Abstract
Objectives
To develop radiomics-based nomograms for preoperative microvascular invasion (MVI) and recurrence-free survival (RFS) prediction in patients with solitary hepatocellular carcinoma (HCC) ≤ 5 cm.
Methods
Between March 2012 and September 2019, 356 patients with pathologically confirmed solitary HCC ≤ 5 cm who underwent preoperative gadoxetate disodium–enhanced MRI were retrospectively enrolled. MVI was graded as M0, M1, or M2 according to the number and distribution of invaded vessels. Radiomics features were extracted from DWI, arterial, portal venous, and hepatobiliary phase images in regions of the entire tumor, peritumoral area ≤ 10 mm, and randomly selected liver tissue. Multivariate analysis identified the independent predictors for MVI and RFS, with nomogram visualized the ultimately predictive models.
Results
Elevated alpha-fetoprotein, total bilirubin and radiomics values, peritumoral enhancement, and incomplete or absent capsule enhancement were independent risk factors for MVI. The AUCs of MVI nomogram reached 0.920 (95% CI: 0.861–0.979) using random forest and 0.879 (95% CI: 0.820–0.938) using logistic regression analysis in validation cohort (n = 106). With the 5-year RFS rate of 68.4%, the median RFS of MVI-positive (M2 and M1) and MVI-negative (M0) patients were 30.5 (11.9 and 40.9) and > 96.9 months (p < 0.001), respectively. Age, histologic MVI, alkaline phosphatase, and alanine aminotransferase independently predicted recurrence, yielding AUC of 0.654 (95% CI: 0.538–0.769, n = 99) in RFS validation cohort. Instead of histologic MVI, the preoperatively predicted MVI by MVI nomogram using random forest achieved comparable accuracy in MVI stratification and RFS prediction.
Conclusions
Preoperative radiomics-based nomogram using random forest is a potential biomarker of MVI and RFS prediction for solitary HCC ≤ 5 cm.
Key Points
• The radiomics score was the predominant independent predictor of MVI which was the primary independent risk factor for postoperative recurrence.
• The radiomics-based nomogram using either random forest or logistic regression analysis has obtained the best preoperative prediction of MVI in HCC patients so far.
• As an excellent substitute for the invasive histologic MVI, the preoperatively predicted MVI by MVI nomogram using random forest (MVI-RF) achieved comparable accuracy in MVI stratification and outcome, reinforcing the radiologic understanding of HCC angioinvasion and progression.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00330-020-07601-2.
Keywords: Hepatocellular carcinoma, Magnetic resonance imaging, Neoplasm recurrence
Introduction
Hepatocellular carcinoma (HCC) is the sixth most prevalent neoplasm and the third leading cause of cancer death [1]. Despite curative therapies, the outcome of HCC patients remains poor, with 5-year recurrence rates reaching 50–70% after hepatectomy and < 35% after liver transplantation [2–6].
Microvascular invasion (MVI), present in 15–57.1% surgical specimens of HCC [7], is a well-established risk factor for postoperative recurrence [8, 9], even for solitary small HCC [10]. To improve the prognosis of MVI-positive patients, a wide resection margin is recommended [11]. Therefore, preoperative diagnosis of MVI is of great importance for treatment strategies.
MVI is defined as the cancer cell nest in vessels lined with endothelium, which is visible only on microscopy [7, 12] and poses a challenge for non-invasive diagnosis. Recently, preoperatively radiologic hallmarks including non-smooth tumor margin, peritumoral enhancement on arterial phase (AP), and peritumoral hypointensity on hepatobiliary phase (HBP) have shown to be conducive to MVI diagnosis but be inferior to radiomics signatures [13]. As a novel and non-invasive tool, radiomics can high-throughput extract quantitative imaging signatures to improve diagnostic or prognostic accuracy [14], which is also applicable to preoperative MVI and outcome prediction. Being related with postoperative recurrence and metastasis, peritumoral area of HCC is rich in highly invasive cells and susceptible to the formation of MVI [12], where it has been neglected in previous radiomics studies [11, 15, 16]. While gadoxetate disodium–enhanced (Gd-EOB-DTPA) MRI offers the identifiability of small or early HCC and the information of tumor heterogeneity and vascularization [17], previous radiomics studies [11, 13] mainly focused on HBP images for predicting MVI. Thus, it is reasonable to investigate whether radiomics signatures extracted from intratumoral and peritumoral regions on multi-parametric images of Gd-EOB-DTPA MRI may allow more effective MVI prediction.
This study aimed to develop and validate nomograms based on multi-scale and multi-parametric radiomics of Gd-EOB-DTPA MRI for the preoperative MVI and outcome prediction in patients with solitary HCC ≤ 5 cm.
Materials and methods
Study design and patients
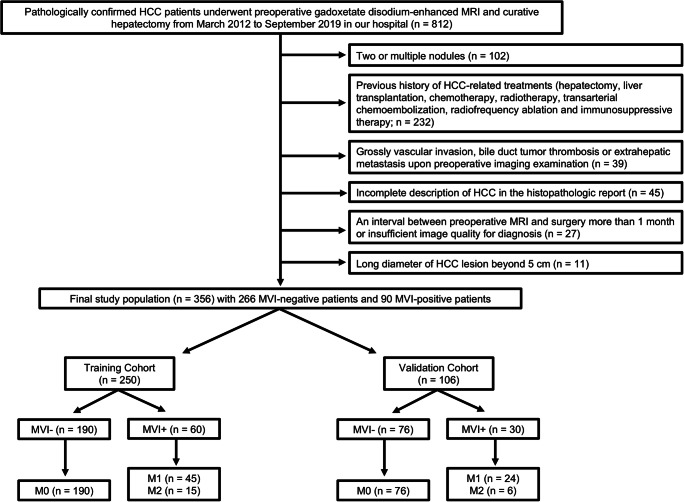
Our hospital ethics committee approved this retrospective study and waived patient informed consent. Between March 2012 and September 2019, 356 pathologically confirmed HCC patients (303 males and 53 females; 54.22 ± 11.40 years) with preoperative Gd-EOB-DTPA MRI met the inclusion criteria (Fig. 1): (a) solitary HCC with the longest diameter ≤ 5 cm; (b) without gross vascular invasion, bile duct tumor thrombosis or extrahepatic metastasis upon preoperative imaging; (c) without previous history of HCC-related treatments (hepatectomy, liver transplantation, chemotherapy, radiotherapy, transarterial chemoembolization, radiofrequency ablation, and immunosuppressive therapy); (d) complete histopathologic description of HCC; (e) MRI with sufficient image quality scanned within 1 month before surgery.
Fig. 1.
Flowchart of the study population
Laboratory examinations and histopathology
Preoperative laboratory indexes (Table 1) comprised serum alpha-fetoprotein (AFP), carcinoembryonic antigen, carbohydrate antigen 19-9, des-gamma-carboxy prothrombin, hepatitis B virus (HBV), anti-hepatitis C virus, HBV-DNA loads, α-L-fucosidase, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin, r-glutamyltransferase, alkaline phosphatase (AKP), total protein, albumin, platelet count, prothrombin time, activated partial thromboplastin time, fibrinogen, thrombin time, prealbumin, hyaluronic acid, procollagen type III, type IV collagen, and laminin. The Child-Pugh and Barcelona Clinic Liver Cancer (BCLC) stages were also included in this study.
Table 1.
