Abstract
The infectious power of coronaviruses is dependent on cholesterol present in the membranes of their target cells. Indeed, the virus enters the infected cell either by fusion or by endocytosis, in both cases involving cholesterol-enriched membrane microdomains. These membrane domains can be disorganized in-vitro by various cholesterol-altering agents, including statins that inhibit cell cholesterol biosynthesis. As a consequence, numerous cell physiology processes, such as signaling cascades, can be compromised. Also, some examples of anti-bacterial and anti-viral effects of statins have been observed for infectious agents known to be cholesterol dependent. In-vivo, besides their widely-reported hypocholesterolemic effect, statins display various pleiotropic effects mediated, at least partially, by perturbation of membrane microdomains as a consequence of the alteration of endogenous cholesterol synthesis. It should thus be worth considering a high, but clinically well-tolerated, dose of statin to treat Covid-19 patients, in the early phase of infection, to inhibit virus entry into the target cells, in order to control the viral charge and hence avoid severe clinical complications. Based on its efficacy and favorable biodisposition, an option would be considering Atorvastatin, but randomized controlled clinical trials are required to test this hypothesis. This new therapeutic proposal takes benefit from being a drug repurposing, applied to a widely-used drug presenting a high efficiency-to-toxicity ratio. Additionally, this therapeutic strategy avoids any risk of drug resistance by viral mutation since it is host-targeted. Noteworthy, the same pharmacological approach could also be proposed to address different animal coronavirus endemic infections that are responsible for heavy economic losses.
Keywords: Covid-19, Coronavirus, Cholesterol, Membrane microdomains, Statins, Drug repurposing
Graphical abstract
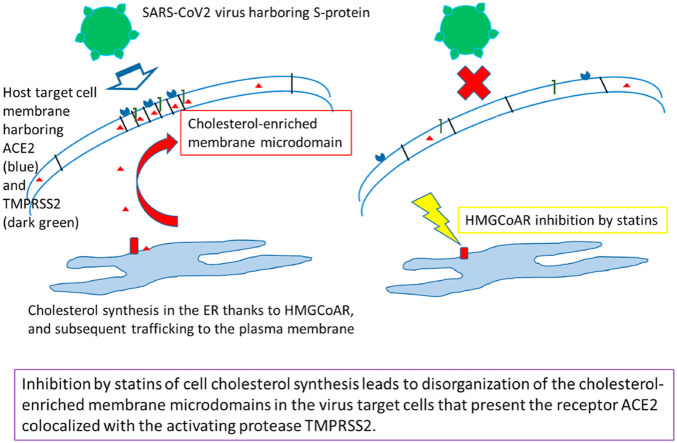
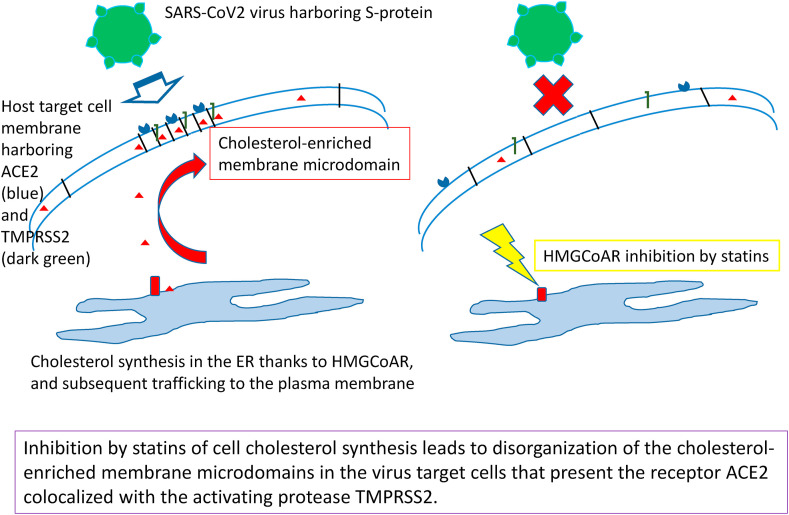
Significance: « short abstract » Covid-19 treatment could benefit from repurposing strategies for already used drugs, allowing a rapid clinical development. SARS-CoV-2 virus enters its target cells by interacting with their plasma membrane receptor, ACE2, localized in raft-type cholesterol-enriched lipid domains. It is here proposed to take benefit from the well-documented anti-cholesterol effect of the statin family, which would allow to disorganize these cholesterol-enriched membrane microdomains. Statins are widely-used safe drugs for long-term prevention of cardiovascular risk, thus presenting a high efficiency-to-toxicity ratio. This new therapeutic, host-targeted approach has the further advantage to avoid any drug resistance due to possible virus mutations.
Abbreviations
- ABC
ATP-binding cassette
- ACE2
angiotensin converting enzyme-2
- AIBV
avian infectious bronchitis virus
- APN
aminopeptidase
- N; BKV, BK
virus
- C/F/HCoV
canine/feline/human coronavirus
- CEACAM1
carcinoembryonic antigen cell adhesion molecule-1
- coronaV
coronaviruses
- CYP
cytochrome P450
- DPP4
dipeptidyl peptidase-4
- EGF(R)
epidermal growth factor (receptor)
- eNOS
endothelial NO synthase
- ER
endoplasmic reticulum
- ERGIC
endoplasmic reticulum-Golgi intermediate compartment
- FIPV
feline infectious peritonitis virus
- GPCR
G protein-coupled receptor
- HCV
hepatitis C virus
- HoFH
homozygous familial hypercholesterolemia
- HIV
human immunodeficiency virus
- HMGCoAR
hydroxymethylglutaryl coenzyme A reductase
- LDL(R)
low-density lipoproteins (receptor)
- LPS
lipopolysaccharides
- LOX1
lectin-like oxidized LDL-1
- MβCD
methyl-β-cyclodextrin
- MHV
murine hepatitis virus
- MERS
Middle-East respiratory syndrome
- OATP
organic anion transporting polypeptides
- PEDV
porcine epidemic diarrhea virus
- PHEV
porcine hemagglutinating encephalomyelitis virus
- PRRSV
porcine reproductive and respiratory virus
- PTGEV
porcine transmissible gastro-enteritis virus
- SAMS
statin-associated muscle symptoms
- SARS
severe acute respiratory syndrome
- SLC
small ligand/solute carriers
- SREBP
sterol regulatory element-binding protein; St, statin
- TEM
tetraspanin-enriched microdomains
- TGN
trans-Golgi network
- TLR4
Toll-like receptor-4
- TMPRSS2
transmembrane protease serine subfamily-2
1. Introduction: coronaviruses and the interest of host targeting through a membrane biophysical approach
The present Covid-19 pandemic has revealed to the world the unexpected pathogenicity of the new virus SARS-CoV-2. This has shed light on the whole coronaviridae family, although some members of this family have been rather well previously described. Along with various veterinary infections in wild or farmed animals, essentially mammals, four human strains have been reported to cause common cold and benign flu in young adult (strains OC43 and 229E), with infrequent pulmonary complications (strains NL63 and HKU1), besides rare severe hemorrhagic diarrhea in newborn [1]. In 2003 in China, the serious epidemic episode of atypical pulmonary infections introduced the novel virus SARS-CoV1, followed in 2012 in some other countries by the related MERS-CoV that provoked the “Middle-East respiratory syndrome”, with an even higher fatality rate although remaining fairly limited in case number. These two related viruses, together with SARS-CoV-2, are responsible with a rather high frequency for a typical “severe acute respiratory syndrome”, leading to oxygen therapy and intensive care supports. As a matter of fact, these viruses are capable to conjugate a very high infectiousness with potentially intense clinical alterations, SARS-CoV-2 being the most successful up to now to well adapt to the host by mutagenesis, considering its virulence and worldwide spreading. Virologist community thus started to investigate them more deeply, and began to reveal their biological and molecular characteristics [2,3]. Among their various properties underlying their unusual pathogenic power, there are a noticeable molecular plasticity and adaptability (which has allowed the virus to bypass species barrier) along with a multifunction capability of some viral proteins as well as some possibilities of functional supplementation between different viral proteins, optimizing viral metabolism and giving flexibility and robustness to the replication cycle.
These characteristics point to the difficulty to specifically target a viral protein to inhibit virus replication, taking into account that new mutants possibly inducing resistances to specific pharmacological agents can always emerge. It is therefore worth considering the alternative possibility of targeting the host, focussing on the specific interactions between the virus components and the infected cell during the viral replication cycle. Among the successive steps of the virus cycle, the entry, the intracellular formation of the replication complexes accompanied by membrane remodeling, and the budding of the neo-formed virions are all different membrane phases that are complex molecular events, involving a number of biochemical components with fine regulations. These membrane steps may thus represent valuable pharmacological opportunities [4]. This approach can be related to the concept of “membrane lipid therapy”, as recently proposed in the context of anticancer therapy [5]. Therefore, a membrane biophysical approach could be fruitful when combined with the more classical strategies coming from molecular and cellular biology. Especially, such an approach is expected to avoid the risk of pharmacological resistance that could be due to a pertinent, selection-induced mutation of a specifically targeted viral protein.
We therefore focus this review on the dependence of coronaV cell entry, and hence infectivity, on membrane cholesterol in the target cells, providing a sound basis for considering statins as potential agents able to modulate it. Indeed, these inhibitors of cell cholesterol biosynthesis are well observed in-vitro to disorganize the cholesterol-rich membrane domains, such as rafts and caveolae. The possibility of in-vivo application of this property relies on the widely-reported “pleiotropic effects” of statins (i.e. independent of the decreased circulating LDL-cholesterol), which are attributed, at least in part, to perturbations of cell and membrane cholesterol. This can thus be extrapolated to a promising inhibiting effect on coronaV cell entry, which may provide, thanks to statins repurposing, a new therapeutic strategy that will deserve being tested by preclinical assays and clinical studies.
2. Coronaviruses and cholesterol: the infectivity of the different coronaV depends on membrane cholesterol in the target cells
2.1. Entry: cell-surface fusion and endocytosis, both dependent on plasma membrane cholesterol
Numerous experimental studies have addressed the biology of coronaV infections of various animals, especially farmed species because of the heavy economic losses caused by epidemic events. The remarkable point is that, for all these studied animal strains of coronaV, the sensitivity to infection of in-vitro cultured cells presents a clear dependence on cholesterol. In a first set of pioneering experiments, cholesterol supplementation of the culture medium of cells infected by the murine hepatitis virus (MHV) can induce an increased virus-mediated cell fusion and modulate virus persistence in the cells [[6], [7], [8]]. Alternatively, treatment of cultured cells by the cholesterol-chelating agent methyl-β-cyclodextrin (MβCD) regularly induces a decrease of virus entry and infection when challenged by the MHV [8,9], the porcine transmissible gastro-enteritis virus (PTGEV) [10,11], the porcine reproductive and respiratory syndrome virus (PRRSV), the porcine epidemic diarrhea virus (PEDV) [12,13], the porcine hemagglutinating encephalomyelitis virus (PHEV) [14], the avian infectious bronchitis virus (AIBV) [15], the type I (but not type II) feline infectious peritonitis virus (FIPV or FCoV) [16,17], the canine enteric virus (CECoV) [18] or the canine respiratory virus (CRCoV) [19], as well as by the human HCoV-229E [20], HCoV-OC43 [21] and SARS-CoV1 [[22], [23], [24], [25]]. The analysis of the underlying mechanism strongly indicated the involvement of membrane microdomains enriched in, and dependent on cholesterol, whatever their precise molecular composition [26], whose integrity appears necessary for virus internalization into the target cell. These membrane domains have been identified as lipid rafts in the case of SARS-CoV1 [23,25], and to caveolae (revealed by caveolin-1) in the case of AIBV [15], PEDV [13], PTGEV [27], CRCoV [19], HCoV-229E [20], HCoV-OC43 [21] and SARS-CoV1 [24]. The process for cell entry of the virus via these cholesterol-dependent membrane domains has been attributed to receptor-mediated endocytosis in some cases, either depending on clathrin (MHV, PHEV, PEDV, PTGEV, HCoV-NL63, SARS-CoV1) [13,14,[27], [28], [29], [30]] or not (FCoV, HCoV-OC43, SARS-CoV1) [17,21,25], but most often depending on dynamin [13,17,19,21,27,30]. In the case of such delayed endocytic pathway, the virus entry is due to the fusion of its membrane with that of endosomes induced by the local acidification. However, the cell entry of the virus has also been reported to follow a direct entry pathway thanks to a process of membrane fusion at the cell surface, especially in the cases of the human viruses [31,32].
