Abstract
Objective
To evaluate the evidence of mother-to-child transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Methods
This is a descriptive, multicentre, observational study in nine tertiary care hospitals throughout Spain. The study population was women with coronavirus disease 2019 during pregnancy. Mother-to-child transmission was defined as positive real-time RT-PCR of SARS-CoV-2 in amniotic fluid, cord blood, placenta or neonatal nasopharyngeal swabs taken immediately after birth.
Results
We included 43 women with singleton pregnancies and one with a twin pregnancy, as a result we obtained 45 samples of placenta, amniotic fluid and umbilical cord blood. The median gestational age at diagnosis was 34.7 weeks (range 14–41.3 weeks). The median interval between positive RT-PCR and delivery was 21.5 days (range 0–141 days). Fourteen women (31.8%, 95% CI 18.6%–47.6%) were positive at the time of delivery. There was one singleton pregnancy with SARS-CoV-2 RT-PCR positive in the placenta, amniotic fluid and umbilical cord blood (2.2%, 95% CI 0.1%–11.8%). Nasopharyngeal aspiration was performed on 38 neonates at birth, all of which were negative (0%, 95% CI 0%–9.3%). In 11 neonates the nasopharyngeal aspiration was repeated at 24–48 hours, and one returned positive (9.1%, 95% CI 0.2%–41.3%).
Conclusions
The presence of SARS-CoV-2 in placenta, amniotic fluid and cord blood shows that mother-to-child transmission is possible but uncommon.
Keywords: Congenital infection, Coronavirus disease 2019, Mother-to-child transmission, Pregnancy, Severe acute respiratory syndrome coronavirus 2
Introduction
Physiological changes in pregnancy increase the susceptibility to infections and their severity. Moreover, maternal infections may have consequences for the offspring, as obstetric complications or congenital infections.
Clinical presentation of coronavirus disease 2019 (COVID-19) in pregnancy is similar to the general population [1], but with a significantly higher risk of Intensive Care Unit admission and invasive ventilation than in non-pregnant adults [2,3].
The likelihood of mother-to-child transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is still unknown. Several studies have suggested this possibility [[4], [5], [6]], but most of them did not have enough evidence to demonstrate both maternal and fetal or neonatal infection.
The aim of this study was to evaluate the possibility of mother-to-child transmission of SARS-CoV-2 in a cohort of pregnant women with COVID-19.
Materials and methods
Approval for the study was obtained from the Vall d'Hebron University Hospital Ethics Committee (PR(AMI)181/2020) on 27 March 2020, and subsequently validated in the other hospitals. Informed consent was obtained from pregnant women for the collection of data and biological samples from the mother and newborn.
Study population
This sub-study of the Gesta-Covid Collaborative Group (see Supplementary material, Appendix S1) included only pregnant women with COVID-19 infection for whom amniotic fluid, umbilical cord blood and placenta samples were collected at birth. Exclusion criteria were age under 18 years, difficulty understanding informed consent and refusal to participate.
A COVID-19 confirmed case was defined as laboratory confirmation of real-time RT-PCR for SARS-CoV-2 assay of the nasal and pharyngeal swab. In probable cases (negative RT-PCR), if the symptoms had started in the last 7 days then the RT-PCR was repeated after 24 hours, otherwise a serology test was performed.
Study outcomes
The primary outcome was evidence of mother-to-child transmission, defined as positive RT-PCR of SARS-CoV-2 in amniotic fluid, cord blood, placenta or neonatal nasopharyngeal swabs taken immediately after birth [7].
Clinical data
Medical and obstetric history, exposure history and COVID-19 symptoms in the previous 14 days, physical examination, and laboratory and radiological findings were collected. COVID-19 severity was classified into three groups: mild (not requiring hospital admission), severe (pneumonia) and critical (Intensive Care Unit admission).
Data on pregnancy, gestational age at delivery, mode of delivery, indication for cesarean delivery, maternal complications and neonatal outcomes were also recorded.
