Abstract
Objectives:
While adults with bipolar disorder (BD) often report symptoms starting in childhood, continuity of mania and/or hypomania (mania/hypomania) from childhood to adulthood has been questioned. Using longitudinal data from the Course and Outcome of Bipolar Youth (COBY) study, we assessed threshold mania/hypomania in young adults who manifested BD as youth.
Methods:
COBY is a naturalistic, longitudinal study of 446 youth with BD (84% recruited from outpatient clinics), 7–17 years old at intake, and over 11 years of follow-up. Focusing on youth with BD-I/II (n=297), we examined adult mania/hypomania risk (≥18 years old; mean 7.9 years of follow-up) according to child (<13 years old) vs. adolescent (13–17 years old) onset. We next used penalized regression to test demographic and clinical predictors of young adult mania/hypomania.
Results:
Most participants (64%) had child-onset mania/hypomania, 57% of whom also experienced mania/hypomania in adolescence. Amongst those who experienced an episode in adolescence, over 40% also had mania/hypomania during adulthood; the risk did not differ according to child vs. adolescent onset. In contrast, 7% with mania/hypomania in childhood, but not adolescence, experienced mania/hypomania in adulthood. Family history (of mania and suicide attempts) predicted mania/hypomania in young adulthood (p-values<.05); age of onset was not a significant predictor. Amongst participants with no mania/hypomania during adulthood, 53% (105/198) still experienced subthreshold manic episodes.
Discussion:
We find substantial continuity across developmental stage indicating that, in this carefully characterized sample, children who experience mania/hypomania--particularly those who also experience mania/hypomania in adolescence--are likely to experience mania/hypomania in young adulthood.
Keywords: bipolar disorder, child and adolescent, young adulthood, longitudinal study, mania, hypomania, predictors
1. INTRODUCTION
In clinical studies, over half of adults with bipolar disorder (BD) report onset of their illness prior to 18 years of age, and some report mood disorder onset younger than 13 years old1,2. In retrospective studies of adults with BD, early onset of illness, particularly onset before the age of 13, has been associated with a worse course (e.g. less euthymia, more rapid cycling), more comorbidity, more suicidality, and a stronger family history of mood disorder3–6. Our group previously compared youth with BD-I in the Course and Outcome of Bipolar Youth (COBY) study to adults with BD-I in the Collaborative Depression Study and found that, similar to the adults, BD youths over follow-up mainly have subsyndromal mood symptoms and depression, but youth showed more time symptomatic, more mixed episodes, and more polarity switches7. An updated meta-analysis of epidemiologic studies found the rate of bipolar sub/threshold disorder in children and adolescents to be 3.9%, and the rate of bipolar I disorder to be 0.6%.8 Given the disease burden conferred by BD, the earliest possible identification of BD in youths is a clinical and public health need of great urgency.9
Despite the evidence that early-onset BD exists, and that it portends a worsening course, the continuity between mania and hypomania (hereafter, mania/hypomania) in childhood and adulthood has been questioned10,11. Adding further to the skepticism are the results of population-based prospective studies suggesting that <10% of youth who report subthreshold manic states (and even hypomanic episodes) during adolescence experience classic mania/hypomania in adulthood12,13. However, given methodological limitations, such as the lower sensitivity and specificity of epidemiologic instruments (compared to gold-standard clinical assessments) and the absence of a collateral informant on mood symptoms (which may be key during adolescence and young adulthood14), their ability to fully address the question of mania/hypomania continuity is constrained.
Previous COBY analyses assessed differences between youth with child- and adolescent-onset BD at intake and found that youth with child-onset (vs. adolescent-onset) BD had higher levels of mood lability during manic/hypomanic episodes, higher rates of attention deficit hyperactivity disorder (ADHD) and lower rates of substance use disorder (SUD), and higher rates of family history of both mood and non-mood psychopathology15,16. While analyses from the COBY study indicate that mood symptoms in general persist into young adulthood for these youth17,18, the presence vs. absence of mania/hypomania in young adulthood, and how this may differ for youth with child- vs. adolescent-onset mania/hypomania, have not been directly assessed. In fact, to our knowledge, there have not been any clinical studies to prospectively assess the continuity of mania/hypomania across childhood, adolescence, and young adulthood. The presence vs. absence of mania/hypomania continuity has important clinical implications regarding the assessment, prognosis, and treatment of youth who meet diagnostic criteria for BD.
Using data from the COBY study, with over 11 years of follow-up of a clinical sample of children and adolescents diagnosed with BD, we now have an opportunity to longitudinally assess the continuity of mania/hypomania across developmental stages. In the current analysis, we include only participants diagnosed with BD-I/II before 18 years old, who have at least one follow-up visit during adulthood. The main goals of this analysis are to assess: (1) continuity between mania/hypomania in childhood, adolescence, and adulthood and (2) predictors of mania/hypomania continuity into young adulthood. We report not only on threshold mania/hypomania, but also whether subthreshold manic episodes (i.e. episodes not meeting full symptom and/or duration criteria for hypomania or mania; see definition in Methods) continue into young adulthood, since even subthreshold episodes have previously shown to be associated with high levels of impairment, substance use, and suicidality19–25.
2. METHOD
2.1. Participants
COBY methods have previously been described in detail 26,27 (also see eMethods). Briefly, 446 youths aged 7–17 years old at intake with DSM-IV BD-I or II28, or an operationally defined BD not otherwise specified (BD-NOS), were recruited at Brown University, University of California Los Angeles, and the University of Pittsburgh. BD youths were recruited from outpatient clinics (84.4%), inpatient units (4.4%), advertisements (6.7%), and referrals from other physicians (4.4%), and were enrolled independent of current BD state or treatment status, from October 2000 through July 2006. Youths with schizophrenia, autism, or an Intelligence Quotient (IQ) ≤7029, and mood disorders secondary to use of substances, medications, or medical conditions were excluded. Each university’s Institutional Review Board approved all study procedures. Thus far, youths have been prospectively interviewed approximately every 7 months for a median of 11.7 years, with a retention rate of 83%. The analyses presented in this report are based upon the prospective evaluation of 297 youth who had follow-up through the age of 18 years old; and met criteria for BD-I or -II by the age of 18 years old.
