Abstract
Purpose
Identifying patient characteristics that define a worse disease prognosis or “high tumor burden” (HTB) status is essential for clinical decision-making and treatment selection in metastatic non-small cell lung cancer (mNSCLC). We aimed to define this concept based on the experience of oncologists in clinical practice.
Patients and Methods
A representative sample of Spanish experts was selected and asked to complete an online survey regarding the definition of HTB according to their personal experience.
Results
HTB was identified by the oncologists (N = 81) as one of the principle factors influencing first-line treatment decision-making. According to the experts, HTB is mainly defined by the number of metastatic lesions (n = 45, 56%), location (n = 34, 42%), tumor size (sum of diameters of target lesions; n = 26, 32%) and liver involvement (n = 24, 30). High lactate dehydrogenase (LDH) levels were also associated with HTB. Almost half of respondents (n = 33, 41%) believed that one metastatic lesion was sufficient to consider a patient as presenting HTB, 72% (n = 58) considered that two were necessary and 99% (n = 80) three. Liver (n = 76, 100%) followed by brain (n = 65, 86%) were the main metastatic sites associated with HTB. Tumor size ranging from 6 cm to 10 cm as well as high LDH levels (three times the upper limit) defined the concept for 82% (n = 62) and 100% (n = 76) of oncologists, respectively.
Conclusion
In the real-world setting, according to experts, HTB is defined by the number of metastatic lesions, location of metastases, tumor size and by high LDH levels. Given the relevance of this concept, efforts should be made to unify its definition and to further explore its potential as a prognostic factor for mNSCLC patients.
Keywords: non-small cell lung cancer, “high tumor burden”, number of metastases, tumor size, metastatic locations, lactate dehydrogenase
Introduction
Lung cancer is the leading cause of cancer death among men and the second among women worldwide.1 Non-small cell lung cancer (NSCLC) is the most prevalent subtype, accounting for 80% to 85% of all lung cancer diagnoses.2 Given that it is frequently diagnosed in the advanced stage3 and is associated with poor survival rates,4 accurate prognosis is essential for clinical decision making and treatment selection. Prognostic factors can be used to construct homogenous patient groups, helping guide therapy in some cases, for example by identifying subgroups of individuals according to their therapy requirements. Additionally, they can be used as stratification factors.5
The tumor-nodes-metastasis (TNM) classification system for cancer staging published by the Union for International Cancer Control (UICC)6 has been one of the most reproducible methods for establishing prognosis to date. However, this system classifies patients into different stages, each representing a range of disease extent that may not best reflect individual patient prognosis,7 especially in patients with more advanced stages of the disease (stage IVb). New statistical models that combine multiple variables have recently been developed to improve the prognosis of cancer patients.8,9 In the real-world setting, the identification of patient characteristics that define a worse disease prognosis or “high tumor burden” (HTB) status is essential in these advanced stages. Classifying patients based on this concept may help guide treatment decision making, offering more specific alternatives for patients with metastatic NSCLC (mNSCLC). Given the special medical requirements of this group, our aim was to define the concept of HTB based on the experience of oncologists in clinical practice.
Methods
A representative sample of Spanish experts was surveyed. Inclusion in the survey required the oncologist to be the primary decision maker for the treatment of ≥10 mNSCLC patients per month and to have experience in immunotherapy regimens.
The experts were asked to complete an online survey containing some general questions and specific questions regarding the definition of HTB according to their personal experience in clinical practice (Supplementary Material). Data were collected between 29 November 2019 and 14 January 2020 and processed by an independent market research company (Ipsos Healthcare, London, UK). Categorical data are presented as frequencies and continuous variables as mean ± standard deviation (SD).
Due to the non-interventional nature of the study and in accordance with Spanish legislation, ethics committee approval and informed consent were not required (Ministerial Order 1090/2015, 4 December 2015; available at: https://www.boe.es/eli/es/rd/2015/12/04/1090).