The mechanisms of “fusion” for virus entry into the target cell is made possible by the fact that the coronaV are enveloped viruses, whose viral lipids can mix with the host plasma membrane lipids when they are in proper contact. This mixing step requires the specific interaction of the Spike glycoprotein (“S protein”) harbored by the virion surface with its corresponding cell receptor, ACE2 in case of SARS-CoV1/CoV-2 and HCoV-NL63, DPP4 (=CD26) for MERS-CoV, APN (=CD13) for HCoV-229E and various animal coronaV strains, O-acetylated sialic acid for HCoV-OC43 and HKU1, CEACAM1 for MHV [2,3,8,[33], [34], [35], [36], [37], [38]]. In all cases, the S protein is composed of two subdomains (S1 and S2) with distinct functions, S1 being responsible for the initial recognition and binding to the cell receptor, and S2 for the subsequent step of lipid fusion. This important step of fusion of viral and cell membranes is allowed by a large and complex S2 protein transconformation induced by local cell proteases, including furin-type proprotein convertases for the priming cleavage (S1/S2 site) and the transmembrane serine protease TMPRSS2 for the triggering cleavage (S2’ site) releasing the fusion protein [2,[33], [34], [35], [36],[38], [39], [40], [41]]. The point is that this necessary structural, protein and lipid reorganization is cholesterol dependent [33,42], including when considering SARS-CoV1 [41] and SARS-CoV-2 [43,44]. In addition, the two protein partners, ACE2 and TMPRSS2, of this specific interaction are colocalized in cholesterol-rich membrane domains, giving them their functional specificity, since this connectivity is decisive for virus entry (and hence infectivity) [9,22,45]. Also, the cell proteases required for virus entry have been shown to co-segregate with some tetraspanins, including CD9 for MERS-CoV and HCoV-229E, leading to the proposition that tetraspanin-enriched microdomains (TEM) constitute membrane platforms suited for the virus entry [34,46,47]. This makes sense since these TEM are known to be enriched in cholesterol [48,49].
As a whole, coronaV can enter the target cells both by endocytosis and by “early” membrane fusion, depending on the considered virus strain, the cell type and the host cell factors [33,41], and finally it appears that the fusion mechanism is more efficient, especially in humans [32,34,41]. However, the remarkable point is that these two processes are both dependent on the cholesterol content within the host cell plasma membrane.
2.2. Intracellular membrane remodeling and budding
The viral lipid envelope sometimes contains cholesterol, which suggests that the virus budding can occurs from a cholesterol-enriched membrane [18]. However, treatment before infection of the virus envelope using MβCD has an influence on the infectivity only in some cases, depending on the animal virus strain [12,50]. For the human strains, coronaV are reported as budding at the level of a specific intracellular membrane compartment forming an intermediate between endoplasmic reticulum and Golgi, the “ERGIC” [2,51,52], a region thus containing rather low level of cholesterol. This budding process is accompanied by an important reorganization of these internal membrane compartments, with the formation of a reticulo-vesicular network, “convoluted” membranes, vesicle packets, and double-membrane vesicles, which are thought to nest the viral replication complexes [2,3,51,53,54]. Besides, the trans-Golgi network (TGN), richer in cholesterol, is believed to be an obligatory step for the trafficking of M protein [55], which represents the “driving force” of the viral assembly, due to its oligomerization ability possibly leading to membrane curvature, as well as its association with N protein [52]. In addition, E protein, while contributing to membrane curvature with M protein, and otherwise facilitating the intracellular traffic of S protein [56], can form a pentameric ionic channel, viroporin, whose function and selectivity depend on the local conditions, especially lipid composition (including cholesterol) [57,58]. However, within the current knowledge, it appears that the membrane-linked events involved in the intracellular steps of the replication cycle are not stringently dependent on cholesterol, although some cholesterol influence on the assembly and/or the budding phase cannot be discarded, as observed for various other viruses [59,60].
Otherwise, statins have been reported to have possible antiviral effects on some viruses, but they recover a variety of different mechanisms of action, often involving various signaling cascades, most often downward the virus entry step, and generally independent of cholesterol-containing membrane domains [61]. Alternatively, it has been proposed that a highly sensitive step to be pharmacologically targeted in the replication cycle of an enveloped virus is its cell entry [62], which involves lipid rafts in the case of coronaV [63,64]. It is worth emphazing here that, beyond the presence of ACE2 within the plasma membrane rafts, the meaningful point is the cholesterol dependence of its colocalization with TMPRSS2. In conclusion, the most pertinent effect of cholesterol on coronaV infectivity relies on its entry phase, when the virus interacts with the host cell plasma membrane cholesterol-rich domains, and we thus address the question of a possible action of statins at that level.
3. In-vitro effects of statins on cholesterol-enriched membrane microdomains: rafts can be altered by statins
All the above-mentioned characteristics on the cellular replication cycle of coronaV show that it would be desirable to alter the cell cholesterol, especially in the plasma membrane, in order to control its cell entry and hence the deleterious effects induced by the viral infectious properties. There exists some biochemical agents (e.g. MβCD, filipin, saponin, cholesterol oxidase) aimed at efficiently perturbing membrane cholesterol in-vitro by altering its concentration or distribution in raft-type microdomains, hence altering their associated functions [[65], [66], [67], [68]]; unfortunately, these molecules would be too toxic to be used in-vivo, especially in clinics. However, this is not the case for a family of related molecules that is well-known for a wide use as preventive drugs, thus fairly safe, in cardio-vascular risk patients: the statins (chemical structures in Ref. [69]).
3.1. Cellular effects: alteration of various cell signaling pathways
Statins are biochemically defined as (competitive) inhibitors of HMGCoAR (hydroxy-methyl-glutaryl CoenzymeA reductase), the limiting step enzyme in the biosynthesis pathway of cholesterol. As a consequence of the decreased synthesis rate of cholesterol in cultured cells treated in-vitro by a statin, their membrane lipid rafts, and other cholesterol-enriched microdomains, are disorganized, and hence lose their various functionalities [61]. Noteworthy, at the level of the cell, either cultured in the presence of cholesterol or in in-vivo situation exposed to circulating lipoproteins, the exogenous cholesterol, bound to these lipoproteins and internalized by receptor-mediated endocytosis, mainly accumulates within the internal membranes, while cholesterol in the plasma membrane is mainly of endogenous origin [70,71], and is thus preferentially perturbed by the action of statins.
Among the numerous cellular effects of statins that have been observed involving functional alterations of cholesterol-dependent membrane domains, a few of them will be described here as illustrating examples of signaling platform perturbations, with raft disruption as the proven underlying mechanism. It should be stated at this stage that the inhibition of HMGCoAR also leads to a decreased synthesis rate of the non-cholesterol branches of the mevalonate pathway that produce protein prenylations and other various acylations. Since protein acylation is a common post-transcriptional regulation mechanism of membrane proteins involved in signaling cascades, it can often produce various cellular effects, typically playing a role in cell growth, thus potentially in tumor biology. Thus any statin-induced cell perturbation must be carefully analyzed to ascertain that it is mediated by its action at the level of the membrane rafts or caveolae, since these structures are actually forming active signaling platforms. Several pertinent examples are found in the literature, such as the perturbation of the Akt signaling cascade: SimvaSt lowered raft cholesterol content in prostate cancer cells [72] and in A431 epidermoid carcinoma cells [73], and in leukemia cells where the rescue was obtained by adding mevalonate or squalene, but not isoprenoid compounds [74]. Similarly, in pancreas cancer cells, LovaSt deregulated CD133/FAK signaling by disrupting the lipid rafts, leading to chemosensitization [75]. In EGF signaling pathway, LovaSt and AtorvaSt depleted lipid rafts of cholesterol and altered raft location of EGFR, recovering EGF signaling in breast cancer cells [76], while MevaSt activated EGFR signaling through disruption of caveolae in keratinocytes [77], and SimvaSt suppressed the phosphorylation of EGFR in astrocytes [78]. As another example of a typical situation, AtorvaSt has been shown to modulate the recruitment of signaling molecules (Lck) into the lipid rafts of T cells that constitute the immunological synapse [79]. Similarly, AtorvaSt also inhibited the recruitment of the LPS receptor, TLR4, into the lipid rafts of pro-B cells, leading to an anti-inflammatory response that could be rescued by mevalonate [80]. Also, FluvaSt inhibited Fcγ receptor signaling in monocyte rafts, which was rescued by mevalonate, whereas the use of prenyl transferase inhibitors was without effect, thus indicating the involvement of these cholesterol-rich membrane domains [81]. Finally, PravaSt (at the low concentration of 0.5 μM) counteracted the deleterious effect of exposure to benzo(a)pyrene and ethanol of hepatic cells and of obese zebrafish larvae liver, which was mediated by membrane remodeling altering cholesterol-containing rafts that mediate inflammatory signaling [82].
3.2. Anti-infectious effects: some examples with tentative extrapolations to in-vivo treatments
The lipid rafts have also been reported as membrane platforms mediating cell entry of various, bacterial as well as viral, pathogens [4,59,83].
3.2.1. Anti-bacterial effects
The cell internalization of the obligatory intracellular bacteria, Helicobacter, into gastric cells requires lipid rafts, and statins have been shown to decrease this internalization, as followed by the decreased delivery of the virulence factor, CagA [84]. In addition, treatment by statins of macrophages infested by Listeria [85] or by Mycobacterium [86] leads to an increase of the phagosomal degradation of the bacteria as a consequence of the decreased cell cholesterol. This last case of anti-infectious effect has also been demonstrated in-vivo on a murin model.
3.2.2. Anti-viral effects: HCV, BKV, and animal CoV as the “missing link”
In hepatocytes infected by the HCV, viral replication occurs at the Golgi level, with budding in the TGN that recruits raft-like membrane domains. This reveals the cholesterol dependence of the viral cycle, which can be inhibited by treatment of the infected cells with a statin (5 μM LovaSt) [87]. Among the different statins tested in-vitro to inhibit HCV replication, FluvaSt showed the best efficacy, followed by AtorvaSt and SimvaSt, all in the μM range [88]. On the basis of these in-vitro data, the administration of a conventional dose of AtorvaSt used to reduce hypercholesterolemia has been tested on infected patients who were otherwise hyperlipidemic. However, no antiviral effect could be observed, but it could be objected that the applied dose could have been insufficient taking into account the metabolic profile of these patients [89]. In contrast, a high dose of FluvaSt (20–320 mg/d) has been observed to induce transient reductions of the viral load for periods of few weeks [90].
BKV virus, from the SV40 family, is chronically present in the urinary tract in latent form, and can be reactivated in an opportunistic manner in grafted kidneys as a consequence of the required immunomodulating treatments, questioning the functional prognosis of the transplantation. Virus internalization into the kidney cells occurs by caveolar endocytosis [91]. Treatment of these infected cells by PravaSt, which disorganizes the caveolae by downregulating caveolin-1, either directly or by decreasing membrane cholesterol, has an antiviral effect more marked when the drug is given at an early phase of the infection [92]. This in-vitro effect is the basis for proposing statins as a new therapeutic approach of this infection, for which no satisfactory treatment exists [93].
Regarding coronaV, there is one report of using a statin, MevaSt, to disorganize membrane rafts with an antiviral effect, hence indicating their involvement in the viral cycle of AIBV [15]. This in-vitro effect is similar to that obtained using MβCD, and is focused on the viral cell entry phase, but not the replication or the budding phases. At this stage, this report constitutes the precious “missing link” between the cholesterol dependence of coronaV infectivity, including in particular the SARS-CoV1 [[22], [23], [24], [25]], and the anti-infectious effects displayed by statins against various other cholesterol-dependent pathogens.
For HCV and BKV, these anti-viral in-vitro data have set the bases for proposing statins as a new therapeutic approach possibly applied in-vivo, for preclinical or/and clinical assays (that would need to be further investigated), which thus could be also proposed for coronaV.
4. From the treatment of hypercholesterolemia to the various “pleiotropic” clinical effects: in-vivo effects of statins on cholesterol transport and intramembrane distribution
4.1. Hypocholesterolemic effect and upregulation of LDLR
The in-vivo mechanisms of action of statins have been widely addressed [69,[94], [95], [96]]. Statins are the first-line treatment for hypercholesterolemia in patients at cardio-vascular risk. Their action relies on decreasing the level of circulating LDL-cholesterol, this decrease being the consequence of a positive regulation of the LDLR induced by the cholesterol sensor, SREBP, present in the ER where cholesterol synthesis is inhibited, mainly in the hepatocytes, hence promoting LDL clearance. But this is not the sole mechanism of action, as demonstrated by the only partial, but significant, effect of statins when treating patients with homozygous familial hypercholesterolemia (HoFH) that are devoid of LDLR [[97], [98], [99]], although it could be argued that this is linked to some residual LDLR [100]. Anyway, this specific statin effect points to the relevance of a direct effect of cholesterol synthesis inhibition at the level of the whole cells in the organism.
