Abstract
Asthma is a chronic inflammatory disease of the respiratory tract in which the numerous immune cells, including eosinophils, neutrophils, macrophages, T-lymphocytes, mast cells and epithelial lining play key roles. The numerous anti-asthmatic drugs are available in modern medicine to treat asthma, but they have several disadvantages, including side effects and the cost variations, which compromise treatment compliance. The literature review reveals that traditional herbal medicines have good potential as alternative treatment and management for asthma. However, communities hesitated to use the traditional herbal medicines due to lack of established mechanism of action about their anti-asthmatic potential. The present review aimed to summarise the information stated in the literature about the potential effect of traditional medicinal plants (TMPs) conferring protection against ovalbumin (OVA)-induced asthma model. The literature search was conducted in database like PubMed, Scopus, Google Scholar and ScienceDirect. After screening through the literature from 2011 to date, a total of 27 medicinal plants and two polyherbal extracts have been reported to be used as traditional herbal medicines and also utilised to be tested against OVA-induced asthma, were included. We found them to be an important alternative source of treatment for asthma, since some have comparable efficacies with drugs commonly used in the modern system against asthma. All the reported medicinal plants confirmed their traditional use against asthma or its related inflammation. The present review provides faith in traditional information and also offers new insight into the potential of natural products against asthma.
Keywords: OVA-induced asthma, traditional medicinal plants, natural products, allergic asthma, inflammation, T-helper cells
Introduction
Asthma, one of the chronic inflammatory airway diseases, which affecting 300 million people worldwide and is expected to be nearly 400 million by the next 5 years. The incidence of asthma is high and accounting for 1 out of 250 deaths worldwide.1 Majority of the asthma treatment (as recommended by the Global Initiative for Asthma), target to reduce the symptoms via ameliorating the inflammatory processes.2,3 To date, current asthma therapies are mainly based on pulmonary, followed by oral or intravenous administrations. The drugs of choice include beta-adrenoceptor-2 (β2) agonists, corticosteroids as well as xanthines and their derivatives. For symptomatic relief from asthma β2 agonists are often the drugs of choice.3,4 Nevertheless, given the wide choices of available anti-asthmatic drugs, they produce numerous side effects, comprising headaches, nausea and convulsions (xanthines),5 cardiovascular effects (β2 agonists),6 vomiting (phosphodiesterase type 4 inhibitors),7–9 osteoporosis, myopathies, adrenal suppression and metabolic disturbances, which may compromise the patients growth especially in young children (corticosteroids).5,7,10,11
Traditional medicine encompasses the total skill, knowledge and practices used in the maintenance of health as well as in the prevention, diagnosis, enhancement, or treatment of physical and mental illness which can be based on myths, perceptions, and experiences indigenous to various cultures.12 The use of traditional medicinal plants (TMPs) as herbal medicines which have good potential for treating physiological disorders like asthma or as an adjuvant to modern therapy has been extensively reported since ancient times. In addition, ethnopharmacological studies have been considered as an important tool for bioprospecting new bioactive compounds from medicinal plants.13 Although significant progress has been achieved in the synthetic chemistry that involves the synthesis of new molecules, drugs or active constituents developed from medicinal plants are until preserved.14,15
Traditional herbal medicine is often referred as “complementary and alternative medicine” which includes traditional Chinese medicine, traditional Indian medicine (Ayurveda), traditional Japanese medicine (Kampo) and traditional Korean medicine which are the most widely used systems today. Since all these traditional systems utilise medicinal plants and have been practiced for hundreds or even thousands of years all over the world, some have morphed into orderly regulated medicine systems.16–18 Nevertheless, to date, there is a lack of information on the use of TMPs as well as their experimental evidences.
Rural individuals from developing countries tend to have more trust in traditional herbal medicine due to their considerable experience and lack of access to modern medicines. Hence, in-depth information on traditional and ethnomedical perspectives can facilitate community-centered approaches under the present medical system given that the potential of traditional herbal medicines in the treatment of asthma is not fully utilized. Thus, established preclinical studies are needed. Several experimental animal models for asthma are accessible for preclinical screening. Among them, OVA-induced asthma animal model has been widely used.
OVA is the main protein found in egg white, which is not intrinsically immunogenic and therefore have to be injected into the systemic circulation in the presence of adjuvants (substances that increase the immunogenicity of an antigen) typically aluminium hydroxide (Alum).19 Immunization of OVA along with alum in animals induces T helper (Th) cell (preferably Th2) responses, ie, called sensitisation. Further, immunization of OVA to the sensitized animals synergize the former immune responses, ie, called challenge. Subsequently, challenged animal’s exhibit many of the features seen in asthmatic individuals including inflammation, infiltration of eosinophils, production of Th2 cytokines, increase in serum IgE and airway hyper-responsiveness (AHR). OVA challenge models have been long-established to be worthwhile for the preclinical assessment of potential therapeutic agents for asthma, where specific questions regarding allergic asthma can be addressed.
To gain insight into the potential role of natural products, an overview of various traditionally used medicinal plants against asthma and OVA-induced asthma models has been represented here. The attempt has been made to outline the summary of information gathering the anti-asthmatic potential of medicinal plants against OVA-induced asthma and correlated with their traditional use was performed. This review provides a significant contribution to bridging these evidence gaps, creating a resource for safe and effective implementation of TMPs among the communities, confirming their pharmacological activities, potential efficacies and safety as an alternative medicine for asthma treatment.
Methods
In this review, relevant studies were collected from several scientific databases, including PubMed, Scopus, Google Scholar and ScienceDirect (between 2011 and to date). The keywords used for the search include “Traditional herbal medicine” OR “Traditional Indian medicine” OR “Ayurveda” OR “Traditional Chinese medicine” OR “Traditional Korean medicine” OR “Traditional Japanese Medicine” OR “Kampo” OR “Herbal medicine” OR “Natural products” OR “Medicinal plants” OR “Plant extract” AND “Asthma” AND “Ovalbumin” AND “OVA” AND “OVA-induced allergic asthma” AND Anti-asthmatic AND “OVA-induced lung inflammation”. Studies apart from English language and those that does not have full article were excluded from this review. The medicinal plants studied for OVA-induced asthma model, but without supporting any traditional use related to the management and treatment of asthma has been omitted. After applying the inclusion and exclusion criteria’s and eliminating the duplicates from one to another database, a total of 27 TMPs and two polyherbal extracts investigated against OVA-induced asthma model along with its supportive evidence on its traditional use against asthma or its related inflammation were included in the present review.
Anti-Asthmatic Potential of TMPs Against OVA-Induced Asthma in Animals
Animal Model of OVA-Induced Asthma
The use of an appropriate animal model of allergy can help unravel unanswered questions in human allergic diseases.20–23 Therefore, the following ideal characteristics should be considered for any allergic asthma animal model.
Induced allergic parameters, both clinical and immunologic reactions, should mimic humans.
Animal model of allergy-induced asthma by protein antigen alone is ideal, rather than in combination with adjuvants.
The animal model should be less complicated, with a limited number of sensitisations.
An ideal animal model serves as a powerful tool for understanding the immunopathogenic mechanisms of the various drug candidates.
Among the different species, mouse models are preferred in allergen-induced studies when compared to rat models.24 Although various other mouse models (C57BL/6 and A/J strains) are available, interestingly, BALB/c mice model, which have a high tendency of developing allergic responses, has been widely used.25 The foremost reason for the high allergic responses is due to the Th2 phenotype where BALB/c mice easily develop Th2-biased immune response. In addition, BALB/c successfully develops clinical symptoms, which are relatable to humans, including infiltration of leukocytes, airway inflammation and wall remodeling (goblet cell hyperplasia or metaplasia, epithelial hypertrophy, epithelial basement membrane thickening), mucus secretion, total and allergen-specific serum IgG1 and IgE, cytokine profile, among others.26 As an example, BALB/c mice sensitised with the insect allergen (Forcipomyia taiwana [biting midge]) show a similar type of eosinophil and lymphocyte infiltrations observed in humans.27 Furthermore, BALB/c mice model can also be used for exploration of novel mechanisms independent of cytokines (IL-4 and IL-5) in allergic airway hyperreactivity.28 In addition, many studies have confirmed the utility of BALB/c mice as a model for allergy.24,29–31 Having said that induction of allergen-specific hypersensitivity reactions are also species- and strain-dependent,31,32 indicating that a balance of these factors are required when selecting the best model.
Pathophysiology of OVA-Induced Asthma
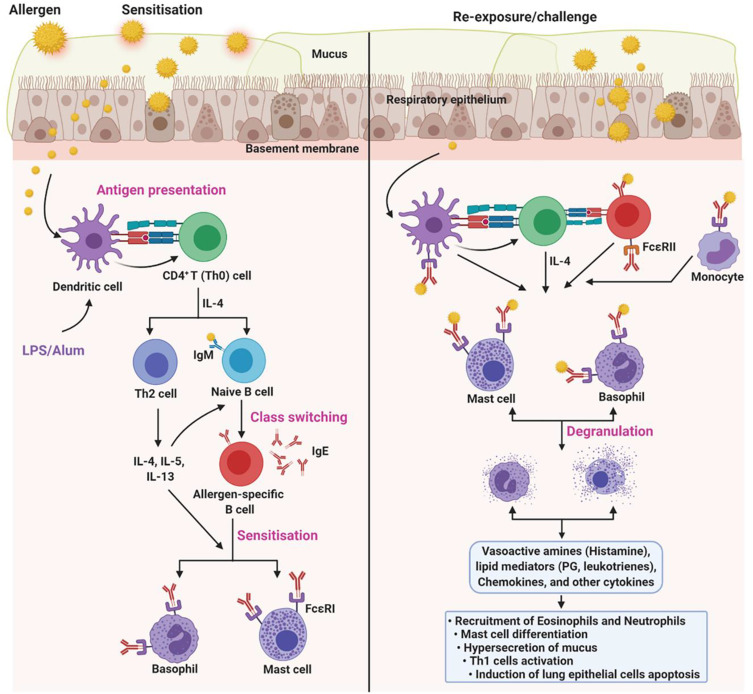
Despite different pathophysiological mechanisms in allergen-induced asthma, OVA-induced classical eosinophilic asthma is common. Moreover, OVA in combination with endotoxin (lipopolysaccharide, LPS) at different sensitisation and challenge schedules were used to induce neutrophilic and mixed-granulocytic asthma.23,33 Although OVA at high doses is sufficient to induce asthma, the use of endotoxin and/or adjuvant can help exacerbate the OVA-induced immune response. For this, several sensitisation and challenge (acute or chronic) protocols have been developed and used. The use of the endotoxin (LPS) and adjuvant (Alum) may overcome the tolerogenic issues of OVA. Moreover, the use of endotoxin in the protocols are preferred in studies focusing on acute neutrophilia. Similarly, an adjuvant such as Alum, in particular, can be used to induce acute asthma models. Of note, Alum induces Th2-biased immune responses, thus synergising and facilitating OVA-induced immune response (Figure 1). Nevertheless, the inclusion of an adjuvant is not necessary for some type of allergy models such as food-induced allergy models.
Figure 1.
Pathophysiology of OVA-induced asthma. OVA-induces different types of allergic asthma, including eosinophilic, neutrophilic, and mixed-granulocytic asthma. Although different sensitisation and challenge protocols has been used, the underlying mechanism is nearly similar. Dendritic cells (DCs) are the first innate immune cells encounters the allergen after crossing the respiratory epithelium. DCs process and present the antigen to the CD4+ T cells, which subsequently polarized into Th2 cells via Th2 inducing factor (IL-4). Th2 cells secrete copious amounts of cytokines (IL-4, IL-5, and IL-13), which later activate the naïve B cells to antigen-specific B cells (class switching). Further, antigen-specific B cells secret IgE, which sequentially recognized by the FcεRI on the mast cells and basophils. This process is called sensitisation. Upon re-exposure, allergen experienced leukocytes accelerate the priming process and activate mast cells and basophils. In addition, sensitized mast cells and basophils directly recognize the IgE antigen conjugates and undergo degranulation, which releases vasoactive amines (histamine), lipid mediators (prostaglandin, leukotrienes), chemokines, and other cytokines. As a net effect, the former mediators recruit the eosinophils and neutrophils, induce the mast cell differentiation, hypersecretion of mucus, Th1 cell activation, lung epithelial cells apoptosis, and others.137 Of note, few protocols use LPS or Alum, which accelerate the process, to induce a specific type of allergic condition. The figure was created with the support of https://biorender.com under the paid subscription.
