Abstract
Coccidioidomycosis is an endemic fungal infection that is typically asymptomatic or associated with pulmonary disease. Extrapulmonary disease may involve the skin, bones, or central nervous system, yet endovascular infections are exceedingly rare. We report the first case, to our knowledge, of coccidioidomycosis of the native aorta in an immunocompromised host.
Keywords: aortitis, coccidioidomycosis, immunocompromised host, mycotic aneurysm
Coccidioidomycosis is an endemic fungal infection caused by inhalation of spores (arthroconidia) from Coccidioides immitis or Coccidioides posadasii. These organisms are found in soil in the Southwestern United States, Northwestern Mexico, and South America [1]. Half to two-thirds of infections are asymptomatic [2–4]. Pulmonary infection is the most common manifestation, with symptoms resembling community-acquired pneumonia such as fevers, pleuritic chest pain, night sweats, or cough [4]. The incidence of extrapulmonary or disseminated infection varies widely, depending on the ancestry and the immune status of the host, with risk factors including African or Filipino ancestry, HIV/AIDS, other immunosuppression, third-trimester pregnancy, and cardiopulmonary disease [4–7]. Extrapulmonary disease most commonly involves the skin, bones, and central nervous system (CNS) [8].
Vascular graft infections due to Coccidioides spp. have been reported in the literature. However, these reports are exceedingly rare [9–11]. We report the first case, to our knowledge, of coccidioidomycosis of the native aorta in an immunocompromised host with HIV infection.
CASE REPORT
A 50-year-old Caucasian man with hypertension, nicotine dependence, chronic kidney disease stage 3, and HIV infection presented with excruciating back pain during the summer of 2020. He was diagnosed with HIV infection in 2003 with an initial CD4 count of 116 cells/mm3. His HIV infection was well controlled on lamivudine/zidovudine/efavirenz until 2014, after which he was lost to follow-up and subsequently progressed to AIDS with a CD4 count <100 cells/mm3 due to nonadherence. He was living in the Southwestern United States during this time. Per limited available documentation of his clinical care, he was diagnosed with pulmonary coccidioidomycosis in 2016 based on computed tomography (CT)–guided biopsy of a pulmonary nodule. Cerebrospinal fluid analysis showed no evidence of CNS involvement, and he was initiated on fluconazole. His Coccidioides complement fixation (CF) titer was 1:8. He resumed combined antiretroviral therapy (cART) with bictegravir/tenofovir alafenamide/emtricitabine. His CD4 count recovered, and he had documented HIV-1 viral suppression. Fluconazole was discontinued 2 years before the current presentation, as the CD4 count had been >250 cells/mm3 for more than a year and his Coccidioides CF titer became undetectable.
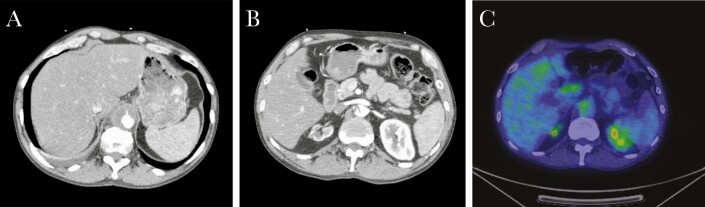
During this current presentation, CT imaging revealed an aortic dissection 15 cm in length and a 3.7-cm diameter thoracoabdominal aortic aneurysm (Figure 1). Transesophageal echocardiogram showed no evidence of valvular vegetations or other findings concerning for endocarditis. Serologies for Treponema pallidum, Bartonella henselae, Bartonella quintana, Brucella spp., Coxiella burnetii, Blastomyces dermatitidis, and Histoplasma capsulatum were negative. Coccidioides serology was reactive with a titer of 1:4 by CF. Due to low suspicion for a fungal aneurysm at that time, he was managed conservatively with blood pressure and heart rate control and discharged from the hospital, with interval monitoring of Coccidioides CF titer.
Figure 1.