2.2. Instruments
At intake, youth and parents (about their children) were directly interviewed for the presence of current and lifetime psychiatric disorders using the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL).30 At intake, parents were also interviewed using the Structured Clinical Interview for DSM-IV disorders (SCID)31. At intake and throughout follow-up, family psychiatric history was obtained using the Family History Screen32. Socioeconomic status (SES) was assessed at intake and throughout follow-up using the Hollingshead Scale.33
During follow-ups, the Longitudinal Interval Follow-up Evaluation (LIFE)34 was used to assess week-by-week changes in mood symptoms, other psychiatric symptoms, and medication use; symptomatology was quantified using the Psychiatric Status Rating (PSR) Scale, which is operationally linked to the DSM-IV criteria. For DSM-IV mood disorder symptoms (including mania/hypomania), the PSR scores ranged from 1 to 2 for no/minimal symptoms, 3 to 4 for clinically relevant subthreshold symptoms and impairment, and 5 to 6 for full threshold criteria with increasing degrees of severity or impairment. For example, to score 5 or 6 on the PSR hypomania scale, a participant would need to meet full DSM-IV symptom criteria (elated mood + 3 associated symptoms; or irritability + four associated symptoms), duration criteria (≥4 days), and a change in function. A score of 3 or 4 on the PSR, corresponding to subthreshold mania, would require a cluster of manic symptoms lasting for at least ½ of the day and causing impairment, but not meeting full duration criteria (<4 days) and/or symptom criteria (i.e. one symptom short) (see eMethods for PSR anchors).
The PSR is not a rating scale, but rather a clinical assessment that takes into account all available data, including functional impairment. Clinicians administering the PSR during follow-up utilize past diagnostic data and mood course (including previous PSR ratings) to anchor questioning about the most recent interval. All assessments were completed by research staff with extensive clinical research experience, who were trained to administer the instruments with a high level of reliability within and between the sites. Importantly, all cases were reviewed with a child psychiatrist/psychologist who was ultimately responsible for the final PSR ratings for the participant. The overall K-SADS kappas for psychiatric disorders were ≥0.818. The PSR Intraclass Correlation (ICC) for full threshold DSM-IV mood episodes was 0.85. The ICC for subsyndromal mood symptoms was 0.82. Reliability for PSR mood disorder ratings over the course of COBY was an average Kendall’s W of 0.826.
2.3. Statistical Analysis
We first assessed univariate differences amongst youth with mania/hypomania during (1) childhood only (<13 years; child-only); (2) both childhood and adolescence (13–17 years; child/adolescent); and (3) adolescence only (adolescent-only). For youth with a threshold manic/hypomanic (including mixed) episode prior to intake, we assessed age of mania/hypomania onset using the KSADS-PL. This was defined retrospectively as the minimum age for a manic, hypomanic, or mixed episode. For youth with an intake diagnosis of BD-NOS, who had a first episode of threshold mania/hypomania during follow-up, we defined age of onset as the presence of the first DSM-IV threshold score for hypomania or mania on the PSR; this could be either a pure manic/hypomanic episode or a mixed episode, depending on whether they had concurrent depressive features. To determine age at most recent threshold manic/hypomanic week (<18 years old), we used PSR data for those with ≥1 mania/hypomania over follow-up. For those without threshold mania/hypomania during child/adolescent follow-up, we used the age of offset for the most recent manic/hypomanic or mixed episode prior to intake; this was interpreted as the last week of mania/hypomania prior to intake.
We next assessed for the presence vs. absence of mania/hypomania during young adulthood, based on the maximum PSR for mania and/or hypomania during this time period. Those with a maximum PSR of 5–6 (on the mania and/or hypomania line) were considered to have threshold mania/hypomania. Those with a PSR 3–4 on the hypomania line were considered to have subthreshold mania. We used logistic regression to compare the risk of mania/hypomania during young adulthood across group (child-only, child/adolescent, and adolescent-only). To account for unequal length of follow-up, all analyses were adjusted for length of follow-up. While our primary analyses focused on mania/hypomania, we conducted a supplemental analysis only including youth diagnosed with BD-I by age 18, assessing the proportion of youth who experienced mania (and temporally associated hospitalization) in adulthood.
Then, we assessed the clinical and demographic predictors of threshold mania/hypomania during young adulthood. For variable selection purposes, we conducted a penalized (least absolute shrinkage and selection operator; lasso) regression in R (package glmnetUtils) to model the outcome (young adult mania/hypomania; modeled using a logistic regression); cross-validation selected the optimal regularization parameter via the one-standard-error rule 35. We report penalized exponentiated beta coefficients. We computed p-values by entering variables selected by the lasso regression model into a standard, unpenalized logistic regression model.
Next, we used logistic regression to assess whether the probability of young adult mania/hypomania was related to the frequency of threshold mood symptoms (mania/hypomania and depression) during adolescence. We specifically assessed the final two years of adolescence and only included in this analysis participants who were recruited prior to the age of 16, so that they had follow-up data throughout this period. Models were adjusted for covariates selected by the lasso regression above.
To address the possibility that maintenance mood stabilizing medication were impacting our findings, we conducted a (post-hoc) analysis comparing the percent time on anti-manic medications (defined as lithium, antimanic anticonvulsants, or antipsychotics) in participants who experienced vs. did not experience a manic/hypomanic episode during young adulthood. Next, during young adult visits of the COBY study, we collected data from an additional informant where possible; this was most often a parent but could also be another family member or significant other with substantial knowledge of the participant’s psychiatric symptoms. However, for many young adult participants, this was not possible. Thus, in a second post-hoc analysis, we tested whether the presence (vs. absence) of an additional informant at each visit (nested within participant) impacted the observed risk of threshold mania/hypomania.
3. RESULTS
3.1. Demographic and Clinical Characteristics for Youth With BD-I/II by Age 18 Years Old
In the COBY sample, 297 youth had a BD-I/II diagnosis by the age of 18 (and follow-up through 18 years old). Participants had a median of 13.3 years of follow-up and 16 follow-up visits (Table 1). Most participants (74.1%, 220/297) were on lithium and/or an antimanic anticonvulsant medication at some point before 18, although we do not know for certain that these medications were prescribed for mania per se. Most participants (71.4%, 212/297) were hospitalized at least once before 18, indicating significant impairment. Demographic and clinical differences between participants who experienced threshold mania/hypomania in childhood (but not adolescence) (child-only), childhood and adolescence (child/adolescent), and adolescent-only are shown in eTable 1.
Table 1.
