Abstract
Cancer progression largely depends on tumor blood vessels as well on immune cell infiltration. In various tumors, vascular cells, namely endothelial cells (ECs) and pericytes, strongly regulate leukocyte infiltration into tumors and immune cell activation, hence the immune response to cancers. Recently, a lot of compelling studies unraveled the molecular mechanisms by which tumor vascular cells regulate monocyte and tumor-associated macrophage (TAM) recruitment and phenotype, and consequently tumor progression. Reciprocally, TAMs and monocytes strongly modulate tumor blood vessel and tumor lymphatic vessel formation by exerting pro-angiogenic and lymphangiogenic effects, respectively. Finally, the interaction between monocytes/TAMs and vascular cells is also impacting several steps of the spread of cancer cells throughout the body, a process called metastasis. In this review, the impact of the bi-directional dialog between blood vascular cells and monocytes/TAMs in the regulation of tumor progression is discussed. All together, these data led to the design of combinations of anti-angiogenic and immunotherapy targeting TAMs/monocyte whose effects are briefly discussed in the last part of this review.
Keywords: Cancer, Endothelial cell, Pericyte, Monocyte/macrophage, Angiogenesis, Metastasis
Introduction
Tumor-associated macrophages
TAMs in the tumor microenvironment
Tumor-associated macrophages (TAMs) are major tumor microenvironment (TME) cells and represent an important part of the cancer immune infiltrate. TAM infiltration and TAM numbers are correlated with poor prognosis in a majority of cancer types [1]. TAMs play an important role in cancer development notably via the promotion of tumor growth, tumor inflammation, angiogenesis, lymphangiogenesis, metastasis, immunosuppression, and chemotherapeutic resistance [2–5]. Macrophages are classified as pro-inflammatory M1 macrophages and anti-inflammatory M2 macrophages. This classification is oversimplified since TAMs can express both M1 and M2 markers, and hence, TAMs are classified onto a M1 and M2 polarization axis in which M1 and M2 macrophages are the two extremes. Basically, and based on in vitro experiments, M1 macrophages are polarized with pro-inflammatory cytokines and bacterial molecules such as interferon γ and lipopolysaccharides. M1 macrophages express high levels of pro-inflammatory cytokines (e.g., IL-12, tumor necrosis factor α (TNFα), and IL-6) and intracellular host response genes (e.g., CD80 and IFIT1) [6, 7]. M2 macrophages are divided into at least three subsets called M2a, M2b, and M2c. This M2 classification in three subsets was firstly proposed in [6]. M2a are activated by IL-4 and/or IL-13 and express high levels of CD206, CD163, and fibronectin [6, 7]. M2b are induced by Toll-like receptors ligands and immune complex activation, whereas M2c are activated by IL-10. Interestingly, macrophage M2 polarization is also induced by the TME [8–14]. Nonetheless, the three classes of M2 macrophages share common features such as IL-12low and IL-10high and arginase-1 (Arg-1)high, whereas M1 macrophages are IL-12high, IL-23high, and IL-10low. In the TME, CD163 and CD206 are commonly used to identify macrophages from the M2 population, whereas CD86 is a common M1 marker.
Origins of TAMs
There exist at least two origins of TAMs. TAMs can originate either from tissue-resident macrophages (TRMs) or from blood vessel inflammatory monocytes (IMs) CCR2+, which are recruited via CCL2 chemotaxis [15–17]. TRMs are present in healthy tissues, hence before cancer initiation [15]. TRMs arise from embryonic progenitor–derived macrophages (e.g., brain macrophages also called microglia) or from blood monocytes (e.g., intestine or dermis). Furthermore, TRMs are able to self-maintain without adult blood monocyte contribution. Although TRMs are known for a while, the implication of TRMs in cancers has only recently been investigated, mostly in murine tumor models. For example, TRMs promote pancreatic ductal adenocarcinoma (PDAC) progression [18]. Indeed, colony-stimulating factor 1 (CSF1) antibodies combined with clodronate liposome followed by 10 days of blood monocyte recovery induce an almost complete TRM depletion without affecting circulating monocyte. In these conditions, tumor burden and high-grade carcinoma development are drastically reduced [18]. Nonetheless, monocyte-derived macrophages represent the major macrophage population in a majority of murine cancer types, such as breast, lung, brain, and hepatocellular carcinoma [15]. Monocytes are classified into 3 subsets in humans and in mice, according to marker expression [19, 20]. There are IMs (CD16−/CD14+/CX3CR1lo in human, Ly6Chigh/CD43lo/CX3CR1lo in mouse), non-classical monocytes (or patrolling, CX3CR1high, CD14lo, CD16+ in human and Ly6Clo/CD43high/CX3CR1high in mouse), and intermediate monocytes (CX3CR1high, CD14+, CD16+ in human, Ly6CintCD43hiCX3CR1hi in mouse). Numerous murine studies showed that IMs are the major source of TAMs in tumors, such as mammary tumors and their associated lung metastases, hepatocellular carcinoma, orthotopic Lewis lung carcinoma (LLc), and PDAC [19]. Furthermore, IMs display pro-tumoral functions such as angiogenesis and metastasis promotion [19]. Non-classical and intermediate monocytes have pro and anti-tumoral functions. Indeed, human intermediate and non-classical CD16+ monocytes promote angiogenesis in vitro [21] and murine Ly6Glo patrolling monocytes are immunosuppressive in vivo [22, 23], whereas murine patrolling monocytes prevent breast to lung metastasis in murine PyMT breast cancer model [24]. Another type of monocyte classification exists, based on the receptor tyrosine kinase Tie2 expression. Indeed, recently, Tie2-expressing monocytes (TEMs) have been discovered by De Palma and colleagues [25, 26]. Before these studies, only ECs were thought to express the angiopoietin (1–4) receptor Tie2 [25]. Nowadays, some cell types have been discovered to express Tie2: endothelial cells (ECs), TEMs, a subset of TAMs, pericyte precursors of mesenchymal origin, a subset of hematopoietic stem cells, and some cancer cell lines [26–28]. Two studies showed that Tie2 is expressed mainly by intermediate monocyte (CD14+ CD16+), whereas one study shows that Tie2 is also expressed in non-classical monocyte (CD14dim CD16+). Hence, Tie2 is expressed mostly but not exclusively in CD16+ monocytes and to a lesser extent in CD16− monocytes [29].
Tumor blood vessels
The onset of angiogenesis or the “angiogenic switch”
During cancer development, the transition from an avascular tumor to a vascularized tumor, called the “angiogenic switch” is a critical step [30, 31]. This switch occurs when the balance between pro-angiogenic factors (e.g., vascular endothelial growth factor (VEGF)-A) and anti-angiogenic factors (e.g., statins) shifts towards angiogenesis [30]. This switch appears during the progression from hyperplasia to neoplasia and coincides with malignant transition in PyMT and RIP1-Tag2 mice models. It is needed for malignant tumor progression [32, 33]. Immune cells such as TAMs and neutrophils are involved in this process. For example, in the PyMT murine breast cancer model, high TAM infiltration precedes the onset of angiogenesis. Furthermore, vasculature development is observed earlier in this model when macrophage infiltration is induced with CSF1 transgenic overexpression specifically in mammary tissues [32]. In the Rip1-Tag2 mouse pancreatic tumor model, neutrophil ablation with anti-Gr1 antibody strongly diminishes tumor vessel development [34].
Lymphatic vasculature and lymphangiogenesis
Lymphatic vasculature is critically involved in fluid homeostasis regulation, immune cell dissemination/surveillance, and lipid reabsorption. Absence or non-functional lymphatic system causes lymphedema, a disease characterized by huge swelling and repeated skin infections.
Lymphangiogenesis is defined as the formation of new lymphatic vessels from existing ones. It occurs during embryonic development and during tumor growth. It is correlated with a bad prognosis in cancer [35]. Lymphatic vessel hyaluronic receptor 1 (LYVE 1), podoplanin, and prospero homeobox 1 (prox1) are lymphatic EC (LEC) markers. Mechanistically, VEGF-C and VEGF-D are the two main lymphangiogenic factors which promote lymphangiogenesis by activating LECs VEGF receptor 3 (VEGFR-3) [5]. Lymphatic vasculature is critically involved in the metastatic spread of cancer cells into lymph nodes and finally to distant organs [36–39]. Lymphatic vessel density and lymph node status (i.e., the presence or the absence of cancer cells) is associated with poor prognosis and metastasis in several cancers [35]. The link between VEGF-C, VEGF-D, lymphatic vessel density, lymph node metastasis, and prognosis is extensively reviewed in [40].
Tumor blood vessels and immune system: endothelial anergy
During cancer progression, the immune system is progressively modified by the TME in a process called immunoediting. This process is composed of three phases, namely elimination, equilibrium, and escape. In the two first phases, the immune system is able to kill cancer cells notably via CD8+ T cells and natural killer (NK) cells. During these stages, TAMs belong mostly to M1 phenotype and are able to kill cancer cells and to activate the immune system. For example, in early-stage human lung tumors, TAMs mostly share both M1 and M2 markers and are able to activate T cell function, and hence are anti-tumoral [41]. In pancreatic pre-cancerous lesions, in gastrointestinal stromal tumors, in ovarian cancer, and in bladder cancer, TAMs mostly belong to the M1 phenotype and are progressively skewed toward the M2 phenotype during disease progression [42–44]. In later stages, TAMs display mostly M2 phenotype and are pro-tumoral and immunosuppressive.
Tumor blood vessels constitute a barrier regulating immune cell recruitment from blood into tumor via extravasation. The regulation of immune cell extravasation into tumor through blood vessels is then crucial in the regulation of tumor progression. This process is highly regulated and is composed of several steps. First, there is leukocyte rolling followed by the arrest and firm adhesion to ECs. Then, leukocytes transmigrate through ECs to extravasate and infiltrate the tissue. This process requires adhesion molecules expressed by ECs such as E-selectin (rolling), ICAM1 and VCAM1 (firm arrest), and VE-cadherin and CD31 (transendothelial migration) [45]. The expression of these proteins is tightly regulated and promoted by inflammatory stimuli such as TNFα. Tumor vessels are modified by TME to induce endothelial anergy, notably via VEGF [46]. In this state, tumor endothelial cells are unresponsive to pro-inflammatory stimuli such as TNFα and hence do not promote anymore leukocyte extravasation [47]. This anergy is crucial in tumor growth promotion, likely more importantly during the elimination and equilibrium phases, because the immune system is anti-tumoral. For example, the overexpression of EGF-like domain–containing protein 7 (Egfl7) in cancer cells, an endothelial activation repressor [48], promotes tumor growth and development by preventing leukocyte infiltration via endothelial E-selectin and ICAM1 and VCAM1 adhesion molecule repression [49].
Tumor blood and lymphatic vessels also modulate the immune system (this is well reviewed in [46]). Indeed, lymphatic ECs (LECs) and ECs both express program death-ligand 1 (PD-L1), which inhibits T cell function [50, 51]. Furthermore, ECs can induce T cell apoptosis by Fas ligand expression [52]. Tumor ECs are modified by the TME. Indeed, IL-6 and IL-10 secretion from lung tumor ECs is strongly increased. Normal lung ECs induce strong NK cell activation, whereas this ability is strongly reduced in ECs from lung tumors [53]. Furthermore, IL-6 and IL-10 cytokines are involved in macrophage polarization towards M2 phenotype and hence promote tumor growth [54, 55].
To recapitulate, tumor blood vessels regulate immune cell infiltration as well as their activation in tumors. In this review, the impact of vascular cells (ECs and pericytes) on monocyte and TAM recruitment into tumors will be discussed. Furthermore, the impact of vascular cells on monocyte and TAM angiogenic phenotype and polarization will also be described. Reciprocally, the impact of TAMs, TEMs, and classical and non-classical monocytes on blood vessels will be emphasized. Their impact on angiogenesis, lymphangiogenesis, and metastasis will be detailed.
Effects of vascular and perivascular cells on macrophages (related to Fig. 1)
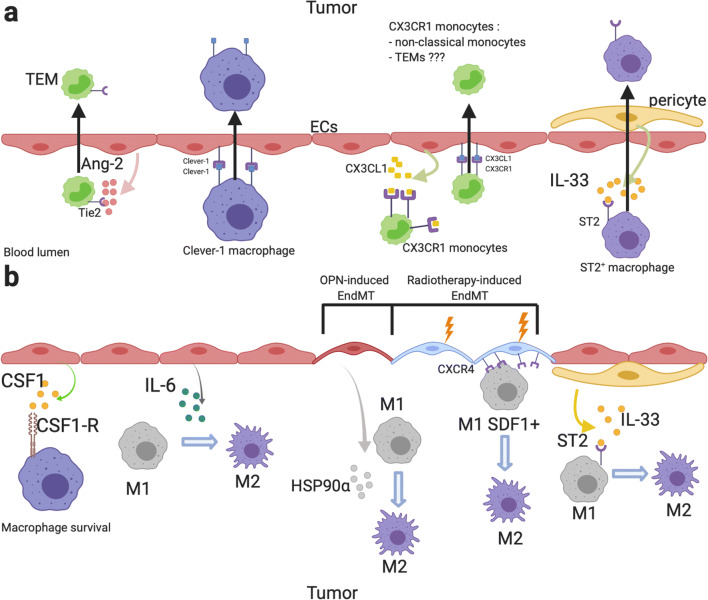
Fig. 1.
Effects of blood vessels on monocyte/macrophage recruitment and polarization. a Effects of ECs and pericytes on monocyte/macrophage recruitment. ECs secrete high dose of Ang-2 which induces TEM recruitment in tumor [56–59]. Furthermore, Ang-2 promotes angiogenic phenotype in TEMs and in Tie2-expressing macrophages [58]. Homophilic interaction between Clever-1 in ECs and TAMs induces TAM infiltration [60]. ECs secrete CX3CL1 which induces CX3CR1-expressing monocyte (non-classical monocyte) chemotaxis toward ECs [21, 61]. CX3CL1/CX3CR1 interaction induces non-classical monocyte recruitment via VEGF-A-dependent CX3CL1 shedding [21, 62, 63]. IL-33 secreted by pericytes promotes TAM recruitment via the IL-33 receptor ST2 activation [64]. b Effects of ECs and pericytes on TAM survival and polarization. CSF1 promotes TAM survival in the TME via CSF1R activation [65]. ECs are high IL-6 producer. EC-derived IL-6 induces TAM M2 polarization [12]. Osteopontin-induced EndMT promotes M2 TAMs polarization via HSP90⍺ secretion [66]. Radiotherapy-induced EndMT induces CXCR4 expression in ECs, which promotes SDF1α-expressing TAM M2 polarization [67]. IL-33 secreted by pericytes promotes M2 polarization in a ST2-dependent manner [68]. This figure was created with BioRender.com
Monocyte and macrophage recruitment by ECs and pericytes (related to Fig. 1a)
TAM recruitment in cancer is involved in the angiogenic switch induction; promotes tumor growth, metastasis, vessel “abnormalization”; and is associated with a bad prognosis in most cancer types. Indeed, macrophage depletion by different ways has a negative impact on these features. TAMs are recruited by different chemokines and cytokines such as chemokine (C-C motif) ligand 2 (CCL2), CCL5, CCL7, angiopoietin-2 (Ang-2), CSF1, VEGF, IL-33, semaphorin 3D, endothelial monocyte–activating polypeptide-II (EMAP-II), endothelin (ET)-1 and 2, stromal cell–derived factor 1α (SDF1α/CXCL12), eotaxin, and oncostatin which are secreted by cancer cells, stromal cells, and perivascular and vascular cells. This is extensively reviewed in [69, 70]. TAMs are classified not only according to their marker expression into M1 or M2 phenotype, but also according to their tumor localization into migratory TAMs or perivascular TAMs [4, 17]. Here, we will focus on the effects of ECs and perivascular cells on TAM and monocyte recruitment as well as on their localization within the tumor.