4.2. Pleiotropic effects and the involvement of cell and membrane cholesterol
When used in clinics, statins also exhibit various biological effects besides the decreased level of circulating cholesterol [69,95]. These widely-reported “pleiotropic effects” mainly include immuno-modulation, anti-inflammatory, anti-thrombotic, and anti-oxidant properties, and are considered for their beneficial side-effects in various therapeutic applications in cardio-vascular diseases. The underlying mechanisms, generally addressed using various cell models, appear rather multiple and entangled, since these pleiotropic effects can be, at least in part, mediated by alteration of the expression and function of various prenylated membrane proteins that are often involved in signaling processes in various cell types. However, based on above-mentioned in-vitro observations [79,80,101,102], the various biological effects of statins, such as immuno-modulating and anti-inflammatory effects, and also anti-oxidant effects, appear often mediated by perturbations of cell signaling relying on cholesterol-enriched membrane domains. Indeed, aside perturbing rafts as signaling platforms, the disruption of these domains was also reported to inhibit cell-surface expression of MHC-II and other immuno-modulatory proteins [103]. Also regarding immunocompetent cells, NK cytotoxic function was decreased by SimvaSt or FluvaSt, and this was correlated with membrane raft alteration, in a similar manner to a treatment by MβCD but not by isoprenyl transferase inhibition [104]. Regarding endothelial cells, AtorvaSt was shown on HUVEC to upregulate anti-oxidative lipid raft proteins, while downregulating CRP associated with inflammation and cell adhesion [105]. Also in endothelial cells, LovaSt and AtorvaSt induced inhibition of LOX-1, the receptor to oxidized LDL, by disruption of caveolin-containing lipid rafts as a consequence of cholesterol reduction, which highly decreased the receptor fraction associated to these membrane domains [101]. Furthermore, in coronary arterial endothelium, PravaSt and SimvaSt altered the redox signaling pathway induced by oxidized LDL thanks to the simultaneous inhibition of the association of the NADPH oxidase subunits and of the eNOS repression by caveolin-1, in both cases by disrupting the membrane rafts due to lowering membrane cholesterol [102].
The various pleiotropic effects of statins can also rely on more general mechanisms of perturbation of various cholesterol-containing membrane domains, such as caveolae and TEM, and even cell cholesterol, with alteration of endocytosis among other consequences. In particular, cholesterol perturbations can induce changes of expression of caveolins that thereby promote various alterations of cell biochemical cascades, thus producing a variety of cellular effects. As a matter of fact, SimvaSt decreases caveolin-1 expression in cultured castration-resistant prostate cancer cells [106], PravaSt induces in-vitro dysregulation of various gene expression in cancer cells correlated with a decreased caveolae density [107], and SimvaSt decreases caveolin-3 expression and caveolae number in cultured cardiac myocytes with accompanying changes in calcium fluxes and NO production [108]. Furthermore, statins cause changes in the cholesterol distribution between different membrane domains: PravaSt is able to shift cholesterol from caveolae to “flat rafts” in monocytes ex-vivo cultured after myocardial infarction to a level similar to that observed in healthy donors [109]. Also, beyond affecting caveolin signaling, statins also perturb various cholesterol-enriched membrane domains, as shown by the induced redistribution of the LPS receptor, CD14, from “classical” rafts to TEM, with as a consequence an anti-inflammatory effect on cultured macrophages [110]. Finally, statins can alter endocytosis, known to be cholesterol-dependent, both morphologically in smooth muscle cells at the level of Golgi with an effect similar to that of cholesterol oxidase [111], and functionally as shown for the GPCR serotonin receptor 1A that switches from a clathrin-mediated to a caveolae-mediated pathway due to cholesterol depletion [112]. More generally regarding the functional importance of cell cholesterol, as an example, MevaStat as well as zaragozic acid (inhibiting only cholesterol synthesis) can resensitize cultured AM leukemia cells to radio and chemotherapy by decreasing the protecting effect of a high level of cell cholesterol [113].
4.3. Tentative extrapolation from in-vitro data to clinical situation of statin action mechanisms involving cholesterol-rich membrane domains
Globally, all these cellular effects reported for statins on various cell models allowed to delineate their underlying processes, and provide convergent indications that cell and/or membrane cholesterol is involved as the common molecular factor, whatever the biological alteration observed downward. It should be acknowledged that, in some cases, such a cholesterol-dependent mechanism is not exclusive from other molecular mechanisms, such as alteration of cell signaling by inhibition of isoprenylation [95,114]. However, the common mechanistic link based on membrane cholesterol provides a pertinent unifying mode of action underlying many pleiotropic statin effects.
As a direct attempt to evidence an in-vitro/in-vivo correlation, functional perturbations of lymphocytes have been assayed on cultured cells and parallel ex-vivo collection after oral statin treatment, and compared for membrane raft alteration in both situations [115]: 40 mg/d SimvaSt induced lower effects than 1 μM in the culture medium, with a quantity of rafts somehow decreased in the ex-vivo determination, although without statistical significance, showing the experimental difficulties of such an approach. However, these data qualitatively show that, in that range, the in-vitro concentration vs in-vivo dose values of statin can produce comparable molecular effects on membrane rafts.
It is finally noteworthy that these multiform statin effects and their underlying mechanisms are clearly dependent on the cell type considered [107,113,116]. In addition, mechanistic analyses are often hampered by the use of various experimental conditions, including in particular different statin concentrations used in-vitro and in-vivo [117], and different weight doses for animals and humans [118]. However, as a whole, it appears that the statins present a complex clinical pharmacology with entangled underlying mechanisms, which are nevertheless more or less mediated by perturbations of cellular cholesterol pools relevant in functional membrane domains, that can be observed even for short duration treatments. Actually, to conclude from the presented data, the existence of such cholesterol-dependent pleiotropic effects witnesses, and provides strong indication, that statins are able to efficiently alter plasma membrane cholesterol in clinical situation.
5. Proposed anti-Covid19 repurposing for statins
5.1. Biological and pharmacological rationale
The analysis of the above-exposed biological and pharmacological data prompt us to consider that statins could be used as a “ready-to-use” pharmacological tool to fight against Covid-19, and it should be desirable to propose it as a drug family to be tested in the early phase of viral infection (see Scheme 1 ). Indeed, as detailed above: (i) SARS-CoV-2 cell entry is cholesterol dependent due to the location of its specific receptor, ACE2, in membrane rafts, (ii) the in-vitro cell entry, and hence infectivity, of a related animal coronaV, AIBV, can be inhibited by a statin, and (iii) statins can display various in-vivo effects that are relied on functional perturbations of cholesterol-enriched plasma membrane domains. It can thus be fairly hypothesized that in-vivo administered statins could exhibit an anti-infectious effect on SARS-CoV-2 by altering its cholesterol-dependent entry into the target cells (see Scheme 2 ). It should be pointed that this therapeutic strategy is designed for the control of the viral dissemination in the target tissues, i.e. essentially the respiratory tract, but not only, as the gastro-intestinal tract [119] and the endothelia [[120], [121], [122], [123]] are also typically concerned. Indeed, it is noteworthy that, besides pulmonary epithelium, endothelium is perhaps the most relevant cellular target for an antiviral action of statins because it is a primary viral target, since it expresses both ACE2 and TMPRSS2 [120], leading to an endotheliitis that can induce the suffering of various organs as secondary complications. Obviously, statins should thus be used as early as possible in the infection course, and of course before secondary organ failures and other various health status alterations and complications of the infection, which may occur in particular in case of delayed dysregulation of the induced immune response (the “cytokine storm”). As above recalled, statins are a family of widely-used drugs, recognized as rather safe and well tolerated for long-term treatments: their few side-effects are well described, and can be addressed and followed conveniently [95]. Thus, statins typically form a pharmacological family that can be easily proposed for a repurposing strategy to treat, using a short-term administration, a new and potentially severe disease like Covid-19 in the absence of any other efficient treatment.
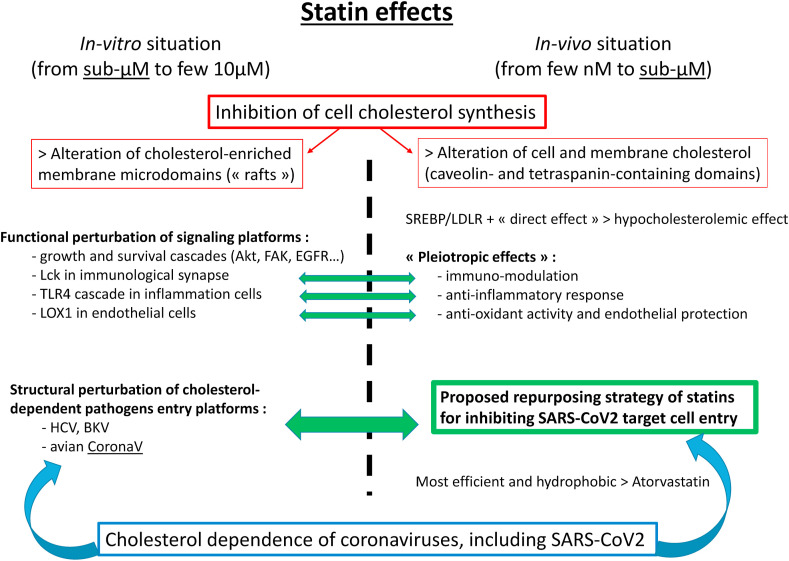
Scheme 1.
The « take-home message »
Overview of the different underlying biological data supporting the proposed repurposing strategy for statins against SARS-CoV-2 infection. Note that in-vitro and in-vivo (preclinical and clinical) situations for statin effects both can occur in the sub-micromolar concentration range.
Scheme 2.
« Graphical abstract »
Simplified cartoon describing the mechanism of action underlying the proposed repurposing of statins for fighting against Covid-19 by inhibiting SARS-CoV-2 entry into the target cells.
5.2. Clinical compatibility
Some in-vivo experiments have shown that statins can induce an up-regulation of the expression level of ACE2 in treated animals [[124], [125], [126]], which may frighten about their sensitivity to the virus. However, the Covid-19 infection can induce by itself a down-regulation of this receptor [119,127], and such possible statin-induced up-regulation probably does not preclude the clinical use of statins under these conditions [119,[127], [128], [129], [130], [131]], especially taking into account the various beneficial cardio-vascular protective effects well acknowledged to statins [69,95,129,[132], [133], [134], [135]]. As a matter of fact, ACE2 hydrolyzes angiotensin II, inducing vasodilatation and decreased oxidative stress, with therefore an endothelium protective action. Globally, statins have been shown to exhibit immuno-modulation properties with anti-inflammatory effects, and to improve vascular endothelial function with an anti-thrombotic effect, two main features that can have positive effects on worse outcome Covid-19 forms (see below §7.1 for such use of statins in the specific frame of symptomatic treatment of the severe complications). In addition, taking into account the large number of cardio-vascular patients receiving long-term statin treatment, some recent retrospective observational clinical studies have been performed to assess a possible repercussion on the infection course. The current evidence from some separate retrospective studies in Covid-19 patients has shown that a baseline statin treatment was associated with a lower severity of disease [[136], [137], [138]] and a reduced mortality [[139], [140], [141], [142], [143], [144], [145], [146]], although two studies did not find any association between exposure to statins and the outcome of Covid-19 infection [147,148]. Furthermore, some meta-analyses have been recently performed, globally revealing an overall 27–50% reduction, with respect to the control patients, in the pooled risk of developing severe form/mortality from Covid-19 [[149], [150], [151], [152]], with the evidence of a marked patient heterogeneity in the various observations [151,152], in particular considering the qualitative difference between pre-admission and in-hospital use of statins [150,153]. Although precious and fortunate, these correlation data are very global, gathering various statin molecules and administered doses (more or less efficient on the treated hypercholesterolemia). And, overall, these data cannot decipher the underlying mechanism and its causality, addressing either the initial viral phase or the subsequent immunological and vascular complications since statins are known for their anti-inflammatory and immuno-modulating properties. However, these preliminary data are encouraging for launching further specifically designed, randomized controlled clinical trials on Covid-19 patients without prior statin treatment.
5.3. Pharmacokinetics data for choosing the best-suited statin administration procedure
Beyond presenting a general paradigm supporting statins as potential inhibitors of coronaV cell entry, we would like to detail here some published data and rational arguments intended to provide guidelines to design and foster specific clinical trials that are demanded.
5.3.1. The recommended dose
As seen above, the pharmacological situation is not the same when comparing in-vitro and in-vivo observations, including the quantitative parameters of the active molecules of statin directed toward the target cells or tissues, i.e. concentration in the cell culture medium and dose administered to the animal or patient, respectively. Indeed, it is difficult to estimate the local concentration within target tissues of a statin administered at a given dose (a reported correspondence is 80 mg per daily dose into ∼0.2 μM in patient serum [127,154], consistent with a border-line effect on lymphocyte rafts observed with 40 mg/d SimvaSt [115]; however, plasma drug concentration is at best an approximate evaluation of the actual target tissue local concentration), and this requires a careful direct measurement when possible. Generally speaking, however, the in-vitro statin concentrations used are usually somehow higher, typically in the μM range (but it may be less [155]), than the corresponding estimated in-vivo efficient concentrations [117]. In order to expect a fair disorganizing effect on the cholesterol-dependent membrane domains sufficient to inhibit ACE2-dependent cell entry of the virus in infected patients, it should be thus desirable to administer the highest statin dose as possible that is compatible with its clinical safety range for a short duration (few days) treatment (see below §5.3.6).
5.3.2. The route of administration
In order to target the whole respiratory tract while limiting a systemic delivery, the drug could theoretically be delivered by a local route using nasal instillation. However, this route has never been considered for statins, and this would need a specific study on healthy volunteers prior to testing this protocol on infected patients. This has nevertheless been very recently proposed as associating nasal spray and mouth wash [156]. Similarly, the parenteral route, although allowing a better drug concentration control than oral route, has never been currently used for statins. The oral route will be thus preferred for a ready-to-use delivery.
