Abstract
COVID-19, the disease responsible for the devastating pandemic that began at the end of 2019, has been associated with a significantly increased risk of pulmonary thrombosis, even in patients receiving prophylactic anticoagulation. The predilection for thrombosis in COVID-19 may be driven by at least two distinct, but interrelated, processes: a hypercoagulable state responsible for large-vessel thrombosis and thromboembolism and direct vascular and endothelial injury responsible for in situ microvascular thrombosis. The presence of pulmonary thrombosis may explain why hypoxemia is out of proportion to impairment in lung compliance in some patients with COVID-19 pneumonia. Because pulmonary embolism (PE) and COVID-19 pneumonia share many signs and symptoms, diagnosing PE in patients with COVID-19 can be challenging. Given the high mortality and morbidity associated with severe COVID-19 and the concern that aspects of the disease may be driven by thrombosis, many hospital systems have instituted aggressive anticoagulation protocols above standard VTE prophylaxis. In this review, the epidemiologic and pathophysiologic features, diagnosis, and treatment of COVID-19 pulmonary thrombosis and thromboembolism are discussed.
Key Words: anticoagulation, COVID-19, immunothrombosis, pulmonary embolism, VTE
Abbreviations: CTPA, CT pulmonary angiography; DIC, disseminated intravascular coagulopathy; PE, pulmonary embolism; PVR, pulmonary vascular resistance; RV, right ventricular
In late December 2019, a cluster of patients with pneumonia of unknown cause was linked to a seafood and wet animal wholesale market in Wuhan, China.1 By early January 2020, a novel coronavirus, SARS-CoV-2, was isolated from these patients with virus-infected pneumonia, and soon after, the clinical syndrome caused by SARS-CoV-2 was labeled as COVID-19 by the World Health Organization.2 Since then, this highly transmissible and virulent disease has devastated the world, overwhelming hospitals with critically ill patients. A few notable observations about COVID-19 were made early in the course of the pandemic: (1) many patients with COVID-19 demonstrate markedly abnormal coagulation parameters, particularly D-dimer elevation, which correlates with mortality3; (2) patients with COVID-19, particularly those in the ICU, show a notably high incidence of thrombotic complications4; (3) small autopsy series of patients with COVID-19 have demonstrated a high incidence of both pulmonary macrothrombi and microthrombi, despite the use of prophylactic anticoagulation5 , 6; and (4) many patients with COVID-19 who experience respiratory failure seemed to have hypoxemia that was out of proportion to the impairment in lung compliance, a disconnect that perhaps could be explained by pulmonary thrombosis.7 Given the high mortality and morbidity associated with severe COVID-198 , 9 and the concern that aspects of the disease may be driven by thrombosis, many hospital systems instituted aggressive anticoagulation protocols beyond standard VTE prophylaxis, despite the absence of randomized clinical trials supporting such practices.10 , 11 In this review, the epidemiologic and pathophysiologic features, diagnosis, and treatment of COVID-19 pulmonary thrombosis and thromboembolism are discussed.
Epidemiologic Features
Accurate assessments of the true incidence of VTE in hospitalized patients with COVID-19 remain elusive, with estimates ranging from 4.8% to 85%.12 The significant variability in the reported incidence is likely a consequence of multiple factors, including assessment setting (eg, ICU vs non-ICU), type of events counted (eg, symptomatic vs asymptomatic), testing strategies (eg, clinical suspicion vs systematic screening), and degree of thromboprophylaxis. Given infection control concerns and strained resources during peak surge times early in the pandemic, the threshold for diagnostic testing with CT pulmonary angiography (CTPA), compression ultrasonography, or both was high, leading to a low frequency of testing.13 In a meta-analysis by Jimenez et al12 comprising 36 studies and more than 11,000 patients, the pooled incidence of VTE in patients with COVID-19 was 17% (12% for DVT, 7.1% pulmonary embolism [PE]).
VTE incidence in patients with COVID-19 is elevated when compared with historical control participants. Using a French National administrative database, Piroth et al14 compared the 89,530 patients admitted to the hospital with COVID-19 in France over a 2-month period with the 45,819 patients admitted with influenza over a similar 2-month period during the prior year. VTE and PE rates were 4.9% and 3.4%, respectively, for patients with COVID-19, but only 1.7% and 0.9%, respectively, for patients with influenza. Poissy et al15 noted a PE incidence of 20.6% in 107 consecutive patients with COVID-19 admitted to the ICU during a 1-month period in 2020, which was significantly higher than the 6.1% incidence of PE for the 196 patients admitted to the ICU during the same interval in 2019, despite similar severity of illness scores. Helms et al16 reported an 11.7% incidence of PE in COVID-19 ARDS compared with a 2.1% incidence of PE in a historical prospective cohort of patients with non-COVID-19 ARDS.
Critically ill patients in the ICU with COVID-19 show significantly higher rates of VTE and thrombosis than patients with COVID-19 on the wards. Klok et al17 reported a 31% incidence of thrombotic events in 184 critically ill patients, 81% of the thrombotic events being PE. Piazza et al18 reported that 35.3% of ICU patients experienced major arterial or VTE, whereas the rate was only 2.6% for patients on the wards. Notably, 77% of the DVTs reported in this study were associated with a catheter or device. In attempts to minimize recurrent health care team exposure, many institutions undertook high use of central venous catheters early in the pandemic, especially in the ICU.19 Helms et al16 noted that 28 of 29 patients with COVID-19 in the ICU who received continuous renal replacement experienced premature circuit clotting.