A, Abdominal aorta aneurysm at maximal diameter (T12 level). B, Aortic dissection seen at the L2 level, at the caudal pole of the aneurysm. C, Fluorodeoxyglucose-avid focus in the abdominal aorta at the L1 level.
Four weeks later, he was readmitted for worsening back pain and was found to have an enlarging aneurysm measuring 4.6 cm in diameter. Positron emission tomography CT (PET-CT) scan showed high-fluorodeoxyglucose (FDG) avidity of the aneurysmal wall concerning for an infected aneurysm, a-12 mm left upper lobe nodule, and innumerable bilateral micronodules concerning for coccidioidomycosis reactivation. Bacterial, fungal, and mycobacterial blood cultures were negative. HIV-1 polymerase chain reaction (PCR) was <48 copies/mL, and CD4 count was 237 cells/mm3. Subsequent Coccidioides serology had increased to 1:64 by CF. He was initiated on ertapenem and daptomycin intravenously for culture-negative infected aortic aneurysm, as well as oral fluconazole at 400 mg daily.
A repeat abdominal CT scan after 4 weeks of antimicrobials showed an increase in the diameter of the aneurysm to 5.5 cm, and he was referred to our institution for further management. The patient had clinically improved and was asymptomatic. Antibacterials were stopped after 8 weeks of therapy, but fluconazole was continued. Plasma microbial cell-free DNA (cfDNA) detection using next-generation sequencing (Karius test, Karius Inc., Redwood City, CA, USA) was positive for Coccidioides posadasii at a level below the statistically significant threshold (41 molecules of microbial cfDNA per microliter), and the company’s report noted that the result was not to be considered a clinically diagnostic laboratory test result.
