Abstract
α-Chiral alkyne is a key structural element of many bioactive compounds, chemical probes, and functional materials, and is a valuable synthon in organic synthesis. Here we report a NiH-catalysed reductive migratory hydroalkynylation of olefins with bromoalkynes that delivers the corresponding benzylic alkynylation products in high yields with excellent regioselectivities. Catalytic enantioselective hydroalkynylation of styrenes has also been realized using a simple chiral PyrOx ligand. The obtained enantioenriched benzylic alkynes are versatile synthetic intermediates and can be readily transformed into synthetically useful chiral synthons.
Subject terms: Asymmetric catalysis, Synthetic chemistry methodology
Chiral alkyne motifs bearing an α stereocentre are often found in many bioactive compounds, chemical probes, and functional materials. Here the authors show NiH-catalysed reductive migratory hydroalkynylation of olefins with bromoalkynes that form benzylic alkynylation products in high yield and with excellent regioselectivity.
Introduction
As a key structural element, chiral alkynes motifs bearing an α stereocentre are often found in many bioactive compounds, chemical probes, and functional materials (Fig. 1a). In addition, they are also valuable synthons as the sp3-hybridized carbons could undergo versatile transformations to deliver useful sp2- or sp3-hybridized carbons1. As a result, efficient strategies for catalytic, enantioselective C(sp3)–C(sp) coupling to generate such stereocentres have long been sought (Fig. 1b). For example, Liu2,3 reported an elegant work on Cu-catalyzed asymmetric Sonogashira C(sp3)–C(sp) coupling4,5. Shi6 and Liu7 have demonstrated that Pd- and Cu-catalyzed C(sp3)–H alkynylation could be achieved in an enantioselective fashion. Liu8 has also used an alkene difunctionalization strategy to produce enantioenriched alkynylation product under copper catalyst9. Suginome10 has reported a pioneering work on Ni-catalyzed asymmetric hydroalkynylation of 1,3-dienes based on their previous hydroalkynylation works11,12. As a continued development of general alternatives for asymmetric C(sp3)–C(sp) coupling, here we report an appealing approach via metal-hydride13–15 catalyzed asymmetric (remote) hydroalkynylation16 from readily available alkene starting materials.
Fig. 1. Ni(I)H-catalyzed migratory hydroalkynylation and enantioselective hydroalkynylation.
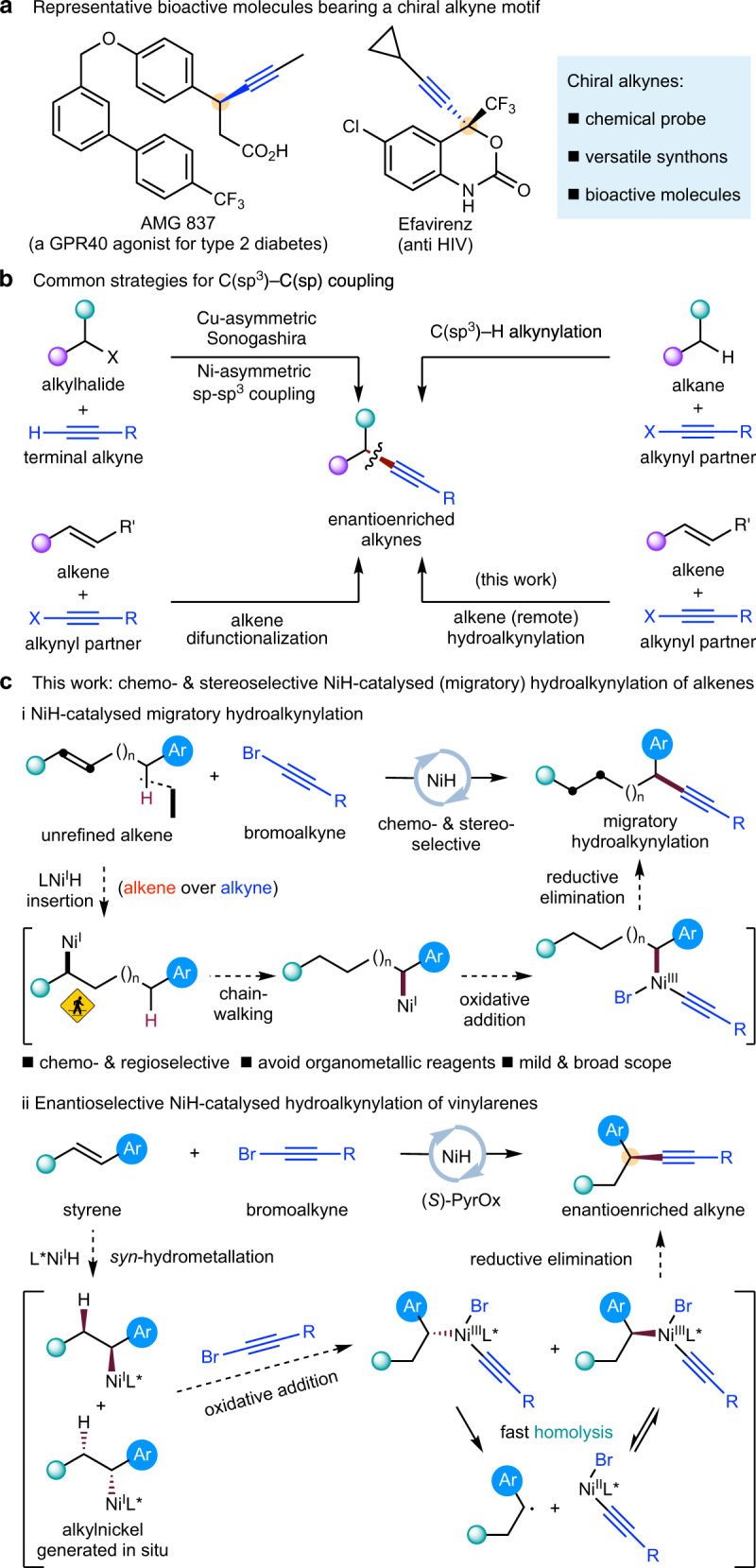
a Representative bioactive molecules bearing a chiral alkyne motif. b Common strategies for C(sp3)–C(sp) coupling. c Chemo- & stereoselective NiH-catalyzed (migratory) hydroalkynylation of alkenes.
