successfully treated with local therapy; however, ~50% of UM cases metastasize with high propensity (90%) to the liver (2). Overt metastases often take years or even decades to develop, highlighting the likelihood of early dissemination of tumor cells from the primary site and cellular dormancy (3). Therapeutic options for advanced-stage disease have been underwhelming to date. Immune checkpoint inhibitors, which have revolutionized treatment in skin melanoma and other tumors, have less efficacy in UM with the best overall response rate (ORR) of 15.6% (4). The need for clinical trials in the field of UM and the exploration of new targets and therapeutic agents was particularly emphasized at this meeting.
Clinical Trials in UM
The first session of the meeting was chaired by Dr. Sapna Patel (MD Anderson Cancer Center) and focused on ongoing clinical trials. Responses of UM to combined immune checkpoint inhibitors (anti-PD1 and anti-CTLA4) are not comparable to those in cutaneous melanoma (4); hence, there is a need to optimize strategies using immune-based therapies. Dr. Suthee Rapisuwon (Georgetown University Medical Center) presented the Phase II trial of adjuvant ipilimumab in combination with nivolumab for high-risk patients with locally treated UM. The primary endpoint is 3-year relapse-free survival with a goal to reduce development of distant metastasis following primary tumor treatment. Adjuvant therapies including immune checkpoint blockades are being tested in high-risk cancers but UM was excluded, hence, this study will be important to compare with other immune checkpoint inhibitor adjuvant studies in other tumor types. There is precedent for the evaluation of agents in the adjuvant setting that demonstrate little benefit in metastastic disease. One example is the previous approved use of recombinant interferon-alfa2a for cutaneous melanoma in the adjuvant setting (5). In tumor types that are not classically responsive to immune therapy in the metastatic setting, it will be important to understand if augmenting immune response in the minimal residual disease setting is beneficial.
Clinical trials by Dr. Takami Sato (Thomas Jefferson University) have shown encouraging results with tebentafusp (IMCgp100), a bispecific antibody that redirects CD3+ T cells to gp100-expressing melanoma cells, such as UM, inducing cytolysis. ORR was 18% in Phase 1 (6). In the Phase 2 expansion cohort studying patients with prior treatment, a major area of focus is whether tebentafusp can restore sensitivity to the immune checkpoint inhibitors in metastatic UM (7). A separate trial of tebentafusp compared to investigator’s choice treatment in previously untreated patients is ongoing. New strategies to overcome resistance to immunotherapy was also discussed by Dr. Filip Janku (MD Anderson Cancer Center). He noted a Type I interferon signature is associated with benefit from ipilimumab in melanoma (8) and then expanded on Phase 1 trials to target a type I interferon response. Such trials include the use of intratumoral stimulator of interferon gamma (STING) agonists and intratumoral toll-like receptor agonists.
Liver metastasis is common in UM and liver resection is often not feasible. Many of the ongoing UM trials focus on liver-directed therapy coupled with anti-cancer and immune-based therapies. Given that the majority of death in UM is from liver failure, and that liver-directed therapy is associated with a survival benefit (9) not only in UM but also in other solid tumors, this is a reasonable approach to continue to explore alone and in combination with systemic therapy (10). Dr. Meredith Pelster (MD Anderson Cancer Center) presented on liver-directed therapy with Yttrium-90 (Y-90) radioembolization in combination with systemic ipilimumab and nivolumab. Potential for synergy with this combination was reported previously where median overall survival (OS) was prolonged to 26.5 months compared to 9.5 months in patients treated with Y-90 only (11,12) The study presented by Dr. Pelster was a further investigaton of this synergy. Initially, the triple combination therapy induced liver toxicity but with Y-90 and immunotherapy dosage adjustments, toxicity was found to be more manageable. Currently, durable responses have been seen including one patient with complete response of 25 months and two patients with partial response of 12 and 16 months. This study continues to recruit patients. Dr. Sapna Patel (MD Anderson Cancer Center) talked about the Phase 1b trial using percutaneous injection of hepatic intratumoral PV-10, a liver-directed therapy with a solution of Rose Bengal, a small molecule which accumulates in tumor lysosomes triggering autolysis. The study allows concomitant use of standard of care immune checkpoint blockade in hopes to propagate the effects of immunogenic cell death. In 13 patients, stable disease was achieved in 62.5% and partial responses in 37.5% of the patients. Further follow-up is needed to calculate the survival benefit with this approach. Dr. Zeynep Eroglu (Moffitt Cancer Center) presented on Percutaneous Hepatic Perfusion (PHP) with melphalan, which delivers a high concentration of chemotherapy (melphalan) into the liver. Contemporary retrospective analysis of outcomes in patients indicated an ORR of 47%, median PFS of 8.1 months, and 1-yr OS of 64.6% (13). The Phase III FOCUS trial, a single-arm and multi-center study (PHP-melphalan administered every 6–8 weeks in patients with metastatic UM) is open and recruiting patients.
