Abstract
Cerebrovascular disease is the most common life-threatening and debilitating condition that often leads to stroke. The multifunctional calcium/calmodulin-dependent protein kinase II (CaMKII) is a key Ca2+ sensor and an important signaling protein in a variety of biological systems within the brain, heart, and vasculature. In the brain, past stroke-related studies have been mainly focused on the role of CaMKII in ischemic stroke in neurons and established CaMKII as a major mediator of neuronal cell death induced by glutamate excitotoxicity and oxidative stress following ischemic stroke. However, with growing understanding of the importance of neurovascular interactions in cerebrovascular diseases, there are clearly gaps in our understanding of how CaMKII functions in the complex neurovascular biological processes and its contributions to cerebrovascular diseases. Additionally, emerging evidence demonstrates novel regulatory mechanisms of CaMKII and potential roles of the less-studied CaMKII isoforms in the ischemic brain, which has sparked renewed interests in this dynamic kinase family. This review discusses past findings and emerging evidence on CaMKII in several major cerebrovascular dysfunctions including ischemic stroke, hemorrhagic stroke, and vascular dementia, focusing on the unique roles played by CaMKII in the underlying biological processes of neuronal cell death, neuroinflammation, and endothelial barrier dysfunction triggered by stroke. We also highlight exciting new findings, promising therapeutic agents, and future perspectives for CaMKII in cerebrovascular systems.
Keywords: Cerebrovascular disease, CaMKII, Stroke, Vascular dementia, Neuronal cell death, Neuroinflammation, Endothelial barrier dysfunction
Introduction
Cerebrovascular disease affects blood vessels and cerebral circulation of the brain and is a common cause of death and disability worldwide. Stoke, either ischemic or hemorrhagic, is a major form of cerebrovascular disease. In the USA, stroke reduces mobility in more than half of stroke survivors age 65 and over, and approximately 3% of males and 2% of females reported that they were disabled because of stroke [1]. Subarachnoid hemorrhage (SAH), a less common type of hemorrhagic stroke, accounts for 5% of stroke cases in the USA [2]. Severe reduction of cerebral blood flow also leads to a complex neurodegenerative disorder known as vascular dementia which associates with memory and cognitive impairment. Stroke as a major form of cerebrovascular disease has been intensely studied with the primary focus on neuronal injury, the main functional deficit of the disease. However, there is growing appreciation in the functional interactions within the multicellular neurovascular unit and their crucial roles in the onset, progression, and recovery after ischemic stroke [3, 4].
CaMKII is a family of calcium (Ca2+) and calmodulin (CaM)–activated multifunctional serine/threonine kinases [5]. It was first discovered in the brain and subsequently shown to play a critical role in brain function, particularly in memory and learning [6]. In ischemic stroke, CaMKII was found to be activated in the early phase of ischemic insult and mediates glutamate excitotoxicity–induced cell death in neurons. However, downstream events leading to cell death remain obscure. Additionally, past studies mainly focus on CaMKII holoenzyme and CaMKIIα (the most abundant CaMKII isoform in the brain) in neurons, but there is sparse information on CaMKII in other cell types within the neurovascular unit (such as endothelial cells, pericytes, smooth muscle cells, astrocytes, microglia, and extracellular matrix) and the functions of other CaMKII isoforms in ischemic stroke are largely unknown. In this review, by revisiting several key biological processes triggered by ischemic or hemorrhagic brain injury, we seek to discuss the diverse functions and signaling mechanisms of CaMKII in cerebrovascular disease in the context of the multicellular neurovascular environment.
Structure and Regulation of CaMKII
Basic Enzyme Structure and Classification
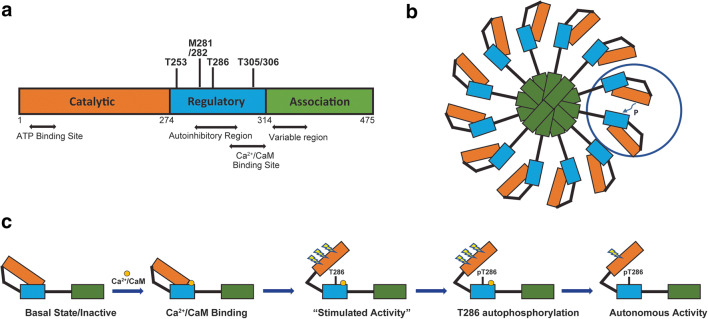
CaMKII is a family of serine/threonine kinases activated by Ca2+ and CaM. Four isoforms (α, β, γ, and δ) have been identified. Each CaMKII holoenzyme typically comprises 12 or 14 subunits of the same or different isoforms [7–11]. The homo- or heteromeric structures confer unique properties to CaMKII based on which isoforms and/or subtypes are present [8]. As shown in Fig. 1, the conserved basic structure of CaMKII includes a N-terminal catalytic domain that contains the ATP binding site, a regulatory domain that encompasses an autoinhibitory region, multiple conserved phosphorylation sites, and a Ca2+/CaM binding site required for activation, and a C-terminal association domain that includes a variable region and an association motif that is essential for oligomerization [7–10, 12]. CaMKII is a key regulator of neuronal function and has been studied extensively in areas of learning, synaptic plasticity, and memory [6, 7].
Fig. 1.
Diagram of CaMKII structure and mechanisms of activation. a The sequence of CaMKII includes a N-terminal catalytic domain that contains the ATP binding and substrate phosphorylation sites, a regulatory domain that contains the autoinhibitory region, the Ca2+/CaM binding site, and multiple post-translational modification sites including phosphorylation sites T253, T286, and T305/306 and the M281/282 oxidation sites, and a C-terminal association domain that mediates multimeric interactions and a variable region that can undergo alternative splicing to generate a variety of CaMKII subtypes. b Schematic depiction of CaMKII holoenzyme with 12 subunits. c Activation mechanism of a CaMKII monomer by the binding of Ca2+/CaM and autophosphorylation at T286
The four isoforms of CaMKII all have conserved domain structures but are derived from different genes [7]. It is a common occurrence for tissues to express more than one isoform of CaMKII [10]. The variable region provides the main structural distinctions between isoforms. Further diversification occurs as a result of alternative splicing in the variable region to produce around 40 different subtypes for the four isoforms [12–14].
All four CaMKII isoforms reside in the brain, though CaMKIIα and CaMKIIβ are known to be more prevalent there, while CaMKIIδ is more abundant in the cardiovascular system and CaMKIIγ is ubiquitously expressed [8–10]. In particular, CaMKIIα is most prevalent in neurons and accounts for more than 1% of protein in some areas of the brain such as the hippocampus and postnatal forebrain neurons [7, 9].
Mechanisms of Activation
In the absence of stimuli, each subunit of CaMKII is kept in a basal/inactive state by the interactions between its catalytic domain and the autoinhibitory region within the regulatory domain [9]. The alpha helical structure of the autoinhibitory region contains two sequences that overlap with each other, an auto-inhibition sequence and a CaM binding sequence [7, 9]. The conserved threonine 286 (T286) (T287 for CaMKIIβ, δ, γ) phosphorylation site resides in the autoinhibition sequence, and two other conserved phosphorylation sites, T305/306, reside in the Ca2+/CaM binding sequence in the regulatory domain (Fig. 1a) [7–9, 12]. The T286 amino acid binds to a conserved site in the catalytic domain to maintain the inactive conformation.
The activation of the CaMKII is triggered by an increase in intracellular Ca2+ levels and the binding of Ca2+ to CaM to form the Ca2+/CaM complex. Ca2+/CaM then binds to the Ca2+/CaM binding site in the regulatory domain to induce a conformational change that allows the catalytic domain to be separated from the autoinhibitory region and produce what is called “stimulated activity” [7, 8]. This will expose the substrate binding site in the catalytic domain to complete the activation [8]. Individual subunits of CaMKII are activated separately in the holoenzyme [7]. The conformational change induced by Ca2+/CaM binding also exposes the conserved T286 phosphorylation site in the autoinhibitory region, allowing it to be phosphorylated by an adjacent, activated subunit. The phosphorylation of T286 prevents the re-binding of the autoinhibitory region to the catalytic domain after Ca2+/CaM dissociation. This leads to “constitutive/autonomous activity” [6]. Autophosphorylation of T286 also creates the circumstance of CaM trapping, meaning that the Ca2+/CaM complex has reduced dissociation, contributing to more activity of the kinase [8].
CaMKII activity is regulated by both phosphorylation and dephosphorylation events. As described above, autophosphorylation of T286 plays a critical role in regulating CaMKII activity. It also regulates the binding capacity of CaMKII, particularly at the post-synaptic density (PSD) [15]. In addition to T286, CaMKII activity can also be regulated by additional autophosphorylations at T305/306 (T306/307 for CaMKII β, γ, and δ) and T253 with CaMKIIα (T254 for CaMKIIβ) [9, 16, 17]. As shown in Fig. 1a, T305/306 is located within the Ca2+/CaM binding site and can only become phosphorylated when the Ca2+/CaM dissociates and autonomous activation is in effect. When this phosphorylation occurs, the Ca2+/CaM complex can no longer bind to cause further stimulation of the kinase, leaving the kinase partially active as a result of autonomous activity due to phosphorylated T286 [16]. The phosphorylation of T253 has no direct effect on the kinase activity or Ca2+/CaM binding of purified CaMKII in vitro, but affects the targeting of CaMKII via interactions with other binding proteins, therefore has functional effects on cell physiology [9, 16–19]. Dephosphorylation returns the enzyme to an inactive state and is catalyzed by protein phosphatase types 1 and 2A [6, 20–23].
Major Regulatory Mechanisms of CaMKII
RNA Splicing
Alternative splicing of the variable region in CaMKII isoforms gives rise to the different subtypes of the kinase [24]. The splicing confers unique properties to certain subtypes of CaMKII isoforms and allows them to regulate distinct cellular processes. The exact numbers of subtypes for each CaMKII isoform and their functions are still an area of active investigation. Based on current knowledge, RNA splicing produces at least three splice variants for CaMKIIα (α, αB, and αKAP). The α and αB subtypes are prevalent in the brain, while the catalytic domain deficient αKAP subtype is predominantly expressed in the skeletal muscle. There are at least six splice variants for CaMKIIβ including β, β’, βe, and βe’, which are mainly distributed in the brain. CaMKIIδ has at least eleven splice variants (CaMKIIδ1–11, including δ1/A, δ3/B, δ2/C, and δ9), and CaMKIIγ has at least eight splice variants (γA–γH) [24, 25]. CaMKIIγ and CaMKIIδ subtypes can be found in various locations such as the brain, skeletal muscle, heart, and lung [26, 27]. Within the heart, different δ subtypes have been shown to function differently, e.g., the nuclear CaMKIIδB controls the expression of the atrial natriuretic factor, and the cytoplasmic CaMKIIδC controls Ca2+ release from the sarcoplasmic reticulum by phosphorylating its cardiac ryanodine receptors [28, 29]. Certain CaMKIIγ subtypes contain a nuclear localization sequence (NLS) signal, which plays a role in shuttling Ca2+/CaM to the nucleus to enable the activation of the cAMP-response element binding protein in the brain [30]. A detailed review about CaMKII alternative splicing is provided by Sloutsky and Stratton [14].
Subcellular Localization
The localization of CaMKII in cells plays a crucial role in regulating CaMKII function. It has been well documented that certain subtypes of the CaMKII isoforms are capable of nuclear localization (αB, δB, γA’) [30–32]. These CaMKII subtypes share a common core NLS sequence, KKRK, in the variable region to mediate nuclear translocation. This nuclear localization event is also negatively regulated by the phosphorylation of a serine residue immediately adjacent to NLS [25, 30, 33]. Another major regulator of CaMKII localization in cells is the influx of Ca2+. In neurons, Ca2+ influx triggered by glutamate binding to N-methyl-D-aspartate (NMDA) receptors causes translocation of CaMKII to post-synaptic sites and extra-synaptic clusters for regulation of synaptic plasticity [7]. CaMKII can also be mobilized to other subcellular locations such as the cytoskeleton due to CaMKIIβ binding to F-actin [34]. Upon Ca2+ influx, CaMKIIβ will dissociate from the F-actin and translocate to the PSD. It is also common to see a heteromeric structure of α/β-CaMKII bound to F-actin through its interaction with the β subunits [35].