Newborns were examined by a paediatrician specialized in congenital infections.
Microbiological samples collection
Nasal and pharyngeal swabs were taken from the mothers on a weekly basis until negativity.
All non-respiratory samples were collected at the time of delivery with strict aseptic techniques to avoid contamination by maternal blood or by respiratory droplets from the mother or birth attendants. The procedure is described in detail in the Supplementary material (Appendix S2).
Results
Forty-four pregnancies with samples of placenta, amniotic fluid and umbilical cord blood collected were included in the study. There was one monochorionic twin pregnancy, in which the samples were taken from both fetuses. As a result, we obtained 45 samples. Table 1 depicts baseline demographic and clinical characteristics of the studied population and Table 2 shows pregnancy outcome.
Table 1.
Baseline demographic and clinical characteristics
| Maternal age (years), median (range) | 33.5 (18–46) |
| Body mass index (kg/m2), median (range) | 26.6 (16.7–47.0) |
| Ethnic group, n (%) | |
| Caucasian | 29 (65.9%) |
| Latin American | 12 (27.3%) |
| Asian | 2 (4.5%) |
| Black-African | 1 (2.3%) |
| Type of pregnancy, n (%) | |
| Single | 43 (97.7%) |
| Dichorionic diamniotic twins | 1 (2.3%) |
| Cigarette smoker, n (%) | 1 (2.3%) |
| Medical condition, n (%) | 8 (18.2%) |
| Autoimmune disease | 4 |
| Asthma | 1 |
| Diabetes | 1 |
| Thrombophilia | 1 |
| Acquired heart disease | 1 |
| Clinical presentation, n (%) | |
| Mild disease | 29 (65.9%) |
| Pneumonia | 12 (27.3%) |
| Severe pneumonia – ICU admission | 3 (6.8%) |
| Diagnosis, n (%) | |
| RT-PCR | 33 (75%) |
| Serology | 11 (25%) |
| Gestational age at diagnosis (weeks), median (range) | 34.7 (14–41.3) |
| Interval RT-PCR diagnosis and delivery (days), median (range) | 21.5 (0–141) |
| RT-PCR positive at delivery, n (%) | 14 (31.8%) |
Abbreviations: ICU, intensive care unit.
Table 2.
Pregnancy outcome
| Gestational age at birth (weeks), median (range) | 39 (28.4–41.4) |
| Labour onset, n (%) | |
| Spontaneous | 16 (36.4%) |
| Elective | 28 (63.6%) |
| For COVID-19 | 2a |
| Other indication | 26 |
| Preterm birth <37 weeks, n (%) | 2 (4.5%) |
| For COVID-19 | 2a |
| Other | 0 |
| Mode of delivery, n (%) | |
| Vaginal delivery | 29 (65.9%) |
| Caesarean section | 15 (34.1%) |
| Pregnancy outcome, n (%) | |
| Live births | 44b |
| Stillbirth | 1c |
| Birthweight (g), median (range) | 3440 (1000–4425) |
Two cases of preterm birth at 28 weeks and 3 days, and 36 weeks and 5 days, respectively, due to COVID-19 infection. In both cases, a caesarean section was performed because of the worsening maternal condition.
One was a twin pregnancy.
The stillbirth occurred in an asymptomatic woman who tested positive for COVID-19 at 20 weeks. The fetus was diagnosed with a macrocephaly, intracranial cyst and polyhydramnios at 30 weeks, and resulted in a stillbirth at 34 weeks. The genetic study did not show any abnormal finding, and the post-mortem examination showed central nervous system anomalies (ventriculomegaly, subependymal cysts).
The median gestational age at COVID-19 diagnosis was 34.7 weeks (range 14–41.3 weeks). The median interval between positive RT-PCR and delivery was 21.5 days (range 0–141 days). Fourteen women (31.8%, 95% CI 18.6%–47.6%) were still positive at the time of delivery.