Owing to its low-cost, facile oxidative addition, and availability of diverse oxidation states, nickel17,18 has emerged as a catalyst complementary to palladium over the past two decades, especially in cross-coupling reaction involving C(sp3) fragments. Reductive migratory hydrofunctionalization19–22 catalyzed by nickel hydride23–25 has recently been recognized as an alternative protocol for selective functionalization of remote C(sp3)–H bonds26–66. Compared to conventional cross-coupling, this process (i) employs readily available, bench-stable alkenes or alkene precursors instead of specially generated organometallic reagents as starting materials and (ii) could also selectively functionalize a remote C(sp3)–H site in addition to the conventional ipso-position. Since its conception, significant progress has been made toward this synthetically useful process26–61, which requires that the cross-coupling partner (e.g., aryl halide or alkyl halide) could selectively capture an alkylnickel species generated through iterative migratory insertion/β-hydride elimination.
To explore this nickel-catalyzed migratory hydrofunctionalization further, we recently investigated if a bromoalkyne, an unsaturated C(sp) cross-coupling partner which is potentially reactive toward NiH, could be used to achieve remote hydroalkynylation (Fig. 1c, i). Successful implementation of this transformation will require (i) a hydrometalation process that can discriminate between alkene and alkyne and (ii) an alkynylation process highly selective for one of the alkylnickel species. A chiral alkyne bearing an α-aryl-substituted stereogenic C(sp3) center2–5,7–10,67,68 would be ultimately obtained from styrene through hydronickellation and subsequent enantioconvergent52,53,55,56,69 alkynylation (Fig. 1c, ii). Here, we show the successful execution of this reaction.
Results
Reaction design and optimization
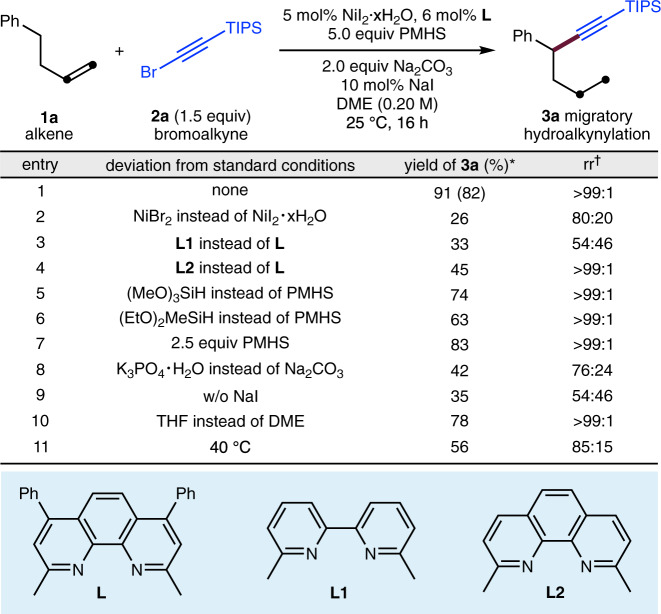
Our initial studies involved the migratory hydroalkynylation of 4-phenyl-1-butene (1a) using 1-bromo-2-(triisopropylsilyl)acetylene (2a) as an alkynylation reagent (Fig. 2). It was determined that NiI2·xH2O and the bathocuproine ligand (L) could generate the desired migratory alkynylation product as a single regioisomer [rr (benzylic product: all other isomers) > 99:1] in 82% yield (entry 1). Other nickel sources such as NiBr2 led to lower yields and a moderate rr (entry 2). Ligand screening revealed that the previously used ligand29, 6,6′-dimethyl-2,2′-bipyridine (L1) resulted in significantly lower yield and rr (entry 3) while a similar ligand neocuproine (L2) led to a similar regioselectivity but a lower yield (entry 4). Other silanes such as trimethoxysilane and diethoxymethylsilane gave diminished yields (entries 5 and 6), and marginally lower yield was obtained when reducing the amount of PMHS to 2.5 equiv (entry 7). K3PO4·H2O was shown to be an unsuitable base (entry 8). The addition of NaI as an additive improves both the yield and rr, presumably by promoting the regeneration of NiH species (entry 9). An evaluation of solvents revealed that THF was less effective than DME (entry 10), and conducting the reaction at 40 °C gave inferior results (entry 11).
Fig. 2. Variation of reaction parameters.
*Yields determined by GC using n-dodecane as the internal standard, the yield in parentheses is the isolated yield. †rr refers to regioisomeric ratio, representing the ratio of the major product to the sum of all other isomers as determined by GC analysis. PMHS polymethylhydrosiloxane, DME dimethoxyethane, TIPS triisopropylsilyl.