EC-derived angiopoietin-2 (Ang-2)
Ang-2 is mainly released by ECs in tumors, but in some cases, Ang-2 is also expressed by cancer cells [71]. Ang-2 is stored in Weibel-Palade bodies in ECs [72, 73], and its expression and release from EC are regulated by CTHRC1/ERK/AP-1 signaling and by neuroligin 2 [74, 75]. In vitro, EC-derived Ang-2 induces chemotaxis of Tie2+ macrophages and monocytes. THP-1 Tie2+ monocytes but not Tie2− migrate towards Ang-2 in the Boyden chamber model [76]. U937 monocytes exposed to Kaposi’s sarcoma EC conditioned media migrate towards the conditioned medium compartment. This migration is abolished with anti-Ang-2 antibody or with Ang-2 shRNA in ECs [56]. Hence, Ang-2 expression and release by EC are tightly regulated and promote TEM migration in vitro.
In vivo, Ang-2 induces Tie2+ TAM and TEM infiltration by stimulating the expression of their Ang-2 receptor Tie2. Indeed, Ang-2 induces macrophage and TEM infiltration that is correlated with metastasis in murine MDA-MB-231-induced breast cancer, in pancreatic cancers, in lung cancer, in Kaposi’s sarcoma, in glioblastomas (GBMs), and in gliomas [56–58, 74, 77]. Indeed, specific EC Ang-2 overexpression increases macrophage and TEM infiltration in murine GBM and LLc lung tumor models [57, 58]. Ang-2 inhibition diminishes TAM and/or TEM infiltration in Kaposi’s sarcoma and breast cancer murine models [56, 59]. Nonetheless, in MMTV-PyMT breast cancer and Rip1-Tag2 pancreatic cancer, Ang-2 inhibition does not modify macrophage or TEM infiltration but rather inhibits their perivascular localization [78]. Ang-2 blockade induces SDF1α overexpression in the MMTV-PyMT model, which can counterbalance the effects of Ang-2 blockade on TAM and TEM infiltration. Ang-2 induces EC ICAM1 and VCAM1 expression that hence increases monocyte and TAM adhesion on EC [77]. Moreover, Ang-2 increases vessel permeability, angiogenesis, and CCL2 expression in ECs that also leads to C-C chemokine receptor type 2 (CCR2)+ monocyte and TAM infiltration [77, 79]. Hence, in tumors, Ang-2 is an important EC-secreted protein that is involved in macrophage and monocyte Tie2+ infiltration and perivascular localization. Moreover, Ang-2-induced EC CCL2 overexpression induces CCR2+ IM recruitment.
In line with the fact that Ang-2 induces TAM and TEM infiltration, Ang-2 expression is correlated with microvascular density and associated with poor prognosis in several cancers [71]. Furthermore, Ang-2 is overexpressed in tumor tissues compared to normal tissues [71]. Ang-2 expression is increased by anti-VEGF therapies in tumor but not in normal tissues [76, 80–82]. This Ang-2 overexpression leads to therapy failure by increasing TEM and TAM infiltration. This TAM recruitment induced by anti-VEGF therapy is blocked by the addition of Ang-2 antibody or soluble Tie2 [76]. This bitherapy has been tested in phase I in human cancer patients and showed acceptable safety and encouraging antitumor activity [83]. To summarize, Ang-2 is involved in tumor resistance against VEGF therapy and anti-Ang-2/VEGF combination shows encouraging results in pre-clinical and clinical studies.
Pericytes and perivascular cancer-associated fibroblasts in TAM recruitment
Pericytes and perivascular cancer-associated fibroblasts (CAFs) are involved in TAM recruitment and their perivascular localization. Platelet-derived growth factor (PDGF)-BB secretion by cancer cells induces IL-33 expression and secretion by pericytes and CAFs via a PDGF receptor β (PDGFRβ)–dependent mechanism. IL-33 further stimulates macrophage migration in vitro and TAM infiltration in vivo via the IL-33 receptor ST2–dependent mechanism [64]. Indeed, pericytes exposed to PDGF-BB in vitro or in vivo in lung tumor model with LLc overexpressing PDGF-BB overexpress IL-33 and this overexpression is abolished by anti-PDGFRβ antibodies. IL33-induced RAW cell migration is abolished by ST2 RAW siRNA. In vivo, TAM infiltration is increased in tumors overexpressing PDGF-BB. This increase is abolished in mice IL-33−/−, ST2−/− or with ST2 soluble factors. These IL-33 recruited TAMs are also involved in tumor growth and in cancer cell stemness via prostaglandin 2 secretion [84]. Milk fat globule-epidermal growth factor 8 (MFG-E8), expressed mostly by pericytes in melanoma tumors, is also involved in TAM infiltration by an unknown mechanism which would be interesting to clarify [85]. Consistently, high MFG-E8 expression is associated with high TAM infiltration in bladder cancer [86]. As said above, TAMs are also classified according to their tumor localization in which there are migratory TAMs and perivascular TAMs [4, 17]. In fact, there is a unidirectional mechanism by which a newly recruited monocyte will differentiate in migratory TAMs which then will be recruited to blood vessels and hence become perivascular [17]. Indeed, in the mammary PyMT model, newly tumor-infiltrated blood CCR2+ monocytes are recruited by cancer cell– and stromal cell–derived CCL2. Then, monocytes differentiate into migratory TAMs and C-X-C chemokine receptor type 4 (CXCR4) expression by TAMs is then promoted by tumor-derived transforming growth factor-β (TGF-β). These migratory TAMs are then recruited near to the blood vessel by SDF1α-derived perivascular CAFs [17]. In summary, perivascular cells are involved in TAM recruitment via IL-33 secretion and in TAM perivascular localization via SDF1α secretion.
Monocyte/TAM recruitment via direct interactions with ECs
Whereas most endothelial-leukocyte adhesion molecules are shared between all leukocyte types [46], monocytes and TAMs are also specifically recruited by tumor ECs [46]. Clever-1/stabilin-1+ is a scavenger receptor and an adhesion molecule regulating macrophage and T regulator lymphocyte transendothelial migration as well as tumor infiltration [60, 87]. Indeed, Clever-1 overexpressing ECs are involved in Clever-1+ monocyte/macrophage and Treg recruitment [60]. Indeed, Clever-1 deletion in mice or specifically in macrophages or in ECs leads to a diminished TAM recruitment, without affecting lymphocyte CD4+ or CD8+ recruitment [60]. Apoptosis signal-regulating kinase 1 (ASK1), a factor involved in the regulation of EC activation, is involved in TAM recruitment in tumor, without affecting lymphocyte CD3+ recruitment [88, 89]. In non-inflammatory conditions, ASK1 is consistently degraded via suppressor of cytokine signaling 1 (SOCS1) by the proteasome and pushes ECs in an inactivated state. In inflammatory condition, ASK1 is stabilized and stimulates EC activation via JNK/p38MAPK activation [89]. EC ASK1 expression induces macrophage infiltration into tumors without affecting lymphocyte recruitment [88]. TAM infiltration in tumors is decreased in ASK1 KO mice or with ASK1 inhibition specifically in EC (via SOCS1 overexpression specifically in EC) or with ASK1 inhibitor. This TAM infiltration prevention by ASK1 inhibition leads to a decrease in tumor growth and in metastasis and to an increased survival in mice [88]. Nonetheless, the mechanism by which ASK1 leads to specific TAM infiltration remains unclear and it would be interesting to be investigated. That could be either by chemotactic factor over-secretion specifically inducing TAM infiltration (e.g., CCL2, Ang-2) or via a direct contact between TAMs and ECs inducing TAM transmigration (e.g., via Clever-1 interaction). In vitro, TAM transmigration is impaired across EC ASK1–specific inhibition, but the lymphocyte transmigration has not been investigated [88]. All these data demonstrate that homophylic interaction between EC and macrophage Clever-1/stabilin-1 is involved in TAM recruitment into tumor without affecting CD4+ or CD8+ lymphocyte recruitment.
CX3CL1/CX3CR1 axis in non-classical monocyte recruitment
Chemokine (C-X3-C motif) ligand 1 (CX3CL1) expression in ECs is involved in the CX3CL1 receptor (CX3CR1)–dependent recruitment of immune cells, such as NK cells, CD8+ T cell, and CX3CR1 non-classical monocytes [90]. CX3CL1 expression in EC specifically regulates CX3CR1-expressing monocyte recruitment into tumors without affecting IM recruitment. This process may be also involved in TEM recruitment since around 50% of TEMs express CX3CR1 [29]. CX3CL1 exists as membrane bound and soluble forms. Soluble CX3CL1 is involved in CX3CR1 monocyte chemotaxis, whereas membrane bound is involved in their adhesion to ECs [61, 91]. Indeed, soluble CX3CL1 induces human peripheral blood mononuclear cell–derived monocyte migration, more effectively than CCL5 [91]. CX3CL1+ monocytes adhere to HEK293 overexpressing membrane bound CX3CR1 but not to WT HEK293. The membrane-bound CX3CL1 promotes human non-classical monocyte crawling and adhesion on endothelium via CX3CR1 activation on non-classical monocytes [21]. The subsequent monocyte transmigration is promoted by angiogenic factors such as VEGF-A. VEGF-A involvement in non-classical monocyte transmigration is due to VEGF-A-induced a disintegrin and metalloproteinase domain–containing protein 10 (ADAM10) and ADAM17 activity stimulation [62, 63], which subsequently promotes CX3CR1 monocyte transmigration via CX3CL1 shedding [92] and hence non-classical monocyte transmigration. Consistently with these results, in vitro transendothelial migration and in vivo infiltration of non-classical monocytes into tumors are critically lower in non-angiogenic tumors, whereas they are increased in angiogenic tumors [21]. Indeed, human non-classical monocytes are recruited mostly in DLD1 or HCT116 tumor expressing high level of VEGF-A, whereas they are less recruited in SKBR1 tumor expressing low level of VEGF-A. Furthermore, treatment of DLD1 tumors with anti-VEGF-A antibody bevacizumab reduces the human CD16+ monocyte recruitment. Nonetheless, the DC101 anti-VEGFR2 antibody increases Ly6Clo monocyte infiltration into orthotopic murine colorectal tumors. Furthermore, non-classical monocytes require CX3CR1 to infiltrate tumors since Ly6Clo monocytes infiltration in murine orthotopic colorectal tumors is abolished in CX3CR1 KO mice [22]. These data suggest that the interaction between CX3CL1 (EC) and CX3CR1 (non-classical monocyte) promotes non-classical monocyte recruitment into tumor. This recruitment is enhanced in angiogenic tumors and it would be interesting to investigate if this process is involved in TEM infiltration since 50% of TEMs express CX3CR1.
Impact of blood vessel cells on macrophage polarization and angiogenic phenotype (related to Fig. 1b)
ECs promote M2 polarization and angiogenic phenotype
ECs are involved in macrophage survival, proliferation, M2-polarization, and angiogenic phenotype acquisition in malignant and non-malignant tissues [12, 54, 65]. The impact of ECs on macrophage survival has been demonstrated by co-culture experiments. The macrophage survival and expansion are mediated by direct contact between ECs and macrophages since macrophage colony formation is observed with direct co-cultures but not with transwell assays. CSF1-membrane bound (EC) and CSF1 receptor (CSF1R) (macrophage) juxtacrine interaction is involved in macrophage survival and expansion, since a CSF1 exclusive inhibitor inhibits macrophage survival and expansion [65]. This survival/proliferation induced by ECs in macrophages is likely due to mechanistic target of rapamycin (mTOR) activation in macrophage since mTOR inhibition with rapamycin inhibits CSF1+IL-6-induced macrophage proliferation [12]. Furthermore, ECs induce M2 polarization in vitro and in vivo, notably via IL-6 secretion [12, 54]. Indeed, the macrophage-EC co-cultures increase M2 marker expression such as Tie2 and decrease M1 marker expression such as major histocompatibility complex II (MHCII) [65]. Furthermore, EC conditioned media induce M2 polarization associated with the enhanced expression of CD206 or Arg-1, which is reduced by anti-IL-6 antibody [12]. This M2 polarization is enhanced in pre-incubated ECs with GBM cells which seems that this EC-induced M2 polarization is amplified by the TME. In human and murine GBM, alternatively activated TAMs are localized proximately to ECs, which are a major source of IL-6. Indeed, in vivo, specific inducible deletion of IL-6 in ECs reveals that ECs are the major source of IL-6 in murine GBM [12]. Furthermore, IL-6 expression is highly detected in ECs cytoplasm of newly formed vessels in human GBM [93]. Specific inducible deletion of IL-6 in ECs strongly decreases M2 macrophage population and slightly increases M1 population, decreases tumor growth, and enhances mice survival [12]. In summary, ECs are involved in macrophage survival and expansion via CSF1-CSF1R juxtacrine loop, and in macrophage M2 polarization, notably via IL-6 secretion in vivo, at least in murine GBM.
ECs induce angiogenic phenotype in macrophages associated with an increase in Tie2 or VEGF-A expression and macrophages co-cultivated with ECs increase murine prostate tumor growth and angiogenesis, when these macrophages are co-injected with cancer cells in mice [65]. EC-derived Ang-2 is not only a chemoattractant for TEMs. Indeed, Ang-2 also promotes M2 polarization and angiogenic profile in TEMs by increasing the expression of M2 markers (IL-10 and MRC1) and of angiogenesis-related gene (cathepsin B and thymidine phosphorylase) expression [58]. Furthermore, in vivo, Ang-2 and Ang-2 + VEGF inhibitions shift macrophage population from M2 towards M1. Anti-Ang-2 increases M1/M2 intermediate macrophage population in murine GBM. Anti-Ang-2 combined with an anti-VEGF increases M1 proportion among total leukocytes in the PyMT model and increases M1 population and decreases M2 population among total macrophages [81, 94]. Hence, Ang-2 is involved in macrophage and TEM M2 polarization and promotes their angiogenesis phenotype.
Endothelial-to-mesenchymal transition (EndMT) promotes macrophage M2 polarization
Endothelial-to-mesenchymal transition (EndMT) is defined as a phenotypic change in ECs characterized by a loss of endothelial features, markers (e.g., CD31), cellular tight junctions, apico-basal polarity, and the acquisition of mesenchymal features and markers such as fibroblast specific protein-1 and α-smooth muscle actin (α-SMA) [95]. EndMT is a source of up to 40% of CAFs; can be induced by radiotherapy, TGFβ-1, or osteopontin; and has an impact on tumorigenesis, metastatic extravasation, and therapy resistance [67, 95–98]. Radiotherapy-induced EndMT is mediated via p53 activation in ECs, whereas it is mediated via transcription factor 12 (TCF12) in osteopontin-induced EndMT since p53 siRNA and TCF12 shRNA inhibit radiotherapy-induced EndMT and osteopontin-induced EndMT, respectively. Furthermore, ECs undergoing EndMT with osteopontin or radiotherapy induce M2 polarization and inhibit M1 polarization [66, 67]. This is mediated via heat shock protein 90 α (HSP90α) secretion by osteopontin-induced EndMT, whereas it is mediated via CXCR4/SDF1α signaling in radiotherapy-induced EndMT [66, 67]. In vitro, osteopontin-induced EndMT conditioned media induce THP-1-derived macrophage M2 polarization which is blocked by anti-HSP90α antibody. On the other hand, bone marrow–derived macrophages (BMDMs) co-cultivated with irradiated tumor ECs display an increased CD206+ M2 macrophage proportion (in total F4/80+ macrophage population) compared with non-irradiated ECs. This effect is abolished in BMDMs co-cultivated with tumor ECs from EC-p53 KO mice. Furthermore, in vivo, subcutaneous co-injection of osteopontin-induced EndMT cells with Panc02 pancreatic cancer cells drastically enhances M2 macrophage population and tumor growth (compared with Panc02 injected alone or injected with ECs). These changes are strongly reduced with intravenously injected anti-HSP90α antibody [66]. Irradiation induces CXCR4 expression in ECs both in vitro and in vivo. This effect is abolished with p53 siRNA and in EC-p53 KO mice. The irradiation-induced CXCR4 expression induces macrophage SDF1α + recruitment and M2 polarization in vivo since this is inhibited with CXCR4 antagonist [67]. Consistently with these results, in human PDAC, there is a correlation between EndMT numbers and M2 macrophage infiltration. Furthermore, M2 macrophages are located close to EndMT cells [66]. All together, these data evidence that ECs undergoing osteopontin- or radiotherapy-induced EndMT induce macrophage M2 polarization in murine tumors via HSP90α secretion and CXCR4/SDF1α signaling, respectively.