Clinical and radiologic hallmarks of the primary cohorts
| Variables | Training cohort (n = 250) | Validation cohort (n = 106) | pInter | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MVI- | MVI+ | pIntra | OR (95% CI) | MVI- | MVI+ | pIntra | OR (95% CI) | ||
| Age, mean (SD), years | 54.12 (12.26) | 53.73 (9.89) | 0.823 | 0.997 (0.973, 1.022) | 54.51 (10.20) | 55.07 (11.73) | 0.900 | 0.913 (0.22, 3.792) | 0.627 |
| Sex (male/female) | 165/25 | 50/10 | 0.496 | 1.320 (0.594, 2.934) | 63/13 | 25/5 | 0.957 | 0.969 (0.313, 3.002) | 0.470 |
| BCLC (0/A stage) | 101/89 | 22/38 | 0.027 | 1.960 (1.079, 3.562) | 49/27 | 6/24 | < 0.001 | 7.259 (2.643, 19.940) | 0.643 |
| Child-Pugh (A / B stage) | 187/3 | 57/3 | 0.153 | 3.281 (0.644, 16.703) | 74/2 | 29/1 | 0.845 | 1.276 (0.111, 14.617) | 1.000 |
| HBV or HCVa | 24/166 | 7/53 | 0.840 | 1.095 (0.446, 2.684) | 9/67 | 5/25 | 0.511 | 0.672 (0.205, 2.198) | 0.834 |
| HBV-DNA loads (≤ 104/> 104) | 156/22 | 49/6 | 0.773 | 0.868 (0.333, 2.263) | 62/10 | 24/3 | 0.716 | 0.775 (0.196, 3.061) | 0.778 |
| AFP (≤ 20, 20–400, > 400 ng/mL) | 107/56/22 | 18/28/10 | 0.004 | 1.827 (1.211, 2.755) | 48/25/3 | 12/7/9 | 0.003 | 2.640 (1.405, 4.959) | 0.610 |
| DCP (≤ 40/> 40 mAU/mL) | 67/34 | 12/18 | 0.011 | 2.956 (1.277, 6.84) | 43/23 | 8/14 | 0.021 | 3.272 (1.197, 8.942) | 0.728 |
| TBIL (≤ 20.4/> 20.4 μmol/L) | 173/17 | 50/10 | 0.098 | 2.035 (0.877, 4.724) | 66/10 | 24/6 | 0.379 | 1.650 (0.541, 5.030) | 0.256 |
| TP (≤ 65/> 65 g/L)b | 46/144 | 21/39 | 0.102 | 0.593 (0.317, 1.109) | 14/62 | 4/26 | 0.531 | 1.468 (0.441, 4.882) | 0.047 b |
| APTT (≤ 31.3/> 31.3 s)b | 164/26 | 48/12 | 0.238 | 1.577 (0.741, 3.358) | 71/5 | 27/3 | 0.551 | 1.578 (0.353, 7.060) | 0.049 b |
| FIB (≤ 200/> 200 mg/dL)b | 58/132 | 23/37 | 0.261 | 0.707 (0.380, 1.295) | 16/60 | 6/24 | 0.904 | 1.067 (0.373, 3.051) | 0.027 b |
| Other laboratory indexes | / | / | > 1.000 | / | / | > 1.000 | > 0.050 | ||
| Tumor size (≤ 2/2–5 cm) | 112/78 | 23/37 | 0.006 | 2.310 (1.274, 4.189) | 51/25 | 7/23 | < 0.001 | 6.703 (2.536, 17.717) | 0.901 |
| T1HBP, mean (SD) | 456.94 (141.61) | 528.39 (173.34) | 0.007 | 1.003 (1.001, 1.005) | 440.28 (123.51) | 522.78 (205.95) | 0.040 | 1.004 (1.000,1.008) | 0.635 |
| T1pre, mean (SD) | 948.65 (260.27) | 1020.73 (270.45) | 0.079 | 1.001 (1.000, 1.002) | 951.96 (286.40) | 975.93 (230.20) | 0.681 | 1.000 (0.999,1.002) | 0.830 |
| Edge roughness, mean (SD) | 0.15 (0.09) | 0.23 (0.15) | < 0.001 | 442.52 (22.78, 8597.07) | 0.13 (0.07) | 0.22 (0.12) | < 0.001 | 118262.81 (197.05, 70979058.40) | 0.227 |
| Typical MRI patterna | 21/169 | 6/54 | 0.819 | 1.118 (0.429, 2.914) | 8/68 | 1/29 | 0.257 | 3.412 (0.408, 28.533) | 0.819 |
| Peritumoral enhancement a | 172/18 | 27/33 | < 0.001 | 11.679 (5.781, 23.593) | 68/8 | 15/15 | < 0.001 | 6.317 (2.370, 16.843) | 0.783 |
| Peritumoral hypointensitya | 180/10 | 36/24 | < 0.001 | 12.0 (5.286, 27.244) | 67/9 | 19/11 | 0.005 | 4.310 (1.558, 11.924) | 0.205 |
| Capsule enhancement (intact/incomplete/absent) | 21/30/139 | 34/20/6 | < 0.001 | 0.180 (0.116, 0.278) | 13/6/57 | 9/13/8 | 0.001 | 0.408 (0.241, 0.689) | 0.836 |
Other laboratory indexes: α-L-fucosidase (≤ 40/> 40 U/L), carcinoembryonic antigen (≤ 5/> 5 ng/mL), carbohydrate antigen 19–9 (≤ 34/> 34 ng/mL), albumin (≤ 35/> 35 g/L), direct bilirubin (≤ 6.8/> 6.8 umol/L), alanine aminotransferase (≤ 50/> 50 U/L), aspartate aminotransferase (≤ 40/> 40 U/L), alkaline phosphatase (≤ 125/> 125 U/L), r-glutamyltransferase (≤ 60/> 60 U/L), total bile acid (≤ 10/> 10 umol/L), platelet count (≤ 100 × 109/L/> 100 × 109/L), prothrombin time (≤ 13/> 13 s), thrombin time (≤ 21/> 21 s), hyaluronic acid (≤ 120/> 120 ng/mL), laminin (≤ 130/> 130 ng/mL), procollagen type III (≤ 15/> 15 ng/mL), type IV collagen (≤ 95/> 95 ng/mL)
Abbreviations: OR, odds ratio; HBV, hepatitis B virus; HCV, hepatitis C virus; HBV-DNA, deoxyribonucleic acid of hepatitis B virus; AFP, alpha-fetoprotein; DCP, des-gamma-carboxy prothrombin; TBIL, total bilirubin: BCLC, Barcelona Clinic Liver Cancer; TP, total protein; APTT, activated partial thromboplastin time; FIB, fibrinogen; T1PRE and T1HBP, defined as the signal intensity of tumor derived from the pre-contrast and hepatobiliary phase T1 maps, respectively
aAbsence/presence
pIntra: p value of univariate logistic regression analysis between the MVI+ and MVI− groups; p Inter: p value of the inter-cohort difference with chi-square test for categorical variables and independent samples t test for numeric variables
bpInter < 0.05: a significant difference between the training and validation cohorts, which was enrolled in the multivariate logistic regression analysis
HCC pathological samples were taken by a 7-point baseline sample collection protocol [12]. Histopathological characteristics (tumor size, number, Edmondson-Steiner grade, MVI status and category, liver fibrosis grade based on the Scheuer scoring system, and Ki-67 protein expression) were assessed in consensus by two experienced abdominal pathologists.
MVI was defined as the presence of tumor in the portal vein, hepatic vein, or a large capsular vessel of the surrounding hepatic tissue lined with endothelium, which was visible only on microscopy [7, 11, 12, 18]. According to the high-risk factors of adverse outcomes [12, 18, 19], the patients were classified into M0 (no MVI), M1 (invaded vessels were no more than five and located at the peritumoral region adjacent to the tumor surface within 1 cm), or M2 (MVI of > 5 or at > 1 cm away from the tumor surface) grades [12], respectively.
Gd-EOB-DTPA MRI
MRI was performed at a 1.5-T scanner (Magnetom Aera, Siemens Healthcare) with intravenous bolus injection of 0.025 mmol/kg gadoxetate disodium (Primovist, Bayer Pharma). MRI sequences were as follows: axial T2-weighted imaging with fat suppression, DWI, in-phase and opposed-phase T1-weighted imaging (T1WI), pre-contrast three-dimensional volumetric-interpolated breath-hold (3D-VIBE) T1WI, post-contrast dynamic 3D-VIBE-T1WI (AP, 20–30 s; portal venous phase: PVP, 60–70 s; transitional phase: TP, 180 s; HBP: 20 min) after the injection of gadoxetate disodium, and automatically reconstructed pre-contrast and HBP T1 maps. Detailed parameters are shown in Table S1.
Qualitative and quantitative analyses of MRI
Morphologic hallmarks (typical MRI pattern of HCC [1, 20], peritumoral enhancement [21], capsule enhancement [22], the longest diameter of tumor [23], and peritumoral hypointensity on HBP images [21]) were independently reviewed by two radiologists (S.X.R. and C.Y., 20 and 15 years of abdominal MRI experience) who were blinded to MVI status. Meanwhile, the average signal intensity of tumor on the pre-contrast and HBP T1 maps were measured and defined as T1PRE and T1HBP, respectively. In case of any discrepancies, a consensus was reached after discussion. Instead of the subjective evaluation of tumor edge, “edge roughness” was automatically and quantitatively computed as the average distance from the actual tumor surface to its convex envelope on HBP images. Namely, edge roughness was a continuous value to measure the non-smoothness of tumor edge.
