Abstract
Purpose
Histopathology method is often used as a gold standard diagnostic for Helicobacter pylori infection in Indonesia. However, it requires an endoscopic procedure which is limited in Indonesia. A non-invasive method, such as 14C Urea Breath Test (UBT), is more favorable; however, this particular method has not been validated yet.
Patients and Methods
A total of 55 dyspeptic patients underwent gastroscopy and 14C-UBT test. We used Heliprobe® UBT for UBT test. As for the histology, May-Giemsa staining of two gastric biopsies (from the antrum and corpus) were evaluated following the Updated Sydney System.
Results
The Receiver Operating Characteristics analysis showed that the optimum cut-off value was 57 with excellence Area under Curve = 0.955 (95% CI = 0.861–1.000). By applying the optimum cut-off value, Heliprobe® UBT showed 92.31% for sensitivity, 97.62% for specificity, 92.31% for positive predictive value, 97.62% for negative predictive value, 38.77 for positive likelihood ratio, 0.0788 for negative likelihood ratio, and 96.36% for the accuracy.
Conclusion
The 14C-UBT is an accurate test for H. pylori diagnosis with excellent sensitivity, specificity, and accuracy. The different optimum cut-off points suggested that a validation is absolutely necessary for new test prior application to the new population.
Keywords: Helicobacter pylori, UBT, diagnostic, infectious disease
Introduction
Helicobacter pylori have infected approximately 50% of the worldwide population, with a higher prevalence in developing countries.1 This bacterium can induce progressive gastric mucosal inflammation leading to several cascades in gastric diseases starting with chronic gastritis, atrophic gastritis until the end phase is gastric adenocarcinoma.2,3 One of the gold standard methods to diagnose H. pylori infection in Indonesia is histopathology examination, which endoscopy examination is necessary to collect the gastric biopsy.4 Unfortunately, there are only less than 400 hospitals with endoscopy system in Indonesia and mostly located in big cities in Java Island.5,6 This number is not sufficient considering the total population of Indonesia is approximately 250 million. Ideally, 2500 consultant gastroenterologists are needed, but in recent years only 10 gastrointestinal endoscopy training centers were established.5 Thus, the use of histopathology examination for H. pylori detection is still a challenge in Indonesia. Thus, the non-invasive method for H. pylori detection is more favorable to cover all Indonesian populations.
Urea Breath Test (UBT) is a non-invasive diagnostic method that is widely used to diagnose H. pylori infection. This test is based on the mechanism that ingested “labeled carbon-containing urea” can be broken down to carbon dioxide (CO2) and ammonia (NH4-OH) by H. pylori as the urease-producing microorganism in the gastric mucosa.7 The tagged carbon within CO2 will be detected in exhaled breath patients.8,9 There are two carbon isotopes (13C and 14C), which are used for the UBT. The 13C isotope need more complex equipment, including a mass spectrophotometer, that makes difficult for conducting the test compare to 14C isotope that only required a portable compact beta-scintillation counter.8 Thus, it is more convenient to perform UBT using 14C isotope. As the requirement of more sophisticated tools on the 13C isotope, it yielded more expensive examination cost compared to the 14C isotope one. The 13C-UBT was reported to cost 36.50–120.00 USD10 while 14C-UBT was only 14 USD.11 In addition, compared to other non-invasive diagnostic methods, UBT has advantages on the less time required to perform the test, quick result, and less technical handling.
Based on national consensus, 13C-UBT still becomes one of the recommended diagnostic tests for H. pylori infection.4,12 Previous studies reported that 13C-UBT method has 99% sensitivity and 98% specificity, respectively.4,12 In contrast, studies in other Asian countries showed that 13C-UBT could have lower sensitivity (64.2%) and lower specificity (89.1%).13,14 Despite many publications had assessed the diagnostic value of 13C-UBT, studies about 14C-UBT remain scarce. Furthermore, to our knowledge, 14C-UBT has not yet been validated in the Indonesian population. A validation study is necessary to get information regarding the diagnostic value of 14C-UBT in Indonesia, so the practitioners can apply this test as an alternative method rather than the invasive methods. Here, we conducted a prospective study to validate 14C-UBT as an alternative H. pylori diagnostic method in Indonesia.
