Abstract
Introduction:
Men diagnosed with localized prostate cancer must navigate a highly preference-sensitive decision between treatment options with varying adverse outcome profiles. We evaluated whether use of a decision support tool previously shown to decrease decisional conflict also impacted the secondary outcome of post-treatment decision regret.
Methods:
Participants were randomized to receive personalized decision support via the Personal Patient Profile-Prostate or usual care prior to a final treatment decision. Symptoms were measured just before randomization and 6 months later; decision regret was measured at 6 months along with records review to ascertain treatment choices. Regression modeling explored associations between baseline variables including race and D’Amico risk, study group, and 6-month variables regret, choice, and symptoms.
Results:
At 6 months, 287 of 392 (73%) men returned questionnaires of which 257 (89%) had made a treatment choice. Of that group, 201/257 (78%) completely answered the regret scale. Regret was not significantly different between participants randomized to the P3P intervention compared to the control group (p=0.360). In univariate analyses, we found that Black men, men with hormonal symptoms, and men with bowel symptoms reported significantly higher decision regret (all p<0.01). Significant interactions were detected between race and study group (intervention vs usual care) in the multivariable model; use of the Personal Patient Profile-Prostate was associated with significantly decreased decisional regret among Black men (p=0.037). Interactions between regret, symptoms and treatment revealed that a) men choosing definitive treatment and reporting no hormonal symptoms reported lower regret compared to all others; and b) men choosing active surveillance and reporting bowel symptoms had higher regret compared to all others.
Conclusion:
The Personal Patient Profile-Prostate decision support tool may be most beneficial in minimizing decisional regret for Black men considering treatment options for newly-diagnosed prostate cancer.
Keywords: decision regret, prostate cancer, decision support techniques
1.0. Background
A diagnosis of localized prostate cancer (LPC) begins a cascade of events in which clinicians help patients navigate a management plan for a highly preference-sensitive decision, given multiple management options. All options are associated with a profile of long-term adverse outcomes. Prostatectomy, both open and laparoscopic/robotic-assisted, can result in erectile dysfunction and incontinence; radiotherapy of various approaches can result in erectile and bowel dysfunction; adjuvant hormonal deprivation is often a cause of decreased libido, loss of muscle mass,[1] cognitive dysfunction[2] and cardiovascular adverse events.[3] Active surveillance can result in anxiety and uncertainty.[4] Clinicians and researchers have been interested in documenting and addressing decision regret that men may experience once a management option is completed or ongoing, as with active surveillance.
Registry[5–7] and retrospective survey[8, 9] studies, as well as prospective, longitudinal[10, 11] and randomized trials[12, 13] all conducted between 2008-2018 yielded mainly consistent results. Younger age at diagnosis and post-management symptoms were associated with higher regret. In studies with racially diverse samples, African American race has been associated with higher regret.[7, 8, 10, 11] However, none of these studies were conducted within the context of a prospective randomized trial within diverse, multi-site settings.
The Ottawa Decision Support Framework[14] identifies regret as an impact outcome of decision making. Decision support is conceptualized as an intervention that can improve decision quality, in this case, lowering post-decision regret. Understanding which men are more likely to regret a particular decision or more likely to experience less regret with decision support may help clinicians during the options review clinic visit. The purpose of this analysis was to compare decisional regret six-months after enrollment in a randomized trial between usual care and the Personal Patient Profile-Prostate (P3P) decision aid and explore relationships between other six-month outcomes.
2.0. Materials and Methods
The study was approved by Dana-Farber Cancer Institute’s institutional review board and review boards at each site. Participants provided informed consent. The design, procedures and primary outcome (decisional conflict) were published previously. [15] In brief, men with a biopsy-proven diagnosis of prostate cancer, cT1 or cT2 of any risk level, no more than one prior post-biopsy clinician contact and an upcoming consult at an enrolling study site were enrolled from urology and radiation clinics in southern California, Massachusetts, Houston, western New York, Georgia and Virginia. The sample was diverse regarding race, ethnicity and income. Participants were randomized immediately after completing the baseline questionnaires to receive personalized decision support via P3P or usual care (UC) alone. The P3P is a self-administered, web-based intervention [16] that queries the user for personal preferences, values and concerns relevant to LPC in order to provide personalized coaching and education based on the user’s priorities. Video vignettes provide patient-provider communication coaching personalized to race and age. Clinicians received a paper printout summarizing the P3P group participants’ reports. Study outcomes were assessed via on-line or paper questionnaires (participant preference) at baseline and 6 months after enrollment.