Substrate scope
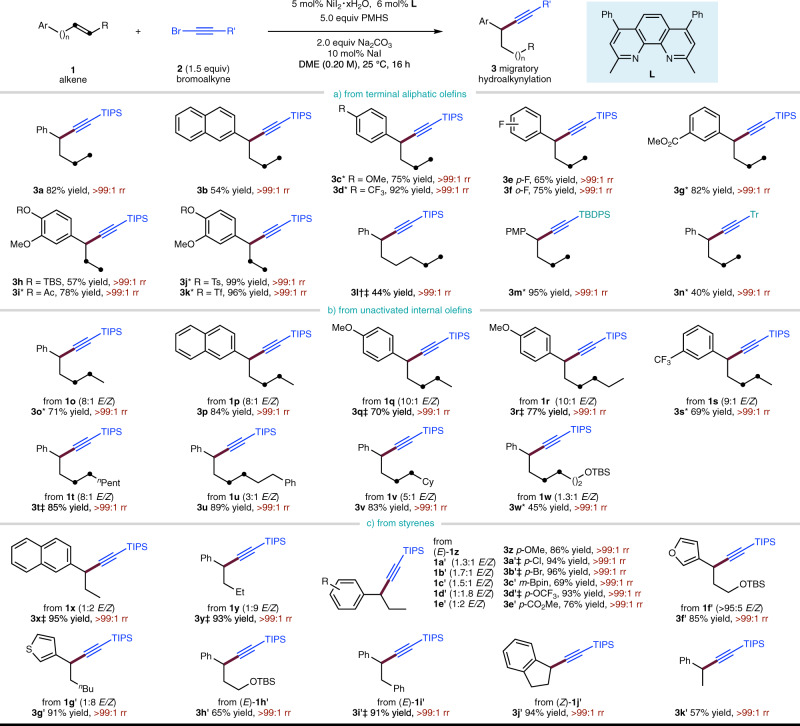
With these optimal reaction conditions, we examined the generality of the reaction. As shown in Fig. 3a, unactivated terminal alkenes bearing electron-donating (3c) or electron-withdrawing (3d–3g) substituents on the remote aryl ring are tolerated. A variety of functional groups are readily accommodated, including ethers (3c, 3h–3k, 3m), a trifluoromethyl group (3d), and esters (3g, 3i). Importantly, tosylates (3j) and triflate (3k) commonly used for further cross-coupling, all remained intact. The reaction could also proceed with olefin substrate having longer chain length between the starting C=C bond and the remote aryl group, producing the benzylic alkynylation product exclusively although with a lower yield (3l). Remarkably, both silyl and sterically hindered alkyl substituted ethynyl bromides work well in this reaction (3m, 3n). Moreover, a variety of unactivated internal alkenes also proved to be competent coupling partners, regardless of the E/Z configuration or the position of the C=C bond (Fig. 3b, 3o–3w). As expected, styrenes themselves smoothly undergo hydroalkynylation to produce the benzylic alkynylation product exclusively (Fig. 3c, 3x–3k′). Under these exceptionally mild reaction conditions, various substituents on the aryl ring (3z–3e′) as well as heteroaromatic styrenes (3f′, 3g′) were also suitable for this reaction.
Fig. 3. NiH-catalyzed migratory hydroalkynylation of alkenes with bromoalkynes.
Yield under each product refers to the isolated yield of purified product (0.20 mmol scale, average of two runs), rr refers to regioisomeric ratio, representing the ratio of the major product to the sum of all other isomers as determined by GC analysis. *Diglyme was used as solvent. †10 mol% NiI2·xH2O, 12 mol% L, and 20 mol% NaI were used. ‡DME (0.10 M) was used. TBS tert-butyldimethylsilyl, TBDPS tert-butyldiphenylsilyl; Tr trityl (triphenylmethyl).
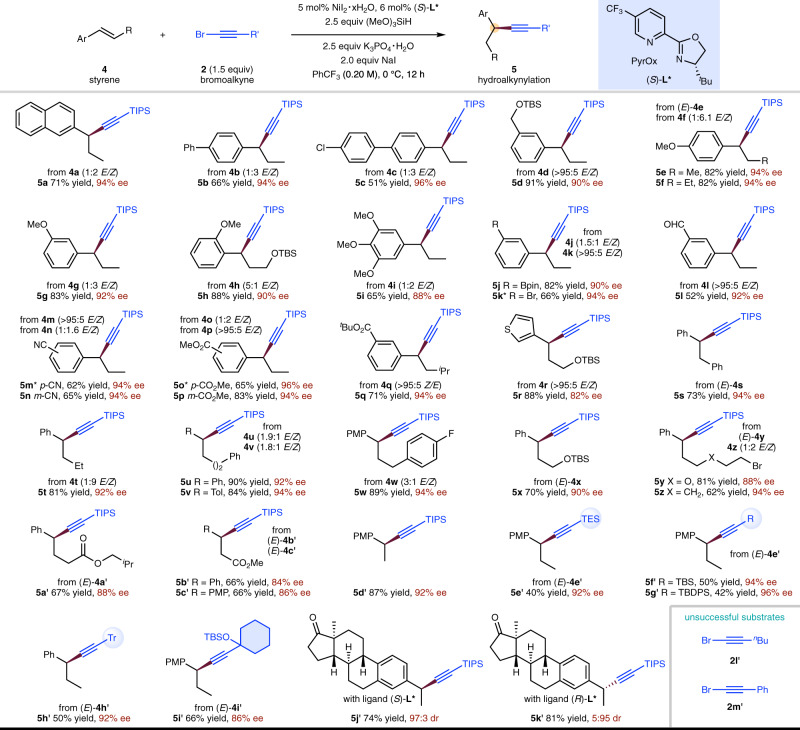
In an effort to obtain enantioenriched benzylic alkynylation products, the asymmetric version of NiH-catalyzed hydroalkynylation of styrenes was explored and the results are in Fig. 4. It was found that a chiral PyrOx ligand (S)-L* under modified reaction conditions could produce the desired hydroalkynylation products in good yields and excellent ee. Styrenes with a variety of substituents on the aromatic ring underwent asymmetric hydroalkynylation smoothly (5a–5q), including ethers (5d–5i), an easily reduced aldehyde (5l), a nitrile (5m, 5n), and esters (5o–5q). Substituents commonly used for further cross-coupling such as aryl chloride (5c), aryl bromide (5k), and boronic acid pinacol ester (5j) all emerged unchanged. The substituents at β-position could also be varied (5r–5c′). Alkyl bromides were compatible with the current reaction, providing a synthetic handle for further derivatization (5y, 5z). β-Unsubstituted styrenes were also compatible (5d′, 5j′, 5k′). The scope of bromoalkynes was also explored and a range of different sterically hindered substituents at the β-position, including silyl and alkyl-substituted ethynyl bromides were shown to be viable substrates (5e′–5i′). However, it should also be noted that the less steric hindered alkyl-substituted ethynyl bromide (2l′) and aryl-substituted ethynyl bromide (2m′) were unsuccessful substrates70 and could easily undergo decomposition under the current conditions.