Oxidative Stress
Oxidative stress, a condition caused by the overproduction of reactive oxygen species (ROS), can induce oxidative damages on lipids and proteins [36]. ROS has been shown to induce oxidative modifications on a conserved methionine pair M281/282 (C281/M282 in CaMKIIα) in the autoinhibitory region of CaMKII (Fig. 1a) to produce an oxidized form of CaMKII [37]. This oxidation event occurs after Ca2+ activation of the kinase when the autoinhibitory region is removed from the catalytic domain. The oxidized methionine pair will keep the autoinhibitory region from re-binding with the catalytic domain after Ca2+/CaM dissociation, which creates an autonomously active state similar to that seen with T286 autophosphorylation [38]. Oxidized CaMKII production is counterbalanced by the activity of methionine sulfoxide reductase A which reduces methionine sulfoxide, product of the first oxidation step. Valine mutation for both methionine oxidation sites of CaMKII reduces atrial fibrillation, similar to methionine sulfoxide reductase overexpression, indicating a pathological role for oxidized CaMKII in cardiac disease [39].
Protein-Protein Interaction
Protein-protein interactions facilitate the recruitment of CaMKII to different subcellular locations where selective sets of CaMKII substrates are available. Cellular membranes are a major site of protein interactions for CaMKII. In the neurons, one of the most important binding partners of CaMKII is the NMDA receptor subunit GluN2B (NR2B) located at post-synaptic sites, which binds and recruits CaMKII to the synapse after its activation by glutamate stimuli [7]. This interaction is mapped to the T286 site in CaMKII where the accessibility of this site is critical for the binding [40, 41]. A variety of other protein binding partners of CaMKII have also been identified such as the connexin36 which binds and recruits CaMKII to gap junctions [42] and the α subunit of L-type Ca2+ channels which interacts with CaMKII at the plasma membrane [43]. Besides membrane association, CaMKII also binds to a F-actin binding protein α-actinin to localize to the cytoskeleton [44].
Non-coding RNAs
Non-coding RNAs (ncRNA) are functional RNAs but do not encode proteins. ncRNAs play important roles in transcriptional and post-transcriptional regulation of gene expression. Two major groups of ncRNAs, microRNAs which are short RNAs less than 200 nucleotides in length and long non-coding RNAs (lncRNAs) which are longer than 200 nucleotides, have both been implicated in regulating CaMKII gene expression [45]. MicroRNA-145 downregulates the expression of CaMKIIδ in cardiomyocytes and reduces Ca2+ overload [46]. Our group identified two novel CAMK2D-associated lncRNAs, CAMK2D-associated transcript 1 (C2dat1) and CAMK2D-associated transcript 2 (C2dat2), that are induced by ischemic insults in the neurons and regulate CaMKIIδ expression in response to ischemia/reperfusion (I/R) [47, 48]. The mechanisms through which these ncRNAs regulate CaMKII gene expression remain to be fully elucidated.
CaMKII and Cerebrovascular Diseases
Cerebrovascular disease refers to a group of conditions or disorders that affect the blood vessels in the brain and the cerebral circulation. It is the major cause of long-term disability and stroke, the fifth leading cause of death for Americans [49]. Current studies on CaMKII in this area are mainly focused on three major cerebrovascular diseases, namely cerebral ischemia, SAH, and vascular dementia (VaD). Although we did not find any reports on CaMKII per se in other cerebrovascular diseases, such as intracranial stenosis, aneurysms, vascular malformations, etc., these diseases are common causes of ischemic or hemorrhagic stroke, for example, intracranial stenosis is the narrowing of an artery in the brain, which often leads to ischemic stroke. Similarly, aneurysms and vascular malformations create weak spots on the vessel wall, which can rupture and cause hemorrhagic stroke. In the following sections, we will discuss in details the activity, expression, signaling mechanisms, and functions of CaMKII in the three major cerebrovascular diseases (see Fig. 2 for the key roles and targets of CaMKII in these diseases).
Fig. 2.
Schematic representation of key roles of CaMKII in the pathogenesis of cerebrovascular diseases. Aberrant activation or expression of CaMKII contributes to the pathophysiology of ischemic stroke by mediating the core signaling events involved in glutamate excitotoxicity, inflammation, oxidative stress, and endothelial barrier dysfunction. CaMKII is also a major regulator of vessel tone and neuronal plasticity in learning and memory and contributes to pathogenesis of subarachnoid hemorrhage (SAH) and vascular dementia
CaMKII and Cerebral Ischemia
Ischemic stroke accounts for 87% of all stroke cases in the USA [1]. It results in severe reduction of cerebral blood flow (CBF), lack of oxygen and nutrients in affected brain tissues, induction of excitotoxicity, and the following oxidative damage and post-ischemic inflammation, which ultimately leads to neuronal cell death and brain infarction. Untimely post-stroke reperfusion leads to secondary neuronal injury and harmful effects such as hemorrhagic transformation and blood-brain barrier (BBB) dysfunction in most stroke patients [50, 51]. Basic and translational scientific efforts have therefore been focused on the development and improvement of diagnostic and therapeutic strategies to limit the burden of ischemic insult–induced neuronal and vascular damages, such as neuronal death, BBB dysfunction, cerebrovascular endothelial injury, and vasogenic brain edema [52, 53]. As previously summarized, all four isoforms of CaMKII are expressed in the brain and regulate a wide range of neuronal and vascular functions [8]. In cerebral ischemia, altered expression and/or activation of CaMKII has been considered as a critical contributor of ischemia-induced neuronal cell death, and CaMKII activation can be targeted for both pro- or anti-neuroprotective therapies depending on timing and duration of treatments [7, 8].
Altered CaMKII Activity and Expression After Ischemic Stroke
Glutamate Excitotoxicity–Induced CaMKII Activation and Translocation
Under ischemic stroke conditions, a dramatic drop in oxygen, glucose, and ATP supply leads to uncontrolled neuronal plasma membrane depolarization, release of K+ into the extracellular space, and a rapid influx of Na+ and Ca2+ into the cells [54]. Neurotransmitters such as glutamate released from depolarized presynaptic and post-synaptic membranes then activate the excitatory NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors [54]. Over-activated NMDA receptors in turn lead to more membrane depolarization and Ca2+ overloading [55]. These alterations will eventually initiate neuronal cell death via programmed cell death (apoptosis) or necrotic pathways and are given the term “glutamate excitotoxicity” [56]. The influx of Ca2+ through NMDA receptors after stroke rapidly activates one of the major Ca2+ sensors in neurons, CaMKII, via binding of Ca2+/CaM to its regulatory domain and leads to T286 autophosphorylation which converts it to an autonomously active and Ca2+/CaM-independent enzyme [57–59]. CaMKII activation and subsequent autophosphorylation can be either a neuronal damaging or pro-survival force in response to ischemic/excitotoxic insults depending on the stroke models and the timing of CaMKII activation which will be discussed in details in the following sections [7, 60].
I/R injury leads to rapid activation and translocation of CaMKII in neuronal tissues. Bing-Ren et al. have shown that isolated neocortex protein from rats after global cerebral ischemia (GCI, 15 min ischemia, followed by 30 min, 4 h, and 24 h reperfusion) exhibited increased CaMKII immunoreactivity and CaM binding that peaked at 30 min after reperfusion in the crude synaptosomal fraction of hippocampal CA1 and CA3/DG (dentate gyrus) regions but decreased in the microsomal and cytosolic fractions, suggesting a translocation of CaMKII to synaptosomes [61]. Interestingly, the translocation of CaMKII in the hippocampal CA1 region is sustained for at least 24 h after reperfusion but partially recovered in CA3/DG [61]. It has been reported by the same group that cerebral ischemia-induced CaMKII translocation to synaptic membranes may enhance neuronal firing rates and Ca2+ influx [62]. Similar observations were reported by Shohei et al. in a rat model of 2 h middle cerebral artery occlusion (MCAO) followed by 2 h reperfusion [63, 64]. In particular, CaMKIIα expression in crude synaptosomal fraction increased in the ischemic core area and the surrounding penumbra during ischemia and reperfusion, whereas CaMKIIα levels in cytosolic fraction decreased by 20–40% in the penumbra and by 80% in the ischemic core area [64]. Meanwhile, CaMKIIα levels remain unchanged in the whole tissue homogenates from different brain regions 2 h after reperfusion [64]. Mechanistically, the active conformation (with autophosphorylation of T286) of CaMKII can be sustained after the transient activation by binding to the NMDA receptor subunit GluN2B (also known as NR2B) [40, 60]. This interaction also leads to translocation of CaMKII from the cytosol to the membrane of PSD upon activation [35, 40, 65–67]. Additionally, it has been reported that CaMKII forms extra-synaptic clustering, a self-aggregating process that leads to reduction of CaMKII activity, under ischemic conditions and when stimulated by pathological glutamate stimuli [66–68]. I/R also results in increased phosphorylation of CaMKII on T253 in a PSD-enriched fraction prepared from rat hippocampus [18]. Although T253 does not activate CaMKII directly, it may indirectly regulate CaMKII activity by binding to other proteins, which may contribute to the persistent activation of CaMKII involved in ischemia-/excitotoxicity-induced neuronal cell death of a human neuroblastoma cell line (SH-SY5Y) [19]. In general, upon ischemic activation, CaMKII is recruited to synaptic membranes where it may modulate cellular functions that affect cell survival and neurotransmission.
Oxidative Stress–Induced CaMKII Activation
In addition to excitotoxicity, cerebral ischemia increases the production of ROS, such as nitric oxide and superoxide, and reperfusion further stimulates ROS generation from neurons and glia cells, resulting in CaMKII oxidation and activation [69]. Erickson et al. reported that M281/282 oxidation leads to a similar conformational change in CaMKII produced by T286 phosphorylation that could maintain CaMKII in an open position to phosphorylate its substrates after Ca2+/CaM dissociation [70]. In another study, extracts from mouse forebrain synaptosomes were treated with different oxidizers and immunoblotted under non-reducing conditions with an antibody against CaMKIIα showing extensive oxidation of CaMKII forms large molecular weight aggregates and oxidized CaMKII is more closely associated with PSD [71]. To better investigate ischemia-induced oxidation of CaMKII under physiological conditions, the authors soaked acutely prepared mouse hippocampal slices in a modified artificial cerebrospinal fluid buffer pre-saturated with 95% N2/5% CO2 to mimic the ischemia condition, and a similar oxidative modification of CaMKII was observed in the hippocampal slices exposed to ischemic insults [71]. It was also noted in this study that oxidation of CaMKII suppressed both total (in the presence of Ca2+/CaM) and autonomous (independent of Ca2+/CaM) kinase activities and synaptic potentiation post ischemia due to the formation of large aggregates [71]. CaMKII oxidation has been extensively studied in cardiovascular and pulmonary diseases where oxidative stress drives disease pathology (see a detailed review by Mark E. Anderson [37]). Contrary to the rapid activation of CaMKII, decreased CaMKII activities were also documented in cultured rat hippocampal neurons treated with glutamate [58] and in the rat neocortex, striatum, and hippocampus after forebrain ischemia [72]. The later study also reported that protein levels of CaMKIIα and β decreased in the cytosol and increased in the particulate fractions of the three brain regions they examined, suggesting an intracellular redistribution of CaMKIIs [72]. Nonetheless, functional outcomes or pathological consequences of excitotoxic/ischemia-induced loss of CaMKII activity were not discussed in these two studies.