Results
A total of 81 experts participated in the survey, resulting in a sampling error of 10.3% with a 95.5% confidence level and p = 0.045. The general characteristics of the respondents are shown in Table 1.
Table 1.
General Characteristics of Respondents
| Characteristics | Total Respondents (N = 81) |
|---|---|
| Origin, n (%) | |
| North of Spain | 17 (21) |
| Northeast/Levante | 25 (31) |
| Center of Spain | 23 (28) |
| South of Spain | 16 (20) |
| Years of experience | |
| ≤15 years | 58% (47) |
| >15 years | 42% (34) |
| Number of cancer patients per month, mean ± SD | 218 ± 107 |
| Lung cancer patients | 101 ± 74 |
| NSCLC patients | 72 ± 53 |
| mNSCLC patients | 50 ± 38 |
Abbreviations: NSCLC, non-small cell lung cancer; mNSCLC, metastatic non-small cell lung cancer; SD, standard deviation.
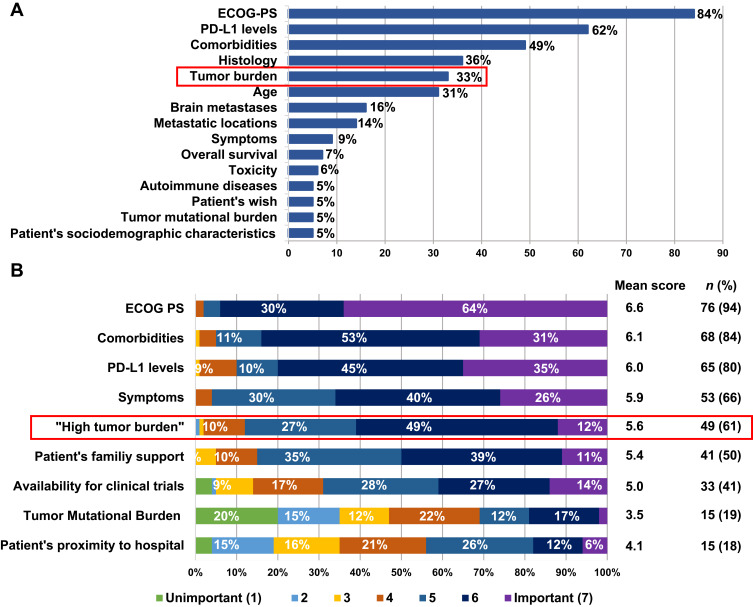
Regarding first-line treatment decision making, oncologists indicated that Eastern Cooperative Oncology Group Performance Status (ECOG PS) at diagnosis (n = 68, 84%), PD-L1 expression levels (n = 50, 62%) and comorbidities (n = 40, 49%) were the main factors influencing their choice of treatment (Figure 1A). Tumor burden (n = 27, 33%) was also included as one of the five main factors considered by the experts when prescribing treatments in the first-line setting. Notably, when assessing the degree of importance of those factors, HTB, considered as a key variable in the decision-making process for the majority of oncologists (n = 49, 61%), was classified as “important” (mean score of 5.6/7) (Figure 1B).
Figure 1.
Factors influencing experts’ first-line treatment decision (A) and degree of importance of those factors evaluated through a scoring system (scale from 1 to 7 where 1 is “Unimportant” and 7 “Important”) (B).
Abbreviations: ECOG PS, Eastern cooperative oncology group performance status; PD-L1, programmed death-ligand 1.