MEK inhibitors were suboptimally efficacious in advanced stage UM, highlighting the need for new targeted therapy trials (14). One target discussed was protein kinase C (PKC) which acts downstream of Gαq/11 proteins (15). Dr. Meredith McKean (Sarah Cannon Research Institute) spoke about a trial with a dedicated UM cohort for a second generation PKC inhibitor, LXS196, which was shown to be effective in preclinical studies. A key future avenue is to analyze the tolerability of LXS196 in combination with additional kinase inhibitors.
Like in other cancers, in UM, the threshold for activity that is considered promising should be deliberated in the context of past results. Benchmarks for a promising therapy should exceed the outcomes of a recent meta-analysis of metastatic UM from 29 trials. In that report analyzing trials from 2000 to 2016, the median PFS was 3.3 months, 1-year OS was 43%, and median OS is 10.2 months (16). Additionally, therapies that induce an objective response rate (ORR) greater than 15% and with consistent benefits (eg. durable responses) have been recognized by federal agencies as worthy of further investigation. As an orphan disease, federal agencies are also willing to consider non-randomized trials for therapeutic approval in UM where benchmarks are clearly surpassed compared to historic reports with confirmation in a randomized study. In light of the National Comprehensive Cancer Network (NCCN) guidelines noting trametinib as a treatment option and recent reports citing nivolumab in combination with ipilimumab with activity (17), these agents could be considered the control arms for a randomized trial design.
In addition to UM, the treatments discussed here may also have general implications to extend survival of patients with liver metastases such as colorectal and pancreatic cancers and/or immunologically ‘cold’ tumors. Pre-clinical studies showing the role of liver stromal-derived HGF and FGF mediates targeted therapy resistance (18,19) may have implications for other cancer types that metastasize to the liver. In UM, however, key challenges include smaller patient cohorts due to rarity of UM and development of therapeutic resistance. Translational research using combination of patient samples from trials and appropriate pre-clinical and animal models is therefore warranted.
Molecular determinants in UM
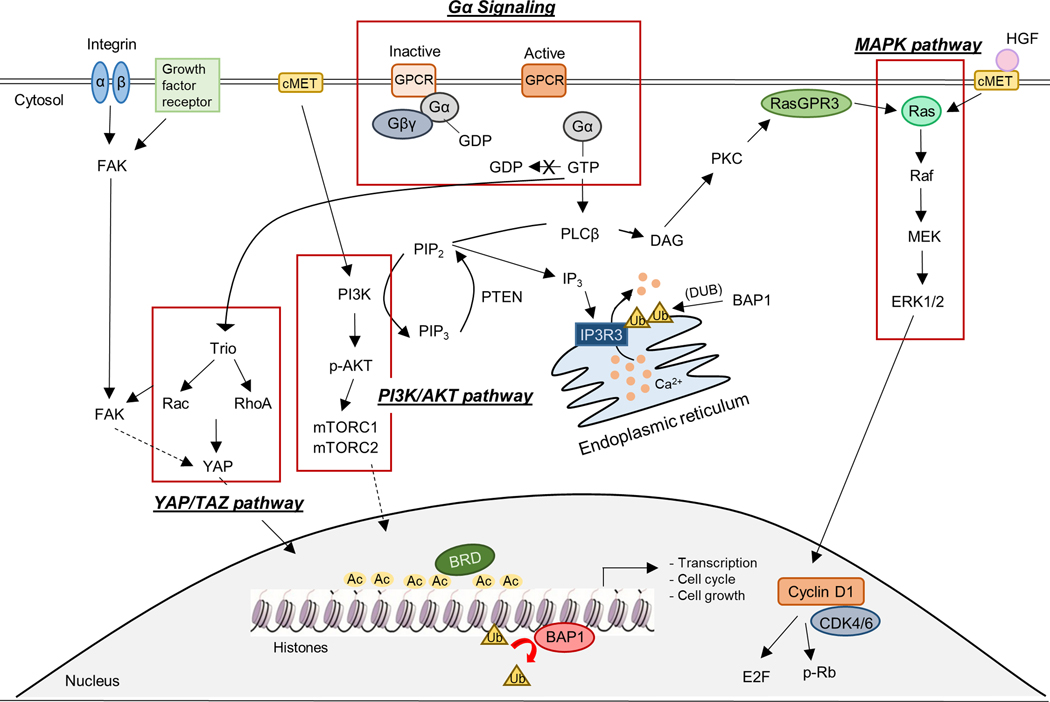
In the Basic Science session chaired by Dr. Andrew Aplin (Thomas Jefferson University), novel findings about the biology and mutations in UM (GNAQ/11, BAP1), strategies for targeting key pathways (Figure 1) and future needs for research were discussed (20,21). Dr. William Harbour (Bascom Palmer Eye Institute) spoke on the key role of BAP1 loss in transcription factor networks. BAP1 promotes progenitor cell differentiation into melanocytes by increasing MITF expression and BAP1 loss may promote metastasis in UM by remodeling the epigenome to resemble that of stem-like migratory neural crest cells. While BAP1 is likely to display context-dependent functions, a more detailed understanding of its role in UM will impact malignant mesothelioma (MM) and renal cell carcinoma (RCC) given its frequent mutation in these cancer types. BAP1 also functions as a tumor suppressor in these tumors. BAP1 knockout in mice leads to MM growth, larger tumors and increased number of metastases and BAP1 protein expression is also correlated with poor prognosis and shorter survival in RCC and cholangiocarcinoma, as well as UM (22–25). Dr. Hunter Shain (University of California San Francisco) presented about sequencing paired primary and metastatic tumors from 35 patients. He posited that Gαq/11 mutations undergo selection early in UM followed by BAP1 loss and then gain of chromosomal arm 8q in evolution to metastasis (26). A striking observation is that UM metastases display more mutations and genomic alterations compared to primary tumors, a feature that is unique to UM. A future avenue will be to determine whether BAP1 loss, 8q gain or additional late-stage alterations offer therapeutic opportunities.