Pericytes and perivascular mesenchymal stem cells (MSCs) induce macrophage M2 polarization
Perivascular cells regulate macrophage polarization in melanoma and pancreatic cancers. In melanoma, pericytes and MSCs influence macrophage polarization notably via MFG-E8 secretion [99]. MFG-E8, also called lactadherin, is a secreted integrin-binding protein which is overexpressed in several tumor types compared to normal tissues [100]. MFG-E8 promotes cancer progression, cancer chemoresistance, and tumor angiogenesis and is associated with poor prognosis in human melanoma. Pericytes and perivascular MSCs are the major sources of MFG-E8 secretion in melanoma tumors [85, 99]. MFG-E8 is involved in macrophage M2 reprograming since macrophage incubation with MFG-E8 induces IL-10, TGF-β, and VEGF-A secretion, and increases the proportion of CD206+ macrophages [101]. MFG-E8 released by apoptotic ECs or MSCs is also involved in M2 polarization [85, 101]. Indeed, in vitro, RAW macrophages co-cultivated with MSCs display higher M2 marker expression, which is not observed in macrophages co-cultivated with MSC MFG-E8 KO. Nonetheless, the way by which MFG-E8 induces M2 polarization still needs to be investigated. In vivo, MFG-E8 enhances tumor angiogenesis and tumor growth. Furthermore, higher vascularization is observed in MFG-E8 WT mice compared to MFG-E8 KO [99]. This angiogenesis enhancement is likely due to MFG-E8-induced macrophage M2 polarization. In pancreatic cancers, pericytes and CAFs are the main cells responsible for IL-33 secretion in the TME [68]. IL-33 causes M2 polarization and matrix metalloprotease-9 (MMP-9) expression in TAMs, which are mediated by the IL-33 receptor ST2 activation. MMP-9 and M2 polarization induce cancer cell intravasation and metastasis in vivo [68]. Furthermore, IL-33 induces TAM prostaglandin-2 secretion which enhances cancer stemness and tumor growth [84]. To conclude, perivascular cells induce TAMs M2 and pro-angiogenic phenotype via MFG-E8 and IL-33 secretion, which impacts tumor growth.
Effects of TAMs and monocytes on tumor blood vessels
Angiogenesis and TAMs (related to Fig. 2)
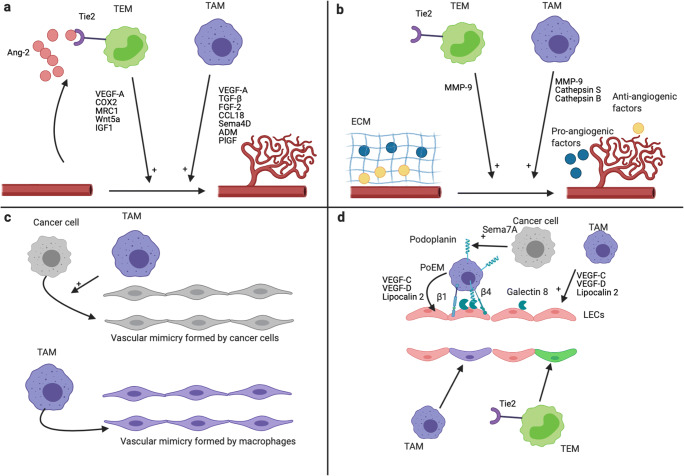
Fig. 2.
Mechanisms of tumor angiogenesis and lymphangiogenesis promotion by TAMs and TEMs. Effects of TAMs and TEMs on angiogenesis (a, b, c) and lymphangiogenesis (d). a TAMs and TEMs promote tumor angiogenesis via secreted factors [5]. EC-derived Ang-2 enhances the pro-angiogenic phenotype of TEMs [58, 102]. b TAMs and TEMs promote tumor angiogenesis via the secretion of protease. TAMs secrete MMP-9, cathepsin B, and cathepsin S, whereas TEMs secrete high level of MMP-9 [58, 103–105]. MMP-9 increases VEGF-A bioavailability via ECM degradation [106, 107]. Cathepsin S is involved in the degradation of anti-angiogenic proteins and in the formation of pro-angiogenic peptides via ECM degradation [108]. The promotion of angiogenesis by cathepsin B occurs via the induction of VEGF expression by cancer cells [109, 110]. All together, these proteases lead to an increase and a decrease of pro-angiogenic factor and anti-angiogenic factor in the TME, respectively, which promote tumor angiogenesis. c Upper panel: Vascular mimicry structures are perfused non-endothelial channels. They are formed by cancer cells in several cancer types, and promote tumor growth, metastasis, and angiogenesis [111, 112]. TAMs promote, at least in vitro, the formation of vascular mimicry channels by cancer cells [113, 114]. Lower panel: TAMs can directly form vascular mimicry structures in tumors [115]. d Upper panel: TAMs promote tumor lymphangiogenesis via the secretion of VEGF-C, VEGF-D, and LCN2 [116–118]. Furthermore, podoplanin-expressing macrophages (PoEMs) are able to interact with tumor LECs and are strongly involved in the promotion of tumor lymphangiogenesis [119–121]. This interaction is dependent on GAL8 (LECs), podoplanin, and β1 and β4 integrins (PoEMs) [119, 121]. The secretion of Semaphorin 7A by cancer cells promote the expression of podoplanin by TAMs [120]. Lower panel: TEMs and a subset of TAMs (called M-LECP) are able to integrate into pre-existing lymphatics, which promotes tumor lymphangiogenesis [122, 123]. This figure was created with BioRender.com
Angiogenesis refers to the formation of new blood vessels from pre-existing ones [124]. Tumor blood vessels are critical in regulating tumor growth via oxygen supply and in supporting metastasis via cancer cell dissemination. Microvessel density corresponds to the small blood vessel density in a tumor and hence is the reflection and a way to assess tumor angiogenesis [125]. It is well described that microvessel density correlates with angiogenic factors, metastasis risk, and prognosis in a huge panel of solid tumors [125, 126]. TAMs are important regulators of tumor angiogenesis [5]. Correlation between TAMs, microvessel density, and poor prognosis is observed in a lot of solid tumors. TAMs are involved in tumor blood vessel development and in the angiogenic switch [32, 127]. Indeed, in the early stage of tumor development, the vessel network development is observed several weeks earlier in CSF1-overexpressing mice than that in WT mice. TAMs promote tumor angiogenesis by pro-angiogenic factor secretion, protease secretion, and transdifferentiating themselves into vessel-like structures in a process called “vascular mimicry.”
Pro-angiogenic factor secretion
Once in the tumor, TAMs secrete pro-angiogenic factors such as VEGF-A, TGF-β, fibroblast growth factor-2 (FGF-2), CCL18, semaphorin 4D (Sema4D), adrenomedullin (ADM), and placental growth factor (PlGF) [128–133]. Macrophage pro-angiogenic phenotype is regulated by hypoxia and lactate. Indeed, in vitro, conditioned media from macrophages exposed to lactate or hypoxia have higher angiogenic capacity than conditioned media from macrophages exposed to normoxia, as shown in rat corneal angiogenesis assays [134]. Hypoxia and lactate induce VEGF-A expression in macrophages via hypoxia-inducible factor-1α (HIF-1α), since this is abolished in macrophage from HIF-1α KO mice [135, 136]. It is strongly suggested in [136] that tumor-derived lactate induces TAM M2 phenotype and promotes their angiogenic phenotype. Very recently, it was shown that the expression of the lactate transporter MCT1 by macrophages is strongly involved in lactate uptake and oxidation by macrophages and in lactate-induced macrophage M2 polarization and VEGF secretion [137]. Furthermore, in vitro, HIF-1α and HIF-2α stability in macrophage is regulated by PI3K/Akt signaling, since HIF-1α, HIF-2α, and VEGF induction by hypoxia is strongly inhibited with PI3K inhibitors or AKT siRNA [138]. In vivo, TAM angiogenic phenotype and microvessel density are reduced in tumors exposed to PI3K inhibitor or in p110γ−/− (a subunit of PI3K) mice. In tumors, TAMs are major VEGF producers and are located mostly in avascular and hypoxic areas [70, 136, 139]. In breast cancer, VEGF-A and TGF-β expression and secretion in TAMs are also regulated by cancer cells, notably via macrophage Fra-1 activation [132]. In vitro, Fra-1, VEGF-A, and TGF-β expression in macrophages from Balb/c mouse peritoneum co-cultivated with 4T1 breast cancer cells is enhanced, whereas Fra-1 siRNA diminish the enhanced VEGF-A and TGF-β expression. In vivo, co-injection of 4T1 and RAW macrophages subjected to Fra-1 knockdown in Balb/c mice induces tumor with less VEGF-A and TGF-β expression and with lower microvessel density than in 4T1 and RAW WT co-injected tumors [132]. FGF-2 expression and secretion in TAMs are regulated by the long non-coding RNA MALAT1. In vitro, MALAT1 knockdown in TAMs inhibits FGF-2 expression and secretion. MALAT1 siRNA diminishes the vascular structure formation induced by TAMs conditioned media in HUVECs and is reversed in TAMs overexpressing FGF-2 [133]. Sema4D expression and CCL18 expression in TAMs are correlated with microvascular density and these two proteins are mainly produced by TAMs [129]. In vitro, CCL18 induces EC tube formation via the CCL18 receptor PITPNM3 activation since this CCL18-induced tube formation is decreased in si-PITPNM3 HUVECs. Microvascular density in tumor xenografts treated with CCL18 is higher than that in the control. High angiogenesis inhibition is observed in Sema4D KO mice. The injection of WT TAMs in sema4D mice enhances angiogenesis to the same extent as that in WT mice, whereas the injection of sema4D KO TAMs does not [128]. In vitro, ADM secretion by macrophages is enhanced by melanoma cancer cells. TAMs promote angiogenesis via ADM secretion in ECs since these TAM conditioned media–induced angiogenesis is abolished by anti ADM. In vivo, colocalization between CD68+ RAW macrophages and ADM indicates that TAMs are a source of ADM in this melanoma murine model [131].
Protease secretion
TAMs also promote angiogenesis via the secretion of proteases such as cathepsins (S and B) and MMPs such as MMP-9. In vitro, cathepsin S and B secretion by macrophages is stimulated by the combination of M2 polarization cytokines such as IL-4, IL-10, and IL-6. This occurs in an inositol-requiring enzyme 1α (IRE1α)–dependent manner since this secretion stimulation is abolished with both IRE1α inhibitor and siRNA [140]. In vivo, TAMs promote angiogenesis in PDAC murine tumor model via cathepsin B and S secretion. Indeed, Rip1-Tag2 tumors inoculated with BMDMs from cathepsin B and S KO mice have a lower average vessel density than Rip1-Tag2 tumors inoculated with BMDMs from WT mice [103]. Furthermore, cathepsin S promotes angiogenesis in pancreatic Rip1-Tag2 tumors via matrix protease activity leading to an increase in pro-angiogenic factor release and in anti-angiogenic factor degradation [108]. Cathepsin B angiogenesis regulation is not fully understood but cathepsin B downregulation in multiple models leads to angiogenesis inhibition. VEGF secretion by cancer cells and in tumor is regulated by cathepsin via an unknown mechanism and could explain the positive impact of cathepsin B on tumor angiogenesis [103, 140–144]. Indeed, cathepsin B inhibition or overexpression in GBM cell lines respectively decreases or increases VEGF secretion by these cells. Furthermore, VEGF protein level is higher in breast tumor from mouse PyMT overexpressing cathepsin B than that in tumor from PyMT WT mice [109, 110, 141]. In Rip1-Tag2 pancreatic tumors, MMP-9 is involved in the angiogenic switch by the VEGF-A bioavailability enhancement [106, 107]. In vitro, M2 macrophages secrete high levels of MMP-9 and low levels of tissue inhibitor of metalloproteinase (TIMP)1, a MMP inhibitor, whereas M1 macrophages secrete both MMP-9 and TIMP1 [145]. Hence, cancer cells by skewing TAMs toward M2 phenotype promotes MMP-9 activity. Accordingly, M2 macrophages favor angiogenesis in vivo in a MMP-9-dependent manner since this ability is decreased in MMP-9 KO macrophages [145]. MMP-9 expression in macrophages is regulated by the M2 polarization marker cyclooxygenase 2 which is activated notably by MMP-1/3 and IL-6 [146, 147]. In vivo, tumor angiogenesis is strongly inhibited in MMP-9 KO mice or by chemical compounds inhibiting MMP-9 [104, 106]. In tumors, MMP-9 is strongly expressed in immune cells [148], mostly by neutrophils [145]. Although neutrophils constitute the major source, TAMs are also high MMP-9 producers, as shown in human colon cancer and in murine cervical cancer [104, 149]. MMP-9 expression and activity in tumors and in TAMs increase during tumor progression of the Rip1-Tag2 cancer model [104].
Vascular mimicry
Vascular mimicry, also called vasculogenic mimicry, refers to vascular channels formed by non-endothelial cells (mostly cancer cells). These structures were firstly described by Maniotis et al., in 1999 [150]. They showed that highly invasive melanoma cells, which notably are expressing high level of tie1, were able to form vascular perfused channels both in vitro and in vivo. Nowadays, it is known that vascular mimicry networks are also observed in numerous cancer types [111, 112, 151]. These vascular mimicry channels are perfused and connected to the general circulation. They are known to increase tumor growth and to be associated with poor prognosis and metastasis in patients [111, 112].
In vitro, M2 macrophages induce vascular mimicry in glioma and GBM cells [113, 114]. The macrophage-induced vascular mimicry in gliomas cells is dependent on IL-6 and COX2 induction in gliomas and GBM cells, respectively. Indeed, IL-6 expression inhibition in glioma cells and COX2 inhibition in GBM cells abolish the impact of macrophages on vascular mimicry formation by these cells. This is consistent with the fact that, in GBM patients, vascular mimicry positive areas display high TAMs infiltration. Furthermore, in glioma patients, vascular mimicry density is correlated with the quantity of M2 macrophages. In uveal melanoma, there are more macrophages in tumors having vascular mimicry than in those without vascular mimicry [152]. The correlation between macrophages and vascular mimicry appearance in tumors may be due to hypoxia since hypoxia promotes the formation of vascular mimicry as well as the infiltration of macrophages [69, 70, 153]. It would hence be interesting to investigate if TAMs induce vascular mimicry formation by cancer cells in vivo.