A significant percentage of the VTEs in patients with COVID-19 are diagnosed early in the hospital presentation. Mouhat et al20 reported a PE incidence of 27% in 349 hospitalized patients with COVID-19, of whom 20% were diagnosed at admission. Lodgiani et al13 noted a 21% cumulative rate of thromboembolic events, half occurring within the first 24 h of hospital admission. For patients hospitalized with COVID-19, rates of PE developing after hospital discharge are low, reported to be 2% within the first 6 weeks after discharge.21 COVID-19 hospitalization does not seem to increase the risk of VTE after discharge compared with hospitalization as a result of other acute medical illnesses.22
Systematic screening for VTE has been known to increase detection rates in patients without COVID-19.23 Voicu et al24 reported that 36% of mechanically ventilated patients with COVID-19 were diagnosed with DVT within 3 days after intubation when screened with compression ultrasonography. In patients with COVID-19 on the wards, Santoliquido et al25 demonstrated a DVT incidence of 12% with systematic screening, although the rate was 2.4% when counting only proximal DVT. Mirsadraee et al26 performed systematic whole-body CT scanning on 72 patients with COVID-19 on admission to the ICU, noting that 34 patients (47%) demonstrated PE, which had been suspected clinically in only 7%.
The presence of VTE in hospitalized patients with COVID-19 is associated with greater disease severity and increased mortality. Patients with PE more frequently require mechanical ventilation and ICU admission and have increased overall hospital length of stay.27 In more than 3,000 consecutive hospitalized patients with COVID-19 in a New York City hospital, after multivariate adjustment, both venous and arterial thrombosis were associated with increased mortality (adjusted hazard ratio, 1.82).28 It is unclear whether thrombosis is a direct cause of these worse outcomes or merely a marker of more severe disease.
Pathophysiologic Features
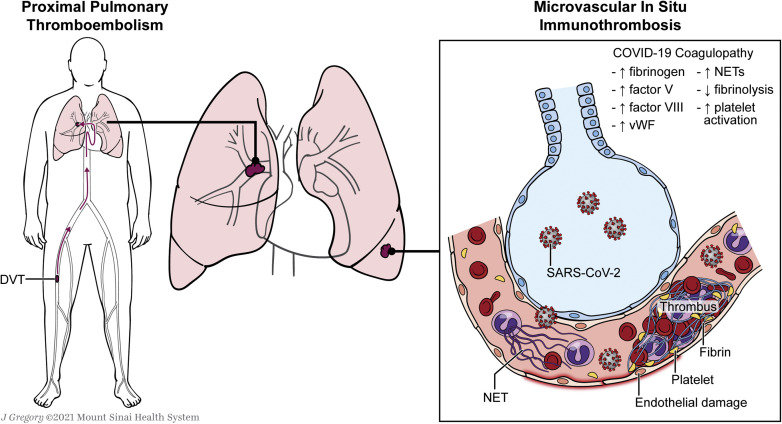
Considering that VTE rates in patients hospitalized with COVID-19 are significantly higher than in historical control participants, likely other thrombotic mechanisms beyond the classic VTE risk factors of immobility and severe illness are a factor.29 The predilection for thrombosis in COVID-19 is driven by at least two distinct, but interrelated, processes: a hypercoagulable state responsible for large-vessel thrombosis and thromboembolism and direct vascular and endothelial injury responsible for in situ microvascular thrombosis (Fig 1 ).30
Figure 1.
Illustrations demonstrating two different mechanisms for pulmonary thrombosis in COVID-19, which include large-vessel occlusion resulting from thromboembolism and microvascular in situ immunothrombosis resulting from direct vascular and endothelial injury. NET = neutrophil extracellular trap; vWF = von Willebrand factor.
Hypercoagulable State
It became evident early in the pandemic that patients with COVID-19 showed abnormal hemostasis profiles, elevated D-dimer being the most frequent abnormality.3 In a study of 2,377 hospitalized patients with COVID-19 in a New York hospital, 76% showed elevated D-dimer at presentation.31 D-dimer is a degradation product of fibrinolysis, and although a multitude of inflammatory processes can influence D-dimer levels, to some extent its elevation likely reflects intravascular thrombosis in patients with COVID-19.32 , 33 Elevated D-dimer has been shown to correlate with rates of thrombosis in COVID-19.20 , 27 , 28 Mouhat et al20 reported that a D-dimer of > 2,590 ng/mL was associated with a 17-fold increase in adjusted risk of PE in patients hospitalized with COVID-19. Li et al34 noted that a > 50% increase in D-dimer level during hospitalization was the strongest independent predictor of symptomatic VTE in patients with COVID-19. Elevated D-dimer levels also are associated independently with more severe disease and increased mortality in COVID-19.9 , 35
The biochemical coagulation phenotype in COVID-19 likely differs from disseminated intravascular coagulopathy (DIC) and sepsis-induced coagulopathy. DIC and sepsis-induced coagulopathy are consumptive coagulopathies characterized by low platelet counts, decreased plasma levels of clotting factors, and prolongation of prothrombin time.36 In contrast, neither platelet nor clotting factor consumption are common features in COVID-19, suggesting a different mechanism of coagulopathy in COVID-19.37 For example, Huang et al38 reported that in hospitalized patients with COVID-19, only 8% of patients in the ICU and 4% of patients not in the ICU showed platelet counts of < 100 × 109/L on admission. Helms et al16 reported that although > 95% of patients with COVID-19 in the ICU showed elevated D-dimer and fibrinogen levels, none demonstrated a positive International Society of Thrombosis and Haemostasis DIC score. Table 1 summarizes and compares the main coagulation parameters of DIC and COVID-19 coagulopathy.