Radiomics analysis
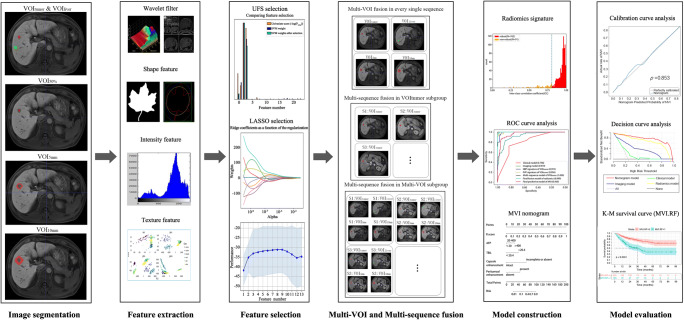
Radiomics was implemented by Python programming language (version 3.7.3, https://www.python.org) with Pyradiomics (version 2.2.0, https://pyradiomics.readthedocs.io/en/latest/index.html) and Scikit-learn (version 2.1.0, https://scikit-learn.org/stable/index.html) packages. Radiomics workflow comprised manual tumor segmentation, feature extraction and selection, multiple sequences and volumetric interests (VOIs) fusion, and model construction and evaluation (Fig. 2).
Fig. 2.
Flowchart of radiomics analysis
First, tumor boundaries were manually delineated on all single sequence images, denoted as VOItumor, by two radiologists (H.H.C. and L.Y., 8 years and 10 years of abdominal imaging experiences) with ITK-SNAP software (http://www.itksnap.org/pmwiki/pmwiki.php). Besides, the two radiologists randomly picked 5 to 10 blocks distributed in different liver lobes sufficiently away from large vessels, artifacts, liver margins, and hepatic lesions, which were used as regions of normal liver tissues (VOIliver) for contrast analysis with tumor. To further explore the intratumoral and peritumoral information, the VOItumor was shrunk 50% (VOI50%) and dilated by 5 mm and 10 mm (VOI5mm and VOI10mm) using standard image morphological erosion and dilation operations, respectively. Please note that VOI5mm and VOI10mm excluded the tumor region and only referred to the peritumoral zone within 5 mm and 10 mm from the tumor surface. Meanwhile, a variety of regional combinations were experimented, including VOItumor + liver which combined the tumor (VOItumor) and the liver background (VOIliver) regions; VOItumor + 5mm and VOItumor + 10mm joined the tumor (VOItumor) with peripheral zones VOI5mm and VOI10mm, respectively, based on which VOItumor + 5mm + liver or VOItumor + 10mm + liver was defined with additional VOIliver merged.
Subsequently, a set of 854 features radiomics features were extracted from the original and three-dimensional wavelet filters images [24], including tumor shape, size, intensity, and texture (Table S2). These features were first selected by the Least Absolute Shrinkage and Selection Operator (LASSO, Table S3) for each VOI of each single sequence. The first selected features were then combined to obtain the optimal multi-VOI models in single sequences (Tables 2 and S4). These multi-VOI features of each sequence were finally joined and selected using LASSO again (Table S5) to derive the ultimate multi-sequence, multi-VOI radiomics model (Table S6), and based on which MVI nomograms were constructed with random forest (RF) and logistic regression (LR) classifiers respectively for comparison. Finally, the receiver operating characteristic, calibration, and decision curves were plotted and the validation data was tested for model evaluation.
Table 2.
Results of single sequences based on multiple volumetric interests for predicting MVI
| Sequence | Classifier and cohort | AUC | ||||||
|---|---|---|---|---|---|---|---|---|
| VOI50% | VOItumor | VOItumor + 5mm | VOItumor + 10mm | VOItumor + liver | VOItumor + 5mm + liver | VOItumor + 10mm + livera | ||
| T2WI | RF (TD/VD) | 0.818/0.722 | 0.832/0.714 | 0.897/0.730 | 0.816/0.742 | 0.841/0.726 | 0.867/0.749 | 0.975/0.755 |
| LR (TD/VD) | 0.641/0.698 | 0.647/0.708 | 0.632/0.725 | 0.650/0.712 | 0.647/0.708 | 0.632/0.725 | 0.638/0.727 | |
| DWI | RF (TD/VD) | 0.830/0.736 | 0.980/0.778 | 0.879/0.793 | 0.828/0.791 | 0.813/0.784 | 0.832/0.793 | 0.978/0.812 |
| LR (TD/VD) | 0.695/0.701 | 0.752/0.703 | 0.663/0.775 | 0.655/0.777 | 0.681/0.731 | 0.664/0.774 | 0.667/0.780 | |
| PRE | RF (TD/VD) | 0.829/0.737 | 0.938/0.765 | 0.898/0.771 | 0.813/0.761 | 0.991/0.782 | 0.878/0.797 | 0.912/0.810 |
| LR(TD/VD) | 0.746/0.749 | 0.730/0.752 | 0.728/0.757 | 0.728/0.757 | 0.730/0.761 | 0.730/0.773 | 0.735/0.782 | |
| Pre-T1 maps | RF (TD/VD) | 0.802/0.793 | 0.720/0.714 | 0.642/0.738 | 0.669/0.758 | 0.677/0.717 | 0.826/0.740 | 0.752/0.766 |
| LR(TD/VD) | 0.633/0.765 | 0.658/0.724 | 0.643/0.714 | 0.631/0.746 | 0.648/0.715 | 0.652/0.714 | 0.637/0.754 | |
| AP | RF (TD/VD) | 0.980/0.685 | 0.873/0.765 | 1.000/0.812 | 0.996/0.802 | 0.948/0.777 | 0.886/0.815 | 0.944/0.830 |
| LR (TD/VD) | 0.701/0.692 | 0.715/0.693 | 0.686/0.746 | 0.731/0.742 | 0.639/0.719 | 0.821/0.761 | 0.715/0.761 | |
| PVP | RF (TD/VD) | 0.920/0.740 | 0.996/0.810 | 0.876/0.832 | 0.808/0.818 | 0.902/0.825 | 0.902/0.836 | 0.912/0.837 |
| LR (TD/VD) | 0.761/0.706 | 0.755/0.768 | 0.728/0.798 | 0.731/0.799 | 0.733/0.796 | 0.732/0.800 | 0.727/0.806 | |
| TP | RF (TD /VD) | 0.900/0.729 | 0.963/0.728 | 0.995/0.738 | 0.854/0.778 | 0.884/0.749 | 0.871/0.762 | 0.802/0.792 |
| LR (TD /VD) | 0.716/0.683 | 0.718/0.716 | 0.720/0.707 | 0.739/0.754 | 0.720/0.725 | 0.736/0.720 | 0.751/0.762 | |
| HBP | RF (TD/VD) | 0.712/0.784 | 0.991/0.799 | 0.874/0.831 | 0.976/0.789 | 1.000/0.808 | 0.866/0.827 | 0.885/0.855 |
| LR (TD/VD) | 0.676/0.723 | 0.744/0.746 | 0.678/0.735 | 0.770/0.759 | 0.743/0.762 | 0.751/0.803 | 0.715/0.805 | |
| HBP-T1 maps | RF (TD/VD) | 0.923/0.718 | 0.808/0.705 | 0.821/0.726 | 0.821/0.726 | 0.822/0.724 | 0.822/0.729 | 0.807/0.731 |
| LR (TD/VD) | 0.705/0.703 | 0.706/0.703 | 0.691/0.708 | 0.684/0.715 | 0.683/0.714 | 0.705/0.715 | 0.702/0.716 | |
Abbreviations: VOI, volumetric interest; AUC, area under the curve; VD, validation dataset; TD, training dataset; RF, random forest; LR, logistic regression; T2WI, T2-weighted imaging with fat suppression; DWI, diffusion-weighted imaging; PRE, pre-contrast phase; AP, arterial phase; PVP, portal venous phase; TP, transitional phase; HBP, hepatobiliary phase
a The sensitivity, specificity, and AUC of VOItumor + 10mm + liver using random forest in each single sequence for predicting histologic MVI are listed in Table S4
Italicized values indicated the highest AUC of validation cohort in each single sequence
Outcome analysis
Follow-up was performed at intervals of 3 to 6 months after curative surgery. The date of surgery, recurrence, metastasis, death, and the last follow-up were recorded for calculating the overall and recurrence-free survival (RFS).
Statistical analyses
Statistical analyses were performed with IBM SPSS Statistics (version 25) and R (version 3.6.1, https://www.r-project.org) software. Patients enrolled in MVI or outcome study were randomly allocated to training and validation cohorts in a ratio of 7:3. The discrimination performance of models was quantified by area under the curve (AUC) and net reclassification index (NRI). NRI > 0 meant a positive improvement, indicating that the predictive ability of the new model precedes the old one. Compared to the histologic MVI, the preoperatively predicted MVI status was calculated by MVI nomogram using RF (MVI-RF) or LR (MVI-LR) in each patient, with prediction probabilities > 50% classified into MVI-positive group and > 90% defined as M2 grade. P < 0.05 was considered statistically significant.