In summary, OVA-induced asthma animal models have been used for 1) classification of allergen types, 2) assessing the efficacy of novel vaccine adjuvants, 3) understanding the molecular mechanisms of different types of allergies, and 4) evaluating potential drug candidates. All the above categories are based on pathophysiological observation, including allergen-specific IgE estimation, anaphylactic symptoms, blood histamine levels, immunophenotyping of mast cells and other subtypes, splenocyte cytokine profiles and histology.
Selective TMPs Conferring Protection Against OVA-Induced Asthma
All the medicinal plants reported in this review have been traditionally used to treat asthma or its related inflammation. Table 1 summarises the details on the family, distribution, traditional uses and chemical constituents of TMPs and two polyherbal extracts. In this review, the data for 27 TMPs and two polyherbal extracts useful against OVA-induced asthma models as well as their potential mechanism of actions are described later.
Table 1.
Information About TMPs Conferring Protection Against OVA-Induced Asthma
| Medicinal Plant | Family | Distribution and Availability | Parts Used | Solvent | Chemical Constituents | Ethnomedical and Traditional Uses | References |
|---|---|---|---|---|---|---|---|
| S. striata | Scrophulariaceae | It is a perennial herbaceous plant found mainly in western Iran. | Aerial parts | 80% Ethanol | Phenylpropanoids, terpenoids, phenolic compounds and flavonoids. | Treatment for gastritis, conjunctivitis, hemorrhoids, otitis, infectious wounds, common cold and burns. | Azadmehr et al;34 Tamri64 |
| L. dentata | Lamiaceae | It is a powerful aromatic and medicinal herb found mainly in Atlantic Islands and the Arabian Peninsula. | Flowers and leaves | Ethanol | Linalool, linalyl acetate, mono and sesquiterpenes, luteolin, ursolic acid and coumarins. | Treatment for diabetes, cold and renal colic. | Almohawes and Alruhaimi35 |
| P. weinmannifolia | Anacardiaceae | It is a shrub widely found in China. | Root | 50% Ethanol | Not identified | Treating enteritis, dysentery, headache, influenza, lung cancer and traumatic bleeding. | Lee et al;36 Zhao et al;89 Chen et al;90 Ci et al91 |
| O. basilicum | Lamiaceae | It is one of the main Ocimum species and is commonly known as a sweet basil. It originates from Asia, and is cultivated in many Mediterranean and tropical countries. | Leaves | 70% Ethanol | 4-Allylphenol, anethole, methyl cinnamate, methyl salicylate, ethyl cinnamate, methyl eugenol, cuminaldehyde and estragole. | Treatment of headaches, coughs, constipation, kidney malfunctions, diarrhea, worms, warts, fevers, throat congestions and stomachache. | Eftekhar et al,37 Javanmardi et al;92 Prakash and Gupta93 |
| P. fruticose | Araliaceae | It is widely distributed in Vietnam, China and in other tropical countries. | Leaves | Ethanol | Saponins, glycosides, sterols and alkaloids. | Treatment of ischemia and inflammation. | Koffuor et al;38 Do;94 Asumeng Koffuor et al;95 Hanh et al96 |
| H. tiubae | Malvaceae | It is a shrub, found in Brazil and is popularly known as “mela bode” and “lava-prato”. | Aerial parts | Ethanol | Kaempherol | Treatment for fever and respiratory diseases. | Mozzini et al;39 Falcão‐Silva et al97 |
| S. xanthocarpum | Solanaceae | It is mainly found in India. | Whole plant | Water | Alkaloids, phenolics, flavonoids, sterol, saponins and glycosides. | Treatment for expectorant, cough, asthma and chest pain. | Gulati;40 Ghani;98 Parmar et al99 |
| C. sativus | Iridaceae | It is a flowering plant and commonly known as saffron. It is widely cultivated in Iran and other countries including India and Greece. | Petal stigma of flowers | 70% Ethanol | Crocin, crocetin and safranal. | Used for antispasmodic and expectorant. | Mahmoudabady et al;41 Rios et al100 |
| C. gigantea | Asclepiadaceae | It is commonly known as milkweed or swallow-wort and is mainly found in wasteland throughout India. | Root | Methanol | α and β-amyrin, β-sitosterol, taraxasterol and stigmasterol. | Treatment of asthma, leprosy, bronchitis and expectorant. | Bulani et al;42 Maheshwari and Singh;101 Warrier et al;102 Kirtikar and Basu103 |
| U. dioica | Urticaceae | It is commonly known as nettle and is mainly found in Europe, much of the temperate Asia and western North Africa regions. | Leaves | Water | Caffeic acid, anthocyanidins, gallic acid, scopoletin, secoisolariciresinol, quercetin and carotenoids. | Anti-asthmatic, depurative and astringent. | Zemmouri et al;43 Randall et al;104 Chrubasik et al;105 Mittman106 |
| W. tinctoria | Apocynaceae | It is commonly known as “Indrajau” and is distributed throughout the world, especially in India. | Leaves | 90% Ethanol | Lupeol, β-amyrin, cycloartenone, β-sitosterol and cycloeucalenol. | Treatment of inflammation, mumps and herpes. Relieve toothache. | Khan and Imtiyaz;44 Srivastava107 |
| A. cochinchinensis | Asparagaceae | The plant usually grows in China, Japan and Korea. | Root | Water | Steroidal saponins, phenolic compounds, flavonoid and protodioscine. | Treating lung and spleen-related diseases. To improve immunologic ability, eliminate superoxide radical effects and delay aging. | Choi et al;45 Xiong et al108 |
| A. yomena | Asteraceae | It is an edible herb mainly found in Korea, China, Japan and Siberia. | Leaves | 70% Ethanol | Phenolic compounds | Treatment of cough, asthma and insect bites | Sim et al46 |
| A. cepa | Liliaceae | It is commonly known as onion. It grows around the world. | Vegetable | Methanol | Flavonoids (Quercetin) | Treatment for allergic and inflammation. | Marefati et al;47 Roldán et al;109 Lee and Jung110 |
| A. argyi | Asteraceae | It is commonly known as mugwort, is distributed in China, Korea, Mongolia, Japan, and Russia. | Aerial parts | Methanol | Phenolic compounds (Dehydromatricarin A) | Control abdominal pain, inflammation, uterine hemorrhage and dysmenorrhea. | Shin et al;48 Kim et al;111 Kim et al;112 Yun et al;113 Lee et al;114 Yao et al115 |
| Z. officinale | Zingiberaceae | It is commonly known as ginger, is consumed worldwide as both spice and flavoring agents. The plant is grown in many parts of the region including in Asia, India and Africa. | Rhizome | 70% Ethanol | Zingerone, shogaols, gingerols, sesquiterpenoids and monoterpenoids. | Treatment for rheumatoid arthritis, inflammation, sore throats, nausea, constipation and indigestion, fever, infectious diseases and helminthiasis. Also used to treat chronic adjuvant arthritis and airway inflammation. | Khan et al;49 Srivastava;107 Ahui et al;116 Townsend et al117 |
| E. japonicum | Liliaceae | It is an indigenous herb found in Korea and several East Asian countries. It is focused in Hokkaido and is also distributed throughout Japan. | Aerial parts | 80% Ethanol | Isothiocyanates | Used as stomachic, anti-diarrheal, detoxification and nourishment. | Seo et al;50 Shin et al118 |
| T. foenum-graecum | Fabaceae | It is commonly known as Fenugreek. It is a legume crop that is used as a spice in cooking. It is found mainly in India. | Seeds | Ethanol | Flavonoids | Laxative, olfactory and galactogogue. | Piao et al;51 Khalil et al119 |
| E. scaber | Alismataceae | It is a sub-aquatic herbaceous plant native to Brazil, is also found in the regions of the Amazon and Pantanal. | Leaves | 70% Ethanol | Phenolic compounds (Vitexin, rutin and gallic acid), flavonoids and alkaloids | Treatment of inflammation, allergies, rheumatism, kidney infections and respiratory diseases. | Rosa et al;52 Bieski et al;120 De la Cruz;121 Messias et al122 |
| S. aethiopicus | Zingiberaceae | It is naturally distributed in many countries including South Africa, Zimbabwe, Malawi and Zambia. | Rhizomes | Diethyl ether/Ethanol/Water | Furanoterpenoid | Treatment of mild asthma, coughs, influenza and colds. | Fouche et al;53 Hutchings;123 Steenkamp;124 Steenkamp et al125 |
| S. uncinata | Selaginellaceae | It is also known as “Cuiyuncao” in China and “Spring blue spike moss” in Europe. It is a perennial herb widely distributed in some Southeast Asian countries. | Whole plant | 70% Ethanol | Flavonoids (Amentoflavone, hinokiflavone and isocryptomerin) | Relieve cough and ameliorate inflammation stasis. Treatment of asthma, pulmonary tuberculosis, jaundice, pertussis and edema. | Yu et al;54 Zou et al;126 Zheng et al;127 Li et al128 |
| R. multiflora | Rosaceae | It is commonly known as “Multiflora Rose,” and is native to Korea, Japan and China. | Fructus | Water | Quercetin glycosides, vitamin E, carotene, multiflorins, minerals and essential fatty acids. | Treatment for rheumatism, edema, joint inflammation and beri-beri. | Bui et al;55 Cheng et al129 |
| M. citrifolia | Rubiaceae | It is commonly known as “noni”, the fruits. It is a small tropical tree that grows widely in Polynesia, Australia, China and Hawaii. | Fruits | Hexane, ethyl acetate and n-butanol | Phenolic compounds, flavonoids, coumarins, phenolic acid, vanillin and iridoids. | Treatment for asthma and inflammation. | Dussossoy et al;56 Whistler130 |
| S. baicalensis | Lamiaceae | It is commonly known as Skullcap and is widely distributed across Asia. | Skullcap | 70% Ethanol | Polyphenolic compounds (baicalin, baicalein and wogonin) | Treatment for allergic, inflammation and viral infections. | Jung et al;57 Li-Weber;131 Zhang et al132 |
| S. ternatum | Ophioglossaceae | It is a common herb and is mainly found in China. | Whole plant | 70% Ethanol | Not identified | Deemed to have anti-toxic effects in the body. Also used in the treatment of asthma and whooping cough. | Yuan et al;58 Wang et al133 |
| C. longa | Zingiberaceae | It is commonly known as turmeric and is native to Southwest India. | Rhizome | Ethanol | Curcumin | Treatment for cancer, arthritic, muscular disorders, inflammations, biliary disorders, anorexia, diabetes, sinusitis cough, diabetic wounds, microbial infections and hepatic disorders. | Shakeri et al;59 Omosa et al134 |
| Z. bungeanum | Rutaceae | It is a popular condiment in cooking and is mainly found in China. | Seeds Oil | – | Alkaloids, terpenoids, flavonoids and fatty acids. | Treatment for digestive disorders, toothache, stomach ache, diarrhea and ascariasis. | Wang et al;60 Zhang et al;135 Rahman et al136 |
| Polyherbal Extracts | |||||||
| Soshiho-tang | – | All the ingredients are common herbs, mainly found in China. | Bupleuri Radix (31.6%), Scutellariae Radix (21.1%), Ginseng Radix, (10.5%) Pinelliae Tuber (10.5%), Glycyrrhizae Radix et Rhizoma (5.3%), Zingiberis Rhizoma Crudus (10.5%), and Zizyphi Fructus (10.5%). | Water | Liquritin, glycyrrhizin and baicalin are commonly found in all the plants included in Soshiho-tang. | Treatment of pulmonary disorders include cold and pneumonitis. | Jeon et al;62 Ohtake et al61 |
| Pentaherbs formula (PHF) | – | All the ingredients are common herbs, mainly found in China. | Lonicerae flos (50 g), Menthae herba (25 g), Phellodendri cortex (50 g), Moutan cortex (50 g) and Atractylodis rhizome (50 g) | Water | Not identified | Treatment for allergic, inflammation and asthma. | Tsang et al63 |
The treatment with ethanolic extract of Scrophularia striata (S. striata) to asthmatic mice shown suppressed Th2 cytokines (including IL-4 and IL-5) in bronchoalveolar lavage fluid (BALF) and inflammatory cells. In addition, it also decreased the level of total immunoglobulin E (IgE) and OVA-specific IgE in serum.34 These results make evident that S. striata extract have good potential in modulating Th2 cytokines and therefore, can be utilised as an immunomodulatory agent for allergic asthma. Almohawes and Alruhaimi35 reported that of hydro-alcoholic extract of Lavandula dentata (L. dentata) reduced the serum levels of IgE, triglycerides, total cholesterol and glucose in guinea pig with OVA-induced asthma model. Moreover, it also attenuated the levels of malondialdehyde (MDA) and restored the glutathione (GSH) levels in the lungs tissue. These results indicate that L. dentata extract ameliorates asthma and the oxidative stress as induced by OVA.
