Abstract
Purpose
Leiomyosarcoma (LMS) is a rare and extremely aggressive malignancy that is derived from or shows evidence of differentiation toward smooth muscle. If LMS occurs in the female reproductive system, preoperative diagnosis can be difficult, as LMS is easily mistaken for a uterine leiomyoma, especially a degenerated uterine fibroid (DUF). Thus, we assessed the diagnostic value of the preoperative serum concentrations of cancer antigen 125 (CA125), lactate dehydrogenase (LDH) and human epididymis protein 4 (HE4) for differentiating LMS from DUF.
Patients and Methods
We enrolled patients with LMS or DUF who were receiving treatment in The First Affiliated Hospital of Chongqing Medical University between 2009 and 2020. If the preoperative serum concentrations of CA125, LDH and HE4 of our study participants had been tested, these data were analyzed. The preoperative serum concentrations of CA125, LDH and HE4 in participants with LMS (n = 37) were compared with those of participants with pathologically diagnosed DUF (n = 102), who served as the control group.
Results
The preoperative serum concentrations of CA125, LDH and HE4 of participants with LMS of the female reproductive system were significantly higher than those of participants with DUF (P = 0.009, P < 0.001, P = 0.001, respectively). The cut-off preoperative serum concentrations of CA125, LDH and HE4 were 30.85 U/mL, 186.50 U/L and 50.50 pmol/L, respectively. When these three parameters were used for an analysis of their combined diagnostic utility, the area under the curve (AUC) was 0.892, the sensitivity was 68.4% and the specificity was 95.1% (P < 0.001).
Conclusion
A combined analysis of the preoperative serum concentrations of CA125, LDH and HE4 could be a promising method for diagnostically differentiating LMS of the female reproductive system from DUF.
Keywords: leiomyosarcoma of the female reproductive system, degenerated uterine fibroid, cancer antigen 125, human epididymis protein 4, lactate dehydrogenase
Introduction
Leiomyosarcoma (LMS) is a rare and extremely aggressive malignancy derived from or showing evidence of differentiation toward smooth muscle.1 It can occur in the female reproductive system, especially in the uterus (which is rich in smooth muscle), or in other parts of the pelvic area such as the ovaries, fallopian tube, uterine ligaments, vulva, vagina and other extrauterine sites. Uterine leiomyosarcoma (ULMS) accounts for 1–2% of uterine malignancy.2 LMS has non-specific symptoms, is aggressive and has high recurrence rates, and thus, patients with LMS have a poor prognosis, even if the disease is detected and treated at an early stage.2–4
Uterine leiomyomas (uterine fibroids) are the most common solid, benign gynecological tumor.5 In conventional magnetic resonance imaging (MRI), a typical leiomyoma exhibits low signal intensities in T1-weighted images (T1WIs) and T2-weighted images (T2WIs). LMS of the female reproductive system can easily be distinguished from a typical leiomyoma in MRI, as LMS exhibits areas of necrosis and hemorrhage, and high signal intensities in T1WIs and T2WIs.6 However, most large leiomyomas display various types of degeneration and are known as degenerated uterine fibroids (DUFs). DUF exhibits hyperintense T1WIs and T2WIs in conventional MRI, which are similar in appearance to the T1WIs and T2WIs of LMS.6–8 This similarity of LMS with DUF in MRI, combined with the fact that the clinical manifestation of LMS is similar to that of DUF, and that there are no validated serum biomarkers of LMS, means that LMS is easily misdiagnosed as DUF.
Treatment for LMS includes total hysterectomy and debulking of the tumor if it is present outside the uterus,2–4 whereas DUF is managed conservatively, such as by follow-up and myomectomy.5,9 LMS is rarely suspected before surgery and is often detected by histopathology after hysterectomy or myomectomy, with or without morcellation.4 Unintended morcellation of occult LMS results in a grave prognosis.10 Preoperative diagnostic differentiation between LMS and DUF is therefore very important.