Altered CaMKII Expression at Transcriptional Level
Besides post-translational regulation by phosphorylation or oxidation, we recently showed that CaMKII could also be regulated at transcriptional level by two lncRNAs, C2dat1 and C2dat2. Wild-type C57BL/6J mice were subjected to 1 h of MCAO and 6–24 h of reperfusion. C2dat1 expression was significantly increased in the cortical penumbra at 6–24 h post reperfusion compared to the sham-operated mice. This was also accompanied by an increased expression of CaMKIIδ at transcript and protein levels [47]. Similar expression patterns can be observed in primary mouse neuron cultures under in vitro ischemic condition [47]. Moreover, knockdown of C2dat1 and 2 by siRNAs significantly blocked in vitro ischemia-induced expression of CaMK2D in N2a cells and primary neurons. The knockdowns also exacerbated ischemia-induced neuronal cell death [47, 48]. However, knockdown of C2dat1 and C2dat2 had no effects on oxygen-glucose deprivation and reoxygenation (OGD/R)–induced expression of CaMKIIγ, indicating specific targeting of CAMK2D by these lncRNAs [48]. The upregulation of CaMKIIγ was also interesting as both CaMKIIδ and γ were thought to be absent or low in neurons. Knockdown of CaMKIIδ and γ exacerbated OGD/R-induced neuronal cell death, implying that both isoforms promote neuronal survival. This is distinct from its pro-death effects observed in the early phase of I/R. It is possible that increased CaMKIIδ and γ may alter the functionality of CaMKII holoenzyme since their upregulation could change several properties of CaMKII including the ratio of subunit composition in the holoenzyme, stimulated/autonomous activity, localization, and protein interactions.
Contributory Roles of CaMKII in the Pathogenesis of Ischemic Stroke
Cerebral ischemia triggers a cascade of pathological events that ultimately result in irreversible neuronal injury in stroke-affected brain tissues within minutes of stroke insult [54]. The pathophysiological order of the events ranges from excitotoxicity within minutes, robust inflammatory responses within hours, to programmed cell death and tissue loss within hours and days of stroke onset [54, 73, 74]. Based on our literature search, it appears that most studies have been focusing on the involvement of CaMKII in excitotoxicity-induced neuronal death [7, 60, 75, 76]. Only a few reported functional roles of CaMKII in inflammation [77], endothelial barrier integrity [3], and astrocyte dysfunction [78] after experimental stroke (summarized in Table 1). In this section, we review the functional inputs of CaMKII in these biological processes.
Table 1.
Contributory roles of CaMKII in ischemic stroke: a summary of pathological events, their cellular targets, and the overall pathological outcome of CaMKII
| Pathological events in stroke | Targeting sites | Cellular targets/outcomes | |
|---|---|---|---|
| CaMKII | Increased pCaMKII/CaMKII ratio after OGD/R | Neurons | Pro-apoptosis [79] |
| CaMKII | Prolonged CaMKII activity inhibition exacerbates excitotoxicity | Neurons | Pro-apoptosis [75] |
| CaMKIIɑ | siRNA targeting CaMKIIɑ reduces oxidative stress after MCAO |
Neurons Astrocytes |
Glucose 6-phosphate dehydrogenase [80]/pro-death |
| CaMKII | CaMKII inhibitor KN93 reduces inflammatory marker in organ culture | Cerebral arteries | TNF-α receptor 1 (77)/pro-death (implied) |
| CaMKIIδ | OGD induces increased RIPK3-CaMKIIδ interaction | Oligodendrocyte progenitor cells | Pro-necroptosis [81] |
| CaMKIIɑ | Increased p-T286 CaMKIIɑ in cerebellar vermis after GCI | Cerebellar vermis | Decrease Purkinje cell density [82]/pro-death |
| CaMKIIδ, γ | Increased potassium currents, results in hypoxia-induced cell swelling of brain endothelial cells | ECs | Voltage-gated K+ channels [3]/pro-death |
| CaMKIIɑ | CaMKIIɑ−/− rat presents larger infarction after 2.5 h MCAO and 21 h reperfusion | Whole body | Neuroprotection [83] |
| CaMKII | CaMKII activity inhibition at the time of glutamate insult can be neuroprotective | Neurons | Neuroprotection [60] |
| CaMKII | CaMKII inhibition by tat-CN21 induces calcium oscillations in cortical astrocytes | Astrocytes | Neuroprotection [84] |
| CaMKIIδ, γ | I/R-induced CaMKIIδ, γ promotes neuronal survival | Neurons | NF-κB [48]/neuroprotection |
Excitotoxicity-Induced Cell Death
Cerebral ischemia–induced neuronal damage is largely caused by glutamate-mediated excitotoxicity. Synaptic glutamate released from the damaged neurons leads to the activation of NMDA and AMPA receptors [85, 86]. NMDA receptors are Ca2+ permeable, and the opening of these channels leads to further membrane depolarization and greater Ca2+ influx which exacerbates intracellular Ca2+ overload [86]. Oxygen deprivation–induced acidosis disrupts Na+/Ca2+ exchanger, prevents the efflux of Ca2+, and further contributes to Ca2+ overload. It is known that Ca2+, at a lower concentration (~ 10−6 M), mediates neuroprotective processes, whereas at higher concentration about 10−4–10−3 M, Ca2+ induces apoptosis or necrosis [87, 88]. Prolonged Ca2+ increases during ischemia result in pathophysiological activation of many intracellular signaling cascades, including Ca2+/CaM/CaMKII [88]. CaMKII also phosphorylates NMDA and AMPA glutamate receptors, which further increases Ca2+ influx and aggravates excitotoxicity [88]. These processes contribute to neuronal death during I/R but may also contribute to the death of endothelial cells (ECs) [74], astrocytes [89, 90], and oligodendrocytes [81] after cerebral I/R insult or OGD/R. Excitotoxicity may induce cell death mainly by necrotic, apoptotic, or autophagic pathways.
Necrosis
Necrosis is generally identified as the cells present swollen organelle phenotypes, which subsequently burst to release their contents into the extracellular space, whereas apoptotic cells undergo nuclear and cytoplasmic condensation, followed by cell fragmentation and phagocytosis by phagocytes [91]. Necrosis occurs during first post-stroke minutes, primarily in the ischemic core [92, 93], whereas apoptosis develops later, for hours or days, mainly in the surrounding penumbra. Overloaded cytosolic Ca2+ can induce necrosis via activation of proteases, phospholipases, and mitochondrial permeability transition [94]. The best-characterized form of regulated necrosis is necroptosis. Necroptosis is defined as a necrotic cell death dependent on the kinase activity of Receptor Interacting Protein 1 (RIP1) and RIP3 and expression of mixed lineage kinase–like protein [94, 95]. Increased RIPK3 expression and RIPK3-CaMKIIδ interaction have been reported in a study in which necroptosis is induced in vitro in oligodendrocyte progenitor cells (OPCs) by OGD plus caspase inhibitor zVAD treatment to facilitate cell death from apoptosis to necroptosis [81], whereas RIPK3 siRNA treatment significantly reduced RIPK3 expression, the RIPK3-CaMKIIδ interaction, and OPC cell death at 24 h after OGD [81]. Moreover, activated CaMKIIδ (p-T287) levels were increased in OPCs at 12 and 24 h after OGD, whereas total CaMKIIδ levels were not altered. Furthermore, RIPK3 inhibition via siRNA decreased p-T287 CaMKIIδ levels [81]. These results suggest that RIPK3 mediates oligodendrocyte necroptosis through interacting with and activating CaMKIIδ. In another study by Lixuan et al., they found in a rat model of transient global cerebral ischemia, pretreatment of KN93 (a CaMK inhibitor) before the surgery significantly reduced RIP1 and RIP3 interaction and protected hippocampal neurons from global cerebral ischemia–induced necroptosis [96].
Apoptosis
Apoptosis plays a major role in tissue loss in ischemic lesions. Unlike in the ischemic core area of the stroke where neuronal death is generally mediated by necrosis and is considered beyond rescue, apoptosis is the dominating cell death modality in the penumbra [97]. The therapeutic time window between the onset of stroke insult and apoptosis-induced tissue loss surrounding the ischemic site makes it a very attractive target for stroke therapy [97–99]. Diverse apoptotic initiation and regulation pathways are induced in penumbra from different initial triggers and various anti-apoptotic proteins are upregulated as well. CaMKII is found to be upregulated in the penumbra surrounding the infarction core in the rat cerebral cortex at 1, 4, and 24 h after stroke [99]. Our group has also discovered increased CaMKIIδ fluorescence intensity in the peri-infarct region after I/R [47]. Nicholas et al. reported an increase in CaMKIIɑ activity (p-T286) in mouse cerebellar vermis at 6 h after global I/R condition. This is believed to contribute to decreased Purkinje cell (a type of neuron located in the cerebellum) densities at 7 days after surgery because it can be inhibited with a single dose of a peptide inhibitor of CaMKII activity, tat-CN19, at 30 min after surgery [82]. In another in vitro study in which PC12 cells were incubated under OGD condition for 2 h and then cultured under normal conditions for 1, 2, 4, 5, 12, and 24 h, pCaMKII/CaMKII ratio increased from 1 h after incubation in normal conditions, reached maximum at 2 h, and then gradually declined with culturing time but remained higher than non-OGD control group [79]. Cell apoptosis increased in the OGD/R-induced PC12 cells and was blocked by KN93 treatment [79]. These studies suggest that CaMKII activation following I/R contributes to apoptotic cell death. This is consistent with previous studies that have identified a pro-apoptotic role for CaMKII activation following ischemia [18, 19, 100–102] (also summarized by Steven et al. [7]).
Despite the overwhelming evidence supporting a pro-apoptotic function of CaMKII after I/R, some studies suggest that CaMKII can be neuroprotective by phosphorylating and inhibiting several pro-apoptotic proteins such as NO synthase, Bad, and GSK-3 [7]. In these studies, CaMKII was mostly studied as a whole without distinguishing the specific isoforms involved. Therefore, the role that each CaMKII isoform played in neuronal survival after ischemia remains to be defined. In our studies, both CaMKIIδ and CaMKIIγ were upregulated in the penumbral tissue from 1 to 4 days after MCAO surgery [47, 48]. Overexpression of CaMKIIδ promoted neuronal survival in the OGD/R model, while knockdown of CaMKIIγ resulted in significant neuronal death after OGD/R [48]. We also demonstrated that I/R-induced CaMKIIδ and CaMKIIγ promoted neuronal survival through activating the canonical NF-κB signaling pathway [48]. It is notable that the induction of CaMKIIδ by lncRNAs was gradual and persistent which is distinct from the acute activation of CaMKII immediately after I/R that often associates with neuronal death, highlighting the dynamic regulation and functional diversity of the CaMKII kinases in the course of I/R injury in the brain. In line with the pro-survival function of CaMKII, there are also studies that suggest that prolonged inhibition or knockout of CaMKII exacerbates ischemic injuries [60, 75, 83]. For example, genetic knockout of CaMKIIɑ resulted in larger infarction compared to wild-type littermates in a MCAO model [83]. This finding could be explained by the developmental defects caused by lacking CaMKIIɑ since it was reported by another group that CaMKIIɑ knockout mice are epileptic [103], meaning the KO mice are generally more susceptible to ischemic insults. The Hudmon group showed that prolonged pharmacological inhibition of CaMKII (incubate with tat-CN21 for 4–24 h) exacerbated excitotoxicity following glutamate/glycine insult in cultured neurons [60]. They further confirmed that cultured neurons underwent apoptosis in response to prolonged CaMKII inhibition (>4 h) since the prolonged CaMKII inhibition was associated with an increase in TUNEL staining and caspase-3 cleavage [75]. These findings are consistent with the notion that the extent of neuronal damage in the penumbra depends on the level of CaMKII, and loss of CaMKII augments neuronal susceptibility to ischemic insults.