Table 1.
Alterations of Hematologic Parameters in COVID-19 Coagulopathy and DIC
| Hematologic Parameter | COVID-19 Coagulopathy | DIC |
|---|---|---|
| D-dimer | ↑ | ↑ |
| Platelets | ↔ | ↓ |
| PT, aPTT | ↔ | ↑ |
| Fibrinogen | ↑ | ↓ |
| Thrombin | ↑ | ↑ |
| Factor VIII, factor V | ↑ | ↓ |
aPTT = activated partial thromboplastin time; DIC = disseminated intravascular coagulation; PT = prothrombin time.
Other commonly noted coagulation abnormalities in COVID-19 include dramatically increased thrombin production39 and elevated concentrations of both von Willebrand factor and factor V.16 , 40 Factor VIII, one of the more potent triggers of hypercoagulability, has been shown to be increased significantly in COVID-19.41 Thromboelastography studies of severe COVID-19 demonstrate rapid clot formation with impaired fibrinolysis.41 Additionally, platelets from patients with COVID-19 are activated more efficiently than are platelets from both healthy control participants and patients with non-COVID-19 ARDS.42
Immunothrombosis
Elevated markers of systemic inflammation, particularly C-reactive protein and IL-6, are observed commonly in patients with COVID-19.43 Extensive cross talk occurs between the immune and coagulation systems to provide effective host defense.44 Immune cells and inflammatory cytokines incite the development of immunothrombi, which consist of fibrin, monocytes, neutrophils, and platelets. By creating a sterile barrier against further pathogen invasion, these physiologic thrombi initially serve a protective purpose.45 , 46 However, dysregulation of thrombosis and inflammation can devolve into an injurious vicious cycle, leading to exuberant thrombosis with consequent organ dysfunction.33 , 47 The immune and coagulation systems also are linked via neutrophil extracellular traps, weblike structures of DNA decorated with antimicrobial proteins. Neutrophil extracellular traps are expelled from neutrophils to capture and immobilize pathogens physically and also can activate immunothrombosis.48 Neutrophil extracellular trap levels have been shown to be elevated in patients with COVID-19 when compared with control participants and also to correlate with disease severity.49
Endothelial Injury
Autopsy studies early during the pandemic revealed diffuse endothelial inflammation in many organs, including the lung, heart, liver, and kidney, with evidence of direct viral infection of endothelial cells by the SARS-CoV-2 virus.50 Because in vivo biosynthesis of von Willebrand factor is restricted to endothelial cells and megakaryocytes, high plasma von Willebrand factor concentrations in patients with COVID-19 suggest significant endothelial cell derangement.40 , 41 Immune cell arteritis was found in the lungs of nearly half of those who had died of COVID-19 in one autopsy series.51 Endothelial injury, particularly in the context of a hypercoagulable milieu, likely is responsible for the high rates of microthrombosis noted in the pulmonary vasculature. Although pulmonary microthrombosis has been noted previously in classical ARDS,52 the extent evident in COVID-19 is significantly greater. Ackerman et al6 noted that autopsies from patients with COVID-19 showed nine times more alveolar capillary microthrombi compared with autopsies from patients with ARDS secondary to H1N1 influenza. The high rates of microthrombosis are not limited to the lungs; they also have been reported in the heart53 and skin.54 Imaging studies demonstrate that thrombotic lesions in COVID-19 are smaller and more peripherally located compared with those in non-COVID acute PE, suggesting that some filling defects on CTPA, particularly isolated subsegmental PE, may reflect in situ pulmonary thrombosis instead of the typical embolization of thrombi originating from peripheral DVT.12 , 55 Mirsadraee et al26 reported that of the critically ill patients with COVID-19 found to have pulmonary thrombosis via screening CTPA, 77% did not have radiologic evidence of peripheral DVT.