Patients and Methods
Study Population
We conducted a cross-sectional study and recruited patients for endoscopic examination from November 2018 to April 2019 in Surabaya, Indonesia. A total of 55 dyspeptic patients with endoscopy indications were included. This sample number was calculated based on this equation.
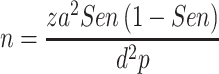 |
With the expected sensitivity of 97%, power of statistic (D = 0.1) and previous reported H. pylori infection rate 22.1%15 it yielded a minimum sample number of 50. Among all participants, none had received antibiotics and bismuth drugs for 4 weeks and 2 weeks before the procedure, respectively. In addition, none of these patients had a history of gastric surgery, endoscopy contraindication, and bleeding gastrointestinal tract 4 weeks prior to the procedure. Patients were fasting before endoscopy and UBT. Before the UBT test, patients were interviewed with a standard questionnaire to obtain demographic data. One day after the UBT test, upper-endoscopy was performed by experienced endoscopists. During the endoscopic examination procedure, the endoscopist diagnosed the patients based on the endoscopic observation. The superficial gastritis is defined as several hyperemic streaks found on the gastric mucosa and it is not always associated with the H. pylori infection. Whereas the erosive gastritis has similar characteristic to the superficial gastritis with additional observation of raised gastric lesion.16 In addition, we obtained two gastric specimens (one from the lesser curvature of the antrum, approximately 3 cm from the gastric pyloric ring, and one more from the greater curvature of the corpus). Those two specimens were used for the histological examination by professional histopathologist. This study was approved by Dr. Soetomo General Hospital Ethics Committee (0803/KEPK/XI/2018) and written informed consent obtained from all patients. This study was conducted in accordance with the Declaration of Helsinki.
Histology Evaluation of H. pylori Infection
The H. pylori status was based on histopathological examination by May-Giemsa staining as the reference standard to classify the H. pylori-positive and the H. pylori-negative groups.17 Briefly, all biopsy materials for histological examination were fixed in 10% buffered formalin and embedded inside paraffin. Serial sections were stained with May–Giemsa stain. The stained specimens then were evaluated using the Updated Sydney System.18 Samples with bacterial load score ≥1 would be considered as positive for H. pylori.
14C-Urea Breath Test (UBT)
Patients had to avoid smoking and sparkling water or soft drinks on the day of the test and fast at least 4 hours before the test. For the UBT examination, we used Heliprobe® 14C-UBT (Heliprobe, Stockholm, Sweden), and all the procedures we followed the manufacturer’s instructions. The patient swallowed a HeliCap® capsule containing 14C urea (1 μCi) and wait 10 minutes before exhaling into Heliprobe® Breath Card where the reactivity filters absorb the CO2. The indicator changes color from orange to yellow to indicate when the reactivity filters are saturated and sampling completed. Then the Heliprobe® Breath Card was inserted into the Heliprobe® Analyzer to obtain the measurement value.
Statistical Analysis
Discrete variables were tested using Pearson’s chi-squared test. The normality of the continuous variables was analyzed by the Shapiro–Wilk normality test. Receiver-operating characteristic (ROC) curves were determined to calculate the best cut-off values, including the area under the curve for discriminating H. pylori positivity. The statistical analysis was performed by R. Studio (R. Studio, Inc., Boston, USA) and SPSS statistical software version 23.0 (IBM Corp., Armonk, NY, USA) to calculate diagnostic test value: sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio, as well as their accuracy.
Results
Patients Characteristic
We included 55 subjects, consisted of 36 males (65.5%) and 19 females (34.5%) with a mean age of 48.27 ± 11.4 years old. The majority of the patients were aged more than 40 years old with age group between 40–49 years old became the predominant age group (17/55, 27.3%). Most of the patients were Javanese (38/55, 69.0%) because the patients were from Surabaya city that is located on Java Island.
Diagnostic Value of 14C-UBT and Cut-off Determination
The manufacturer cut-off recommendation for the 14C-UBT was 50. The analysis using this cut-off showed the value of sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 92.31%, 95.24%, 85.71%, 85.71%, and 94.54%, respectively. The value of positive possibility ratio and negative possibility ratio were 19.380 and 0.079, respectively.