Demographic, Clinical, and Family History Characteristics of Youth with BD-I/II Diagnosed Before 18 Years Old (n=297)
| Demographics | |
| Sex: n (%) Female | 143 (48.15%) |
| Intake Age: Mean (SD) | 12.85 (3.26) |
| Race: n (%) Non-White | 46 (15.49%) |
| SES: Mean (SD) | 3.41 (1.21) |
| Total Follow-Up Duration (years): Median (Q1,Q3) | 13.32 (12.00,14.81) |
| Number of Total Follow-Up Visits: Median (Q1,Q3) | 16 (12,20) |
| Adult Follow-up Duration (years): Median (Q1,Q3) | 7.76 (2.48,6.70) |
| Number of Adult Follow-Up Visits: Median (Q1,Q3) | 8 (5,13) |
| Clinical Variables (up to age 18 years) | |
| BD Subtype | |
| BD-I: n (%) | 252 (84.85%) |
| BD-II: n (%) | 45 (15.15%) |
| Major Depressive Episode: n (%) | 256 (86.20%) |
| Age at Mania/hypomania Onset: Median (Q1,Q3) | 11.43 (7.89,14.27) |
| Age at Oldest Mania/hypomania: Median (Q1,Q3) | 15.61 (12.62,17.19) |
| >1 Manic/hypomanic Episode: n (%) | 199 (67.00%) |
| Anxiety: n (%) | 196 (65.99%) |
| ADHD: n (%) | 194 (65.32%) |
| DBD: n (%) | 180 (60.61%) |
| SUD: n (%) | 72 (24.24%) |
| Treatment and Hospitalization (up to age 18 years) | |
| Medication History | |
| Antipsychotic: n (%) | 223 (75.08%) |
| Antimanic Anticonvulsant: n (%) | 139 (46.80%) |
| Lithium: n (%) | 137 (46.13%) |
| Lamictal: n (%) | 75 (25.25%) |
| ≥1 Inpatient Hospitalization: n (%) | 212 (71.38%) |
| Family History (1st or 2nd Degree Relative) | |
| Mania: n(%) | 183 (61.62%) |
| Suicide Attempt: n(%) | 134 (45.12%) |
| Anxiety: n(%) | 224 (75.42%) |
| SUD: n(%) | 214 (72.05%) |
| Psychosis : n(%) | 58 (19.53%) |
| Depression: n(%) | 260 (87.54%) |
3.2. Probability of Threshold Mania/Hypomania in Childhood, Adolescence, and Young Adulthood (Figure 1)
Figure 1.
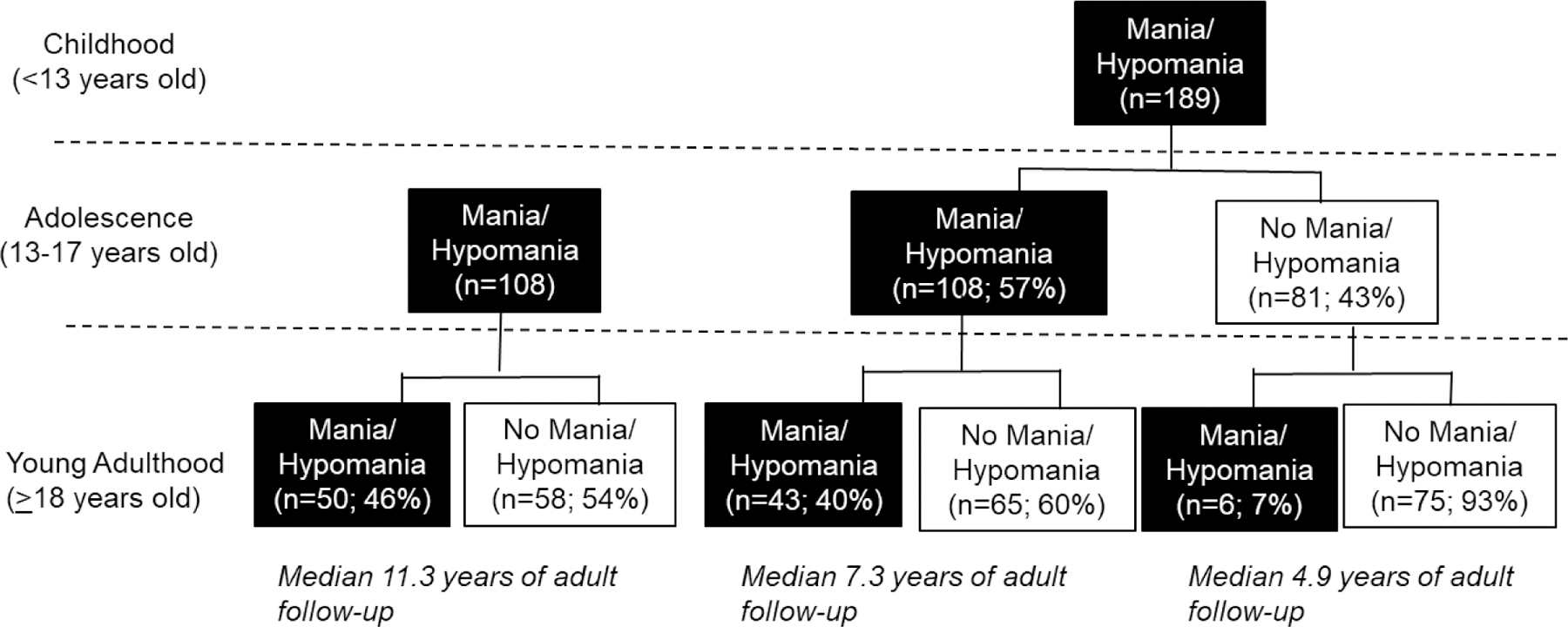
Proportion of COBY participants with threshold mania/hypomania in childhood, adolescence, and young adulthood.
Most COBY participants (189/297; 64%) had child-onset mania/hypomania, and 57% (108/189) of these participants continued to experience mania/hypomania in adolescence (Figure 1). Approximately two-thirds of our participants had more than one manic/hypomanic episode prior to age 18 (Table 1); this percentage was higher in the child/adolescent group (88%; 94/108) (eTable 1).