TAMs can also form vascular mimicry structures in vitro and in vivo in melanoma tumor model, in multiple myeloma, in human anaplastic thyroid carcinoma, in human meningioma, and benign melanoma tumor [115, 154, 155]. In vitro, exposure of multiple myeloma macrophages to VEGF and FGF-2 induces a capillary-like network associated with increased EC marker expression (factor VIII–related antigen, VE-cadherin, and VEGFR2) [154]. In the murine melanoma tumor model, these channels are functional, perfused, and connected to the vasculature since dextran is detected in these structures upon its injection in the tail vein [115]. Hypoxia is a key factor involved in vascular mimicry formation since less vascular mimicry channels are formed with HIF-1α KO macrophages [115]. Consistently with these results, in human anaplastic thyroid carcinoma, cancer cells that are closed to these macrophage channels are not necrotic or hypoxic, even at long distance from blood vessels [155]. This indicates that these channels are perfused or at least lead to tumor cell oxygenation. Additionally, vascular mimicry is observed in human malignant meningioma and benign melanoma tumors [115]. The functional significance of these TAM-derived vascular mimicry structures for tumor growth, angiogenesis, metastasis as well as for prognosis is thus worth investigating.
Communication with pericytes
The effects of TAMs on blood vessel angiogenesis rely on communication not only with ECs but also with pericytes. This communication between macrophages and pericytes occurs notably via Notch signaling and PDGFB-PDGFRβ-induced pericyte migration and periostin expression [156, 157]. In vitro, HUVEC cells co-cultivated with macrophages or pericytes enhance the formation of microvessels. Furthermore, the triple co-culture of macrophages, pericytes, and ECs is synergic in promoting angiogenesis. Notch signaling is involved in this process since Notch inhibition in each cell type inhibits angiogenesis [157]. In vitro, the secretion of PDGF-BB by macrophages induces pericyte PDGFRβ+ migration and secretion of VEGF-A and pro-angiogenic extracellular matrix component (ECM) periostin by pericytes which enhance angiogenesis [156]. The expression and secretion of PDGF-BB by macrophages are promoted by IL-4 and IL-13 but not by IL-10 [158]. The induction of PDGF-BB expression by IL-4 is mediated at least by PI3Kγ since this induction is diminished in macrophages from p110γ KO mice [159]. Accordingly, PDGF-BB expression and secretion are higher in M2 macrophages compared to M1 macrophages [158, 160]. PDGF-BB expression and secretion in macrophages are also stimulated by cancer cells. In vitro, macrophages exposed to U87 GBM cancer cells show higher PDGF-BB expression. This induction occurs via cat eye syndrome critical region protein 1 (CECR1) induction since it is abolished in siRNA CECR1-treated macrophages [156]. Furthermore, the stimulation of TAMs with CECR1 induces PDGF-BB expression in TAMs. Consistently with this, CECR1 expression in GBM is highly produced by TAMs and is correlated with human GBM microvascular density [156, 161]. In vivo, in the early steps of murine brain tumors, macrophages are involved in pericyte-endothelial interaction and thereby in tumor angiogenesis. Indeed, neural glial antigen 2 (NG2) KO specifically in macrophages strongly decreases macrophage recruitment during the beginning of murine brain tumors. Then, macrophage recruitment returns to the same level as in WT tumors in the later stages of tumor growth [162]. Interestingly, macrophage recruitment is correlated with the level of tumor blood vessel covered with pericyte and tumor angiogenesis, indicating that macrophages are most likely involved in these processes. Indeed, 10 days after the development of NG2 macrophage KO tumor, macrophage infiltration is reduced by 90% compared to WT tumors. This decrease in macrophage infiltration is associated with a lower pericyte coverage of tumor blood vessels. After 16-day tumor development, macrophage infiltration and pericyte coverage of tumor blood vessel are comparable in NG2 macrophages KO mice and in WT mice. In conclusion, TAM communication with pericytes promotes angiogenesis in vitro, via Notch signaling and secretion of PDGF-BB which induces pericyte recruitment. In the early steps of murine brain tumors, TAMs promote angiogenesis and the pericyte coverage of tumor blood vessels. Hence, it would then be interesting to investigate the impact of TAM-derived PDGF-BB and Notch signaling involvement in the regulation of tumor angiogenesis and pericyte recruitment in the early steps of other cancer types.
TAMs promote tumor lymphangiogenesis (related to Fig. 2)
Tumor lymphatic vessels are formed via tumor lymphangiogenesis process and are involved in the spread of cancer cells. In tumors, VEGF-C and VEGF-D are the main factors involved in tumor lymphangiogenesis, via VEGFR3 activation in LECs. In murine tumor models, overexpression of VEGF-C/D increases tumor lymphangiogenesis. Accordingly, VEGF-C/D inhibition or VEGFR3 inhibition decreases lymph node metastasis [163]. In the TME, TAMs are major VEGF-C and VEGF-D producers [5, 116, 117]. There exists a correlation between lymphatic vessel density and VEGF-C/D production by TAMs. Furthermore, there is a correlation between TAM density, lymphatic vessel density, and lymph node metastasis in several cancers (reviewed in [39]). Recently, TAM-derived lipocalin 2 (LCN2) was observed to induce lymphangiogenesis [118, 164]. In vitro, TAM-derived LCN2 induces LEC proliferation, which is abolished in TAMs transfected with LCN2 siRNA. LCN2 induces lymphangiogenesis via VEGF-C expression induction in LECs, which induces VEGFR3 activation in LECs. In vivo, LCN2 is involved in tumor lymphangiogenesis and its associated metastases since there are less lung metastases and lower lymphatic vessel density in PyMT LCN2 KO mice than those in WT mice. Consistently with these results, LCN2 expression is correlated with lymph node metastasis in human breast and colorectal cancers [165, 166].
Other mechanisms by which TAMs promote lymphangiogenesis are by their abilities to become perilymphatic and to integrate into pre-existing lymphatics [39, 122]. These mechanisms occur in a subset of TAMs, called myeloid-lymphatic endothelial cell progenitors (M-LECP) [167]. These cells co-express macrophage markers such as CD68 (human) or CD11b (mouse) and lymphatic markers such as podoplanin and LYVE 1. These TAMs colocalize around lymphatic structures and compose macrophage-derived lymphatic structures which thereby promote lymphangiogenesis [39, 119–121]. Indeed, TAMs can transdifferentiate into LEC progenitors and acquire LEC markers such as LYVE 1 and podoplanin in murine and human tumors [39, 167, 168]. The adhesion between TAMs and LECs depends on podoplanin expression in TAMs and galectin 8 (GAL8) expression in LECs [119]. Podoplanin expression in TAMs is induced by semaphorin 7A both in vitro and in vivo during tumorigenesis and during physiological postpartum mammary gland involution [120]. Semaphorin 7A is also involved in macrophage motility, chemotaxis towards lymphatics, and TAM incorporation in lymphatics during lymphangiogenesis in vitro [120]. Podoplanin-expressing macrophages (PoEMs) are located near lymphatic vessels in murine breast cancer. The perilymphatic localization of PoEMs is mediated by interaction with GAL8-expressing LECs [119]. Indeed, GAL8-specific deletion in LECs or GAL8 pharmacological inhibition impairs PoEM perilymphatic localization in vivo. Furthermore, this interaction between PoEMs and GAL8 induces TAMs β1 integrin clustering which is needed for TAM-LEC adhesion. Another team showed that TAM location around lymphatic structures is also dependent on TAMs β4 integrin interaction with laminin 5 in murine triple-negative breast cancer [121]. Finally, PoEMs secrete high amounts of MMPs (and VEGF-C and VEGF-D) which increases VEGF-C and VEGF-D bioavailability and hence promotes lymphangiogenesis [119]. In conclusion, TAMs favor tumor lymphangiogenesis and their subsequent lymph node metastasis, either by secreted factors (VEGF-C, VEGF-D, and LCN2) or by integration of a subset of TAMs, called M-LECP, into lymphatic vessels.
Angiogenesis and lymphangiogenesis promotion by Tie2-expressing monocytes (TEMs) (related to Fig. 2)
In vitro, TEMs secrete more pro-angiogenic factors such as VEGF-A, TNFα, cyclooxygenase 2, MRC1, and Wnt5a than Tie2− monocytes. They are a major source of MMP-9 [58, 105]. These TEMs are recruited into tumors by EC-derived Ang-2 (see above) [56–58, 74, 77]. Furthermore, these Ang-2-activated TEMs secrete higher levels of insulin-like growth factor 1 (IGF1), cathepsin B, and thymidine phosphorylase and are more pro-angiogenic in vitro [58, 102]. TEMs are also pro-angiogenic in vivo. For example, the co-injection of glioma or ovarian cancer cells with TEMs in mice induces more vascularized tumors compared to injection of tumor cells alone or of tumor cells co-injected with CD11b+ myeloid cells without TEMs [102, 123, 169]. Ang-2-induced TEM IGF1 secretion induces angiogenesis and tumor growth via an IGF1 receptor–dependent activation of ECs [102]. Indeed, Ang-2-treated TEMs are more pro-angiogenic in vitro and in vivo and this increase is abolished by anti-IGF1 antibodies. Consistently with these results, the proportion of TEMs amongst total tissue TAMs is correlated with total tumor microvascular density in human ovarian, renal cell carcinoma, hepatocellular carcinoma, and non-small cell lung cancer, but not in colorectal cancer [29, 102, 170, 171]. High TEM infiltration or high number of circulating TEMs is correlated with poor prognosis in ovarian cancer and in hepatocellular carcinoma, respectively, whereas it is surprisingly correlated with good prognosis in hilar cholangiocarcinoma [102, 172–175]. These TEMs are also found in hypoxic and tumor areas enriched in small immature non-pericytic blood vessels [123, 176]. Less TAM-expressing Tie2 infiltration in murine GBM tumors is observed in HIF-1 KO mice [176]. Interestingly, in murine and human breast cancers, TEMs express lymphatic markers (e.g., LYVE 1, VEGFR-3, and podoplanin) and lymphangiogenic factors (VEGF-C and VEGF-D) and are associated with lymphatic structures [123]. These isolated breast cancer TEMs induce lymphangiogenesis both in vitro and in vivo (as shown with corneal vascularization assays) by Tie2- and VEGFR-1-dependent mechanism. Indeed, TEMs isolated from breast cancer induce lymphangiogenesis. This process is slightly inhibited by Tie2 or VEGFR inhibitors while it is abolished by the combination of both inhibitors. Interestingly, TEMs are involved in chemotherapy relapse and vessel reconstruction after chemotherapy [177]. Indeed, in murine fibrosarcoma tumors, chemotherapy (doxorubicin) firstly decreases vessel density and tumor volume which is followed by a strong increase in tumor growth and vessel density (tumor relapse). These features are correlated with TEM accumulation in the tumors. Vessel density and tumor growth promotion by doxorubicin are strongly diminished in mice with Tie2 deletion specifically in the myeloid cells. In summary, TEMs promote angiogenesis and lymphangiogenesis in several cancer types and are involved in chemotherapy relapse of murine fibrosarcoma tumors [177].
TAMs promote metastasis (related to Fig. 3)
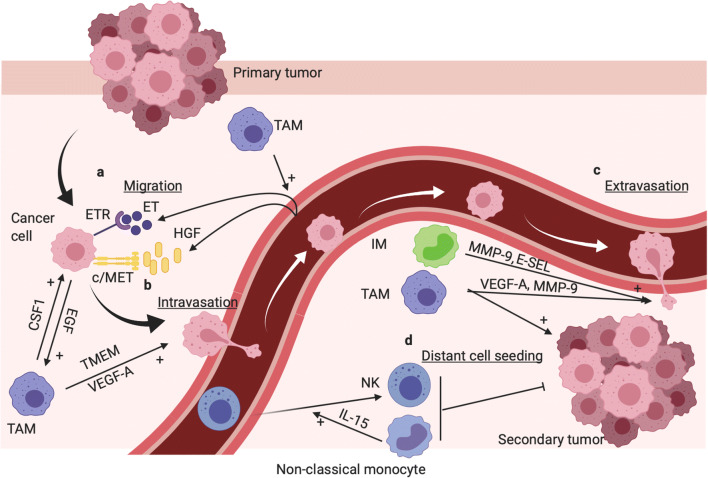
Fig. 3.
TAMs and inflammatory monocytes (IMs) promote tumor metastasis, whereas murine non-classical monocytes prevent metastasis. a TAMs promote cancer cell migration from primary tumor site towards blood vessel via CSF1 (TAMs) EGF (cancer cell) paracrine loop [178]. TAMs promote endothelin secretion (ET) by ECs; moreover, ET and HGF secretion by ECs induce cancer cell chemotaxis toward blood vessel via ET receptor and c-Met receptor activation, respectively [179, 180]. b In breast cancer, specific area called TMEM composed of a cancer cell, a TAM, and an EC promote cancer cell intravasation. Basically, TAMs induce invadopodia formation in the cancer cell which is involved in ECM breaking during EC transendothelial migration [181]. Furthermore, Tie2high TAM-derived VEGF-A promote transient and local vascular leakage which favor cancer cell transendothelial migration [182]. c TAMs and IMs promote cancer cell extravasation notably via MMP-9 and VEGF-A-dependent vascular leakage [16, 183]. d TAMs and IMs promote metastasis and distant cancer cell seeding [16, 184]. Murine non-classical monocytes prevent distant cell seeding, notably via IL-15-induced NK cell recruitment [24, 185, 186]. This figure was created with BioRender.com
Metastasis process is defined as the dissemination of cancer cells from a primary tumor site into a secondary site [187]. This process is responsible for up to 90% of cancer deaths [188]. It is composed of different steps including cancer cell migration/invasion through ECM, cancer cell intravasation, cancer cell circulation and survival into the blood, cancer cell extravasation, and metastasis formation. The effects of perivascular TAMs on blood vessels are involved in cancer cell migration/invasion, intravasation, extravasation, and metastatic formation. TAM deletion in 3 different ways (CD11b+ TAM deletion, CSF1R mice KO, or clodronate liposomes) and monocyte recruitment inhibition into the lung by CCL2 blockade all inhibit metastatic spread from primary murine PyMT mammary tumor to lungs [16, 184].
TAMs and tumor ECs promote cancer cell migration toward blood vessels
Cancer cell migration toward blood vessel is enhanced by a paracrine loop between TAMs and cancer cells, ECs and cancer cells, and the three cell types. Indeed, cancer cell migration is enhanced by perivascular TAMs involving a paracrine loop of TAMs CSF1 secretion and epidermal growth factor (EGF) secretion by cancer cells. Indeed, the inhibition of either EGF or CSF1 results in strong cancer cell migration diminution [178]. The migration of cancer cells toward blood vessels is also stimulated both in vitro and in vivo with EC-derived HGF which promotes cancer cell migration in a c-Met receptor–dependent manner [179]. Breast cancer cell motility towards HUVEC conditioned medium is impaired by cancer cell c-Met knockdown or by HUVECs HGF knockdown. Furthermore, cancer cell migration towards blood vessel is impaired by c-Met inhibition in vivo in breast murine cancer. A paracrine loop between TAMs, ECs, and cancer cells is involved in breast cancer cell migration/invasion toward blood vessels. Indeed, macrophage conditioned media induce ET secretion by HUVECs and ET receptor activation in cancer cells. These effects create cancer cell chemotaxis toward blood vessels which is blocked by ET-1 blocking antibody both in vitro and in vivo [180]. This paracrine loop is also responsible for tumor cell transendothelial migration and for metastasis.