Following the recommendation for validating the new diagnostic modality for a new population,8,19 we investigated the best cut-off value for the Indonesian population. The ROC analysis showed the area under the curve of the 14C-UBT was 0.955 (95% CI=0.861–1.000). The optimum cut-off value was 57, with sensitivity, specificity, positive predictive value, negative predictive value, and accuracy values of 92.31%, 97.62%, 92.31%, 97.62%, and 96.36%, respectively (Table 1). The value of positive likelihood ratio and negative likelihood ratio were 38.770 and 0.0788, respectively. We could obtain similar sensitivity but slightly higher specificity by applying a new cut-off point of 57, suggesting the cut-off value of 57 is more suitable to be applied in the Indonesian population. Therefore, further analysis in this study was using cut-off 57.
Table 1.
The Diagnostic Value Between Two Different Cut-Offs
| Diagnostic Parameter | Diagnostic Value | |
|---|---|---|
| Cut-Off 50 | Cut-Off 57 | |
| Sensitivity | 92.31% | 92.31% |
| Specificity | 95.24% | 97.62% |
| PPV | 85.71% | 92.31% |
| NPV | 97.56% | 97.62% |
| Accuracy | 94.54% | 96.36% |
Diagnostic Test results and Patients’ Demographic Data
We compared the prevalence of H. pylori infection by using 14C-UBT with a new cut-off 57 and histopathology analysis (Table 2). A similar prevalence of H. pylori (13/55, 23.6%) was observed between these methods. The characteristic of sex and age were similar between these methods. The number of males (7/13, 53.8%) was more than female (6/13, 46.2%) in the H. pylori-positive patients’ group. The predominant age group of H. pylori-positive patients was 40–49 years old (5/13, 38.5%). In addition, Javanese people become the most dominant in the positive case of the H. pylori group between these methods.
Table 2.
Diagnostic Test Results and Patients’ Demographic Data
| Characteristics | 14C-UBT* | Histology | ||||||
|---|---|---|---|---|---|---|---|---|
| Hp Positive (%) | Hp Negative (%) | Hp Positive (%) | Hp Negative (%) | |||||
| Total patients | 13 | (23.6) | 42 | (76.4) | 13 | (23.6) | 42 | (76.4) |
| Sex | ||||||||
| Male | 7 | (53.8) | 29 | (69.0) | 7 | (53.8) | 29 | (69.0) |
| Female | 6 | (46.2) | 13 | (31.0) | 6 | (46.2) | 13 | (31.0) |
| Age | ||||||||
| <29 | 0 | (0.0) | 4 | (9.5) | 0 | (0.0) | 4 | (9.5) |
| 30–39 | 0 | (0.0) | 7 | (16.7) | 0 | (0.0) | 7 | (16.7) |
| 40–49 | 5 | (38.5) | 12 | (28.6) | 5 | (38.5) | 12 | (28.6) |
| 50–59 | 3 | (23.1) | 12 | (28.6) | 3 | (23.1) | 12 | (28.6) |
| >60 | 5 | (38.5) | 7 | (16.7) | 5 | (38.5) | 7 | (16.7) |
| Ethnic | ||||||||
| Ambon | 0 | (0.0) | 2 | (4.8) | 0 | (0.0) | 2 | (4.8) |
| Bugis | 0 | (0.0) | 1 | (2.4) | 0 | (0.0) | 1 | (2.4) |
| Javanese | 11 | (84.6) | 27 | (64.3) | 10 | (76.9) | 28 | (66.7) |
| Dayak | 0 | (0.0) | 1 | (2.4) | 0 | (0.0) | 1 | (2.4) |
| Madura | 1 | (7.7) | 5 | (11.9) | 2 | (15.4) | 4 | (9.5) |
| Sunda | 0 | (0.0) | 1 | (2.4) | 0 | (0.0) | 1 | (2.4) |
| Chinese | 1 | (7.7) | 5 | (11.9) | 1 | (7.7) | 5 | (11.9) |
Note: *Diagnosis based on UBT was using the cut-off value of 57.