Of the 189 COBY participants with child-onset mania/hypomania, 49 (26%) experienced mania/hypomania in young adulthood. Within the child-only group, the re-emergence of mania/hypomania in young adulthood was rare (6/81;7%). In the child/adolescent group, 43/108 (40%) had mania/hypomania during young adulthood. In the adolescent-only group, 50/108 (46%) had threshold mania/hypomania in young adulthood. Years of adult follow-up time differed across groups, due to differences in intake age (Figure 1, eTable1; H=87.9, p<.0001). After adjustment for adult follow-up time, child-only vs. adolescent-only were less likely to have threshold mania/hypomania in young adulthood (OR=0.17, 95% CI=0.06, 0.45; p=.0004); however, there were no significant differences between the child/adolescent vs. adolescent-only groups (OR=1.06, 95% CI=0.58, 1.91; p=.86). Those who experienced mania/hypomania during young adulthood experienced a median of 1.6 weeks per year (Q1=0.46 weeks; Q3=3.9 weeks). The median number of weeks per year did not differ across group (i.e. child-only, child/adolescent, and adolescent-only), even at a trend level (H=1.04, p=.60).
3.3. Predictors of Threshold Mania/Hypomania in Young Adulthood
Lasso Regression (Table 2):
Table 2:
Full model: Selected Predictors from the Lasso Regression for Mania/hypomania in Young Adulthood
| Variable | Lasso exp(B) | p-valuea |
|---|---|---|
| Age at BD Onset | . | . |
| Age at Mania/hypomania Onset | . | . |
| Oldest Age (<18) of Mania/hypomania | 1.62 | <0.001 |
| BD Subtype (BDI vs. BDII) | . | . |
| >1 manic/hypomanic episode (Y/n) | . | . |
| Depression Under 18 (Y/n) | . | . |
| Demographics: Sex (M/F) | . | . |
| Demographics: SES | . | . |
| Demographics: Race (white/non-white) | . | . |
| Family History: Depression (Y/n) | . | . |
| Family History: Mania (Y/n) | 1.99 | <0.001 |
| Family History: Suicide Attempt (Y/n) | 1.15 | 0.01 |
| Family History: Psychosis (Y/n) | . | . |
| Family History: Anxiety (Y/n) | . | . |
| Family History: SUD (Y/n) | . | . |
| Family History: ADHD (Y/n) | . | . |
| Family History: Conduct Disorder (Y/n) | . | . |
| Comorbidity: Anxiety Under 18 (Y/n) | . | . |
| Comorbidity: ADHD Under 18 (Y/n) | . | . |
| Comorbidity: DBD Under 18 (Y/n) | . | . |
| Comorbidity: SUD Under 18 (Y/n) | . | . |
| Nuisance: Intake Age | 1.08 | 0.07 |
| Nuisance: Length of Adult Follow-up | . | . |
| Nuisance: # Adult Follow-up Visits | 1.06 | 0.05 |
p-values calculated by fitting an unpenalized logistic regression model using lasso-selected predictors
The predictors of young adult mania/hypomania selected by the Lasso regression were family history of mania, family history of suicide attempts, oldest age of mania/hypomania under 18, and number of adult follow-up visits. Intake age was also selected by the lasso regression but was not a significant predictor. The most important predictor of mania/hypomania in young adulthood was an older age of mania/hypomania under 18 (OR=1.62, p<.001). Participants with family history of mania (1st or 2nd degree relative) were twice as likely to experience mania/hypomania in young adulthood (OR=1.99; p=.01); increased risk was also observed with a family history of suicide attempts (OR=1.15; p=.01). Of note, age of BD onset and age of mania/hypomania onset were not selected by the lasso regression as predictors of young adult threshold mania/hypomania.
In participants with intake before 16 years old, we assessed whether number of weeks with threshold depression and/or mania/hypomania from 16–17 years old predicted adult mania/hypomania. After adjusting for predictors selected in the above lasso regression (age at oldest mania/hypomania <18 years old, family history of mania, family history of suicide attempts, intake age, and adult follow-up visits), the number of weeks with mania/hypomania did not predict adult mania/hypomania. The number of weeks with major depression was not significant but did show a trend level relationship; each week with MDE during adolescence was associated with a 2% increase in the odds of adult mania/hypomania (OR=1.02, 95% CI=1.00, 1.03, p=.06).
3.4. Probability of Threshold Mania in Childhood, Adolescence, and Young Adulthood (Figure 2):
Figure 2.
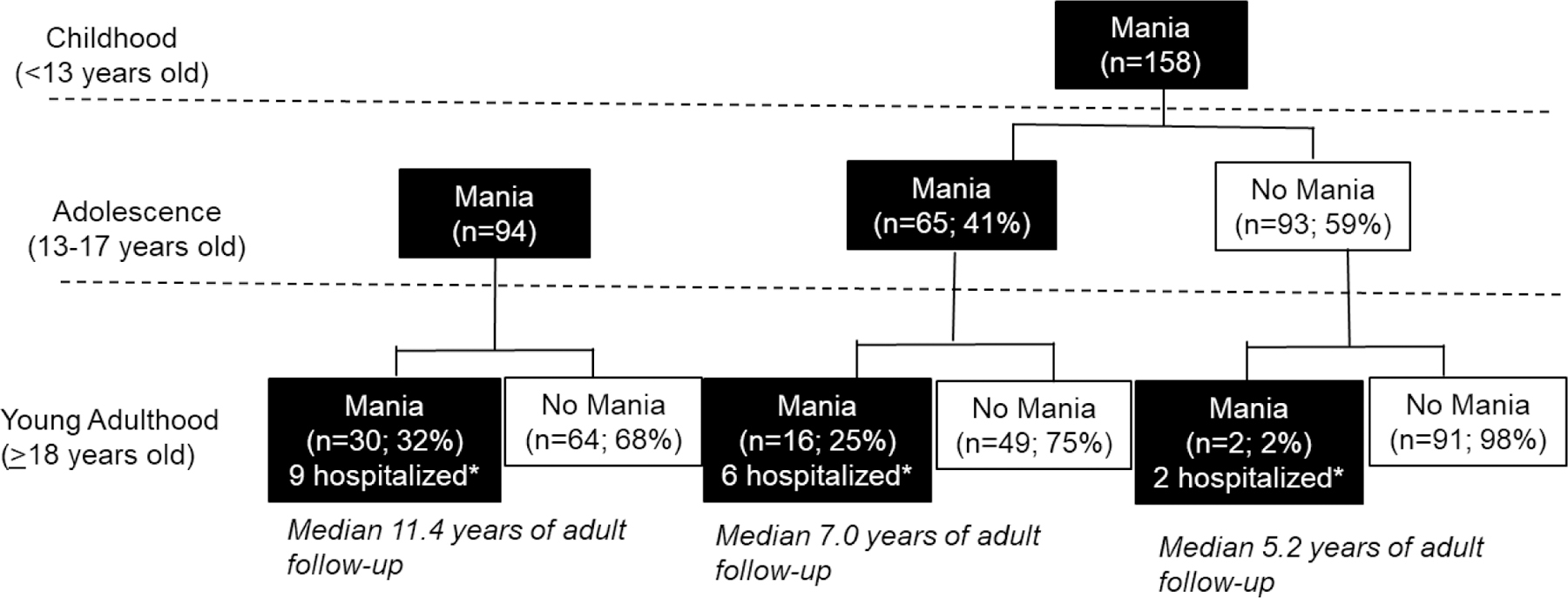
Proportion of COBY participants with threshold mania in childhood, adolescence, and young adulthood.