There is currently no consensus on the use of serological markers for LMS. In related work, cancer antigen 125 (CA125) has been widely used as a tumor marker in ovarian cancer.11 The serum concentrations of CA125 in patients with uterine sarcoma are significantly higher than those in patients with leiomyomas.12 Nonetheless, the diagnostic efficacy of the serum concentrations of CA125 in this context remains controversial and has yet to be clinically validated. Human epididymis protein 4 (HE4) has been approved by the Food and Drug Administration (FDA) as a serum biomarker for the diagnosis of ovarian cancers,13 but its diagnostic value for LMS and DUF patients remains unclear. Lactate dehydrogenase (LDH) is an important coenzyme in glycolysis and is closely associated with malignant tumors. The serum concentrations of LDH are increased in patients with malignant tumors of the genital tract and, particularly, in patients with uterine sarcomas.14,15
There are many studies on ULMS, but few reports on LMS that occurs in other parts of the female reproductive system. There is currently no unified staging standard and diagnosis and treatment guidelines for LMS of the female reproductive system. So, simpler and more objective studies are clearly needed to develop diagnostic tests to distinguish between LMS of the female reproductive system patients and DUF patients. Thus, our aim was to explore whether the serum concentrations of CA125, HE4 and LDH could be used to diagnostically distinguish between LMS and DUF.
Patients and Methods
Patients
This study was approved by the Institutional Review Board of The First Affiliated Hospital of Chongqing Medical University (2020–425), and informed consent was obtained from all patients. This study also complied with the Declaration of Helsinki.
Clinical information was collected from the electronic medical record system of The First Affiliated Hospital of Chongqing Medical University. The medical records of 50 participants with LMS of the female reproductive system who were receiving treatment between January 2009 and August 2020 in the Department of Obstetrics and Gynecology at this hospital were retrospectively reviewed. In addition, 102 participants diagnosed with DUF and who were receiving treatment between September 2013 and December 2019 at this hospital were selected as controls. All patients had histologically confirmed LMS and DUF. The clinical features of these participants, including their age, menstrual status, abdominal pain and abnormal vaginal bleeding, were reviewed. The preoperative serum concentrations of CA125, LDH and HE4 of participants were analyzed from their medical records if these parameters had been evaluated. Patients were excluded from the study if they demonstrated other organ insufficiencies (eg renal dysfunction) or if they had experienced other pathologies (eg adenomyosis, endometrial cancer, ovarian cancer, lung cancer or hematological diseases), acute infection or major trauma.
Five patients with LMS who had associated adenomyosis, which could potentially lead to increases in the serum concentrations of CA125, were excluded. Five patients with recurrent LMS were excluded. Three patients with LMS were excluded because they had undergone subtotal or total hysterectomy in other hospitals. Finally, during the selection of matched controls, patients who had DUF and associated pelvic or non-pelvic pathologies were also excluded.
Statistical Analysis
All calculations were performed with Statistical Product and Service Solutions (SPSS) 25.0 statistical software. The Wilcoxon-Mann–Whitney test was used to compare serum marker distributions between the two groups of participants. A chi-square analysis was used to assess the qualitative data. The sensitivities and specificities of CA125, HE4, LDH and combined detection modes for LMS were calculated. The predicted probabilities for each indicator were used to construct receiver operating characteristic (ROC) curves. The area under the curve (AUC) values and the optimal cut-off values were calculated. Differences were considered statistically significant if their P value was <0.05.