2.1. Measures
Demographic data were collected by self-report at baseline. The Expanded Prostate Cancer Index Composite-Clinical Practice (EPIC-CP)[17] is a 16-item version of the 26-item EPIC[18] developed for measuring prostate cancer symptoms in clinical settings. We chose the shorter version as it has performed well with regard to internal consistency and correlations with the EPIC 26 while decreasing respondent burden and administered the 16-item version at baseline and six months after study enrollment. About one year into the study, we added a decision regret (DR)[19] measure as a 6-month outcome. Originally validated in 2003, the DR scale has been used in over 60 published studies.[20] Research coordinators at each clinical site reviewed medical records and documented the baseline D’Amico risk, final treatment choice, as defined by each provider, and the date on which the treatment or surveillance began.
2.2. Statistical Considerations
Baseline characteristics were compared between groups with the Fisher’s exact test and Wilcoxon rank sum test for categorical and continuous variables, respectively. The 6-month treatment choice was summarized descriptively to include exclusive groupings of active surveillance, prostatectomy, external beam radiation, brachytherapy, cryotherapy, and other. EPIC scores were compared between cases with different treatment choices with a Wilcoxon rank sum test. The 5-item DR scale has Likert-type agreement response options ranging from (1) strongly agree to (5) strongly disagree and is scored by calculating the mean of the items and converting to 0-100; higher scores indicate more decision regret.[19] A complete case analysis was conducted including participants with complete DR data. Cronbach’s alpha was calculated to evaluate internal consistency. Due to the high proportion of men with no regret (DR score of 0), a Tobit regression model[21] was used to explore the association between DR, 6-month symptoms and baseline characteristics. Variables of interest included study group (UC, P3P) plus demographics and measures previously known to influence decision regret: race (Black, other), treatment choice (active surveillance, definitive treatment), D’Amico risk (high/intermediate, low), EPIC symptom presence at 6-month. Due to frequent zeros in the EPIC subscale scores, these data were dichotomized to 0 and >0. Univariate analysis first was used to explore the potential association with each variable and DR, then the final multivariable model was selected with stepwise selection considering all possible 2-way interactions. P-values at the 0.20 level were entered in the model and only those at the 0.05 level were retained; study group was required to remain in the model. P-values were 2-sided and considered significant at the <0.05 level. All analyses were performed in SAS 9.4 (SAS Institute, Cary NC) and R version 3.5.1.[22]
3.0. Results
A total of 392 men, including 113 (29%) self-identified black/African American men, were randomized to the P3P group or the UC group. A full description of the primary sample was reported elsewhere.[15] A total of 287 (73%) men returned the 6-month follow-up questionnaire, of whom 257 (89%) had made a treatment choice (included active surveillance). The chosen approach was initiated at a median of four months (range 3-5) prior to returning the 6 month questionnaire.
Of the 257 with a confirmed treatment choice by 6-months, 76 (30%) began active surveillance and 181 (70%) received definitive treatment. EPIC scores were balanced at baseline between active surveillance and definitive treatment groups. At 6-months, men who chose active surveillance reported significantly less severe adverse symptoms including urinary incontinence (p<0.0001), urinary irritation (p=0.03), bowel symptoms (p=0.005), and sexual symptoms (p<0.0001) than men choosing definitive treatments (Table 1). Within the definitive treatment groups, adverse sexual (p<.0001) and incontinence (p<.0001) outcomes were significantly worse for those with surgical treatment compared to radiation. Irritative (p<.0001) and hormonal (p<.0001) symptoms were significantly worse for men with radiation.
Table 1.