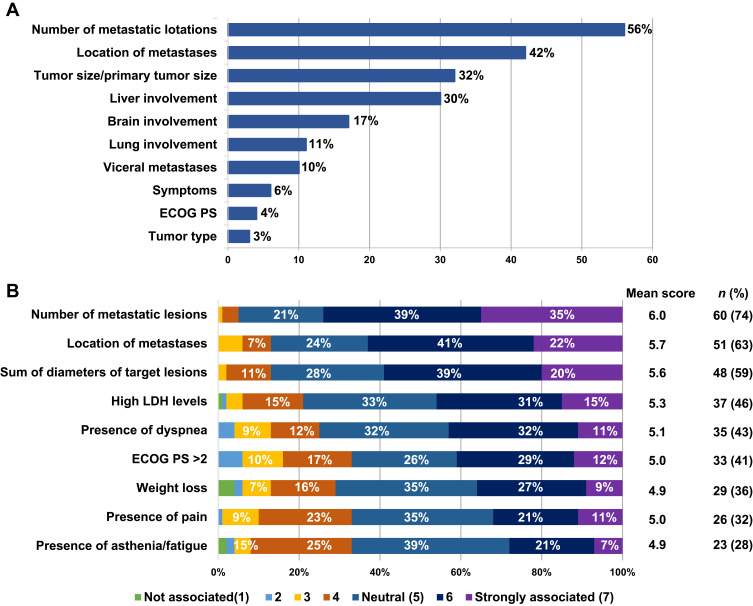
According to the experts, the factors that mainly defined the concept of HTB were the number of metastatic lesions (n = 45, 56%); location of metastases (n = 34, 42%); tumor size (sum of diameters of a maximum of five target lesions, considering no more than two lesions/organ; n = 26, 32%); and liver involvement (n = 24, 30%) (Figure 2A). These factors, along with high lactate dehydrogenase (LDH) levels, were moderately-strongly associated with the concept of HTB by most participants (Figure 2B).
Figure 2.
Factors defining “high tumor burden” (A) and degree of association of those factors with this concept evaluated through a scoring system (scale from 1 to 7 where 1 is “I do not associate it with ‘high tumor burden’ at all” and 7 “I strongly associate it with ‘high tumor burden’”) (B).
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; LDH, lactate dehydrogenase.
Regarding the minimum number of metastatic lesions needed to consider HTB, almost half of respondents (n = 33, 41%) considered that patients with one metastatic lesion present HTB; this percentage increased to 72% (n = 58) for patients with two metastatic lesions and to 99% (n = 80) for patients with three metastatic lesions.
Liver (n = 76, 100%) followed by brain (n = 65, 86%) are the main metastatic sites associated with the concept of HTB. Lung, bone and other locations were indicated by 46% (n = 35), 40% (n = 30) and 17% (n = 13) of the experts, respectively (participants who did not consider metastatic sites as a relevant factor were excluded; N = 76).
In terms of tumor size, 82% (n = 62) of oncologists identified HTB when the sum of diameters of target lesions ranges between 6 cm and 10 cm (participants who did not consider tumor size as a relevant factor were excluded; N = 76).
In the case of LDH, 46% (n = 35) of respondents consider HTB when LDH levels are twice the upper limit of normal (ULN), and 100% (n = 76) consider HTB when levels are three times the ULN (participants who did not consider LDH levels as a relevant factor were excluded; N = 76). Finally, 68% considered the patient had HTB when they had weight loss of at least 15% (n = 48) (participants who did not consider LDH levels or weight loss as relevant factors were excluded; N = 76 and N = 70, respectively).
Discussion
The concept of “high tumor burden” remains unclear. Its definition and implementation as a prognostic factor in the landscape of lung cancer could help to design individualized treatment plans that would not only improve efficacy outcomes and patient quality of life, but also reduce the incidence of adverse effects.10
Our survey found, first, that in the real-world setting, HTB is identified by oncologists as an important variable to consider when choosing first-line treatment. This concept is mainly defined based on the number of metastatic lesions, location of metastases, and tumor size; these are factors previously demonstrated to have independent prognostic value in NSCLC.11–15 According to the experts, high LDH levels, which are associated with poor outcomes in several cancer types and specifically in mNSCLC,16–18 also allow patients with HTB to be identified.