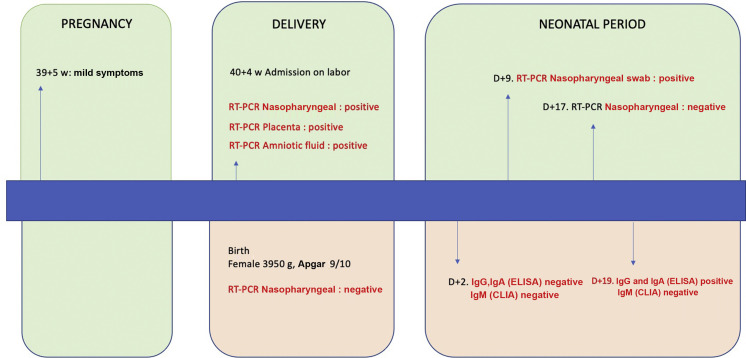
One singleton pregnancy was SARS-CoV-2 RT-PCR-positive in the placenta, amniotic fluid and umbilical cord blood (2.2%, 95% CI 0.1%–11.8%) Fig. 1
Fig. 1.
Summary of the case.
Nasopharyngeal aspiration was performed on 38 neonates at birth, all of which were negative (0%, 95% CI 0%–9.3%). In 11 neonates the nasopharyngeal aspiration was repeated at 24–48 hours, and one was positive (9.1%, 95% CI 0.2%–41.3%).
Discussion
Of the 45 neonates analysed, one was SARS-CoV-2 RT-PCR-positive in the placenta, amniotic fluid and umbilical cord blood, but negative in nasopharyngeal aspirate.
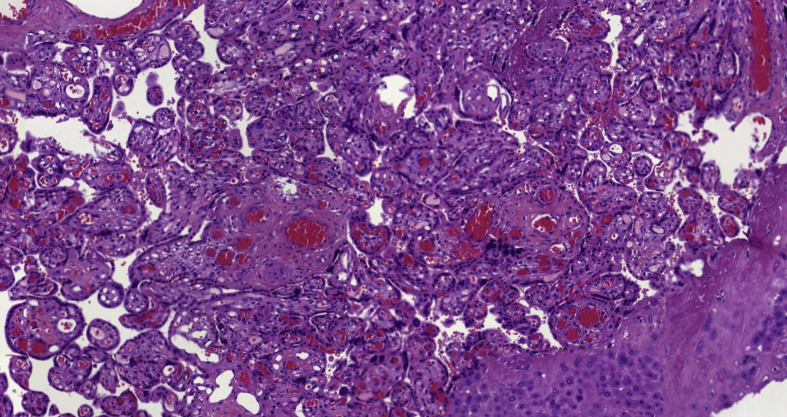
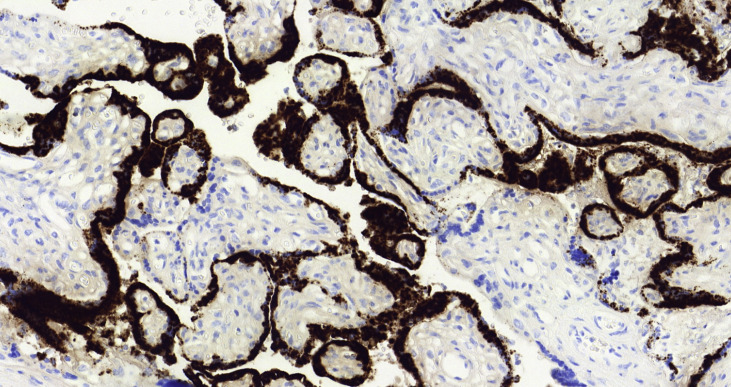
To accept the possibility of mother-to-child transmission of SARS-CoV-2, there are two requirements: a confident diagnostic test to confirm maternal and fetal or neonatal infection and an adequate exclusion of contamination of the samples. In our case, both conditions were met. The mother had COVID-19 confirmed by RT-PCR with clinical symptoms at delivery, and neonatal infection was proven by the detection of SARS-CoV-2 in placenta, amniotic fluid and umbilical cord blood. Strict aseptic measures were taken to collect the samples. Contamination of the placenta can be excluded by the cytoplasmic positivity of trophoblastic cells observed with antibody against SARS-CoV-2 Fig. 2, Fig. 3 . Amniotic fluid and cord blood samples are not easily susceptible to contamination by vaginal fluid or by respiratory droplets from the mother or attendants if strict sterile collection measures are taken. Moreover, the possibility of contamination of both, taken at different times, is extremely unlikely.