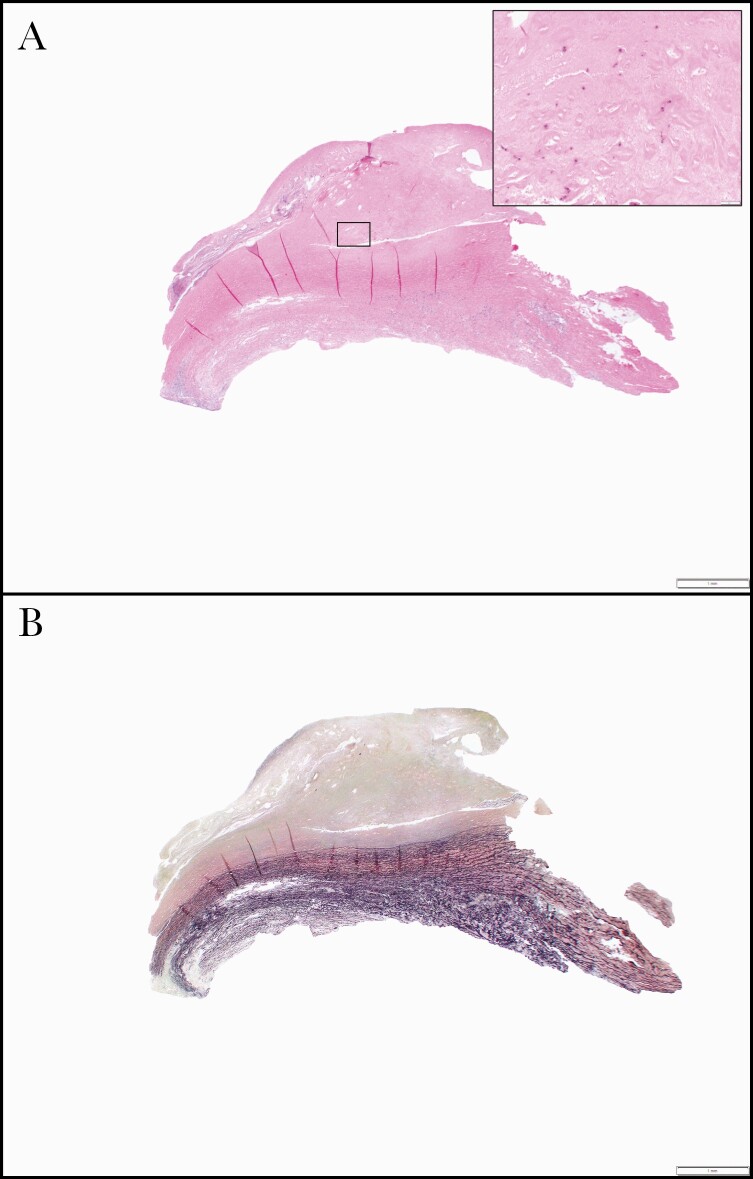
The patient remained asymptomatic 4 weeks after stopping antibacterials. A surveillance PET scan showed that the aneurysm size had increased to 5.7 cm in diameter with unchanged FDG avidity suspicious for persistent infection. He was restarted on daptomycin and ertapenem, and 4 months after his initial presentation, he underwent repair of his aortic aneurysm with a cryopreserved descending thoracic aortic conduit and antegrade debranching of the celiac, superior mesenteric, and left renal arteries from the descending thoracic aorta with aortoiliac cryopreserved conduit. The aortic wall showed marked intimal atherosclerosis, laminar medial necrosis, and adventitial fibrosis, including lymphoplasmacytic inflammation with giant cells (Figure 2). Fragments of mural thrombus were also seen. Within the intimal atherosclerotic plaque, pleomorphic fungal fragments were highlighted with special stains (Figures 3 and 4).
Figure 2.
Photomicrograph of aortic resection. The aorta showed intimal fibroplasia and lymphoplasmacytic inflammation with rare multinucleated giant cells (A, hematoxylin and eosin stain, 12.5× original magnification). Spores can be appreciated on hematoxylin and eosin stain at higher magnification (A inset, 200× original magnification). An elastic stain shows damage to the aortic media, consistent with the patient’s history of chronic dissection and aneurysm formation (B, 12.5× original magnification).
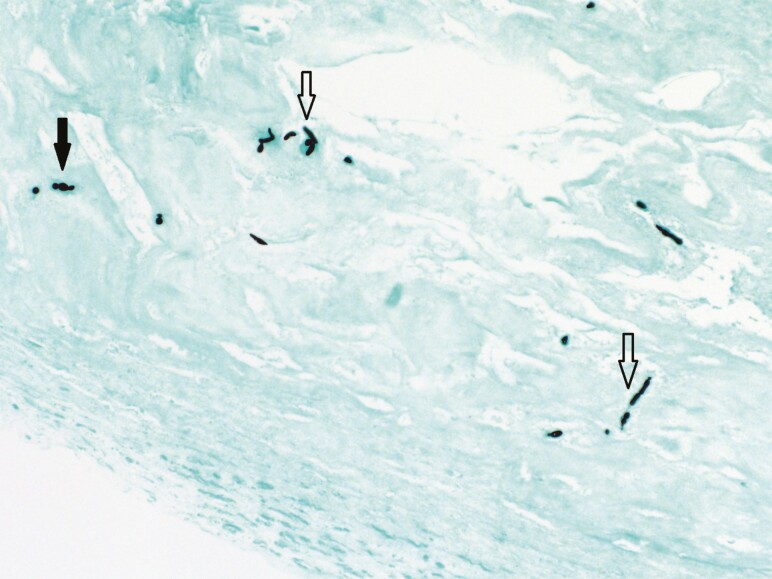
Figure 3.
Grocott methenamine silver stain highlighting fungal elements consisting of short hyphae (open arrowheads) and spores (closed arrowhead) consistent with Coccidioides posadasii/immitis (200× magnification).