Fig. 4. NiH-catalyzed enantioselective hydroalkynylation of styrenes with bromoalkynes.
Yield under each product refers to the isolated yield of purified product (0.20 mmol scale, average of two runs), single regioisomer was obtained unless otherwise noted. Enantioselectivities were determined by chiral HPLC analysis. *NiBr2·diglyme used as catalyst, 1,2-dichloroethane used as solvent, 3.0 equiv NaI used. TES triethylsilyl.
Discussion
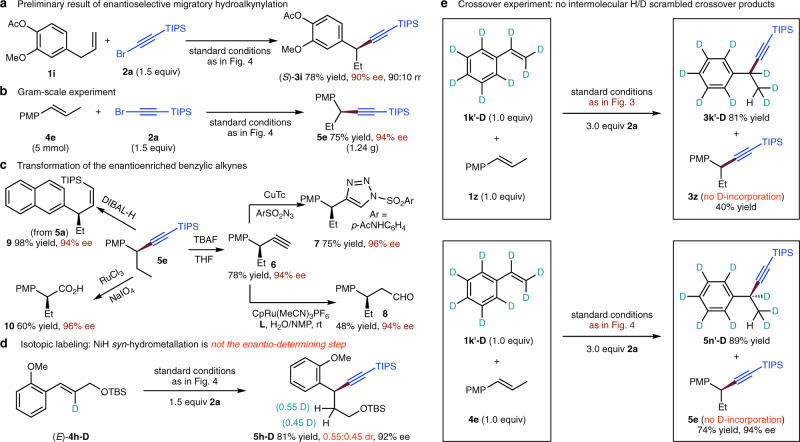
The asymmetric migratory hydroalkynylation could also be realized. In a preliminary experiment with 3-aryl-1-propene (1i) as substrate (Fig. 5a), chain-walking and subsequent asymmetric alkynylation at benzylic position product ((S)-3i) was obtained with excellent ee (90% ee) as major isomer (90:10 rr). When the reaction was conducted on a 5 mmol scale, the functionalized chiral benzylic alkyne (5e) was obtained in high yield and with excellent enantioselectivity (Fig. 5b). To highlight the synthetic utility of the method, subsequent derivatizations were carried out (Fig. 5c). Desilylation of 5e yielded the enantioenriched terminal alkyne (6), which could further undergo a click reaction to form 7 or a hydration reaction to form 8. The semi-hydrogenation of alkyne (5a) by DIBAL-H (diisobutylaluminum hydride) could be highly stereoselective, giving the Z-alkene (9). In addition, oxidative cleavage of the triple bond in 5e could afford the corresponding chiral carboxylic acid (10).
Fig. 5. Enantioselective migratory hydroalkynylation, gram-scale, derivatization, and isotopic labeling experiments.
a Preliminary result of enantioselective migratory hydroalkynylation. b Gram-scale experiment. c Transformation of the enantioenriched benzylic alkynes. d Isotopic labeling experiment. e Crossover experiment.
To gain further insight into the mechanism of the hydrometallation process, isotope labeling experiments were conducted. As shown in Fig. 5d, the use of the deuterated trans-alkene (E-4h-D) led to the formation of both diastereomers in approximately equal amounts (0.55:0.45 dr), which indicated that the syn-hydrometallation is not the enantio-determining step because if it was, a diastereomerically pure 5h-D should be formed. This observation is consistent with our initial mechanistic proposal that the benzylic stereocentre is formed through rapid homolysis of the alkyl-Ni(III) bond and subsequently enantioconvergent process, reforming only one Ni(III) enantiomer from Ni(II) and benzylic radical (see Fig. 1c, ii). Furthermore, no intermolecular H/D scrambled crossover products were obtained in both migratory and asymmetric hydroalkynylation conditions, revealing that hydrometallation of NiH/NiD species to styrene is irreversible (Fig. 5e).
In conclusion, we report a NiH-catalyzed strategy to form functionalized benzylic alkynylation products, which are versatile synthetic intermediates. Both migratory hydroalkynylation and asymmetric hydroalkynylation can be realized. These two mild, efficient, and straightforward processes tolerate a wide range of functional groups on both the alkene and bromoalkyne components. A broad substrate scope as well as synthetic utility of this protocol have been demonstrated. An investigation of the mechanism and the development of a migratory enantioselective version of this transformation are currently in progress.