Figure 1: Crosstalk between key and emerging signaling pathways that are therapeutically targetable in UM.
Key pathways in UM include the Gαq signaling and its downstream cascades such as the MAPK (MEK/ERK), YAP/TAZ, and PI3K/AKT pathways. The MAPK and PI3K/AKT pathways are also activated by the hepatocyte growth factor (HGF) and its receptor, cMET. Cyclin D1 and CDK4/6 are downstream of ERK1/2. FAK is often activated by integrins or growth factor receptors (eg. platelet-derived growth factor receptor (PDGFR) and epidermal growth factor receptor (EGFR)) and directs signals, in part, through YAP/TAZ. These pathways ultimately regulate gene transcription, cell growth and cell cycle progression. Major roles of BAP1 in the nucleus and cytoplasm are also shown. BAP1 has been shown to deubiquitinate histones in the nucleus and also the calcium transporter, IP3R3, on the endoplasmic reticulum. The BET protein, BRD4, binds to acetylation of histones and regulates gene transcription and is also a druggable therapeutic target in UM.
Dr. Michael Onken (Washington University) presented data on the cyclic depsipeptide, FR900359, a Gαq inhibitor which shifts and traps Gαq into a GDP-bound (inactive) state (27). On-going questions regarding Gαq inhibitors are whether they will be sufficient to elicit durable responses in liver metastatic in vivo UM models and the tolerability of these agents. The development of more selective inhibitors would be a major advance for the field. These studies with G-protein inhibitors will also likely impact skin melanoma and other non-melanoma cancers that harbor GPCR or G-protein alterations such as meningeal, biliary tract and lung tumors (28). Ms. Amanda Truong (University of Utah) showed that combination of an autophagy inhibitor, hydroxychloroquine (HCQ) with a MEK inhibitor, synergistically delays GNAQ/11 mutant UM growth. These findings extend previous observations in pancreatic cancer where MEK inhibitors induce autophagy, which was also seen in UM (29). Dr. Keiran Smalley (Moffitt Cancer Center) showed activation of oncogenic pathways such as PI3K/AKT and YAP/TAZ following MEK inhibitor treatment of GNAQ/11 mutant UM cell lines, with the pathways and UM growth in vitro and in vivo further inhibited by combining MEK and histone deacetylase inhibitors (30). The next step for this combination is to determine whether BAP1 status influences the response and its translational testing in both the high-risk adjuvant and metastatic settings. These findings will add to our understanding of crosstalk between the MAPK and compensatory pathways, as well as, combination therapeutic strategies involving MEK inhibitors that could be tested in melanomas as well as in cancers driven by the MAPK pathway (31). Whilst preclinical in vitro findings seem promising, a challenge in UM research is the lack of representative animal models. At this meeting, we learnt about a zebrafish model for UM by Ms. Grace Phelps (Massachusetts Institute of Technology) who showed that expression of GNAQ Q209L leads to tumor growth which was accelerated by a concomitant loss of MITF (32).