Results
The basic demographic features of the study population are presented in Table 1. The LMS group and DUF group participants were matched with respect to age, menstrual status, abdominal pain and abnormal vaginal bleeding. The LMS group comprised participants with ULMS (n = 17; 46.0%), LMS of the cervix (n = 2; 5.4%), Ovarian LMS (n = 1; 2.7%), LMS of uterine ligaments (n = 3; 8.1%) and pelvic LMS (n = 14; 37.8%). As the preoperative serum concentrations of CA125, HE4 and LDH were not normally distributed, non-parametric analysis methods were used. The preoperative serum concentrations of CA125 of 35 and 102 participants in the LMS and DUF groups, respectively, had been measured. The preoperative serum concentrations of LDH of 21 and 102 participants in the LMS and DUF groups, respectively, had been measured. The preoperative serum concentrations of HE4 of 26 and 102 participants in the LMS and DUF groups, respectively, had been measured. As can be seen in Table 2, there was a statistically significant difference between the preoperative serum concentrations of CA125, LDH and HE4 of the LMS group and those of the DUF group (P = 0.009, P < 0.001, P = 0.001, respectively).
Table 1.
Patients’ Characteristics
| Variables | LMS of the Female Reproductive System (n=37) Mean (±SD) or n (%) |
DUF (n=102) Mean (±SD) or n (%) |
P |
|---|---|---|---|
| Age (years) | 49(±13) | 44 (±11) | 0.044 |
| Menopause (n) | 16(43.2) | 17(16.7) | 0.001 |
| Abnormal vaginal bleeding (n) | 11(29.7) | 14(13.7) | 0.030 |
| Lower abdominal pain (n) | 19(51.4) | 32(31.4) | 0.031 |
Abbreviation: SD, standard deviation.
Table 2.
The CA125, LDH and HE4 Values of the LMS of the Female Reproductive System and DUF
| Variables | LMS of the Female Reproductive System (n=37) Mean (±SD) or n (%) |
DUF (n=102) Mean (±SD) or n (%) |
P |
|---|---|---|---|
| CA125(U/mL) | 41.48(±54.03) | 18.60(±9.56) | 0.009 |
| LDH(U/L) | 248.95(±151.23) | 157.08(±34.01) | <0.001 |
| HE4(pmol/L) | 118.69(±269.85) | 43.02(±14.13) | 0.001 |
Abbreviation: SD, standard deviation.
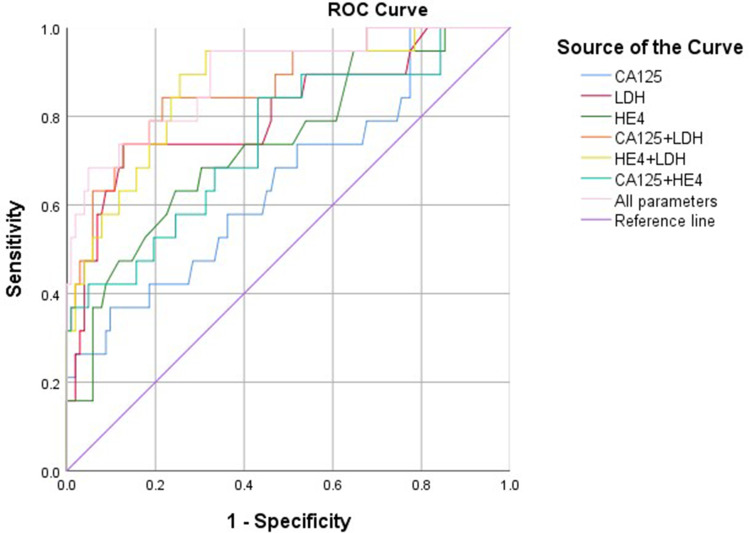
To evaluate whether these three serum markers of tumors could be used as diagnostic markers for the clinical disease, ROC curves were generated (Figure 1) and results are presented in Table 3. The AUC of a combination of the above three serum markers was 0.892 (95% confidence interval: 0.809–0.976, P < 0.001), with a sensitivity of 68.4% and a specificity of 95.1%.
Figure 1.
The receiver operating characteristic curves of preoperative serum CA125, LHD, HE4 and the model combination of above three markers.
Table 3.