Fig. 2.
Detection of SARS-CoV-2 by immunohistochemistry.
Fig. 3.
Detection of SARS-CoV-2 by in situ hybridisation.
Several studies reported SARS-CoV-2 RT-PCR-positive results in neonatal samples within the first hours after birth [[4], [5], [6]], but they did not report placenta, amniotic fluid or umbilical cord blood positive samples. The link between mother and neonate infections is during labour or postnatal, but there is no evidence of longer exposition for the fetus during pregnancy. Only six studies [[8], [9], [10], [11], [12], [13]] and ours support the possibility of intrauterine exposure and transmission to the child.
According to Shah et al. [14] classification system, only the studies by Vivanti et al. [12], Fenizia et al. [13] and us are confirmed cases of congenital infection in a live-born neonate. Other studies [[4], [5], [6],[8], [9], [10], [11]] may only consider possible or even unlikely congenital infection because there are not enough specimens from the mother or the newborn and contamination during labour or caesarean cannot be ruled out.
Our newborn nasopharyngeal RT-PCR was negative. In Vivanti et al., neonatal respiratory sample was obtained from non-bronchoscopic bronchoalveolar lavage before extubation, which is more sensitive. Testing RT-PCR in other tissues may improve the detection of the virus in neonates [15].
Neonatal antibodies were negative at birth and became positive 49 days later. It could be the response of the newborn's immune system to SARS-CoV-2 infection. However, maternal origin postnatally or cross-reactivity with non-specific antibodies can never be excluded entirely.
The sample size is small. As a result of the epidemiological time when the patients were recruited, personal protective equipment was lacking, and ethical approval was substantially delayed, making sample collection challenging. Besides, in some cases, amniocentesis was not possible because of premature rupture of membranes.
The findings of this study support the possibility of mother-to-child transmission, even it seems to be rare. A larger cohort would be necessary to accurately evaluate the rate of congenital transmission and assess the newborn's potential consequences.
Transparency declaration
The authors declare that they have no conflicts of interest.
This study was supported by a grant from Instituto de Salud Carlos III (ISCIII) COV20/00188. The funding sources were not involved in the study design, collection, analysis or interpretation of data, in the writing of the report or in the submission for publication.
Contribution to authorship
This study was conceived by IG, ES, NM and AS, who contributed to the design of the study. Collection and analysis of the data was performed by IG, BS, IF, LR, DS, MF, FC, NF, NM and AS. Analysis of the samples was performed by ES, AA, JE and AN. Drafting the article was performed by IG, ES, NM, EC, NF and AS. All authors, IG, ES, BS, IF, LR, DS, AA, JE, MF, FC, AN, NF, NM, EC, AS and Gestacovid Collaborative Group, reviewed and agreed to the final version of the manuscript.
Details of ethics approval
This study was approved by the Vall d'Hebron University Hospital Ethics Committee (PR(AMI)181/2020) on 27 March 2020.
Acknowledgements
We are grateful to PerkinElmer® for providing the RT-PCR reagents and SYNLAB GmbH for performing the tests of the samples from Hospital Universitario de Torrejón.
Editor: M. Cevik
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.06.016.
Contributor Information
GESTACOVID Collaborative Group:
Jorge Burgos, Vicente Diago, María de la Calle, Marta Muner, Sara Ruiz, Daniel Orós, Olga Ocón, Francisca Sonia Molina García, Mar Gil, and Juan Luis Delgado
Appendix.