*Hospitalized within 4 weeks of manic episode
Above analyses were repeated only including participants with BD-I diagnosed before age 18 years old. Approximately 63% (158/252) of BD-I participants in COBY had child-onset mania and, of these, 41% (65/158) experienced a manic episode in adolescence and 11% (18/158) experienced a manic episode in adulthood. As with mania/hypomania, re-emergence of threshold mania during young adulthood in the child-only group was very rare (2/93; 2%). In the child/adolescent group, 25% (16/65) also had threshold mania in young adulthood; and in the adolescent-only group, 32% (30/94) experienced threshold mania in young adulthood. After adjusting for duration of adult follow-up, the risk of mania during young adulthood was less in the child-only vs. adolescent-only group (OR=0.09, 95% CI=0.02, 0.43; p<.0001); but there were no significant differences between the child/adolescent vs. adolescent-only group (OR=1.07, 95% CI=0.49, 2.37; p=.86). As above, we used a lasso regression to assess for predictors of mania. Results are similar to mania/hypomania, except that family history of suicide was not selected as a significant predictor (eTable 2).
Of the 48 young adults with mania during adulthood, 17 (35%) were hospitalized (partial or inpatient) within four weeks of at least one manic episode, indicating substantial functional impairment. The proportion of manic episodes temporally associated with hospitalization according to child- vs. adolescent-onset is shown in Figure 2.
3.5. Probability of Subthreshold Mania in Young Adulthood
Of 198 participants with no threshold mania/hypomania during young adulthood, 105 (53%) still experienced subthreshold mania during young adulthood. Of the 75 in the child-only group without threshold mania/hypomania, 29 (39%) experienced at least one episode of subthreshold mania in adulthood. In the child/adolescent group, 59% (38/65) with no threshold mania/hypomania experienced subthreshold mania in adulthood; in adolescent-only group, this percentage was 66% (38/58). After adjusting for duration of adult follow-up, adult subthreshold mania did not differ across group. Those who experienced subthreshold mania only during young adulthood experienced a median rate of 3.1 weeks per year with subthreshold mania (Q1=1.0 weeks; Q3=9.2 weeks). The median number of weeks per year did not differ across group (H=2.38, p=.30). Most participants experienced subthreshold mania during adolescence (173/198; 87.4%); subthreshold mania in adolescence was not associated with an increased risk of subthreshold mania in young adulthood, after adjusting for adult follow-up time (p=.62).
3.6. Supplemental Analyses
Effect of Medications:
We assessed the possibility that medications could be preventing manic episodes by first assessing the percent time on antimanic medications (including antipsychotics, anticonvulsants, and lithium) during the young adult time period in those with and without threshold mania/hypomania. There were no visual or statistical differences in the distributions (eFigure 1). Importantly, this does not mean that medications did not have any effect at the individual level, but rather that the effects of medication were likely cancelled out by the propensity for individuals with more manic symptoms to take medication (i.e. “confounding by indication”).
Effect of Informant:
Of 297 participants, 261 (88%) had an additional informant for at least one visit during young adulthood; participants had an informant for a mean of 55% of their young adult visits. We assessed whether the presence of a secondary informant was associated with increased reporting of manic/hypomanic episodes. Using mixed models (clustering within participant), we found no association between the presence of a secondary informant and whether mania/hypomania was reported during that time period. In addition, after taking into account number of adult follow-up visits, we did not find an association between presence of or percentage of visits with secondary informant and reported mania/hypomania during adulthood.
3.7. Narratives
Because PSR ratings alone may be insufficient to convey continuity of BD, included in the eSupplement are three narratives that describe cases of participants who experienced mania during childhood, adolescence, and adulthood. These narratives clarify that symptoms of mania were episodic and functionally impairing throughout these developmental stages.
4. DISCUSSION
In this sample of youths with BD-I/II, we investigated the prospectively observed continuity of mania/hypomania into adulthood and identified predictors of this trajectory. We observed substantial continuity, with 57% of child-onset youth also experiencing mania/hypomania in adolescence, and approximately 40% of those with mania/hypomania in adolescence having an episode in young adulthood. The numbers are slightly lower for mania: 41% of youth with child-onset mania also experience mania in adolescence, and just under 30% of youth with adolescent mania (regardless of age of onset) having an episode in young adulthood. When considering subthreshold mania in particular, continuity is even greater, indicating that even in those without threshold mania/hypomania, subthreshold manic episodes still exist. The most important predictors of mania/hypomania continuity are older age at most recent mania/hypomania under 18, family history of suicide attempts, and family history of mania. Importantly, after accounting for age of most recent mania/hypomania, age of onset was not an important predictor of mania/hypomania (or mania) continuity, indicating similar continuity of child- and adolescent-onset mania/hypomania into young adulthood.
4.1. Possible Reasons for the Absence of Mania/Hypomania in Young Adulthood
While there was substantial continuity of mania/hypomania across development, not everyone who met DSM-IV criteria for mania/hypomania in childhood and adolescence continued to do so in young adulthood. There are many possible reasons for this.
First, it is possible that for a minority of participants, threshold manic/hypomanic episodes are developmentally limited. This could be the natural course of BD in these youth or a positive outcome of early diagnosis and treatment. Such improvement has also been observed in other adolescent symptoms and diagnoses, including depression, psychotic symptoms, Tourette’s, and ADHD, where child/adolescent onset confers increased risk in adulthood but is not deterministic36–38. Developmentally limited disorders are also found in other areas of medicine, for example, certain childhood epilepsy syndromes or juvenile rheumatoid arthritis39,40.