TAMs promote cancer cell intravasation in areas called tumor microenvironment of metastasis
In breast cancer, cancer cell intravasation is enhanced in areas called tumor microenvironment of metastasis (TMEM) which is composed of one TAM, one cancer cell overexpressing the invasive isoform of “mammalian enabled” protein (MenaINV; an actin regulatory protein), and one EC, all three in direct contact [189]. Mechanistically, direct contact of macrophages with breast cancer cells induces Notch1-dependent MenaINV expression in breast cancer cells [190]. Then, this interaction induces Rhoa GTPase–mediated invadopodia which helps cancer cells to break ECM during transendothelial migration [181]. Furthermore, VEGF-A released by Tie2high TAMs enhances local and transient vascular leakiness and hence cancer cell transendothelial migration in vitro and in vivo [182]. TAM-derived TNF-α also enhances cancer cell migration toward ECs, endothelial permeability, and cancer cell intravasation in 3D fibrosarcoma and breast cancer models [191]. TAM-derived IL-1β enhances cancer cell adhesion and transendothelial migration throughout blood and lymphatic cells in vitro [192]. TMEM structures are observed in early tumor lesions from breast cancer of MMTV-PyMT and MMTV-HER2 mice [193]. Furthermore, in human breast cancer, TMEMs are restricted to the blood vessels (not seen in lymphatic vessels) and a high number of TMEMs are associated with increased risk of distant metastasis and correlated with breast cancer grade [194–196]. In conclusion, TAMs, as a crucial part of TMEMs, are involved in breast cancer cell intravasation and thereby involved in breast cancer metastasis. Nonetheless, since this effect of macrophages on cancer cell intravasation is restricted to breast cancer, it would be interesting to investigate if macrophages could promote cancer cell intravasation or if TMEM structures are observed in other cancer types.
TAMs promote cancer cell extravasation, cancer cell seeding, and distant metastasis
The extravasation step is enhanced by TAMs and monocytes, notably via blood vessel permeabilization [16, 183]. Blood vessel permeabilization is mostly promoted by TAM and monocyte VEGF-A secretion and monocyte-induced endothelial retraction in an E-selectin-dependent manner. In a 3D transmigration assay, cancer cell transmigration is diminished by 5-fold in the absence of macrophages. Interestingly, the effects of TAMs are inhibited by CCL2 blocking antibody and totally ablated in VEGF-A KO TAMs. TAM-secreted VEGF-A also enhances vascular permeability [16]. VEGF-A-induced vascular permeability is mediated by tyrosine phosphatase density-enhanced phosphatase-1 (DEP-1)–dependent Src kinase activation, which then mediates VE-cadherin uncoupling, thereby creating endothelial gaps [197, 198]. In vivo, VEGF-A-induced tumor vascular permeability is diminished in DEP1 KO mice or with Src inhibitor. Indeed, there is less Evans blue diffusion in healthy and tumor tissues (Miles assay) upon its injection in the tail vein of DEP-1 KO mice or in Src inhibitor–treated mice than that in WT mice or untreated mice, respectively [197, 198]. TAM-derived VEGF-A-induced vascular leakiness is a key factor involved in distant seeding of cancer cells and metastatic spread [16]. Indeed, VEGF-A deletion specifically in monocytes inhibits the efficiency of mammary cancer cell seeding in the lung, without affecting monocyte recruitment into the secondary site. Furthermore, SRC KO and DEP-1 KO mice have less metastatic spread than WT mice [198]. Vascular leakage is also enhanced by monocyte-derived MMP-9 and via monocyte-induced EC retraction in an E-selectin-dependent manner [183, 199]. In 3D in vitro model, monocyte MMP-9 secretion induces EC tight junction zonula occludens-1 (ZO-1) and occludin disruption, thus enhancing cancer cell extravasation [183]. Accordingly, in murine breast cancers, monocyte/macrophages are major MMP-9 producers and have a strong impact on cancer cell extravasation since MMP-9 expression and cancer extravasation are strongly reduced in tumor mice ablated of CCR2+ inflammatory monocytes. Moreover, in co-culture experiments, monocytes promote EC permeability and VE-cadherin dephosphorylation, which sustains cancer cell extravasation. This is dependent on monocyte interaction with EC E-selectin since this is not observed with monocytes lacking E-selectin ligands or with ECs from E-selectin KO mice. Cancer cell injection into mice induces lung vessel permeability, which depends on monocytes since this is not observed in mice depleted of monocytes. Furthermore, upon extravasation, TAMs are involved in cancer cell invasion and seeding in the ECM. Indeed, in 3D in vitro model, cancer cell invasion and migration are enhanced by pre-invaded macrophages [183]. Furthermore, in vivo, breast cancer cell pulmonary seeding is blocked by three different methods of macrophage depletion and by monocyte recruitment inhibition by CCL2 blockade [16, 184]. In conclusion, TAMs and monocytes are strong factors involved in the promotion of cancer cell extravasation, cancer cell seeding, and thereby distant metastasis.
Murine non-classical monocytes prevent lung metastasis
Murine non-classical monocyte (CX3CR1high/Ly6Clo) differentiation and survival depend on the orphan nuclear receptor Nr4a1, and hence, Nr4a1 KO mice have drastically less non-classical monocytes without affecting IM or macrophage population. Non-classical monocytes prevent lung metastasis formation in the PyMT breast cancer murine model or induced by LLc or B16-F10 cancer cells injected intravenously [24, 185, 186, 200]. Indeed, more lung metastases are observed in non-classical monocyte–depleted Nr4a1 KO mice upon cancer cells injected intravenously, and this is counteracted by Ly6Clo monocyte injection [24]. Furthermore, PyMT mice transplanted with bone marrow from Nr4a1 KO mice show drastically more lung metastases than mice transplanted with WT bone marrow, without affecting primary tumor growth [24]. Upon cancer cell injection, non-classical monocytes interact with cancer cells in a CX3CR1-dependent manner, infiltrate the lung, engulf cancer cell material, and promote NK cell recruitment. These processes are responsible for the inhibition of lung metastases [24]. Non-classical monocyte infiltration into the lungs depends on Kindlin-3 since specific Kindlin-3 deletion in non-classical monocytes diminished their lung infiltration after cancer cell injection. This diminution is associated with an increase in lung metastases [200]. The interactions between cancer cells and non-classical monocytes and subsequent cancer cell material engulfment by non-classical monocytes depend on CX3CR1 expression in non-classical monocytes since these processes are decreased in CX3CR1 KO mice [24]. The NK cell recruitment is induced by non-classical monocytes via IL-15 secretion. Indeed, B16F-10 primary melanoma tumors induce NK cell recruitment into the lungs which is abolished with non-classical monocyte depletion or IL-15 inhibition [186]. Non-classical monocytes are high IL-15 producers, and this secretion is enhanced by primary tumors [186]. Moreover, non-classical monocytes enhance NK cell activation, notably by increasing their stimulatory receptor expression and by diminishing their inhibitory receptor expression [185]. Non-classical monocytes prevent lung metastases also via targeting exosomal content from primary tumors [201, 202]. Non-classical monocyte infiltration in lungs is enhanced by BAG6-presenting exosomes and by non-metastatic A375 melanoma cell line exosomes [201, 202].
In conclusion, several murine studies showed that murine non-classical monocytes are involved in the prevention of metastasis. Since some differences are observed between human and mouse monocytes [203, 204], it would be very interesting to confirm/correlate the results with studies performed with human monocytes.
TAMs promote tumor vessel abnormalization
Tumor blood vessels are abnormal, which means that they have higher permeability, less pericytes, and poor architectural network, functionality, and perfusion enhancing tumor hypoxia and acidosis. Furthermore, the decrease in blood perfusion observed in abnormal tumor vessels is responsible for the decrease in the delivery of chemotherapeutic drugs within the tumor. Tumor vessel “abnormalization” is the process by which blood vessels become abnormal. TAM promotion of vessel “abnormalization” is involved in metastasis notably by promoting cancer cell intravasation and extravasation. M2 TAMs and VEGF and PlGF secretion by TAMs are involved in the tumor blood vessel abnormalization. This abnormalization is characterized by a decrease in pericyte-covered vessels and vessel perfusion, associated with an increase in EC gaps and tumor hypoxia [130, 205]. The deletion of VEGF specifically in myeloid cells (i.e., TAMs and neutrophils) decreases tumor angiogenesis and promotes vascular normalization characterized by an increase in pericyte coverage associated with a decrease in vessel permeability. Furthermore, histidin-rich glycoprotein (HRG) drastically reduces hepatocellular carcinoma (HCC) metastasis via the inhibition of M2 TAM polarization and PlGF expression in TAMs. HRG has no effect on TAM-depleted tumors or on tumors with PlGF KO TAMs. Tumor blood vessel abnormalization and metastasis are markedly inhibited in mice with PlGF KO TAMs [130]. Interestingly, the blood vessel normalization is proposed as an emerging concept in antiangiogenic therapy since 2005 [206]. In conclusion, TAMs are strongly involved in angiogenesis induction and promotion. Blood vessels whose creation is induced by TAMs are abnormal, notably because of TAM-derived VEGF and PlGF which decrease the coverage of tumor blood vessel with pericytes, and thus promoting tumor vessel permeability and tumor metastasis.
Interestingly, the pro-metastatic activity of TAMs in hypoxia areas is regulated by metabolism. Indeed, the glycolysis and glucose uptake by TAMs are regulated by DNA damage responses 1 (REDD1). REDD1 deletion specifically in TAMs enhances the glucose uptake as well as glycolysis in TAMs. This effect induces glucose competition with ECs leading to vessel normalization and metastasis inhibition [207]. Indeed, REDD1 deletion in TAMs increases glycolytic metabolism in TAMs in vitro, inhibits metastasis in multiple mouse tumor models, and induces vessel normalization characterized by an increase in tumor blood vessel pericyte coverage and tumor perfusion. These effects of REDD1 deletion depend on the increase in glycolytic metabolism in TAMs since they are abolished when the increase in TAM glycolytic metabolism is abolished with the glycolytic activator PFKB3 deletion [207]. In conclusion, glucose competition between tumor ECs and TAMs regulates blood vessel features. High glucose consumption by TAMs reduces glucose availability to ECs and allows the formation of a mature and poorly metastatic vascular network. On the other hand, low glucose consumption by TAMs allows high glucose uptake by ECs which allows the formation of immature, abnormal, and pro-metastatic leaky vessels.
Discussion
There are strong reciprocal interactions between tumor monocytes/macrophages and tumor blood/lymphatic vessels. TAMs and TEMs are involved in angiogenesis, in lymphangiogenesis, and in multiple metastasis steps, whereas blood vessels are involved in the recruitment of monocytes/macrophages/TEMs into tumors and in macrophage polarization into M2 pro-tumoral phenotype. In the last few years, many discoveries have been made about the effects of blood vessels on the polarization of macrophages, although research is still needed. IMs promote tumor growth and metastasis. Conversely, murine non-classical monocytes prevent lung metastasis, whereas human non-classical monocytes promote angiogenesis in vitro. Since human and murine monocytes have functional differences, it would be interesting to better understand how these monocytes prevent metastasis and to confirm that human non-classical monocytes have similar effects on tumor metastasis. Furthermore, it would be interesting to know the impact of non-classical monocytes on tumor angiogenesis in vivo and to better understand mechanisms regulating their infiltration into tumors. The improvement of the knowledge of the physiology of tumor blood vessels and TAMs led to the development of several therapies. Some therapy targets only TAMs with the aims to diminish TAM survival and TAM recruitment (CCL2/CCR2 or CSF1/CSF1R inhibition) or to induce a reprogramming of TAMs from M2 phenotype towards M1 phenotype [208, 209]. On the other hand, some therapies target only tumor blood vessels and aim to inhibit angiogenesis, to improve endothelial junctional integrity, to improve tumor perfusion, or to promote vascular normalization [210]. More recently, a lot of researches have been performed about the combination of anti-angiogenic drugs and immunotherapies, and some of them are currently in clinical trials (reviewed in [211, 212]). Anti-angiogenic therapies have beneficial effects on immunotherapy, and inversely. More related to this review, the combination of Ang-2 and VEGF inhibition induces the normalization of the tumor vasculature and promotes TAM reprogramming from M2 toward M1 phenotype and hence increases the M1/M2 ratio and the overall survival in sarcoma and GBM murine models [81, 213, 214]. More recently, the dual Ang-2/VEGF inhibition has been combined with CD40 or PD-1 immune therapies and showed strong synergistic effects in terms of tumor growth, overall survival, and immune cell activation in several murine tumor models [94, 215]. Interestingly, the combination of Ang-2/VEGF with PD-L1 or CD40 immunotherapies are currently in clinical trials (NCT01688206; NCT02665416). In conclusion, the improved knowledge in tumor-associated monocyte/macrophage and tumor blood vessels leads to the development of new promising and innovative therapeutic strategies which could enhance patient overall survival. Nonetheless, research on this topic is still needed in order to improve patient outcome and to diminish adverse effects of the treatments.
Abbreviations
- ADAM
a disintegrin and metalloproteinase domain–containing protein
- ADM
adrenomedullin
- Ang-2
angiopoietin-2
- AP-1
activator protein-1
- Arg-1
arginase-1
- ASK1
apoptosis signal-regulating kinase 1
- BMDM
bone marrow–derived macrophages
- CAF
cancer-associated fibroblast
- CCL
chemokine (C-C motif) ligand
- CCR2
C-C chemokine receptor type 2
- CD
cluster of differentiation
- CSF1
colony-stimulating factor 1
- CSF1R
CSF1 receptor
- CTHRC1
collagen triple helix repeat containing 1
- CX3CL1
chemokine (C-X3-C motif) ligand 1
- CX3CR1
CX3CL1 receptor
- CXCR4
C-X-C chemokine receptor type 4
- EC
endothelial cell
- ECM
extracellular matrix
- Egfl7
EGF-like domain–containing protein 7
- EMAP-II
endothelial monocyte–activating polypeptide-II
- EndMT
endothelial-to-mesenchymal transition
- ERK
extracellular signal–regulated kinase
- ET
endothelin
- FGF-2
fibroblast growth factor-2
- GAL8
galectin 8
- GBM
glioblastoma
- HIF-1α
hypoxia-inducible factor-1α
- HIF-2α
hypoxia-inducible factor-2α
- HRG
histidin-rich glycoprotein
- HSP90α
heat shock protein 90 α
- ICAM1
intercellular adhesion molecule 1
- IFIT1
interferon-induced protein with tetratricopeptide repeats 1
- IGF1
insulin-like growth factor 1
- IL
interleukin
- IM
inflammatory monocyte
- IRE1α
inositol-requiring enzyme 1α
- JNK
c-Jun N-terminal kinases
- KO
knockout
- LCN2
lipocalin 2
- LEC
lymphatic endothelial cell
- LLc
Lewis lung carcinoma
- LYVE 1
lymphatic vessel hyaluronic receptor 1
- MCT1
monocarboxylate transporter 1
- MenaINV
invasive isoform of mammalian enabled protein
- MFG-E8
milk fat globule-epidermal growth factor 8
- MHCII
major histocompatibility complex class II
- M-LECP
myeloid-lymphatic endothelial cell progenitors
- MMP
matrix metalloprotease
- MSC
mesenchymal stem cell
- mTOR
mechanistic target of rapamycin
- NG2
neural glial antigen 2
- NK
natural killer
- p38MAPK
p38 mitogen–activated protein kinase
- PDAC
pancreatic ductal adenocarcinoma
- PDGF
platelet-derived growth factor
- PDGFRβ
PDGF receptor β
- PD-1
programmed cell death 1
- PD-L1
programmed death-ligand 1
- PI3K
phosphoinositide 3-kinase
- PlGF
placental growth factor
- PoEM
podoplanin-expressing macrophage
- REDD1
regulated in development and DNA damage responses 1
- SDF1α
stromal cell–derived factor 1α
- Sema4D
semaphoring 4D
- SOCS1
suppressor of cytokine signaling 1
- TAM
tumor-associated macrophage
- TCF12
transcription factor 12
- TEM
Tie2-expressing monocyte
- TGF-β
transforming growth factor β
- TIMP
tissue inhibitor of metalloproteinase
- TME
tumor microenvironment
- TMEM
tumor microenvironment of metastasis
- TNFα
tumor necrosis factor α
- TRM
tissue-resident macrophage
- VCAM1
vascular cell adhesion molecule 1
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
- WT
wild-type
- ZO-1
zonula occudens-1
Author contribution
V.D wrote the whole manuscript and designed the figures. C.M supervised the entire work and critically revised the paper.