Descriptive statistics on 6-month EPIC-CP symptoms by treatment choice
| Definitive treatment type |
||||||
|---|---|---|---|---|---|---|
| Total (n=257) | Active Surv (n=76) | Definitive Tx (n=181) | Other (n=2) | Radiation (n=77) | Surgery (n=102) | |
| Urinary incontinence | ||||||
| Median (IQR) | 2.0 (0.0 - 4.0) | 1.0 (0.0 - 2.0)* | 2.0 (1.0 - 5.0) | - | 1.0 (0.0 - 2.0) | 4.0 (2.0 - 7.0)+ |
| 0 | 75 (29.2%) | 35 (46.1%) | 40 (22.1%) | 0 (0.0%) | 33 (42.9%) | 7 (6.9%) |
| >0 | 172 (66.9%) | 37 (48.7%) | 135 (74.6%) | 2 (100.0%) | 42 (54.5%) | 91 (89.2%) |
| Missing | 10 (3.9%) | 4 (5.3%) | 6 (3.3%) | 0 (0.0%) | 2 (2.6%) | 4 (3.9%) |
| Urinary irritation | ||||||
| Median (IQR) | 3.0 (1.0 - 5.0) | 2.0 (0.2 - 4.0) | 3.0 (1.0 - 5.0) | - | 4.0 (2.0 - 6.0)~ | 2.0 (1.0 - 4.0) |
| 0 | 44 (17.1%) | 18 (23.7%) | 26 (14.4%) | 1 (50.0%) | 9 (11.7%) | 16 (15.7%) |
| >0 | 200 (77.8%) | 52 (68.4%) | 148 (81.8%) | 1 (50.0%) | 67 (87.0%) | 80 (78.4%) |
| Missing | 13 (5.1%) | 6 (7.9%) | 7 (3.9%) | 0 (0.0%) | 1 (1.3%) | 6 (5.9%) |
| Hormonal | ||||||
| Median (IQR) | 1.0 (0.0 - 4.0) | 1.0 (0.0 - 3.0) | 2.0 (0.0 - 4.0) | - | 3.0 (1.0 - 5.0)~ | 1.0 (0.0 - 3.0) |
| 0 | 82 (31.9%) | 26 (34.2%) | 56 (30.9%) | 2 (100.0%) | 12 (15.6%) | 42 (41.2%) |
| >0 | 159 (61.9%) | 44 (57.9%) | 115 (63.5%) | 0 (0.0%) | 62 (80.5%) | 53 (52.0%) |
| Missing | 16 (6.2%) | 6 (7.9%) | 10 (5.5%) | 0 (0.0%) | 3 (3.9%) | 7 (6.9%) |
| Bowel | ||||||
| Median (IQR) | 0.0 (0.0 - 2.0) | 0.0 (0.0 - 1.0)* | 0.0 (0.0 - 3.0) | - | 1.0 (0.0 - 4.0) | 0.0 (0.0 - 2.0) |
| 0 | 145 (56.4%) | 52 (68.4%) | 93 (51.4%) | 2 (100.0%) | 36 (46.8%) | 55 (53.9%) |
| >0 | 104 (40.5%) | 21 (27.6%) | 83 (45.9%) | 0 (0.0%) | 40 (51.9%) | 43 (42.2%) |
| Missing | 8 (3.1%) | 3 (3.9%) | 5 (2.8%) | 0 (0.0%) | 1 (1.3%) | 4 (3.9%) |
| Sexual | ||||||
| Median (IQR) | 6.0 (3.0 - 9.0) | 3.0 (1.0 - 5.0)* | 8.0 (5.0 - 10.0) | - | 6.0 (3.8 - 8.0) | 8.0 (6.0 - 10.0)+ |
| 0 | 19 (7.4%) | 14 (18.4%) | 5 (2.8%) | 0 (0.0%) | 4 (5.2%) | 1 (1.0%) |
| >0 | 219 (85.2%) | 55 (72.4%) | 164 (90.6%) | 2 (100.0%) | 68 (88.3%) | 94 (92.2%) |
| Missing | 19 (7.4%) | 7 (9.2%) | 12 (6.6%) | 0 (0.0%) | 5 (6.5%) | 7 (6.9%) |
Note: EPIC-CP=Expanded Prostate Cancer Index Composite-Clinical Practice; Surv=Surveillance; Tx=Treatment IQR=Interquartile range
Significantly different between active surveillance and active treatments
Surgery significantly worse than radiation
Radiation significantly worse than surgery
DR questionnaires were completed fully by 201 of the 207 (97%) participants who had made a decision and also received the DR questionnaire (Figure 1). The DR scale had good internal consistency with a Cronbach’s alpha of 0.86. Overall, 76 (38%) indicated no DR with score 0; these were split evenly, 38 (39%) and 38 (36%), in the P3P and UC groups, respectively. The median DR score was 10 (range 0-25) and 15 (range 0-25), and the mean for those with a non-0 DR score was 14.38 (SD=16.32) and 17.07 (SD=19.04), in the P3P and UC groups, respectively. Regret was not significantly different between participants randomized to the P3P intervention compared to the control group (p=0.36). Additional univariate analyses revealed black/African American men reported an estimated 10 points higher DR score (p=0.02) compared with others, men with any hormonal symptoms reported an estimated 11.3 points higher DR score (p=0.009) compare with those without any hormonal symptom, and men with any bowel symptoms had an estimated 8.3 points higher DR score (p=0.03) compared with those without any bowel symptom. Men who chose active surveillance had marginally higher DR scores (est=7.96, p=0.07) (Table 2).