Second, it may be the case that BD is still present, but not currently presenting with threshold mania/hypomania. As reported here and in previous analyses, many of these participants continued to struggle into adulthood with depression and subthreshold manic symptoms18. Moreover, depressive states, especially those characterized by psychomotor and psychotic features, have been prospectively linked to mania in youths41. Thus, while we did not assess these depressive states in this analysis, the absence of threshold mania/hypomania does not imply that BD is no longer present. Also, some of these participants may have entered into periods of extended remission, as was noted by Kraepelin 42. For example, one patient in our clinic had a recent recurrence of mania in her late twenties, after not having mania since adolescence, in the context of pregnancy/post-partum. While we followed participants for a mean of eight years into adulthood, many participants had a shorter follow-up time than this, and may experience a re-emergence of threshold mania/hypomania after this window; indeed, the absence of symptoms within the observed follow-up period may not indicate a permanent recovery.
Third, it is possible that medications and/or therapy are protective for many of our participants. While on the group level, it does not appear that medications are having any effects, this may reflect a balancing of the fact that individuals with a more severe course are more likely to be prescribed medication (i.e. “confounding by indication”) with beneficial effects of medication. It is possible, within participants on medication, that risk of mania/hypomania would in fact be higher in if they were not on medication. Fourth, while we explored the effects of additional informants, it is possible that these informants are not as aware of participants’ symptoms as they were in adolescence, given normative independence of young adulthood. Thus, even participants with additional informants might be under-reporting symptoms. Fifth, while the K-SADS-PL is a “gold standard” instrument for clinical assessment and all diagnoses were confirmed by each site’s PI, no diagnostic measure has 100% accuracy; thus, the possibility of incorrect diagnoses must be acknowledged.
4.2. Importance of Subthreshold Mania
While our focus was the continuity of threshold mania/hypomania, the importance of subthreshold mania deserves consideration for the following reasons. First, in COBY and other studies, subthreshold mania is associated with suicidality, substance abuse, poorer functioning, and poor quality of life19,20,25,43,44. As previously described, the subthreshold episodes reported in COBY are, in most cases, meeting full symptom criteria and lasting 2–3 days.19,45 Thus, while these subthreshold episodes do not meet full duration criteria for DSM-IV threshold mania/hypomania, they are still clinically significant and important when considering continuity of diagnosis. From the perspective of Kraepelin and many others, these subthreshold episodes point to a bipolar diathesis that illustrates continuity across the lifespan25,42,46, even if participants are not meeting full threshold criteria for mania/hypomania during a particular period of time. From a clinical perspective, such subthreshold events in a patient’s illness course bears importantly on decisions on the risk/benefit trade-off of maintaining long-term maintenance therapies.
4.3. Predictors of Threshold Mania/Hypomania: Summary
Amongst the most important predictors of young adult mania/hypomania were family history of mania and, to a lesser extent, suicide attempts. This is not surprising given the familial nature of BD, and the fact that individuals with a family history of the disorder are at elevated risk of the disorder themselves47–51. A family history of suicide attempts may also reflect a greater family loading for BD, and severe mood disorder in general, which may increase the risk that youth will continue to experience threshold mania/hypomania into adulthood. More depressive weeks during late adolescence was also associated with mania/hypomania in young adulthood, but this was only significant at a trend level.
4.4. Study Limitations
Our study has several limitations. First, most of our participants were white and non-Hispanic (reflecting the race distribution of the general population in the metropolitan areas surrounding each study site at the time of original enrollment), and were recruited from university settings, possibility limiting the generalizability of our results. Second, we had variable follow-up time in adulthood that differed according child vs. adolescent onset. However, findings remained similar even after adjusting for duration of adult follow-up. Third, we did not have in-person interviews with all family members and thus, similar to other studies, we rely on the informant (participant and parent) to tell us about family history. This may differentially impact our ability to observe effects of some disorders, which may be reported with more accuracy (e.g. suicide attempts), vs. others which may be predicted with more error (e.g. mania). Fourth, we did not record whether medications and hospitalizations were in response to a manic episode or for other aspects of bipolar illness or comorbidity, so it is difficult to disentangle the specific effects of mania on functional impairment in this sample. To address this somewhat, we have included narratives in the eSupplement that describe the impact of mania across development. Fifth, we have somewhat arbitrarily used a cut-point of 18 years old to define adulthood; while this is well-established in the literature, it may oversimplify the developmental course of disorder, which may continue to progress and evolve during young adulthood. Sixth, we have used DSM-IV criteria to define the continuity of disorder across developmental stage. While DSM-IV criteria “index” disorder, it has been argued that that these criteria do not “constitute” disorder52. However, these criteria are reliable and allow us to compare our findings to other pediatric and adult studies; we have supplemented our report with narratives to provide some indication of the presentation of BD across development.
4.5. Conclusions
Despite these limitations, COBY offers an unprecedented opportunity to use over a decade of follow-up data on a large sample of children and adolescents with BD to answer an extremely important clinical question regarding continuity of mania/hypomania across developmental stage. While not everyone diagnosed in childhood or adolescence reported a threshold manic/hypomanic episode during early adulthood, a substantial minority did, and even more experienced subthreshold mania. Even more importantly, after accounting for age of most recent threshold mania/hypomania, age of onset (child vs. adolescent) did not have any effect on mania/hypomania continuity into young adulthood. In other words, a participant with child-onset mania/hypomania (recurring in adolescence) was equally likely to have young adult mania/hypomania as a participant with adolescent-onset mania/hypomania. This provides important evidence that prepubertal mania/hypomania, while it may be difficult to assess and rare, has important diagnostic continuity with young adult mania/hypomania.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the National Institute of Mental Health (NIMH), Course and Outcome of Bipolar Youth (COBY) study grants MH059929 (PI Boris Birmaher, MD), MH059691 (PIs Martin B., Keller, MD, and Shirley Yen, PhD), and MH05977 (PI Michael Strober, PhD), and Predicting Adult Outcomes in Bipolar Youth (PROBY) study grants MH112544 (PI Boris Birmaher, MD), and MH112543 (PI Shirley Yen, PhD). The authors wish to thank John Merranko, MA, and Fangzi Liao, MS, for their statistical expertise in this research, the study participants and their families for their participation, the Course and Outcome of Bipolar Youth (COBY) research team, and the faculty and staff of the Child and Adolescent Bipolar Spectrum Services clinic at the University of Pittsburg. The authors would also like to acknowledge Stacia Friedman-Hill, PhD, and Shelli Avenevoli, PhD, of NIMH, for their continued encouragement and support.