More details (T1 maps and morphologic hallmarks, feature extraction and selection, and statistical analyses) are available in the Supplementary Materials and Methods.
Results
Clinicoradiologic characteristics and performances for predicting MVI
Among the 356 solitary HCC patients, only 90 patients suffered from MVI, 347 patients underwent hepatectomy, and 9 patients received liver transplantation. The univariate LR results of clinicoradiologic characteristics are summarized in Table 1. In multivariate LR analysis of the training cohort, AFP > 20 ng/mL (p = 0.006, OR = 7.683, 95% CI: 1.776–33.245) and TBIL > 20.4 μmol/L (p = 0.010, OR = 8.420, 95% CI: 1.658–42.766) were independent risk factors for MVI in the clinical model.
Edge roughness was significantly different between MVI-negative and MVI-positive patients (0.147 ± 0.080 vs 0.224 ± 0.137, p < 0.001), indicating higher value of edge roughness (less spheroid of tumor) was positively correlated with MVI. In multivariate LR analysis, absent or incomplete capsule enhancement (p < 0.001, OR = 18.678, 95% CI: 6.129–56.925), higher value of edge roughness (p = 0.05, OR = 68.886, 95% CI: 0.974–4874.172), and peritumoral enhancement (p < 0.001, OR = 5.721, 95% CI: 2.161–15.151) were independent risk factors for MVI in the imaging model. Representative MVI images are shown in Fig. 3. The MVI predictive performances of clinical and imaging models are summarized in Table 3.
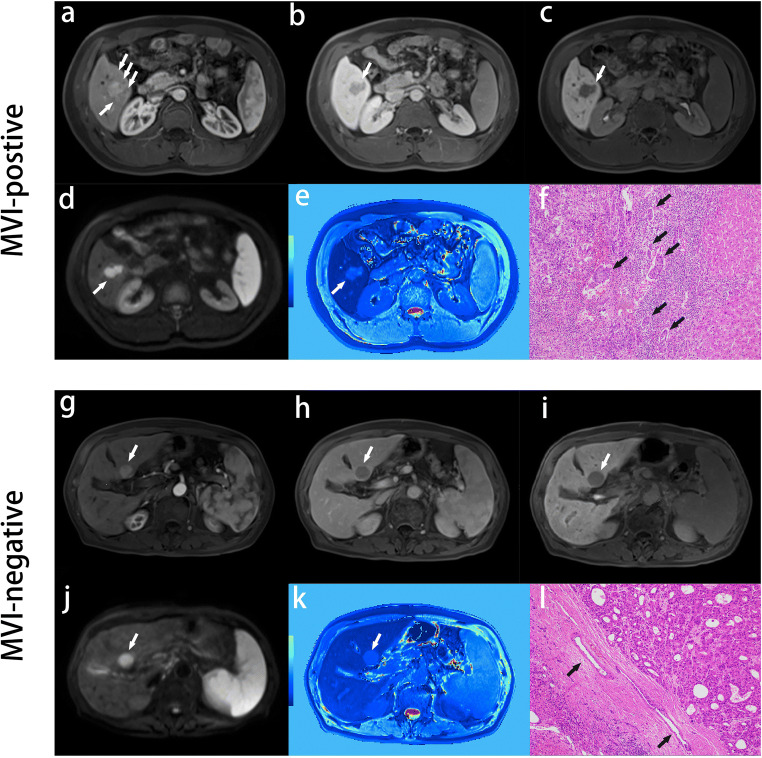
Fig. 3.
Representative images of MVI-positive and MVI-negative patients. MVI-positive case: A 51-year-old male with elevated AFP, TBIL, and AKP levels (320 ng/mL, 32.6 μmol/L, and 131 U/L) was admitted to our department for abdominal discomfort and yellow sclera and identified intrahepatic recurrence at 11 months after hepatectomy. Gd-EOB-DTPA MRI detected a solid lesion (2.9 × 1.9 cm) in hepatic segment V, with the architectures of wedge-shaped peritumoral enhancement on arterial phase images (a, arrows), absent capsule enhancement on transitional phase images (b, arrows), non-smooth tumor edge on HBP, DWI, and HBP T1 maps (c–e, arrows), and typical MRI pattern of HCC (non-rim arterial phase enhancement and non-peripheral transitional phase hypointensity). M2 grade was diagnosis by postoperative pathological specimens with standard hematoxylin and eosin (HE, × 100): multiple tumor thrombi of microvasculature (f, black arrow; MVI > 5) were distributed in the widespread inflammatory cells, which were located at the region between the normal liver tissue in the right side and the infiltrating HCC lesion without tumor capsule in the upper left corner. MVI-negative case: A 77-year-old male with normal levels of AFP, TBIL, and AKP (3.4 ng/mL, 11.7 μmol/L, and 90 U/L) was admitted to our hospital for a liver lesion in health examination, and identified recurrence-free until April 2020 (18 months after hepatectomy). Gd-EOB-DTPA MRI detected a well-circumscribed solid lesion (2.3 × 2.0 cm) in hepatic segment II, with the architectures of absent peritumoral enhancement (g, arrows), intact capsule enhancement (h, arrows), smooth tumor margin (i–k, arrow), and typical MRI pattern of HCC. M0 grade was diagnosed by pathologic HE (× 100) sample: no tumor thrombus was detected in microvascular system (l, black arrow), which were located at the region between the normal liver tissue in the lower left corner and the HCC lesion with intact capsule in the upper right corner
Table 3.
The performance of the clinical, imaging, radiomics model and the nomogram for predicting MVI
| Models | Classifier | Training cohort (n = 205) | Validation cohort (n = 106) | Cutoff | ||||
|---|---|---|---|---|---|---|---|---|
| Sen | Spe | AUC (95% CI) | Sen | Spe | AUC (95% CI) | |||
| Clinical | RF | 0.72 | 0.83 | 0.798 (0.739–0.857) | 0.73 | 0.59 | 0.725 (0.647–0.803) | 0.25 |
| LR | 0.73 | 0.72 | 0.779 (0.719–0.837) | 0.70 | 0.55 | 0.668 (0.570–0.766) | 0.17 | |
| Imaging | RF | 0.83 | 0.88 | 0.919 (0.880–0.958) | 0.77 | 0.87 | 0.876 (0.816–0.934) | 0.31 |
| LR | 0.82 | 0.84 | 0.894 (0.855–0.933) | 0.83 | 0.67 | 0.792 (0.713–0.869) | 0.13 | |
| Radiomics a | RF | 1.00 | 0.97 | 0.999 (0.999–0.999) | 0.96 | 0.86 | 0.918 (0.859–0.977) | 0.26 |
| LR | 0.70 | 0.69 | 0.773 (0.714–0.832) | 0.63 | 0.88 | 0.809 (0.731–0.887) | 0.27 | |
| Nomogram | RF | 0.87 | 0.94 | 0.960 (0.940–0.980) | 0.93 | 0.85 | 0.920 (0.861–0.979) | 0.23 |
| LR | 0.92 | 0.84 | 0.934 (0.895–0.973) | 0.93 | 0.75 | 0.879 (0.820–0.938) | 0.19 | |
Abbreviations: RF, random forest; LR, logistic regression; Sen, sensitivity; Spe, specificity; AUC, area under the curve; CI, confidence interval
Radiomics a: the final radiomics model based on the multi-parametric (arterial phase, portal venous phase, hepatobiliary phase T1-weighted image, and diffusion-weighted imaging) fusion in VOItumor + 10mm + liver
Performance of radiomics features from single sequences
The AUCs of each VOI in single sequences are displayed in Table 2. For the vast majority of VOIs and sequences, RF outperformed LR classifier, HBP, and PVP were superior to other sequences, and the VOItumor + 10mm + liver yielded the best multi-VOI fusion for predicting MVI. Interestingly, the AUCs of VOI50%, VOItumor, VOItumor + 5mm, VOItumor + 10mm, and VOItumor + 10mm + liver subgroups approximately kept increasing almost in all sequences regardless of the choice of classifiers. Notably, VOItumor + 10mm + liver showed consistent performance improvements compared to VOItumor in HBP and PVP sequences on the validation cohort (NRIs > 0, Table 4).
Table 4.