Methods
NiH-catalyzed migratory hydroalkynylation of alkenes
In a nitrogen-filled glove box, to an oven-dried 8 mL screw-cap vial equipped with a magnetic stir bar were added NiI2·xH2O (3.8 mg, 5.0 mol%), L (4.3 mg, 6.0 mol%), Na2CO3 (42.4 mg, 2.0 equiv), NaI (3.0 mg, 10.0 mol%) and anhydrous DME (1.0 mL). The mixture was stirred for 20 min at room temperature (stirred at 800 rpm) before the addition of PMHS (60 μL, 1.0 mmol, 5.0 equiv). Stirring was continued for an additional 5 min before the addition of olefin 1 (0.20 mmol, 1.0 equiv) and bromoalkyne 2 (0.30 mmol, 1.5 equiv). The tube was sealed with a teflon-lined screw cap, removed from the glove box and the reaction was stirred at 25 °C for up to 16 h (the mixture was stirred at 1000 rpm). After the reaction was complete, the reaction was quenched upon the addition of H2O, and the mixture was extracted with EtOAc. The organic layer was concentrated to give the crude product. n-Dodecane (20 μL) was added as an internal standard for GC analysis. The product was purified by flash column chromatography (petroleum ether/EtOAc) for each substrate. See Supplementary Information for more detailed experimental procedures and characterization data for all products.
Enantioselective NiH-catalyzed hydroalkynylation of styenes
In a nitrogen-filled glove box, to an oven-dried 8 mL screw-cap vial equipped with a magnetic stir bar were added NiI2·xH2O (3.8 mg, 5.0 mol%), (S)-L* (3.3 mg, 6.0 mol%), K3PO4·H2O (115.1 mg, 2.5 equiv), NaI (60.0 mg, 2.0 equiv) and anhydrous PhCF3 (1.0 mL). The mixture was stirred for 20 min at room temperature (stirred at 800 rpm) before the addition of (MeO)3SiH (64 μL, 0.50 mmol, 2.5 equiv). Stirring was continued for an additional 5 min before the addition of olefin 4 (0.20 mmol, 1.0 equiv) and bromoalkyne 2 (0.30 mmol, 1.5 equiv). The tube was sealed with a teflon-lined screw cap, removed from the glove box and the reaction was stirred at 0 °C for up to 12 h (the mixture was stirred at 800 rpm). After the reaction was complete, the reaction was quenched upon the addition of H2O, and the mixture was extracted with EtOAc. The organic layer was concentrated to give the crude product. n-Dodecane (20 μL) was added as an internal standard for GC analysis. The product was purified by flash column chromatography (petroleum ether/EtOAc) for each substrate. The enantiomeric excesses (% ee) were determined by HPLC analysis using chiral stationary phases. See Supplementary Information for more detailed experimental procedures and characterization data for all products.
Supplementary information
Acknowledgements
Research reported in this publication was supported by NSFC (21822105, 21772087), NSF of Jiangsu Province (BK20190281, BK20201245), Six Kinds of Talents Project of Jiangsu Province (JNHB-003), programs for high-level entrepreneurial and innovative talents introduction of Jiangsu Province (group program), and Fundamental Research Funds for the Central Universities (020514380263).
Author contributions
X.J., Y.W., and S.Z. designed the project. X.J., B.H., Y.X., M.D., Z.G., Y.W., and S.Z. co-wrote the manuscript, analyzed the data, discussed the results, and commented on the manuscript. X.J., B.H., Y.X., M.D., and Z.G. performed the experiments. All authors contributed to discussions.
Data availability
The authors declare that the main data supporting the findings of this study, including experimental procedures and compound characterization, are available within the article and its supplementary information files, or from the corresponding author upon reasonable request.
Competing interests
The authors declare the following competing interest(s): a patent for the synthesis of AMG 837 using this method has been filed.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
You Wang, Email: wangyou@nju.edu.cn.
Shaolin Zhu, Email: shaolinzhu@nju.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-24094-9.
References
- 1.Trost, B. M. & Li, C.-J. Modern AlkyneChemistry: Catalytic and Atom-Economic Transformations (Wiley-VCH, Weinheim, 2015).
- 2.Dong X-Y, et al. A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling. Nat. Chem. 2019;11:1158–1166. doi: 10.1038/s41557-019-0346-2. [DOI] [PubMed] [Google Scholar]
- 3.Xia H-D, et al. Photoinduced copper-catalyzed asymmetric decarboxylative alkynylation with terminal alkynes. Angew. Chem. Int. Ed. 2020;59:16926–16932. doi: 10.1002/anie.202006317. [DOI] [PubMed] [Google Scholar]
- 4.Caeiro J, Sestelo JP, Sarandeses LA. Enantioselective nickel-catalyzed cross-coupling reactions of trialkynylindium reagents with racemic secondary benzyl bromides. Chemistry. 2008;14:741–746. doi: 10.1002/chem.200701035. [DOI] [PubMed] [Google Scholar]
- 5.Fang H, et al. Transmetal-catalyzed enantioselective cross-coupling reaction of racemic secondary benzylic bromides with organoaluminum reagents. Org. Lett. 2016;18:6022–6025. doi: 10.1021/acs.orglett.6b02933. [DOI] [PubMed] [Google Scholar]