Figure 1.
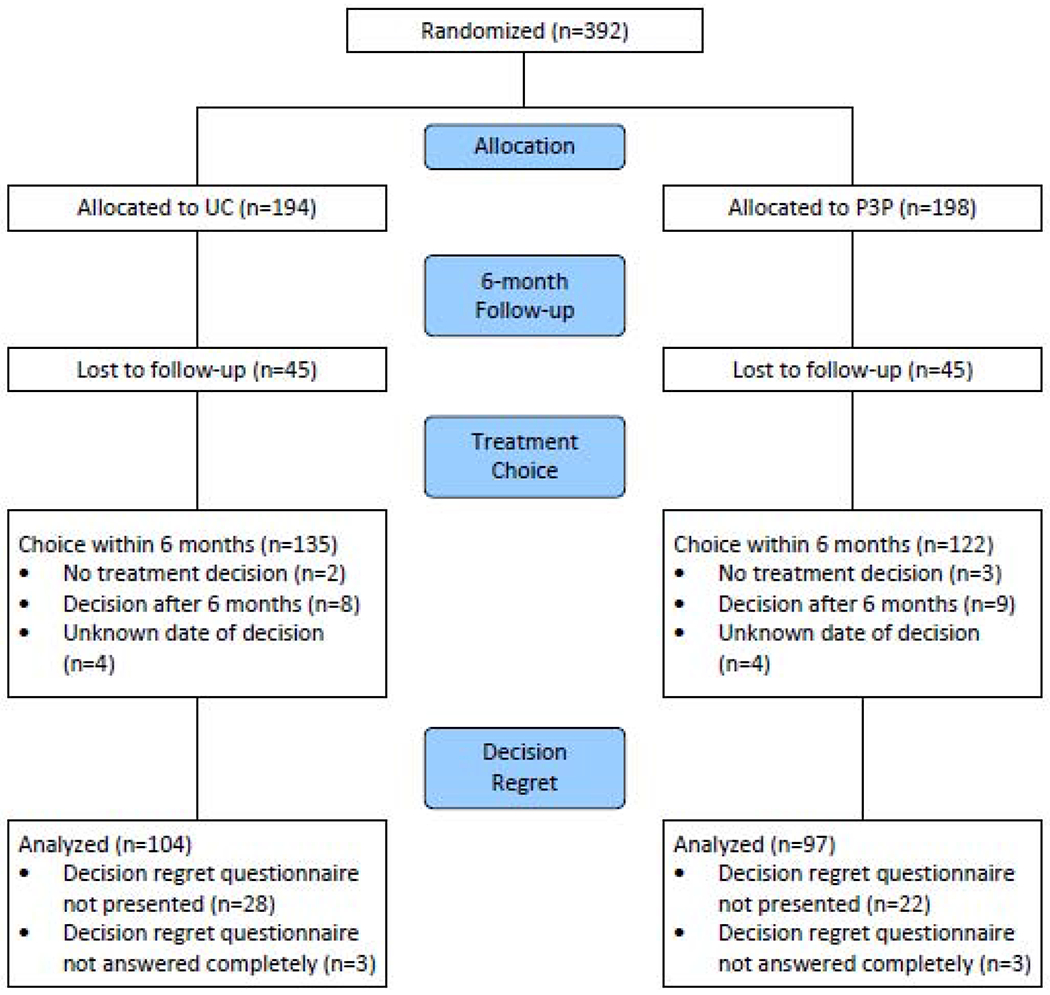
CONSORT diagram for Personal Patient Profile-Prostate II trial
Table 2.