Gas Exchange vs Lung Compliance
Early in the pandemic, Gattinoni et al7 noted that although many patients with COVID-19 technically fulfilled the Berlin criteria for ARDS, many showed marked hypoxemia and elevated shunt fraction with only minimally affected lung compliance, particularly early in the course of disease. Chiumello et al56 noted that venous admixture was unrelated to the fraction of nonaerated lung tissue in COVID-19 ARDS, yet was correlated to the fraction of nonaerated lung tissue in a historical cohort of patients with non-COVID ARDS who were matched for both Pao 2 to Fio 2 ratio and compliance. Additionally, many nonintubated patients with COVID-19 demonstrate dramatic hypoxemia, yet lack proportional signs of respiratory distress, a condition coined happy hypoxemia.57 Some have hypothesized that the presence of pulmonary thrombi, both microthrombi and macrothrombi, may help to explain the disconnect between gas exchange and lung compliance in severe COVID-19.58
Hemodynamic Perturbations
Pulmonary emboli increase pulmonary vascular resistance (PVR) and pulmonary artery pressure, with higher thrombotic burden correlating with higher PVR and pulmonary artery pressure.59 With sufficiently elevated right ventricular (RV) afterload, pulmonary emboli can induce RV dilation and dysfunction.60 Somewhat strikingly, patients with COVID-19 requiring mechanical ventilation show invasive hemodynamic profiles that are characterized by low, not high, PVR.61 This finding is surprising given the high prevalence of PE in patients with COVID-19 in the ICU, as well as the high prevalence of elevated PVR in non-COVID ARDS.62
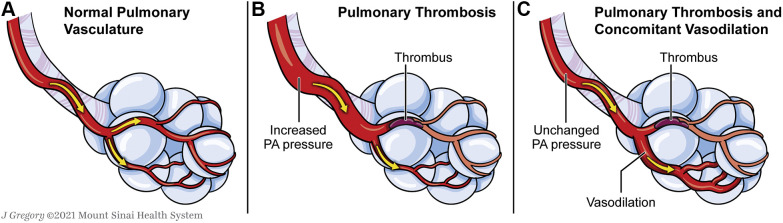
It is possible that the hemodynamic effect of pulmonary thrombosis is mitigated by a primary pulmonary vasodilatory process in some patients with COVID-19. Dual-energy CT imaging has demonstrated pulmonary vessel dilation in COVID-19 pneumonia.63 Ackerman et al,6 in addition to demonstrating high rates of pulmonary microthrombosis in COVID-19, also noted high rates of intussusceptive and sprouting angiogenesis. Reynolds et al64 reported that 83% of mechanically ventilated patients with COVID-19 showed positive bubble study findings as assessed by contrast-enhanced transcranial Doppler imaging, likely indicative of abnormal pulmonary capillary dilation, pulmonary arteriovenous malformations, or both. Additionally, the degree of transpulmonary bubble transit correlated with Pao 2 to Fio 2 ratio, suggesting that these pulmonary vascular dilations may be a significant cause of hypoxemia in COVID-19 ARDS. Whereas pulmonary macrothrombi and microthrombi increase PVR, pulmonary vasodilation decreases PVR; when both processes occur simultaneously, each can “cancel out” the hemodynamic effect of the other (Fig 2 ).65 The coexistence of both obliterative and vasodilatory processes in the pulmonary vasculature is reminiscent of what can occur in chronic liver disease, specifically portopulmonary hypertension (obliterative) and hepatopulmonary syndrome (vasodilatory).66 Ultimately, in COVID-19 ARDS, the obliterative processes may dominate, leading to severe RV failure and cardiogenic shock.67
Figure 2.
Illustration showing how concomitant vasodilation can mitigate the hemodynamic effects of pulmonary thrombosis. A, Normal pulmonary vasculature. B, Pulmonary thrombosis, which increases pulmonary vascular resistance and leads to increased PA pressure. C, Concomitant pulmonary vasodilation potentially can “cancel out” the increases in pulmonary vascular resistance and PA pressure caused by pulmonary thrombosis. PA = pulmonary arterial.
Although the vasodilatory and obliterative processes may offset each other hemodynamically, their coexistence may amplify hypoxemia in COVID-19. Vasodilated regions experience increased blood flow, creating low / ratios. Microthrombi and vasoconstriction in other areas of the lung reroute additional blood flow to the vasodilated regions and drive down the / ratio further.65 Mathematical modeling demonstrates that the large amount of pulmonary venous admixture in the setting of relatively minor parenchymal involvement observed in early COVID-19 can be explained reasonably by a combination of pulmonary thrombosis and vasodilation.68
Diagnosis
In the absence of systematic screening, the diagnosis of PE begins with clinical suspicion. Unexplained dyspnea and hypoxemia, particularly in the setting of normal chest radiography findings, raises the clinical suspicion for PE.69 Clinical suspicion of PE in a patient with COVID-19 pneumonia often is diminished because the signs and symptoms of COVID-19 pneumonia mimic those of PE; a patient’s dyspnea and hypoxemia may be attributed solely to COVID-19 pneumonia, and further diagnostic testing for potential PE may be deferred. Although clinical probability scores, such as the Wells score,70 are helpful in raising clinical suspicion of PE in patients, they have not been validated in patients with COVID-19 and likely underestimate the probability of PE in COVID-19.71
D-dimer, in conjunction with clinical probability assessment, has great usefulness in ruling out PE in patients with low or intermediate probability of PE, although its usefulness in COVID-19 is unclear.72 Although D-dimer levels in COVID-19 correlate with rates of thrombosis, it is not clear whether a particular D-dimer value “rules in” or “rules out” PE. In the study by Mirsadraee et al26 in which screening CTPA was performed for patients with COVID-19 on admission to the ICU, D-dimer levels did not discriminate between patients with and without PE. Li et al34 developed a three-factor score consisting of admission fibrinogen, admission D-dimer, and D-dimer increment > 1.5 fold, the score performing with a sensitivity of 0.93 and specificity of 0.71 for symptomatic VTE.