Diagnostic Performance of Different Groups Assessment by CA125, LDH and HE4
| Variables | Cut-off Value | Sensitivity(%) | Specificity(%) | Accuracy(%) | AUC(%95CI) | P |
|---|---|---|---|---|---|---|
| CA125(U/mL) | 30.85 | 36.8 | 90.2 | 76.6 | 0.655(0.515,0.796) | 0.009 |
| LDH(U/L) | 186.50 | 72.7 | 87.3 | 83.7 | 0.808(0.686,0.929) | <0.001 |
| HE4(pmol/L) | 50.50 | 63.2 | 75.5 | 72.7 | 0.739(0.613,0.864) | 0.001 |
| CA125+LDH | - | 84.2 | 78.4 | - | 0.867(0.774,0.960) | <0.001 |
| HE4+LDH | - | 89.5 | 74.5 | - | 0.869(0.779,0.959) | <0.001 |
| CA125+HE4 | - | 84.2 | 56.9 | - | 0.747(0.618,0.875) | 0.001 |
| All parameters | - | 68.4 | 95.1 | - | 0.892(0.809,0.976) | <0.001 |
Abbreviations: AUC, area under the curve; CI, confidence interval.
Discussion
In this study, we determined that there were significantly higher preoperative serum concentrations of CA125, LDH and HE4 in participants with LMS of the female reproductive system than in participants with DUF. In addition, a combination of all three of these serum markers afforded better diagnostic efficacy than that afforded by any marker alone or by any pairwise combination of markers.
LMS is a major health problem worldwide due to its low rate of preoperative diagnosis and poor prognosis and the limited range of treatment options. For example, ULMS patients have post-5-year relapse rates of 53–71%.4 A Norwegian study confirmed this poor prognosis of ULMS patients, as its participants had a 5-year overall survival of 51% at International Federation of Gynecology and Obstetrics (FIGO) stage I and 25% at stage II, and all those participants with ULMS that had spread outside the pelvis had died within 5 years.16
Recent research from the United States showed that approximately 70% of women develop uterine leiomyomas during their lifetime.5 Degenerative changes occur when these leiomyomas outgrow their blood supply, and the type of degenerative change depends on the rapidity of onset and the extent of vascular insufficiency.17 The various types of degeneration are hyaline degeneration, calcification, cystic degeneration, red degeneration and sarcomatous change.17 Pathologically, an accumulation of proteinaceous tissue results in hyaline degeneration; venous thrombosis and arterial rupture results in red degeneration; and the accumulation of a gelatinous fluid results in myxoid degeneration. These different types of degeneration can represent different histopathological features. For example, when leiomyoma develops red degeneration, hemorrhagic infarction may be present in necrotic areas.18 A typical leiomyoma usually exhibits a low mitotic rate, which is generally 0–3 mitotic figures per 10 high-power fields (HFPs), but mitotic rate can be up to 10 mitotic figures per 10 HFPs in DUF.19 LMS is thus diagnosed based on the histological findings of tumor cell necrosis, moderate to severe nuclear atypia and a high mitotic rate (greater than or equal to 10 mitotic figures per 10 HFPs).8
The rapid development of minimally invasive surgery means that power morcellation is often required for tumor-tissue reduction. However, when a sarcoma is morcellated, cancerous tissue may be released into the abdominal cavity, which leads to local spread and recurrence.20 Indeed, occult LMS in patients who have previously been treated for presumed benign leiomyomas has been found to represent 0.07–0.49% of LMS cases.21–23 Moreover, a Norwegian cohort study of women diagnosed with ULMS found that in up to 52% of patients, LMS was not suspected at the time of operation.24 Efforts have been made to detect LMS at an early stage, but most patients present at advanced stages. Therefore, novel diagnostic methods are required to enable sarcomas to be distinguished from leiomyomas.