Tobit regression for 6-month decisional regret
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Est. Coeff | Std. Error | P | Est. Coeff | Std. Error | P | |
| Def Tx vs Act Surv | −7.96 | 4.32 | 0.07 | −18.11 | 7.35 | 0.01 |
| B/AA vs other race | 10.03 | 4.29 | 0.02 | 3.37 | 6.34 | 0.60 |
| UC vs P3P | 3.55 | 3.88 | 0.36 | 0.04 | 4.26 | 0.99 |
| Hormone >0 vs 0 | 11.31 | 4.31 | 0.009 | −7.95 | 7.59 | 0.30 |
| Bowel >0 vs 0 | 8.30 | 3.86 | 0.03 | 27.26 | 7.31 | <0.001 |
| B/AA*UC † | - | - | - | 15.90 | 8.22 | 0.05 |
| DefTx*Hormone >0† | - | - | - | 26.64 | 9.22 | 0.004 |
| DefTx*Bowel >0 † | - | - | - | −30.59 | 8.91 | <0.001 |
Note: Est. Coeff= Estimated coefficient; Def Tx=Definitive treatment; Act Surv=Active Surveillance; B/AA=Black/African American; UC=Usual care; P3P=Personal Patient Profile-Prostate; Surv=Surveillance; Tx=Treatment
Figure 2 illustrates interaction effects
In the final multivariable Tobit model, (Table 2), significant interactions were detected between race and study group; Black men in the UC group had higher DR compared to all others (Figure 2A, p=0.05). Men who chose definitive treatment and reported no hormonal symptoms at 6-month reported lower DR compared to all others (Figure 2B, p=0.004). Men in active surveillance and reporting bowel symptoms at 6-month had higher DR compared to all others (Figure 2C, p <0.001). Due to possible influential variables that could have impacted model results, the DR score was ranked and the Tobit model was fit for a sensitivity analysis. The same factors were found to be associated with the ranking of DR.
Figure 2: Mean predicted decisional regret score for cases that chose active surveillance or definite treatment, and (A) by race (black vs others), (B) by hormonal symptom at 6-month (presence vs absence), (C) by bowel symptom at 6-month (presence vs absence).
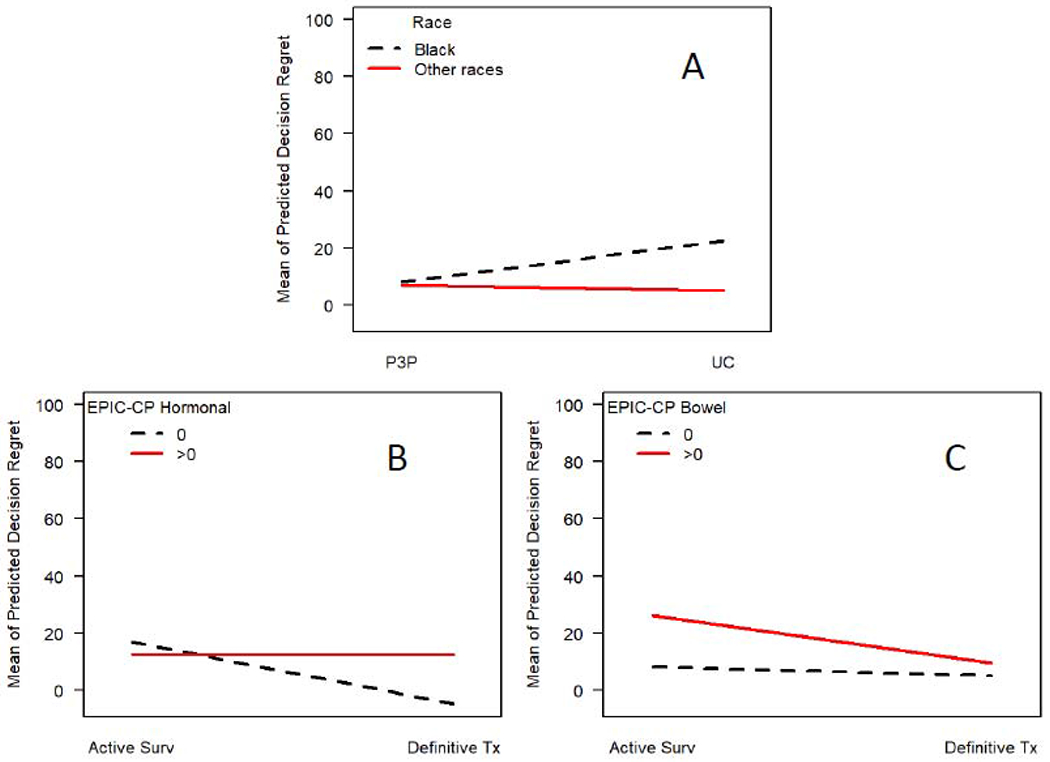
Note: P3P=Personal Patient Profile-Prostate; UC=Usual Care EPIC-CP=Expanded Prostate Cancer Index Composite-Clinical Practice; Surv=Surveillance; Tx=Treatment
4.0. Discussion
In a diverse sample of men recently diagnosed with localized prostate cancer, 6-month outcome measures revealed differential decision regret based on study group interacting with race, symptoms and treatment choice. The significant interaction between study group and Black race for regret at 6 months in our trial suggested particular benefit of P3P for Black men in comparison to other races. Feldman-Stewart and colleagues[23] demonstrated significantly lower regret with use of a values clarification decision aid in a sample of Canadian men, yet race and ethnicity were neither reported or analyzed. No other randomized intervention trial of a decision aid for localized prostate cancer has published differential race effects on regret. However, Diefenbach et al. [24] did establish race as a significant moderator of a decision aid’s effect on decisional support in that African American men reported greater decision support with the intervention than white men. These findings also are consistent with a meta-analysis describing the ability of shared decision making to reduce health inequalities.[25]
Associations between adverse outcomes and decision regret in men with localized prostate cancer have been confirmed by a number of investigators. The first systematic review of regret[26] following treatment for localized prostate cancer, published in 2015, concluded that post-treatment adverse symptomatology was the dominant reason for increased decision regret. While P3P educates and coaches men regarding treatments, outcomes and communication with clinicians, the intervention does not dictate to the user what an optimal choice would be. Therefore, our findings of higher rates of active surveillance in low risk levels without a significant difference by study group is not surprising. These findings were similar to those in a 2019 publication of randomized trial of a decision aid for localized prostate cancer completed in the metropolitan Philadelphia region.[27]