Endoscopy Finding Among H. Pylori-Infected Patients
The endoscopy finding revealed that among H. pylori-uninfected patients (Table 3), the most predominant observation was superficial gastritis (52.4%), followed by gastro-duodenitis (23.8%) and erosive gastritis (19.0%). Meanwhile, among H. pylori-infected patients, erosive gastritis was predominant (46.2%), followed by peptic ulcer (30.8%) and superficial gastritis (15.4%). We observed a significant relationship between H. pylori positivity with the endoscopic findings (P = 0.002).
Table 3.
Endoscopy Results Among H. pylori-Infected Patients
| Endoscopic Finding | H. pylori-Negative (%) | H. pylori-Positive (%) | ||
|---|---|---|---|---|
| N = 42 | N = 13 | |||
| Normal | 1 | (2.4) | 0 | (0.0) |
| Superficial gastritis | 22 | (52.4) | 2 | (15.4) |
| Erosive Gastritis | 8 | (19.0) | 6 | (46.2) |
| Gastroduodenitis | 10 | (23.8) | 1 | (7.7) |
| Peptic Ulcer | 1 | (2.4) | 4 | (30.8) |
Discussion
This is the first study to determine the diagnostic performance of 14C-UBT in the Indonesian population. The standard measurement according to the manufacturer recommendation had yielded a good value of sensitivity, specificity, positive predictive value, negative predictive value, positive possibility ratio, negative possibility ratio, and accuracy compared to gold standard histopathology. However, even in this state a validation of new test prior application to the new population is necessary. Our ROC analysis resulted in the optimum cut-off point was 57, which is slightly higher than the manufacturer recommendation. By applying this new cut-off point we could get similar sensitivity, but slightly better specificity, suggesting the ability to determine the true negative patients is increasing. Our result was in line with a systematic review study that insisted all results of UBT have to be adjusted for cut-off value for a new population.8,19 The adjustment of cut-off value may increase the performance of the diagnostic test.19 A review study revealed that overall the specificity and sensitivity of the UBT are mostly more than 95% and better than when using higher cut-off.20 In addition, a study from Iran and Europe showed a better diagnostic performance was obtained when applied a higher cut-off point,8,21 suggesting this method combining with higher cut-off value would give accurate, reliable, and useful for the diagnosis of H. pylori infection in routine clinical practice. The higher Heliprobe® cut-off value should depend on the population, because different population has different prevalence of H. pylori due to several factors, including ethnicity, host genetic background, lifestyle, eradication related antibiotic resistance and virulence of circulating H. pylori.22,23 Therefore, different population has different optimum cut-off value. Further study is interesting to do because Indonesia has many kinds of population with different characteristic and variety of H. pylori prevalence.
The new cut-off application of 14C-UBT and histopathology method yielded the same positive and negative results in diagnosing H. pylori infection. A previous study showed that around 20% to 30% of the H. pylori-infected individuals may develop peptic ulcer disease, and then end-stage of the infection might be developed to gastric cancer.2,3 Endoscopy following by histopathology method has been widely performed for gastrointestinal disorders and become a pivotal approach for H. pylori infection diagnosis,20 but this method still becomes a challenge in Indonesia because the gastroenterology and endoscopic facilities remain limited and not enough to cover total population.5,6 Moreover, this invasive method also spends a relatively long time, higher cost, and more inconvenient compared to the non-invasive method. In addition, our UBT validation result showed an excellent AUC value of 0.955, suggesting only less than 5% case was no concordance between test and gold standard diagnostic method. This value was greater than other diagnostic methods that had been validated in Indonesia, such as serology anti-IgG (AUC = 0.913).24 The 14C-UBT has higher cost with more labor and difficult to handle compared to serology examination. Nevertheless, serology examination has a major drawback of slightly lower performance over low prevalence H. pylori population such as Indonesia and unable to distinguish current active and past infection. As another comparison to RAPIRUN®, the overall accuracy of Heliprobe® in Indonesia showed better performance.25 In addition, a systematic review comparing non-invasive methods was also resulting an excellent performance of UBT with reaching sensitivity of 94% and 93% for 13C-UBT and 14C-UBT, respectively.26 These results confirmed that UBT is an excellent non-invasive H. pylori infection diagnostic method, even compared to other non-invasive modalities.