Footnotes
CONFLICT OF INTEREST
Dr. Hafeman has reported grants from the National Institute of Mental Health (NIMH), Brain and Behavior Research Foundation (BBRF), and the Klingenstein Third Generation Foundation. Dr. T.R. Goldstein has reported grants from NIMH, the American Foundation for Suicide Prevention (AFSP), the University of Pittsburgh Clinical and Translational Science Institute (CTSI), and the BBRF, and royalties from Guilford Press, outside the submitted work. Dr. Diler has received research support from NIMH. Dr. Ryan has received grant or research support from NIMH and was a scientific consultant to Axsome Therapeutics. Dr. B.I. Goldstein has received grant or research support from NIMH, the BBRF, Brain Canada, the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Canada, the Ontario Ministry of Research and Innovation, and the University of Toronto. Dr. Axelson has received grants from NIMH during the conduct of the study, and personal fees from Janssen Research and Development, LLC and UpToDate, outside the submitted work. Dr. Strober has received research support from NIMH and support from the Resnick Endowed Chair in Eating Disorders. Dr. Keller has received research support from NIMH. Ms. Hower has received research support from NIMH, and honoraria from the Department of Defense (DOD) and the University of California at San Diego (UCSD) Eating Disorders Center for Treatment and Research. Dr. Hunt receives honoraria from J. Wiley Publishers and has received research support from NIMH. Dr. Weinstock has received research support from NIMH. Dr. Yen has received research support from NIMH and the AFSP, and has served as a consultant at Janssen Research and Development, LLC. Dr. Birmaher has reported grants from NIMH, during the conduct of the study, and royalties from Random House, UpToDate, and Lippincott, Williams, and Wilkins, outside of the submitted work. Mr. Merranko, Ms. Gill, and Ms. Liao have reported no biomedical financial interests or potential conflicts of interest.
DATA AVAILABILITY STATEMENT
Data from the COBY study were uploaded to NIMH RDOC database to share with the public. It is accessible at https://data-archive.nimh.nih.gov/rdocdb/.
References
- 1.Lish JD, Dime-Meenan S, Whybrow PC, Price RA, Hirschfeld RM. The National Depressive and Manic-depressive Association (DMDA) survey of bipolar members. J Affect Disord. 1994;31(4):281–294. [DOI] [PubMed] [Google Scholar]
- 2.Perlis RH, Dennehy EB, Miklowitz DJ, et al. Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: results from the STEP-BD study. Bipolar Disord. 2009;11(4):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holtzman JN, Miller S, Hooshmand F, et al. Childhood-compared to adolescent-onset bipolar disorder has more statistically significant clinical correlates. Journal of Affective Disorders. 2015;179:114–120. [DOI] [PubMed] [Google Scholar]
- 4.Propper L, Ortiz A, Slaney C, et al. Early-onset and very-early-onset bipolar disorder: distinct or similar clinical conditions? Bipolar Disorders. 2015;17(8):814–820. [DOI] [PubMed] [Google Scholar]
- 5.Leverich GS, Post RM, Keck PE Jr., et al. The poor prognosis of childhood-onset bipolar disorder. The Journal of pediatrics. 2007;150(5):485–490. [DOI] [PubMed] [Google Scholar]
- 6.Baldessarini RJ, Tondo L, Vazquez GH, et al. Age at onset versus family history and clinical outcomes in 1,665 international bipolar-I disorder patients. World Psychiatry. 2012;11(1):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Meter A, Moreira ALR, Youngstrom E. Updated Meta-Analysis of Epidemiologic Studies of Pediatric Bipolar Disorder. J Clin Psychiatry. 2019;80(3). [DOI] [PubMed] [Google Scholar]
- 9.Conus P, Macneil C, McGorry PD. Public health significance of bipolar disorder: implications for early intervention and prevention. Bipolar Disorders. 2014;16(5):548–556. [DOI] [PubMed] [Google Scholar]
- 10.Duffy A Debate: Pediatric bipolar disorder – the elephant in the room. Child and adolescent mental health. 2019;24(1):99–100. [DOI] [PubMed] [Google Scholar]
- 11.Duffy A, Carlson G, Dubicka B, Hillegers MHJ. Pre-pubertal bipolar disorder: origins and current status of the controversy. International Journal of Bipolar Disorders. 2020;8(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stringaris A, Santosh P, Leibenluft E, Goodman R. Youth meeting symptom and impairment criteria for mania-like episodes lasting less than four days: an epidemiological enquiry. J Child Psychol Psychiatry. 2010;51(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paaren A, von Knorring AL, Olsson G, von Knorring L, Bohman H, Jonsson U. Hypomania spectrum disorders from adolescence to adulthood: a 15-year follow-up of a community sample. J Affect Disord. 2013;145(2):190–199. [DOI] [PubMed] [Google Scholar]
- 14.Youngstrom E, Meyers O, Youngstrom JK, Calabrese JR, Findling RL. Diagnostic and measurement issues in the assessment of pediatric bipolar disorder: implications for understanding mood disorder across the life cycle. Dev Psychopathol. 2006;18(4):989–1021. [DOI] [PubMed] [Google Scholar]
- 15.Rende R, Birmaher B, Axelson D, et al. Childhood-onset bipolar disorder: Evidence for increased familial loading of psychiatric illness. J Am Acad Child Adolesc Psychiatry. 2007;46(2):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birmaher B, Axelson D, Strober M, et al. Comparison of manic and depressive symptoms between children and adolescents with bipolar spectrum disorders. Bipolar Disorders. 2009;11(1):52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birmaher B, Merranko JA, Gill MK, et al. Predicting Personalized Risk of Mood Recurrences in Youths and Young Adults With Bipolar Spectrum Disorder. J Am Acad Child Adolesc Psychiatry. 2020. [DOI] [PMC free article] [PubMed]
- 18.Birmaher B, Gill MK, Axelson DA, et al. Longitudinal Trajectories and Associated Baseline Predictors in Youths With Bipolar Spectrum Disorders. The American journal of psychiatry. 2014;171(9):990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Axelson D, Birmaher B, Strober M, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(10):1139–1148. [DOI] [PubMed] [Google Scholar]