Several features that are visible in MRI, ultrasonography or computed tomography are suggestive of sarcomas and leiomyomas, but there are currently no diagnostic criteria to distinguish between them.25 Although MRI can show tumor characteristics, LMS imaging findings vary in general MRI settings. In addition, hypercellularity or necrosis can be recognized by MRI, but DUF may exhibit similar MRI features.9,26 Serum markers that can enable a differential diagnosis to be made between LMS and DUF are therefore needed to improve the rates of early diagnosis and survival of LMS patients.
CA125, a glycoprotein encoded by the MUC16 gene,27 is used commonly to diagnose cancer and monitor the effects of cancer treatment, particularly for epithelial-origin ovarian and endometrial tumors.11,27 The upper limit of CA125 is 35 U/mL, and a reference range is used to assist in the diagnosis of ovarian cancer. An increasing number of researchers are also exploring the role of CA125 in uterine sarcoma. For example, Juang and colleagues found that the preoperative serum concentrations of CA125 were significantly higher in a group of 42 LMS patients than in a group of 84 leiomyoma patients.28 In addition, Kim and colleagues reported that there are certain intrinsic associations between the serum concentrations of CA125 and uterine sarcoma.12 However, there are too few studies to support the use of the serum concentrations of CA125 for diagnostically differentiating between LMS and DUF. We therefore performed this study to examine whether the preoperative serum concentrations of CA125 could be used to differentiate between participants with LMS and those with DUF. Notably, we found that the preoperative serum concentrations of CA125 were significantly higher in participants with LMS than in those with DUF. Their sensitivity and specificity were 36.8% and 90.2%, respectively, with an optimal cut-off value of 30.85 U/mL, which was within the normal range. However, there is no reliable reference range for CA125 concentrations in Asian women, with respect to LMS and DUF. One study measured CA125 concentrations in 68 Chinese women with benign gynecological disorders and found a median concentration of 22.1 U/mL, which is less than our result.29
The activation of glycolytic metabolism30 is an important chemical feature of malignant tumor cells, and LDH is a key enzyme in this process as it catalyzes the reduction of free pyruvate to lactate. Aerobic glycolysis occurs in cancer cells, and its increase is a hallmark of most cancer cells, denoted as the Warburg effect.31 If tumor cell membranes are damaged, intracellular LDH is released into the blood.32 Moreover, LDH plays an important role in tumor occurrence, development, invasion, metastasis and prognosis.31 Increased serum concentrations of LDH are commonly seen in myocardial infarction, liver disease and blood system disease, in addition to malignant tumors. Furthermore, prior studies have found significantly higher pretreatment serum concentrations of LDH in patients with sarcoma than in those with leiomyomas.9,33,34 In our study, we found that the preoperative serum concentrations of LDH were significantly higher in patients with LMS than in patients with DUF, and that the LDH cut-off concentration was 186.50U/L, with a reference range of 125–250 U/L. Although this cut-off concentration was within the normal range, this reference range may be not suitable for distinguishing between patients with LMS and those with DUF, particularly because there are few studies on reference serum concentrations of LDH in Asian populations.
HE4, a glycoprotein encoded by the WFDC2 gene, has been approved by the FDA as a serum biomarker for use in the diagnosis of ovarian cancers.13 Recent studies have shown that HE4 is not expressed in the ovaries and myometrium of healthy women, but is overexpressed in those of women with ovarian cancer.35 However, there have been no studies on the expression of HE4 in women with sarcoma. We therefore examined the relationship between HE4 and LMS by comparing the preoperative serum concentrations of HE4 of a group with LMS with those of a group with DUF. This revealed that there were significantly higher preoperative serum concentrations of HE4 in the LMS group than in the DUF group. The upper limit of the serum concentration of HE4 is 70 pmol/L for pre-menopausal women and 140 pmol/L for post-menopausal women, and a reference range is commonly used to assist in the diagnosis of ovarian cancer. However, there is no reliable reference range for the HE4-based diagnosis of LMS and DUF in Asian women. Mokhtar and colleagues36 analyzed HE4 reference ranges in 300 healthy Asian women and found that the median serum concentration of HE4 was 36.60 pmol/L for the total cohort, while it was 33.8 pmol/L and 40.3 pmol/L for pre and postmenopausal women, respectively; the mean serum HE4 concentration was 42.80 pmol/L for the total cohort. However, our study found that the cut-off value of serum HE4 concentration was 50.50 pmol/L for all of the participants, with a sensitivity of 63.2% and a specificity of 75.5%. These findings suggest that HE4 is a feasible serum marker for the preoperative differentiation between cases of LMS and DUF. In the future, reference ranges for the serum concentrations of CA125, LDH and HE4 in the Asian female population must be defined by analyzing large sample sizes.