In this study, we found a significant association between H. pylori infection with the endoscopic findings. This association might be due to the fact that most cases found from H. pylori-uninfected patients were superficial gastritis. Meanwhile, erosive gastritis was predominant in the H. pylori-infected patients. Comparison between these two groups showed that the prevalence of superficial gastritis and gastroduodenitis was more dominant in H. pylori-uninfected patients than H. pylori-infected patients, while the more severe condition that is a peptic ulcer, was more dominant in H. pylori-infected patients. This result can be explained from H. pylori infection pathogenesis. H. pylori can make inflammation and further damage that create erosive gastritis.27 If this condition continues, erosive gastritis might become a gastric ulcer, gastric atrophy, and even develop into gastric adenocarcinoma.28
There were several limitations to this study. The sample number of this study might be not enough to represent the country needed since data are taken from only one city. Therefore, further study with a higher number of samples collected from several different locations in Indonesia might be needed. In addition, the subjects were only taken from dyspepsia patients who underwent endoscopy in the hospital. This could not fully represent H. pylori infection prevalence because infected patients also can be asymptomatic.
Conclusion
The 14C-UBT is an accurate test for H. pylori diagnosis with excellent sensitivity, specificity, and accuracy. The different optimum cut-off points applied in Indonesian samples suggested that validation is absolutely necessary for new test prior application to the new population.
Funding Statement
This report is based on work supported in part by grants from the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (15H02657, 16H05191, 16H06279, 18KK0266, and 19H03473) (YY) and the National Institutes of Health (DK62813) (YY). This work was also supported by the Japan Society for the Promotion of Science (JSPS) Institutional Program for Core-to-Core Program; B. Asia-Africa Science Platform (YY). DD, KAF and RIA are PhD students supported by the Japanese Government (MEXT) scholarship program for 2016, 2017, and 2019, respectively. This study was also funded by the Program Penelitian Kolaborasi Indonesia Grant Tahun 2021 to MM.
Author Contributions
All authors contributed to conception, study design, execution, acquisition of data, data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Salih B. Helicobacter pylori infection in developing countries: the burden for how long? Saudi J Gastroenterol. 2009;15(3):201–207. doi: 10.4103/1319-3767.54743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19(3):449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basso D, Plebani M, Kusters JG. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2010;15(SUPPL. 1):14–20. doi: 10.1111/j.1523-5378.2010.00781.x [DOI] [PubMed] [Google Scholar]
- 4.Syam AF, Simadibrata M, Makmun D, et al. National consensus on management of dyspepsia and Helicobacter pylori infection. Acta Med Indones. 2017;49(3):279. [PubMed] [Google Scholar]
- 5.Makmun D. Present status of endoscopy, therapeutic endoscopy and the endoscopy training system in Indonesia. Dig Endosc. 2014;26(S2):2–9. [DOI] [PubMed] [Google Scholar]
- 6.Miftahussurur M, Doohan D, Nusi IA, et al. Gastroesophageal reflux disease in an area with low Helicobacter pylori infection prevalence. PLoS One. 2018;13(11):e0205644. doi: 10.1371/journal.pone.0205644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham DY, Miftahussurur M. Helicobacter pylori urease for diagnosis of Helicobacter pylori infection: a mini review. J Adv Res. 2018;13:51–57. doi: 10.1016/j.jare.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansour-Ghanaei F, Sanaei O, Joukar F. Clinical validation of an office-based C-UBT (Heliprobe) for H. pylori diagnosis in Iranian dyspeptic patients. Gastroenterol Res Pract. 2011;2011:930941.:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moosavi SSL. Urea breath test [Internet]. StatPearls Publishing LLC; 2019. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542286/. Accessed May25, 2021. [PubMed] [Google Scholar]