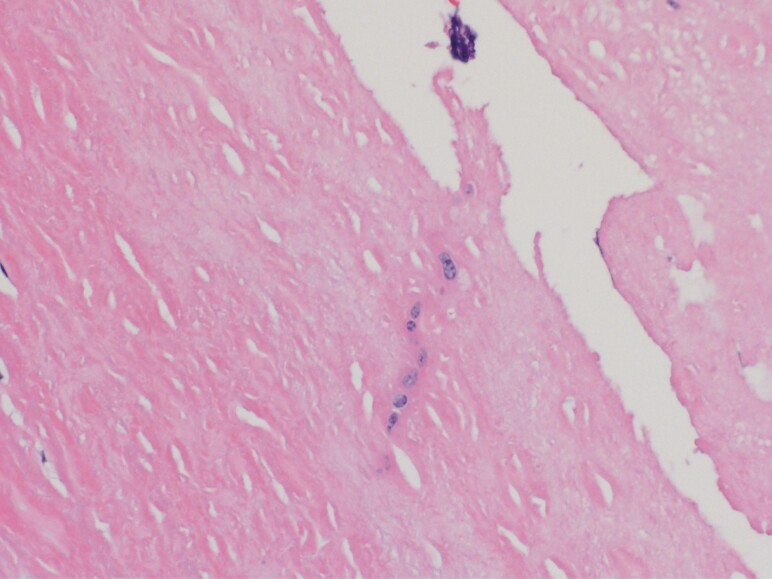
Figure 4.
High-power view of fungal elements seen on hematoxylin and eosin stain (400× magnification).
The patient was empirically started on liposomal amphotericin B at 5 mg/kg intravenously daily. Coccidioides PCR of a fresh tissue sample from the aortic aneurysm was positive, and multiple intraoperative fungal cultures of the aneurysm grew Coccidioides posadasii/immitis, which was confirmed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Postoperative bacterial and fungal blood cultures were negative. Antibacterials were discontinued after 48 hours of negative bacterial cultures. His creatinine trended from a baseline of 1.4 mg/dL to a peak of 2.1 mg/dL on day 11 of liposomal amphotericin B therapy, after which he was transitioned to fluconazole 800 mg orally daily. His creatinine started to improve by hospital discharge.
Magnetic resonance imaging (MRI) of the brain revealed a single peripherally enhancing lesion in the right inferior frontal gyrus, and he was instructed to continue lifelong suppression with fluconazole 800 mg orally daily due to suspicion for CNS involvement. Due to lack of symptoms suggestive of CNS involvement, lumbar puncture was deferred with the intention to repeat MRI of the brain at follow-up, to avoid an invasive procedure that would not alter management. The patient’s remaining hospital course was uncomplicated, and he was discharged on postoperative day 15 with outpatient follow-up.
DISCUSSION
Coccidioidomycosis is acquired through inhalation of arthroconidia. The majority of Coccidioides infections are asymptomatic or minimally symptomatic [3, 4]. In those with clinical evidence of infection, mild to moderate respiratory symptomatology predominates and improves irrespective of antifungal treatment. Fewer than 1% of these cases disseminate beyond the thorax, most commonly to the skin, bones, and central nervous system. However, in immunocompromised hosts the risk of progressive disease and dissemination increases by several fold [12, 13]. Risks factors associated with a higher risk of dissemination include African or Filipino ancestry (including immunocompetent hosts) as well as conditions associated with depressed adaptive immunity including HIV/AIDS, organ transplantation, immunosuppressive medication use, and third-trimester pregnancy [4]. In people with HIV infection, a CD4 cell count of <250 cells/mm3 is a risk factor for severe disease and extrathoracic dissemination after primary infection, as well as for possible reactivation months to years after primary infection [13–17]. Of note, disseminated disease can occur in the absence of pulmonary symptoms. Before this episode, our patient had demonstrated improvement in his Coccidioides CF titers to undetectable levels and had a recovery of his CD4 count before discontinuation of fluconazole. Based on the above, his current episode was thought to represent relapse rather than a progression of underlying disease.
Vascular coccidioidomycosis has rarely been reported, with 2 reported cases of vascular graft infections [9–11]. Based on our literature search, extrathoracic coccidioidomycosis presenting as an infected vascular aneurysm of the native vasculature has never been reported.