The protective effect of 50% ethanolic extract of Pistacia weinmannifolia (P. weinmannifolia) root (EEPWR) on inflammation and hypersecretion of mucus in respiratory tract in BALB/c mice was studied by Lee et al.36 Treatment with EEPWR decreased the eosinophil count and Th2 cytokines (IL-4, IL-5 and IL-13) levels in the BALF of OVA-exposed mice. Moreover, EEPWR inhibited total and OVA-specific IgE levels in serum. Additionally, EEPWR also efficiently inhibited the accumulation of inflammatory cells into the lungs, as well as respiratory tract mucus hypersecretion. Moreover, the monocyte chemoattractant protein-1 was significantly reduced following EEPWR treatment in the BALF of OVA-exposed mice. The protective effects of EEPWR on OVA-induced inflammation of respiratory tract were accompanied by the downregulation of mitogen associated protein kinases and activation of nuclear factor-κB. Overall, the studies indicate that EEPWR is a valuable armamentarium for asthma treatment.36
The anti-inflammatory and immunomodulatory activity of hydro-ethanolic extract of Ocimum basilicum (O. basilicum) leaves in OVA-challenged rats were examined by Eftekhar et al.37 Administration of O. basilicum extract decreased the levels of IL-4, IgE, phospholipase A2 and total protein and increased interferon-γ (IFN-γ)/IL-4 ratio in OVA-challenged rats. Additionally, O. basilicum extract showed improvements in OVA-induced changes in immunological, pathological and inflammatory markers in OVA-sensitised rats. The results were comparable and can even be more potent than that for standard dexamethasone, suggesting its therapeutic potential in asthma.
The anti-inflammatory actions of an ethanolic extract of Polyscias fruticosa (P. fruticosa) leaves in OVA-induced asthma was tested by Koffuor et al.38 Administration of P. fruticosa extract reduced the OVA-induced elevation of white blood cells (WBC) and C-reactive protein to normalcy. Moreover, administration of P. fruticosa extract reduced the rate of erythrocyte sedimentation. In the recent study, OVA-sensitized BALB/c mice were treated with ethanolic extract of aerial parts of Herissantia tiubae (H. tiubae) 1 day before evaluating the anxiety and respiratory parameters. Increase in plasma IL-13, which was restored with the treatment of H. tiubae extract was observed. Additionally, there was a rise in total BALF cell count with prominent eosinophilia has been seen. It was determined that H. tiubae extract can show similar action on respiratory parameters to that of aminophylline and behavioral changes as that of diazepam. Suppression of inflammation was as efficient as that for dexamethasone.39
The effects of aqueous extract of Solanum xanthocarpum (S. xanthocarpum) on respiratory tract inflammation and oxidative stress in experimental asthmatic rats were assessed by Gulati.40 The administration of S. xanthocarpum extract (50 mg/kg), reduced the levels of blood and BALF OVA-specific IgE in Ova-immunised rats by 37% and 20%, respectively. These effects were comparable with prednisolone (10 mg/kg), which reduced IgE level by 43% and 31% in blood and BALF respectively. Similarly, administration of S. xanthocarpum extract at the dose of 100 mg/kg attenuated the levels of tumor necrosis factor-α (TNF-α), IL-6 and IL-4 and elevated the levels of IFN-γ when compared with control. These data indicate improved immunostimulatory/immunomodulatory effects. In addition, administration of S. xanthocarpum extract (100 mg/kg) ameliorates oxidative stress in the animals and it is indicated by reduced MDA levels and elevated GSH levels. It was hypothesised that the beneficial effects of S. xanthocarpum in bronchial asthma are attributed to the balancing influence on pro-oxidant-antioxidant status and reduction in airway inflammation.
The effect of 70% ethanolic extract of Crocus sativus (C. sativus) flowers on differential and total WBC count in lung lavage fluid of Ova-sensitised rats were reported by Mahmoudabady et al41 C. sativus extract treatment decreased percentage of neutrophils and eosinophils and total WBC count and the in OVA-sensitised animals. Therefore, the findings indicated that C. sativus extract is effective in alleviating lung inflammatory cells in experimentally induced asthmatic animals.41 Bulani et al42 reported the effect of methanolic extract of Calotropis gigantea (C. gigantea) root on OVA-induced asthma in rats. C. gigantea extract-treatment showed significant inhibition of neutrophil, lymphocyte, eosinophil and total leukocyte count in BALF of animals treated with OVA. Moreover, C. gigantea extract reduced the oxidative stress in OVA treated animals and it was evident by reduced nitric oxide (NO) levels in BALF and lipid peroxidation (LPO) in the lungs and restored levels of GSH, superoxide dismutase (SOD) in lungs. C. gigantea extract also significantly improved the OVA-induced histological changes. These results suggest the potential effect of C. gigantea in OVA-induced asthma.
The anti-asthmatic and antioxidant activities of aqueous extract of Urtica dioica (U. dioica) leaves in OVA-sensitised rats were examined by Zemmouri et al43 The aqueous extract of U. dioica leaves significantly inhibited serum eosinophilia (by 60%), leucocyte (by 32.75%) and lymphocytes (by 29.22%) in BALF of asthmatic rats. Besides, LPO generated following allergen administration was significantly diminished by U. dioica treatment in the lung tissues (by 48.58%), thus confirming that U. dioica aqueous leaves extract confer some protective effects against airway inflammation. Khan and Imtiyaz44 reported the ethanolic extract of Wrightia tinctoria (W. tinctoria) leaves treatment decreased leukocyte count when compared to OVA-induced animals. The findings indicate that ethanolic leaves extract of W. tinctoria possess some anti-asthmatic effects, possibly mediated via inhibition of histamine release.
The administration of Asparagus cochinchinensis (A. cochinchinensis) root extract lowered the bronchial thickness and infiltration of inflammatory cells, also showed a reduction in macrophages, eosinophils, IgE and Th2 cytokines in OVA-induced animals.45 In addition, there was a significant recovery in goblet cell hyperplasia, matrix metallopeptidase-9 (MMP-9) expression and vascular endothelial growth factor (VEGF) signaling pathway upon airway remodelling in the extract-treated group. Overall, these findings provide strong evidence that A. cochinchinensis root extract prevents airway inflammation of a chronic asthma model.45 Sim et al46 reported that 70% ethanolic extract of Aster yomena (A. yomena) decreased Th2 type cytokines and eosinophils in the BALF and OVA-specific IgE in serum. In addition to that, A. yomena extract reduced AHR and histopathology of lungs returned to normalcy in the OVA-challenged animals.46
The aqueous extract of Allium cepa (A. cepa) normalised the levels of nitrogen dioxide (NO2), nitrate (NO3), MDA, IL-4 and IgE, and the antioxidant markers such as SOD, catalase (CAT), thiol and IFN-γ as well as the IFN-γ/IL-4 ratio in OVA-sensitised animals.47 The OVA-induced animals when treated with Artemisia argyi (A. argyi), it has reduced inflammatory cells, cytokines and AHR in asthmatic animals.48 Furthermore, A. argyi decreased extracellular receptor kinase (ERK) phosphorylation as well as the expression of MMP-9 in asthmatic animal models, indicating its potential against allergic asthma. The aqueous and ethanolic extracts of rhizomes of Zingiber officinale (Z. officinale) treated animals showed a reduction of total WBC, eosinophils and neutrophils in BALF.49 Moreover, Z. officinale extracts significantly inhibited Th2-mediated immune response, as evidenced by a decrease in mRNA expression levels of IL-4 and IL-5. Furthermore, there was a significant reduction in protein levels of IL-4 and IL-5 in BALF, as well as total serum IgE level by both extracts.
Seo et al50 reported that the 80% ethanolic extract of Erythronium japonicum (E. japonicum) treatment suppressed the number of WBC and decreased the IgE level in the BALF in mice treated with OVA. Furthermore, treatment with E. japonicum extract modulated the expression of both Th2 and Th1 cell-related factor. Trigonella foenum-graecum (T. foenum-graecum) treatment decreased Th2 cytokines and increased Th1 cytokines in BALF and lung homogenates.51 Furthermore, T. foenum-graecum significantly inhibited serum IgE and anti-OVA IgG1, indicated that it is a useful agent against allergic asthma.51 Treatment of 75% hydroethanolic extract of Echinodorus scaber (E. scaber) leaves reduced total eosinophil, neutrophil, leukocyte and mononuclear cell counts in OVA-induced allergic asthma.52 Further, E. scaber extract ameliorated the increased in Th2 cytokines and IgE levels, which confirmed the utility of the leaves in treating allergic inflammation.
Treatment of Siphonochilus aethiopicus (S. aethiopicus) to OVA-induced mice reduced allergic lung inflammation and eosinophils percentage in BALF, although it did not influence airway hyper-reactivity.53 Yu et al54 investigated the protective effects of total flavonoids from 70% ethanolic extract of Selaginella uncinata (S. uncinata) on airway hyper-responsiveness, cytokine release and bitter taste receptors (T2Rs) signalling, emphasising on inflammatory responses in OVA-induced asthma in rats.
Treatment with S. uncinata extract attenuated AHR and goblet cell hyperplasia. Additionally, serum levels of Th2-related cytokines include IL-4, IL-5 and IL-13, total and OVA-specific IgE were reduced and IFN-γ was improved in S. uncinata-treated rats. They have also reported that S. uncinata utilises its anti-inflammatory role via T2R10/IP3R1/NFAT1 dependent signaling pathway.54 Rosa multiflora (R. multiflora) extract inhibited eosinophil accumulation in nasal lavage fluid (NALF), the goblet cells and nasal mucosa in the nasal epithelium and mast cells in the respiratory region of the nasal cavity.55 Furthermore, R. multiflora extract suppressed Th2-related cytokines in NALF, splenocytes and nasal-associated lymphoid tissue (NALT), whereas Th1-associated cytokine IL-12 was up-regulated, indicating that R. multiflora has therapeutic potential in treating allergic rhinitis by moderating the associations between Th1/Th2 responses.
Dussossoy et al56 found that Morinda citrifolia (M. citrifolia) fruit juice reduced the inflammation in OVA-sensitised animals with regards to the decreased number of inflammatory cells in lung lymphocytes, macrophages, neutrophils and eosinophils. In a study, n-hexane fraction of 70% Scutellaria baicalensis (S. baicalensis) ethanolic extract has significantly suppressed the production of Th2-mediated cytokines and increased Th1-mediated cytokines against OVA-induced animals.57 The 70% aqueous ethanolic extract of Sceptridium ternatum (S. ternatum) treatment significantly reduced AHR, raised the ratio of Th1/Th2 and reduced Cyslt1 mRNA level in a dose-dependent manner.58 Moreover, high-dose of S. ternatum had similar efficacy as the investigated standard drug (Montelukast) in a mouse asthma model and therefore is a good potential anti-asthmatic agent.