Net reclassification indexes and p values of diverse combinations
| Subgroups | Diverse combinations | Classifier and cohort | NRI (%) | p (NRI) | p (AUC) |
|---|---|---|---|---|---|
| Single sequence | VOItumor + 10mm + liver vs. VOItumor on HBP | RF (TD/VD) | - 31.03%/17.70% | 1.000/0.072 | 0.960/0.313 |
| LR (TD/VD) | - 10.34%/6.44% | 0.971/0.169 | 0.700/0.245 | ||
| VOItumor + 10mm + liver vs. VOItumor on PVP | RF (TD/VD) | - 24.35%/7.81% | 1.000/0.187 | 0.915/0.371 | |
| LR (TD/VD) | - 3.38%/0.44% | 0.770/0.486 | 0.672/0.334 | ||
| VOItumor | Multi-parametric a vs. HBP | RF (TD/VD) | 5.68%/19.28% | 0.002/0.046 | 0.441/0.206 |
| LR (TD/VD) | 10.96%/3.24% | 0.049/0.408 | 0.134/0.294 | ||
| Multi-parametric a vs. PVP | RF (TD/VD) | 6.49%/20.90% | 0.021/0.017 | 0.467/0.238 | |
| LR (TD/VD) | 2.76%/1.35% | 0.307/0.410 | 0.165/0.393 | ||
| VOItumor + 10mm + liver | Multi-parametric b vs. HBP | RF (TD/VD) | 35.04%/19.44% | < 0.001/0.008 | 0.030/0.192 |
| LR (TD/VD) | 11.14%/3.24% | 0.031/0.391 | 0.173/0.482 | ||
| Multi-parametric b vs. PVP | RF (TD/VD) | 27.99%/24.54% | < 0.001/0.003 | 0.075/0.180 | |
| LR (TD/VD) | - 0.11%/4.63% | 0.507/0.349 | 0.229/0.486 | ||
| Model | Radiomics vs. Clinical model | RF (TD/VD) | 41.8%/54.1% | 0.001/0.002 | 0.012/0.050 |
| LR (TD/VD) | - 16.1%/11.1% | 0.856/0.298 | 0.527/0.116 | ||
| Radiomics vs. Imaging model | RF (TD/VD) | 25.7%/22.2% | < 0.001/0.029 | 0.095/0.321 | |
| LR (TD/VD) | - 26.1%/2.3% | 0.997/0.442 | 0.977/0.426 | ||
| Nomogram vs. Clinical model | RF (TD/VD) | 19.7%/56.8% | 0.091/< 0.001 | 0.004/0.013 | |
| LR (TD/VD) | 14.6%/47.7% | 0.133/0.005 | 0.005/0.009 | ||
| Nomogram vs. Imaging model | RF (TD/VD) | 9.1%/14.0% | 0.038/0.075 | 0.249/0.309 | |
| LR (TD/VD) | 9.5%/78.9% | 0.070/0.041 | 0.254/0.163 | ||
| Nomogram vs. Radiomics model | RF (TD/VD) | - 16.3%/- 2.8% | 0.999/0.647 | 0.790/0.491 | |
| LR (TD/VD) | 35.7%/19.4% | < 0.001/0.054 | 0.004/0.217 |
Net reclassification index (NRI): NRI > 0 was a positive improvement, indicating that the predictive ability of the new model was better than the old one
Abbreviations: AUC, area under curve; VD, validation dataset; TD, training dataset; RF, random forest; LR, logistic regression
Multi-parametric a: the best combination (portal venous phase, hepatobiliary phase, arterial phase T1-weighted image, and pre-contrast T1 map) in the VOItumor subgroup
Multi-parametric b or Radiomics model: the optimal radiomics model based on the best combination (portal venous phase, hepatobiliary phase, arterial phase T1-weighted image, and diffusion-weighted imaging) in the VOItumor + 10mm + liver subgroup
Italicized values: p < 0.05 in the validation cohort
Performance of radiomics features from multiple sequences
In the VOItumor subgroup, the MVI predictive efficacies of two best single sequences (HBP and PVP) were worse than any of the multi-sequence models either using RF or LR (Table S7), especially inferior to that of the best combination (PVP, HBP, AP, and pre-contrast T1 maps; AUCs of validation cohort: 0.871 using RF and 0.792 using LR; Fig. 4). Concretely, this four-sequence model showed significant improvements compared to the two best single sequences (HBP: NRI 19.28%, p = 0.046; PVP: NRI 20.90%, p = 0.017; Table 4 ) in the validation cohort using RF.
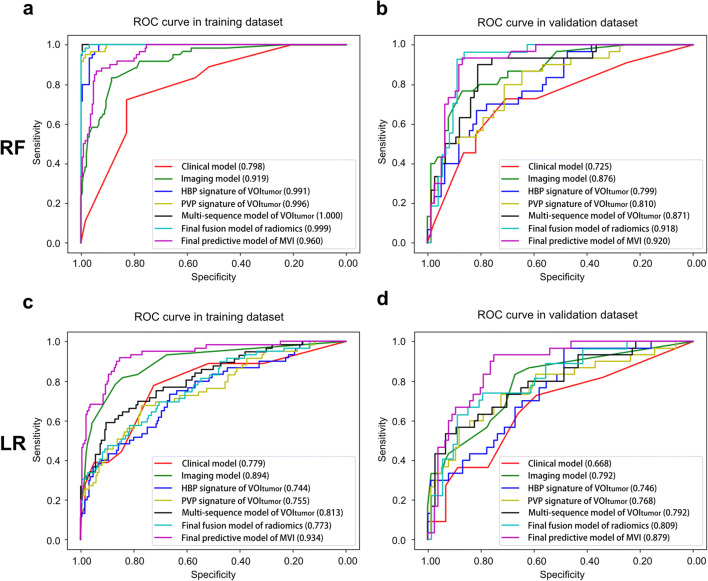
Fig. 4.
Receiver operating characteristic curves of different models for predicting MVI. Receiver operating characteristic curves of different models for predicting MVI were plotted by random forest (a: training cohort, b: validation cohort) and logistic regression (c: training cohort, d: validation cohort) to crossly validate the robustness of models
In the VOItumor + 10mm + liver subgroup, the optimal multi-sequence fusion was the integration of PVP, HBP, AP, and DWI, with AUCs of 0.918 using RF and 0.809 using LR in the validation cohort (Tables 3 and S6). Meanwhile, the predictive performance of this four-sequence fusion—the final radiomics model—was also significantly superior to those of the two best single sequences (HBP: NRI 19.44%, p = 0.008; PVP: NRI 24.54%, p = 0.003; Table 4) as well as those of the clinical (NRI 54.1%, p = 0.002) and imaging models (NRI 22.2%, p = 0.029) using RF in the validation cohort. The details of the top six most discriminating features in the final radiomics model are provided in Table S8.
Performance of MVI nomograms
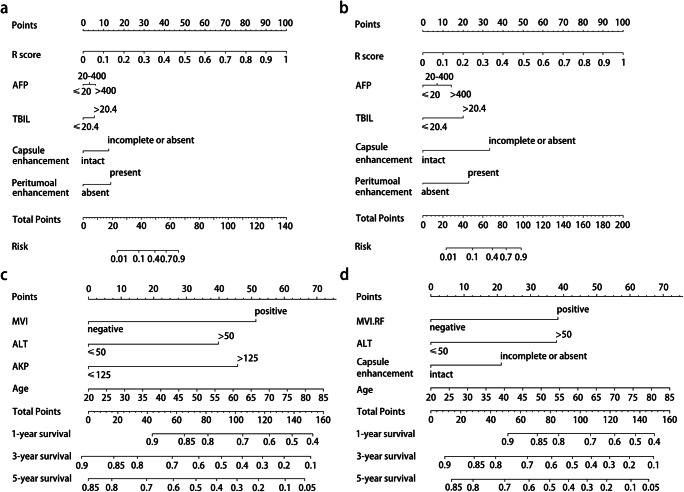
Based on the clinical, imaging, and final radiomics predictors, the ultimate MVI predictive model incorporated the independent risk factors of TBIL > 20.4 μmol/L, AFP > 20 ng/mL, incomplete or absent capsule enhancement, peritumoral enhancement, and higher score of radiomics (R-score) into visualized nomograms (Fig. 5a–b) as follows:
- the nomogram using RF:
- the nomogram using LR:
Fig. 5.