Autophagy
Excitotoxic insults also lead to neuronal autophagy, and accumulating studies have indicated autophagy is involved in the pathophysiological changes in ischemic stroke [104, 105]. Interestingly, autophagy can play both protective and detrimental roles in ischemic stroke which are summarized in a review article by Pei et al. [106]. CaMKII has been shown to regulate autophagy in neuroblastoma cells. Li et al. reported that CaMKII can directly phosphorylate Beclin-1 (a critical regulator of autophagy) to promote the activation of autophagy [107]. CaMKII has also been shown to regulate autophagy in myocardial I/R injury. Kong et al. demonstrated that CaMKII inhibition attenuated autophagic flux through mitigating the phosphorylation of Beclin-1 [108]. In addition, Kulbe et al. found that tat-CN21, a highly selective peptide inhibitor of CaMKII with neuroprotective function [109], inhibited basal autophagy in primary hippocampal neurons [110]. However, in the presence of excitotoxic glutamate insults that blocked autophagy, no further effects were observed after tat-CN21 treatment [110]. Taken together, although a promising target, more investigations are needed to define the role of CaMKII and its regulatory mechanisms in autophagy in cerebral I/R injury.
Neuroinflammation
Cerebral ischemia induces a cascade of molecular events that transform the cerebrovascular endothelium from a quiescent to a proinflammatory state [111]. Activated cerebral endothelium have the capacity to produce and/or secrete many inflammatory mediators, most important of which are the selectins, proinflammatory cytokines, and integrins, thus becoming a source of inflammation themselves in the cerebral vasculature [112]. Tumor necrosis factor alpha (TNF-α) is one of the proinflammatory cytokines that initiates inflammation in many cell types via its receptor (TNF-α receptor 1) and plays an essential role in cerebrovascular inflammation. Using the method of organ culture for inducing ischemic-like vascular wall changes, Roya et al. incubated rat basilar arteries in serum-free medium for 0, 3, 6, or 24 h in the presence or absence of CaMKII inhibitor KN93 [77]. They found CaMKII activation in the non-incubated (0 h) arteries was gradually decreased with time. The TNF-α receptor 1 expression was elevated after organ culture and can be blocked by treatment of CaMKII inhibitor KN93 [77]. Their findings suggest that inhibiting CaMKII activation attenuates neuroinflammatory mediators and could contribute to neuroprotection. The elevation of another proinflammatory mediator, endothelin-1 (ET-1), has been indicated in the regions of vascular injuries and inflammation [113]. In mouse brain microvascular endothelial cells bEnd.3, Chih-Chung et al. found that ET-1 stimulated increases of intracellular Ca2+. This contributed to ET-1–induced CaMKII activation and ET-1/ET receptor–mediated cyclooxygenase-2/prostaglandin E2 release (both are known to aggravate brain inflammation) through regulating mitogen-activated protein kinases and the downstream activating transcription factor 2 [114]. Both studies suggest that strategies targeting CaMKII may be beneficial for brain injury and inflammatory disease.
Oxidative Stress–Induced Tissue Damage
Many pathological events occur during the ischemia reperfusion procedure, including the release of free radicals that bring damages to the brain and aggravate neuronal injuries. These free radicals represented by ROS could spark oxidative stress in the brain. With the help of glutathione reductase and nicotinamide adenine dinucleotide phosphate (NADPH), oxidized glutathione can be transformed to reduced glutathione, which is necessary to eliminate excess ROS [115]. Previous studies indicate that CaMKII can be activated by ROS, and activated CaMKII also regulates redox regulators such as NADPH and reduced glutathione to further exacerbate oxidative stress–induced tissue damage [37], providing a potential feed-forward cycle for CaMKII to infuse the oxidative stress–induced tissue damage. In a rat ischemic stroke model, Yamin et al. performed intracerebroventricular injection of siRNA that targets CaMKIIα at 48 h pre-MCAO surgery. They found rats that received siRNA injection showed milder neurological deficits and smaller infarct volume compared to those that received scramble control injection [80]. In addition, CaMKIIα siRNA decreased ROS content and increased the reduced glutathione/oxidized glutathione and NADPH/NADP+ ratios in the rat cortex after MCAO [80], suggesting that CaMKIIα inhibition could suppress oxidative stress through upregulating NADPH and reduced glutathione. This study provides evidence that CaMKII inhibition can serve as anti-oxidant therapy in cerebrovascular disease.
Endothelial Barrier Dysfunction
Endothelium plays a critical role in the regulation of vascular function. Untimely reperfusion after ischemic stroke often times leads to harmful consequences including blood-brain barrier (BBB) dysfunction. BBB is centrally positioned within the neurovascular unit, and several elements contribute to the physical barrier of BBB, including cerebrovascular ECs, junctional proteins, pericytes, astrocytes, and basement membrane [53]. Among those, cerebrovascular ECs are the primary barrier between circulation and tissue that play an essential role in the maintenance of BBB integrity/permeability and cerebral homeostasis. CaMKIIα was found in the perinuclear region of the cells in primary cerebral EC culture [116]. In a separate study, glutamate treatment induced CaMKIIα activation in primary cultures of rat cerebral ECs even after 10- and 60-min recovery [59]. Besides CaMKIIα, both CaMKIIδ and γ are expressed in rat brain capillary ECs. Moreover, activation of CaMKIIδ and γ was found upstream of voltage-gated potassium channels, resulting in hypoxia-induced cell swelling that may precede barrier dysfunction [3]. In addition, CaMKIIδ and γ were found in human central nervous system pericytes, and their activation is required for extracellular acidosis–induced cAMP-response element binding protein activation and proinflammatory cytokine interleukin-6 upregulation [4]. CaMKII is also found expressed in cultured cortical astrocytes using pan-CaMKII antibody [84]. To mimic the rapid loss of CaMKII activity in brain tissue during an ischemic insult, cultured cortical astrocytes were treated with CaMKII inhibitor tat-CN21. Inhibiting CaMKII activity in neuronal support cells (astrocytes) induces ATP release from astrocytes and contributes to Ca2+ oscillations in astrocytes and induces neuronal death [84]. However, functional roles of CaMKII in regulating endothelial barrier function in vivo remain to be explored.
Inhibitors of CaMKII and Their Effects on Post-insult Neuroprotection
Given the well-documented CaMKII activation immediately after ischemic insults and its role in mediating neuronal death in experimental models of stroke, it appears to be a promising target for acute clinical intervention to limit brain damage after stroke. Small molecule CaMKII inhibitors KN62 and KN93 have been widely used to study CaMKII function in vitro, as they are membrane penetrating [7]. However, KN inhibitors are not selective inhibitors for CaMKII activation [117]. A more selective CaMKII inhibitor is CN21, which is a 21mer peptide derived from the natural CaMKII inhibitory protein CaM-KIIN [118]. Peptide-based inhibitors are further made cell penetrating by fusion with a sequence motif such as tat [119]. Inhibiting stimulated and autonomous CaMKII activity with tat-CN21 attenuated neuronal cell death after a glutamate insult in vitro or in vivo and was effective even when administered 1 h after the MCAO surgery, which is within a clinically relevant window of therapeutic opportunity [109]. In a separate study in which KN93, tat-AIP, and tat-CN21 were applied either immediately before or after the excitotoxic insult, post-insult neuroprotection by CaMKII inhibition was confirmed using both tat-CN21 and tat-AIP, but not KN93 [60]. This study also confirmed significant neuroprotection in cortical cultures when tat-CN21 was administered 2 h after excitotoxic challenge. On the contrary, KN93 was neuroprotective only when administered during but not after the insult in both studies [60, 109]. In a rat GCI model, tat-CN21 administered 3 h after reperfusion exerted neuroprotective effects against delayed hippocampal CA1 pyramidal neuronal cell death 10 days post GCI through decreasing GCI-induced phosphorylation, translocation, and membrane targeting of CaMKIIɑ, as well as CaMKIIɑ-NR2B interaction [102]. Mechanistically, tat-CN21 and tat-AIP can both block Ca2+/CaM-stimulated activity of CaMKII and the Ca2+-independent autonomous CaMKII activity during the insult, whereas KN93 only inhibits Ca2+/CaM–stimulated CaMKII activity effectively, suggesting that the autonomous activation of CaMKII may be a promising drug target for post-stroke neuroprotection. However, both KN93 and AIP have been shown to have off-target effectors. KN93 is an inhibitor of several CaMK family members including CaMKI, CaMKII, CaMKIV, and voltage-gated K+ and Ca2+ channels [60]. The peptide inhibitor AIP has also been shown to inhibit other CaMK family members [60].
Interestingly, using a competitive inhibitor of CaMKII kinase activity to inhibit ischemia-induced sustained activation of CaMKII may lead to opposite effects. For example, tat-CN21 treatment for 8 h or more before the glutamate insult resulted in a significant increase of neuronal death in cortical cultures compared to glutamate insult alone [60]. In an in vitro study, tat-CN21 treatment in cortical astrocytes decreased glutamate uptake, increased ATP release, and dysregulated astrocyte-neuron signaling thereby compromising neuronal survival after ischemic insult [84]. These studies are again in line with the previous observation that CaMKIIɑ−/− mice had larger infarction than the wild-type mice after MCAO [83]. Overall, CaMKII inhibition–mediated neuroprotection was achieved when inhibitors were administered at the acute phase of ischemic/excitotoxic insults, whereas sustained CaMKII inhibition long (> 4 h) before ischemic insult could exacerbate neuronal death by enhancing neuronal vulnerability to the insults.
CaMKII and Subarachnoid Hemorrhage
Hemorrhagic stroke is caused by rupture of weakened arterial blood vessels, causing bleeding and damage to the surrounding brain tissue. There are two types of hemorrhagic stroke; the most common type is intracerebral hemorrhage (ICH) that occurs within the brain tissue, and the less common type is subarachnoid hemorrhage (SAH). There have not been any studies on CaMKII in ICH. Therefore, we mainly discuss the role of CaMKII in SAH in this section. Approximately 80% of non-traumatic, spontaneous SAH is due to the rupture of an intracranial aneurysm leading to the extravasation of arterial blood into subarachnoid space [120]. The initial mortality in SAH patients before receiving medical attention is high (12%) [121]. After several days, the development of delayed cerebral ischemia and cerebral vasospasm contributes to a prolonged CBF reduction in approximately 40% of SAH patients [122]. Of those patients who survive SAH, many of them suffer from long-term memory and cognitive defects that severely impact their quality of life [123].