Funding
Victor Delprat is financed by a Televie grant (FNRS- National Funds for Scientific Research, Belgium).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aras S, Zaidi MR. TAMeless traitors: macrophages in cancer progression and metastasis. British Journal of Cancer. 2017;117(11):1583–1591. doi: 10.1038/bjc.2017.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6(3):1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez LR, Borriello L, Entenberg D, Condeelis JS, Oktay MH, Karagiannis GS. The emerging roles of macrophages in cancer metastasis and response to chemotherapy. Journal of Leukocyte Biology. 2019;106(2):259–274. doi: 10.1002/JLB.MR0218-056RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis CE, Harney AS, Pollard JW. The Multifaceted role of perivascular macrophages in tumors. Cancer Cell. 2016;30(2):365. doi: 10.1016/j.ccell.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Frontiers in Physiology. 2014;5:75. doi: 10.3389/fphys.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Delprat V, Tellier C, Demazy C, Raes M, Feron O, Michiels C. Cycling hypoxia promotes a pro-inflammatory phenotype in macrophages via JNK/p65 signaling pathway. Scientific Reports. 2020;10(1):882. doi: 10.1038/s41598-020-57677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. 2019;35(4):588–602. doi: 10.1016/j.ccell.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature Reviews. Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 10.Wang HW, Joyce JA. Alternative activation of tumor-associated macrophages by IL-4: priming for protumoral functions. Cell Cycle. 2010;9(24):4824–4835. doi: 10.4161/cc.9.24.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, et al. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kgamma to promote pancreatic cancer metastasis. Cancer Research. 2018;78(16):4586–4598. doi: 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, He Z, Huang M, Liu T, Wang Y, Xu H, et al. Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2alpha. Nature Communications. 2018;9(1):559. doi: 10.1038/s41467-018-03050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tripathi C, Tewari BN, Kanchan RK, Baghel KS, Nautiyal N, Shrivastava R, et al. Macrophages are recruited to hypoxic tumor areas and acquire a pro-angiogenic M2-polarized phenotype via hypoxic cancer cell derived cytokines Oncostatin M and Eotaxin. Oncotarget. 2014;5(14):5350–5368. doi: 10.18632/oncotarget.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dehne N, Mora J, Namgaladze D, Weigert A, Brune B. Cancer cell and macrophage cross-talk in the tumor microenvironment. Current Opinion in Pharmacology. 2017;35:12–19. doi: 10.1016/j.coph.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Franklin RA, Li MO. Ontogeny of tumor-associated macrophages and its implication in cancer regulation. Trends Cancer. 2016;2(1):20–34. doi: 10.1016/j.trecan.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arwert EN, Harney AS, Entenberg D, Wang Y, Sahai E, Pollard JW, et al. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Reports. 2018;23(5):1239–1248. doi: 10.1016/j.celrep.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, et al. Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity. 2017;47(3):597. doi: 10.1016/j.immuni.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. Journal of Leukocyte Biology. 2019;106(2):309–322. doi: 10.1002/JLB.4RI0818-311R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf AA, Yanez A, Barman PK, Goodridge HS. The ontogeny of monocyte subsets. Frontiers in Immunology. 2019;10:1642. doi: 10.3389/fimmu.2019.01642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidibe A, Ropraz P, Jemelin S, Emre Y, Poittevin M, Pocard M, et al. Angiogenic factor-driven inflammation promotes extravasation of human proangiogenic monocytes to tumours. Nature Communications. 2018;9(1):355. doi: 10.1038/s41467-017-02610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung K, Heishi T, Khan OF, Kowalski PS, Incio J, Rahbari NN, et al. Ly6Clo monocytes drive immunosuppression and confer resistance to anti-VEGFR2 cancer therapy. The Journal of Clinical Investigation. 2017;127(8):3039–3051. doi: 10.1172/JCI93182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung K, Heishi T, Incio J, Huang Y, Beech EY, Pinter M, et al. Targeting CXCR4-dependent immunosuppressive Ly6C(low) monocytes improves antiangiogenic therapy in colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(39):10455–10460. doi: 10.1073/pnas.1710754114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, et al. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350(6263):985–990. doi: 10.1126/science.aac9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nature Medicine. 2003;9(6):789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 26.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8(3):211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Drescher, F., Juarez, P., Arellano, D. L., Serafin-Higuera, N., Olvera-Rodriguez, F., Jimenez, S., et al. (2020). TIE2 induces breast cancer cell dormancy and inhibits the development of osteolytic bone metastases. Cancers (Basel), 12(4). 10.3390/cancers12040868. [DOI] [PMC free article] [PubMed]
- 28.Wang J, Wu K, Zhang D, Tang H, Xie H, Hong L, et al. Expressions and clinical significances of angiopoietin-1, -2 and Tie2 in human gastric cancer. Biochemical and Biophysical Research Communications. 2005;337(1):386–393. doi: 10.1016/j.bbrc.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 29.Matsubara T, Kanto T, Kuroda S, Yoshio S, Higashitani K, Kakita N, et al. TIE2-expressing monocytes as a diagnostic marker for hepatocellular carcinoma correlates with angiogenesis. Hepatology. 2013;57(4):1416–1425. doi: 10.1002/hep.25965. [DOI] [PubMed] [Google Scholar]
- 30.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nature Reviews. Cancer. 2003;3(6):401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 31.Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Seminars in Cancer Biology. 2009;19(5):329–337. doi: 10.1016/j.semcancer.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Research. 2006;66(23):11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 33.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 34.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(33):12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nature Reviews. Cancer. 2014;14(3):159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 36.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nature Medicine. 2001;7(2):186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 37.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nature Medicine. 2001;7(2):192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 38.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. The EMBO Journal. 2001;20(4):672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ran S, Montgomery KE. Macrophage-mediated lymphangiogenesis: the emerging role of macrophages as lymphatic endothelial progenitors. Cancers (Basel) 2012;4(3):618–657. doi: 10.3390/cancers4030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mumprecht V, Detmar M. Lymphangiogenesis and cancer metastasis. Journal of Cellular and Molecular Medicine. 2009;13(8A):1405–1416. doi: 10.1111/j.1582-4934.2009.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singhal, S., Stadanlick, J., Annunziata, M. J., Rao, A. S., Bhojnagarwala, P. S., O'Brien, S., et al. (2019). Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Science Translational Medicine, 11(479). 10.1126/scitranslmed.aat1500. [DOI] [PMC free article] [PubMed]
- 42.Liou GY, Bastea L, Fleming A, Doppler H, Edenfield BH, Dawson DW, et al. The presence of interleukin-13 at pancreatic ADM/PanIN lesions alters macrophage populations and mediates pancreatic tumorigenesis. Cell Reports. 2017;19(7):1322–1333. doi: 10.1016/j.celrep.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;7:19. doi: 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeuchi H, Tanaka M, Tanaka A, Tsunemi A, Yamamoto H. Predominance of M2-polarized macrophages in bladder cancer affects angiogenesis, tumor grade and invasiveness. Oncology Letters. 2016;11(5):3403–3408. doi: 10.3892/ol.2016.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goswami, D., & Vestweber, D. (2016). How leukocytes trigger opening and sealing of gaps in the endothelial barrier. F1000Res, 5. 10.12688/f1000research.9185.1. [DOI] [PMC free article] [PubMed]
- 46.De Sanctis F, Ugel S, Facciponte J, Facciabene A. The dark side of tumor-associated endothelial cells. Seminars in Immunology. 2018;35:35–47. doi: 10.1016/j.smim.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Wu NZ, Klitzman B, Dodge R, Dewhirst MW. Diminished leukocyte-endothelium interaction in tumor microvessels. Cancer Research. 1992;52(15):4265–4268. [PubMed] [Google Scholar]
- 48.Pinte S, Caetano B, Le Bras A, Havet C, Villain G, Dernayka R, et al. Endothelial cell activation is regulated by epidermal growth factor-like domain 7 (Egfl7) during inflammation. The Journal of Biological Chemistry. 2016;291(46):24017–24028. doi: 10.1074/jbc.M116.731331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delfortrie S, Pinte S, Mattot V, Samson C, Villain G, Caetano B, et al. Egfl7 promotes tumor escape from immunity by repressing endothelial cell activation. Cancer Research. 2011;71(23):7176–7186. doi: 10.1158/0008-5472.CAN-11-1301. [DOI] [PubMed] [Google Scholar]
- 50.Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ, Qiao H, Fahl SP, et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood. 2012;120(24):4772–4782. doi: 10.1182/blood-2012-04-427013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. European Journal of Immunology. 2003;33(11):3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 52.Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nature Medicine. 2014;20(6):607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mulligan JK, Young MR. Tumors induce the formation of suppressor endothelial cells in vivo. Cancer Immunology, Immunotherapy. 2010;59(2):267–277. doi: 10.1007/s00262-009-0747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nature Immunology. 2014;15(5):423–430. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 56.Yu X, Sha J, Xiang S, Qin S, Conrad P, Ghosh SK, et al. Suppression of KSHV-induced angiopoietin-2 inhibits angiogenesis, infiltration of inflammatory cells, and tumor growth. Cell Cycle. 2016;15(15):2053–2065. doi: 10.1080/15384101.2016.1196303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scholz A, Harter PN, Cremer S, Yalcin BH, Gurnik S, Yamaji M, et al. Endothelial cell-derived angiopoietin-2 is a therapeutic target in treatment-naive and bevacizumab-resistant glioblastoma. EMBO Mol Med. 2016;8(1):39–57. doi: 10.15252/emmm.201505505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Research. 2010;70(13):5270–5280. doi: 10.1158/0008-5472.CAN-10-0012. [DOI] [PubMed] [Google Scholar]
- 59.Huang H, Lai JY, Do J, Liu D, Li L, Del Rosario J, et al. Specifically targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing monocyte infiltration, and tumor growth. Clinical Cancer Research. 2011;17(5):1001–1011. doi: 10.1158/1078-0432.CCR-10-2317. [DOI] [PubMed] [Google Scholar]
- 60.Karikoski M, Marttila-Ichihara F, Elima K, Rantakari P, Hollmen M, Kelkka T, et al. Clever-1/stabilin-1 controls cancer growth and metastasis. Clinical Cancer Research. 2014;20(24):6452–6464. doi: 10.1158/1078-0432.CCR-14-1236. [DOI] [PubMed] [Google Scholar]
- 61.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, et al. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. The Journal of Experimental Medicine. 2003;197(12):1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donners MM, Wolfs IM, Olieslagers S, Mohammadi-Motahhari Z, Tchaikovski V, Heeneman S, et al. A disintegrin and metalloprotease 10 is a novel mediator of vascular endothelial growth factor-induced endothelial cell function in angiogenesis and is associated with atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(11):2188–2195. doi: 10.1161/ATVBAHA.110.213124. [DOI] [PubMed] [Google Scholar]
- 63.Swendeman S, Mendelson K, Weskamp G, Horiuchi K, Deutsch U, Scherle P, et al. VEGF-A stimulates ADAM17-dependent shedding of VEGFR2 and crosstalk between VEGFR2 and ERK signaling. Circulation Research. 2008;103(9):916–918. doi: 10.1161/CIRCRESAHA.108.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Y, Andersson P, Hosaka K, Zhang Y, Cao R, Iwamoto H, et al. The PDGF-BB-SOX7 axis-modulated IL-33 in pericytes and stromal cells promotes metastasis through tumour-associated macrophages. Nature Communications. 2016;7:11385. doi: 10.1038/ncomms11385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He H, Xu J, Warren CM, Duan D, Li X, Wu L, et al. Endothelial cells provide an instructive niche for the differentiation and functional polarization of M2-like macrophages. Blood. 2012;120(15):3152–3162. doi: 10.1182/blood-2012-04-422758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fan CS, Chen LL, Hsu TA, Chen CC, Chua KV, Li CP, et al. Endothelial-mesenchymal transition harnesses HSP90alpha-secreting M2-macrophages to exacerbate pancreatic ductal adenocarcinoma. Journal of Hematology & Oncology. 2019;12(1):138. doi: 10.1186/s13045-019-0826-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi SH, Kim AR, Nam JK, Kim JM, Kim JY, Seo HR, et al. Tumour-vasculature development via endothelial-to-mesenchymal transition after radiotherapy controls CD44v6(+) cancer cell and macrophage polarization. Nature Communications. 2018;9(1):5108. doi: 10.1038/s41467-018-07470-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andersson, P., Yang, Y., Hosaka, K., Zhang, Y., Fischer, C., Braun, H., et al. (2018). Molecular mechanisms of IL-33-mediated stromal interactions in cancer metastasis. JCI Insight, 3(20). 10.1172/jci.insight.122375. [DOI] [PMC free article] [PubMed]
- 69.Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. The Journal of Clinical Investigation. 2016;126(10):3672–3679. doi: 10.1172/JCI84427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104(8):2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 71.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nature Reviews. Molecular Cell Biology. 2009;10(3):165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 72.Saharinen P, Eklund L, Alitalo K. Therapeutic targeting of the angiopoietin-TIE pathway. Nature Reviews. Drug Discovery. 2017;16(9):635–661. doi: 10.1038/nrd.2016.278. [DOI] [PubMed] [Google Scholar]
- 73.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103(11):4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 74.Lee J, Song J, Kwon ES, Jo S, Kang MK, Kim YJ, et al. CTHRC1 promotes angiogenesis by recruiting Tie2-expressing monocytes to pancreatic tumors. Experimental & Molecular Medicine. 2016;48(9):e261. doi: 10.1038/emm.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pergolizzi M, Bizzozero L, Riccitelli E, Pascal D, Samarelli AV, Bussolino F, et al. Modulation of angiopoietin 2 release from endothelial cells and angiogenesis by the synaptic protein Neuroligin 2. Biochemical and Biophysical Research Communications. 2018;501(1):165–171. doi: 10.1016/j.bbrc.2018.04.204. [DOI] [PubMed] [Google Scholar]
- 76.Cortes-Santiago N, Hossain MB, Gabrusiewicz K, Fan X, Gumin J, Marini FC, et al. Soluble Tie2 overrides the heightened invasion induced by anti-angiogenesis therapies in gliomas. Oncotarget. 2016;7(13):16146–16157. doi: 10.18632/oncotarget.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Srivastava K, Hu J, Korn C, Savant S, Teichert M, Kapel SS, et al. Postsurgical adjuvant tumor therapy by combining anti-angiopoietin-2 and metronomic chemotherapy limits metastatic growth. Cancer Cell. 2014;26(6):880–895. doi: 10.1016/j.ccell.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 78.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19(4):512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 79.Rathnakumar K, Savant S, Giri H, Ghosh A, Fisslthaler B, Fleming I, et al. Angiopoietin-2 mediates thrombin-induced monocyte adhesion and endothelial permeability. Journal of Thrombosis and Haemostasis. 2016;14(8):1655–1667. doi: 10.1111/jth.13376. [DOI] [PubMed] [Google Scholar]