5.3.3. The most efficient statin
When used in-vitro, the efficiencies of statins are rarely compared, except in one report showing that FluvaSt, AtorvaSt, SimvaSt and LovaSt display comparable potencies, in the 1–2 μM range [88]. Under the very different conditions prevailing in-vivo, the different statins display well-reported ranking for their seminal property of decreasing the circulating cholesterol level, the most efficient ones being RosuvaSt and AtorvaSt [69,95,157]. This clinical effect is also partly observed in HoFH patients devoid of LDLR, witnessing in that case the contribution of the “direct” biological effect of statins on decreasing cell cholesterol level, which is the effect that is expected to perturb the membrane domains responsible for SARS-CoV-2 infectious ability. Interestingly, the most efficient statins in that case are reported to be also RosuvaSt and AtorvaSt [99], and this observation could be attributed to the same underlying mechanism of HMGCoAR inhibition. This common molecular action is thus determining for ranking the different statins for their respective efficiencies to perturb membrane cholesterol. However, the influence of treatment duration is uncertain, and a short-term use could mitigate this ranking.
5.3.4. The less toxic statin
Since statins are to be delivered to infected patients at high doses, although for much shorter periods than for the “classical” chronic preventive treatments against cardio-vascular risks, the different statins should also be compared for their respective potencies to induce toxic side effects, in order to choose the safest one(s). The most often reported side-effect is myopathy (the so-called “statin-associated muscle symptoms”, SAMS), going from myalgia to rhabdomyolysis [95], and appearing in a wide time range after treatment start (with a median time of 1 month) [158]. This is attributed to an alteration of the intracellular level of ubiquinone (or coenzyme Q10), a non-cholesterol end-product of the mevalonate pathway, leading to inhibition of the respiratory chain and ultimately to mitochondrial suffering [159]. This mechanism, among others, establishes a basis for the tissue specificity, and overall allows to ascertain that statins can penetrate these cells. However, the different statins display various rankings, either correlated with their efficacy in blood cholesterol lowering (the lowest being FluvaSt), or with their hydrophobicity, or with their hepatic metabolisms, depending on the study [95], and hence there is no relevant basis for choosing one versus another from that property.
5.3.5. The target tissues
Hepatic toxicity, the other potential side-effect reported with possible myopathy risks, generally comes from statin-drug metabolic interactions coming from a common handling by cytochrome P450-3A4 (CYP-3A4), typically for LovaSt, SimvaSt, and AtorvaSt, or by CYP-2C9 for FluvaSt [95,160]. But statin-drug interactions can also be mediated by the influx transporters OATP-1B1 and 1B3, two related multidrug transporters of the SLC superfamily [161], essentially for the most hydrophilic statins, RosuvaSt and PravaSt [69,162], and also PitavaSt [163]. The involvement of these transporters indicates that cell internalization of statins could be limited in some cases, depending on the expression level of these transporters. Thus, the question of the possible access of statins to cells different from hepatocytes is raised since these transporters are reported to be mainly expressed in the liver. Otherwise, in the hepatocyte, the expression at the biliary pole of efflux transporters, such as the ABC multidrug transporters B1 (for LovaSt, SimvaSt, and AtorvaSt) [164] and G2 (for RosuvaSt) [162], providing another possible level for statin-drug interactions [95], could also contribute to limit the intracellular statin concentration. However, the above-mentioned possible toxic effects in muscle cells, as well as the description of the various clinical pleiotropic effects, show that statin biodisposition is not restricted to liver, but can reach various tissues and cell types, such as immunocompetent cells and endothelial cells. It can thus be finally expected that statins, at least the more hydrophobic/lipophilic ones that display fair passive diffusion capacity across cell membranes, can reach the intracellular pools of HMGCoAR in the various cell types that are targeted by SARS-CoV-2 in order to control its invasiveness and the ensuing clinical signs.
5.3.6. Tentative rational choice and practical recommendations
As a whole, the above considerations lead us to elaborate tentative recommendations for the most rational procedure suited for a clinical use. A high-efficacy and hydrophobic statin appears to be the optimal choice. This would lead for example to prefer AtorvaSt to RosuvaSt. Also, the highest available dose for the chosen statin should be prescribed, i.e. AtorvaSt 80 mg/day [157]. Practically, taking into account its half-life of 7–17 h [69,154,165] (but that of active metabolites may be much longer [166]) and the fact that the highest well-tolerated dose is superior to 80 mg/d [167], with even a possibility to administer up to 320 mg/d for few weeks [90], a regimen consisting in AtorvaSt 120 mg/d in two daily per os intakes (80 + 40 mg) could be proposed. The administration of a high-efficacy statin must be reserved to patients without significant liver function alteration, nor concomitant medications that may increase the risk of any drug-drug interaction. Finally, such administration should be accompanied by a close monitoring of any possible clinical and biochemical side-effects. Well-designed randomized clinical trials are necessary to test the safety and the efficiency of this new therapeutic procedure (see below §7).
6. Preliminary and complementary studies on animals
6.1. Preclinical animal models: macaques, Syrian hamsters, and mice
Launching clinical trials is not always easy, for various reasons, and a possible alternative is to turn to animal models allowing to test new therapeutic approaches. In the case of SARSCoV/CoV2 infection, there exists a non-human primate (macaque) model for the viral infection, without the further immunological alterations seen with some human patients [[168], [169], [170], [171], [172]]. So this model appears convenient for testing the possible antiviral effect of statins targeting the early infection phase. In addition, CYP-related statin metabolism is rather similar in macaque and in man [173,174], which renders this model fairly reliable considering its clinical pharmacological characteristics. This model has the further advantage to allow to test somehow higher statin doses in order to determine the acceptable upper limit to be administered, giving hence a better opportunity to detect a dose-dependence of the antiviral effects. This model has already been used for statin treatment, with AtorvaSt 10 mg/d, for studying as a pleiotropic effect the prevention by anti-inflammatory effect of the pulmonary arterial hypertension induced by HIV [175]. This animal model thus provides a valuable means for testing statin effects on Covid-19 before further clinical trials on infected patients.
In addition, it has been very recently reported that another relevant animal model is provided by Syrian hamsters [[176], [177], [178], [179]]. Unexpectedly, this is the animal (along with common marmoset) presenting the most analogous ACE2 amino acid sequence to the human one, just after the macaque [176,179]. The clinical, radiological and histological manifestations of the infection are very similar to the human form, except that there is no lethal issue to the spontaneous evolution of the disease. This small animal model has the advantage to be less expensive than the non-human primate models, and thus provides the possibility to test more easily new therapeutic options and new antiviral molecules, i.e. various administration regimens and various statin derivatives. Like for other rodent models, hamsters could most probably tolerate higher doses of the potentially toxic compounds to be evaluated. In the case of statins, the availability of this preclinical model should appear thus to be a fair opportunity to establish the “proof of concept” for its anti-Covid repurposing strategy.
Finally, it should be mentioned the recent reports of a mouse model for Covid-19, based on “humanized” transgenic mice expressing the human ACE2 to become sensitive to SARS-CoV-2 [[180], [181], [182]]. Infected mice, but not wild-type control mice, develop a pulmonary disease reminiscent of the human clinics. However, the detailed histological alterations depend on the expression level and tissue distribution of the exogenous hACE2. Therefore, this model could be proposed for preliminary preclinical assays to evaluate the potential antiviral effect of statins.
6.2. Related coronaviruses and veterinary clinics
Alternatively, the antiviral effect of statins on other animal models infected by related coronaV, whose cell entry also depends on cell membrane cholesterol, would be worth being evaluated. For example, MHV could give such a fair opportunity for addressing in-vivo potentialities of statins as a preliminary assay on mice. However, this model presents some difficulties for extrapolating the pharmacological data to human clinics: (i) in mice, cholesterol metabolism and transport in lipoproteins is different from humans [183]; (ii) MHV entry into target cells is realized by receptor-mediated endocytosis, not cell-surface membrane fusion, and the viral receptor is not ACE2 [3]; (iii) administered statin doses are much higher than in humans (in mg/kg) [117]. However, some preclinical trials of statin effects on cholesterol have been performed on mice [184], which could be considered as a first basis.
Nevertheless, in a veterinary clinic context, it could be proposed that the statin-based antiviral approach should be theoretically considered to fight against various farmed animal (e.g. piglets, poultry) epidemic infections due to different coronaV, which cause heavy economic losses in breeding industry.
7. Proposed clinical recommendations and trials
In order to decrease the viral load (thanks to the fact that statins are expected to be active on the cell entry step of the virus), and hence diminish the potential clinical signs, we may predict a higher statin efficiency if used at the early phase of the viral contamination. As a consequence, a high dose of a high-efficacy statin should be administered as soon as possible after a suspected contact with an infected person, especially if the subject has comorbidity, and this treatment delivered for the short duration necessary to limit the viral infection. This also applies for the patients already on a long-term preventive statin treatment for cardio-vascular risks, provided there is no contra-indication to switch to a high-dose statin, based on previous absence of SAMS and no concomitent medications prone to drug-drug interactions.
However, these clinical recommendations obviously require a first, careful and rigorous validation based on well-designed clinical trials. They must be randomized controlled trials, typically against a placebo. The potential benefit of the tested treatment should be evaluated by the infection outcome by comparison to the control group. A high-efficacy hydrophobic statin, such as AtorvaSt, would be preferred based on the previously exposed arguments regarding its efficiency and pharmacokinetic properties, but other statins would also be tested for comparison in this frame of short-term delivery, and the determination of the optimized dose to be administered for the short duration of this antiviral treatment would also be addressed.
8. Conclusion: present background and future prospects
8.1. Another (complementary) therapeutic use of statins: fighting against the late-phase complications (the “secondary cytokine storm”) of Covid-19
Within another pharmacological and clinical frame, statins have already raised some consideration for addressing the Covid-19 clinical complications, essentially at the pulmonary and cardio-vascular levels [119,127,[129], [130], [131], [132],134,135,185]. Indeed, it has been proposed to take benefit of the various pleiotropic clinical effects of statins, which mainly include: (i) an immuno-modulatory effect with anti-inflammatory action, mediated by inhibition of the NLRP3 inflammasome pathway via depression of the TLR4/MyD88/NF-κB/IL-6 cascade; (ii) an anti-thrombotic effect with endothelium protection, by inhibition of the ROCK pathway and thrombomodulin up-regulation. These conjugated effects delivered by statins could thus be positive and desirable in the context of SARS-CoV-2-induced clinical complications, and they would deserve to be clinically tested in intensive care units, although caution should be given to the risks of drug-drug interactions in this context. However, the non-specificity of this therapeutic approach is shown by the fact that it has already been proposed and tested on the rather similar complications caused by some other viral infections [186], such as MERS [187], and also Influenza [188,189] and Ebola [190,191].
8.2. A challenge for linking basic research and public health
The scope of this work is to provide a rational basis for proposing a new therapeutic approach for treating Covid-19 at an early phase of viral infection, thanks to a repurposing strategy conveniently available for the statin pharmacological family. This should be considered as a “working hypothesis”, but we feel it is supported by a set of published biological data sound enough so that it should inspire some trust. Actually, such a new use for statins has already been suggested or mentioned by others [119,130,131], but not addressed per se and only besides the more frequently proposed use, as mentioned above, for treating the secondary complications induced by the viral infection, which is another interesting and important, yet distinct issue. Here, we put the emphasis on the potential interest of developing a host-targeting strategy directed against the SARS-CoV-2, which consists in counteracting the virus entry into the infected cells by destabilizing and disrupting the cholesterol-enriched membrane domains forming the ACE2 receptor-mediated recognition and internalization platforms due to TMPRSS2 colocalization. The point is to take a therapeutic benefit of the “ménage à trois” configuration presented by coronaV, cholesterol and statins. In addition, since it is host-targeted, this strategy has the important advantage to preclude any risk of viral mutations of specific “druggable” proteins. However, we are aware that the only point that could be limiting for this pharmacological application is the relevancy of the local drug concentration aimed at efficiently inhibiting cell cholesterol synthesis. Extrapolations between in-vitro and in-vivo data are always difficult because of the multiparametric nature of the experimental conditions, and this is even more difficult when considering clinical situations. Indeed, some factors are poorly known and controlled, such as, for example, the tissue pattern of statin transporters expression and the pharmacological roles of the various statin metabolites (efficacy, potency, half-life …). This is the reason for recommending the administration of the highest possible dose of the most efficient, non-hydrophilic statin, AtorvaSt, to be likely in position to obtain at once a favorable action. Finally, the point is to consider the balance between the fairly acceptable “pharmacological risk” of statin intake and the “viral infection risk” that should be addressed. In particular, the practical question will be to delineate the patient sub-populations (i.e. asymptomatics, or with light signs, or hospitalized without or with severe signs) that will specifically benefit from such a statin treatment, taking into account the complication probabilities due to the infection. As a conclusion, it remains now to take the opportunity of “accepting the risk” to test in-vivo, in preclinical animal models or/and in clinical assays, this new pharmacological application, taking into account that the clinical pharmacology properties of statins are well known, hence leading to consider that this risk is actually minor compared to the world-wide public health concern we are presently facing.