Nomograms for predicting MVI and recurrence-free survival. The final predictive model of MVI was visualized as nomograms (a: random forest, b: logistic regression). The independent predictors of recurrence were graphically shown as nomograms in the histologic MVI (c) and the predicted MVI-RF (d) subgroups, respectively
The predictive performances of MVI nomograms (Tables 3 and 4) demonstrated moderately or dramatically enhancements compared to those of clinical models (NRIs: 14.6–56.8%), imaging models (NRIs: 9.1–78.9%), and radiomics model using LR (NRIs: 19.4–35.7%), with a slightly negative improvement contrasted to radiomics model using RF (NRI < 0, p > 0.05). Furthermore, the AUCs (Fig. 4) of HBP sequence in VOItumor, PVP sequence in VOItumor, multi-sequence fusion in VOItumor, the final radiomics in VOItumor + 10mm + liver, and the ultimate predictive model of MVI (MVI nomogram) presented a gradual upward trend in validation cohort using RF or LR classifier. Being highly consistent with the actual MVI status in the calibration curves (Fig. S1a–d), MVI nomograms obtained the best net clinical benefit, followed by the radiomics and imaging models, with the clinical model worst in the decision curves (Fig. S1e–h).
Clinical outcome
Until April 2020, 329 patients had completed follow-up. While 1-, 3-, and 5-year overall survival rates reached 99.0%, 95.4%, and 93.6%, 1-, 3-, and 5-year RFS rates were 85.4%, 72%, and 68.4%, respectively. Therefore, this study only focused on the RFS analysis.
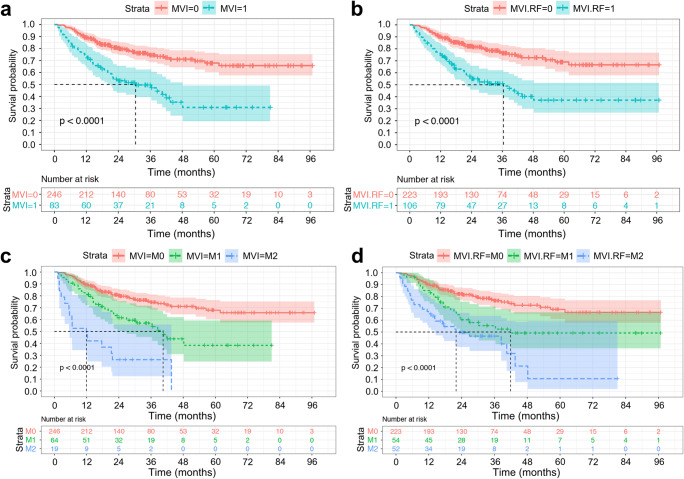
The median RFS of histologic MVI-positive (M2, M1) and MVI-negative (M0) patients were 30.5 months (M2, 11.9 months; M1, 40.5 months) and > 96.9 months (log-rank test, p < 0.001, Fig. 6), respectively. Similarly, the median RFS of positive and negative MVI-RF groups were 36.4 months (M2, 22.0 months; M1, 41.9 months) and > 96.9 months (p < 0.001), respectively. However, MVI-LR failed to satisfy the proportional hazard assumption in the discrimination and stratification of MVI for predicting RFS (log-rank test, p = 0.735, 0.224; Fig. S2).
Fig. 6.
Kaplan-Meier curves of recurrence-free survival. With the Kaplan-Meier analysis and 2-sided log-rank test, recurrence-free survival curves were scaled by the histologic MVI status (a) and the predicted MVI status (b) by MVI nomogram using random forest (MVI-RF) and were further stratified by the histologic MVI (c) and MVI-RF grades (d), respectively
The results of multivariate Cox regression (Table 5) presented that histologic MVI, AKP > 125 U/L, ALT > 50 U/L, and the elderly were independent risk factors for recurrence, with C-indexes of 0.704 (95% CI: 0.645–0.764; n = 230) in the training cohort and 0.654 (95% CI: 0.538–0.769; n = 99) in the validation cohort. Similarly, the positive MVI-RF status, incomplete or absent capsule enhancement, ALT > 50 U/L and the elderly were independent risk factors of recurrence in MVI-RF subgroup, with C-indexes of 0.700 (95% CI: 0.638–0.763) in the training cohort and 0.673 (95% CI: 0.570–0.776) in the validation cohort. The RFS nomograms of the histologic and predicted MVI subgroups are showed in Fig. 5c, d, and their calibration curves are available in Fig. S3.
Table 5.
Variables associated with recurrence-free survival according to the Cox proportional hazards model
| Variables | Univariate analysis | Multivariate analysis (histologic MVI subgroup) | Multivariate analysis (predicted MVI-RF subgroup) | |||
|---|---|---|---|---|---|---|
| p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | |
| Age | 0.020 | 1.025 (1.004, 1.046) | 0.044 | 1.022 (1.001, 1.044) | 0.047 | 1.022 (1.000, 1.044) |
| Ki-67 | 0.077 | 1.010 (0.999, 1.021) | ||||
| Cirrhosisa | 0.505 | 1.175 (0.731, 1.887) | ||||
| ES (III–IV/I–II) | 0.085 | 1.489 (0.946, 2.343) | ||||
| HBV or HCV a | 0.076 | 0.581 (0.319, 1.058) | ||||
| LD (2–5 cm / ≤ 2 cm) | 0.922 | 1.023 (0.650, 1.608) | ||||
| Histologic MVI a | < 0.001 | 2.703 (1.702, 4.293) | < 0.001 | 2.733 (1.712, 4.362) | / | / |
| Predicted MVI-RF a | < 0.001 | 2.593 (1.652, 4.072) | / | / | < 0.001 | 2.258 (1.416, 3.601) |
| BCLC (A/0 stage) | 0.622 | 1.120 (0.714, 1.758) | ||||
| Child-Pugh (B/A class) | 0.009 | 3.382 (1.362, 8.396) | ||||
|
Alpha-fetoprotein (> 20/≤ 20 ng/ml); (> 400/≤ 400 ng/ml) |
0.261 0.878 | 1.302 (0.822, 2.064); 1.051 (0.554, 1.997) | ||||
| ALB (≤ 35/> 35 g/L) | 0.038 | 0.295 (0.093, 0.937) | ||||
| AST (> 40/≤ 40 U/L) | 0.072 | 1.627 (0.958, 2.766) | ||||
| GGT (> 60/≤ 60 U/L) | 0.016 | 1.787 (1.117, 2.860) | 0.058 | 1.614 (0.984, 2.648) | ||
| ALT (> 50/≤ 50 U/L) | 0.001 | 2.528 (1.484, 4.305) | 0.003 | 2.241 (1.307, 3.843) | 0.012 | 2.067 (1.176, 3.635) |
| TBA (> 10/≤ 10 umol/L) | 0.091 | 1.485 (0.939, 2.350) | ||||
| AKP (> 125/≤ 125 U/L) | 0.023 | 2.653 (1.145, 6.149) | 0.045 | 1.022 (1.001, 1.044) | ||
| Ascites a | 0.008 | 2.714 (1.302, 5.656) | ||||
| Typical MRI patterna | 0.698 | 1.180 (0.512, 2.723) | ||||
| Edge non-smoothness | 0.087 | 5.868 (0.755, 44.428) | ||||
| Capsule enhancement b | 0.002 | 1.861 (1.265, 2.739) | 0.041 | 1.662 (1.021, 2.706) | ||
| Peritumoral enhancement a | 0.001 | 1.995 (1.319, 3.015) | ||||
| Peritumoral hypointensity a | < 0.001 | 2.330 (1.510, 3.595) | ||||
| Other indexes | > 0.100 | |||||
Other indexes: sex (male/female), α-L-fucosidase (≤ 40/> 40 U/L), carcinoembryonic antigen (≤ 5/> 5 ng/mL), carbohydrate antigen 19–9 (≤ 34/> 34 ng/mL), platelet count (≤ 100 × 109/L/> 100 × 109/L), total bilirubin (≤ 20.4/> 20.4 μmol/L), direct bilirubin (≤ 6.8/> 6.8 umol/L), total protein (≤ 65/> 65 ng/mL), prealbumin (≤ 180/> 180 mg/L), hyaluronic acid (≤ 120/> 120 ng/mL), procollagen type III (≤ 15 /> 15 ng/mL), type IV collagen (≤ 95/> 95 ng/mL), laminin (≤ 130/> 130 ng/mL), prothrombin time (≤ 13/> 13 s), activated partial thromboplastin time (≤ 31.3/> 31.3 s), fibrinogen (≤ 200/> 200 mg/dlL), thrombin time (≤ 21/> 21 s); portal hypertension (present/absent); T1PRE and T1HBP, defined as the signal intensity of tumor derived from the pre-contrast and hepatobiliary phase T1 maps, respectively
Abbreviations: ES, Edmondson-Steiner grades; LD, the longest diameter of tumor; BCLC, Barcelona Clinic Liver Cancer: TBA, total bile acids; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; AKP, alkaline phosphatase; GGT, r-glutamyltransferase; HR, hazard ratio;CI, confidence interval
a Present/absent; b Incomplete-absent/intact capsule enhancement
Discussion
Our study developed radiomics-based nomograms for preoperatively predicting MVI and outcome in patients with solitary HCC ≤ 5 cm. The results demonstrated that AFP > 20 ng/mL, TBIL > 20.4 μmol/L, absent or incomplete capsule enhancement, peritumoral enhancement, and higher R-score were independent risk factors for MVI. Mainly based on radiomics signatures of PVP, HBP, AP, and DWI in VOItumor + 10mm + liver, the nomogram using RF or LR excellently identified MVI-positive patients. Furthermore, histologic MVI, ALT > 50 U/L, AKP > 125 U/L, and the elderly independently impaired RFS, with a relatively favorable prediction for recurrence. Histologic M0, M1, and M2 grades were significantly inverse correlated with RFS. Intriguingly, contrasted to histologic MVI, MVI-RF achieved comparable accuracy in MVI stratification and prognostic analyses.