CTPA is the first-choice method for the diagnosis of PE because of its high accuracy, wide availability, and ability to assess for other pulmonary pathologic features. Its use may be limited in critically ill patients with COVID-19 who are not stable enough for transfer and in patients with renal failure, a common complication in severe COVID-19.73 / scanning can be used for patients in whom CTPA is contraindicated or inconclusive. Scans performed on patients with abnormal chest radiography findings, as is often the case in patients with COVID-19 pneumonia, are more likely to result in false-positive results because the images rarely appear as normal or showing a low probability of PE in such patients. Compression ultrasonography can be performed to assess for DVT when chest imaging is contraindicated or indeterminate; however, the absence of DVT does not imply the absence of pulmonary thrombosis, especially because in situ pulmonary thrombosis is a potential mechanism in COVID-19. A diagnosis of DVT may eliminate the need to evaluate for PE because the indication for therapeutic anticoagulation will have been established. Echocardiography can raise suspicion for the diagnosis of PE with the presence of clot in the right side of the heart or new right heart strain. Although it has limited diagnostic value, echocardiography is most useful for risk stratification of confirmed PE.74
Prophylaxis
Considering that PE is one of the most common preventable causes of hospital death, thromboprophylaxis is a crucial component in the care of hospitalized patients,75 and COVID-19 is no exception. A retrospective study by Rentsch et al76 demonstrated that the administration of prophylactic anticoagulation within 24 h of admission in patients with COVID-19 was associated with decreased mortality when compared with no prophylactic anticoagulation. Multiple society guidelines recommend prophylactic anticoagulation for hospitalized patients with COVID-19 who do not have a contraindication to treatment.77 , 78 However, standard doses of prophylactic anticoagulation likely are insufficient for the prevention of VTE in patients with COVID-19; many VTE events are diagnosed within the first 24 h after admission,13 and the reported rates of VTE are notably high despite the use of anticoagulant thromboprophylaxis.15 , 17 , 24
As a result of the high rates of VTE despite standard-dose thromboprophylaxis, many institutions have implemented protocols using higher doses, including an intermediate dose and even a therapeutic dose. Tacquard et al11 reported in a study of 538 patients with COVID-19 in eight French ICUs that high-dose prophylactic anticoagulation (intermediate or therapeutic dose) was associated with a significant reduction in thrombotic complications (hazard ratio, 0.81) without an increase in bleeding risk. In more than 4,000 patients with COVID-19 at a New York hospital with an aggressive anticoagulation protocol, Nadkarni et al10 noted a trend toward a mortality reduction with therapeutic anticoagulation compared with prophylactic anticoagulation, a finding that did not meet statistical significance (P = .08).
However, recent randomized controlled trials do not support the use of higher than standard doses for prophylactic anticoagulation in critically ill patients with COVID-19. In 600 critically ill patients, intermediate-dose anticoagulation with enoxaparin 1 mg/kg daily was not superior to standard prophylactic anticoagulation with enoxaparin 40 mg daily in reducing the composite outcome of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 days. Although bleeding events were rare, major and clinically relevant nonmajor bleeding events were nonsignificantly more frequent with intermediate-dose anticoagulation (6.2% for intermediate dose, 3.1% for standard dose; P = .08).79 A large National Institutes of Health multiplatform randomized controlled trial incorporating three global networks (A Randomised, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired Pneumonia [REMAP-CAP], Anti-Thrombotic Therapy to Ameliorate Complications of COVID-19 [ATTACC], and Accelerating COVID-19 Therapeutic Interventions and Vaccines-4A [ACTIV-4A]) examining the benefit of therapeutic dose vs standard dose prophylactic anticoagulation in more than 1,000 critically ill patients with COVID-19 discontinued enrollment because statistical criteria for futility were met. Importantly, despite therapeutic anticoagulation decreasing major thrombotic events, an 89% probability was found that therapeutic anticoagulation was inferior to standard dose prophylactic anticoagulation in achieving the primary outcome of survival or days free of organ support.80 The mechanism for this likely harm is unclear, given that major bleeding was increased only mildly with therapeutic anticoagulation (3.1% vs 2.4%). These findings suggest that initiating therapeutic anticoagulation after severe COVID-19 has developed may be too late to alter the clinical course beneficially. In contrast, for moderately ill hospitalized patients with COVID-19, the National Institutes of Health recently announced via press release that therapeutic-dose anticoagulation was superior to standard-dose prophylactic anticoagulation in reducing the need for organ support and mortality.81 The full results and official publication of these studies are awaited anxiously because they undoubtedly will help to establish the optimal anticoagulation dosing strategies for patients with COVID-19.