Funding
This work did not receive any specific grant.
Author contributions
SO conceived and wrote the manuscript; JJM, AG, and EB reviewed and corrected the manuscript. All authors have approved the final text.
Declaration of competing interest
JJM has consulting activity for Mylan, Servier and Pfizer; AG has received personal fees for public speaking or consultancy support from Akcea Therapeutics, Amgen, MSD, Mylan, Novartis, Sanofi and Regeneron, Unilever; EB declares having received honoraria from Amgen, MSD, Sanofi and Regeneron, Danone, Aegerion, Chiesi, Rottapharm-MEDA, Servier, Ionis-pharmaceuticals, AKCEA, Mylan, GENFIT and Silence Therapeutic; the other author has no competing interests to declare.
Acknowledgements
Many thanks to J Bezin, F Bonnet, and JL Montastruc for electronic correspondance leading to interesting and helpful discussions in the early phase of this project. We gratefully acknowledge N Jamin (caveolae and caveolins) for careful reading of the manuscript providing useful comments and valuable linguistic corrections. We are also indebted to our other colleagues, F André (drug metabolism) and G Gaibelet (cell cholesterol), for critical reading the manuscript and further discussions.
References
- 1.Mammette A. Crouan & Roques; Lille, France: 1980. Virologie Médicale. [Google Scholar]
- 2.Hartenian E., Nandakumar D., Lari A., Ly M., Tucker J.M., Glaunsinger B.A. The molecular virology of coronaviruses. J. Biol. Chem. 2020;295:12910–12934. doi: 10.1074/jbc.REV120.013930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Oliva A., Ortega-Gonzalez P., Risco C. Targeting host lipid flows: exploring new antiviral and antibiotic strategies. Cell Microbiol. 2019;21 doi: 10.1111/cmi.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preta G. New insights into targeting membrane lipids for cancer therapy. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.571237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervin M., Anderson R. Modulation of coronavirus-mediated cell fusion by homeostatic control of cholesterol and fatty acid metabolism. J. Med. Virol. 1991;35:142–149. doi: 10.1002/jmv.1890350213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daya M., Cervin M., Anderson R. Cholesterol enhances mouse hepatitis virus-mediated cell fusion. Virology. 1988;163:276–283. doi: 10.1016/0042-6822(88)90267-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorp E.B., Gallagher T.M. Requirements for CEACAMs and cholesterol during murine coronavirus cell entry. J. Virol. 2004;78:2682–2692. doi: 10.1128/JVI.78.6.2682-2692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi K.S., Aizaki H., Lai M.M. Murine coronavirus requires lipid rafts for virus entry and cell-cell fusion but not for virus release. J. Virol. 2005;79:9862–9871. doi: 10.1128/JVI.79.15.9862-9871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren X., Glende J., Yin J., Schwegmann-Wessels C., Herrler G. Importance of cholesterol for infection of cells by transmissible gastroenteritis virus. Virus Res. 2008;137:220–224. doi: 10.1016/j.virusres.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin J., Glende J., Schwegmann-Wessels C., Enjuanes L., Herrler G., Ren X. Cholesterol is important for a post-adsorption step in the entry process of transmissible gastroenteritis virus. Antivir. Res. 2010;88:311–316. doi: 10.1016/j.antiviral.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon J.H., Lee C. Cellular cholesterol is required for porcine nidovirus infection. Arch. Virol. 2017;162:3753–3767. doi: 10.1007/s00705-017-3545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei X., She G., Wu T., Xue C., Cao Y. PEDV enters cells through clathrin-, caveolae-, and lipid raft-mediated endocytosis and traffics via the endo-/lysosome pathway. Vet. Res. 2020;51:10. doi: 10.1186/s13567-020-0739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z., Zhao K., Lan Y., Lv X., Hu S., Guan J., Lu H., Zhang J., Shi J., Yang Y., Song D., Gao F., He W. Porcine hemagglutinating encephalomyelitis virus enters neuro-2a cells via clathrin-mediated endocytosis in a Rab5-, cholesterol-, and pH-dependent manner. J. Virol. 2017;91 doi: 10.1128/JVI.01083-17. e01083-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo H., Huang M., Yuan Q., Wei Y., Gao Y., Mao L., Gu L., Tan Y.W., Zhong Y., Liu D., Sun S. The important role of lipid raft-mediated attachment in the infection of cultured cells by coronavirus infectious bronchitis virus beaudette strain. PloS One. 2017;12 doi: 10.1371/journal.pone.0170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takano T., Satomi Y., Oyama Y., Doki T., Hohdatsu T. Differential effect of cholesterol on type I and II feline coronavirus infection. Arch. Virol. 2016;161:125–133. doi: 10.1007/s00705-015-2655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Hamme E., Dewerchin H.L., Cornelissen E., Verhasselt B., Nauwynck H.J. Clathrin- and caveolae-independent entry of feline infectious peritonitis virus in monocytes depends on dynamin. J. Gen. Virol. 2008;89:2147–2156. doi: 10.1099/vir.0.2008/001602-0. [DOI] [PubMed] [Google Scholar]
- 18.Pratelli A., Colao V. Role of the lipid rafts in the life cycle of canine coronavirus. J. Gen. Virol. 2015;96:331–337. doi: 10.1099/vir.0.070870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szczepanski A., Owczarek K., Milewska A., Baster Z., Rajfur Z., Mitchell J.A., Pyrc K. Canine respiratory coronavirus employs caveolin-1-mediated pathway for internalization to HRT-18G cells. Vet. Res. 2018;49:55. doi: 10.1186/s13567-018-0551-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nomura R., Kiyota A., Suzaki E., Kataoka K., Ohe Y., Miyamoto K., Senda T., Fujimoto T. Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J. Virol. 2004;78:8701–8708. doi: 10.1128/JVI.78.16.8701-8708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owczarek K., Szczepanski A., Milewska A., Baster Z., Rajfur Z., Sarna M., Pyrc K. Early events during human coronavirus OC43 entry to the cell. Sci. Rep. 2018;8:7124. doi: 10.1038/s41598-018-25640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glende J., Schwegmann-Wessels C., Al-Falah M., Pfefferle S., Qu X., Deng H., Drosten C., Naim H.Y., Herrler G. Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2. Virology. 2008;381:215–221. doi: 10.1016/j.virol.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li G.M., Li Y.G., Yamate M., Li S.M., Ikuta K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microb. Infect. 2007;9:96–102. doi: 10.1016/j.micinf.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Y., Liu D.X., Tam J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 2008;369:344–349. doi: 10.1016/j.bbrc.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaibelet G., Tercé F., Allart S., Lebrun C., Collet X., Jamin N., Orlowski S. Fluorescent probes for detecting cholesterol-rich ordered membrane microdomains : entangled relationships between structural analogies in the membrane and functional homologies in the cell. AIMS Biophys. 2017;4:121–151. [Google Scholar]
- 27.Wang J., Li Y., Wang S., Liu F. Dynamics of transmissible gastroenteritis virus internalization unraveled by single-virus tracking in live cells. Faseb. J. 2020;34:4653–4669. doi: 10.1096/fj.201902455R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burkard C., Verheije M.H., Wicht O., van Kasteren S.I., van Kuppeveld F.J., Haagmans B.L., Pelkmans L., Rottier P.J., Bosch B.J., de Haan C.A. Coronavirus cell entry occurs through the endo-lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue Y., Tanaka N., Tanaka Y., Inoue S., Morita K., Zhuang M., Hattori T., Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 2007;81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milewska A., Nowak P., Owczarek K., Szczepanski A., Zarebski M., Hoang A., Berniak K., Wojarski J., Zeglen S., Baster Z., Rajfur Z., Pyrc K. Entry of human coronavirus NL63 into the cell. J. Virol. 2018;92 doi: 10.1128/JVI.01933-17. e01933-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millet J.K., Whittaker G.R. Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virology. 2018;517:3–8. doi: 10.1016/j.virol.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirato K., Kawase M., Matsuyama S. Wild-type human coronaviruses prefer cell-surface TMPRSS2 to endosomal cathepsins for cell entry. Virology. 2018;517:9–15. doi: 10.1016/j.virol.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Haan C.A., Rottier P.J. Hosting the severe acute respiratory syndrome coronavirus: specific cell factors required for infection. Cell Microbiol. 2006;8:1211–1218. doi: 10.1111/j.1462-5822.2006.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hantak M.P., Qing E., Earnest J.T., Gallagher T. Tetraspanins: architects of viral entry and exit platforms. J. Virol. 2019;93 doi: 10.1128/JVI.01429-17. e01429-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv. Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wedrowska E., Wandtke T., Senderek T., Piskorska E., Kopinski P. Coronaviruses fusion with the membrane and entry to the host cell. Ann. Agric. Environ. Med. 2020;27:175–183. doi: 10.26444/aaem/122079. [DOI] [PubMed] [Google Scholar]
- 39.Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitra P. Inhibiting fusion with cellular membrane system: therapeutic options to prevent severe acute respiratory syndrome coronavirus-2 infection. Am. J. Physiol. Cell Physiol. 2020;319:C500–C509. doi: 10.1152/ajpcell.00260.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meher G., Bhattacharjya S., Chakraborty H. Membrane cholesterol modulates oligomeric status and peptide-membrane interaction of severe acute respiratory syndrome coronavirus fusion peptide. J. Phys. Chem. B. 2019;123:10654–10662. doi: 10.1021/acs.jpcb.9b08455. [DOI] [PubMed] [Google Scholar]
- 43.Wang H., Yuan Z., Pavel M.A., Hansen S.B. bioRxiv; 2020. The Role of High Cholesterol in Age-Related COVID19 Lethality; p. 2020. 05.09.086249. [Google Scholar]
- 44.Wang S., Li W., Hui H., Tiwari S.K., Zhang Q., Croker B.A., Rawlings S., Smith D., Carlin A.F., Rana T.M. Cholesterol 25-Hydroxylase inhibits SARS-CoV-2 and other coronaviruses by depleting membrane cholesterol. EMBO J. 2020;39 doi: 10.15252/embj.2020106057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85:873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Earnest J.T., Hantak M.P., Li K., McCray P.B., Jr., Perlman S., Gallagher T. The tetraspanin CD9 facilitates MERS-coronavirus entry by scaffolding host cell receptors and proteases. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Earnest J.T., Hantak M.P., Park J.E., Gallagher T. Coronavirus and influenza virus proteolytic priming takes place in tetraspanin-enriched membrane microdomains. J. Virol. 2015;89:6093–6104. doi: 10.1128/JVI.00543-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemler M.E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 49.Yanez-Mo M., Barreiro O., Gordon-Alonso M., Sala-Valdes M., Sanchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19:434–446. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Jeon J.H., Lee C. Cholesterol is important for the entry process of porcine deltacoronavirus. Arch. Virol. 2018;163:3119–3124. doi: 10.1007/s00705-018-3967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stertz S., Reichelt M., Spiegel M., Kuri T., Martinez-Sobrido L., Garcia-Sastre A., Weber F., Kochs G. The intracellular sites of early replication and budding of SARS-coronavirus. Virology. 2007;361:304–315. doi: 10.1016/j.virol.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ujike M., Taguchi F. Incorporation of spike and membrane glycoproteins into coronavirus virions. Viruses. 2015;7:1700–1725. doi: 10.3390/v7041700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gosert R., Kanjanahaluethai A., Egger D., Bienz K., Baker S.C. RNA replication of mouse hepatitis virus takes place at double-membrane vesicles. J. Virol. 2002;76:3697–3708. doi: 10.1128/JVI.76.8.3697-3708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knoops K., Kikkert M., Worm S.H., Zevenhoven-Dobbe J.C., van der Meer Y., Koster A.J., Mommaas A.M., Snijder E.J. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6:e226. doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perrier A., Bonnin A., Desmarets L., Danneels A., Goffard A., Rouille Y., Dubuisson J., Belouzard S. The C-terminal domain of the MERS coronavirus M protein contains a trans-Golgi network localization signal. J. Biol. Chem. 2019;294:14406–14421. doi: 10.1074/jbc.RA119.008964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westerbeck J.W., Machamer C.E. The infectious bronchitis coronavirus envelope protein alters Golgi pH to protect the spike protein and promote the release of infectious virus. J. Virol. 2019;93:e00015–19. doi: 10.1128/JVI.00015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westerbeck J.W., Machamer C.E. A coronavirus E protein is present in two distinct pools with different effects on assembly and the secretory pathway. J. Virol. 2015;89:9313–9323. doi: 10.1128/JVI.01237-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chazal N., Gerlier D. Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. 2003;67:226–237. doi: 10.1128/MMBR.67.2.226-237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heaton N.S., Randall G. Multifaceted roles for lipids in viral infection. Trends Microbiol. 2011;19:368–375. doi: 10.1016/j.tim.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gorabi A.M., Kiaie N., Bianconi V., Jamialahmadi T., Al-Rasadi K., Johnston T.P., Pirro M., Sahebkar A. Antiviral effects of statins. Prog. Lipid Res. 2020;79 doi: 10.1016/j.plipres.2020.101054. [DOI] [PubMed] [Google Scholar]