In our study, we observed that the combination of the serum concentrations of CA125, LDH and HE4 had the highest area under the ROC curve, with a sensitivity of 68.4% and a specificity of 95.1%. This revealed that a combined analysis of these three parameters was more accurate for diagnostically differentiating between LMS and DUF than an analysis of any single parameter or a pairwise combination thereof.
The limitations of our study include that it was retrospective, that the number of participants was very small and that some data could not be obtained.
Conclusion
In conclusion, a combined analysis of the preoperative serum concentrations of CA125, LDH and HE4 could be a new promising method for diagnostically differentiating LMS from DUF. Further studies are required to confirm these findings.
Acknowledgments
We would like to thank all the patients who voluntarily participated in this study and research assistants who helped with data collection.
Funding Statement
There were no funding sources.
Abbreviations
LMS, leiomyosarcoma; DUF, degenerated uterine fibroid; CA125, cancer antigen 125; LDH, lactate dehydrogenase; HE4, human epididymis protein 4; AUC, area under the curve; ULMS, uterine leiomyosarcoma; MRI, magnetic resonance imaging; T1WI, T1-weighted image; T2WI, T2-weighted image; HFP, high power field; FDA, Food and Drug Administration; SPSS, Statistical Product and Service Solutions; ROC, receiver operating characteristic; FIGO, International Federation of Gynecology and Obstetrics; SD, standard deviation; CI, confidence interval.
Data Sharing Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study was approved by the institutional review board of The First Affiliated Hospital of Chongqing Medical University (2020-425), and informed consents were obtained from all patients. And this study complied with the Declaration of Helsinki.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vujosevic S, Krnjevic D, Bogojevic M, et al. Primary leiomyosarcoma of the thyroid gland with prior malignancy and radiotherapy: a case report and review of literature. World J Clin Cases. 2019;7(4):473–481. doi: 10.12998/wjcc.v7.i4.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mbatani N, Olawaiye AB, Prat J. Uterine sarcomas. Int J Gynaecol Obstet. 2018;143(Suppl 2):51–58. doi: 10.1002/ijgo.12613 [DOI] [PubMed] [Google Scholar]
- 3.Gockley AA, Rauh-Hain JA, Del Carmen MG. Uterine leiomyosarcoma: a review article. Int J Gynecol Cancer. 2014;24(9):1538–1542. doi: 10.1097/IGC.0000000000000290 [DOI] [PubMed] [Google Scholar]
- 4.Juhasz-Boss I, Gabriel L, Bohle RM, Horn LC, Solomayer EF, Breitbach GP. Uterine Leiomyosarcoma. Oncol Res Treat. 2018;41(11):680–686. doi: 10.1159/000494299 [DOI] [PubMed] [Google Scholar]
- 5.Giuliani E, As-Sanie S, Marsh EE. Epidemiology and management of uterine fibroids. Int J Gynaecol Obstet. 2020;149(1):3–9. doi: 10.1002/ijgo.13102 [DOI] [PubMed] [Google Scholar]
- 6.Li HM, Liu J, Qiang JW, Zhang H, Zhang GF, Ma F. Diffusion-weighted imaging for differentiating uterine leiomyosarcoma from degenerated leiomyoma. J Comput Assist Tomogr. 2017;41(4):599–606. doi: 10.1097/RCT.0000000000000565 [DOI] [PubMed] [Google Scholar]
- 7.Okizuka H, Sugimura K, Takemori M, Obayashi C, Kitao M, Ishida T. MR detection of degenerating uterine leiomyomas. J Comput Assist Tomogr. 1993;17(5):760–766. doi: 10.1097/00004728-199309000-00018 [DOI] [PubMed] [Google Scholar]
- 8.Sun S, Bonaffini PA, Nougaret S, et al. How to differentiate uterine leiomyosarcoma from leiomyoma with imaging. Diagn Interv Imaging. 2019;100(10):619–634. 10.1016/j.doi.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 9.Nagamatsu A, Umesaki N, Li L, Tanaka T. Use of 18F-fluorodeoxyglucose positron emission tomography for diagnosis of uterine sarcomas. Oncol Rep. 2010;23(4):1069–1076. doi: 10.3892/or_00000734 [DOI] [PubMed] [Google Scholar]
- 10.Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. Available from: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Accessed November24, 2014.