- 6.Han Y-Q, et al. Pd(II)-catalyzed enantioselective alkynylation of unbiased methylene C(sp3)–H bonds using 3,3′-fluorinated-BINOL as a chiral ligand. J. Am. Chem. Soc. 2019;141:4558–4563. doi: 10.1021/jacs.9b01124. [DOI] [PubMed] [Google Scholar]
- 7.Fu L, Zhang Z, Chen P, Lin Z, Liu G. Enantioselective copper-catalyzed alkynylation of benzylic C−H bonds via radical relay. J. Am. Chem. Soc. 2020;142:12493–12500. doi: 10.1021/jacs.0c05373. [DOI] [PubMed] [Google Scholar]
- 8.Fu L, Zhou S, Wan X, Chen P, Liu G. Enantioselective trifluoromethylalkynylation of alkenes via copper-catalyzed radical relay. J. Am. Chem. Soc. 2018;140:10965–10969. doi: 10.1021/jacs.8b07436. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, et al. Copper-catalyzed photoinduced enantioselective dual carbofunctionalization of alkenes. Org. Lett. 2020;22:1490–1494. doi: 10.1021/acs.orglett.0c00071. [DOI] [PubMed] [Google Scholar]
- 10.Shirakura M, Suginome M. Nickel-catalyzed asymmetric addition of alkyne C–H bonds across 1,3-dienes using taddol-based chiral phosphoramidite ligands. Angew. Chem. Int. Ed. 2010;49:3827–3829. doi: 10.1002/anie.201001188. [DOI] [PubMed] [Google Scholar]
- 11.Shirakura M, Suginome M. Nickel-catalyzed regioselective hydroalkynylation of styrenes: improved catalyst system, reaction scope, and mechanism. Org. Lett. 2009;11:523–526. doi: 10.1021/ol802475h. [DOI] [PubMed] [Google Scholar]
- 12.Shirakura M, Suginome M. Nickel-catalyzed, regio- and stereoselective hydroalkynylation of methylenecyclopropanes with retention of the cyclopropane ring, leading to the synthesis of 1-methyl-1-alkynylcyclopropanes. J. Am. Chem. Soc. 2009;131:5060–5061. doi: 10.1021/ja900876w. [DOI] [PubMed] [Google Scholar]
- 13.Liu RY, Buchwald SL. CuH-Catalyzed olefin functionalization: from hydroamination to carbonyl addition. Acc. Chem. Res. 2020;53:1229–1243. doi: 10.1021/acs.accounts.0c00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberhardt NA, Guan H. Nickel hydride complexes. Chem. Rev. 2016;116:8373–8426. doi: 10.1021/acs.chemrev.6b00259. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Guo J, Lu Z. Recent advances in hydrometallation of alkenes and alkynes via the first row transition metal catalysis. Chin. J. Chem. 2018;36:1075–1109. doi: 10.1002/cjoc.201800314. [DOI] [Google Scholar]
- 16.Zhang W, Wang Z, Bai X, Li B. Substrate-directed catalytic asymmetric hydroalkynylation of alkenes. Chin. J. Org. Chem. 2020;40:1087–1095. doi: 10.6023/cjoc202002017. [DOI] [Google Scholar]
- 17.Tasker SZ, Standley EA, Jamison TF. Recent advances in homogeneous nickel catalysis. Nature. 2014;509:299–309. doi: 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogoshi, S. Nickel Catalysis in Organic Synthesis (Wiley-VCH, Weinheim, 2020).
- 19.Larionov E, Li H, Mazet C. Well-defined transition metal hydrides in catalytic isomerizations. Chem. Commun. 2014;50:9816–9826. doi: 10.1039/C4CC02399D. [DOI] [PubMed] [Google Scholar]
- 20.Vasseur A, Bruffaerts J, Marek I. Remote functionalization through alkene isomerization. Nat. Chem. 2016;8:209–219. doi: 10.1038/nchem.2445. [DOI] [PubMed] [Google Scholar]
- 21.Sommer H, Juliá-Hernández F, Martin R, Marek I. Walking metals for remote functionalization. ACS Cent. Sci. 2018;4:153–165. doi: 10.1021/acscentsci.8b00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen-Müller D, Sahoo B, Sun S-Z, Martin R. Tackling remote sp3 C−H functionalization via Ni-catalyzed “chain-walking” reactions. Isr. J. Chem. 2020;60:195–206. doi: 10.1002/ijch.201900072. [DOI] [Google Scholar]
- 23.Cornella J, Gómez-Bengoa E, Martin R. Combined experimental and theoretical study on the reductive cleavage of inert C–O bonds with silanes: ruling out a classical Ni(0)/Ni(II) catalytic couple and evidence for Ni(I) intermediates. J. Am. Chem. Soc. 2013;135:1997–2009. doi: 10.1021/ja311940s. [DOI] [PubMed] [Google Scholar]
- 24.Pappas I, Treacy S, Chirik PJ. Alkene hydrosilylation using tertiary silanes with α-diimine nickel catalysts redox-active ligands promote a distinct mechanistic pathway from platinum catalysts. ACS Catal. 2016;6:4105–4109. doi: 10.1021/acscatal.6b01134. [DOI] [Google Scholar]
- 25.Kuang Y, et al. Ni(I)-catalyzed reductive cyclization of 1,6-dienes: mechanism-controlled trans selectivity. Chem. 2017;3:268–280. doi: 10.1016/j.chempr.2017.07.010. [DOI] [Google Scholar]
- 26.Busolv I, Becouse J, Mazza S, Montandon-Clerc M, Hu X. Chemoselective alkene hydrosilylation catalyzed by nickel pincer complexes. Angew. Chem. Int. Ed. 2015;54:14523–14526. doi: 10.1002/anie.201507829. [DOI] [PubMed] [Google Scholar]
- 27.Lu X, et al. Practical carbon–carbon bond formation from olefins through nickel-catalyzed reductive olefin hydrocarbonation. Nat. Commun. 2016;7:11129–11136. doi: 10.1038/ncomms11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green SA, Matos JLM, Yagi A, Shenvi RA. Branch-selective hydroarylation: iodoarene-olefin cross-coupling. J. Am. Chem. Soc. 2016;138:12779–12782. doi: 10.1021/jacs.6b08507. [DOI] [PubMed] [Google Scholar]