Treatment
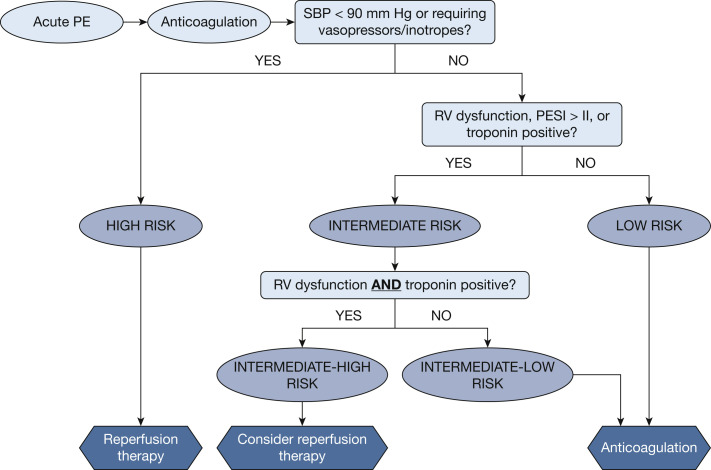
Anticoagulation is the mainstay of the treatment for acute PE, both for patients with and without COVID-19, to prevent further thrombosis and thromboembolism.74 Initial treatment options for anticoagulation include unfractionated heparin, low-molecular-weight heparin, fondaparinux, and, in low-risk patients, direct oral anticoagulants. As is the case with non-COVID-19 PE, risk stratification is the central tool used to identify patients at increased risk of early death who may benefit from reperfusion therapy (ie, thrombolysis or embolectomy), mechanical circulatory support, or both. Per the European Society of Cardiology guidelines,74 high-risk PE is characterized by cardiac arrest, systolic BP < 90 mm Hg, or requiring vasopressors, inotropes, or both. Intermediate-risk PE is characterized by normotension with signs of RV dysfunction on echocardiography or CTPA, elevated troponin levels, or an elevated PE severity index score. Of note, the role of troponin levels as a prognostic biomarker for PE in COVID-19 is confounded by the fact that troponin frequently is elevated in patients with COVID-1982; in that context, it likely reflects myocardial inflammation, cardiac microthrombosis, or both, rather than RV pressure overload. Low-risk PE is characterized by normotension, lack of RV dysfunction, and a low PE severity index score. Figure 3 summarizes the European Society of Cardiology risk stratification algorithm and treatment strategy.
Figure 3.
Risk-stratification algorithm and treatment strategy, adapted from the European Society of Cardiology Guidelines.75 Reperfusion therapy includes thrombolysis and embolectomy. PE = pulmonary embolism; PESI = pulmonary embolism severity index; RV = right ventricular; SBP = systolic BP.
If possible, patients with high-risk PE should undergo reperfusion therapy, mechanical circulatory support, or both. Patients with intermediate-risk PE should be monitored closely for signs of clinical deterioration, with select patients proceeding to reperfusion therapy.74 Given the risk of viral transmission from transporting patients with COVID-19 to operating rooms and invasive laboratories, the use of procedural-based therapies (eg, surgical embolectomy, catheter-based therapies) may be limited in patients with PE and COVID-19. Ultimately, the use of PE response teams can aid in providing multidisciplinary recommendations and rapid mobilization of resources.83
Given the potential pathophysiologic role of pulmonary microthrombosis, thrombolysis has been used in small case series of COVID-19 ARDS. Although these reports note improvement in hypoxemia, dead-space ventilation, and hemodynamics in patients with COVID-19 ARDS, the therapeutic responses seem to be short-lived.58 , 84 It is possible that concomitant anticoagulation during the administration of a thrombolytic is necessary to prevent immediate rethrombosis.58 Currently, randomized trials evaluating the use of tissue plasminogen activator (ClinicalTrials.gov Identifier: NCT04357730) and tenecteplase (ClinicalTrials.gov Identifier: NCT045055920) in patients with COVID-19 ARDS are underway.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Other contributions: The author thanks Jill Gregory, MFA, Icahn School of Medicine at Mount Sinai, for creating the illustrations in this article.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phelan A.L., Katz R., Gostin L.O. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323(8):709–710. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 3.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lax S.F., Skok K., Zechner P., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadkarni G.N., Lala A., Bagiella E., et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(16):1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tacquard C., Mansour A., Godon A., et al. Impact of high dose prophylactic anticoagulation in critically ill patients with COVID-19 pneumonia. Chest. 2021;159(6):2417–2427. doi: 10.1016/j.chest.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jimenez D., García-Sanchez A., Rali P., et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2021;159(3):1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piroth L., Cottenet J., Mariet A.-S., et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9(3):251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poissy J., Goutay J., Caplan M., et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 16.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piazza G., Campia U., Hurwitz S., et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilonzo N., Rao A., Soundararajan K., et al. The importance of a centralized line service during the COVID-19 pandemic. J Vasc Surg. 2020;72(2):403–404. doi: 10.1016/j.jvs.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mouhat B., Besutti M., Bouiller K., et al. Elevated D-dimers and lack of anticoagulation predict PE in severe COVID-19 patients. Eur Respir J. 2020;56(4):2001811. doi: 10.1183/13993003.01811-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall J., Myall K., Lam J.L., et al. Identifying patients at risk of post-discharge complications related to COVID-19 infection. Thorax. 2021;76(4):408–411. doi: 10.1136/thoraxjnl-2020-215861. [DOI] [PubMed] [Google Scholar]
- 22.Roberts L.N., Whyte M.B., Georgiou L., et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136(11):1347–1350. doi: 10.1182/blood.2020008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierce C.A., Haut E.R., Kardooni S., et al. Surveillance bias and deep vein thrombosis in the national trauma data bank: the more we look, the more we find. J Trauma. 2008;64(4):932–936. doi: 10.1097/TA.0b013e318166b808. discussion 936-937. [DOI] [PubMed] [Google Scholar]