The hydro-ethanolic extract of Curcuma longa (C. longa) significantly decreased tracheal responsiveness as well as pathological lung features in OVA-sensitised rats. Additionally, the extract decreased tracheal responsiveness to methacholine. Moreover, C. longa significantly decreased interstitial fibrosis compared to OVA-sensitized group. The C. longa treated animals showed similar or even more than that conferred by standard dexamethasone at 250 µg/kg.59 The administration of Zanthoxylum bungeanum (Z. bungeanum) seed oil lessened airway remodelling and lung tissue injury and inhibited the infiltration of eosinophils and leukocytes into the airway by decreasing the expression levels of inflammatory chemokines and cytokines compared with OVA-treated animals.60 Moreover, Z. bungeanum seed oil also reduced the levels of inflammatory adhesion molecules and chemokine via downregulation of extracellular signal-regulated kinase and activation of c-JUN N-terminal kinase (JNK).
Soshiho-tang, also known as “Xiao-Chai-Hu-Tang” (Chinese) and “Sho-Saiko-to” (Japanese), is composed of seven herbs (Table 1).61 The administration of Soshiho-tang water extract suppressed eosinophil influx into BALF while decreasing the levels of Th2-type cytokines.62 Moreover, Soshiho-tang water extract exhibited a marked decrease in mucus hypersecretion, IgE levels while significantly induced the expression of HO-1 protein. Therefore, it was determined that Soshiho-tang water extract might be another useful armamentarium against allergic asthma. In one another study, the pentaherbs formula (PHF) comprised of five traditional Chinese herbal medicines (Table 1). The water extract of PHF showed significant suppression of pulmonary eosinophilia and asthma-related cytokines IL-4 and IL-33 in BALF T.63
Overall Potential Mechanism of Action of TMPs Against OVA-Induced Asthma
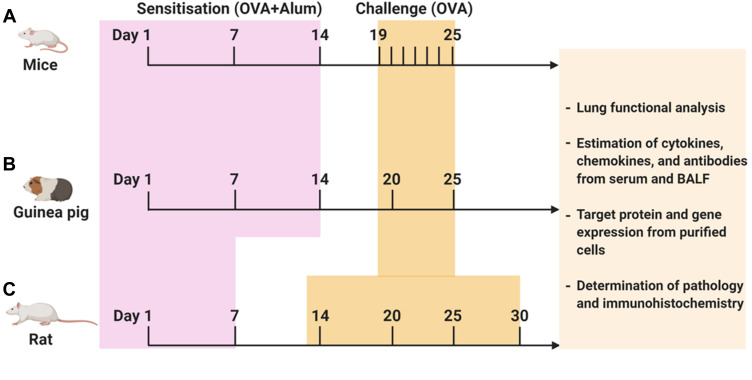
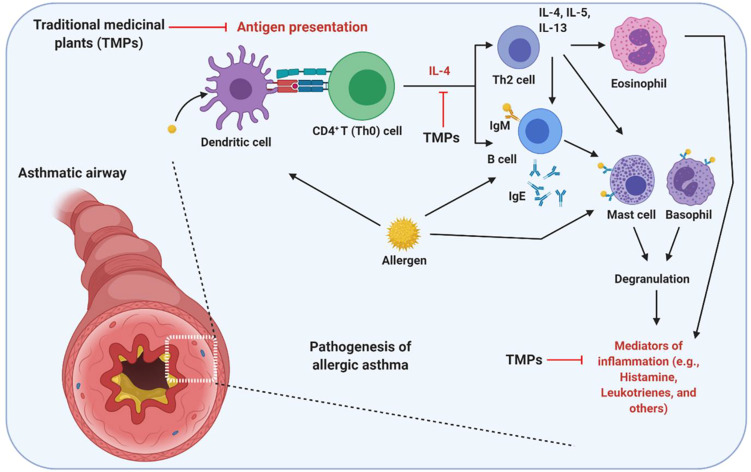
Due to the ethical and moral issues subjecting asthma patients to traditional herbal medicines which may exacerbate their conditions in clinical trials, the development of preclinical animal model is of great importance. OVA-induced asthma is one of the most studied allergic asthma model across the world. In this review, it was noteworthy to state that there are several variations of the model in which OVA-sensitization and OVA-challenge have been conducted via different delivery modes including repeated intraperitoneal, subcutaneous and intranasal or intratracheal administrations. The overview of the detailed protocol followed to investigate the effect of TMPs against OVA-induced asthma has been summarized in Figure 2 and Table 2. When allergen/OVA enter the lungs, antigen-presenting cells (dendritic cells) captures and present the processed antigen to the CD4+ T cells. Activated T cells produce copious amounts of Th2-biased cytokines (IL-4, IL5, and IL-13), which further activates the Th2 cells and B cells. Sequentially activated B cells secrete IgE, which is a stimulating factor for mast cells to produce inflammatory mediators. Furthermore, T cell activates the other innate immune cells, including eosinophils which aggravate the inflammatory situation. TMPs which composed of heterogenous components inhibit the allergen/OVA-induced asthma by inhibiting either the antigen presentation, cytokine secretion or the release of inflammatory mediators. The detailed mechanism of TMPs against OVA-induced asthma was shown in Figure 3.
Figure 2.
Protocol followed to investigate TMPs against OVA-induced asthma. OVA-induced animal asthma model: (A) mice, (B) Guinea pig, (C) Rat. The above figure depicts the timeline of OVA sensitization and challenge followed by the end readouts. Schedules of sensitization and challenge depend on the type of animal model and routes of administration. In addition, varying OVA doses (with or without adjuvant) have been used both in sensitization and challenge (for more information, see Table 1).
Table 2.
Study Protocol and the Protective Mechanism of TMPs Against OVA-Induced Asthma
| Medicinal Plant | Treatment Dose | Strain | OVA-Sensitization Route and Protocol | OVA-Challenge Route and Protocol | Mechanism of Action | Reference |
|---|---|---|---|---|---|---|
| S. striata | 100 and 200 mg/kg**, i.p., 7 days | Balb/c mice | OVA (100 μg) + 1 mg of Al(OH)3, in 200 μL of PBS (Days 1–7, s.c.). | OVA (10 μg) in 200 μL of PBS on day 14 (i.p.). | ↓ Th2-related cytokines (IL-4 and IL-5), ↓ IgE, eosinophils and total inflammatory cells in BALF, ↓ reactive oxygen species (ROS). | Azadmehr et al34 |
| L. dentata | 300 mg/kg*, P.O., 21 days | Guinea pigs | OVA (0.1 mg) + 1 mg of Al(OH)3, in 0.5 mL of normal saline (Day 0, 7 and 14, i.p.). | On days 21–23, aerosolised with 1 mg/mL of OVA in daily basis via nebuliser. | ↓ Total plasma IgE, ↑ SOD, GSH and γ-glutamyl transferase (γ-GT). | Almohawes and Alruhaimi35 |
| P. weinmannifolia | 7.5 and 15 mg/kg**, P.O., 6 days | Balb/c mice | OVA (30 μg) + 3 mg of Al(OH)3, in 0.2 mL of PBS. (Twice daily on Day 0 and 14, i.p.). | On days 21‑23, aerosolised with 1% OVA (Al(OH)3‑free saline solution, 60 min/day) via nebuliser. | ↓ Th2-related cytokines (IL-4, IL-5 and IL-5), ↓ IgE, inhibit the influx of inflammatory cells into the lungs, as well as airway mucus hypersecretion. | Lee et al36 |
| O. basilicum | 0.75, 1.50 and 3.00 mg/mL**, P.O., 21 days | Wistar rats | OVA (1 mg/kg) + 100 mg of Al(OH)3, administered by i.p. and rats were exposed to OVA (2%) aerosol with air flow of 8 L/min for 20 min/day in a 0.8 m3 chamber. | No OVA-challenge | ↓ IL-4, IgE and PLA2. ↑ IFN-γ/IL-4 ratio and ↓ Total protein. | Eftekhar et al37 |
| P. fruticose | 100, 250* and 500 mg/kg, P.O. day 16 | Guinea pigs | OVA (150 μg) + 100 mg Al(OH)3, in 1 mL of normal saline (Twice daily on day 1 and 7, i.p.). | On day 15, aerosolised with 1% saline solution of OVA for 30 min. | ↓ Leucocytes and serum C-reactive protein. | Koffuor et al38 |
| H. tiubae | 50, 100 and 200 mg/kg**, P.O., 1 day | Balb/c mice | OVA (10 mg) + 2 mg Al(OH)3, in normal saline (Day 1 and 10, s.c.). | On days 19–24, aerosolised with after 1% OVA for 20 min daily via nebuliser. | ↓ Inflammatory cell count in BALF. ↓ IL-13 and IgE. | Mozzini et al39 |
| S. xanthocarpum | 50, 100** and 200 mg/kg, P.O., 22 days | Wistar rats | OVA (40 mg) + 2 mg Al(OH)3, in normal saline (Day 0, i.p.). | 14 days after immunisation, aerosolised with 1% OVA for 8 consecutive days via nebuliser. | ↓ IL-4, IL-6, TNF-α and IgE. ↑ IFN-γ/IL-4 ratio, ↓ oxidative stress and MDA, ↑ GSH. | Gulati40 |
| C. sativus | 50, 100* and 200 mg/kg, P.O., 32 days | Wistar rats | OVA (1 mg) + 50 mg Al(OH)3, in 0.5 mL normal saline (i.p.). One week later, they were administered with 0.02 mg OVA + 50 mg Al(OH)3, in 0.5 mL saline (i.p.) as a booster dose. | On days 14–32, aerosolised with 4% OVA for 18±1 days, 5 min daily via nebuliser. | Inhibit eosinophils count in BALF, ↓ leucocytes and neutrophils. | Mahmoudabady et al41 |
| C. gigantea | 100, 200 and 400 mg/kg**, P.O., 14 days | Wistar rats | OVA (20 μg) + 8 mg Al(OH)3, in 1 mL normal saline (i.p.). A booster injection of the same amount was administered for 7 days (i.p.). | Seven days after (Day 15), aerosolised with 1% OVA for 30 min in a closed plexiglass chamber. | Inhibit eosinophils, neutrophils and lymphocytes and total leukocyte count in BALF, ↑ GSH, SOD and catalase (CAT), ↓ LPO, ROS and oxidative stress. | Bulani et al42 |
| U. dioica | 1.5 g/kg*, P.O., 25 days | Wistar rats | OVA (1 mg/mL) + 1 mg/mL of Al(OH)3, in normal saline (Day 0 and 14, i.p.). | On days 21–23, aerosolised with OVA (5 mg/mL) for 30 min via a nebuliser. | Inhibit eosinophils in BALF. Suppress the inflammatory cells and ↓ LPO and ROS in lung tissues. | Zemmouri et al43 |
| W. tinctoria | 100, 150 and 250 mg/kg**, s.c., 24 days | Wistar rats | OVA (0.2 mL) mixed with Al(OH)3 (Days 0, 7, and 14, i.p.). | On days 22 and 24, aerosolised with 1 mL OVA in 100 mL PBS for 1 h daily via nebuliser. | ↓ Total leukocyte count. | Khan et al49 |
| A. cochinchinensis | 250 and 500 mg/kg**, P.O., 6 days | Balb/c mice | OVA (20 µg) + 200 µL Al(OH)3, in PBS solution (Day 1 and 14, i.p.). | On days 21–23, aerosolised with 2% OVA for 30 min daily via nebuliser. | ↓ Macrophages, eosinophils, IgE and expression of Th2 cytokines (IL-4, IL-13, IL-1β) and TNF-α. | Choi et al45 |
| A. yomena | (1g/kg*, P.O., 10 days) | Balb/c mice | OVA (75 µg) + 2 mg Al(OH)3, in 200 µL of PBS (Day 0 and 7, i.p.). | Days 14–16 and 21–23, 50 µg OVA in 20 µL PBS (i.n.). | ↓ Th2 type cytokines (IL-4, IL-5 and IL-13), eosinophils in the BALF and IgE in serum, ↓ transforming growth factor β (TGF-β) and COX-2. | Sim et al46 |
| A. cepa | 35, 70 and 140 mg/kg**, P.O., 21 days | Wistar rats | OVA (1 mg/kg) + 100 mg of Al(OH)3, in normal saline (3 days, i.p.). | On days 6, 9, 12, 15, 18 and 21, aerosolised with 1% OVA for 20 min/day via nebuliser with an air flow of 8 L/min. | ↓ Th2-related cytokines (IL-4), ↓ IgE, ↓ ROS, MDA and oxidative stress, ↑ GSH, SOD and CAT, ↑ IFN-γ and IFN-γ/IL-4 ratio. | Marefati et al47 |
| A. argyi | 50 and 100 mg/kg**, P.O., 6 days | Balb/c mice | OVA (20 μg) + 2 mg Al(OH)3, in normal saline (Day 0 and 14, i.p.). | On days 21–23, aerosolised with 1% w/v of OVA for 1 h daily via nebuliser. | ↓ Inflammatory cells, cytokines (IL-4, IL-5 and IL-13) and AHR. ↓ MMP-9 and p-ERK/t-ERK. | Shin et al48 |
| Z. officinale | 500*/720* mg/kg (ethanol/water extract), i.p., 7 days | Balb/c mice | OVA (20 mg) + 2 mg Al(OH)3, in 0.1 mL PBS (Day 0 and 14, i.p.). | Days 21–27, aerosolised with OVA/PBS (1 c/o), in daily basis via nebuliser. | ↓ Th2-related cytokines (IL-4, IL-5 and IL-13). ↓ IgE in serum. | Khan et al49 |
| E. japonicum | 60 and 600 mg/kg**, P.O., 5 days | Balb/c mice | OVA (20 μg) + 1 mg Al(OH)3, in 500 μL of normal saline (Day 1 and 8, i.p.). | On days 21–25, aerosolised with 5% OVA for 30 min daily via nebuliser (3 mL/min). | ↓ Secretion of mucus in the bronchioles, eosinophil infiltration around the bronchioles and vessels, and goblet cell and epithelial cell hyperplasia. ↓ Inflammatory cells, cytokines (IL-4, IL-5, IL-13), TNF-α and GATA-3, ↑ IFN-γ, ↓ IL-12p35 and IL-12p40. | Seo et al50 |