- 20.Hafeman D, Axelson D, Demeter C, et al. Phenomenology of bipolar disorder not otherwise specified in youth: a comparison of clinical characteristics across the spectrum of manic symptoms. Bipolar Disord. 2013;15(3):240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein BI, Shamseddeen W, Axelson DA, et al. Clinical, demographic, and familial correlates of bipolar spectrum disorders among offspring of parents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(4):388–396. [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein TR, Obreja M, Shamseddeen W, et al. Risk for suicidal ideation among the offspring of bipolar parents: results from the Bipolar Offspring Study (BIOS). Arch Suicide Res. 2011;15(3):207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaudreuil CAH, Faraone SV, Di Salvo M, et al. The morbidity of subthreshold pediatric bipolar disorder: A systematic literature review and meta-analysis. Bipolar Disorders. 2019;21(1):16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hafeman DM, Spada M. Assessment, Prognosis, and Treatment of Subthreshold Mood Symptoms. In: Singh MK, ed. Clinical Handbook for the Diagnosis and Treatment of Pediatric Mood Disorders: American Psychiatric Association; 2019. [Google Scholar]
- 25.Angst J, Gamma A, Benazzi F, Ajdacic V, Eich D, Rossler W. Toward a re-definition of subthreshold bipolarity: epidemiology and proposed criteria for bipolar-II, minor bipolar disorders and hypomania. J Affect Disord. 2003;73(1–2):133–146. [DOI] [PubMed] [Google Scholar]
- 26.Axelson DA, Birmaher B, Strober MA, et al. Course of subthreshold bipolar disorder in youth: diagnostic progression from bipolar disorder not otherwise specified. J Am Acad Child Adolesc Psychiatry. 2011;50(10):1001–1016e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition). Washinton, DC: American Psychiatric Association; 2004. [Google Scholar]
- 29.Wechsler D Weschler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: Harcourt Brace and Company; 1999. [Google Scholar]
- 30.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- 31.First MB, Gibbon M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). Comprehensive handbook of psychological assessment, Vol. 2: Personality assessment. Hoboken, NJ, US: John Wiley & Sons Inc; 2004:134–143. [Google Scholar]
- 32.Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57(7):675–682. [DOI] [PubMed] [Google Scholar]
- 33.Hollingshead AB. Four-Factor Index of Social Status. New Haven, Connecticut: Yale University Department of Sociology; 1975. [Google Scholar]
- 34.Keller MB, Lavori PW, Friedman B, et al. The Longitudinal Interval Follow-up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Archives of general psychiatry. 1987;44(6):540–548. [DOI] [PubMed] [Google Scholar]
- 35.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning. 2nd ed. New York: Springer; 2009. [Google Scholar]
- 36.Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children’s Self-Reported Psychotic Symptoms and Adult Schizophreniform Disorder: A 15-Year Longitudinal Study. Archives of General Psychiatry. 2000;57(11):1053–1058. [DOI] [PubMed] [Google Scholar]
- 37.Weissman MM, Wolk S, Goldstein RB, et al. Depressed Adolescents Grown Up. JAMA. 1999;281(18):1707–1713. [DOI] [PubMed] [Google Scholar]
- 38.Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. The lancet Psychiatry. 2016;3(12):1157–1165. [DOI] [PubMed] [Google Scholar]
- 39.Berg AT, Testa FM, Levy SR. Complete remission in nonsyndromic childhood-onset epilepsy. Annals of neurology. 2011;70(4):566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fantini F, Gerloni V, Gattinara M, Cimaz R, Arnoldi C, Lupi E. Remission in juvenile chronic arthritis: a cohort study of 683 consecutive cases with a mean 10 year followup. J Rheumatol. 2003;30(3):579–584. [PubMed] [Google Scholar]
- 41.Strober M, Carlson G. Bipolar illness in adolescents with major depression: clinical, genetic, and psychopharmacologic predictors in a three- to four-year prospective follow-up investigation. Arch Gen Psychiatry. 1982;39(5):549–555. [DOI] [PubMed] [Google Scholar]
- 42.Kraepelin E Manic-depressive insanity and paranoia. Edinburgh: E. & S. Livingstone; 1921. [Google Scholar]
- 43.Bonnin CM, Sanchez-Moreno J, Martinez-Aran A, et al. Subthreshold symptoms in bipolar disorder: impact on neurocognition, quality of life and disability. J Affect Disord. 2012;136(3):650–659. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein BI, Strober M, Axelson D, et al. Predictors of First-Onset Substance Use Disorders During the Prospective Course of Bipolar Spectrum Disorders in Adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(10):1026–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Towbin K, Axelson D, Leibenluft E, Birmaher B. Differentiating bipolar disorder-not otherwise specified and severe mood dysregulation. J Am Acad Child Adolesc Psychiatry. 2013;52(5):466–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Judd LL, Schettler PJ, Akiskal HS, et al. Residual symptom recovery from major affective episodes in bipolar disorders and rapid episode relapse/recurrence. Arch Gen Psychiatry. 2008;65(4):386–394. [DOI] [PubMed] [Google Scholar]
- 47.Mesman E, Nolen WA, Reichart CG, Wals M, Hillegers MH. The Dutch bipolar offspring study: 12-year follow-up. Am J Psychiatry. 2013;170(5):542–549. [DOI] [PubMed] [Google Scholar]
- 48.Duffy A, Goodday S, Keown-Stoneman C, Grof P. The emergent course of bipolar disorder: observations over two decades from the Canadian high-risk offspring cohort. American Journal of Psychiatry. 2019;176(9):720–729. [DOI] [PubMed] [Google Scholar]
- 49.Preisig M, Strippoli M-PF, Castelao E, et al. The specificity of the familial aggregation of early-onset bipolar disorder: A controlled 10-year follow-up study of offspring of parents with mood disorders. Journal of Affective Disorders. 2016;190:26–33. [DOI] [PubMed] [Google Scholar]
- 50.Hafeman DM, Merranko J, Axelson D, et al. Toward the Definition of a Bipolar Prodrome: Dimensional Predictors of Bipolar Spectrum Disorders in At-Risk Youths. American Journal of Psychiatry. 2016;173(7):695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Axelson D, Goldstein B, Goldstein T, et al. Diagnostic Precursors to Bipolar Disorder in Offspring of Parents With Bipolar Disorder: A Longitudinal Study. Am J Psychiatry. 2015;172(7):638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kendler KS. DSM disorders and their criteria: how should they inter-relate? Psychol Med. 2017;47(12):2054–2060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the COBY study were uploaded to NIMH RDOC database to share with the public. It is accessible at https://data-archive.nimh.nih.gov/rdocdb/.