Interestingly, liver involvement was highly associated with the concept of HTB. This is not surprising considering that, among patients with mNSCLC, those with liver metastases show the lowest median overall survival rates,12,19–21 and present a 1.55-fold higher mortality risk compared to patients with other distant metastases.20 The physicians’ answers reveal the negative effect associated with the presence of liver metastases, and the relevance of this factor as a poor prognostic factor in NSCLC patients, as previously reported.12,22 Given its clear negative impact on survival, therapeutic strategies seeking to improve outcomes in this population are desirable. In this respect, the addition of an antiangiogenic agent in the immunotherapy combination has been especially beneficial for these patients.23,24
This analysis has also some limitations. First, our sample only includes a small percentage of Spanish oncologists and, consequently, our findings are limited by the possibility of selection bias. However, it should be taken into account that, as shown, the origin of the participants was well-balanced. Most importantly, we relied on oncologists’ opinions for our results. Thus, caution must be exercised when interpreting our findings, bearing in mind that experts’ perceptions are not likely to be a completely accurate surrogate for patient disease status. Despite these limitations, we believe our study is powerful enough to highlight the relevance of HTB as a concept for defining a worse prognosis for mNSCLC patients, although we stress the need for further research to support this notion.
In summary, according to the findings of our survey, HTB, a concept frequently used by oncologists, is mainly defined as having at least two metastatic lesions, mainly liver and brain metastases, a minimum tumor size ranging from 6–10 cm (corresponding to the sum of diameters) and LDH levels ≥2 times the ULN. Given the importance of this concept, efforts should be made to unify its definition and to further explore its potential as a prognostic factor for patients with mNSCLC.
Acknowledgments
The authors would like to thank Dr. Almudena Fuster-Matanzo from Statistics Consulting S.L. (Valencia) for providing scientific support and medical writing services. The abstract of this paper was presented at the 2020 World Conference on Lung Cancer as a poster presentation with interim findings. The poster’s abstract was published in ‘Poster Abstracts’ in Journal of Thoracic Oncology (2021), Vol. 16 No. 3S: S521-S522: [https://www.jto.org/article/S1556-0864(21)00961-8/fulltext#secsectitle0020].
Funding Statement
This work was sponsored by Roche Farma S.A., Spain. Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
Disclosure
O.H.G. reports advisory and consultancy honoraria from Roche, AstraZeneca, Amgen, Boehringer and Sanofi, speaker honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Pfizer and Amgen, as well as travel/accommodation/expenses support from Roche, Merck, and AstraZeneca. A.M.P reports advisory/consultancy, speaker´s honoraria, and/or travel/accommodation expenses from Roche, MSD, and BMS. A-L.O.G. reports advisory and consultancy honoraria from Roche, Merck, Bristol-Myers Squibb and Boehringer, speaker honoraria from Roche, Merck, Bristol-Myers Squibb and Boehringer, as well as travel/accommodation/expenses support from Roche, Merck, and Bristol-Myers Squibb. L.V. reports advisory and consultancy honoraria from Roche, speaker honoraria from Roche and Bristol-Myers Squibb, research grant funding from AstraZeneca, as well as travel/accommodation/expenses support from Merck, Pfizer and Boehringer. D.P.P., P.R-G. and L.S.C-A. were full-time employees of Roche Farma S.A. at the time the survey was conducted. The authors report no other conflicts of interest in this work.