- 80.Rigamonti N, Kadioglu E, Keklikoglou I, Wyser Rmili C, Leow CC, De Palma M. Role of angiopoietin-2 in adaptive tumor resistance to VEGF signaling blockade. Cell Reports. 2014;8(3):696–706. doi: 10.1016/j.celrep.2014.06.059. [DOI] [PubMed] [Google Scholar]
- 81.Kloepper J, Riedemann L, Amoozgar Z, Seano G, Susek K, Yu V, et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(16):4476–4481. doi: 10.1073/pnas.1525360113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chae SS, Kamoun WS, Farrar CT, Kirkpatrick ND, Niemeyer E, de Graaf AM, et al. Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas. Clinical Cancer Research. 2010;16(14):3618–3627. doi: 10.1158/1078-0432.CCR-09-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hidalgo M, Martinez-Garcia M, Le Tourneau C, Massard C, Garralda E, Boni V, et al. First-in-human phase I study of single-agent vanucizumab, a first-in-class bispecific anti-angiopoietin-2/anti-VEGF-A antibody, in adult patients with advanced solid tumors. Clinical Cancer Research. 2018;24(7):1536–1545. doi: 10.1158/1078-0432.CCR-17-1588. [DOI] [PubMed] [Google Scholar]
- 84.Fang M, Li Y, Huang K, Qi S, Zhang J, Zgodzinski W, et al. IL33 promotes colon cancer cell stemness via JNK activation and macrophage recruitment. Cancer Research. 2017;77(10):2735–2745. doi: 10.1158/0008-5472.CAN-16-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamada K, Uchiyama A, Uehara A, Perera B, Ogino S, Yokoyama Y, et al. MFG-E8 drives melanoma growth by stimulating mesenchymal stromal cell-induced angiogenesis and M2 polarization of tumor-associated macrophages. Cancer Research. 2016;76(14):4283–4292. doi: 10.1158/0008-5472.CAN-15-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sugano G, Bernard-Pierrot I, Lae M, Battail C, Allory Y, Stransky N, et al. Milk fat globule--epidermal growth factor--factor VIII (MFGE8)/lactadherin promotes bladder tumor development. Oncogene. 2011;30(6):642–653. doi: 10.1038/onc.2010.446. [DOI] [PubMed] [Google Scholar]
- 87.Kzhyshkowska J, Gratchev A, Goerdt S. Stabilin-1, a homeostatic scavenger receptor with multiple functions. Journal of Cellular and Molecular Medicine. 2006;10(3):635–649. doi: 10.1111/j.1582-4934.2006.tb00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yin, M., Zhou, H. J., Zhang, J., Lin, C., Li, H., Li, X., et al. (2017). ASK1-dependent endothelial cell activation is critical in ovarian cancer growth and metastasis. JCI Insight, 2(18). 10.1172/jci.insight.91828. [DOI] [PMC free article] [PubMed]
- 89.He Y, Zhang W, Zhang R, Zhang H, Min W. SOCS1 inhibits tumor necrosis factor-induced activation of ASK1-JNK inflammatory signaling by mediating ASK1 degradation. The Journal of Biological Chemistry. 2006;281(9):5559–5566. doi: 10.1074/jbc.M512338200. [DOI] [PubMed] [Google Scholar]
- 90.Conroy MJ, Lysaght J. CX3CL1 signaling in the tumor microenvironment. Advances in Experimental Medicine and Biology. 2020;1231:1–12. doi: 10.1007/978-3-030-36667-4_1. [DOI] [PubMed] [Google Scholar]
- 91.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385(6617):640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 92.Schwarz N, Pruessmeyer J, Hess FM, Dreymueller D, Pantaler E, Koelsch A, et al. Requirements for leukocyte transmigration via the transmembrane chemokine CX3CL1. Cellular and Molecular Life Sciences. 2010;67(24):4233–4248. doi: 10.1007/s00018-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang CY, Li MC, Liao SL, Huang YL, Shen CC, Pan HC. Prognostic and clinical implication of IL-6 expression in glioblastoma multiforme. Journal of Clinical Neuroscience. 2005;12(8):930–933. doi: 10.1016/j.jocn.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 94.Schmittnaegel, M., Rigamonti, N., Kadioglu, E., Cassara, A., Wyser Rmili, C., Kiialainen, A., et al. (2017). Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Science Translational Medicine, 9(385). 10.1126/scitranslmed.aak9670. [DOI] [PubMed]
- 95.Platel V, Faure S, Corre I, Clere N. Endothelial-to-mesenchymal transition (EndoMT): roles in tumorigenesis, metastatic extravasation and therapy resistance. Journal of Oncology. 2019;2019:8361945. doi: 10.1155/2019/8361945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Research. 2007;67(21):10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 97.Krizbai IA, Gasparics A, Nagyoszi P, Fazakas C, Molnar J, Wilhelm I, et al. Endothelial-mesenchymal transition of brain endothelial cells: possible role during metastatic extravasation. PLoS One. 2015;10(3):e0123845. doi: 10.1371/journal.pone.0123845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fan CS, Chen WS, Chen LL, Chen CC, Hsu YT, Chua KV, et al. Osteopontin-integrin engagement induces HIF-1alpha-TCF12-mediated endothelial-mesenchymal transition to exacerbate colorectal cancer. Oncotarget. 2018;9(4):4998–5015. doi: 10.18632/oncotarget.23578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Motegi S, Leitner WW, Lu M, Tada Y, Sardy M, Wu C, et al. Pericyte-derived MFG-E8 regulates pathologic angiogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(9):2024–2034. doi: 10.1161/ATVBAHA.111.232587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li BZ, Zhang HY, Pan HF, Ye DQ. Identification of MFG-E8 as a novel therapeutic target for diseases. Expert Opinion on Therapeutic Targets. 2013;17(11):1275–1285. doi: 10.1517/14728222.2013.829455. [DOI] [PubMed] [Google Scholar]
- 101.Brissette MJ, Lepage S, Lamonde AS, Sirois I, Groleau J, Laurin LP, et al. MFG-E8 released by apoptotic endothelial cells triggers anti-inflammatory macrophage reprogramming. PLoS One. 2012;7(4):e36368. doi: 10.1371/journal.pone.0036368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang X, Zhu Q, Lin Y, Wu L, Wu X, Wang K, et al. Crosstalk between TEMs and endothelial cells modulates angiogenesis and metastasis via IGF1-IGF1R signalling in epithelial ovarian cancer. British Journal of Cancer. 2017;117(9):1371–1382. doi: 10.1038/bjc.2017.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes & Development. 2010;24(3):241–255. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. The Journal of Clinical Investigation. 2004;114(5):623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gabrusiewicz K, Liu D, Cortes-Santiago N, Hossain MB, Conrad CA, Aldape KD, et al. Anti-vascular endothelial growth factor therapy-induced glioma invasion is associated with accumulation of Tie2-expressing monocytes. Oncotarget. 2014;5(8):2208–2220. doi: 10.18632/oncotarget.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature Cell Biology. 2000;2(10):737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hawinkels LJ, Zuidwijk K, Verspaget HW, de Jonge-Muller ES, van Duijn W, Ferreira V, et al. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. European Journal of Cancer. 2008;44(13):1904–1913. doi: 10.1016/j.ejca.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 108.Wang B, Sun J, Kitamoto S, Yang M, Grubb A, Chapman HA, et al. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. The Journal of Biological Chemistry. 2006;281(9):6020–6029. doi: 10.1074/jbc.M509134200. [DOI] [PubMed] [Google Scholar]
- 109.Malla RR, Gopinath S, Gondi CS, Alapati K, Dinh DH, Gujrati M, et al. Cathepsin B and uPAR knockdown inhibits tumor-induced angiogenesis by modulating VEGF expression in glioma. Cancer Gene Therapy. 2011;18(6):419–434. doi: 10.1038/cgt.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sevenich L, Werner F, Gajda M, Schurigt U, Sieber C, Muller S, et al. Transgenic expression of human cathepsin B promotes progression and metastasis of polyoma-middle-T-induced breast cancer in mice. Oncogene. 2011;30(1):54–64. doi: 10.1038/onc.2010.387. [DOI] [PubMed] [Google Scholar]
- 111.Seftor RE, Hess AR, Seftor EA, Kirschmann DA, Hardy KM, Margaryan NV, et al. Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. The American Journal of Pathology. 2012;181(4):1115–1125. doi: 10.1016/j.ajpath.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Treps L, Faure S, Clere N. Vasculogenic mimicry, a complex and devious process favoring tumorigenesis - interest in making it a therapeutic target. Pharmacology & Therapeutics. 2021;223:107805. doi: 10.1016/j.pharmthera.2021.107805. [DOI] [PubMed] [Google Scholar]
- 113.Zhang L, Xu Y, Sun J, Chen W, Zhao L, Ma C, et al. M2-like tumor-associated macrophages drive vasculogenic mimicry through amplification of IL-6 expression in glioma cells. Oncotarget. 2017;8(1):819–832. doi: 10.18632/oncotarget.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rong X, Huang B, Qiu S, Li X, He L, Peng Y. Tumor-associated macrophages induce vasculogenic mimicry of glioblastoma multiforme through cyclooxygenase-2 activation. Oncotarget. 2016;7(51):83976–83986. doi: 10.18632/oncotarget.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barnett FH, Rosenfeld M, Wood M, Kiosses WB, Usui Y, Marchetti V, et al. Macrophages form functional vascular mimicry channels in vivo. Scientific Reports. 2016;6:36659. doi: 10.1038/srep36659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. The American Journal of Pathology. 2002;161(3):947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ji RC. Macrophages are important mediators of either tumor- or inflammation-induced lymphangiogenesis. Cellular and Molecular Life Sciences. 2012;69(6):897–914. doi: 10.1007/s00018-011-0848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jung M, Oren B, Mora J, Mertens C, Dziumbla S, Popp R, et al. Lipocalin 2 from macrophages stimulated by tumor cell-derived sphingosine 1-phosphate promotes lymphangiogenesis and tumor metastasis. Sci Signal. 2016;9(434):ra64. doi: 10.1126/scisignal.aaf3241. [DOI] [PubMed] [Google Scholar]
- 119.Bieniasz-Krzywiec P, Martin-Perez R, Ehling M, Garcia-Caballero M, Pinioti S, Pretto S, et al. Podoplanin-expressing macrophages promote lymphangiogenesis and lymphoinvasion in breast cancer. Cell Metabolism. 2019;30(5):917–936. doi: 10.1016/j.cmet.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Elder AM, Tamburini BAJ, Crump LS, Black SA, Wessells VM, Schedin PJ, et al. Semaphorin 7A promotes macrophage-mediated lymphatic remodeling during postpartum mammary gland involution and in breast cancer. Cancer Research. 2018;78(22):6473–6485. doi: 10.1158/0008-5472.CAN-18-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Evans R, Flores-Borja F, Nassiri S, Miranda E, Lawler K, Grigoriadis A, et al. Integrin-mediated macrophage adhesion promotes lymphovascular dissemination in breast cancer. Cell Reports. 2019;27(7):1967–1978. doi: 10.1016/j.celrep.2019.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Volk-Draper L, Patel R, Bhattarai N, Yang J, Wilber A, DeNardo D, et al. Myeloid-derived lymphatic endothelial cell progenitors significantly contribute to lymphatic metastasis in clinical breast cancer. The American Journal of Pathology. 2019;189(11):2269–2292. doi: 10.1016/j.ajpath.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bron S, Henry L, Faes-Van't Hull E, Turrini R, Vanhecke D, Guex N, et al. TIE-2-expressing monocytes are lymphangiogenic and associate specifically with lymphatics of human breast cancer. Oncoimmunology. 2016;5(2):e1073882. doi: 10.1080/2162402X.2015.1073882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cellular and Molecular Life Sciences. 2020;77(9):1745–1770. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. British Journal of Cancer. 2002;86(10):1566–1577. doi: 10.1038/sj.bjc.6600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zetter BR. Angiogenesis and tumor metastasis. Annual Review of Medicine. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 127.Shieh YS, Hung YJ, Hsieh CB, Chen JS, Chou KC, Liu SY. Tumor-associated macrophage correlated with angiogenesis and progression of mucoepidermoid carcinoma of salivary glands. Annals of Surgical Oncology. 2009;16(3):751–760. doi: 10.1245/s10434-008-0259-6. [DOI] [PubMed] [Google Scholar]
- 128.Sierra JR, Corso S, Caione L, Cepero V, Conrotto P, Cignetti A, et al. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. The Journal of Experimental Medicine. 2008;205(7):1673–1685. doi: 10.1084/jem.20072602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lin L, Chen YS, Yao YD, Chen JQ, Chen JN, Huang SY, et al. CCL18 from tumor-associated macrophages promotes angiogenesis in breast cancer. Oncotarget. 2015;6(33):34758–34773. doi: 10.18632/oncotarget.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19(1):31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 131.Chen P, Huang Y, Bong R, Ding Y, Song N, Wang X, et al. Tumor-associated macrophages promote angiogenesis and melanoma growth via adrenomedullin in a paracrine and autocrine manner. Clinical Cancer Research. 2011;17(23):7230–7239. doi: 10.1158/1078-0432.CCR-11-1354. [DOI] [PubMed] [Google Scholar]
- 132.Luo YP, Zhou H, Krueger J, Kaplan C, Liao D, Markowitz D, et al. The role of proto-oncogene Fra-1 in remodeling the tumor microenvironment in support of breast tumor cell invasion and progression. Oncogene. 2010;29(5):662–673. doi: 10.1038/onc.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang JK, Ma L, Song WH, Lu BY, Huang YB, Dong HM, et al. LncRNA-MALAT1 promotes angiogenesis of thyroid cancer by modulating tumor-associated macrophage FGF2 protein secretion. Journal of Cellular Biochemistry. 2017;118(12):4821–4830. doi: 10.1002/jcb.26153. [DOI] [PubMed] [Google Scholar]
- 134.Constant JS, Feng JJ, Zabel DD, Yuan H, Suh DY, Scheuenstuhl H, et al. Lactate elicits vascular endothelial growth factor from macrophages: a possible alternative to hypoxia. Wound Repair and Regeneration. 2000;8(5):353–360. doi: 10.1111/j.1524-475x.2000.00353.x. [DOI] [PubMed] [Google Scholar]
- 135.Staples KJ, Sotoodehnejadnematalahi F, Pearson H, Frankenberger M, Francescut L, Ziegler-Heitbrock L, et al. Monocyte-derived macrophages matured under prolonged hypoxia transcriptionally up-regulate HIF-1alpha mRNA. Immunobiology. 2011;216(7):832–839. doi: 10.1016/j.imbio.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 136.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang J, Muri J, Fitzgerald G, Gorski T, Gianni-Barrera R, Masschelein E, et al. Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metabolism. 2020;31(6):1136–1153. doi: 10.1016/j.cmet.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Joshi S, Singh AR, Zulcic M, Durden DL. A macrophage-dominant PI3K isoform controls hypoxia-induced HIF1alpha and HIF2alpha stability and tumor growth, angiogenesis, and metastasis. Molecular Cancer Research. 2014;12(10):1520–1531. doi: 10.1158/1541-7786.MCR-13-0682. [DOI] [PubMed] [Google Scholar]
- 139.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192(2):150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 140.Yan D, Wang HW, Bowman RL, Joyce JA. STAT3 and STAT6 signaling pathways synergize to promote cathepsin secretion from macrophages via IRE1alpha activation. Cell Reports. 2016;16(11):2914–2927. doi: 10.1016/j.celrep.2016.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yanamandra N, Gumidyala KV, Waldron KG, Gujrati M, Olivero WC, Dinh DH, et al. Blockade of cathepsin B expression in human glioblastoma cells is associated with suppression of angiogenesis. Oncogene. 2004;23(12):2224–2230. doi: 10.1038/sj.onc.1207338. [DOI] [PubMed] [Google Scholar]