- 11.Weiland F, Fritz K, Oehler MK, Hoffmann P. Methods for identification of CA125 from ovarian cancer ascites by high resolution mass spectrometry. Int J Mol Sci. 2012;13(8):9942–9958. doi: 10.3390/ijms13089942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HS, Han KH, Chung HH, et al. Neutrophil to lymphocyte ratio for preoperative diagnosis of uterine sarcomas: a case-matched comparison. Eur J Surg Oncol. 2010;36(7):691–698. doi: 10.1016/j.ejso.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 13.Moore RG, Hill EK, Horan T, et al. HE4 (WFDC2) gene overexpression promotes ovarian tumor growth. Sci Rep. 2014;4:3574. doi: 10.1038/srep03574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyriazoglou A, Liontos M, Ziogas DC, et al. Management of uterine sarcomas and prognostic indicators: real world data from a single-institution. BMC Cancer. 2018;18(1):1247. doi: 10.1186/s12885-018-5156-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mollo A, Raffone A, Travaglino A, et al. Increased LDH5/LDH1 ratio in preoperative diagnosis of uterine sarcoma with inconclusive MRI and LDH total activity but suggestive CT scan: a case report. BMC Womens Health. 2018;18(1):169. doi: 10.1186/s12905-018-0662-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abeler VM, Royne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009;54(3):355–364. doi: 10.1111/j.1365-2559.2009.03231.x [DOI] [PubMed] [Google Scholar]
- 17.Deshmukh SP, Gonsalves CF, Guglielmo FF, Mitchell DG. Role of MR imaging of uterine leiomyomas before and after embolization. Radiographics. 2012;32(6):E251–281. doi: 10.1148/rg.326125517 [DOI] [PubMed] [Google Scholar]
- 18.Barral M, Place V, Dautry R, et al. Magnetic resonance imaging features of uterine sarcoma and mimickers. Abdom Radiol. 2017;42(6):1762–1772. doi: 10.1007/s00261-017-1076-9 [DOI] [PubMed] [Google Scholar]
- 19.Devereaux KA, Schoolmeester JK. Smooth muscle tumors of the female genital tract. Surg Pathol Clin. 2019;12(2):397–455. doi: 10.1016/j.path.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 20.Glorie N, Baert T, Van Den Bosch T, Coosemans AN. Circulating protein biomarkers to differentiate uterine sarcomas from leiomyomas. Anticancer Res. 2019;39(8):3981–3989. doi: 10.21873/anticanres.13553 [DOI] [PubMed] [Google Scholar]
- 21.Saridogan E, Cutner A. Endoscopic management of uterine fibroids. Hum Fertil. 2006;9(4):201–208. doi: 10.1080/14647270600560204 [DOI] [PubMed] [Google Scholar]
- 22.Worldwide, AAGL Advancing Minimally Invasive Gynecology. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21(4):517–530. doi: 10.1016/j.jmig.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 23.Harlow BL, Weiss NS, Lofton S. The epidemiology of sarcomas of the uterus. J Natl Cancer Inst. 1986;76(3):399–402. [PubMed] [Google Scholar]
- 24.Skorstad M, Kent A, Lieng M. Preoperative evaluation in women with uterine leiomyosarcoma. A nationwide cohort study. Acta Obstet Gynecol Scand. 2016;95(11):1228–1234. doi: 10.1111/aogs.13008 [DOI] [PubMed] [Google Scholar]