- 29.He Y, Cai Y, Zhu S. Mild and regioselective benzylic C–H functionalization: Ni-catalyzed reductive arylation of remote and proximal olefins. J. Am. Chem. Soc. 2017;139:1061–1064. doi: 10.1021/jacs.6b11962. [DOI] [PubMed] [Google Scholar]
- 30.Juliá-Hernández F, Moragas T, Cornella J, Martin R. Remote carboxylation of halogenated aliphatic hydrocarbons with carbon dioxide. Nature. 2017;545:84–88. doi: 10.1038/nature22316. [DOI] [PubMed] [Google Scholar]
- 31.Gaydou M, Moragas T, Juliá-Hernández F, Martin R. Site-selective catalytic carboxylation of unsaturated hydrocarbons with CO2 and water. J. Am. Chem. Soc. 2017;139:12161–12164. doi: 10.1021/jacs.7b07637. [DOI] [PubMed] [Google Scholar]
- 32.Chen F, et al. Remote migratory cross-electrophile coupling and olefin hydroarylation reactions enabled by in situ generation of NiH. J. Am. Chem. Soc. 2017;139:13929–13935. doi: 10.1021/jacs.7b08064. [DOI] [PubMed] [Google Scholar]
- 33.Zhou F, Zhu J, Zhang Y, Zhu S. NiH-catalyzed reductive relay hydroalkylation: a strategy for the remote C(sp3)–H alkylation of alkenes. Angew. Chem. Int. Ed. 2018;57:4058–4062. doi: 10.1002/anie.201712731. [DOI] [PubMed] [Google Scholar]
- 34.Xiao J, He Y, Ye F, Zhu S. Remote sp3 C–H amination of alkenes with nitroarenes. Chem. 2018;4:1645–1657. doi: 10.1016/j.chempr.2018.04.008. [DOI] [Google Scholar]
- 35.Sun S-Z, Börjesson M, Martin-Montero R, Martin R. Site-selective Ni-catalyzed reductive coupling of α-haloboranes with unactivated olefins. J. Am. Chem. Soc. 2018;140:12765–12769. doi: 10.1021/jacs.8b09425. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Xu X, Zhu S. Nickel-catalysed selective migratory hydrothiolation of alkenes and alkynes with thiols. Nat. Commun. 2019;10:1752. doi: 10.1038/s41467-019-09783-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou L, Zhu C, Bi P, Feng C. Ni-catalyzed migratory fluoro-alkenylation of unactivated alkyl bromides with gem-difluoroalkenes. Chem. Sci. 2019;10:1144–1149. doi: 10.1039/C8SC04162H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bera S, Hu X. Nickel-catalyzed Regioselective hydroalkylation and hydroarylation of alkenyl boronic esters. Angew. Chem. Int. Ed. 2019;58:13854–13859. doi: 10.1002/anie.201907045. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Han B, Zhu S. Rapid access to highly functionalized alkylboronates via NiH-catalyzed remote hydroarylation of boron-containing alkenes. Angew. Chem. Int. Ed. 2019;58:13860–13864. doi: 10.1002/anie.201907185. [DOI] [PubMed] [Google Scholar]
- 40.Sun S-Z, Romano C, Martin R. Site-selective catalytic deaminative alkylation of unactivated olefins. J. Am. Chem. Soc. 2019;141:16197–16201. doi: 10.1021/jacs.9b07489. [DOI] [PubMed] [Google Scholar]
- 41.Qian C, Tang W. NiH-catalyzed migratory defluorinative olefin cross-coupling: trifluoromethyl-substituted alkenes as acceptor olefins to form gem-difluoroalkenes. Chin. J. Org. Chem. 2020;40:1076–1077. doi: 10.6023/cjoc202000017. [DOI] [PubMed] [Google Scholar]
- 42.Gao Y, et al. Visible-light-induced nickel-catalyzed cross-coupling with alkylzirconocenes from unactivated alkenes. Chem. 2020;6:675–688. doi: 10.1016/j.chempr.2019.12.010. [DOI] [Google Scholar]
- 43.Kumar GS, et al. Nickel-catalyzed chain-walking cross-electrophile coupling of alkyl and aryl halides and olefin hydroarylation enabled by electrochemical reduction. Angew. Chem. Int. Ed. 2020;59:6513–6519. doi: 10.1002/anie.201915418. [DOI] [PubMed] [Google Scholar]
- 44.Jiao K-J, et al. Nickel-catalyzed electrochemical reductive relay cross-coupling of alkyl halides to aryl halides. Angew. Chem. Int. Ed. 2020;59:6520–6524. doi: 10.1002/anie.201912753. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, He J, Song P, Wang Y, Zhu S. Ligand-enabled NiH-catalyzed migratory hydroamination: chain walking as a strategy for regiodivergent/regioconvergent remote sp3 C–H amination. CCS Chem. 2020;2:2259–2268. doi: 10.31635/ccschem.020.202000490. [DOI] [Google Scholar]
- 46.Jeon J, Lee C, Seo H, Hong S. NiH-Catalyzed proximal-selective hydroamination of unactivated alkenes. J. Am. Chem. Soc. 2020;142:20470–20480. doi: 10.1021/jacs.0c10333. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, Yin H, Fu GC. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature. 2018;563:379–383. doi: 10.1038/s41586-018-0669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou F, Zhang Y, Xu X, Zhu S. NiH-catalyzed remote asymmetric hydroalkylation of alkenes with racemic α-bromo amides. Angew. Chem. Int. Ed. 2019;58:1754–1758. doi: 10.1002/anie.201813222. [DOI] [PubMed] [Google Scholar]
- 49.He S-J, et al. Nickel-catalyzed enantioconvergent reductive hydroalkylation of olefins with α-heteroatom phosphorus or sulfur alkyl electrophiles. J. Am. Chem. Soc. 2020;142:214–221. doi: 10.1021/jacs.9b09415. [DOI] [PubMed] [Google Scholar]