Infected vascular aneurysms are most often caused by bacteria, with fungal vascular aneurysms being extremely rare [18]. Of these, vascular infections secondary to Candida spp. and Aspergillus spp. are the most frequently reported [19, 20]. Common mechanisms of infected vascular aneurysms include septic emboli to the vasa vasorum of the vasculature, extension from the contiguous focus of infection, hematogenous spread, and traumatic inoculation. Atherosclerotic vasculature is more prone to be seeded by microorganisms, as an intact intima is ordinarily resistant to invading microorganisms [21]. In our patient, active smoking, peripheral arterial disease, and chronic kidney disease were highly predictive of underlying aortic atherosclerosis, which may have increased the risk of intravascular seeding of the aortic vasculature with Coccidioides spp., most likely during reactivation after cessation of fluconazole. Imaging did not reveal any evidence of a contiguous focus of infection, and he presented with simultaneous aortic dissection and aneurysm, which suggests that he had intravascular seeding of the intimal layer resulting in the above processes.
The progression of aneurysm size despite appropriate antifungal therapy most likely represented lack of source control, as opposed to failure of therapy. The atypical hyphal forms seen in this case were accompanied by more typical spores (Figure 2A). Atypical fungal hyphal and other forms of Coccidioides spp. have been reported from various sites [22]. It is hypothesized that formation of hyphae may be favored, with low partial pressure of oxygen or carbon dioxide or presence of eosinophils [23]. Prior research has also shown that the presence of neutrophils may promote spherule formation and that elevated carbon dioxide and temperature promote spherule formation in vitro [24]. In patients with pulmonary coccidioidomycosis, risk factors for development of hyphae include infection lasting >8 months, type 2 diabetes mellitus, cough, hemoptysis, and radiologic evidence of a cavitary lung lesion [25]. In our patient, atypical hyphal forms were postulated to be due to long-term exposure to fluconazole and restricted penetration leading to subinhibitory concentrations of fluconazole at the site of infection.
Treatment of disseminated infection involves azoles including fluconazole, itraconazole, or voriconazole. Amphotericin B is recommended if lesions are rapidly worsening and are in critical locations [4]. Pharmacodynamics of antifungal medications vary between fungicidal and fungistatic mechanisms depending on the organism being treated [26]. Azoles are fungistatic against Coccidioides spp., while amphotericin B has a higher minimum fungicidal concentration [27]. After cessation of the drug, there is a risk of relapse and delayed dissemination lasting for many years [28–31]. This risk is accentuated in immunosuppressed patients. The risk of relapse varies based on the site of infection, with up to 80% relapse of CNS infections after cessation of medication [32]. In our patient, fluconazole was discontinued 2 years before the current presentation in accordance with Centers for Disease Control and Prevention/National Institutes of Health/Infectious Diseases Society of America guidelines after receiving more than 12 months of fluconazole therapy following a rise in CD4 count to >250 cells/mm3 and virological suppression with cART [14, 33]. Although underlying immunosuppression may have played a significant role in relapse, the potential for relapse in immunocompetent patients remains [34].
A combination of surgical and medical management is essential for patients with infected vascular aneurysms. Our patient was started on 400 mg per day of oral fluconazole when the antibody titers against Coccidioides spp. increased to 1:64. The rapid progression of the aortic aneurysm despite fluconazole therapy likely represented ongoing vascular damage from active inflammation against persistent organisms, as opposed to failure of fluconazole. A large-scale evaluation of in vitro activity of various antifungals tested against Coccidioides isolates (n = 581) showed that MICs to fluconazole were elevated (≥16 µg/mL in 37.3% of isolates) more often than those of itraconazole, voriconazole, posaconazole (≥1–2 µg/mL in only 1%) or amphotericin B (≥2 µg/mL in only 2.8%), though the clinical relevance is unknown [35]. Given that the progression of this patient’s disease was related to source control, antifungal susceptibility testing was not performed on this patient’s isolates. After surgery, the patient was treated with liposomal amphotericin B for 11 days, followed by a transition to lifelong suppression with fluconazole at 800 mg per day.
CONCLUSIONS
Coccidioidomycosis is an exceedingly rare cause of infected vascular aneurysms. These infections may have catastrophic consequences if not identified and treated promptly with a combination of surgery and antifungal therapy. A high index of suspicion is essential in patients residing in endemic areas or with a remote history of exposure, especially in patients with risk factors for relapse and delayed dissemination, rising antibody titers against Coccidioides spp., or blood culture–negative infected vascular aneurysms.