- 25.Van den Bosch T, Coosemans A, Morina M, Timmerman D, Amant F. Screening for uterine tumours. Best Pract Res Clin Obstet Gynaecol. 2012;26(2):257–266. doi: 10.1016/j.bpobgyn.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 26.Shah SH, Jagannathan JP, Krajewski K, O’Regan KN, George S, Ramaiya NH. Uterine sarcomas: then and now. AJR Am J Roentgenol. 2012;199(1):213–223. doi: 10.2214/AJR.11.7287 [DOI] [PubMed] [Google Scholar]
- 27.Huang GS, Chiu LG, Gebb JS, et al. Serum CA125 predicts extrauterine disease and survival in uterine carcinosarcoma. Gynecol Oncol. 2007;107(3):513–517. doi: 10.1016/j.ygyno.2007.08.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juang CM, Yen MS, Horng HC, Twu NF, Yu HC, Hsu WL. Potential role of preoperative serum CA125 for the differential diagnosis between uterine leiomyoma and uterine leiomyosarcoma. Eur J Gynaecol Oncol. 2006;27(4):370–374. [PubMed] [Google Scholar]
- 29.Zhang Y, Qiao C, Li L, Zhao X, Li Y. Serum HE4 is more suitable as a biomarker than CA125 in Chinese women with benign gynecologic disorders. Afr Health Sci. 2014;14(4):913–918. doi: 10.4314/ahs.v14i4.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayley JP, Devilee P. The Warburg effect in 2012. Curr Opin Oncol. 2012;24(1):62–67. doi: 10.1097/CCO.0b013e32834deb9e [DOI] [PubMed] [Google Scholar]
- 31.Song KJ, Yu XN, Lv T, et al. Expression and prognostic value of lactate dehydrogenase-A and -D subunits in human uterine myoma and uterine sarcoma. Medicine. 2018;97(14):e0268. doi: 10.1097/MD.0000000000010268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jurisic V, Radenkovic S, Konjevic G. The actual role of LDH as tumor marker, biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:115–124. doi: 10.1007/978-94-017-7215-0_8 [DOI] [PubMed] [Google Scholar]
- 33.Kusunoki S, Terao Y, Ujihira T, et al. Efficacy of PET/CT to exclude leiomyoma in patients with lesions suspicious for uterine sarcoma on MRI. Taiwan J Obstet Gynecol. 2017;56(4):508–513. doi: 10.1016/j.tjog.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 34.Nagai T, Takai Y, Akahori T, et al. Highly improved accuracy of the revised PREoperative sarcoma score (rPRESS) in the decision of performing surgery for patients presenting with a uterine mass. Springerplus. 2015;4:520. doi: 10.1186/s40064-015-1318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlsen NS, Karlsen MA, Hogdall CK, Hogdall EV. HE4 tissue expression and serum HE4 levels in healthy individuals and patients with benign or malignant tumors: a systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2285–2295. doi: 10.1158/1055-9965.EPI-14-0447 [DOI] [PubMed] [Google Scholar]
- 36.Mokhtar N, Thevarajah M, Ma N. Human epididymis protein 4 reference intervals in a multiethnic asian women population. Asian Pac J Cancer Prev. 2012;13(12):6391–6395. doi: 10.7314/APJCP.2012.13.12.6391 [DOI] [PubMed] [Google Scholar]