- 142.Joyce JA, Baruch A, Chehade K, Meyer-Morse N, Giraudo E, Tsai FY, et al. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell. 2004;5(5):443–453. doi: 10.1016/s1535-6108(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 143.Mijanovic O, Brankovic A, Panin AN, Savchuk S, Timashev P, Ulasov I, et al. Cathepsin B: a sellsword of cancer progression. Cancer Letters. 2019;449:207–214. doi: 10.1016/j.canlet.2019.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C, et al. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes & Development. 2006;20(5):543–556. doi: 10.1101/gad.1407406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Deryugina EI, Zajac E, Juncker-Jensen A, Kupriyanova TA, Welter L, Quigley JP. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia. 2014;16(10):771–788. doi: 10.1016/j.neo.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Steenport M, Khan KM, Du B, Barnhard SE, Dannenberg AJ, Falcone DJ. Matrix metalloproteinase (MMP)-1 and MMP-3 induce macrophage MMP-9: evidence for the role of TNF-alpha and cyclooxygenase-2. Journal of Immunology. 2009;183(12):8119–8127. doi: 10.4049/jimmunol.0901925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kothari P, Pestana R, Mesraoua R, Elchaki R, Khan KM, Dannenberg AJ, et al. IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. Journal of Immunology. 2014;192(1):349–357. doi: 10.4049/jimmunol.1301906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nielsen BS, Timshel S, Kjeldsen L, Sehested M, Pyke C, Borregaard N, et al. 92 kDa type IV collagenase (MMP-9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. International Journal of Cancer. 1996;65(1):57–62. doi: 10.1002/(SICI)1097-0215(19960103)65:1<57::AID-IJC10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 150.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe'er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. The American Journal of Pathology. 1999;155(3):739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Paulis YW, Soetekouw PM, Verheul HM, Tjan-Heijnen VC, Griffioen AW. Signalling pathways in vasculogenic mimicry. Biochimica et Biophysica Acta. 2010;1806(1):18–28. doi: 10.1016/j.bbcan.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 152.Stalhammar G, See TRO, Phillips SS, Grossniklaus HE. Density of PAS positive patterns in uveal melanoma: correlation with vasculogenic mimicry, gene expression class, BAP-1 expression, macrophage infiltration, and risk for metastasis. Molecular Vision. 2019;25:502–516. [PMC free article] [PubMed] [Google Scholar]
- 153.Wei X, Chen Y, Jiang X, Peng M, Liu Y, Mo Y, et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Molecular Cancer. 2021;20(1):7. doi: 10.1186/s12943-020-01288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Scavelli C, Nico B, Cirulli T, Ria R, Di Pietro G, Mangieri D, et al. Vasculogenic mimicry by bone marrow macrophages in patients with multiple myeloma. Oncogene. 2008;27(5):663–674. doi: 10.1038/sj.onc.1210691. [DOI] [PubMed] [Google Scholar]
- 155.Caillou B, Talbot M, Weyemi U, Pioche-Durieu C, Al Ghuzlan A, Bidart JM, et al. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PLoS One. 2011;6(7):e22567. doi: 10.1371/journal.pone.0022567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhu C, Chrifi I, Mustafa D, van der Weiden M, Leenen PJM, Duncker DJ, et al. CECR1-mediated cross talk between macrophages and vascular mural cells promotes neovascularization in malignant glioma. Oncogene. 2017;36(38):5356–5368. doi: 10.1038/onc.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tattersall IW, Du J, Cong Z, Cho BS, Klein AM, Dieck CL, et al. In vitro modeling of endothelial interaction with macrophages and pericytes demonstrates Notch signaling function in the vascular microenvironment. Angiogenesis. 2016;19(2):201–215. doi: 10.1007/s10456-016-9501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–4488. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kaneda MM, Cappello P, Nguyen AV, Ralainirina N, Hardamon CR, Foubert P, et al. Macrophage PI3Kgamma drives pancreatic ductal adenocarcinoma progression. Cancer Discovery. 2016;6(8):870–885. doi: 10.1158/2159-8290.CD-15-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.van der Kroef M, Carvalheiro T, Rossato M, de Wit F, Cossu M, Chouri E, et al. CXCL4 triggers monocytes and macrophages to produce PDGF-BB, culminating in fibroblast activation: implications for systemic sclerosis. Journal of Autoimmunity. 2020;111:102444. doi: 10.1016/j.jaut.2020.102444. [DOI] [PubMed] [Google Scholar]
- 161.Zhu C, Mustafa D, Zheng PP, van der Weiden M, Sacchetti A, Brandt M, et al. Activation of CECR1 in M2-like TAMs promotes paracrine stimulation-mediated glial tumor progression. Neuro-Oncology. 2017;19(5):648–659. doi: 10.1093/neuonc/now251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yotsumoto F, You WK, Cejudo-Martin P, Kucharova K, Sakimura K, Stallcup WB. NG2 proteoglycan-dependent recruitment of tumor macrophages promotes pericyte-endothelial cell interactions required for brain tumor vascularization. Oncoimmunology. 2015;4(4):e1001204. doi: 10.1080/2162402X.2014.1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140(4):460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 164.Syed SN, Jung M, Weigert A, Brune B. S1P Provokes tumor lymphangiogenesis via macrophage-derived mediators such as IL-1beta or lipocalin-2. Mediators of Inflammation. 2017;2017:7510496. doi: 10.1155/2017/7510496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hu C, Yang K, Li M, Huang W, Zhang F, Wang H. Lipocalin 2: a potential therapeutic target for breast cancer metastasis. Oncotargets and Therapy. 2018;11:8099–8106. doi: 10.2147/OTT.S181223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maier HT, Aigner F, Trenkwalder B, Zitt M, Vallant N, Perathoner A, et al. Up-regulation of neutrophil gelatinase-associated lipocalin in colorectal cancer predicts poor patient survival. World Journal of Surgery. 2014;38(8):2160–2167. doi: 10.1007/s00268-014-2499-x. [DOI] [PubMed] [Google Scholar]
- 167.Ran S, Volk-Draper L. Lymphatic endothelial cell progenitors in the tumor microenvironment. Advances in Experimental Medicine and Biology. 2020;1234:87–105. doi: 10.1007/978-3-030-37184-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schledzewski K, Falkowski M, Moldenhauer G, Metharom P, Kzhyshkowska J, Ganss R, et al. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. The Journal of Pathology. 2006;209(1):67–77. doi: 10.1002/path.1942. [DOI] [PubMed] [Google Scholar]
- 169.Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109(12):5276–5285. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 170.Goede V, Coutelle O, Shimabukuro-Vornhagen A, Holtick U, Neuneier J, Koslowsky TC, et al. Analysis of Tie2-expressing monocytes (TEM) in patients with colorectal cancer. Cancer Investigation. 2012;30(3):225–230. doi: 10.3109/07357907.2011.636114. [DOI] [PubMed] [Google Scholar]
- 171.Ji J, Zhang G, Sun B, Yuan H, Huang Y, Zhang J, et al. The frequency of tumor-infiltrating Tie-2-expressing monocytes in renal cell carcinoma: its relationship to angiogenesis and progression. Urology. 2013;82(4):974 e979-913. doi: 10.1016/j.urology.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 172.Fan S, Yuan J, Deng S, Chen Y, Xie B, Wu K, et al. Activation of interleukin-1beta release by the classical swine fever virus is dependent on the NLRP3 inflammasome, which affects virus growth in monocytes. Frontiers in Cellular and Infection Microbiology. 2018;8:225. doi: 10.3389/fcimb.2018.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Atanasov G, Hau HM, Dietel C, Benzing C, Krenzien F, Brandl A, et al. Prognostic significance of TIE2-expressing monocytes in hilar cholangiocarcinoma. Journal of Surgical Oncology. 2016;114(1):91–98. doi: 10.1002/jso.24249. [DOI] [PubMed] [Google Scholar]
- 174.He YF, Wang CQ, Yu Y, Qian J, Song K, Sun QM, et al. Tie2-expressing monocytes are associated with identification and prognoses of hepatitis B virus related hepatocellular carcinoma after resection. PLoS One. 2015;10(11):e0143657. doi: 10.1371/journal.pone.0143657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xue, R., Sheng, Y., Duan, X., Yang, Y., Ma, S., Xu, J., et al. (2020). Tie2-expressing monocytes as a novel angiogenesis-related cellular biomarker for non-small cell lung cancer. International Journal of Cancer. 10.1002/ijc.33381. [DOI] [PubMed]
- 176.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen L, Li J, Wang F, Dai C, Wu F, Liu X, et al. Tie2 expression on macrophages is required for blood vessel reconstruction and tumor relapse after chemotherapy. Cancer Research. 2016;76(23):6828–6838. doi: 10.1158/0008-5472.CAN-16-1114. [DOI] [PubMed] [Google Scholar]
- 178.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Research. 2004;64(19):7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 179.Leung E, Xue A, Wang Y, Rougerie P, Sharma VP, Eddy R, et al. Blood vessel endothelium-directed tumor cell streaming in breast tumors requires the HGF/C-Met signaling pathway. Oncogene. 2017;36(19):2680–2692. doi: 10.1038/onc.2016.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chen CC, Chen LL, Hsu YT, Liu KJ, Fan CS, Huang TS. The endothelin-integrin axis is involved in macrophage-induced breast cancer cell chemotactic interactions with endothelial cells. The Journal of Biological Chemistry. 2014;289(14):10029–10044. doi: 10.1074/jbc.M113.528406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Roh-Johnson M, Bravo-Cordero JJ, Patsialou A, Sharma VP, Guo P, Liu H, et al. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene. 2014;33(33):4203–4212. doi: 10.1038/onc.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, et al. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discovery. 2015;5(9):932–943. doi: 10.1158/2159-8290.CD-15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kim H, Chung H, Kim J, Choi DH, Shin Y, Kang YG, et al. Macrophages-triggered sequential remodeling of endothelium-interstitial matrix to form pre-metastatic niche in microfluidic tumor microenvironment. Adv Sci (Weinh) 2019;6(11):1900195. doi: 10.1002/advs.201900195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4(8):e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Narasimhan PB, Eggert T, Zhu YP, Marcovecchio P, Meyer MA, Wu R, et al. Patrolling monocytes control NK cell expression of activating and stimulatory receptors to curtail lung metastases. Journal of Immunology. 2020;204(1):192–198. doi: 10.4049/jimmunol.1900998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kubo H, Mensurado S, Goncalves-Sousa N, Serre K, Silva-Santos B. Primary tumors limit metastasis formation through induction of IL15-mediated cross-talk between patrolling monocytes and NK Cells. Cancer Immunology Research. 2017;5(9):812–820. doi: 10.1158/2326-6066.CIR-17-0082. [DOI] [PubMed] [Google Scholar]
- 187.Anderson RL, Balasas T, Callaghan J, Coombes RC, Evans J, Hall JA, et al. A framework for the development of effective anti-metastatic agents. Nature Reviews. Clinical Oncology. 2019;16(3):185–204. doi: 10.1038/s41571-018-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Critical Reviews in Oncogenesis. 2013;18(1-2):43–73. doi: 10.1615/critrevoncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Research. 2007;67(6):2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 190.Pignatelli J, Bravo-Cordero JJ, Roh-Johnson M, Gandhi SJ, Wang Y, Chen X, et al. Macrophage-dependent tumor cell transendothelial migration is mediated by Notch1/Mena(INV)-initiated invadopodium formation. Scientific Reports. 2016;6:37874. doi: 10.1038/srep37874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(34):13515–13520. doi: 10.1073/pnas.1210182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Storr SJ, Safuan S, Ahmad N, El-Refaee M, Jackson AM, Martin SG. Macrophage-derived interleukin-1beta promotes human breast cancer cell migration and lymphatic adhesion in vitro. Cancer Immunology, Immunotherapy. 2017;66(10):1287–1294. doi: 10.1007/s00262-017-2020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Linde N, Casanova-Acebes M, Sosa MS, Mortha A, Rahman A, Farias E, et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nature Communications. 2018;9(1):21. doi: 10.1038/s41467-017-02481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Roussos ET, Goswami S, Balsamo M, Wang Y, Stobezki R, Adler E, et al. Mena invasive (Mena(INV)) and Mena11a isoforms play distinct roles in breast cancer cell cohesion and association with TMEM. Clinical & Experimental Metastasis. 2011;28(6):515–527. doi: 10.1007/s10585-011-9388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ginter, P. S., Karagiannis, G. S., Entenberg, D., Lin, Y., Condeelis, J., Jones, J. G., et al. (2019). Tumor Microenvironment of Metastasis (TMEM) Doorways are restricted to the blood vessel endothelium in both primary breast cancers and their lymph node metastases. Cancers (Basel), 11(10). 10.3390/cancers11101507. [DOI] [PMC free article] [PubMed]
- 196.Robinson BD, Jones JG. Tumor microenvironment of metastasis (TMEM): a novel tissue-based assay for metastatic risk in breast cancer. Future Oncology. 2009;5(7):919–921. doi: 10.2217/fon.09.79. [DOI] [PubMed] [Google Scholar]
- 197.Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. The Journal of Cell Biology. 2004;167(2):223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fournier P, Dussault S, Fusco A, Rivard A, Royal I. Tyrosine phosphatase PTPRJ/DEP-1 is an essential promoter of vascular permeability, angiogenesis, and tumor progression. Cancer Research. 2016;76(17):5080–5091. doi: 10.1158/0008-5472.CAN-16-1071. [DOI] [PubMed] [Google Scholar]
- 199.Hauselmann I, Roblek M, Protsyuk D, Huck V, Knopfova L, Grassle S, et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade. Cancer Research. 2016;76(18):5302–5312. doi: 10.1158/0008-5472.CAN-16-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Marcovecchio, P. M., Zhu, Y. P., Hanna, R. N., Dinh, H. Q., Tacke, R., Wu, R., et al. (2020). Kindlin-3 is essential for patrolling and phagocytosis functions of nonclassical monocytes during metastatic cancer surveillance. Journal of Leukocyte Biology. 10.1002/JLB.4HI0420-098R. [DOI] [PMC free article] [PubMed]
- 201.Schuldner M, Dorsam B, Shatnyeva O, Reiners KS, Kubarenko A, Hansen HP, et al. Exosome-dependent immune surveillance at the metastatic niche requires BAG6 and CBP/p300-dependent acetylation of p53. Theranostics. 2019;9(21):6047–6062. doi: 10.7150/thno.36378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Plebanek MP, Angeloni NL, Vinokour E, Li J, Henkin A, Martinez-Marin D, et al. Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nature Communications. 2017;8(1):1319. doi: 10.1038/s41467-017-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115(3):e10–e19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ziegler-Heitbrock L. Monocyte subsets in man and other species. Cellular Immunology. 2014;289(1-2):135–139. doi: 10.1016/j.cellimm.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 205.Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456(7223):814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 207.Wenes M, Shang M, Di Matteo M, Goveia J, Martin-Perez R, Serneels J, et al. Macrophage metabolism controls tumor blood vessel morphogenesis and metastasis. Cell Metabolism. 2016;24(5):701–715. doi: 10.1016/j.cmet.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 208.Zheng X, Turkowski K, Mora J, Brune B, Seeger W, Weigert A, et al. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget. 2017;8(29):48436–48452. doi: 10.18632/oncotarget.17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Genard G, Lucas S, Michiels C. Reprogramming of tumor-associated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. Frontiers in Immunology. 2017;8:828. doi: 10.3389/fimmu.2017.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Viallard C, Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409–426. doi: 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- 211.Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nature Reviews. Clinical Oncology. 2018;15(5):310–324. doi: 10.1038/nrclinonc.2018.9. [DOI] [PubMed] [Google Scholar]
- 212.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nature Reviews. Clinical Oncology. 2018;15(5):325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Peterson TE, Kirkpatrick ND, Huang Y, Farrar CT, Marijt KA, Kloepper J, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(16):4470–4475. doi: 10.1073/pnas.1525349113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhu X, Yang J, Gao Y, Wu C, Yi L, Li G, et al. The dual effects of a novel peptibody on angiogenesis inhibition and M2 macrophage polarization on sarcoma. Cancer Letters. 2018;416:1–10. doi: 10.1016/j.canlet.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 215.Kashyap AS, Schmittnaegel M, Rigamonti N, Pais-Ferreira D, Mueller P, Buchi M, et al. Optimized antiangiogenic reprogramming of the tumor microenvironment potentiates CD40 immunotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(1):541–551. doi: 10.1073/pnas.1902145116. [DOI] [PMC free article] [PubMed] [Google Scholar]