| T. foenum-graecum | 20 mg/mL*, P.O., 12 days | Balb/c mice | OVA (50 mg) + 1 mg Al(OH)3, 2 mL of normal saline (Day 1, i.p.). On day 14, they were administered with 50 mg OVA (i.p.) as a booster dose. | On days 27–29, aerosolised with 5% OVA for 20 min daily via nebuliser. | ↓ IL-5, IL-6, IL-1β and TNF-α. ↓ Inflammatory and goblet cells, ↓ anti-OVA IgG1 | Piao et al51 |
| E. scaber | 1, 5 and 30 mg/kg**, P.O., twice a day for 6 days | Swiss Webster mice | OVA (100 μg/mL) + 10 mg/mL Al(OH)3, in 200 μL of normal saline (Day 1 and 10, i.p.). | On days 19–24, aerosolised with 3% OVA for 20 min for 20 min daily via nebuliser. | ↓ Inflammatory cells and Th2-related cytokines (IL-4, IL-5 and IL-13) and IgE. | Rosa et al52 |
| S. aethiopicus | 500 mg/kg*, i.p./P.O., two times daily, 4 days | Balb/c mice | OVA (50 µg) + 1.3% Al(OH)3, in normal saline (Day 0, 7 and 14, i.p.). | On days 21–23, challenged intranasally (i.n.) with 1 mg of OVA. | ↓ Lung inflammation and the percentage of eosinophils in BALF. | Fouche et al53 |
| S. uncinata | 500 mg/kg*, i.p., two times daily, 4 days | Sprague Dawley rats | OVA (20 μg) + 1 mg Al(OH)3, in normal saline (Day 1 and 14, i.p.). | On days 21–27, aerosolised with 5% OVA for 1 h daily via nebuliser. | ↓ Th2-related cytokines (IL-4, IL-5 and IL-13), ↓ IgE. ↓ Inflammatory and goblet cells. Up-regulate T2R10 gene expression and down-regulate IP3R1. Suppress NFAT1, ↑ IFN-γ. | Yu et al54 |
| R. multiflora | 100, 200 and 400** mg/kg, P.O., 13 days | Balb/c mice | OVA (50 μg) + 1 mg Al(OH)3, in 200 μL of normal saline (Day 0, 7 and 14, i.p.) | On days 21–27, challenged intranasally (i.n.) with 20 μL OVA (1 mg/mL) in each nasal cavity. | ↓ Th2-related cytokines (IL-4, IL-5 and IL-13) in NALT, NALF, and splenocytes. Up-regulate IL-12. | Bui et al55 |
| M. citrifolia | 2.17 mL/kg*, i.p., 8 days or 4.55 mL/kg*, P.O., 8 days | Brown Norway rats | OVA (1 mg/mL) + 100 mg/mL Al(OH)3, in normal saline (1 mL/day on Day 1, 2, 3 and 16, i.p.). | On days 22–29, aerosolised with OVA (1% w/v) for 20 min daily via nebuliser. | ↓ Inflammatory cells in lung (macrophages, lymphocytes, eosinophils and neutrophils), ↓ ROS and NO levels. | Dussossoy et al56 |
| S. baicalensis | – | Balb/c mice | OVA (20 µg) + 2 mg/mL of Al(OH)3, in normal saline (Day 0 and 14, i.p.). | – | ↓ Th2-related cytokines (IL-4, 5, 10 and 13) and ↑ Th1-mediated cytokines (IFN-γ and IL-12), ↑ IFN-γ. | Jung et al57 |
| S. ternatum | 1.935 g/kg, 9.675 g/kg and 19.350 g/kg**, P.O., 10 days | Balb/c mice | OVA (0.08%, 0.1 mL) mixed with equal volume of Inject Al(OH)3 (Day 1 and 14, i.p.). | On days 24–26, aerosolised with 10 mL of 1% OVA for 20 min daily via nebuliser. | ↓ Airway responsiveness, elevated the ratio of Th1/Th2, and ↓ Cyslt1 mRNA. | Yuan et al58 |
| C. longa | 150, 300 and 600 mg/kg**, P.O., 21 days | Wistar rats | OVA (1 mg) + 100 mg of Al(OH)3, in normal saline (Day 1–3, i.p.). | On days 6, 9, 12, 15, 18 and 21, aerosolised with 1% OVA 20 min daily via nebuliser. | ↓ Tracheal contractile response, Interstitial fibrosis and inflammation. | Shakeri et al59 |
| Z. bungeanum | 2 g/kg*, i.p., day 1, 2, 3, 7 and 14 | Balb/c mice | OVA (20 mg) + 40 mg of Al(OH)3, in 200 μL of normal saline (Day 1, i.p.). On day 9, 20 mg OVA + 10 mg of Al(OH)3, in 200 μL of normal saline (Day 1, i.p.). | On day 1–3, 7 and 14, aerosolised with 20 μg/50 μL OVA in normal saline via nebuliser. | ↓ Pro-inflammatory cytokines, Inflammatory chemokines and adhesion molecules via down-regulation of extracellular signal-regulated kinase and activation of JNK. | Wang et al60 |
| Polyherbal Extracts | ||||||
| Soshiho-tang | 100 and 200 mg/kg**, P.O., 6 days | Balb/c mice | OVA (20 μg) + 2 mg of Al(OH)3, in 200 μL of PBS (Day 0 and 14, i.p.). | On days 21–23, aerosolised with OVA (1% w/v) for 1 h via nebuliser. | ↓ Eosinophils and inflammatory cells. ↓ Th2-related cytokines (IL-4, IL-5, IL-13, IL-17 and IL-33) and ↓ chemokine (eotaxin) in BALF, ↓ IgE and induce heme oxygenase (HO)-1 protein expression. | Jeon et al62 |
| Pentaherbs formula (PHF) | 9.2 mg*, P.O., 14 days | Balb/c mice | OVA (1%) in Al(OH)3 (Day 1 and 8, i.p.). | On days 21–23, aerosolised with OVA (10 mg/mL) twice daily via nebuliser. | Suppresses pulmonary eosinophilia and ↓ IL-4 and IL-33 in BALF. In addition, modulate T cells population and up-regulate IL-10 in serum, ↓ TGF-β. | Tsang et al63 |
Notes: ↑ Indicates increased response; ↓ Indicates decreased response; *Most effective dose; **Dose-dependent; All the other abbreviations are available in the main text.
Figure 3.
Possible mechanism of action of TMPs against OVA-induced asthma. As an allergen, OVA-induces airway inflammation and hyperresponsiveness. As a protein OVA recognized, processed and presented by the antigen-presenting cells. Upon presentation, CD4+ T helper (Th0) cells are activated and polarized toward Th2 phenotype, which activates eosinophils (Eosinophilic asthma), mast cells, and basophils to secret inflammatory mediators. OVA directly or indirectly (via Th cells mediated) activates the B lymphocytes to secret IgE, which is the main correlative factor in allergen-induced asthma. TMPs may either act by inhibiting antigen presentation or cytokine secretion or other inflammatory mediators (histamine, leukotrienes, and others). The figure was created with the support of https://biorender.com under the paid subscription.
Challenges and Opportunities of TMPs for the Treatment of Asthma
Since asthma is a chronic disease, conventional management for asthma generally requires institution of extensive bronchodilators and corticosteroids treatment. Nevertheless, drugs, especially steroids, are allied with various side effects and refractory responses. Therefore, TMPs may provide a better alternative strategy against asthma. Nevertheless, scientific validation remains lacking and is urgently required to confirm their utility either as a complementary alternative therapy or as an adjunct to conventional therapies.64
Based on the established literatures, administration of the reported medicinal plant extracts to OVA-induced animals can effectively inhibit the entry of inflammatory cells into the lungs, where a significant reduction in the total eosinophil and leucocyte populations in the lung tissue can be seen. All the medicinal plants stated in this review are used to treat respiratory inflammation, asthma or its related diseases for many years based on the traditional Indian medicine (Ayurveda), traditional Chinese medicine and other indigenous medicinal systems. The scientific reports reviewed have demonstrated that all these medicinal plants have potential anti-asthmatic effect against OVA-induced asthma model, which is similar to asthma experienced by humans which strongly support their traditional uses. However, further studies are required since most are established in the earlier phases of study and clinical trials are encouraged. Additionally, the safety, toxicity and side effect data associated with TMPs are severely lacking and have to be addressed. TMPs have several chemical constituents which are responsible for the management of asthma or its relevant complications. Thus, the exact active constituent responsible for its anti-asthmatic effects should be evaluated. Furthermore, its pharmacokinetic, pharmacological and clinical data has to be investigated to confirm its potential as a lead compound against asthma.
Moreover, an archive of information on herbal medicine from various cultural backgrounds (Indian, Chinese, Japanese, Korean, African and Arabian) for the management and treatment of asthma.65–81 To date, knowledge on the medicinal plants used for asthma medication and their mode of preparations are limited to some herbal medicine practitioners although some clinical and pharmacological evidences are available.68 Therefore, there is insufficient evidence to make recommendations to use of these TMPs in clinical practice since the global acceptance and utilisation of herbal medicines is dependent on their safety and efficacy.
Our review suggests that OVA-induced animal model may be used to confirm the effectiveness of the TMPs. The model confirms the effectiveness of herbal medicines on typical inflammatory processes as seen in asthma inflammation which confirms the clinical biological plausibility of herbal medicines effects. Several new animal models are currently available for preclinical screening to confirm the efficacy of the anti-asthmatic drugs. Thakur et al82 developed a new model to confirm the anti-asthmatic effects in which the combination of OVA with LPS demonstrated the phenotypes of severe lymphocytic inflammation, neutrophilic, eosinophilic and bronchoconstriction, when compared to a single allergen. Few other studies have also shown that pulmonary neutrophilia in mouse is triggered by the combination of LPS with commercial OVA.83,84 These new models can be exploited to evaluate the severe anti-asthmatic effect of TMPs.
Very recently, several clinical studies have reported the use of TMPs for asthma treatment. Dulla and Jahan70 indicated that some traditional Chinese medicines indicated their effectiveness against asthma in children when compared to modern medicines such as salbutamol and montelukast. In another research, some of the traditional herbal medicines in East-Asia were used as adjuncts in improving acute and chronic asthma outcomes85 which boosts the evaluation of TMPs for clinical trials. Wang et al86 reported that the clinical efficacy of traditional Chinese medicine, which binds to proteins, are potential targets for asthma therapy. Another approach is a combination therapy, when herbal medicines used together with modern medicines enhance asthma outcomes as compared to the use of pharmacotherapies alone.87 Zhou et al88 indicated that acupuncture ameliorates asthmatic pulmonary function associated to metallothionein-2. Based on all the above studies, it is hypothesised that the combination of acupuncture with TMPs treatments also provide better therapeutic outcomes. Future research on asthma treatment should be focused in this direction and more advanced studies are required to confirm the use of TMPs in combination with conventional therapies.