- 62.Mazzon M., Marsh M. Targeting viral entry as a strategy for broad-spectrum antivirals. F1000Res. 2019;8:1628. doi: 10.12688/f1000research.19694.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fecchi K., Anticoli S., Peruzzu D., Iessi E., Gagliardi M.C., Matarrese P., Ruggieri A. Coronavirus interplay with lipid rafts and autophagy unveils promising therapeutic targets. Front. Microbiol. 2020;11:1821. doi: 10.3389/fmicb.2020.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sorice M., Misasi R., Riitano G., Manganelli V., Martellucci S., Longo A., Garofalo T., Mattei V. Targeting lipid rafts as a strategy against coronavirus. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.618296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gimpl G., Burger K., Fahrenholz F. Cholesterol as modulator of receptor function. Biochemistry. 1997;36:10959–10974. doi: 10.1021/bi963138w. [DOI] [PubMed] [Google Scholar]
- 66.Hao M., Mukherjee S., Maxfield F.R. Cholesterol depletion induces large scale domain segregation in living cell membranes. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13072–13077. doi: 10.1073/pnas.231377398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orlowski S., Martin S., Escargueil A. P-glycoprotein and 'lipid rafts': some ambiguous mutual relationships (floating on them, building them or meeting them by chance?) Cell. Mol. Life Sci. 2006;63:1038–1059. doi: 10.1007/s00018-005-5554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Subtil A., Gaidarov I., Kobylarz K., Lampson M.A., Keen J.H., McGraw T.E. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oesterle A., Laufs U., Liao J.K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 2017;120:229–243. doi: 10.1161/CIRCRESAHA.116.308537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gaibelet G., Allart S., Terce F., Azalbert V., Bertrand-Michel J., Hamdi S., Collet X., Orlowski S. Specific cellular incorporation of a pyrene-labelled cholesterol: lipoprotein-mediated delivery toward ordered intracellular membranes. PloS One. 2015;10 doi: 10.1371/journal.pone.0121563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mukherjee S., Zha X., Tabas I., Maxfield F.R. Cholesterol distribution in living cells: fluorescence imaging using dehydroergosterol as a fluorescent cholesterol analog. Biophys. J. 1998;75:1915–1925. doi: 10.1016/S0006-3495(98)77632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhuang L., Kim J., Adam R.M., Solomon K.R., Freeman M.R. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J. Clin. Invest. 2005;115:959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y.C., Park M.J., Ye S.K., Kim C.W., Kim Y.N. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am. J. Pathol. 2006;168:1107–1118. doi: 10.2353/ajpath.2006.050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vilimanovich U., Bosnjak M., Bogdanovic A., Markovic I., Isakovic A., Kravic-Stevovic T., Mircic A., Trajkovic V., Bumbasirevic V. Statin-mediated inhibition of cholesterol synthesis induces cytoprotective autophagy in human leukemic cells. Eur. J. Pharmacol. 2015;765:415–428. doi: 10.1016/j.ejphar.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 75.Gupta V.K., Sharma N.S., Kesh K., Dauer P., Nomura A., Giri B., Dudeja V., Banerjee S., Bhattacharya S., Saluja A., Banerjee S. Metastasis and chemoresistance in CD133 expressing pancreatic cancer cells are dependent on their lipid raft integrity. Canc. Lett. 2018;439:101–112. doi: 10.1016/j.canlet.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 76.Irwin M.E., Mueller K.L., Bohin N., Ge Y., Boerner J.L. Lipid raft localization of EGFR alters the response of cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J. Cell. Physiol. 2011;226:2316–2328. doi: 10.1002/jcp.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sawaya A.P., Jozic I., Stone R.C., Pastar I., Egger A.N., Stojadinovic O., Glinos G.D., Kirsner R.S., Tomic-Canic M. Mevastatin promotes healing by targeting caveolin-1 to restore EGFR signaling. JCI Insight. 2019;4 doi: 10.1172/jci.insight.129320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu H., Mahmood A., Lu D., Jiang H., Xiong Y., Zhou D., Chopp M. Attenuation of astrogliosis and modulation of endothelial growth factor receptor in lipid rafts by simvastatin after traumatic brain injury. J. Neurosurg. 2010;113:591–597. doi: 10.3171/2009.9.JNS09859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jury E.C., Isenberg D.A., Mauri C., Ehrenstein M.R. Atorvastatin restores Lck expression and lipid raft-associated signaling in T cells from patients with systemic lupus erythematosus. J. Immunol. 2006;177:7416–7422. doi: 10.4049/jimmunol.177.10.7416. [DOI] [PubMed] [Google Scholar]
- 80.Chansrichavala P., Chantharaksri U., Sritara P., Ngaosuwankul N., Chaiyaroj S.C. Atorvastatin affects TLR4 clustering via lipid raft modulation. Int. Immunopharm. 2010;10:892–899. doi: 10.1016/j.intimp.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 81.Hillyard D.Z., Jardine A.G., McDonald K.J., Cameron A.J. Fluvastatin inhibits raft dependent Fcgamma receptor signalling in human monocytes. Atherosclerosis. 2004;172:219–228. doi: 10.1016/j.atherosclerosis.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 82.Imran M., Sergent O., Tete A., Gallais I., Chevanne M., Lagadic-Gossmann D., Podechard N. Membrane remodeling as a key player of the hepatotoxicity induced by Co-exposure to benzo[a]pyrene and ethanol of obese zebrafish larvae. Biomolecules. 2018;8:E26. doi: 10.3390/biom8020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lafont F., van der Goot F.G. Bacterial invasion via lipid rafts. Cell Microbiol. 2005;7:613–620. doi: 10.1111/j.1462-5822.2005.00515.x. [DOI] [PubMed] [Google Scholar]
- 84.Lin C.J., Liao W.C., Lin H.J., Hsu Y.M., Lin C.L., Chen Y.A., Feng C.L., Chen C.J., Kao M.C., Lai C.H., Kao C.H. Statins attenuate Helicobacter pylori CagA translocation and reduce incidence of gastric cancer: in vitro and population-based case-control studies. PloS One. 2016;11 doi: 10.1371/journal.pone.0146432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parihar S.P., Guler R., Lang D.M., Suzuki H., Marais A.D., Brombacher F. Simvastatin enhances protection against Listeria monocytogenes infection in mice by counteracting Listeria-induced phagosomal escape. PloS One. 2013;8 doi: 10.1371/journal.pone.0075490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parihar S.P., Guler R., Khutlang R., Lang D.M., Hurdayal R., Mhlanga M.M., Suzuki H., Marais A.D., Brombacher F. Statin therapy reduces the mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J. Infect. Dis. 2014;209:754–763. doi: 10.1093/infdis/jit550. [DOI] [PubMed] [Google Scholar]
- 87.Aizaki H., Lee K.J., Sung V.M., Ishiko H., Lai M.M. Characterization of the hepatitis C virus RNA replication complex associated with lipid rafts. Virology. 2004;324:450–461. doi: 10.1016/j.virol.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 88.Ikeda M., Abe K., Yamada M., Dansako H., Naka K., Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117–125. doi: 10.1002/hep.21232. [DOI] [PubMed] [Google Scholar]
- 89.O'Leary J.G., Chan J.L., McMahon C.M., Chung R.T. Atorvastatin does not exhibit antiviral activity against HCV at conventional doses: a pilot clinical trial. Hepatology. 2007;45:895–898. doi: 10.1002/hep.21554. [DOI] [PubMed] [Google Scholar]
- 90.Bader T., Fazili J., Madhoun M., Aston C., Hughes D., Rizvi S., Seres K., Hasan M. Fluvastatin inhibits hepatitis C replication in humans. Am. J. Gastroenterol. 2008;103:1383–1389. doi: 10.1111/j.1572-0241.2008.01876.x. [DOI] [PubMed] [Google Scholar]
- 91.Moriyama T., Marquez J.P., Wakatsuki T., Sorokin A. Caveolar endocytosis is critical for BK virus infection of human renal proximal tubular epithelial cells. J. Virol. 2007;81:8552–8562. doi: 10.1128/JVI.00924-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moriyama T., Sorokin A. Repression of BK virus infection of human renal proximal tubular epithelial cells by pravastatin. Transplantation. 2008;85:1311–1317. doi: 10.1097/TP.0b013e31816c4ec5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mbianda C., El-Meanawy A., Sorokin A. Mechanisms of BK virus infection of renal cells and therapeutic implications. J. Clin. Virol. 2015;71:59–62. doi: 10.1016/j.jcv.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blaha M.J., Martin S.S. How do statins work?: changing paradigms with implications for statin allocation. J. Am. Coll. Cardiol. 2013;62:2392–2394. doi: 10.1016/j.jacc.2013.08.1626. [DOI] [PubMed] [Google Scholar]
- 95.Davies J.T., Delfino S.F., Feinberg C.E., Johnson M.F., Nappi V.L., Olinger J.T., Schwab A.P., Swanson H.I. Current and emerging uses of statins in clinical therapeutics: a review. Lipid Insights. 2016;9:13–29. doi: 10.4137/LPI.S37450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sirtori C.R. The pharmacology of statins. Pharmacol. Res. 2014;88:3–11. doi: 10.1016/j.phrs.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 97.Belanger A.M., Akioyamen L., Alothman L., Genest J. Evidence for improved survival with treatment of homozygous familial hypercholesterolemia. Curr. Opin. Lipidol. 2020;31:176–181. doi: 10.1097/MOL.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 98.Gidding S.S., Champagne M.A., de Ferranti S.D., Defesche J., Ito M.K., Knowles J.W., McCrindle B., Raal F., Rader D., Santos R.D., Lopes-Virella M., Watts G.F., Wierzbicki A.S. The agenda for familial hypercholesterolemia: a scientific statement from the American heart association. Circulation. 2015;132:2167–2192. doi: 10.1161/CIR.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 99.Marais A.D., Raal F.J., Stein E.A., Rader D.J., Blasetto J., Palmer M., Wilpshaar W. A dose-titration and comparative study of rosuvastatin and atorvastatin in patients with homozygous familial hypercholesterolaemia. Atherosclerosis. 2008;197:400–406. doi: 10.1016/j.atherosclerosis.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 100.Gossios T., Zografou I., Simoulidou V., Pirpassopoulou A., Christou K., Karagiannis A. Multimodal treatment of homozygous familial hypercholesterolemia. Curr. Pharmaceut. Des. 2018;24:3616–3621. doi: 10.2174/1381612824666181009095522. [DOI] [PubMed] [Google Scholar]
- 101.Matarazzo S., Quitadamo M.C., Mango R., Ciccone S., Novelli G., Biocca S. Cholesterol-lowering drugs inhibit lectin-like oxidized low-density lipoprotein-1 receptor function by membrane raft disruption. Mol. Pharmacol. 2012;82:246–254. doi: 10.1124/mol.112.078915. [DOI] [PubMed] [Google Scholar]
- 102.Wei Y.M., Li X., Xiong J., Abais J.M., Xia M., Boini K.M., Zhang Y., Li P.L. Attenuation by statins of membrane raft-redox signaling in coronary arterial endothelium. J. Pharmacol. Exp. Therapeut. 2013;345:170–179. doi: 10.1124/jpet.112.201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuipers H.F., Biesta P.J., Groothuis T.A., Neefjes J.J., Mommaas A.M., van den Elsen P.J. Statins affect cell-surface expression of major histocompatibility complex class II molecules by disrupting cholesterol-containing microdomains. Hum. Immunol. 2005;66:653–665. doi: 10.1016/j.humimm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 104.Hillyard D.Z., Nutt C.D., Thomson J., McDonald K.J., Wan R.K., Cameron A.J., Mark P.B., Jardine A.G. Statins inhibit NK cell cytotoxicity by membrane raft depletion rather than inhibition of isoprenylation. Atherosclerosis. 2007;191:319–325. doi: 10.1016/j.atherosclerosis.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 105.Gu M.X., Fu Y., Sun X.L., Ding Y.Z., Li C.H., Pang W., Pan S., Zhu Y. Proteomic analysis of endothelial lipid rafts reveals a novel role of statins in antioxidation. J. Proteome Res. 2012;11:2365–2373. doi: 10.1021/pr300098f. [DOI] [PubMed] [Google Scholar]