Acknowledgments
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. The patient’s written consent was obtained, and information was anonymized as far as possible. The design of this work conforms to standards currently applied in the United States of America.
References
- 1. Centers for Disease Control and Prevention. Valley fever (coccidioidomycosis). 2020. Available at: https://www.cdc.gov/fungal/diseases/coccidioidomycosis/index.html. Accessed 22 January 2021.
- 2. Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health 1946; 36:1394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am 2003; 17:41–57, viii. [DOI] [PubMed] [Google Scholar]
- 4. Galgiani JN, Ampel NM, Blair JE, et al. ; Infectious Diseases Society of America . Coccidioidomycosis. Clin Infect Dis 2005; 41:1217–23. [DOI] [PubMed] [Google Scholar]
- 5. Brown J, Benedict K, Park BJ, Thompson GR 3rd. Coccidioidomycosis: epidemiology. Clin Epidemiol 2013; 5:185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Odio CD, Marciano BE, Galgiani JN, Holland SM. Risk factors for disseminated coccidioidomycosis, United States. Emerg Infect Dis 2017; 23:308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen IM, Galgiani JN, Potter D, Ogden DA. Coccidioidomycosis in renal replacement therapy. Arch Intern Med 1982; 142:489–94. [PubMed] [Google Scholar]
- 8. Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine (Baltimore) 2004; 83:149–75. [DOI] [PubMed] [Google Scholar]
- 9. Schwartz DN, Fihn SD, Miller RA. Infection of an arterial prosthesis as the presenting manifestation of disseminated coccidioidomycosis: control of disease with fluconazole. Clin Infect Dis 1993; 16:486–8. [DOI] [PubMed] [Google Scholar]
- 10. Bardwell J, August J, Farran S, et al. Infection of aortic endograft caused by coccidioidomycosis. Am J Med 2020; 133:e1–2. [DOI] [PubMed] [Google Scholar]
- 11. Kaur M, Mian S. Case report: coccidioides immitis infects a patient’s vascular graft. Rheumatologist 2019. [Google Scholar]
- 12. Cohen IM, Galgiani JN, Potter D, Ogden DA. Coccidioidomycosis in renal replacement therapy. Arch Intern Med 1982; 142:489–94. [PubMed] [Google Scholar]
- 13. Fish DG, Ampel NM, Galgiani JN, et al. Coccidioidomycosis during human immunodeficiency virus infection. A review of 77 patients. Medicine (Baltimore) 1990; 69:384–91. [DOI] [PubMed] [Google Scholar]
- 14. Masannat FY, Ampel NM. Coccidioidomycosis in patients with HIV-1 infection in the era of potent antiretroviral therapy. Clin Infect Dis 2010; 50:1–7. [DOI] [PubMed] [Google Scholar]
- 15. Ampel NM. Delayed-type hypersensitivity, in vitro T-cell responsiveness and risk of active coccidioidomycosis among HIV-infected patients living in the coccidioidal endemic area. Med Mycol 1999; 37:245–50. [PubMed] [Google Scholar]
- 16. Ampel NM. Coccidioidomycosis in persons infected with HIV type 1. Clin Infect Dis 2005; 41:1174–8. [DOI] [PubMed] [Google Scholar]
- 17. Stevens DA. Coccidioidomycosis. N Engl J Med 1995; 332:1077–82. [DOI] [PubMed] [Google Scholar]
- 18. Smeds MR, Duncan AA, Harlander-Locke MP, et al. ; Vascular Low-Frequency Disease Consortium . Treatment and outcomes of aortic endograft infection. J Vasc Surg 2016; 63:332–40. [DOI] [PubMed] [Google Scholar]
- 19. Brunner S, Engelmann MG, Näbauer M. Thoracic mycotic pseudoaneurysm from Candida albicans infection. Eur Heart J 2008; 29:1515. [DOI] [PubMed] [Google Scholar]