Conclusion and Future Perspectives
In this review, we highlighted 27 traditional medicinal plant species and two polyherbal extracts that have been investigated for their anti-asthmatic potential using OVA-induced models. Our review revealed that most of the investigated medicinal plants and their extracts promote attenuation of asthma symptoms in experimental animals and supports its traditional information. Nevertheless, the toxicological profiles of the plant extracts remain unreported since most of the studies focused only on their anti-asthmatic potentials. Additionally, there is a severe lack of information on the efficacy, safety and the required dosage to induce the in vivo anti-asthma activity which should be the focus in future studies. The lack of scientific reports and limited information on the isolated compounds responsible for anti-asthmatic actions is another negative factor that should be addressed in future studies. In conclusion, the vast potentials of the TMPs should be explored for the development of new drugs to enhance existing anti-asthmatic treatment as an adjuvant or to be used as a new armamentarium against asthma.
Acknowledgments
The authors would like to thank Universiti Kuala Lumpur Royal College of Medicine Perak, Malaysia for providing necessary facilities and resources to complete this study for publication.
Consent for Publication
The final version of the manuscript was reviewed by all the authors and consented to its submission.
Author Contributions
S.A., and M.S. conceived the idea. S.A., M.S., and S.R.B. designed, collected the literature, interpreted the data, analyzed the data, drafted and revised the manuscript. All the authors have made noteworthy contributions to the study design, data collection, review and interpretation; have engaged in the drafting or revision of the article; have agreed to submit to the current journal; have given final approval of the version to be published; and have agreed to be responsible for all aspects of the work.
Disclosure
The authors have no conflict of interest associated with this work or publication. There was no significant financial support for this work.
References
- 1.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Vol. 2020. Global Initiative for Asthma; 2016. [Google Scholar]
- 2.Kaufman G. Asthma: pathophysiology, diagnosis and management. Nurs Stand. 2011;26(5):48. doi: 10.7748/ns.26.5.48.s55 [DOI] [PubMed] [Google Scholar]
- 3.Pedersen SE, Hurd SS, Lemanske JRF, et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46(1):1–17. doi: 10.1002/ppul.21321 [DOI] [PubMed] [Google Scholar]
- 4.Mohammed S, Goodacre S. Intravenous and nebulised magnesium sulphate for acute asthma: systematic review and meta-analysis. Emerg Med. 2007;24(12):823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams BK, Cydulka RK. Asthma evaluation and management. Emerg Med Clin N Am. 2003;21(2):315–330. doi: 10.1016/S0733-8627(03)00015-4 [DOI] [PubMed] [Google Scholar]
- 6.Schaneberg BT, Crockett S, Bedir E, et al. The role of chemical fingerprinting: application to Ephedra. Phytochemistry. 2003;62(6):911–918. doi: 10.1016/S0031-9422(02)00716-1 [DOI] [PubMed] [Google Scholar]
- 7.Barnes PJ. Drugs for asthma. Br J Pharmacol. 2006;147(S1):S297–S303. doi: 10.1038/sj.bjp.0706437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118(11):3546–3556. doi: 10.1172/JCI36130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109(3):366–398. [DOI] [PubMed] [Google Scholar]
- 10.DiMartino SJ. Idiopathic inflammatory myopathy: treatment options. Curr Rheumatol Rep. 2008;10(4):321. doi: 10.1007/s11926-008-0051-4 [DOI] [PubMed] [Google Scholar]
- 11.Kesler SM, Sprenkle MD, David WS, et al. Severe weakness complicating status asthmaticus despite minimal duration of neuromuscular paralysis. Intensive Care Medicine. 2009;35(1):157–160. doi: 10.1007/s00134-008-1267-5 [DOI] [PubMed] [Google Scholar]
- 12.Che C-T, George V, Ijinu T, et al. Traditional Medicine. Pharmacognosy: Elsevier; 2017:15–30. [Google Scholar]
- 13.Albuquerque UPD, Hanazaki N. As pesquisas etnodirigidas na descoberta de novos fármacos de interesse médico e farmacêutico: fragilidades e pespectivas. Revista Brasileira De Farmacognosia. 2006;16:678–689. doi: 10.1590/S0102-695X2006000500015 [DOI] [Google Scholar]
- 14.Yuan G, Wahlqvist ML, He G, et al. Natural products and anti-inflammatory activity.. Asia Pac J Clin Nutr. 2006;15(2):143–152. [PubMed] [Google Scholar]
- 15.Bonam SR, Wu YS, Tunki L, et al. What has come out from phytomedicines and herbal edibles for the treatment of cancer? Chem Med Chem. 2018;13(18):1854–1872. doi: 10.1002/cmdc.201800343 [DOI] [PubMed] [Google Scholar]
- 16.Yuan H, Ma Q, Ye L, et al. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559. doi: 10.3390/molecules21050559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery.. Environ Health Perspect. 2001;109 Suppl 1(suppl 1):69–75. doi: 10.1289/ehp.01109s169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alves RR, Rosa IM. Biodiversity, traditional medicine and public health: where do they meet? Journal of Ethnobiology and Ethnomedicine. 2007;3(1):14. doi: 10.1186/1746-4269-3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonam SR, Partidos CD, Halmuthur SKM, et al. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol Sci. 2017;38(9):771–793. doi: 10.1016/j.tips.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 20.Chen C, Sun N, Li Y, et al. A BALB/c mouse model for assessing the potential allergenicity of proteins: comparison of allergen dose, sensitization frequency, timepoint and sex. Food Chem Toxicol. 2013;62:41–47. doi: 10.1016/j.fct.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 21.Shin YS, Takeda K, Gelfand EW. Understanding asthma using animal models. Allergy Asthma Immunol Res. 2009;1(1):10–18. doi: 10.4168/aair.2009.1.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schülke S, Albrecht M. Mouse models for food allergies: where do we stand? Cells. 2019;8(6):546. doi: 10.3390/cells8060546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Q-L, Chen Z. Establishment of different experimental asthma models in mice.. Exp Ther Med. 2018;15(3):2492–2498. doi: 10.3892/etm.2018.5721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C, Lianhua L, Nana S, et al. Development of a BALB/c mouse model for food allergy: comparison of allergy-related responses to peanut agglutinin, β-lactoglobulin and potato acid phosphatase. Toxicol Res. 2017;6(2):251–261. doi: 10.1039/C6TX00371K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aun MV, Bonamichi-Santos R, Arantes-Costa FM, et al. Animal models of asthma: utility and limitations. J Asthma Allergy. 2017;10:293. doi: 10.2147/JAA.S121092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res. 2017;367(3):551–569. doi: 10.1007/s00441-016-2566-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M-F, Yang K-J, Wang NM, et al. The development of a murine model for Forcipomyia taiwana (biting midge) allergy. PLoS One. 2014;9(3):e91871. doi: 10.1371/journal.pone.0091871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogan SP, Matthaei KI, Young JM, et al. A novel T cell-regulated mechanism modulating allergen-induced airways hyperreactivity in BALB/c mice independently of IL-4 and IL-5.. J Immunol. 1998;161(3):1501–1509. [PubMed] [Google Scholar]
- 29.Zhou C, Ludmila T, Sun N, et al. BALB/c mice can be used to evaluate allergenicity of different food protein extracts. Food Agric Immunol. 2016;27(5):589–603. doi: 10.1080/09540105.2015.1129600 [DOI] [Google Scholar]
- 30.Bodinier M, Leroy M, Ah-Leung S, et al. Sensitization and elicitation of an allergic reaction to wheat gliadins in mice. J Agric Food Chem. 2009;57(4):1219–1225. doi: 10.1021/jf802898u [DOI] [PubMed] [Google Scholar]
- 31.Morafo V, Srivastava K, Huang C-K, et al. Genetic susceptibility to food allergy is linked to differential TH2-TH1 responses in C3H/HeJ and BALB/c mice. J Allergy Clin Immunol. 2003;111(5):1122–1128. doi: 10.1067/mai.2003.1463 [DOI] [PubMed] [Google Scholar]
- 32.Hylkema M, Hoekstra M, Luinge M, et al. The strength of the OVA-induced airway inflammation in rats is strain dependent. Clin Exp Immunol. 2002;129(3):390–396. doi: 10.1046/j.1365-2249.2002.01938.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson JL, Scott R, Boyle MJ, et al. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11(1):54–61. doi: 10.1111/j.1440-1843.2006.00784.x [DOI] [PubMed] [Google Scholar]
- 34.Azadmehr A, Hajiaghaee R, Zohal MA, et al. Protective effects of Scrophularia striata in Ovalbumin-induced mice asthma model. DARU J Pharmaceutical Sci. 2013;21(1):56–62. doi: 10.1186/2008-2231-21-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almohawes Z, Alruhaimi H. Effect of Lavandula dentata extract on Ovalbumin-induced Asthma in Male Guinea Pigs. Brazilian Journal of Biology. 2020;80(1):87–96. doi: 10.1590/1519-6984.191485 [DOI] [PubMed] [Google Scholar]
- 36.Lee J-W, Min J-H, Kim M-G, et al. Pistacia weinmannifolia root exerts a protective role in ovalbumin‑induced lung inflammation in a mouse allergic asthma model.. Int J Mol Med. 2019;44(6):2171–2180. doi: 10.3892/ijmm.2019.4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eftekhar N, Moghimi A, Roshan NM, et al. Immunomodulatory and anti-inflammatory effects of hydro-ethanolic extract of Ocimum basilicum leaves and its effect on lung pathological changes in an ovalbumin-induced rat model of asthma. BMC Complement Altern Med. 2019;19(1):349. doi: 10.1186/s12906-019-2765-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koffuor GA, Boye A, Ofori-Amoah J, et al. Anti-inflammatory and safety assessment of Polyscias fruticosa (L.) Harms (Araliaceae) leaf extract in ovalbumin-induced asthma. J Phytopharm. 2014;3(5):337–342. [Google Scholar]
- 39.Mozzini MT, Ferrera Costa H, Carvalho Vieira G, et al. Anti-asthmatic and anxiolytic effects of Herissantia tiubae, a Brazilian medicinal plant. Immun Inflamm Dis. 2016;4(2):201–212. doi: 10.1002/iid3.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gulati K. Evaluation of Anti-Inflammatory and Immunomodulatory Effects of Aqueous Extract of Solanum Xanthocarpum in Experimental Models of Bronchial Asthma. EC Pharmacol Toxicol. 2016;2:241–250. [Google Scholar]
- 41.Mahmoudabady M, Neamati A, Vosooghi S, et al. Hydroalcoholic extract of Crocus sativus effects on bronchial inflammatory cells in ovalbumin sensitized rats. Avicenna J Phytomed. 2013;3(4):356. [PMC free article] [PubMed] [Google Scholar]
- 42.Bulani V, Biyani K, Kale R, et al. Inhibitory effect of Calotropis gigantea extract on ovalbumin-induced airway inflammation and Arachidonic acid induced inflammation in a murine model of asthma. Int J Cur Bio Med Sci. 2011;1(2):19–25. [Google Scholar]