- 106.Gao Y., Li L., Li T., Ma L., Yuan M., Sun W., Cheng H.L., Niu L., Du Z., Quan Z., Fan Y., Fan J., Luo C., Wu X. Simvastatin delays castrationresistant prostate cancer metastasis and androgen receptor antagonist resistance by regulating the expression of caveolin1. Int. J. Oncol. 2019;54:2054–2068. doi: 10.3892/ijo.2019.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garnett D.J., Greenhough T.J. Statins cause profound effects on gene expression in human cancer cells in vitro: the role of membrane microdomains. Gene Expr. 2013;15:225–234. doi: 10.3727/105221613x13571653093240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pugh S.D., MacDougall D.A., Agarwal S.R., Harvey R.D., Porter K.E., Calaghan S. Caveolin contributes to the modulation of basal and beta-adrenoceptor stimulated function of the adult rat ventricular myocyte by simvastatin: a novel pleiotropic effect. PloS One. 2014;9 doi: 10.1371/journal.pone.0106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salvary T., Gambert-Nicot S., Brindisi M.C., Meneveau N., Schiele F., Seronde M.F., Lorgis L., Zeller M., Cottin Y., Kantelip J.P., Gambert P., Davani S. Pravastatin reverses the membrane cholesterol reorganization induced by myocardial infarction within lipid rafts in CD14(+)/CD16(-) circulating monocytes. Biochim. Biophys. Acta. 2012;1821:1287–1294. doi: 10.1016/j.bbalip.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 110.Jin Y., Tachibana I., Takeda Y., He P., Kang S., Suzuki M., Kuhara H., Tetsumoto S., Tsujino K., Minami T., Iwasaki T., Nakanishi K., Kohmo S., Hirata H., Takahashi R., Inoue K., Nagatomo I., Kida H., Kijima T., Ito M., Saya H., Kumanogoh A. Statins decrease lung inflammation in mice by upregulating tetraspanin CD9 in macrophages. PloS One. 2013;8 doi: 10.1371/journal.pone.0073706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thyberg J. Cholesterol oxidase and the hydroxymethylglutaryl coenzyme A reductase inhibitor mevinolin perturb endocytic trafficking in cultured vascular smooth muscle cells. J. Submicr. Cytol. Pathol. 2003;35:457–468. [PubMed] [Google Scholar]
- 112.Kumar G.A., Chattopadhyay A. Statin-induced chronic cholesterol depletion switches GPCR endocytosis and trafficking: insights from the Serotonin1A receptor. ACS Chem. Neurosci. 2020;11:453–465. doi: 10.1021/acschemneuro.9b00659. [DOI] [PubMed] [Google Scholar]
- 113.Li H.Y., Appelbaum F.R., Willman C.L., Zager R.A., Banker D.E. Cholesterol-modulating agents kill acute myeloid leukemia cells and sensitize them to therapeutics by blocking adaptive cholesterol responses. Blood. 2003;101:3628–3634. doi: 10.1182/blood-2002-07-2283. [DOI] [PubMed] [Google Scholar]
- 114.Kuipers H.F., van den Elsen P.J. Immunomodulation by statins: inhibition of cholesterol vs. isoprenoid biosynthesis. Biomed. Pharmacother. 2007;61:400–407. doi: 10.1016/j.biopha.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 115.Hillyard D.Z., Cameron A.J., McDonald K.J., Thomson J., MacIntyre A., Shiels P.G., Panarelli M., Jardine A.G. Simvastatin inhibits lymphocyte function in normal subjects and patients with cardiovascular disease. Atherosclerosis. 2004;175:305–313. doi: 10.1016/j.atherosclerosis.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 116.Menter D.G., Ramsauer V.P., Harirforoosh S., Chakraborty K., Yang P., Hsi L., Newman R.A., Krishnan K. Differential effects of pravastatin and simvastatin on the growth of tumor cells from different organ sites. PloS One. 2011;6 doi: 10.1371/journal.pone.0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bjorkhem-Bergman L., Lindh J.D., Bergman P. What is a relevant statin concentration in cell experiments claiming pleiotropic effects? Br. J. Clin. Pharmacol. 2011;72:164–165. doi: 10.1111/j.1365-2125.2011.03907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suh J.W., Choi D.J., Chang H.J., Cho Y.S., Youn T.J., Chae I.H., Kim K.I., Kim C.H., Kim H.S., Oh B.H., Park Y.B. HMG-CoA reductase inhibitor improves endothelial dysfunction in spontaneous hypertensive rats via down-regulation of caveolin-1 and activation of endothelial nitric oxide synthase. J. Kor. Med. Sci. 2010;25:16–23. doi: 10.3346/jkms.2010.25.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Katsiki N., Banach M., Mikhailidis D.P. Lipid-lowering therapy and renin-angiotensin-aldosterone system inhibitors in the era of the COVID-19 pandemic. Arch. Med. Sci. 2020;16:485–489. doi: 10.5114/aoms.2020.94503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gustafson D., Raju S., Wu R., Ching C., Veitch S., Rathnakumar K., Boudreau E., Howe K.L., Fish J.E. Overcoming barriers: the endothelium as a linchpin of coronavirus disease 2019 pathogenesis? Arterioscler. Thromb. Vasc. Biol. 2020;40:1818–1829. doi: 10.1161/ATVBAHA.120.314558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Libby P., Luscher T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nagele M.P., Haubner B., Tanner F.C., Ruschitzka F., Flammer A.J. Endothelial dysfunction in COVID-19: current findings and therapeutic implications. Atherosclerosis. 2020;314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Y.H., Wang Q.X., Zhou J.W., Chu X.M., Man Y.L., Liu P., Ren B.B., Sun T.R., An Y. Effects of rosuvastatin on expression of angiotensin-converting enzyme 2 after vascular balloon injury in rats. J Geriatr Cardiol. 2013;10:151–158. doi: 10.3969/j.issn.1671-5411.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020;318:H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tikoo K., Patel G., Kumar S., Karpe P.A., Sanghavi M., Malek V., Srinivasan K. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem. Pharmacol. 2015;93:343–351. doi: 10.1016/j.bcp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 127.Castiglione V., Chiriaco M., Emdin M., Taddei S., Vergaro G. Statin therapy in COVID-19 infection. Eur. Heart J. Cardiovasc. Pharmacother. 2020;6:258–259. doi: 10.1093/ehjcvp/pvaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dashti-Khavidaki S., Khalili H. Considerations for statin therapy in patients with COVID-19. Pharmacotherapy. 2020;40:484–486. doi: 10.1002/phar.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Minz M.M., Bansal M., Kasliwal R.R. Statins and SARS-CoV-2 disease: current concepts and possible benefits. Diabetes Metab Syndr. 2020;14:2063–2067. doi: 10.1016/j.dsx.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rodrigues-Diez R.R., Tejera-Munoz A., Marquez-Exposito L., Rayego-Mateos S., Santos Sanchez L., Marchant V., Tejedor Santamaria L., Ramos A.M., Ortiz A., Egido J., Ruiz-Ortega M. Statins: could an old friend help in the fight against COVID-19? Br. J. Pharmacol. 2020;177:4873–4886. doi: 10.1111/bph.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Subir R., Jagat J.M., Kalyan K.G. Pros and cons for use of statins in people with coronavirus disease-19 (COVID-19) Diabetes Metab Syndr. 2020;14:1225–1229. doi: 10.1016/j.dsx.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barkas F., Milionis H., Anastasiou G., Liberopoulos E. Statins and PCSK9 inhibitors: what is their role in coronavirus disease 2019? Med. Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bifulco M., Endo A. Statin: new life for an old drug. Pharmacol. Res. 2014;88:1–2. doi: 10.1016/j.phrs.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 134.Ganjali S., Bianconi V., Penson P.E., Pirro M., Banach M., Watts G.F., Sahebkar A. Commentary: statins, COVID-19, and coronary artery disease: killing two birds with one stone. Metab. Clin. Exp. 2020;113 doi: 10.1016/j.metabol.2020.154375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kashour T., Halwani R., Arabi Y.M., Sohail M.R., O'Horo J.C., Badley A.D., Tleyjeh I.M. Statins as an adjunctive therapy for COVID-19: the biological and clinical plausibility. Immunopharmacol. Immunotoxicol. 2021;43:37–50. doi: 10.1080/08923973.2020.1863984. [DOI] [PubMed] [Google Scholar]
- 136.Daniels L.B., Sitapati A.M., Zhang J., Zou J., Bui Q.M., Ren J., Longhurst C.A., Criqui M.H., Messer K. Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients. Am. J. Cardiol. 2020;136:149–155. doi: 10.1016/j.amjcard.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.De Spiegeleer A., Bronselaer A., Teo J.T., Byttebier G., De Tre G., Belmans L., Dobson R., Wynendaele E., Van De Wiele C., Vandaele F., Van Dijck D., Bean D., Fedson D., De Spiegeleer B. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID-19 infection among nursing home residents. J. Am. Med. Dir. Assoc. 2020;21:909–914. doi: 10.1016/j.jamda.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tan W.Y.T., Young B.E., Lye D.C., Chew D.E.K., Dalan R. Statin use is associated with lower disease severity in COVID-19 infection. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-74492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chacko S.R., DeJoy R., 3rd, Lo K.B., Albano J., Peterson E., Bhargav R., Gul F., Salacup G., Pelayo J., Azmaiparashvili Z., Rangaswami J., Patarroyo-Aponte G., Benzaquen S., Gupta E. Association of pre-admission statin use with reduced in-hospital mortality in COVID-19. Am. J. Med. Sci. 2021 doi: 10.1016/j.amjms.2021.03.001. S0002-9629(21)00089-6 (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fan Y., Guo T., Yan F., Gong M., Zhang X.A., Li C., He T., Luo H., Zhang L., Chen M., Wu X., Wang H., Deng K.Q., Bai J., Cai L., Lu Z. Association of statin use with the in-hospital outcomes of 2019-coronavirus disease patients: a retrospective study. Front. Med. 2020;7 doi: 10.3389/fmed.2020.584870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gupta A., Madhavan M.V., Poterucha T.J., DeFilippis E.M., Hennessey J.A., Redfors B., Eckhardt C., Bikdeli B., Platt J., Nalbandian A., Elias P., Cummings M.J., Nouri S.N., Lawlor M., Ranard L.S., Li J., Boyle C., Givens R., Brodie D., Krumholz H.M., Stone G.W., Sethi S.S., Burkhoff D., Uriel N., Schwartz A., Leon M.B., Kirtane A.J., Wan E.Y., Parikh S.A. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat. Commun. 2020;12:1325. doi: 10.1038/s41467-021-21553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee H.Y., Ahn J., Park J., Kyung Kang C., Won S.H., Wook Kim D., Park J.H., Chung K.H., Joh J.S., Bang J.H., Hee Kang C., Bum Pyun W., Oh M.D., N.C.f.C.M.o.E.I.D. Korean Society of Hypertension beneficial effect of statins in COVID-19-related outcomes-brief report: a national population-based cohort study. Arterioscler. Thromb. Vasc. Biol. 2021;41:e175–e182. doi: 10.1161/ATVBAHA.120.315551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maric I., Oskotsky T., Kosti I., Le B., Wong R.J., Shaw G.M., Sirota M., Stevenson D.K. Decreased mortality rate among COVID-19 patients prescribed statins: data from electronic health records in the US. Front. Med. 2021;8 doi: 10.3389/fmed.2021.639804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Masana L., Correig E., Rodriguez-Borjabad C., Anoro E., Arroyo J.A., Jerico C., Pedragosa A., Miret M., Naf S., Pardo A., Perea V., Perez-Bernalte R., Plana N., Ramirez-Montesinos R., Royuela M., Soler C., Urquizu-Padilla M., Zamora A., Pedro-Botet J., Group O. Effect of statin therapy on SARS-CoV-2 infection-related. Eur. Heart J. Cardiovasc. Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa128. pvaa128 (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rodriguez-Nava G., Trelles-Garcia D.P., Yanez-Bello M.A., Chung C.W., Trelles-Garcia V.P., Friedman H.J. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study. Crit. Care. 2020;24:429. doi: 10.1186/s13054-020-03154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang X.J., Qin J.J., Cheng X., Shen L., Zhao Y.C., Yuan Y., Lei F., Chen M.M., Yang H., Bai L., Song X., Lin L., Xia M., Zhou F., Zhou J., She Z.G., Zhu L., Ma X., Xu Q., Ye P., Chen G., Liu L., Mao W., Yan Y., Xiao B., Lu Z., Peng G., Liu M., Yang J., Yang L., Zhang C., Lu H., Xia X., Wang D., Liao X., Wei X., Zhang B.H., Zhang X., Yang J., Zhao G.N., Zhang P., Liu P.P., Loomba R., Ji Y.X., Xia J., Wang Y., Cai J., Guo J., Li H. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metabol. 2020;32:176–187. doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Butt J.H., Gerds T.A., Schou M., Kragholm K., Phelps M., Havers-Borgersen E., Yafasova A., Gislason G.H., Torp-Pedersen C., Kober L., Fosbol E.L. Association between statin use and outcomes in patients with coronavirus disease 2019 (COVID-19): a nationwide cohort study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-044421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Oh T.K., Song I.A., Jeon Y.T. Statin therapy and the risk of COVID-19: a cohort study of the national health insurance service in South Korea. J. Personalized Med. 2021;11 doi: 10.3390/jpm11020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kow C.S., Hasan S.S. Meta-analysis of effect of statins in patients with COVID-19. Am. J. Cardiol. 2020;134:153–155. doi: 10.1016/j.amjcard.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Onorato D., Pucci M., Carpene G., Henry B.M., Sanchis-Gomar F., Lippi G. Protective effects of statins administration in European and north American patients infected with COVID-19: a meta-analysis. Semin. Thromb. Hemost. 2021;47:392–399. doi: 10.1055/s-0040-1722307. [DOI] [PubMed] [Google Scholar]