Early brain injury (first 72 h after SAH) could happen within minutes after the initial bleeding, mostly due to ischemia, based on a large body of animal studies [124, 125]. A study by Parker et al. utilized quantitative mass spectrometry–based phosphoproteomic analysis to dissect early SAH-induced phosphorylation events (0.5 h and 1 h post SAH) in cerebral artery tissue lysates from SAH rats [126]. Among those protein kinases significantly altered by SAH, they found CaMKII activation at its autophosphorylation site T286 which could be blocked by treating the rat with KN93 prior to SAH. However, the total CaMKII expression remained unchanged [126], implying that CaMKII might play a role in SAH-induced cerebral vasculopathy. In agreement with Benjamin’s study, Lars et al. also observed a rapid activation of CaMKII at its autophosphorylation site T286 at 1 h post SAH in rat cerebral artery lysates, and the activated CaMKII reduced to sham-operated levels at 6 and 24 h post SAH [127]. They also observed an increased overall CaMKII protein expression at 72 h post SAH by immunohistochemistry in cerebral vascular smooth muscle cells. Interestingly, this can be blocked by KN93 treatment prior and immediately after SAH operation [127]. Moreover, KN93 treatment attenuated endothelin and serotonin receptor–mediated vasoconstriction and improved sensorimotor function at 48 h post SAH. Therefore, inhibiting CaMKII activation by KN93 can be beneficial through reducing cerebral vasoconstriction and improving neurological outcomes after SAH [127]. The beneficial effects through inhibiting CaMKII activity were further affirmed by another group using siRNA that targets CaMKIIα [128]. The expression of activated CaMKIIα in rat brain increased from 3 to 24 h post SAH (total CaMKIIα expression did not change), and p-CaMKIIα (T286) mainly localized within the neurons and microglia [128]. Meanwhile, CaMKIIα knockdown by siRNA injected intracerebroventricularly at 48 h prior to surgery, markedly improved neurobehavior after SAH [128] and significantly decreased p-TAK1, p-JNK, and nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome expression (indicating reduced inflammatory response). Sang et al. reported that 6 days after SAH injury, CaMKII overall expression was reduced in the dendritic layer of the CA1 area of the hippo-pyramidal cells. This was accompanied by a significant decrease of synapses in neurons in the CA1 area that could be responsible for loss of long-term potentiation (a persistent strengthening of synapses following high levels of neuronal stimulation) after SAH [129]. Taken together, SAH-induced CaMKII activation or overall expression can vary depending on different brain regions, cell types, SAH models, and time after SAH surgery. Nonetheless, inhibition of CaMKII activity mostly associates with improved neurological outcome after SAH.
CaMKII and Vascular Dementia
Vascular dementia (VaD) is a complex neurodegenerative disorder that is manifested as the functional consequence of reduced blood flow (acutely or chronically) in the brain [130]. VaD associates with a progressive cognitive and memory impairment, and the instances increase with age [131]. While CaMKII is highly enriched in neuronal tissue (especially in hippocampal pyramidal cells), the autophosphorylation of CaMKII is deemed essential for the hippocampus-dependent memory formation and the long-lasting increase in synaptic efficacy following long-term potentiation [132]. The activation and functional involvement of CaMKII signaling in the hippocampus and other brain regions has been well documented in different animal VaD models.
Most studies focus on the neuroprotective function of CaMKII in hippocampal CA1 pyramidal neurons and the restored learning and memory ability in VaD animals through activating CaMKII. For example, in a VaD mouse model, Yui et al. reported a cerebral ischemia–induced contextual memory deficit, and both activated CaMKII (including α and β isoforms) and the total CaMKIIα and CaMKIIβ expressions were significantly decreased in the hippocampal CA1 region at 12 days in bilateral common carotid artery occlusion (BCCAO) mice [133]. Under certain BCCAO conditions, they found nobiletin (a compound for Alzheimer’s disease therapy) treatment inhibited delayed neuronal death and restored both CaMKII (α and β) activation and the overall expression in the hippocampal CA1 region. Nobiletin also improved BCCAO-induced memory deficits and hippocampal long-term potentiation impairment [133]. Xiao-Juan et al. reported similar observations that cerebral ischemia led to reduced p-CaMKII (T286) in the hippocampal CA1 pyramidal neurons, which associates with decreased spatial learning and memory capacity of mice subjected to right unilateral common carotid artery occlusion (a chronic VaD model) at 30 days after surgery [134]. These deficits can be partially rescued by CaM antagonist loaded micelle treatment [134]. The same group reported in another study using a bilateral common carotid artery stenosis mouse model (BCCAS, a common model of cerebral hypoperfusion–induced cognitive impairment) that CaM inhibitor DY-9836 restored the decrease of p-CaMKII (T286) in hippocampal pyramidal neurons and improved learning impairment after BCCAS surgical operation [135]. Similar results were reported in a rat permanent BCCAO model [136, 137]. In addition, VaD is an age-related disease. Qi et al. explored aging-induced cognitive deficits in a senescence-accelerated mouse (SAMP8) model [138], in which the cognitive deficits can be observed as early as 4 months after birth [139]. A significant decrease in p-CaMKIIα (T286) but not the non-phosphorylated CaMKIIα expression, was observed in the prefrontal cortex region of older SAMP8 mice compared to the younger SAMR1 mice [138], and oral administration of a traditional Chinese prescription, Kangen-karyu, ameliorated aging-induced memory deficits and restored p-CaMKIIα expression in the cerebral cortex of older SAMP8 mice [138]. Despite the variety of VaD models employed in different studies, it appears that they all come to the same conclusion that restoring p-CaMKII (T286) or CaMKII overall expression is beneficial for cerebral ischemia–/age-induced cognitive deficits.
Summary and Future Prospects
In this article, we reviewed the important roles of CaMKII serine/threonine kinases in cerebrovascular diseases. There are both well-studied and under-studied areas of CaMKII functions in stroke, subarachnoid hemorrhage, and vascular dementia (summarized in Table 2). The four isoforms of CaMKII, although highly conserved in domain structures, vary widely in expression patterns in different tissues and exhibit significant pathobiological functional diversity in cerebrovascular diseases. In light of all the literature on this topic, we have come to the conclusion that CaMKIIs significantly influence the pathological processes of cerebrovascular diseases by regulating a whole host of events and neurovascular cells including neurons, ECs, smooth muscle cells, pericytes, and astrocytes. In addition to the most extensively studied isoform CaMKIIɑ, which is a neuron-specific isoform, the understudied CaMKII isoforms (CaMKIIδ and γ) have also emerged as new players in neuronal recovery after ischemic insults [47, 48, 81]. Overall, in ischemic stroke, there is compelling evidence supporting rapid CaMKII activation immediately after I/R insults, and activated CaMKII plays a crucial role in mediating excitotoxicity-induced neuronal death through necrotic, apoptotic, and possibly autophagic pathways and contributes to secondary oxidative damage, neuroinflammation, and BBB dysfunction, indicating that inhibition of CaMKII may be a promising therapeutic strategy to limit brain damage in acute phase of ischemic injury. However, there remain discrepancies in the role of CaMKII in later phases of ischemic stroke with evidence showing that prolonged inhibition or loss of CaMKII compromises neuronal function and survival, suggesting a positive role of CaMKII in neuronal recovery. In SAH, despite the varied CaMKII activity and expression in different brain regions after hemorrhagic injury, inhibition of CaMKII activity has consistently resulted in improved neurological outcome after SAH, supporting a pro-death function of CaMKII in SAH. In contrast, results from various VaD models have consistently demonstrated that increased p-CaMKII (T286) or CaMKII overall expression reduces cerebral ischemia–/age-induced cognitive deficits, implying that restoration of CaMKII activity and expression may benefit VaD patients. From a therapeutic perspective, the timing and duration of CaMKII inhibition will need to be calibrated carefully since inhibitors administered at the acute phase of I/R may be neuroprotective, while those resulted in long periods of sustained CaMKII inhibition could exacerbate neuronal death. Additionally, CaMKII inhibitors that block autonomous activation of CaMKII clearly provide added advantage for post-stroke neuroprotection, such as tat-CN21 which is a selective and efficacious CaMKII inhibitor that blocks both stimulated and autonomous activity of CaMKII in vitro and in vivo.
Table 2.
CaMKII studies in cerebral vascular diseases: a summary of well-studied and under-studied areas of CaMKII in major cerebral vascular diseases
| CaMKII | Well-studied | Under-studied |
|---|---|---|
| Ischemic stroke |
• Changes of CaMKII activity, expression, and localization before and after ischemic stroke • Ischemia-induced CaMKII oxidation • Role of CaMKII holoenzyme or CaMKIIα in ischemia-induced apoptotic neuronal cell death • CaMKII in excitotoxicity-induced neuronal death in ischemic stroke • Effects of CaMKII inhibitors on post-insult neuroprotection |
• Signaling mechanisms of CaMKII in neurons and non-neuronal cells in ischemic stroke • Functions of CaMKII in non-neuronal cells such as vascular endothelial cells • Involvement of CaMKII in neuroinflammation and endothelial barrier dysfunction induced by ischemic insults • Roles of under-studied CaMKII isoforms such as δ and γ in ischemic stroke • CaMKII regulation by non-coding RNAs • Translation of CaMKII-targeted agents to clinical settings |
| Subarachnoid hemorrhage |
• Changes of CaMKII expression and activity before and after SAH • Impact of CaMKII activation on neurological outcome after SAH |
• How CaMKII contributes to improved neurological outcome after SAH • Functional involvement and signaling mechanisms of CaMKII in neurons and non-neuronal cells after SAH |
| Vascular dementia | • Activation and neuroprotective function of CaMKII in hippocampal neurons in different animal VaD models |
• Mechanisms of CaMKII activation/upregulation in VaD • Functional involvement of CaMKII in non-neuronal cells in VaD models |
Although previous studies have implied the effectiveness of neuroprotectant in animal stroke models, there has not been much progress made by far in regard to the successful translation of neuroprotective strategies to clinical settings [73], suggesting that focusing only on neuroprotection is insufficient. In other words, non-neuronal cells and the local microenvironment of the surviving neurons could serve as potential therapeutic targets to alleviate ischemic brain injuries as well. For example, cerebrovascular ECs play an essential role in the maintenance of BBB integrity and cerebral homeostasis under physiological conditions [140, 141]. Ischemia-induced endothelial injury increases cerebrovascular permeability and compromises BBB integrity. Proinflammatory factors may then pass through the compromised BBB to attract circulating immune cells to the injured brain which leads to secondary ischemic brain parenchymal injuries [142]. Studies by Ferenc group indicated that CaMKIIɑ, δ, and γ are all expressed in murine cerebrovascular ECs [59, 116], and Blasig group further reported that hypoxia induced activation of CaMKIIδ and γ in rat brain capillary ECs associated with cell swelling that may precede barrier dysfunction [3]. Based on this previous evidence, it would be of great interest to explore whether CaMKII is one of the upstream molecular regulators of BBB stability, dysfunction, or protection after cerebral ischemia. Moreover, studies in rodent cerebral ischemia models have demonstrated that cerebral vascular ECs begin to proliferate in the peri-infarct regions as early as 12h after stroke onset [143]. Importantly, the extent of angiogenesis is often closely associated with reduced cerebral infarction and improved neurological recovery [143]. Thus, studying whether CaMKII activity is involved in post-ischemic angiogenesis may be a useful therapeutic strategy for treatment of ischemic stroke in clinical settings.
With the help of advanced techniques such as genetic manipulation, genomic editing, and proteomics, more comprehensive and in-depth understanding of CaMKII’s roles in cerebrovascular diseases will be conducive to develop novel therapeutic approaches to effectively reduce the risk of cerebrovascular diseases, as well as improve prognosis and decrease complications.
Abbreviations
- CaMKII
Ca2+/calmodulin–dependent protein kinase II
- CaM
calmodulin
- CBF
cerebral blood flow
- BBB
blood-brain barrier
- MCAO
middle cerebral artery occlusion
- OGD
oxygen and glucose deprivation
- NLS
nuclear localization sequence
- PSD
post-synaptic density
- ROS
reactive oxygen species
- lncRNA
long non-coding RNA
- GCI
global cerebral ischemia
- EC
endothelial cell
- SAH
subarachnoid hemorrhage
- VaD
vascular dementia
- BCCAO
bilateral common carotid arteries occlusion
- NMDA
N-methyl-D-aspartate
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NADPH
nicotinamide adenine dinucleotide phosphate
- TNF-α
tumor necrosis factor alpha
- ET-1
endothelin-1
Author Contribution
Q. Jane Wang had the idea for the article and directed the drafting and revising of the work, Xuejing Zhang and Jaclyn Connelly performed the literature search and data analysis and drafted the work, and Edwin S. Levitan and Dandan Sun participated in drafting and critically revising the manuscript.