Novel Targets in UM
The Novel Targets session, chaired by Dr. Richard Carvajal (Columbia University), covered new targets (Figure 1) in UM that are likely translated to clinical trials. Dr. Stefan Kurtenbach (Bascom Palmer Eye Institute) presented functions of PRAME (33), which is associated with aggressive forms of UM but it’s functional contribution in UM is not well understood. Dr. Kurtenbach showed that PRAME expression maintains cells in a stem-like state, is associated with chromosomal instability and aneuploidy in uveal melanocytes, suggesting that PRAME is not just a biomarker but a promoter of UM metastasis.
Dr. Grazia Ambrosini (Columbia University) presented on BRD4, a bromodomain and extra-terminal (BET) protein (34,35). The combination of BET inhibitors and MEK inhibitors induces synergistic growth inhibitory on UM growth and is currently being proposed for a clinical trial. Next generation BET inhibitors such as PLX2853 (NCT03297424), have been developed and their shorter half lives may overcome the tolerability of first-generation BET inhibitors and enhable combination studies in UM and other cancers.
Ms. Nadia Arang (University of California San Diego) showed that focal adhesion kinase (FAK) is a druggable therapeutic target in UM. The combination of FAK and MEK inhibitors synergistically promotes apoptotic cell death and reduces tumor burden in vivo. This study underscored the utility of synthetic lethality screens to identify for precision oncology. The FAK inhibitor, defactinib, has been combined with the MEK inhibitor, VS-6766, in several cancers (NCT03875820) and in UM, FAK/MEK co-targeting is slated to start investigation clinically in 2020.
Dr. Prabhjot Mundi (Columbia University) discussed the OncoTreat clinical pipeline which is a re-conceptualization of cancer as orchestrated chaos. OncoTreat identifies aberrantly activated nodes and profiles the effects of different drugs on nodal activity. A future goal is to apply this platform to UM and further develop in vivo models such as patient-derived xenografts (PDX) in which predictions can then be validated. Dr. Moony Tseng (The Broad Institute) talked about the Cancer Cell Line Factory and Cancer Dependency Map (https://depmap.org/portal/) whose goal is to identify rare tumor vulnerabilities using the drug repurposing library and genome-scale CRISPR screens. Patients with rare tumor diseases in United States and Canada can donate fresh tissue to support the generation of laboratory models that can be widely shared.
Summary and Future Directions
UM is biologically and clinically distinct from cutaneous melanoma and, regardless of the success of primary tumor therapy, about 50% of patients experience metastasis (2,36). The outcome of patients with metastatic disease remains discouraging with a median survival after metastasis of 12 months (37–39). The MRF CURE OM Science Meeting highlighted increased efforts in preclinical and translational research with dedicated UM clinical trial opportunities.
The inclusion of patients with UM in clinical trials early in the drug development process is critical to accelerate access to active therapies. In the Clinical Trials session at the meeting, several of the studies focused on strategies to improve the efficacy of liver-directed therapies and immune checkpoint inhibitors. The management of liver metastases from UM continues to be a significant challenge and there is little consensus on how to manage this other than recommendations for participation on a clinical trial (https://www.nccn.org/professionals/physician_gls/pdf/uveal.pdf). Studies suggest regional liver-directed therapy may achieve disease control that is more durable than that achieved with the available systemic therapeutic options (40,41). But increasing survival benefit warrants innovation in terms of strategies to overcome immune resistance such as promotion of a Type I interferon response. Intratumoral approaches are underway to promote this mechanism. Tebentafusp is a leading candidate to make inroads into UM survival via T-cell redirection. The ideal patient population (treatment-naïve, post-checkpoint blockade) to benefit from this therapy is being further delineated. Translational studies of responders versus non-responders will be critical to our understanding of how these class of agents provide benefit, produce toxicity, and redirect the immune system.