- 43.Zemmouri H, Sekiou O, Ammar S, et al. Urtica dioica attenuates ovalbumin-induced inflammation and lipid peroxidation of lung tissues in rat asthma model. Pharm Biol. 2017;55(1):1561–1568. doi: 10.1080/13880209.2017.1310905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan Z, Ansari I. Antiasthmatic activity of the ethanol extract of leaves of Wrightia tinctoria.. Asian J Pharm Clin Res. 2018;11(9):136–139. doi: 10.22159/ajpcr.2018.v11i9.26151 [DOI] [Google Scholar]
- 45.Choi JY, Kim J, Park JJ, et al. The anti-inflammatory effects of fermented herbal roots of Asparagus cochinchinensis in an ovalbumin-induced asthma model. J Clin Med. 2018;7(10):377. doi: 10.3390/jcm7100377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sim JH, Lee HS, Lee S, et al. Anti-Asthmatic Activities of an Ethanol Extract of Aster yomena in an Ovalbumin-Induced Murine Asthma Model. J Med Food. 2014;17(5):606–611. doi: 10.1089/jmf.2013.2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marefati N, Eftekhar N, Kaveh M, et al. The effect of Allium cepa extract on lung oxidant, antioxidant, and immunological biomarkers in ovalbumin-sensitized rats. Med Princ Pract. 2018;27(2):122–128. doi: 10.1159/000487885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin N-R, Ryu H-W, Ko J-W, et al. Artemisia argyi attenuates airway inflammation in ovalbumin-induced asthmatic animals. J Ethnopharmacol. 2017;209:108–115. doi: 10.1016/j.jep.2017.07.033 [DOI] [PubMed] [Google Scholar]
- 49.Khan AM, Shahzad M, Raza Asim M, et al. Zingiber officinale ameliorates allergic asthma via suppression of Th2-mediated immune response. Pharm Biol. 2015;53(3):359–367. doi: 10.3109/13880209.2014.920396 [DOI] [PubMed] [Google Scholar]
- 50.Seo J-H, Bang M, Kim G, et al. Erythronium japonicum attenuates histopathological lung abnormalities in a mouse model of ovalbumin-induced asthma. Int J Mol Med. 2016;37(5):1221–1228. doi: 10.3892/ijmm.2016.2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piao CH, Bui TT, Song CH, et al. Trigonella foenum-graecum alleviates airway inflammation of allergic asthma in ovalbumin-induced mouse model. Biochem Biophys Res Commun. 2017;482(4):1284–1288. doi: 10.1016/j.bbrc.2016.12.029 [DOI] [PubMed] [Google Scholar]
- 52.Rosa SIG, Rios-Santos F, Balogun SO, et al. Hydroethanolic extract from Echinodorus scaber Rataj leaves inhibits inflammation in ovalbumin-induced allergic asthma. J Ethnopharmacol. 2017;203:191–199. doi: 10.1016/j.jep.2017.03.025 [DOI] [PubMed] [Google Scholar]
- 53.Fouche G, Nieuwenhuizen N, Maharaj V, et al. Investigation of in vitro and in vivo anti-asthmatic properties of Siphonochilus aethiopicus. J Ethnopharmacol. 2011;133(2):843–849. doi: 10.1016/j.jep.2010.11.014 [DOI] [PubMed] [Google Scholar]
- 54.Yu B, Cai W, Zhang -H-H, et al. Selaginella uncinata flavonoids ameliorated ovalbumin-induced airway inflammation in a rat model of asthma. J Ethnopharmacol. 2017;195:71–80. doi: 10.1016/j.jep.2016.11.049 [DOI] [PubMed] [Google Scholar]
- 55.Bui TT, Kwon D-A, Choi DW, et al. Rosae multiflorae fructus extract and its four active components alleviate ovalbumin-induced allergic inflammatory responses via regulation of Th1/Th2 imbalance in BALB/c rhinitis mice. Phytomedicine. 2019;55:238–248. doi: 10.1016/j.phymed.2018.06.044 [DOI] [PubMed] [Google Scholar]
- 56.Dussossoy E, Bichon F, Bony E, et al. Pulmonary anti-inflammatory effects and spasmolytic properties of Costa Rican noni juice (Morinda citrifolia L.). J Ethnopharmacol. 2016;192:264–272. doi: 10.1016/j.jep.2016.07.038 [DOI] [PubMed] [Google Scholar]
- 57.Jung SY, Lee S-Y, Choi DW, et al. Skullcap (Scutellaria baicalensis) hexane fraction inhibits the permeation of ovalbumin and regulates Th1/2 immune responses. Nutrients. 2017;9(11):1184. doi: 10.3390/nu9111184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan Y, Yang B, Ye Z, et al. Sceptridium ternatum extract exerts antiasthmatic effects by regulating Th1/Th2 balance and the expression levels of leukotriene receptors in a mouse asthma model. J Ethnopharmacol. 2013;149(3):701–706. doi: 10.1016/j.jep.2013.07.032 [DOI] [PubMed] [Google Scholar]
- 59.Shakeri F, Roshan NM, Boskabady MH. Hydro-ethanolic extract of Curcuma longa affects tracheal responsiveness and lung pathology in ovalbumin-sensitized rats. Int J Vitamin Nutrition Res. 2020;90(1–2):141–150. doi: 10.1024/0300-9831/a000524 [DOI] [PubMed] [Google Scholar]
- 60.Wang J-Q, Li X-W, Liu M, et al. Inhibitory effect of Zanthoxylum bungeanum seed oil on ovalbumin-induced lung inflammation in a murine model of asthma. Mol Med Rep. 2016;13(5):4289–4302. doi: 10.3892/mmr.2016.5050 [DOI] [PubMed] [Google Scholar]
- 61.Ohtake N, Nakai Y, Yamamoto M, et al. The herbal medicine Shosaiko-to exerts different modulating effects on lung local immune responses among mouse strains. Int Immunopharmacol. 2002;2(2–3):357–366. doi: 10.1016/S1567-5769(01)00161-8 [DOI] [PubMed] [Google Scholar]
- 62.Jeon W-Y, Shin H-K, Shin I-S, et al. Soshiho-tang water extract inhibits ovalbumin-induced airway inflammation via the regulation of heme oxygenase-1. BMC Complement Altern Med. 2015;15(1):1–10. doi: 10.1186/s12906-015-0857-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsang MS, Jiao D, Chan BC, et al. Anti-inflammatory activities of pentaherbs formula, berberine, gallic acid and chlorogenic acid in atopic dermatitis-like skin inflammation. Molecules. 2016;21(4):519. doi: 10.3390/molecules21040519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamri P. A mini-review on phytochemistry and pharmacological activities of Scrophularia striata. J Herbmed Pharmacol. 2019;8(2):85–89. doi: 10.15171/jhp.2019.14 [DOI] [Google Scholar]
- 65.Ullah R, Alqahtani AS, Noman OMA, et al. A review on ethno-medicinal plants used in traditional medicine in the Kingdom of Saudi Arabia. Saudi J Biol Sci. 2020;27(10):2706. doi: 10.1016/j.sjbs.2020.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dash G, Mohanty KKGR, Sahoo D, et al. Traditional medicinal plants used for the treatment of asthma in Bhubaneswar, Odisha. Int J Herb Med. 2018;6(5):57–60. [Google Scholar]
- 67.Javadi B, Sahebkar A, Emami SA. Medicinal plants for the treatment of asthma: a traditional Persian medicine perspective. Curr Pharm Des. 2017;23(11):1623–1632. doi: 10.2174/1381612822666161021143332 [DOI] [PubMed] [Google Scholar]
- 68.Savithramma N, Sulochana C, Rao K. Ethnobotanical survey of plants used to treat asthma in Andhra Pradesh, India. J Ethnopharmacol. 2007;113(1):54–61. doi: 10.1016/j.jep.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 69.Younis A, Younis W, Asif H, et al. Traditional medicinal plants used for respiratory disorders in Pakistan: a review of the ethno-medicinal and pharmacological evidence. Chinese Medicine. 2018;13(1):48. doi: 10.1186/s13020-018-0204-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dulla O, Jahan FI. Ethnopharmacological survey on traditional medicinal plants at Kalaroa Upazila, Satkhira District, Khulna Division, Bangladesh. J Intercult Ethnopharmacol. 2017;6(3):316. doi: 10.5455/jice.20170719010256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Napagoda MT, Sundarapperuma T, Fonseka D, et al. An ethnobotanical study of the medicinal plants used as anti-inflammatory remedies in Gampaha District, Western Province, Sri Lanka. Scientifica. 2018;2018:1–8. doi: 10.1155/2018/9395052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panthong A, Kanjanapothi D, Taylor W. Ethnobotanical review of medicinal plants from Thai traditional books, Part I: plants with anti-inflammatory, anti-asthmatic and antihypertensive properties. J Ethnopharmacol. 1986;18(3):213–228. doi: 10.1016/0378-8741(86)90001-2 [DOI] [PubMed] [Google Scholar]
- 73.Gbekley HE, Katawa G, Karou SD, et al. Ethnobotanical study of plants used to treat asthma in the maritime region in Togo. Afr J Tradit Complement Altern Med. 2016;14(1):196–212. doi: 10.21010/ajtcam.v14i1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mali RG, Dhake AS. A review on herbal antiasthmatics. Orient Pharm Exp Med. 2011;11(2):77–90. doi: 10.1007/s13596-011-0019-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh BB, Khorsan R, Vinjamury SP, et al. Herbal treatments of asthma: a systematic review. J Asthma. 2007;44(9):685–698. doi: 10.1080/02770900701247202 [DOI] [PubMed] [Google Scholar]
- 76.Maema LP, Potgieter M, Mahlo SM. Invasive alien plant species used for the treatment of various diseases in Limpopo Province, South Africa. Afr J Tradit Complement Altern Med. 2016;13(4):223–231. doi: 10.21010/ajtcam.v13i4.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaladhar D. Traditional and Ayurvedic medicinal plants from India: practices and treatment for human diseases. LAP. 2012;176. [Google Scholar]
- 78.Eisenbrand G, Tang W. Handbook of Chinese Medicinal Plants: Chemistry, Pharmacology, Toxicology. John Wiley & Sons; 2010. [Google Scholar]
- 79.Wiart C. Medicinal Plants of China, Korea, and Japan: Bioresources for Tomorrow’s Drugs and Cosmetics. CRC press; 2012. [Google Scholar]
- 80.Khare C. World Healing Plants for Tomorrow. Daya Publishing House; 2018. [Google Scholar]
- 81.Kim H, Song M-J. Traditional plant-based therapies for respiratory diseases found in North Jeolla Province, Korea. J Altern Complement Med. 2012;18(3):287–293. doi: 10.1089/acm.2010.0848 [DOI] [PubMed] [Google Scholar]
- 82.Thakur VR, Khuman V, Beladiya JV, et al. An experimental model of asthma in rats using ovalbumin and lipopolysaccharide allergens. Heliyon. 2019;5(11):e02864. doi: 10.1016/j.heliyon.2019.e02864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao S, Jiang Y, Yang X, et al. Lipopolysaccharides promote a shift from Th2-derived airway eosinophilic inflammation to Th17-derived neutrophilic inflammation in an ovalbumin-sensitized murine asthma model. J Asthma. 2017;54(5):447–455. doi: 10.1080/02770903.2016.1223687 [DOI] [PubMed] [Google Scholar]
- 84.Lowe A, Thomas RS, Nials A, et al. LPS exacerbates functional and inflammatory responses to ovalbumin and decreases sensitivity to inhaled fluticasone propionate in a guinea pig model of asthma. Br J Pharmacol. 2015;172(10):2588–2603. doi: 10.1111/bph.13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clyne A, Yang AWH, Li M, et al. Traditional medicines for asthma in children and adults: a systematic review of placebo-controlled studies. Int J Clin Pract. 2019;73(12):e13433. doi: 10.1111/ijcp.13433 [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, Chen Y-J, Xiang C, et al. Discovery of potential asthma targets based on the clinical efficacy of Traditional Chinese Medicine formulas. J Ethnopharmacol. 2020;252:112635. doi: 10.1016/j.jep.2020.112635 [DOI] [PubMed] [Google Scholar]
- 87.Shergis JL, Wu L, Zhang AL, et al. Herbal medicine for adults with asthma: a systematic review. J Asthma. 2016;53(6):650–659. doi: 10.3109/02770903.2015.1101473 [DOI] [PubMed] [Google Scholar]