Funding
This work was supported in part by the American Heart Association award 19TPA34850096 (Wang) and NIHR01NS48216 (Sun).
Data Availability
All data and materials are available and support the published claims and comply with field standards.
Code Availability
Not applicable. No coding in the study.
Declarations
Ethics Approval
This is a review paper. No ethical approval is required.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
This is a review paper. No participants involved in the study.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dandan Sun, Email: sund@upmc.edu.
Jane Q. Wang, Email: qjw1@pitt.edu
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Roux PD, Winn HR. Management of cerebral aneurysms. How can current management be improved? Neurosurg Clin N Am. 1998;9(3):421–433. doi: 10.1016/S1042-3680(18)30241-9. [DOI] [PubMed] [Google Scholar]
- 3.Balla Z, Hoch B, Karczewski P, Blasig IE. Calcium/calmodulin-dependent protein kinase IIdelta 2 and gamma isoforms regulate potassium currents of rat brain capillary endothelial cells under hypoxic conditions. J Biol Chem. 2002;277(24):21306–21314. doi: 10.1074/jbc.M200553200. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura K, Kamouchi M, Arimura K, Nishimura A, Kuroda J, Ishitsuka K, Tokami H, Sugimori H, Ago T, Kitazono T. Extracellular acidification activates cAMP responsive element binding protein via Na+/H+ exchanger isoform 1-mediated Ca(2)(+) oscillation in central nervous system pericytes. Arterioscler Thromb Vasc Biol. 2012;32(11):2670–2677. doi: 10.1161/ATVBAHA.112.254946. [DOI] [PubMed] [Google Scholar]
- 5.Bayer KU, Schulman H. CaM kinase: still inspiring at 40. Neuron. 2019;103(3):380–394. doi: 10.1016/j.neuron.2019.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3(3):175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 7.Coultrap SJ, Vest RS, Ashpole NM, Hudmon A, Bayer KU. CaMKII in cerebral ischemia. Acta Pharmacol Sin. 2011;32(7):861–872. doi: 10.1038/aps.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toussaint F, Charbel C, Allen BG, Ledoux J. Vascular CaMKII: heart and brain in your arteries. Am J Physiol Cell Physiol. 2016;311(3):C462–CC78. doi: 10.1152/ajpcell.00341.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rostas JAP, Spratt NJ, Dickson PW, Skelding KA. The role of Ca(2+)-calmodulin stimulated protein kinase II in ischaemic stroke - a potential target for neuroprotective therapies. Neurochem Int. 2017;107:33–42. doi: 10.1016/j.neuint.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Ebenebe OV, Heather A, Erickson JR. CaMKII in vascular signalling: "friend or foe"? Heart Lung Circ. 2018;27(5):560–567. doi: 10.1016/j.hlc.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharyya M, Stratton MM, Going CC, McSpadden ED, Huang Y, Susa AC, et al. Molecular mechanism of activation-triggered subunit exchange in Ca(2+)/calmodulin-dependent protein kinase II. Elife. 2016;5:e13405. doi: 10.7554/eLife.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharyya M, Karandur D, Kuriyan J. Structural Insights into the regulation of Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) Cold Spring Harb Perspect Biol. 2020;12(6):a035147. doi: 10.1101/cshperspect.a035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tombes RM, Faison MO, Turbeville JM. Organization and evolution of multifunctional Ca(2+)/CaM-dependent protein kinase genes. Gene. 2003;322:17–31. doi: 10.1016/j.gene.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Sloutsky R, Stratton MM. Functional implications of CaMKII alternative splicing. Eur J Neurosci. 2020. 10.1111/ejn.14761. [DOI] [PMC free article] [PubMed]
- 15.Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/ calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1998;273(33):20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- 16.Skelding KA, Rostas JAP Regulation of multifunctional calcium/calmodulin stimulated protein kinases by molecular targeting Ch. (2020) 26.(Springer Nature Switzerland AG). [DOI] [PubMed]
- 17.Migues PV, Lehmann IT, Fluechter L, Cammarota M, Gurd JW, Sim AT, et al. Phosphorylation of CaMKII at Thr253 occurs in vivo and enhances binding to isolated postsynaptic densities. J Neurochem. 2006;98(1):289–299. doi: 10.1111/j.1471-4159.2006.03876.x. [DOI] [PubMed] [Google Scholar]
- 18.Gurd JW, Rawof S, Zhen Huo J, Dykstra C, Bissoon N, Teves L, Wallace MC, Rostas JAP. Ischemia and status epilepitcus result in enhanced phosphorylation of calcium and calmodulin-stimulated protein kinase II on threonine 253. Brain Res. 2008;1218:158–165. doi: 10.1016/j.brainres.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 19.Rostas JA, Hoffman A, Murtha LA, Pepperall D, McLeod DD, Dickson PW, et al. Ischaemia- and excitotoxicity-induced CaMKII-mediated neuronal cell death: the relative roles of CaMKII autophosphorylation at T286 and T253. Neurochem Int. 2017;104:6–10. doi: 10.1016/j.neuint.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J Neurochem. 1997;68(5):2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- 21.Patton BL, Miller SG, Kennedy MB. Activation of type II calcium/calmodulin-dependent protein kinase by Ca2+/calmodulin is inhibited by autophosphorylation of threonine within the calmodulin-binding domain. J Biol Chem. 1990;265(19):11204–11212. doi: 10.1016/S0021-9258(19)38577-1. [DOI] [PubMed] [Google Scholar]
- 22.Schworer CM, Colbran RJ, Soderling TR. Reversible generation of a Ca2+-independent form of Ca2+(calmodulin)-dependent protein kinase II by an autophosphorylation mechanism. J Biol Chem. 1986;261(19):8581–8584. doi: 10.1016/S0021-9258(19)84416-2. [DOI] [PubMed] [Google Scholar]
- 23.Lai Y, Nairn AC, Greengard P. Autophosphorylation reversibly regulates the Ca2+/calmodulin-dependence of Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1986;83(12):4253–4257. doi: 10.1073/pnas.83.12.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zalcman G, Federman N, Romano A. CaMKII isoforms in learning and memory: localization and function. Front Mol Neurosci. 2018;11:445. doi: 10.3389/fnmol.2018.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray CB, Heller BJ. CaMKIIdelta subtypes: localization and function. Front Pharmacol. 2014;5:15. doi: 10.3389/fphar.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayer KU, Löhler J, Schulman H, Harbers K. Developmental expression of the CaM kinase II isoforms: ubiquitous gamma- and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain Res Mol Brain Res. 1999;70(1):147–154. doi: 10.1016/S0169-328X(99)00131-X. [DOI] [PubMed] [Google Scholar]
- 27.Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem. 1989;264(30):17907–17912. doi: 10.1016/S0021-9258(19)84658-6. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez MT, Zhao XL, Schulman H, Brown JH. The nuclear deltaB isoform of Ca2+/calmodulin-dependent protein kinase II regulates atrial natriuretic factor gene expression in ventricular myocytes. J Biol Chem. 1997;272(49):31203–31208. doi: 10.1074/jbc.272.49.31203. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS, Olson EN, Brown JH. CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J Biol Chem. 2007;282(48):35078–35087. doi: 10.1074/jbc.M707083200. [DOI] [PubMed] [Google Scholar]
- 30.Ma H, Groth RD, Cohen SM, Emery JF, Li B, Hoedt E, Zhang G, Neubert TA, Tsien RW. γCaMKII shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell. 2014;159(2):281–294. doi: 10.1016/j.cell.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brocke L, Srinivasan M, Schulman H. Developmental and regional expression of multifunctional Ca2+/calmodulin-dependent protein kinase isoforms in rat brain. J Neurosci. 1995;15(10):6797–6808. doi: 10.1523/JNEUROSCI.15-10-06797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivasan M, Edman CF, Schulman H. Alternative splicing introduces a nuclear localization signal that targets multifunctional CaM kinase to the nucleus. J Cell Biol. 1994;126(4):839–852. doi: 10.1083/jcb.126.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heist EK, Srinivasan M, Schulman H. Phosphorylation at the nuclear localization signal of Ca2+/calmodulin-dependent protein kinase II blocks its nuclear targeting. J Biol Chem. 1998;273(31):19763–19771. doi: 10.1074/jbc.273.31.19763. [DOI] [PubMed] [Google Scholar]
- 34.Lin YC, Redmond L. CaMKIIbeta binding to stable F-actin in vivo regulates F-actin filament stability. Proc Natl Acad Sci U S A. 2008;105(41):15791–15796. doi: 10.1073/pnas.0804399105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284(5411):162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 36.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson ME. Oxidant stress promotes disease by activating CaMKII. J Mol Cell Cardiol. 2015;89(Pt B):160–167. doi: 10.1016/j.yjmcc.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev. 2011;91(3):889–915. doi: 10.1152/physrev.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purohit A, Rokita AG, Guan X, Chen B, Koval OM, Voigt N, Neef S, Sowa T, Gao Z, Luczak ED, Stefansdottir H, Behunin AC, Li N, el-Accaoui RN, Yang B, Swaminathan PD, Weiss RM, Wehrens XHT, Song LS, Dobrev D, Maier LS, Anderson ME. Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation. 2013;128(16):1748–1757. doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411(6839):801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- 41.Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2000;275(31):23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- 42.Alev C, Urschel S, Sonntag S, Zoidl G, Fort AG, Höher T, et al. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Proc Natl Acad Sci U S A. 2008;105(52):20964–20969. doi: 10.1073/pnas.0805408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171(3):537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hell JW. CaMKII: claiming center stage in postsynaptic function and organization. Neuron. 2014;81(2):249–265. doi: 10.1016/j.neuron.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vemuganti R. All's well that transcribes well: non-coding RNAs and post-stroke brain damage. Neurochem Int. 2013;63(5):438–449. doi: 10.1016/j.neuint.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cha MJ, Jang JK, Ham O, Song BW, Lee SY, Lee CY, Park JH, Lee J, Seo HH, Choi E, Jeon WM, Hwang HJ, Shin HT, Choi E, Hwang KC. MicroRNA-145 suppresses ROS-induced Ca2+ overload of cardiomyocytes by targeting CaMKIIδ. Biochem Biophys Res Commun. 2013;435(4):720–726. doi: 10.1016/j.bbrc.2013.05.050. [DOI] [PubMed] [Google Scholar]