- 20. Mettananda KC, De Silva ST, Premawardhena AP. Mycotic aneurysm of the descending aorta due to Aspergillus species. Ceylon Med J 2010; 55:20–1. [DOI] [PubMed] [Google Scholar]
- 21. Wilson WR, Bower TC, Creager MA, et al. Vascular graft infections, mycotic aneurysms, and endovascular infections: a scientific statement from the American Heart Association. Circulation 2016; 134:e412–60. [DOI] [PubMed] [Google Scholar]
- 22. Schuetz AN, Pisapia D, Yan J, Hoda RS. An atypical morphologic presentation of Coccidioides spp. in fine-needle aspiration of lung. Diagn Cytopathol 2012; 40:163–7. [DOI] [PubMed] [Google Scholar]
- 23. Dolan MJ, Lattuada CP, Melcher GP, et al. Coccidioides immitis presenting as a mycelial pathogen with empyema and hydropneumothorax. J Med Vet Mycol 1992; 30:249–55. [DOI] [PubMed] [Google Scholar]
- 24. Baker O, Braude AI. A study of stimuli leading to the production of spherules in coccidioidomycosis. J Lab Clin Med 1956; 47:169–81. [PubMed] [Google Scholar]
- 25. Muñoz-Hernández B, Martínez-Rivera MA, Palma Cortés G, et al. Mycelial forms of Coccidioides spp. in the parasitic phase associated to pulmonary coccidioidomycosis with type 2 diabetes mellitus. Eur J Clin Microbiol Infect Dis 2008; 27:813–20. [DOI] [PubMed] [Google Scholar]
- 26. Manavathu EK, Cutright JL, Chandrasekar PH. Organism-dependent fungicidal activities of azoles. Antimicrob Agents Chemother 1998; 42:3018–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li RK, Ciblak MA, Nordoff N, et al. In vitro activities of voriconazole, itraconazole, and amphotericin B against blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum. Antimicrob Agents Chemother 2000; 44:1734–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winn WA. The use of amphotericin B in the treatment of coccidioidal disease. Am J Med 1959; 27:617–35. [DOI] [PubMed] [Google Scholar]
- 29. Galgiani JN, Catanzaro A, Cloud GA, et al. Comparison of oral fluconazole and itraconazole for progressive, nonmeningeal coccidioidomycosis. A randomized, double-blind trial. Mycoses Study Group. Ann Intern Med 2000; 133:676–86. [DOI] [PubMed] [Google Scholar]
- 30. Graybill JR, Stevens DA, Galgiani JN, et al. Itraconazole treatment of coccidioidomycosis. NAIAD Mycoses Study Group. Am J Med 1990; 89:282–90. [DOI] [PubMed] [Google Scholar]
- 31. Catanzaro A, Galgiani JN, Levine BE, et al. Fluconazole in the treatment of chronic pulmonary and nonmeningeal disseminated coccidioidomycosis. NIAID Mycoses Study Group. Am J Med 1995; 98:249–56. [DOI] [PubMed] [Google Scholar]
- 32. Dewsnup DH, Galgiani JN, Graybill JR, et al. Is it ever safe to stop azole therapy for Coccidioides immitis meningitis? Ann Intern Med 1996; 124:305–10. [DOI] [PubMed] [Google Scholar]
- 33. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at: https://clinicalinfo.hiv.gov/sites/default/files/inline-files/adult_oi.pdf. Accessed 3 February 2021.
- 34. Ampel NM, Giblin A, Mourani JP, Galgiani JN. Factors and outcomes associated with the decision to treat primary pulmonary coccidioidomycosis. Clin Infect Dis 2009; 48:172–8. [DOI] [PubMed] [Google Scholar]
- 35. Thompson GR 3rd, Barker BM, Wiederhold NP. Large-scale evaluation of in vitro amphotericin B, triazole, and echinocandin activity against Coccidioides species from U.S. institutions. Antimicrob Agents Chemother 2017; 61:e02634-16. [DOI] [PMC free article] [PubMed] [Google Scholar]