- 88.Zhou -D-D, Ran J, Li -C-C, et al. Metallothionein-2 is associated with the amelioration of asthmatic pulmonary function by acupuncture through protein phosphorylation. Biomed Pharmacother. 2020;123:109785. doi: 10.1016/j.biopha.2019.109785 [DOI] [PubMed] [Google Scholar]
- 89.Zhao X, Sun H, Hou A, et al. Antioxidant properties of two gallotannins isolated from the leaves of Pistacia weinmannifolia. Biochimica Et Biophysica Acta (BBA) - General Subjects. 2005;1725(1):103–110. doi: 10.1016/j.bbagen.2005.04.015 [DOI] [PubMed] [Google Scholar]
- 90.Chen S, Wu X, Ji Y, et al. Isolation and characterization of microsatellite loci in Pistacia weinmannifolia (Anacardiaceae). Int J Mol Sci. 2011;12(11):7818–7823. doi: 10.3390/ijms12117818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ci X, Chu X, Chen C, et al. Oxytetracycline attenuates allergic airway inflammation in mice via inhibition of the NF-κB pathway. J Clin Immunol. 2011;31(2):216–227. doi: 10.1007/s10875-010-9481-7 [DOI] [PubMed] [Google Scholar]
- 92.Javanmardi J, Khalighi A, Kashi A, et al. Chemical Characterization of Basil (Ocimum basilicum L.) Found in Local Accessions and Used in Traditional Medicines in Iran. J Agric Food Chem. 2002;50(21):5878–5883. doi: 10.1021/jf020487q [DOI] [PubMed] [Google Scholar]
- 93.Prakash P, Gupta N. Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review.. Indian J Physiol Pharmacol. 2005;49(2):125. [PubMed] [Google Scholar]
- 94.Do T. Vietnamese Medicinal Plants and Remedies. Hanoi. Medicine Publisher; 2000:828–830. [Google Scholar]
- 95.Asumeng Koffuor G, Boye A, Kyei S, et al. Anti-asthmatic property and possible mode of activity of an ethanol leaf extract of Polyscias fruticosa. Pharm Biol. 2016;54(8):1354–1363. doi: 10.3109/13880209.2015.1077465 [DOI] [PubMed] [Google Scholar]
- 96.Hanh TTH, Dang NH, Dat NT. α-Amylase and α -Glucosidase Inhibitory Saponins from Polyscias fruticosa Leaves. J Chem. 2016;2016:1–5. doi: 10.1155/2016/2082946 [DOI] [Google Scholar]
- 97.Falcão-Silva VS, Silva DA, Souza V, et al. Modulation of drug resistance in staphylococcus aureus by a kaempferol glycoside from Herissantia tiubae (Malvaceae). Phytother Res. 2009;23(10):1367–1370. doi: 10.1002/ptr.2695 [DOI] [PubMed] [Google Scholar]
- 98.Ghani A. Medicinal Plants of Bangladesh: Chemical Constituents and Uses. Vol. 2. Asiatic society of Bangladesh; 1998:81. [Google Scholar]
- 99.Parmar S, Gangwal A, Sheth N. Solanum xanthocarpum (yellow berried night shade): a review. Der Pharm Lett. 2010;2(4):373–383. [Google Scholar]
- 100.Rios J, Recio M, Giner R, et al. An update review of saffron and its active constituents. Phytother Res. 1996;10(3):189–193. doi: [DOI] [Google Scholar]
- 101.Maheshwari P, Singh U. Dictionary of Economic Plants in India. Dictionary of Economic Plants in India. New Delhi: Indian Council of Agricultural Research; 1965:38–39. [Google Scholar]
- 102.Warrier P, Nambiar V, Ramankutty C, et al. Indian Medicinal Plants. New Delhi: Orient Longman; 1995:341–343. [Google Scholar]
- 103.Kirtikar K, Basu B. Indian medicinal plants. Phytother Res. 1935;1607–1609. [Google Scholar]
- 104.Randall C, Randall H, Dobbs F, et al. Randomized controlled trial of nettle sting for treatment of base-of-thumb pain. J R Soc Med. 2000;93(6):305–309. doi: 10.1177/014107680009300607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chrubasik S, Enderlein W, Bauer R, et al. Evidence for antirheumatic effectiveness of Herba Urticae dioicae in acute arthritis: a pilot study. Phytomedicine. 1997;4(2):105–108. doi: 10.1016/S0944-7113(97)80052-9 [DOI] [PubMed] [Google Scholar]
- 106.Mittman P. Randomized, Double-Blind Study of Freeze-Dried Urtica dioica in the Treatment of Allergic Rhinitis. Planta Med. 1990;56(01):44–47. doi: 10.1055/s-2006-960881 [DOI] [PubMed] [Google Scholar]
- 107.Srivastava R. A review on phytochemical, pharmacological, and pharmacognostical profile of Wrightia tinctoria: adulterant of kurchi. Pharmacogn Rev. 2014;8(15):36. doi: 10.4103/0973-7847.125528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xiong D, Yu L-X, Yan X, et al. Effects of Root and Stem Extracts of Asparagus cochinchinensis on Biochemical Indicators Related to Aging in the Brain and Liver of Mice. Am J Chin Med. 2011;39(04):719–726. doi: 10.1142/S0192415X11009159 [DOI] [PubMed] [Google Scholar]
- 109.Roldán E, Sanchez-Moreno C, de Ancos B, et al. Characterisation of onion (Allium cepa L.) by-products as food ingredients with antioxidant and antibrowning properties. Food Chem. 2008;108(3):907–916. doi: 10.1016/j.foodchem.2007.11.058 [DOI] [PubMed] [Google Scholar]
- 110.Lee BK, Jung Y-S. Allium cepa extract and quercetin protect neuronal cells from oxidative stress via PKC-ε inactivation/ERK1/2 activation. Oxid Med Cell Longev. 2016;2016:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim AR, Ko HJ, Chowdhury MA, et al. Chemical constituents on the aerial parts of Artemisia selengensis and their IL-6 inhibitory activity. Arch Pharm Res. 2015;38(6):1059–1065. doi: 10.1007/s12272-014-0543-x [DOI] [PubMed] [Google Scholar]
- 112.Kim JK, Shin E-C, Lim H-J, et al. Characterization of nutritional composition, antioxidative capacity, and sensory attributes of Seomae mugwort, a native Korean variety of Artemisia argyi H. J Anal Methods Chem. 2015;1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yun C, Jung Y, Chun W, et al. Anti-Inflammatory Effects of Artemisia Leaf Extract in Mice with Contact Dermatitis In Vitro and In Vivo. Mediators Inflamm. 2016;2016:1–8. doi: 10.1155/2016/8027537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee NY, Chung K-S, Jin JS, et al. Effect of chicoric acid on mast cell-mediated allergic inflammation in vitro and in vivo. J Nat Prod. 2015;78(12):2956–2962. doi: 10.1021/acs.jnatprod.5b00668 [DOI] [PubMed] [Google Scholar]
- 115.Yao X, Wu D, Dong N, et al. Moracin C, A Phenolic Compound Isolated from Artocarpus heterophyllus, Suppresses Lipopolysaccharide-Activated Inflammatory Responses in Murine Raw264.7 Macrophages. Int J Mol Sci. 2016;17(8):1199. doi: 10.3390/ijms17081199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ahui MLB, Champy P, Ramadan A, et al. Ginger prevents Th2-mediated immune responses in a mouse model of airway inflammation. Int Immunopharmacol. 2008;8(12):1626–1632. doi: 10.1016/j.intimp.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 117.Townsend EA, Siviski ME, Zhang Y, et al. Effects of ginger and its constituents on airway smooth muscle relaxation and calcium regulation. Am J Respir Cell Mol Biol. 2013;48(2):157–163. doi: 10.1165/rcmb.2012-0231OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shin YJ, Jung DY, Ha HK, et al. Anticancer effect of Erythronium japonicum extract on ICR mouse and L1210 cells with alteration of antioxidant enzyme activities. Korean J Food Sci Technol. 2004;36:968–973. [Google Scholar]
- 119.Khalil MI, Ibrahim MM, El-Gaaly GA, et al. Trigonella foenum (Fenugreek) induced apoptosis in hepatocellular carcinoma cell line, HepG2, mediated by upregulation of p53 and proliferating cell nuclear antigen. Biomed Res Int. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bieski IGC, Rios Santos F, De Oliveira RM, et al. Ethnopharmacology of medicinal plants of the Pantanal region (Mato Grosso, Brazil). Evid Based Complement Altern Med. 2012;2012:1–36. doi: 10.1155/2012/272749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De la Cruz MG. Plantas Medicinais De Mato Grosso: A Farmacopéia Popular Dos Raizeiros. Plantas Medicinais De Mato Grosso: A Farmacopéia Popular Dos Raizeiros. Brazil: Carlini Caniato; 2008:224. [Google Scholar]
- 122.Messias MCTB, Menegatto M, Prado ACC, et al. Uso popular de plantas medicinais e perfil socioeconômico dos usuários: um estudo em área urbana em Ouro Preto, MG, Brasil. Revista Brasileira De Plantas Medicinais. 2015;17(1):76–104. doi: 10.1590/1983-084X/12_139 [DOI] [Google Scholar]
- 123.Hutchings A. Zulu Medicinal Plants: An Inventory. Pietermaritzburg: University of Natal press; 1996:64. [Google Scholar]
- 124.Steenkamp V. Traditional herbal remedies used by South African women for gynaecological complaints. J Ethnopharmacol. 2003;86(1):97–108. doi: 10.1016/S0378-8741(03)00053-9 [DOI] [PubMed] [Google Scholar]
- 125.Steenkamp V, Grimmer H, Semano M, et al. Antioxidant and genotoxic properties of South African herbal extracts. Mutat Res Genet Toxicol Environ Mutagen. 2005;581(1–2):35–42. doi: 10.1016/j.mrgentox.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 126.Zou H, Yi M-L, Xu K-P, et al. Two new flavonoids from Selaginella uncinata. J Asian Nat Prod Res. 2016;18(3):248–252. doi: 10.1080/10286020.2015.1063617 [DOI] [PubMed] [Google Scholar]
- 127.Zheng J-X, Zheng Y, Zhi H, et al. New 3′,8′′-Linked Biflavonoids from Selaginella uncinata Displaying Protective Effect against Anoxia. Molecules. 2011;16(8):6206–6214. doi: 10.3390/molecules16086206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen K-L, Li J, Lei X. Comparison of cytotoxic activities of extracts from Selaginella species. Pharmacogn Mag. 2014;10(40):529. doi: 10.4103/0973-1296.141794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheng BCY, Yu H, Su T, et al. A herbal formula comprising Rosae Multiflorae Fructus and Lonicerae Japonicae Flos inhibits the production of inflammatory mediators and the IRAK-1/TAK1 and TBK1/IRF3 pathways in RAW 264.7 and THP-1 cells. J Ethnopharmacol. 2015;174:195–199. doi: 10.1016/j.jep.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 130.Whistler WA. Tongan Herbal Medicine. Isle Botanica, Honolulu, Hawaii: University of Hawaii Press; 1992:89–90. [Google Scholar]
- 131.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35(1):57–68. doi: 10.1016/j.ctrv.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 132.Zhang Z, Lian X, Li S, et al. Characterization of chemical ingredients and anticonvulsant activity of American skullcap (Scutellaria lateriflora). Phytomedicine. 2009;16(5):485–493. doi: 10.1016/j.phymed.2008.07.011 [DOI] [PubMed] [Google Scholar]
- 133.Wang S, Ruan J, Zhuang J. Small Chunhua oral mouse model of chronic bronchitis and pathological forms of influence and expectorant effects. Fujian Chin Med. 2001;32:18–19. [Google Scholar]
- 134.Omosa L, Midiwo J, Kuete V. Curcuma Longa. Medicinal Spices and Vegetables from Africa. Elsevier; 2017:425–435. [Google Scholar]
- 135.Zhang M, Wang J, Zhu L, et al. Zanthoxylum bungeanum Maxim. (Rutaceae): a Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology. Int J Mol Sci. 2017;18(10):2172. doi: 10.3390/ijms18102172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rahman M, Alimuzzaman M, Ahmad S, et al. Antinociceptive and antidiarrhoeal activity of Zanthoxylum rhetsa. Fitoterapia. 2002;73(4):340–342. doi: 10.1016/S0367-326X(02)00083-7 [DOI] [PubMed] [Google Scholar]
- 137.Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6(10):761–771. doi: 10.1038/nri1934 [DOI] [PubMed] [Google Scholar]