Elevated AFP level [7, 11, 16], incomplete capsule enhancement [7, 8], and peritumoral enhancement [8, 9, 11] have been reported to be independent risk factors for MVI, which are consistent with our results. Independently facilitating MVI in this study, elevated TBIL level may secondary to the existence or obstruction of MVI in the biliary system [12, 18, 25]. This is partly due to the fact that cancerous thrombus in the newly formed bile ducts of tumor capsule [26], bile canaliculus, or interlobular bile ducts, rather than in gross or intrahepatic bile ducts, are difficult to be identified by preoperative imaging and excluded from the study population.
Peritumoral tissue is the first and most frequently vulnerable to MVI [21, 27], the vessels of which further serve as the main hematogenous dissemination pathway of portal vein tumor thrombosis and metastasis [21]. Therefore, we constructed multi-VOI models for exploring this highly aggressive region. Interestingly, the AUCs of VOI50%, VOItumor, VOItumor + 5mm, VOItumor + 10mm, and VOItumor + 10mm + liver signatures approximately kept increasing almost in all sequences irrespective of classifiers. These preponderances of VOItumor over VOI50% and VOItumor + 10mm over VOItumor for predicting MVI were consistent with the CT results of Xu et al [8] and HBP results of Feng et al [13], respectively. Meanwhile, the AUCs of VOItumor, VOItumor + liver and VOItumor + 5mm + liver (VOItumor + 10mm + liver) features, as well as those of VOItumor, VOItumor + 5mm (VOItumor + 10mm) and VOItumor + 5mm + liver (VOItumor + 10mm + liver) signatures, also showed an increasing trend. Notably, the performance of VOItumor + 10mm + liver signatures preceded that of VOItumor + 10mm features either in this paper or in Feng et al study [13]. Besides, the optimal multi-sequence fusion outperformed the two best single sequences both in VOItumor and in VOItumor + 10mm + liver subgroups. These results signified the superiority of tumor periphery compared with tumor interior, the significance of texture and intensity difference between normal liver and intra-/peritumoral tissue, and the synergistic effect of multi-sequence and multi-VOI fusion for predicting MVI, which have been neglected in and might be the reason why our MVI nomograms obtained better performances than previous radiomics studies [8, 11, 13, 15, 16, 28].
Likewise, the top 6 most discriminating signatures of the final radiomics model also indicated the importance of peritumoral and intratumoral fusion. Being partly coincided with previous studies [8, 11], the six signatures included tumor size, shape, and intratumoral and peritumoral texture heterogeneity. By definition, HBP_VOI5mm_wavelet-HHL_firstorder_Energy and HBP_VOI5mm_wavelet-HLL_glszm_GrayLevelNonUniformity involved the texture heterogeneity of the peritumoral tissue within 5 mm, which might reflect an aggressive tendency to invade the tumor capsule and protrude into the peritumoral non-neoplastic parenchyma [27]. In addition, HBP_VOItumor_original_shape_Sphericity and DWI_VOItumor_original_shape_MajorAxisLength represented the spherical disproportion and the largest axis length of tumor, respectively. These were analogue to the well-known independent hallmarks “non-smooth edge and the longest diameter of tumor” of MVI [7–9, 11]. Furthermore, HBP_VOItumor_wavelet-HLL_glszm_SizeZoneNonUniformity and HBP_VOItumor_original_glszm_GrayLevelNonUniformity concerning intratumoral texture heterogeneity might be induced by tumor cellularity, micronecrosis and inflammation, for which further facilitated MVI [11, 29]. Coincidentally, five-sixths features were extracted from HBP, suggesting the significance of Gd-EOB-DTPA MRI in MVI diagnosis.
Histologic MVI [8, 10, 30], the elderly [30–32], incomplete or absent capsule enhancement [33, 34], and elevated ALT [30–32] and AKP [35–37] levels have been reported to impair outcomes of HCC patients, which were corresponded to our results. Conforming to the outcomes of few studies with histologic MVI grades [19, 38], our histologic MVI stratification, especially the novel and non-invasive MVI-RF classifications, showed significantly inverse correlations with RFS. Hence, the MVI-RF—an excellent substitute of histologic MVI—may be employed in patients with solitary HCC ≤ 5 cm, especially for those who underwent ablation without histologic MVI data. Namely, MVI-positive or even M2-grade patients diagnosed by MVI-RF before ablation might require more active clinical treatment and intense follow-up. Nevertheless, the AUCs of RFS nomograms around 0.66 for histologic MVI and MVI-RF subgroups, the unsatisfactory results may be induced by (1) the paucity of postoperative characteristics (e.g., preventive transarterial chemoembolization, immunosuppression therapy); (2) the absence of robust radiomics analysis in terms of recurrence instead of MVI; (3) the exclusion of well-established key predictors of recurrence (e.g., tumor size beyond 5 cm, satellite nodules or multifocal HCC, cancerous thrombus in gross bile ducts or vessels) in our study population.
This study has several limitations. Firstly, this paper is a retrospective single-centre study in China and needs to be validated by the external cohort. Secondly, we did not incorporate genomics with radiologic hallmarks, just as Banerjee et al [39]. Thirdly, this study focused on the solitary HCC within 5 cm, leading to a slightly lower frequency of MVI in our population than those of previous MVI studies with macrovascular invasive, larger, or multifocal HCC [7–9]. Fourthly, the radiomics results may slightly vary between different radiomics or statistical analysis software from feature selection to model evaluation. Hence, the well-recognized LASSO algorithm of R software [13, 40], Pyradiomics [40–42], and Scikit-learn [43, 44] packages of Python software were also employed to this paper, for facilitating the future study to verify the robustness of our findings. Finally, HCC has a strong male preponderance [45], and thus, the sex ratio imbalance—the inherent selection bias—cannot be completely avoided in this study.
In summary, mainly based upon multi-parametric radiomics in VOItumor + 10mm + liver of Gd-EOB-DTPA MRI, the nomogram using random forest is a potential biomarker for preoperatively predicting MVI and RFS in patients with solitary HCC ≤ 5 cm.
Supplementary Information
(DOCX 1639 kb)
Acknowledgements
We sincerely thank Jian-Feng Zhang, Huan Yuan, and Qing Zhou for the assistance of radiomics analysis.
Abbreviations
- AFP
Alpha-fetoprotein
- AKP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- AP
Arterial phase
- AUC
Area under the curve
- HBP
Hepatobiliary phase
- HCC
Hepatocellular carcinoma
- LASSO
Least Absolute Shrinkage and Selection Operator
- LR
Logistic regression
- MVI
Microvascular invasion
- NRI
Net reclassification index
- OR
Odds ratio
- PVP
Portal venous phase
- RF
Random forest
- TBIL
Total bilirubin
- TP
Transitional phase
- VOI
Volumetric interest
Funding
This study has received funding by the National Natural Science Foundation of China (No.91859107), Shanghai Science and Technology Committee (Nos.18DZ1930102 and 19411965500), Zhongshan Hospital, Fudan University (No.2018ZSLC22), Shanghai Municipal Key Clinical Specialty (No.W2019-018).
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Meng-Su Zeng
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Two of the authors (Huan-Huan Chong and Ruo-Fan Sheng) have significant statistical expertise.
Informed consent
Our hospital ethics committee approved this retrospective study and waived patient informed consent.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• case-control study/diagnostic or prognostic study
• performed at one institution
Footnotes
H.H.C and L.Y share the first authorship of this article.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chun Yang, Email: dryangchun@hotmail.com.
Meng-Su Zeng, Email: mengsu_zeng@163.com.