- 24.Voicu S., Bonnin P., Stépanian A., et al. High prevalence of deep vein thrombosis in mechanically ventilated COVID-19 patients. J Am Coll Cardiol. 2020;76(4):480–482. doi: 10.1016/j.jacc.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santoliquido A., Porfidia A., Nesci A., et al. Incidence of deep vein thrombosis among non-ICU patients hospitalized for COVID-19 despite pharmacological thromboprophylaxis. J Thromb Haemost. 2020;18(9):2358–2363. doi: 10.1111/jth.14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirsadraee S., Gorog D.A., Mahon C.F., et al. Prevalence of thrombotic complications in ICU-treated patients with coronavirus disease 2019 detected with systematic CT scanning. Crit Care Med. 2021;49(5):804–815. doi: 10.1097/CCM.0000000000004890. [DOI] [PubMed] [Google Scholar]
- 27.Bompard F., Monnier H., Saab I., et al. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J. 2020;56(1):2001365. doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilaloglu S., Aphinyanaphongs Y., Jones S., et al. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson F.A., Jr., Spencer F.A. Risk factors for venous thromboembolism. Circulation. 2003;107(23 suppl 1):I9–I16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 30.Iba T., Levy J.H., Levi M., Connors J.M., Thachil J., et al. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020;48(9):1358–1364. doi: 10.1097/CCM.0000000000004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger J.S., Kunichoff D., Adhikari S., et al. Prevalence and outcomes of D-dimer elevation in hospitalized patients with COVID-19. Arterioscler Thromb Vasc Biol. 2020;40(10):2539–2547. doi: 10.1161/ATVBAHA.120.314872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linkins L.A., Takach Lapner S. Review of D-dimer testing: good, bad, and ugly. Int J Lab Hematol. 2017;39(suppl 1):98–103. doi: 10.1111/ijlh.12665. [DOI] [PubMed] [Google Scholar]
- 33.Price L.C., McCabe C., Garfield B., Wort J. Thrombosis and COVID-19 pneumonia: the clot thickens! Eur Respir J. 2020;56(1):2001608. doi: 10.1183/13993003.01608-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J.Y., Wang H.F., Yin P., et al. Clinical characteristics and risk factors for symptomatic venous thromboembolism in hospitalized COVID-19 patients: a multicenter retrospective study. J Thromb Haemost. 2021;19(4):1038–1048. doi: 10.1111/jth.15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iba T., Levy J.H., Raj A., Warkentin T.E. Advance in the management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Clin Med. 2019;8(5):728. doi: 10.3390/jcm8050728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umemura Y., Yamakawa K., Kiguchi T., Nishida T., Kawada M., Fujimi S. Hematological phenotype of COVID-19-induced coagulopathy: far from typical sepsis-induced coagulopathy. J Clin Med. 2020;9(9):2875. doi: 10.3390/jcm9092875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranucci M., Sitzia C., Baryshnikova E., et al. Covid-19-associated coagulopathy: biomarkers of thrombin generation and fibrinolysis leading the outcome. J Clin Med. 2020;9(11):3487. doi: 10.3390/jcm9113487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panigada M., Bottino N., Tagliabue P., et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaid Y., Guessous F., Puhm F., et al. Platelet reactivity to thrombin differs between patients with COVID-19 and those with ARDS unrelated to COVID-19. Blood Adv. 2021;5(3):635–639. doi: 10.1182/bloodadvances.2020003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamilton D.O., Pizzi R., Ageno W., Welters I.D. Hypercoagulopathy in severe COVID-19: implications for acute care. Thromb Haemost. 2020;120(12):1654–1667. doi: 10.1055/s-0040-1721487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antoniak S., Mackman N. Multiple roles of the coagulation protease cascade during virus infection. Blood. 2014;123(17):2605–2613. doi: 10.1182/blood-2013-09-526277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thachil J., Srivastava A. SARS-2 coronavirus-associated hemostatic lung abnormality in COVID-19: is it pulmonary thrombosis or pulmonary embolism? Semin Thromb Hemost. 2020;46(7):777–780. doi: 10.1055/s-0040-1712155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colling M.E., Kanthi Y. COVID-19-associated coagulopathy: an exploration of mechanisms. Vasc Med. 2020;25(5):471–478. doi: 10.1177/1358863X20932640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217(6):e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Middleton E.A., He X.-Y., Denorme F., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dorward D.A., Russell C.D., Um I.H., et al. Tissue-specific Immunopathology in Fatal COVID-19. Am J Respir Crit Care Med. 2021;203(2):192–201. doi: 10.1164/rccm.202008-3265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vesconi S., Rossi G.P., Presenti A., Fumagalli R., Gattinoni L. Pulmonary microthrombosis in severe adult respiratory distress syndrome. Crit Care Med. 1988;16(2):111–113. doi: 10.1097/00003246-198802000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Bois M.C., Boire N.A., Layman A.J., et al. COVID-19-associated nonocclusive fibrin microthrombi in the heart. Circulation. 2021;143(3):230–243. doi: 10.1161/CIRCULATIONAHA.120.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shehi E., Chilimuri S., Shin D., Patel M., Ali N., Niazi M. Microthrombi in skin biopsy of a patient with COVID-19. JAAD Case Rep. 2020;6(12):1327–1329. doi: 10.1016/j.jdcr.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Dam L.F., Kroft L.J.M., van der Wal L.I., et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb Res. 2020;193:86–89. doi: 10.1016/j.thromres.