GESTACOVID COLLABORATIVE GROUP
Jorge Burgos, MD, PhD. Biocruces Bizkaia Health Research Institute. Osakidetza. Department of Obstetrics and Gynaecology. Hospital Universitario Cruces. UPV/EHU. Barakaldo, Bizkaia, Spain.
Vicente Diago, MD, PhD. Department of Obstetrics, La Fe University Hospital, Valencia, Spain.
Maria de la Calle, MD, PhD. Department of Obstetrics, Hospital Universitario La Paz, Madrid, Spain.
Marta Muner, MD. Department of Obstetrics, Hospital Universitario La Paz, Madrid, Spain.
Sara Ruiz-Martinez, MD. IISA Aragón, Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain.
Daniel Oros, MD, PhD. IISA Aragón, Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain.
Olga Ocón Hernandez, MD, PhD. San Cecilio University Hospital. Granada. Spain. Instituto de Investigación Biosanitaria ibs.GRANADA, Granada, Spain.
Francisca Sonia Molina García, MD, PhD. San Cecilio University Hospital. Granada. Spain. Instituto de Investigación Biosanitaria ibs.GRANADA, Granada, Spain.
Mar Gil, MD, PhD. University Hospital of Torrejon. Madrid. Spain. School of Medicine. Universidad Francisco de Vitoria. Pozuelo de Alarcón, Madrid, Spain.
Juan Luis Delgado, MD, PhD. Virgen de la Arrixaca University Hospital. Murcia. Spain.
Appendix B. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Yan J., Guo J., Fan C., Juan J., Yu X., Li J., et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zambrano L.D., Ellington S., Strid P., Galang R.R., Oduyebo T., Tong V.T., et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status — United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knight M., Bunch K., Vousden N., Morris E., Simpson N., Gale C., et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez-Perez O., Vouga M., Cruz Melguizo S., Forcen Acebal L., Panchaud A., Muñoz-Chápuli M., et al. Association between mode of delivery among pregnant women with COVID-19 and maternal and neonatal outcomes in Spain. JAMA. 2020;324:296–299. doi: 10.1001/jama.2020.10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan S., Jun L., Nawsherwan, Siddique R., Li Y., Han G., et al. Association of COVID-19 with pregnancy outcomes in health-care workers and general women. Clin Microbiol Infect. 2020;26:788–790. doi: 10.1016/j.cmi.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahyuddin A., Kanneganti A., Wong J., Dimri P.S., Su L., Biswas A., et al. Mechanisms and evidence of vertical transmission of infections in pregnancy including SARS-CoV-2. Prenatal Diagn. 2020 doi: 10.1002/pd.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baud D., Greub G., Favre G., Gengler C., Jaton K., Dubruc E., et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020;323:2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penfield C.A., Brubaker S.G., Limaye M.A., Lighter J., Ratner A.J., Thomas K.M., et al. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am JObstet Gynecol MFM. 2020:100133. doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Algarroba G.N., Rekawek P., Vahanian S.A., Khullar P., Palaia T., Peltier M.R., et al. Visualisation of SARS-CoV-2 virus invading the human placenta using electron microscopy. Am JObstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patanè L., Morotti D., Giunta M.R., Sigismondi C., Piccoli M.G., Frigerio L., et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am JObstet Gynecol MFM. 2020:100145. doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J., et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenizia C., Biasin M., Cetin I., Vergani P., Mileto D., Spinillo A., et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun. 2020;11:5128. doi: 10.1038/s41467-020-18933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah P.S., Diambomba Y., Acharya G., Morris S.K., Bitnun A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020;99:565–568. doi: 10.1111/aogs.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeffelholz M.J., Tang Y.W. Laboratory diagnosis of emerging human coronavirus infections–the state of the art. Emerg Microb Infect. 2020;9:747–756. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.