The influence of having any hormonal symptoms on regret in this current trial was not different between treatment choices overall. The four items included in the hormonal EPIC-CP subscale (hot flashes/breast enlargement, depression & energy) may have conceptually conflated symptoms that can be associated with a variety of conditions. Depressive symptoms and lack of energy are not exclusively associated with hormonal therapy and are certain to have occurred in some men making choices of active surveillance or surgery. Using the same regret scale as in our study and the Prostate 25 (PR25)[28] for symptoms in a multivariable analysis of post-choice regret in men with localized prostate cancer, van Siam et al.[29] reported that hormonal/masculinity-related symptoms were significantly associated with regret. However, the hormonal/masculinity-related symptom scores were driven by one item, feeling less masculine as a result of illness or treatment, not measured on the EPIC-CP. The measures of hormonal symptoms may be conceptually distinct between the PR25 and the EPIC-CP.
Participant report of bowel symptoms at 6 months was associated with higher regret in both univariate and multivariate models. In our previous P3P trial,[12] the presence of 6-month bowel symptoms was a significant predictor of decision regret. Bowel symptoms interacted with whether men had chosen definitive treatment or not, with higher regret in men experiencing bowel symptoms while undergoing active surveillance. It is possible that these men regretted the choice when they experienced a side effect of radiation without ever having radiation.
Our analysis of DR has several limitations. The proportion of Black men in our sample was lower than known diagnosis rates in the United States, where more Black men are diagnosed more often than any other racial or ethnic group.[30] The sample size for the randomized trial was not computed based on regret, an outcome added partially through the trial. Clinicians were aware which participants were in the intervention group; this may have changed clinician behavior and perhaps could have influenced future regret. Six months after baseline may not be the ideal time point for measuring regret given that some men may not have recovered fully from all active treatment. And, as discussed above, the EPIC-CP may not be a conceptually parsimonious measure for research analyses.
4.1. Conclusion
The P3P decision aid did not directly impact regret for the entire sample. However, there may be benefit for Black men in preparation for localized prostate cancer treatment decisions by facilitating less regretful decisions.
Highlights.
Regret was not significantly different between the intervention and control group.
Regret was significantly associated with Black race, hormonal and bowel symptoms.
The intervention was associated with significantly decreased regret in Black men.
Acknowledgements
The authors wish to thank the men who volunteered to enroll in this trial and the research coordinators at each site, the development team at the University of Washington and central office staff at Dana-Farber.
Funding: National Institutes of Health, National Institute for Nursing Research R01NR009692
Abbreviations
- EPIC-CP
Expanded Prostate Cancer Index Composite-Clinical Practice
- DR
decision regret
- UC
usual care
- P3P
Personal Patient Profile-Prostate
- SD
standard deviation
- est
estimate
- PR25
Prostate 25
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: NCT01844999
Ethics approval and consent to participate
The study was approved by Dana-Farber Cancer Institute’s institutional review board and review boards at each site. All participants provided informed consent.
Consent to publish: N/A
Availability of data and materials
Supporting de-identified data may be shared; please contact the first author.