Several research groups have identified evolutionary events in genetic and chromosomal alterations in UM tumors from pre-tumorigenic lesions to metastases, leading us to further understand the role of key molecular determinants in driving UM development and progression. Key questions to address relate to the heterogeneity of primary tumors versus metastatic alterations. Single cell sequencing data will also further characterize the immune cell populations present in UM. Basic science advancements have uncovered mechanisms of mutant Gαq/11 signaling, MEK1/2 resistance via autophagy, and compensatory pathway signaling with direct therapeutic implications. An important development for targeted therapy strategies in UM would be the generation of selective YAP/TAZ-TEAD pathway inhibitors and their testing in UM models given the evidence for this pathway playing a key role downstream of mutant Gαq/11 signaling. These druggable therapeutic clues must now be pursued in the clinic in well-designed studies. Targeted therapy with 2nd generation PKC inhibitors is also being investigated and translational research on compensatory pathway activation is imperative to further this agent in combinatorial approaches. Progress is on the horizon as a number of novel targets are making their way into clinical trial development. PRAME as a target is being addressed in at least two trials to increase T-cell recognition of this antigen (NCT03686124, NCT04262466). BET inhibitors have been studied and are being proposed in combination with MEK inhibition due to evidence of preclinical synergy. FAK inhibition as monotherapy and in combination with MEK inhibition is a novel clinical trial that opened for enrollment in the year 2020. As these and other targeted therapy strategies also reach the clinic pre-clinical modeling and patient sample material should be carefully paired to define charateristics of drug tolerant persisters cells and mechanisms of therapy resistance. Comparison of UM to cancers that are molecularly similar will be important in future studies such as by analysis of epigenetics, metabolic profiles and their plasticity. There is an ongoing project studying adenosine signaling and immune suppression in UM and pancreatic adenocarcinoma. However, differences may exist. For example, MM cells are more sensitive to EZH2 inhibitors following loss of BAP1 but this has not been shown in UM (42). These studies could also lead to context-selective discovery of novel therapeutic strategies and understanding whether BAP1 loss promotes metastasis.
High-throughput and large scale tools such as OncoTreat and the Cancer Cell Line Factory are key instruments in helping predict drug-augmented pathways and provide a tumor dependency map. We must carry on contributions to these important model generating systems with patient-derived tumor tissue to increase our understanding of mechanisms driving metastatic UM. In vivo models for UM need continued refinement with key insights emerging from zebrafish and chick chorioallantoic membrane models (32,43). Zebrafish models are important given their application to high-through put screen and that they can be used in large numbers, supporting statistical analysis. Another need in the field is for a mouse model that faithfully represents the genetic and biological features of UM. Such a model would enable pre-clinical testing of immune-based therapies. Additional PDX models that represent the diversity of UM both at the genetic level are also required.
The information presented at this meeting showed that there has been progress in UM research on the clinical trials, basic science, and novel targets fronts. These advancements would not have been possible without a multidisciplinary team invested in optimizing outcomes for UM patients, and the combined efforts of philanthropy, the organizers at the MRF, and the UM patient community.
Acknowledgements:
This meeting was organized by MRF and Cure OM. MRF is the largest independent organization devoted to melanoma. Committed to the support of medical research in finding effective treatments and eventually a cure for all forms of melanoma, the MRF also educates patients and physicians about prevention, diagnosis and the treatment of melanoma. The CURE OM initiative was founded in 2011 to increase awareness, education and research funding for ocular melanoma. To date, the MRF’s CURE OM initiative has funded over $1.5 million in ocular melanoma research and pioneered international collaborations, groundbreaking scientific initiatives and innovative patient support resources. Further details can be found at: https://melanoma.org/about-us/programs-initiatives/cure-ocular-melanoma-cure-om/. The authors of this work were supported by a Melanoma Research Alliance team science award (#559058) and by the Dr. Ralph and Marian Falk Medical Research Trust Bank of America, N.A. Trustee grants (A.E. Aplin) and American Association for Cancer Research (AACR)/Ocular Melanoma Foundation (OMF) grant (V. Chua).