- 47.Xu Q, Deng F, Xing Z, Wu Z, Cen B, Xu S, Zhao Z, Nepomuceno R, Bhuiyan MIH, Sun D, Wang QJ, Ji A. Long non-coding RNA C2dat1 regulates CaMKIIdelta expression to promote neuronal survival through the NF-kappaB signaling pathway following cerebral ischemia. Cell Death Dis. 2016;7:e2173. doi: 10.1038/cddis.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye J, Das S, Roy A, Wei W, Huang H, Lorenz-Guertin JM, Xu Q, Jacob TC, Wang B, Sun D, Wang QJ. Ischemic injury-induced CaMKIIdelta and CaMKIIgamma confer neuroprotection through the NF-kappaB signaling pathway. Mol Neurobiol. 2019;56(3):2123–2136. doi: 10.1007/s12035-018-1198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.George PM, Steinberg GK. Novel Stroke Therapeutics: Unraveling stroke pathophysiology and its impact on clinical treatments. Neuron. 2015;87(2):297–309. doi: 10.1016/j.neuron.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma F, Sun P, Zhang X, Hamblin MH, Yin KJ. Endothelium-targeted deletion of the miR-15a/16-1 cluster ameliorates blood-brain barrier dysfunction in ischemic stroke. Sci Signal. 2020;13(626):eaay5686. doi: 10.1126/scisignal.aay5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun P, Zhang K, Hassan SH, Zhang X, Tang X, Pu H, Stetler RA, Chen J, Yin KJ. Endothelium-targeted deletion of microRNA-15a/16-1 promotes poststroke angiogenesis and improves long-term neurological recovery. Circ Res. 2020;126(8):1040–1057. doi: 10.1161/CIRCRESAHA.119.315886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Hamblin MH, Yin KJ. Noncoding RNAs and stroke. Neuroscientist. 2019;25(1):22–26. doi: 10.1177/1073858418769556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma F, Zhang X, Yin KJ. MicroRNAs in central nervous system diseases: a prospective role in regulating blood-brain barrier integrity. Exp Neurol. 2020;323:113094. doi: 10.1016/j.expneurol.2019.113094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55(3):310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snider BJ, Gottron FJ, Choi DW. Apoptosis and necrosis in cerebrovascular disease. Ann N Y Acad Sci. 1999;893:243–253. doi: 10.1111/j.1749-6632.1999.tb07829.x. [DOI] [PubMed] [Google Scholar]
- 57.Zalewska T, Bialynicka-Birula K, Domanska-Janik K. Autophosphorylation as a possible mechanism of calcium/calmodulin-dependent protein kinase II inhibition during ischemia. Neurochem Int. 1996;28(2):175–181. doi: 10.1016/0197-0186(95)00072-0. [DOI] [PubMed] [Google Scholar]
- 58.Churn SB, Limbrick D, Sombati S, DeLorenzo RJ. Excitotoxic activation of the NMDA receptor results in inhibition of calcium/calmodulin kinase II activity in cultured hippocampal neurons. J Neurosci. 1995;15(4):3200–3214. doi: 10.1523/JNEUROSCI.15-04-03200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krizbai IA, Deli MA, Pestenacz A, Siklos L, Szabo CA, Andras I, et al. Expression of glutamate receptors on cultured cerebral endothelial cells. J Neurosci Res. 1998;54(6):814–819. doi: 10.1002/(SICI)1097-4547(19981215)54:6<814::AID-JNR9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 60.Ashpole NM, Hudmon A. Excitotoxic neuroprotection and vulnerability with CaMKII inhibition. Mol Cell Neurosci. 2011;46(4):720–730. doi: 10.1016/j.mcn.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Hu BR, Wieloch T. Persistent translocation of Ca2+/calmodulin-dependent protein kinase II to synaptic junctions in the vulnerable hippocampal CA1 region following transient ischemia. J Neurochem. 1995;64(1):277–284. doi: 10.1046/j.1471-4159.1995.64010277.x. [DOI] [PubMed] [Google Scholar]
- 62.Hu BR, Kurihara J, Wieloch T. Persistent translocation and inhibition of Ca2+/calmodulin-dependent protein kinase II in the crude synaptosomal fraction of the vulnerable hippocampus following hypoglycemia. J Neurochem. 1995;64(3):1361–1369. doi: 10.1046/j.1471-4159.1995.64031361.x. [DOI] [PubMed] [Google Scholar]
- 63.Matsumoto S, Shamloo M, Isshiki A, Wieloch T. Persistent phosphorylation of synaptic proteins following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2002;22(9):1107–1113. doi: 10.1097/00004647-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 64.Matsumoto S, Shamloo M, Matsumoto E, Isshiki A, Wieloch T. Protein kinase C-gamma and calcium/calmodulin-dependent protein kinase II-alpha are persistently translocated to cell membranes of the rat brain during and after middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2004;24(1):54–61. doi: 10.1097/01.WCB.0000095920.70924.F5. [DOI] [PubMed] [Google Scholar]
- 65.Bayer KU, LeBel E, McDonald GL, O'Leary H, Schulman H, De Koninck P. Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J Neurosci. 2006;26(4):1164–1174. doi: 10.1523/JNEUROSCI.3116-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hudmon A, Aronowski J, Kolb SJ, Waxham MN. Inactivation and self-association of Ca2+/calmodulin-dependent protein kinase II during autophosphorylation. J Biol Chem. 1996;271(15):8800–8808. doi: 10.1074/jbc.271.15.8800. [DOI] [PubMed] [Google Scholar]
- 67.Hudmon A, Lebel E, Roy H, Sik A, Schulman H, Waxham MN, de Koninck P. A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. J Neurosci. 2005;25(30):6971–6983. doi: 10.1523/JNEUROSCI.4698-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vest RS, O'Leary H, Bayer KU. Differential regulation by ATP versus ADP further links CaMKII aggregation to ischemic conditions. FEBS Lett. 2009;583(22):3577–3581. doi: 10.1016/j.febslet.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olmez I, Ozyurt H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem Int. 2012;60(2):208–212. doi: 10.1016/j.neuint.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 70.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133(3):462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shetty PK, Huang FL, Huang KP. Ischemia-elicited oxidative modulation of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2008;283(9):5389–5401. doi: 10.1074/jbc.M708479200. [DOI] [PubMed] [Google Scholar]
- 72.Shackelford DA, Yeh RY, Hsu M, Buzsaki G, Zivin JA. Effect of cerebral ischemia on calcium/calmodulin-dependent protein kinase II activity and phosphorylation. J Cereb Blood Flow Metab. 1995;15(3):450–461. doi: 10.1038/jcbfm.1995.56. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X, Tang X, Ma F, Fan Y, Sun P, Zhu T, Zhang J, Hamblin MH, Chen YE, Yin KJ. Endothelium-targeted overexpression of Kruppel-like factor 11 protects the blood-brain barrier function after ischemic brain injury. Brain Pathol. 2020;30:746–765. doi: 10.1111/bpa.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang X, Tang X, Liu K, Hamblin MH, Yin KJ. Long noncoding RNA Malat1 regulates cerebrovascular pathologies in ischemic stroke. J Neurosci. 2017;37(7):1797–1806. doi: 10.1523/JNEUROSCI.3389-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ashpole NM, Song W, Brustovetsky T, Engleman EA, Brustovetsky N, Cummins TR, Hudmon A. Calcium/calmodulin-dependent protein kinase II (CaMKII) inhibition induces neurotoxicity via dysregulation of glutamate/calcium signaling and hyperexcitability. J Biol Chem. 2012;287(11):8495–8506. doi: 10.1074/jbc.M111.323915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Z, Xu J, Shen X, Lv C, Xu T, Pei D. CaMKII antisense oligodeoxynucleotides protect against ischemia-induced neuronal death in the rat hippocampus. J Neurol Sci. 2012;314(1-2):104–110. doi: 10.1016/j.jns.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 77.Waldsee R, Eftekhari S, Ahnstedt H, Johnson LE, Edvinsson L. CaMKII and MEK1/2 inhibition time-dependently modify inflammatory signaling in rat cerebral arteries during organ culture. J Neuroinflammation. 2014;11:90. doi: 10.1186/1742-2094-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Z, Huang Y, Liu L, Zhang L. Inhibitions of PKC and CaMK-II synergistically rescue ischemia-induced astrocytic dysfunction. Neurosci Lett. 2017;657:199–203. doi: 10.1016/j.neulet.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 79.Li J, Zhang S, Liu X, Han D, Xu J, Ma Y. Neuroprotective effects of leonurine against oxygen-glucose deprivation by targeting Cx36/CaMKII in PC12 cells. PLoS One. 2018;13(7):e0200705. doi: 10.1371/journal.pone.0200705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei Y, Wang R, Teng J. Inhibition of calcium/calmodulin-dependent protein kinase IIalpha suppresses oxidative stress in cerebral ischemic rats through targeting glucose 6-phosphate dehydrogenase. Neurochem Res. 2019;44(7):1613–1620. doi: 10.1007/s11064-019-02785-6. [DOI] [PubMed] [Google Scholar]
- 81.Qu Y, Tang J, Wang H, Li S, Zhao F, Zhang L, Richard Lu Q, Mu D. RIPK3 interactions with MLKL and CaMKII mediate oligodendrocytes death in the developing brain. Cell Death Dis. 2017;8(2):e2629. doi: 10.1038/cddis.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Chalmers NE, Yonchek J, Steklac KE, Ramsey M, Bayer KU, Herson PS, Quillinan N. Calcium/calmodulin-dependent kinase (CaMKII) inhibition protects against Purkinje cell damage following CA/CPR in mice. Mol Neurobiol. 2020;57(1):150–158. doi: 10.1007/s12035-019-01765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Waxham MN, Grotta JC, Silva AJ, Strong R, Aronowski J. Ischemia-induced neuronal damage: a role for calcium/calmodulin-dependent protein kinase II. J Cereb Blood Flow Metab. 1996;16(1):1–6. doi: 10.1097/00004647-199601000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Ashpole NM, Chawla AR, Martin MP, Brustovetsky T, Brustovetsky N, Hudmon A. Loss of calcium/calmodulin-dependent protein kinase II activity in cortical astrocytes decreases glutamate uptake and induces neurotoxic release of ATP. J Biol Chem. 2013;288(20):14599–14611. doi: 10.1074/jbc.M113.466235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23(9):1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 86.Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460(2):525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 87.Berliocchi L, Bano D, Nicotera P. Ca2+ signals and death programmes in neurons. Philos Trans R Soc Lond B Biol Sci. 2005;360(1464):2255–2258. doi: 10.1098/rstb.2005.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 89.Cao X, Zhang Y, Zou L, Xiao H, Chu Y, Chu X. Persistent oxygen-glucose deprivation induces astrocytic death through two different pathways and calpain-mediated proteolysis of cytoskeletal proteins during astrocytic oncosis. Neurosci Lett. 2010;479(2):118–122. doi: 10.1016/j.neulet.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 90.Chu X, Fu X, Zou L, Qi C, Li Z, Rao Y, Ma K. Oncosis, the possible cell death pathway in astrocytes after focal cerebral ischemia. Brain Res. 2007;1149:157–164. doi: 10.1016/j.brainres.2007.02.061. [DOI] [PubMed] [Google Scholar]
- 91.Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC. Neuronal cell death. Physiol Rev. 2018;98(2):813–880. doi: 10.1152/physrev.00011.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu C, Zhang K, Shen H, Yao X, Sun Q, Chen G. Necroptosis: a novel manner of cell death, associated with stroke (Review) Int J Mol Med. 2018;41(2):624–630. doi: 10.3892/ijmm.2017.3279. [DOI] [PubMed] [Google Scholar]
- 93.Hribljan V, Lisjak D, Petrovic DJ, Mitrecic D. Necroptosis is one of the modalities of cell death accompanying ischemic brain stroke: from pathogenesis to therapeutic possibilities. Croat Med J. 2019;60(2):121–126. doi: 10.3325/cmj.2019.60.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A. Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol. 2014;35:24–32. doi: 10.1016/j.semcdb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 95.Ling WY, Cui Y, Gao JL, Jiang XH, Wang KJ, Tian YX, Sheng HX, Cui JZ. Long-term chemogenetic activation of M1 glutamatergic neurons attenuates the behavioral and cognitive deficits caused by intracerebral hemorrhage. Biochem Biophys Res Commun. 2020;527(1):22–28. doi: 10.1016/j.bbrc.2020.04.083. [DOI] [PubMed] [Google Scholar]