References
- 1.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 2.Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 Model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154:128–139. doi: 10.1053/j.gastro.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Miltiadous O, Sia D, Hoshida Y, et al. Progenitor cell markers predict outcome of patients with hepatocellular carcinoma beyond Milan criteria undergoing liver transplantation. J Hepatol. 2015;63:1368–1377. doi: 10.1016/j.jhep.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Kardashian A, Florman SS, Haydel B et al (2020) Liver transplantation outcomes in a U.S. multicenter cohort of 789 patients with hepatocellular carcinoma presenting beyond Milan criteria. Hepatology. 10.1002/hep.31210 [DOI] [PubMed]
- 5.Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 6.Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:238–255. doi: 10.1093/annonc/mdy308. [DOI] [PubMed] [Google Scholar]
- 7.Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016;151:356–363. doi: 10.1001/jamasurg.2015.4257. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Zhang HL, Liu QP, et al. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. 2019;70:1133–1144. doi: 10.1016/j.jhep.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol. 2017;67:526–534. doi: 10.1016/j.jhep.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZH, Zhang XP, Wang H, et al. Effect of microvascular invasion on the postoperative long-term prognosis of solitary small HCC: a systematic review and meta-analysis. HPB (Oxford) 2019;21:935–944. doi: 10.1016/j.hpb.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Gu D, Wei J, et al. A radiomics nomogram for preoperative prediction of microvascular invasion in hepatocellular carcinoma. Liver Cancer. 2019;8:373–386. doi: 10.1159/000494099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cong WM, Bu H, Chen J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22:9279–9287. doi: 10.3748/wjg.v22.i42.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng ST, Jia Y, Liao B, et al. Preoperative prediction of microvascular invasion in hepatocellular cancer: a radiomics model using Gd-EOB-DTPA-enhanced MRI. Eur Radiol. 2019;29:4648–4659. doi: 10.1007/s00330-018-5935-8. [DOI] [PubMed] [Google Scholar]
- 14.Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Wei J, Gu D, et al. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. 2019;29:3595–3605. doi: 10.1007/s00330-018-5985-y. [DOI] [PubMed] [Google Scholar]
- 16.Peng J, Zhang J, Zhang Q, Xu Y, Zhou J, Liu L. A radiomics nomogram for preoperative prediction of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma. Diagn Interv Radiol. 2018;24:121–127. doi: 10.5152/dir.2018.17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitao A, Matsui O, Yoneda N et al (2020) Gadoxetic acid-enhanced MR imaging for hepatocellular carcinoma: molecular and genetic background. Eur Radiol. 10.1007/s00330-020-06687-y [DOI] [PubMed]
- 18.Rodriguez-Peralvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325–339. doi: 10.1245/s10434-012-2513-1. [DOI] [PubMed] [Google Scholar]
- 19.Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 21.Hu HT, Shen SL, Wang Z, et al. Peritumoral tissue on preoperative imaging reveals microvascular invasion in hepatocellular carcinoma: a systematic review and meta-analysis. Abdom Radiol (NY) 2018;43:3324–3330. doi: 10.1007/s00261-018-1646-5. [DOI] [PubMed] [Google Scholar]
- 22.Wei Y, Huang Z, Tang H, et al. IVIM improves preoperative assessment of microvascular invasion in HCC. Eur Radiol. 2019;29:5403–5414. doi: 10.1007/s00330-019-06088-w. [DOI] [PubMed] [Google Scholar]
- 23.American College of Radiology (2018) CT/MRI LI-RADS® v2018 core. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018. Accessed Jul 2018
- 24.Aerts HJ, Velazquez ER, Leijenaar RT, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu G, Yang HY, Xu HF. Prediction of microvascular invasion in hepatocellular carcinoma with preoperative imaging radiomic analysis: is it ready for prime time? Hepatobiliary Pancreat Dis Int. 2019;18:289–290. doi: 10.1016/j.hbpd.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Cho ES, Choi JY. MRI features of hepatocellular carcinoma related to biologic behavior. Korean J Radiol. 2015;16:449–464. doi: 10.3348/kjr.2015.16.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu H, Zheng Q, Huang Y, et al. A non-smooth tumor margin on preoperative imaging assesses microvascular invasion of hepatocellular carcinoma: a systematic review and meta-analysis. Sci Rep. 2017;7:15375. doi: 10.1038/s41598-017-15491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang R, Xu L, Wen X, et al. A nomogram based on bi-regional radiomics features from multimodal magnetic resonance imaging for preoperative prediction of microvascular invasion in hepatocellular carcinoma. Quant Imaging Med Surg. 2019;9:1503–1515. doi: 10.21037/qims.2019.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang WT, Yang L, Yang ZX, et al. Assessment of microvascular invasion of hepatocellular carcinoma with diffusion kurtosis imaging. Radiology. 2018;286:571–580. doi: 10.1148/radiol.2017170515. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Wu MC, Cong WM. Microvascular invasion predicts a poor prognosis of solitary hepatocellular carcinoma up to 2 cm based on propensity score matching analysis. Hepatol Res. 2019;49:344–354. doi: 10.1111/hepr.13241. [DOI] [PubMed] [Google Scholar]
- 31.Li T, Fan J, Qin LX, et al. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol. 2011;18:1955–1963. doi: 10.1245/s10434-010-1540-z. [DOI] [PubMed] [Google Scholar]
- 32.Pompili M, Saviano A, de Matthaeis N, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59:89–97. doi: 10.1016/j.jhep.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Ahn SJ, Kim JH, Park SJ, Kim ST, Han JK. Hepatocellular carcinoma: preoperative gadoxetic acid-enhanced MR imaging can predict early recurrence after curative resection using image features and texture analysis. Abdom Radiol (NY) 2019;44:539–548. doi: 10.1007/s00261-018-1768-9. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Lai SL, Chen J, et al. Validated preoperative computed tomography risk estimation for postoperative hepatocellular carcinoma recurrence. World J Gastroenterol. 2017;23:6467–6473. doi: 10.3748/wjg.v23.i35.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pang Q, Zhang JY, Xu XS, et al. Significance of platelet count and platelet-based models for hepatocellular carcinoma recurrence. World J Gastroenterol. 2015;21:5607–5621. doi: 10.3748/wjg.v21.i18.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnaoutakis DJ, Mavros MN, Shen F, et al. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: a multi-institutional analysis. Ann Surg Oncol. 2014;21:147–154. doi: 10.1245/s10434-013-3211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu MC, Chan KM, Lee CF, et al. Alkaline phosphatase: does it have a role in predicting hepatocellular carcinoma recurrence? J Gastrointest Surg. 2011;15:1440–1449. doi: 10.1007/s11605-011-1537-3. [DOI] [PubMed] [Google Scholar]
- 38.Sumie S, Nakashima O, Okuda K, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21:1002–1009. doi: 10.1245/s10434-013-3376-9. [DOI] [PubMed] [Google Scholar]
- 39.Banerjee S, Wang DS, Kim HJ, et al. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology. 2015;62:792–800. doi: 10.1002/hep.27877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji GW, Zhu FP, Xu Q, et al. Machine-learning analysis of contrast-enhanced CT radiomics predicts recurrence of hepatocellular carcinoma after resection: a multi-institutional study. EBioMedicine. 2019;50:156–165. doi: 10.1016/j.ebiom.2019.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Gu D, Wei J, et al. A radiomics-based biomarker for cytokeratin 19 status of hepatocellular carcinoma with gadoxetic acid-enhanced MRI. Eur Radiol. 2020;30:3004–3014. doi: 10.1007/s00330-019-06585-y. [DOI] [PubMed] [Google Scholar]
- 42.Kim J, Choi SJ, Lee SH, Lee HY, Park H. Predicting survival using pretreatment CT for patients with hepatocellular carcinoma treated with transarterial chemoembolization: comparison of models using radiomics. AJR Am J Roentgenol. 2018;211:1026–1034. doi: 10.2214/AJR.18.19507. [DOI] [PubMed] [Google Scholar]
- 43.Chen S, Feng S, Wei J, et al. Pretreatment prediction of immunoscore in hepatocellular cancer: a radiomics-based clinical model based on Gd-EOB-DTPA-enhanced MRI imaging. Eur Radiol. 2019;29:4177–4187. doi: 10.1007/s00330-018-5986-x. [DOI] [PubMed] [Google Scholar]
- 44.Graña M, Aguilar-Moreno M, De Lope Asiain J, Araquistain IB, Garmendia X. Improved Activity Recognition Combining Inertial Motion Sensors and Electroencephalogram Signals. Int J Neural Syst. 2020;30:2050053. doi: 10.1142/S0129065720500537. [DOI] [PubMed] [Google Scholar]
- 45.European Association for the Study of the Liver EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1639 kb)