Footnotes
Conflicts of interest: A.E. Aplin reports receiving a commercial research grant from Pfizer Inc. (2013–2017) and has ownership interest in patent number 9880150. S.P. Patel reports institutional clinical trial support from Bristol Myers-Squibb, Novartis, Provectus, and InxMed, has had an advisory role with Cardinal Health, Castle Biosciences, Immunocore, and Incyte, and has received personal honoraria from Merck as a non-promotional speaker.
References
- 1.Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology 2011;118:1881–5. [DOI] [PubMed] [Google Scholar]
- 2.Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol 2005;123:1639–43. [DOI] [PubMed] [Google Scholar]
- 3.Blanco PL, Lim LA, Miyamoto C, Burnier MN. Uveal melanoma dormancy: an acceptable clinical endpoint? Melanoma Res 2012;22:334–40. [DOI] [PubMed] [Google Scholar]
- 4.Heppt MV, Amaral T, Kahler KC, Heinzerling L, Hassel JC, Meissner M, et al. Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multi-center study. J Immunother Cancer 2019;7:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7–17. [DOI] [PubMed] [Google Scholar]
- 6.Sato T, Nathan PD, Hernandez-Aya L, Sacco JJ, Orloff MM, Visich J, et al. Redirected T cell lysis in patients with metastatic uveal melanoma with gp100-directed TCR IMCgp100: Overall survival findings. Journal of Clinical Oncology 2018;36:9521-. [Google Scholar]
- 7.Yang J, Orloff MM, Sacco JJ, Hernandez-Aya LF, Lee K, Merrick S, et al. Resensitization of uveal melanoma (UM) to immune checkpoint inhibition (ICI) by IMCgp100 (IMC). Journal of Clinical Oncology 2019;37:9592-. [Google Scholar]
- 8.Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell 2015;162:974–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowcroft A, Loveday BPT, Thomson BNJ, Banting S, Knowles B. Systematic review of liver directed therapy for uveal melanoma hepatic metastases. HPB (Oxford) 2020;22:497–505. [DOI] [PubMed] [Google Scholar]
- 10.Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AO, Elsayed M, Lawson DH, Ermentrout RM, Kudchadkar RR, Bercu ZL, et al. Predictors of Overall and Progression-Free Survival in Patients with Ocular Melanoma Metastatic to the Liver Undergoing Y90 Radioembolization. Cardiovasc Intervent Radiol 2020;43:254–63. [DOI] [PubMed] [Google Scholar]
- 12.Zheng J, Irani Z, Lawrence D, Flaherty K, Arellano RS. Combined Effects of Yttrium-90 Transarterial Radioembolization around Immunotherapy for Hepatic Metastases from Uveal Melanoma: A Preliminary Retrospective Case Series. J Vasc Interv Radiol 2018;29:1369–75. [DOI] [PubMed] [Google Scholar]
- 13.Karydis I, Gangi A, Wheater MJ, Choi J, Wilson I, Thomas K, et al. Percutaneous hepatic perfusion with melphalan in uveal melanoma: A safe and effective treatment modality in an orphan disease. J Surg Oncol 2018;117:1170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvajal RD, Piperno-Neumann S, Kapiteijn E, Chapman PB, Frank S, Joshua AM, et al. Selumetinib in Combination With Dacarbazine in Patients With Metastatic Uveal Melanoma: A Phase III, Multicenter, Randomized Trial (SUMIT). J Clin Oncol 2018;36:1232–9. [DOI] [PubMed] [Google Scholar]
- 15.Chua V, Lapadula D, Randolph C, Benovic JL, Wedegaertner PB, Aplin AE. Dysregulated GPCR Signaling and Therapeutic Options in Uveal Melanoma. Mol Cancer Res 2017;15:501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoja L, Atenafu EG, Suciu S, Leyvraz S, Sato T, Marshall E, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol 2019;30:1370–80. [DOI] [PubMed] [Google Scholar]
- 17.Najjar YG, Navrazhina K, Ding F, Bhatia R, Tsai K, Abbate K, et al. Ipilimumab plus nivolumab for patients with metastatic uveal melanoma: a multicenter, retrospective study. J Immunother Cancer 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng H, Chua V, Liao C, Purwin TJ, Terai M, Kageyama K, et al. Co-targeting HGF/cMET Signaling with MEK Inhibitors in Metastatic Uveal Melanoma. Mol Cancer Ther 2017;16:516–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chua V, Orloff M, Teh JL, Sugase T, Liao C, Purwin TJ, et al. Stromal fibroblast growth factor 2 reduces the efficacy of bromodomain inhibitors in uveal melanoma. EMBO Mol Med 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Decatur CL, Ong E, Garg N, Anbunathan H, Bowcock AM, Field MG, et al. Driver Mutations in Uveal Melanoma: Associations With Gene Expression Profile and Patient Outcomes. JAMA Ophthalmol 2016;134:728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010;330:1410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One 2014;9:e115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalirai H, Dodson A, Faqir S, Damato BE, Coupland SE. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing. Br J Cancer 2014;111:1373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pena-Llopis S, Vega-Rubin-de-Celis S, Liao A, Leng N, Pavia-Jimenez A, Wang S, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet 2012;44:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph RW, Kapur P, Serie DJ, Eckel-Passow JE, Parasramka M, Ho T, et al. Loss of BAP1 protein expression is an independent marker of poor prognosis in patients with low-risk clear cell renal cell carcinoma. Cancer 2014;120:1059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shain AH, Bagger MM, Yu R, Chang D, Liu S, Vemula S, et al. The genetic evolution of metastatic uveal melanoma. Nat Genet 2019;51:1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onken MD, Makepeace CM, Kaltenbronn KM, Kanai SM, Todd TD, Wang S, et al. Targeting nucleotide exchange to inhibit constitutively active G protein alpha subunits in cancer cells. Sci Signal 2018;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer 2013;13:412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinsey CG, Camolotto SA, Boespflug AM, Guillen KP, Foth M, Truong A, et al. Protective autophagy elicited by RAF-->MEK-->ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat Med 2019;25:620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faiao-Flores F, Emmons MF, Durante MA, Kinose F, Saha B, Fang B, et al. HDAC Inhibition Enhances the In Vivo Efficacy of MEK Inhibitor Therapy in Uveal Melanoma. Clin Cancer Res 2019;25:5686–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Dong Q, Cui Y. Synergistic inhibition of MEK and reciprocal feedback networks for targeted intervention in malignancy. Cancer Biol Med 2019;16:415–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez DE, Henle AM, Amsterdam A, Hagen HR, Lees JA. Uveal melanoma driver mutations in GNAQ/11 yield numerous changes in melanocyte biology. Pigment Cell Melanoma Res 2018;31:604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costessi A, Mahrour N, Tijchon E, Stunnenberg R, Stoel MA, Jansen PW, et al. The tumour antigen PRAME is a subunit of a Cul2 ubiquitin ligase and associates with active NFY promoters. EMBO J 2011;30:3786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambrosini G, Do C, Tycko B, Realubit RB, Karan C, Musi E, et al. Inhibition of NF-kappaB-Dependent Signaling Enhances Sensitivity and Overcomes Resistance to BET Inhibition in Uveal Melanoma. Cancer Res 2019;79:2415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ambrosini G, Sawle AD, Musi E, Schwartz GK. BRD4-targeted therapy induces Myc-independent cytotoxicity in Gnaq/11-mutatant uveal melanoma cells. Oncotarget 2015;6:33397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci 2003;44:4651–9. [DOI] [PubMed] [Google Scholar]
- 37.Virgili G, Gatta G, Ciccolallo L, Capocaccia R, Biggeri A, Crocetti E, et al. Survival in patients with uveal melanoma in Europe. Arch Ophthalmol 2008;126:1413–8. [DOI] [PubMed] [Google Scholar]
- 38.Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol 2006;124:1684–93. [DOI] [PubMed] [Google Scholar]
- 39.Diener-West M, Hawkins BS, Markowitz JA, Schachat AP. A review of mortality from choroidal melanoma. II. A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988. Arch Ophthalmol 1992;110:245–50. [DOI] [PubMed] [Google Scholar]
- 40.Sato T Locoregional management of hepatic metastasis from primary uveal melanoma. Semin Oncol 2010;37:127–38. [DOI] [PubMed] [Google Scholar]
- 41.Gonsalves CF, Eschelman DJ, Adamo RD, Anne PR, Orloff MM, Terai M, et al. A Prospective Phase II Trial of Radioembolization for Treatment of Uveal Melanoma Hepatic Metastasis. Radiology 2019;293:223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaFave LM, Beguelin W, Koche R, Teater M, Spitzer B, Chramiec A, et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat Med 2015;21:1344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalirai H, Shahidipour H, Coupland SE, Luyten G. Use of the Chick Embryo Model in Uveal Melanoma. Ocul Oncol Pathol 2015;1:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]