- 50.Yang Z-P, Fu GC. Convergent catalytic asymmetric synthesis of esters of chiral dialkyl carbinols. J. Am. Chem. Soc. 2020;142:5870–5875. doi: 10.1021/jacs.0c01324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bera S, Mao R, Hu X. Enantioselective C(sp3)–C(sp3) cross-coupling of non-activated alkyl electrophiles via nickel hydride catalysis. Nat. Chem. 2021;13:270–277. doi: 10.1038/s41557-020-00576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He Y, Liu C, Yu L, Zhu S. Enantio- and regioselective NiH-catalyzed reductive hydroarylation of vinylarenes with aryl iodides Angew. Chem. Int. Ed. 2020;59:21530–21534. doi: 10.1002/anie.202010386. [DOI] [PubMed] [Google Scholar]
- 53.Liu J, Gong H, Zhu S. Nickel-catalyzed, regio- and enantioselective benzylic alkenylation of olefins with alkenyl bromide. Angew. Chem. Int. Ed. 2021;60:4060–4064. doi: 10.1002/anie.202012614. [DOI] [PubMed] [Google Scholar]
- 54.Shi L, Xing L-L, Hu W-B, Shu W. Regio- and enantioselective Ni-catalyzed formal hydroalkylation, hydrobenzylation and hydropropargylation of acrylamides to α-tertiary amides. Angew. Chem. Int. Ed. 2021;60:1599–1604. doi: 10.1002/anie.202011339. [DOI] [PubMed] [Google Scholar]
- 55.Cuesta-Galisteo S, Schörgenhumer J, Wei X, Merino E, Nevado C. Nickel-catalyzed asymmetric synthesis of α-arylbenzamides. Angew. Chem. Int. Ed. 2021;60:1605–1609. doi: 10.1002/anie.202011342. [DOI] [PubMed] [Google Scholar]
- 56.He Y, Song H, Zhu S. NiH-Catalyzed asymmetric hydroarylation of N-acyl enamines: Practical access to chiral benzylamines. Nat. Commun. 2021;12:638. doi: 10.1038/s41467-020-20888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qian D, Bera S, Hu X. Chiral alkyl amine synthesis via catalytic enantioselective hydroalkylation of enecarbamates. J. Am. Chem. Soc. 2021;143:1959–1967. doi: 10.1021/jacs.0c11630. [DOI] [PubMed] [Google Scholar]
- 58.Wang J-W, et al. Catalytic asymmetric reductive hydroalkylation of enamides and enecarbamates to chiral aliphatic amines. Nat. Commun. 2021;12:1313. doi: 10.1038/s41467-021-21600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X-X, et al. NiH-Catalyzed reductive hydrocarbonation of enol esters and ethers. CCS Chem. 2021;3:727–737. doi: 10.31635/ccschem.021.202000760. [DOI] [Google Scholar]
- 60.Wang S, et al. Enantioselective access to chiral aliphatic amines and alcohols via Ni-catalyzed hydroalkylations. Nat. Commun. 2021;12:2771. doi: 10.1038/s41467-021-22983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou, F. & Zhu, S. Enantioselective hydroalkylation of α,β-unsaturated amides through reversed syn-hydrometallation of NiH. ChemRxiv 10.26434/chemrxiv.13681579.v1 (2021).
- 62.Lv X-Y, Fan C, Xiao L-J, Xie J-H, Zhou Q-L. Ligand-enabled Ni-catalyzed enantioselective hydroarylation of styrenes and 1,3-dienes with arylboronic acids. CCS Chem. 2019;1:328–334. doi: 10.31635/ccschem.019.20190026. [DOI] [Google Scholar]
- 63.Chen Y-G, et al. Nickel-catalyzed enantioselective hydroarylation and hydroalkenylation of styrenes. J. Am. Chem. Soc. 2019;141:3395–3399. doi: 10.1021/jacs.8b13524. [DOI] [PubMed] [Google Scholar]
- 64.Zhang W-B, Yang X-T, Ma J-B, Su Z-M, Shi S-L. Regio- and enantioselective C–H cyclization of pyridines with alkenes enabled by a nickel/N-heterocyclic carbene catalysis. J. Am. Chem. Soc. 2019;141:5628–5634. doi: 10.1021/jacs.9b00931. [DOI] [PubMed] [Google Scholar]
- 65.He Y, Liu C, Yu L, Zhu S. Ligand-enabled nickel-catalyzed redox-relay migratory hydroarylation of alkenes with arylborons. Angew. Chem. Int. Ed. 2020;59:9186–9191. doi: 10.1002/anie.202001742. [DOI] [PubMed] [Google Scholar]
- 66.Yu R, Rajasekar S, Fang X. Enantioselective nickel-catalyzed migratory hydrocyanation of nonconjugated dienes. Angew. Chem. Int. Ed. 2020;59:21436–21441. doi: 10.1002/anie.202008854. [DOI] [PubMed] [Google Scholar]
- 67.Schley ND, Fu GC. Nickel-catalyzed Negishi arylations of propargylic bromides: a mechanistic investigation. J. Am. Chem. Soc. 2014;136:16588–16593. doi: 10.1021/ja508718m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu F-L, et al. Enantioselective copper-catalyzed decarboxylative propargylic alkylation of propargyl β-ketoesters with a chiral ketimine P,N,N-ligand. Angew. Chem. Int. Ed. 2014;53:1410–1414. doi: 10.1002/anie.201309182. [DOI] [PubMed] [Google Scholar]
- 69.Gutierrez O, Tellis JC, Primer DN, Molander GA, Kozlowski MC. Nickel-catalyzed cross-coupling of photoredox-generated radicals: uncovering a general manifold for stereoconvergence in nickel-catalyzed cross-couplings. J. Am. Chem. Soc. 2015;137:4896–4899. doi: 10.1021/ja513079r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ziadi A, Correa A, Martin R. Formal γ-alkynylation of ketones via Pd-catalyzed C–C cleavage. Chem. Commun. 2013;49:4286–4288. doi: 10.1039/C2CC37281A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the main data supporting the findings of this study, including experimental procedures and compound characterization, are available within the article and its supplementary information files, or from the corresponding author upon reasonable request.