- 10.Masucci L, Blackhouse G, Goeree R. Cost-effectiveness of the carbon-13 urea breath test for the detection of Helicobacter pylori: an economic analysis. Ont Health Technol Assess Ser. 2013;13(20):1. [PMC free article] [PubMed] [Google Scholar]
- 11.Rasool S, Abid S, Jafri W. Validity and cost comparison of 14 carbon urea breath test for diagnosis of H. pylori in dyspeptic patients. World J Gastroenterol. 2007;13(6):925–929. doi: 10.3748/wjg.v13.i6.925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection – a critical review. Aliment Pharmacol Ther. 2004;20(10):1001–1017. doi: 10.1111/j.1365-2036.2004.02203.x [DOI] [PubMed] [Google Scholar]
- 13.Aklil Abd Rahim M, Hafiz Johani F, Azhar Shah S, Rohaizat Hassan M, Rizal Abdul Manaf M. 13C-urea breath test accuracy for Helicobacter pylori infection in the Asian population: a meta-analysis. Ann Glob Health. 2019;85(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng NJ, Lai KH, Liu RS, et al. Capsule 13C-urea breath test for the diagnosis of Helicobacter pylori infection. World J Gastroenterol. 2005;11(9):1361–1364. doi: 10.3748/wjg.v11.i9.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syam AF, Miftahussurur M, Makmun D, Nusi IA, Zain LH. Risk factors and prevalence of Helicobacter pylori in five largest islands of Indonesia: a preliminary study. PLoS One. 2015;10(11):e0140186. doi: 10.1371/journal.pone.0140186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S-Y. Endoscopic gastritis, serum pepsinogen assay, and Helicobacter pylori infection. Korean J Intern Med. 2016;31(5):835–844. doi: 10.3904/kjim.2016.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JY, Kim N. Diagnosis of Helicobacter pylori by invasive test: histology. Ann Transl Med. 2015;3(1):10. doi: 10.3978/j.issn.2305-5839.2014.11.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis: the updated Sydney system. Am J Surg Pathol. 1996;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001 [DOI] [PubMed] [Google Scholar]
- 19.Leal YA, Flores LL, Fuentes-Panana EM, Cedillo-Rivera R, Torres J. 13C-urea breath test for the diagnosis of Helicobacter pylori infection in children: a systematic review and meta-analysis. Helicobacter. 2011;16(4):327–337. doi: 10.1111/j.1523-5378.2011.00863.x [DOI] [PubMed] [Google Scholar]
- 20.Talebi Bezmin Abadi A. Diagnosis of Helicobacter pylori using invasive and noninvasive approaches. J Pathog. 2018;2018:9064952. doi: 10.1155/2018/9064952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonaitis LV, Kiudelis G, Kupcinskas L. Evaluation of a novel 14C-urea breath test “Heliprobe” in diagnosis of Helicobacter pylori infection. Med. 2007;43(1):32–35.:. [PubMed] [Google Scholar]
- 22.Yamaoka Y, Kato M, Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med. 2008;47(12):1077–1083. doi: 10.2169/internalmedicine.47.0975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vakil N. Are there geographical and regional differences in Helicobacter pylori eradication? Can J Gastroenterol. 2003;17(Suppl B):30B–32B. doi: 10.1155/2003/903494 [DOI] [PubMed] [Google Scholar]
- 24.Miftahussurur M, Yamaoka Y. Diagnostic methods of Helicobacter pylori infection for epidemiological studies: critical importance of indirect test validation. Biomed Res Int. 2016;2016:4819423. doi: 10.1155/2016/4819423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syam AF, Miftahussurur M, Uwan WB, Simanjuntak D, Uchida T, Yamaoka Y. Validation of urine test for detection of Helicobacter pylori infection in Indonesian population. Biomed Res Int. 2015;2015:152823. doi: 10.1155/2015/152823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Best LMJ, Takwoingi Y, Siddique S, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;2018. Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012080.pub2/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee I. Critical pathogenic steps to high risk Helicobacter pylori gastritis and gastric carcinogenesis. World J Gastroenterol. 2014;20(21):6412–6419. doi: 10.3748/wjg.v20.i21.6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishaq S, Nunn L. Helicobacter pylori and gastric cancer: a state of the art review. Gastroenterol Hepatol Bed Bench. 2015;8(Suppl1):S6–14. [PMC free article] [PubMed] [Google Scholar]