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiumello D., Busana M., Coppola S., et al. Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: a matched cohort study. Intensive Care Med. 2020;46(12):2187–2196. doi: 10.1007/s00134-020-06281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tobin M.J., Laghi F., Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202(3):356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poor H.D., Ventetuolo C.E., Tolbert T., et al. COVID-19 critical illness pathophysiology driven by diffuse pulmonary thrombi and pulmonary endothelial dysfunction responsive to thrombolysis. Clin Transl Med. 2020;10(2):e44. doi: 10.1002/ctm2.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azarian R., Wartski M., Collignon M.A., et al. Lung perfusion scans and hemodynamics in acute and chronic pulmonary embolism. J Nucl Med. 1997;38(6):980–983. [PubMed] [Google Scholar]
- 60.Matthews J.C., McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev. 2008;4(1):49–59. doi: 10.2174/157340308783565384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caravita S., Baratto C., Di Marco F., et al. Haemodynamic characteristics of COVID-19 patients with acute respiratory distress syndrome requiring mechanical ventilation. An invasive assessment using right heart catheterization. Eur J Heart Fail. 2020;22(12):2228–2237. doi: 10.1002/ejhf.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bull T.M., Clark B., McFann K., Moss M., National Institutes of Health/National Heart, Lung, and Blood Institute ARDS Network Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2010;182(9):1123–1128. doi: 10.1164/rccm.201002-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lang M., Som A., Mendoza D.P., et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. 2020;20(12):1365–1366. doi: 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reynolds A.S., Lee A.G., Renz J., et al. Pulmonary vascular dilatation detected by automated transcranial Doppler in COVID-19 pneumonia. Am J Respir Crit Care Med. 2020;202(7):1037–1039. doi: 10.1164/rccm.202006-2219LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reynolds A.S., Lee A.G., Renz J., et al. Reply to Cherian et al.: positive bubble study in severe COVID-19 indicates the development of anatomical intrapulmonary shunts in response to microvascular occlusion. Am J Respir Crit Care Med. 2021;203(2):265–266. doi: 10.1164/rccm.202009-3404LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krowka M.J. Hepatopulmonary syndrome and portopulmonary hypertension: the pulmonary vascular enigmas of liver disease. Clin Liver Dis (Hoboken) 2020;15(suppl 1):S13–S24. doi: 10.1002/cld.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Creel-Bulos C., Hockstein M., Amin N., Melhem S., Truong A., Sharifpour M., et al. Acute cor pulmonale in critically ill patients with Covid-19. N Engl J Med. 2020;382(21):e70. doi: 10.1056/NEJMc2010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herrmann J., Mori V., Bates J.H.T., Suki B. Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia. Nat Commun. 2020;11(1):4883. doi: 10.1038/s41467-020-18672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stein P.D., Terrin M.L., Hales C.A., et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest. 1991;100(3):598–603. doi: 10.1378/chest.100.3.598. [DOI] [PubMed] [Google Scholar]
- 70.Wells P.S., Anderson D.R., Rodger M., et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416–420. [PubMed] [Google Scholar]
- 71.Kirsch B., Aziz M., Kumar S., et al. Wells score to predict pulmonary embolism in patients with coronavirus disease 2019. Am J Med. 2021;134(5):688–690. doi: 10.1016/j.amjmed.2020.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Righini M., Van Es J., Den Exter P.L., et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311(11):1117–1124. doi: 10.1001/jama.2014.2135. [DOI] [PubMed] [Google Scholar]
- 73.Hirsch J.S., Ng J.H., Ross D.W., et al. Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Konstantinides S.V., Meyer G., Becattini C., et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41(4):543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 75.Kahn S.R., Lim W., Dunn A.S., et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rentsch C.T., Beckman J., Tomlinson L., et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372:n311. doi: 10.1136/bmj.n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spyropoulos A.C., Levy J.H., Ageno W., et al. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moores L.K., Tritschler T., Brosnahan S., et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158(3):1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.INSPIRATION Investigators. Sadeghipour P., Talasaz A.H., Rashidi F., et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goligher E.C., Bradbury C.A., McVery B.J. Therapeutic anticoagulation in critically ill patients with Covid-19—preliminary report. medRxiv. 2021 doi: 10.1101/2021.03.10.21252749. [DOI] [Google Scholar]
- 81.National Institutes of Health Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. January 22, 2021. National Institutes of Health website. https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients Accessed February 26, 2021.
- 82.Lala A., Johnson K.W., Januzzi J.L., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kabrhel C., Rosovsky R., Channick R., et al. A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest. 2016;150(2):384–393. doi: 10.1016/j.chest.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 84.Wang J., Hajizadeh N., Moore E.E., et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18(7):1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]