Competing interests
None
References
- [1].Chou R et al. , “U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews,” in Treatments for Localized Prostate Cancer: Systematic Review to Update the 2002 U.S. Preventive Services Task Force Recommendation. Rockville (MD): Agency for Healthcare Research and Quality (US), 2011. [PubMed] [Google Scholar]
- [2].Sun M et al. , “Cognitive Impairment in Men with Prostate Cancer Treated with Androgen Deprivation Therapy: A Systematic Review and Meta-Analysis,” (in eng), The Journal of urology, February 1 2018, doi: 10.1016/j.juro.2017.11.136. [DOI] [PubMed] [Google Scholar]
- [3].Gupta D, Lee Chuy K, Yang JC, Bates M, Lombardo M, and Steingart RM, “Cardiovascular and Metabolic Effects of Androgen-Deprivation Therapy for Prostate Cancer,” (in eng), Journal of oncology practice / American Society of Clinical Oncology, vol. 14, no. 10, pp. 580–587, October 2018, doi: 10.1200/jop.18.00178. [DOI] [PubMed] [Google Scholar]
- [4].Parker PA et al. , “Relationship between illness uncertainty, anxiety, fear of progression and quality of life in men with favourable-risk prostate cancer undergoing active surveillance,” (in eng), BJU international, vol. 117, no. 3, pp. 469–77, March 2016, doi: 10.1111/bju.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hoffman RM et al. , “Treatment Decision Regret Among Long-Term Survivors of Localized Prostate Cancer: Results From the Prostate Cancer Outcomes Study,” (in eng), Journal of clinical oncology : official journal of the American Society of Clinical Oncology, vol. 35, no. 20, pp. 2306–2314, July 10 2017, doi: 10.1200/jco.2016.70.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Albkri A, Girier D, Mestre A, Costa P, Droupy S, and Chevrot A, “Urinary Incontinence, Patient Satisfaction, and Decisional Regret after Prostate Cancer Treatment: A French National Study,” (in eng), Urologia internationalis, vol. 100, no. 1, pp. 50–56, 2018, doi: 10.1159/000484616. [DOI] [PubMed] [Google Scholar]
- [7].Mahal BA et al. , “The association between race and treatment regret among men with recurrent prostate cancer,” (in eng), Prostate cancer and prostatic diseases, vol. 18, no. 1, pp. 38–42, March 2015, doi: 10.1038/pcan.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schroeck FR et al. , “Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy,” (in eng), European urology, vol. 54, no. 4, pp. 785–93, October 2008, doi: S0302-2838(08)00764-1 [pii] 10.1016/j.eururo.2008.06.063. [DOI] [PubMed] [Google Scholar]
- [9].Lavery HJ et al. , “Baseline functional status may predict decisional regret following robotic prostatectomy,” (in eng), The Journal of urology, vol. 188, no. 6, pp. 2213–8, December 2012, doi: 10.1016/j.juro.2012.08.016. [DOI] [PubMed] [Google Scholar]
- [10].Morris BB et al. , “Treatment decisional regret among men with prostate cancer: Racial differences and influential factors in the North Carolina Health Access and Prostate Cancer Treatment Project (HCaP-NC),” (in eng), Cancer, vol. 121, no. 12, pp. 2029–35, June 15 2015, doi: 10.1002/cncr.29309. [DOI] [PubMed] [Google Scholar]
- [11].Hurwitz LM et al. , “Longitudinal regret after treatment for low- and intermediate-risk prostate cancer,” (in eng), Cancer, vol. 123, no. 21, pp. 4252–4258, November 1 2017, doi: 10.1002/cncr.30841. [DOI] [PubMed] [Google Scholar]
- [12].Berry DL, Wang Q, Halpenny B, and Hong F, “Decision preparation, satisfaction and regret in a multi-center sample of men with newly diagnosed localized prostate cancer,” (in eng), Patient education and counseling, Multicenter Study Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t vol. 88, no. 2, pp. 262–7, August 2012, doi: 10.1016/j.pec.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].van Tol-Geerdink JJ et al. , “Does a decision aid for prostate cancer affect different aspects of decisional regret, assessed with new regret scales? A randomized, controlled trial,” (in eng), Health expectations : an international journal of public participation in health care and health policy, vol. 19, no. 2, pp. 459–70, April 2016, doi: 10.1111/hex.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].“Ottawa Decision Support Framework.” Ottawa Hospital Research Institute. https://decisionaid.ohri.ca/odsf.html (accessed 28 July, 2020). [Google Scholar]