- 151.Pal R., Banerjee M., Yadav U., Bhattacharjee S. Statin use and clinical outcomes in patients with COVID-19: an updated systematic review and meta-analysis. Postgrad. Med. 2021:2020–139172. doi: 10.1136/postgradmedj-2020-139172. [DOI] [PubMed] [Google Scholar]
- 152.Scheen A.J. Statins and clinical outcomes with COVID-19: meta-analyses of observational studies. Diabetes Metab. 2020;47 doi: 10.1016/j.diabet.2020.101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Permana H., Huang I., Purwiga A., Kusumawardhani N.Y., Sihite T.A., Martanto E., Wisaksana R., Soetedjo N.N.M. In-hospital use of statins is associated with a reduced risk of mortality in coronavirus-2019 (COVID-19): systematic review and meta-analysis. Pharmacol. Rep. 2021:1–12. doi: 10.1007/s43440-021-00233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stern R.H., Yang B.B., Hounslow N.J., MacMahon M., Abel R.B., Olson S.C. Pharmacodynamics and pharmacokinetic-pharmacodynamic relationships of atorvastatin, an HMG-CoA reductase inhibitor. J. Clin. Pharmacol. 2000;40:616–623. [PubMed] [Google Scholar]
- 155.Chansrichavala P., Chantharaksri U., Sritara P., Chaiyaroj S.C. Atorvastatin attenuates TLR4-mediated NF-kappaB activation in a MyD88-dependent pathway. Asian Pac. J. Allergy Immunol. 2009;27:49–57. [PubMed] [Google Scholar]
- 156.Abdulrab S., Alkadasi B., Al-Maweri S., Halboub E., Alhadainy H., Geerts G. Statins-based prophylactic mouthwash and nasal spray may protect against coronavirus disease 2019. New Microbes New Infections. 2020;37 doi: 10.1016/j.nmni.2020.100751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fan A.L., Fenske J.N., Van Harrison R., Jackson E.A., Marcelino M.A. UMHS; 2014. Lipid Therapy Guideline. [Google Scholar]
- 158.Bruckert E., Hayem G., Dejager S., Yau C., Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc. Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 159.Stroes E.S., Thompson P.D., Corsini A., Vladutiu G.D., Raal F.J., Ray K.K., Roden M., Stein E., Tokgozoglu L., Nordestgaard B.G., Bruckert E., De Backer G., Krauss R.M., Laufs U., Santos R.D., Hegele R.A., Hovingh G.K., Leiter L.A., Mach F., Marz W., Newman C.B., Wiklund O., Jacobson T.A., Catapano A.L., Chapman M.J., Ginsberg H.N. Statin-associated muscle symptoms: impact on statin therapy-European atherosclerosis society consensus panel statement on assessment, aetiology and management. Eur. Heart J. 2015;36:1012–1022. doi: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Talbert R.L. Safety issues with statin therapy. J. Am. Pharmaceut. Assoc. 2006;46:479–488. doi: 10.1331/154434506778073637. (2003) [DOI] [PubMed] [Google Scholar]
- 161.Alam K., Crowe A., Wang X., Zhang P., Ding K., Li L., Yue W. Regulation of organic anion transporting polypeptides (OATP) 1B1- and OATP1B3-mediated transport: an updated review in the context of OATP-mediated drug-drug interactions. Int. J. Mol. Sci. 2018;19:855. doi: 10.3390/ijms19030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kitamura S., Maeda K., Wang Y., Sugiyama Y. Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab. Dispos. 2008;36:2014–2023. doi: 10.1124/dmd.108.021410. [DOI] [PubMed] [Google Scholar]
- 163.Hirano M., Maeda K., Shitara Y., Sugiyama Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J. Pharmacol. Exp. Therapeut. 2004;311:139–146. doi: 10.1124/jpet.104.068056. [DOI] [PubMed] [Google Scholar]
- 164.Holtzman C.W., Wiggins B.S., Spinler S.A. Role of P-glycoprotein in statin drug interactions. Pharmacotherapy. 2006;26:1601–1607. doi: 10.1592/phco.26.11.1601. [DOI] [PubMed] [Google Scholar]
- 165.Lennernas H. Clinical pharmacokinetics of atorvastatin. Clin. Pharmacokinet. 2003;42:1141–1160. doi: 10.2165/00003088-200342130-00005. [DOI] [PubMed] [Google Scholar]
- 166.Mason R.P., Walter M.F., Day C.A., Jacob R.F. Intermolecular differences of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors contribute to distinct pharmacologic and pleiotropic actions. Am. J. Cardiol. 2005;96:11F–23F. doi: 10.1016/j.amjcard.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 167.Posvar E.L., Radulovic L.L., Cilla D.D., Jr., Whitfield L.R., Sedman A.J. Tolerance and pharmacokinetics of single-dose atorvastatin, a potent inhibitor of HMG-CoA reductase, in healthy subjects. J. Clin. Pharmacol. 1996;36:728–731. doi: 10.1002/j.1552-4604.1996.tb04242.x. [DOI] [PubMed] [Google Scholar]
- 168.Maisonnasse P., Guedj J., Contreras V., Behillil S., Solas C., Marlin R., Naninck T., Pizzorno A., Lemaitre J., Goncalves A., Kahlaoui N., Terrier O., Fang R.H.T., Enouf V., Dereuddre-Bosquet N., Brisebarre A., Touret F., Chapon C., Hoen B., Lina B., Calatrava M.R., van der Werf S., de Lamballerie X., Le Grand R. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:584–587. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 169.Munster V.J., Feldmann F., Williamson B.N., van Doremalen N., Perez-Perez L., Schulz J., Meade-White K., Okumura A., Callison J., Brumbaugh B., Avanzato V.A., Rosenke R., Hanley P.W., Saturday G., Scott D., Fischer E.R., de Wit E. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020;585:268–272. doi: 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rockx B., Kuiken T., Herfst S., Bestebroer T., Lamers M.M., Oude Munnink B.B., de Meulder D., van Amerongen G., van den Brand J., Okba N.M.A., Schipper D., van Run P., Leijten L., Sikkema R., Verschoor E., Verstrepen B., Bogers W., Langermans J., Drosten C., Fentener van Vlissingen M., Fouchier R., de Swart R., Koopmans M., Haagmans B.L. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368:1012–1015. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Smits S.L., de Lang A., van den Brand J.M., Leijten L.M., van I.W.F., Eijkemans M.J., van Amerongen G., Kuiken T., Andeweg A.C., Osterhaus A.D., Haagmans B.L. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yu P., Qi F., Xu Y., Li F., Liu P., Liu J., Bao L., Deng W., Gao H., Xiang Z., Xiao C., Lv Q., Gong S., Liu J., Song Z., Qu Y., Xue J., Wei Q., Liu M., Wang G., Wang S., Yu H., Liu X., Huang B., Wang W., Zhao L., Wang H., Ye F., Zhou W., Zhen W., Han J., Wu G., Jin Q., Wang J., Tan W., Qin C. Age-related rhesus macaque models of COVID-19. Anim. Model Exp. Med. 2020;3:93–97. doi: 10.1002/ame2.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Uno Y., Uehara S., Yamazaki H. Utility of non-human primates in drug development: comparison of non-human primate and human drug-metabolizing cytochrome P450 enzymes. Biochem. Pharmacol. 2016;121:1–7. doi: 10.1016/j.bcp.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 174.Uno Y., Uehara S., Yamazaki H. Genetic polymorphisms of drug-metabolizing cytochrome P450 enzymes in cynomolgus and rhesus monkeys and common marmosets in preclinical studies for humans. Biochem. Pharmacol. 2018;153:184–195. doi: 10.1016/j.bcp.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 175.Rabacal W., Schweitzer F., Rayens E., Tarantelli R., Whang P., Jimenez V.C., Outwater J.A., Norris K.A. Statin treatment prevents the development of pulmonary arterial hypertension in a nonhuman primate model of HIV-associated PAH. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-55301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chan J.F.W., Zhang A.J., Yuan S., Poon V.K.M., Chan C.C.S., Lee A.C.Y., Chan W.M., Fan Z., Tsoi H.W., Wen L., Liang R., Cao J., Chen Y., Tang K., Luo C., Cai J.P., Kok K.H., Chu H., Chan K.H., Sridhar S., Chen Z., Chen H., To K.K.W., Yuen K.Y. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 2020:10. doi: 10.1093/cid/ciaa325. 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Imai M., Iwatsuki-Horimoto K., Hatta M., Loeber S., Halfmann P.J., Nakajima N., Watanabe T., Ujie M., Takahashi K., Ito M., Yamada S., Fan S., Chiba S., Kuroda M., Guan L., Takada K., Armbrust T., Balogh A., Furusawa Y., Okuda M., Ueki H., Yasuhara A., Sakai-Tagawa Y., Lopes T.J.S., Kiso M., Yamayoshi S., Kinoshita N., Ohmagari N., Hattori S.I., Takeda M., Mitsuya H., Krammer F., Suzuki T., Kawaoka Y. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. U.S.A. 2020;117:16587–16595. doi: 10.1073/pnas.2009799117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rosenke K., Meade-White K., Letko M., Clancy C., Hansen F., Liu Y., Okumura A., Tang-Huau T.L., Li R., Saturday G., Feldmann F., Scott D., Wang Z., Munster V., Jarvis M.A., Feldmann H. Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection. Emerg. Microb. Infect. 2020;9:2673–2684. doi: 10.1080/22221751.2020.1858177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sia S.F., Yan L.M., Chin A.W.H., Fung K., Choy K.T., Wong A.Y.L., Kaewpreedee P., Perera R., Poon L.L.M., Nicholls J.M., Peiris M., Yen H.L. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583:834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., Wei Q., Yu P., Xu Y., Qi F., Qu Y., Li F., Lv Q., Wang W., Xue J., Gong S., Liu M., Wang G., Wang S., Song Z., Zhao L., Liu P., Zhao L., Ye F., Wang H., Zhou W., Zhu N., Zhen W., Yu H., Zhang X., Guo L., Chen L., Wang C., Wang Y., Wang X., Xiao Y., Sun Q., Liu H., Zhu F., Ma C., Yan L., Yang M., Han J., Xu W., Tan W., Peng X., Jin Q., Wu G., Qin C. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583:830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 181.Jiang R.D., Liu M.Q., Chen Y., Shan C., Zhou Y.W., Shen X.R., Li Q., Zhang L., Zhu Y., Si H.R., Wang Q., Min J., Wang X., Zhang W., Li B., Zhang H.J., Baric R.S., Zhou P., Yang X.L., Shi Z.L. Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell. 2020;182:50–58. doi: 10.1016/j.cell.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sun S.H., Chen Q., Gu H.J., Yang G., Wang Y.X., Huang X.Y., Liu S.S., Zhang N.N., Li X.F., Xiong R., Guo Y., Deng Y.Q., Huang W.J., Liu Q., Liu Q.M., Shen Y.L., Zhou Y., Yang X., Zhao T.Y., Fan C.F., Zhou Y.S., Qin C.F., Wang Y.C. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe. 2020;28:124–133. doi: 10.1016/j.chom.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fazio S., Linton M.F. Mouse models of hyperlipidemia and atherosclerosis. Front. Biosci. 2001;6:D515–D525. doi: 10.2741/fazio. [DOI] [PubMed] [Google Scholar]
- 184.Pecoraro V., Moja L., Dall'Olmo L., Cappellini G., Garattini S. Most appropriate animal models to study the efficacy of statins: a systematic review. Eur. J. Clin. Invest. 2014;44:848–871. doi: 10.1111/eci.12304. [DOI] [PubMed] [Google Scholar]
- 185.Fajgenbaum D.C., Rader D.J. Teaching old drugs new tricks: statins for COVID-19? Cell Metabol. 2020;32:145–147. doi: 10.1016/j.cmet.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bifulco M., Gazzerro P. Statins in coronavirus outbreak: it's time for experimental and clinical studies. Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yuan S. Statins may decrease the fatality rate of Middle East Respiratory Syndrome infection. mBio. 2015;6 doi: 10.1128/mBio.01120-15. e01120-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fedson D.S. Treating influenza with statins and other immunomodulatory agents. Antivir. Res. 2013;99:417–435. doi: 10.1016/j.antiviral.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 189.Mehrbod P., Omar A.R., Hair-Bejo M., Haghani A., Ideris A. Mechanisms of action and efficacy of statins against influenza. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/872370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fedson D.S., Opal S.M., Rordam O.M. Hiding in plain sight: an approach to treating patients with severe COVID-19 infection. mBio. 2020;11 doi: 10.1128/mBio.00398-20. e00398-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fedson D.S., Rordam O.M. Treating Ebola patients: a 'bottom up' approach using generic statins and angiotensin receptor blockers. Int. J. Infect. Dis. 2015;36:80–84. doi: 10.1016/j.ijid.2015.04.019. [DOI] [PubMed] [Google Scholar]