- 96.Zhan L, Lu Z, Zhu X, Xu W, Li L, Li X, Chen S, Sun W, Xu E. Hypoxic preconditioning attenuates necroptotic neuronal death induced by global cerebral ischemia via Drp1-dependent signaling pathway mediated by CaMKIIalpha inactivation in adult rats. FASEB J. 2019;33(1):1313–1329. doi: 10.1096/fj.201800111RR. [DOI] [PubMed] [Google Scholar]
- 97.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40(5):e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 98.Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener. 2011;6(1):11. doi: 10.1186/1750-1326-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uzdensky AB. Apoptosis regulation in the penumbra after ischemic stroke: expression of pro- and antiapoptotic proteins. Apoptosis. 2019;24(9-10):687–702. doi: 10.1007/s10495-019-01556-6. [DOI] [PubMed] [Google Scholar]
- 100.Takano H, Fukushi H, Morishima Y, Shirasaki Y. Calmodulin and calmodulin-dependent kinase II mediate neuronal cell death induced by depolarization. Brain Res. 2003;962(1-2):41–47. doi: 10.1016/S0006-8993(02)03932-X. [DOI] [PubMed] [Google Scholar]
- 101.Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48(4):635–646. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 102.Ahmed ME, Dong Y, Lu Y, Tucker D, Wang R, Zhang Q. Beneficial effects of a CaMKIIalpha inhibitor TatCN21 peptide in global cerebral ischemia. J Mol Neurosci. 2017;61(1):42–51. doi: 10.1007/s12031-016-0830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Butler LS, Silva AJ, Abeliovich A, Watanabe Y, Tonegawa S, McNamara JO. Limbic epilepsy in transgenic mice carrying a Ca2+/calmodulin-dependent kinase II alpha-subunit mutation. Proc Natl Acad Sci U S A. 1995;92(15):6852–6855. doi: 10.1073/pnas.92.15.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E, Tanaka K, Uchiyama Y. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol. 2008;172(2):454–469. doi: 10.2353/ajpath.2008.070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gabryel B, Kost A, Kasprowska D. Neuronal autophagy in cerebral ischemia--a potential target for neuroprotective strategies? Pharmacol Rep. 2012;64(1):1–15. doi: 10.1016/S1734-1140(12)70725-9. [DOI] [PubMed] [Google Scholar]
- 106.Wang P, Shao BZ, Deng Z, Chen S, Yue Z, Miao CY. Autophagy in ischemic stroke. Prog Neurobiol. 2018:163-164:98-117. [DOI] [PubMed]
- 107.Li X, Wu XQ, Deng R, Li DD, Tang J, Chen WD, Chen JH, Ji J, Jiao L, Jiang S, Yang F, Feng GK, Senthilkumar R, Yue F, Zhang HL, Wu RY, Yu Y, Xu XL, Mai J, Li ZL, Peng XD, Huang Y, Huang X, Ma NF, Tao Q, Zeng YX, Zhu XF. CaMKII-mediated Beclin 1 phosphorylation regulates autophagy that promotes degradation of Id and neuroblastoma cell differentiation. Nat Commun. 2017;8(1):1159. doi: 10.1038/s41467-017-01272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kong L, Xiong F, Sun N, Xu C, Chen Y, Yang J, Su X. CaMKIIdelta inhibition protects against myocardial ischemia/reperfusion injury: Role of Beclin-1-dependent autophagy. Eur J Pharmacol. 2020;886:173539. doi: 10.1016/j.ejphar.2020.173539. [DOI] [PubMed] [Google Scholar]
- 109.Vest RS, O'Leary H, Coultrap SJ, Kindy MS, Bayer KU. Effective post-insult neuroprotection by a novel Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) inhibitor. J Biol Chem. 2010;285(27):20675–20682. doi: 10.1074/jbc.M109.088617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kulbe JR, Mulcahy Levy JM, Coultrap SJ, Thorburn A, Bayer KU. Excitotoxic glutamate insults block autophagic flux in hippocampal neurons. Brain Res. 2014;1542:12–19. doi: 10.1016/j.brainres.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ruetzler CA, Furuya K, Takeda H, Hallenbeck JM. Brain vessels normally undergo cyclic activation and inactivation: evidence from tumor necrosis factor-alpha, heme oxygenase-1, and manganese superoxide dismutase immunostaining of vessels and perivascular brain cells. J Cereb Blood Flow Metab. 2001;21(3):244–252. doi: 10.1097/00004647-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 112.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66(3):232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 113.Bohm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76(1):8–18. doi: 10.1016/j.cardiores.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 114.Lin CC, Hsieh HL, Chi PL, Yang CC, Hsiao LD, Yang CM. Upregulation of COX-2/PGE2 by ET-1 mediated through Ca2+-dependent signals in mouse brain microvascular endothelial cells. Mol Neurobiol. 2014;49(3):1256–1269. doi: 10.1007/s12035-013-8597-1. [DOI] [PubMed] [Google Scholar]
- 115.Kirsch M, De Groot H. NAD(P)H, a directly operating antioxidant? FASEB J. 2001;15(9):1569–1574. doi: 10.1096/fj.00-0823hyp. [DOI] [PubMed] [Google Scholar]
- 116.Deli MA, Joo F, Krizbai I, Lengyel I, Nunzi MG, Wolff JR. Calcium/calmodulin-stimulated protein kinase II is present in primary cultures of cerebral endothelial cells. J Neurochem. 1993;60(5):1960–1963. doi: 10.1111/j.1471-4159.1993.tb13429.x. [DOI] [PubMed] [Google Scholar]
- 117.Enslen H, Sun P, Brickey D, Soderling SH, Klamo E, Soderling TR. Characterization of Ca2+/calmodulin-dependent protein kinase IV. Role in transcriptional regulation. J Biol Chem. 1994;269(22):15520–15527. doi: 10.1016/S0021-9258(17)40710-1. [DOI] [PubMed] [Google Scholar]
- 118.Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci U S A. 1998;95(18):10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schwartz JJ, Zhang S. Peptide-mediated cellular delivery. Curr Opin Mol Ther. 2000;2(2):162–167. [PubMed] [Google Scholar]
- 120.Abraham MK, Chang WW. Subarachnoid hemorrhage. Emerg Med Clin North Am. 2016;34(4):901–916. doi: 10.1016/j.emc.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 121.Kolias AG, Sen J, Belli A. Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: putative mechanisms and novel approaches. J Neurosci Res. 2009;87(1):1–11. doi: 10.1002/jnr.21823. [DOI] [PubMed] [Google Scholar]
- 122.Vergouwen MD, Ilodigwe D, Macdonald RL. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke. 2011;42(4):924–929. doi: 10.1161/STROKEAHA.110.597914. [DOI] [PubMed] [Google Scholar]
- 123.Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41(8):e519–e536. doi: 10.1161/STROKEAHA.110.581975. [DOI] [PubMed] [Google Scholar]
- 124.Bederson JB, Levy AL, Ding WH, Kahn R, DiPerna CA, Jenkins AL, 3rd, et al. Acute vasoconstriction after subarachnoid hemorrhage. Neurosurgery. 1998;42(2):352–360. doi: 10.1097/00006123-199802000-00091. [DOI] [PubMed] [Google Scholar]
- 125.Bederson JB, Connolly ES, Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40(3):994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 126.Parker BL, Larsen MR, Edvinsson LI, Povlsen GK. Signal transduction in cerebral arteries after subarachnoid hemorrhage-a phosphoproteomic approach. J Cereb Blood Flow Metab. 2013;33(8):1259–1269. doi: 10.1038/jcbfm.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Edvinsson L, Povlsen GK, Ahnstedt H, Waldsee R. CaMKII inhibition with KN93 attenuates endothelin and serotonin receptor-mediated vasoconstriction and prevents subarachnoid hemorrhage-induced deficits in sensorimotor function. J Neuroinflammation. 2014;11:207. doi: 10.1186/s12974-014-0207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou K, Enkhjargal B, Xie Z, Sun C, Wu L, Malaguit J, Chen S, Tang J, Zhang J, Zhang JH. Dihydrolipoic acid inhibits lysosomal rupture and NLRP3 through lysosome-associated membrane protein-1/calcium/calmodulin-dependent protein kinase II/TAK1 pathways after subarachnoid hemorrhage in Rat. Stroke. 2018;49(1):175–183. doi: 10.1161/STROKEAHA.117.018593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Han SM, Wan H, Kudo G, Foltz WD, Vines DC, Green DE, Zoerle T, Tariq A, Brathwaite S, D'Abbondanza J, Ai J, Macdonald RL. Molecular alterations in the hippocampus after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2014;34(1):108–117. doi: 10.1038/jcbfm.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry. 1997;63(6):749–753. doi: 10.1136/jnnp.63.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.O'Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C, Gorelick PB, Rockwood K, Burns A, Gauthier S, DeKosky ST. Vascular cognitive impairment. Lancet Neurol. 2003;2(2):89–98. doi: 10.1016/S1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 132.Fukunaga K, Stoppini L, Miyamoto E, Muller D. Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1993;268(11):7863–7867. doi: 10.1016/S0021-9258(18)53037-4. [DOI] [PubMed] [Google Scholar]
- 133.Yamamoto Y, Shioda N, Han F, Moriguchi S, Nakajima A, Yokosuka A, Mimaki Y, Sashida Y, Yamakuni T, Ohizumi Y, Fukunaga K. Nobiletin improves brain ischemia-induced learning and memory deficits through stimulation of CaMKII and CREB phosphorylation. Brain Res. 2009;1295:218–229. doi: 10.1016/j.brainres.2009.07.081. [DOI] [PubMed] [Google Scholar]
- 134.Wang XJ, Gao YP, Lu NN, Li WS, Xu JF, Ying XY, Wu G, Liao MH, Tan C, Shao LX, Lu YM, Zhang C, Fukunaga K, Han F, du YZ. Endogenous polysialic acid based micelles for calmodulin antagonist delivery against vascular dementia. ACS Appl Mater Interfaces. 2016;8(51):35045–35058. doi: 10.1021/acsami.6b13052. [DOI] [PubMed] [Google Scholar]
- 135.Wang R, Yin YX, Mahmood Q, Wang XJ, Gao YP, Gou GJ, Ahmed MM, Kohji F, du YZ, Han F. Calmodulin inhibitor ameliorates cognitive dysfunction via inhibiting nitrosative stress and NLRP3 signaling in mice with bilateral carotid artery stenosis. CNS Neurosci Ther. 2017;23(10):818–826. doi: 10.1111/cns.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li W, Yuan H, Yu Y, Cheong YK, Ren G, Yang Z. Etidronate rescues cognitive deficits through improving synaptic transmission and suppressing apoptosis in 2-vessel occlusion model rats. J Neurochem. 2017;140(3):476–484. doi: 10.1111/jnc.13904. [DOI] [PubMed] [Google Scholar]
- 137.Yang J, Yao Y, Wang L, Yang C, Wang F, Guo J, Wang Z, Yang Z, Ming D. Gastrin-releasing peptide facilitates glutamatergic transmission in the hippocampus and effectively prevents vascular dementia induced cognitive and synaptic plasticity deficits. Exp Neurol. 2017;287(Pt 1):75–83. doi: 10.1016/j.expneurol.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 138.Zhao Q, Yokozawa T, Yamabe N, Tsuneyama K, Li X, Matsumoto K. Kangen-karyu improves memory deficit caused by aging through normalization of neuro-plasticity-related signaling system and VEGF system in the brain. J Ethnopharmacol. 2010;131(2):377–385. doi: 10.1016/j.jep.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 139.Armbrecht HJ, Boltz MA, Kumar VB, Flood JF, Morley JE. Effect of age on calcium-dependent proteins in hippocampus of senescence-accelerated mice. Brain Res. 1999;842(2):287–293. doi: 10.1016/S0006-8993(99)01802-8. [DOI] [PubMed] [Google Scholar]
- 140.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 141.Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Prog Drug Res. 2003;61:39–78. doi: 10.1007/978-3-0348-8049-7_2. [DOI] [PubMed] [Google Scholar]
- 142.Leak RK, Zhang L, Stetler RA, Weng Z, Li P, Atkins GB, Gao Y, Chen J. HSP27 protects the blood-brain barrier against ischemia-induced loss of integrity. CNS Neurol Disord Drug Targets. 2013;12(3):325–337. doi: 10.2174/1871527311312030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25(9):1794–1798. doi: 10.1161/01.STR.25.9.1794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available and support the published claims and comply with field standards.
Not applicable. No coding in the study.