- [15].Berry DL et al. , “Decision Support with the Personal Patient Profile-Prostate: A Multi-Center Randomized Trial,” (in eng), The Journal of urology, vol. 199, pp. 89–97, July 25 2018, doi: 10.1016/j.juro.2017.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Berry DL et al. , “Development and evaluation of the personal patient profile-prostate (P3P), a Web-based decision support system for men newly diagnosed with localized prostate cancer,” (in eng), Journal of medical Internet research, vol. 12, no. 4, p. e67, 2010, doi: v12i4e67 [pii] 10.2196/jmir.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chang P et al. , “Expanded prostate cancer index composite for clinical practice: development and validation of a practical health related quality of life instrument for use in the routine clinical care of patients with prostate cancer,” (in eng), The Journal of urology, Research Support, N.I.H., Extramural Validation Studies vol. 186, no. 3, pp. 865–72, September 2011, doi: 10.1016/j.juro.2011.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Szymanski KM, Wei JT, Dunn RL, and Sanda MG, “Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors,” (in eng), Urology, vol. 76, no. 5, pp. 1245–50, November 2010, doi: S0090-4295(10)00100-7 [pii] 10.1016/j.urology.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brehaut JC et al. , “Validation of a decision regret scale,” (in eng), Medical decision making : an international journal of the Society for Medical Decision Making, vol. 23, no. 4, pp. 281–92, Jul-Aug 2003. [Online]. Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12926578. [DOI] [PubMed] [Google Scholar]
- [20].Becerra Perez MM, Menear M, Brehaut JC, and Legare F, “Extent and Predictors of Decision Regret about Health Care Decisions: A Systematic Review,” (in eng), Medical decision making : an international journal of the Society for Medical Decision Making, vol. 36, no. 6, pp. 777–90, August 2016, doi: 10.1177/0272989x16636113. [DOI] [PubMed] [Google Scholar]
- [21].Yee TW, Vector Generalized Linear and Additive Models: With an Implementation in R. New York, NY: Springer, Inc, 2015. [Google Scholar]
- [22].A language and environment for statistical computing. (2018). R Foundation for Statistical Computing, Vienna, Austria. [Online]. Available: https://www.R-project.org/ [Google Scholar]
- [23].Feldman-Stewart D et al. , “The impact of explicit values clarification exercises in a patient decision aid emerges after the decision is actually made: evidence from a randomized controlled trial,” (in eng), Medical decision making : an international journal of the Society for Medical Decision Making, Multicenter Study Randomized Controlled Trial Research Support, Non-U.S. Gov’t vol. 32, no. 4, pp. 616–26, Jul-Aug 2012, doi: 10.1177/0272989X11434601. [DOI] [PubMed] [Google Scholar]
- [24].Diefenbach MA et al. , “Examining the impact of a multimedia intervention on treatment decision-making among newly diagnosed prostate cancer patients: results from a nationwide RCT,” (in eng), Translational behavioral medicine, vol. 8, no. 6, pp. 876–886, November 21 2018, doi: 10.1093/tbm/iby066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Durand MA et al. , “Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis,” (in eng), PLoS One, vol. 9, no. 4, p. e94670, 2014, doi: 10.1371/journal.pone.0094670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Christie DR, Sharpley CF, and Bitsika V, “Why do patients regret their prostate cancer treatment? A systematic review of regret after treatment for localized prostate cancer,” (in eng), Psycho-oncology, vol. 24, no. 9, pp. 1002–11, September 2015, doi: 10.1002/pon.3776. [DOI] [PubMed] [Google Scholar]
- [27].Jayadevappa R et al. , “Patient-Centered Preference Assessment to Improve Satisfaction With Care Among Patients With Localized Prostate Cancer: A Randomized Controlled Trial,” (in eng), Journal of clinical oncology : official journal of the American Society of Clinical Oncology, p. Jco1801091, March 12 2019, doi: 10.1200/jco.18.01091. [DOI] [PubMed] [Google Scholar]
- [28].van Andel G et al. , “An international field study of the EORTC QLQ-PR25: a questionnaire for assessing the health-related quality of life of patients with prostate cancer,” (in eng), Eur J Cancer, vol. 44, no. 16, pp. 2418–24, November 2008, doi: 10.1016/j.ejca.2008.07.030. [DOI] [PubMed] [Google Scholar]
- [29].van Stam M-A et al. , “Patient-reported Outcomes Following Treatment of Localised Prostate Cancer and Their Association with Regret About Treatment Choices,” European Urology Oncology, In Press, doi: 10.1016/j.euo.2018.12.004. [DOI] [PubMed] [Google Scholar]
- [30].U.S._Cancer_Statistics_Working_Group. “U.S. Cancer Statistics Data Visualizations Tool.” Center_for_Disease_Control_and_Preventio. https://gis.cdc.gov/Cancer/USCS/DataViz.html (accessed 11 Dec, 2019). [Google Scholar]