References
- 1.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. [DOI] [PubMed] [Google Scholar]
- 2.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM classification for lung cancer. J Thoracic Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 4.Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5:288–300. doi: 10.21037/tlcr.2016.06.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paesmans M. Prognostic and predictive factors for lung cancer. Breathe. 2012;9:112. doi: 10.1183/20734735.006911 [DOI] [Google Scholar]
- 6.Brierley JGM, Wittekind CH. TNM Classification of Malignant Tumours. 8th ed. Oxford UK, Hoboken NJ, eds. John Wiley & Sons, Inc; 2017. [Google Scholar]
- 7.Moumtzi D, Lampaki S, Zarogoulidis P, et al. Prognostic factors for long term survival in patients with advanced non-small cell lung cancer. Ann Transl Med. 2016;4:161. doi: 10.21037/atm.2016.05.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang B, Zeng J, Liu Y. Using nomograms to predict prognostic factors in young colorectal mucinous and signet-ring cell adenocarcinoma patients. Biosci Rep. 2019;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng B, Ji P, Chen C, et al. A nomogram from the SEER database for predicting the prognosis of patients with non-small cell lung cancer. Int J Biochem Cell Biol. 2020;127:105825. doi: 10.1016/j.biocel.2020.105825 [DOI] [PubMed] [Google Scholar]
- 10.Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. 2017;377:849–861. doi: 10.1056/NEJMra1703413 [DOI] [PubMed] [Google Scholar]
- 11.Ashour Badawy A, Khedr G, Omar A, et al. Site of metastases as prognostic factors in unselected population of stage iv non-small cell lung cancer. Asian Pacific j Cancer Prevent. 2018;19:1907–1910. doi: 10.22034/APJCP.2018.19.7.1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Zhu H, Sun L, et al. Prognostic value of site-specific metastases in lung cancer: a population based study. J Cancer. 2019;10:3079–3086. doi: 10.7150/jca.30463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motta G, Carbone E, Spinelli E, et al. Considerations about tumor size as a factor of prognosis in NSCLC. Ann Ital Chir. 1999;70:893–897. [PubMed] [Google Scholar]
- 14.Oh Y, Taylor S, Bekele BN, et al. Number of metastatic sites is a strong predictor of survival in patients with nonsmall cell lung cancer with or without brain metastases. Cancer. 2009;115:2930–2938. doi: 10.1002/cncr.24333 [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Gold KA, Lin HY, et al. Relationship between tumor size and survival in non-small-cell lung cancer (NSCLC): an analysis of the surveillance, epidemiology, and end results (SEER) registry. J Thoracic Oncol. 2015;10:682–690. doi: 10.1097/JTO.0000000000000456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DS, Park KR, Kim SJ, et al. Serum lactate dehydrogenase levels at presentation in stage IV non-small cell lung cancer: predictive value of metastases and relation to survival outcomes. Tumour Biol. 2016;37:619–625. doi: 10.1007/s13277-015-3776-5 [DOI] [PubMed] [Google Scholar]
- 17.Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA oncol. 2018;4:351–357. doi: 10.1001/jamaoncol.2017.4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitadai R, Okuma Y, Hakozaki T, et al. The efficacy of immune checkpoint inhibitors in advanced non-small-cell lung cancer with liver metastases. J Cancer Res Clin Oncol. 2020;146:777–785. doi: 10.1007/s00432-019-03104-w [DOI] [PubMed] [Google Scholar]
- 19.Gibson AJW, Li H, D’Silva A, et al. Impact of number versus location of metastases on survival in stage IV M1b non-small cell lung cancer. Med Oncol. 2018;35:117. doi: 10.1007/s12032-018-1182-8 [DOI] [PubMed] [Google Scholar]
- 20.Tamura T, Kurishima K, Nakazawa K, et al. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol clin oncol. 2015;3:217–221. doi: 10.3892/mco.2014.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Zhang Y, Sun X, et al. The prognostic value of multiorgan metastases in patients with non-small cell lung cancer and its variants: a SEER-based study. J Cancer Res Clin Oncol. 2018;144:1835–1842. doi: 10.1007/s00432-018-2702-9 [DOI] [PubMed] [Google Scholar]
- 22.Ren Y, Dai C, Zheng H, et al. Prognostic effect of liver metastasis in lung cancer patients with distant metastasis. Oncotarget. 2016;7:53245–53253. doi: 10.18632/oncotarget.10644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label Phase 3 trial. Lancet Respir Med. 2019;7:387–401. doi: 10.1016/S2213-2600(19)30084-0 [DOI] [PubMed] [Google Scholar]
- 24.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]