Abstract
Objective
Infertility is inadequately acknowledged as a problem in developing countries. Thyroid Autoimmunity (TAI) has been neatly investigated for its association with unfavorable pregnancy and reproductive consequences. We aim to evaluate Clinical Pregnancy Rate (CPR) as a primary outcome following In Vitro Fertilization/Intra Cytoplasmic Sperm Injection (IVF/ICSI) in women with Thyroid Autoimmunity (TAI).
Methods
A Retrospective cohort study included 584 women who underwent IVF/ICSI treatment between November 2012 and April 2017 in Orient Hospital, Damascus, Syria. Patients were tested for TAI before IVF/ICSI procedure.
Results
CPR did not significantly differ between TAI positive and TAI negative groups (p > 0.05). Subgroup analysis for only primary infertility patients showed a statistically significant difference in CPR between TAI positive and TAI negative groups.
Conclusion
Although several arguments were in favor of the relation between IVF/ICSI outcomes and Thyroid autoimmune disease, the presence of TAI positivity did not adversely affect the clinical pregnancy rate.
Keywords: In vitro fertilization (IVF), Intra cytoplasmic sperm injection (ICSI), Thyroid autoimmunity (TAI), Clinical pregnancy rate (CPR), Syria
Highlights
-
•
Thyroid Autoimmunity has been investigated for its association with unfavorable pregnancy and reproductive consequences.
-
•
The prevalence of TAI in Syrian women having IVF/ICSI is 25.3%.
-
•
The positivity of Thyroid Autoimmunity did not adversely affect the clinical pregnancy rate for women underwent IVF/ICSI.
-
•
Screening of Thyroid Autoimmunity constitutes a financial burden on patients more than its benefit.
-
•
The findings highlight the infertility aspects of the Syrian population.
1. Background
Infertility is inadequately acknowledged as a problem in developing countries [1]. Since it was first introduced in 1978, in vitro fertilization-embryo transfer (IVF-ET) still is the most commonly used technique of assisted reproduction with increasing success rates [2]. Thyroid Autoimmunity (TAI) has been neatly investigated for its association with unfavorable consequences for pregnancy in all trimesters such as implantation failure, miscarriage, placental abruption, preterm birth, and increased risk of perinatal mortality [3,4].
Previous studies showed that 5–20% of women of childbearing age are affected by Thyroid Autoimmunity (TAI) which is characterized by the presence of anti-thyroid peroxidase (anti-TPO) and/or anti-thyroglobulin (anti-TG) antibodies [5]. Thyroglobulin (TG) is a molecule synthesized by thyroid cells to produce and store thyroid hormones. Thyroid peroxidase (TPO) is an enzyme involved in the production of thyroid hormones by iodination of surfactant protein A tyrosine residues [6].
TAI diseases are often underdiagnosed because they may be present without overt thyroid dysfunction for several years [7,8]. Accordingly, TAI is more prevalent in women attending fertility clinics compared to the general population [9,10]. However, the relationship between the presence of TAI and infertility continues to be broadly speculative [11]. A previous study investigated the impact of anti-TPO on reproductive biology and concluded that positive anti-TPO adversely affects folliculogenesis, spermatogenesis, fertilization rates (FRs), embryo quality, and pregnancy rates [12]. Moreover, when compared to controls, pregnancy outcomes in women with TAI subjected to In Vitro Fertilization (IVF) were lower [13].
In this study, we aim to evaluate the impact of TAI on IVF/ICSI outcomes such as Clinical Pregnancy Rate (CPR) as a primary outcome. To the best of our knowledge, this is the first study in Syria to investigate TAI in women undergoing IVF/ICSI.
2. Methods
This was a retrospective cohort study carried out using the computerized database at Assisted Reproduction Unit, Orient Hospital, Damascus, Syria. Data were manually checked for any mistakes in thoroughness and accuracy before initiating the study. Orient Hospital is a professional unit affiliated to the Faculty of Medicine, Damascus University, and an approved training facility for Assisted Reproduction Technology (ART) by the Ministry of Health in Syria. Ethical approval was obtained from the ethical and research committees in both Faculty of Medicine, Damascus University and Syrian Private University with the approval of Orient Hospital Board of Directors. The study was conducted in compliance with the STROCSS criteria [14]. Our work was submitted with a research Registry UIN: “researchregistry6786”.
2.1. Patients
All women undergoing IVF/ICSI cycles between November 2012 and April 2017 were tested for thyroid function, including Anti Thyroid Peroxidase Antibodies (anti-TPO), Anti Thyroglobulin Antibodies (anti-TG), Thyroid Stimulating Hormone (TSH), all of which were requested by the referring physician before ovarian stimulation.
2.2. IVF procedure
Women were treated either with the long Gonadotropin-releasing hormone (GnRH) agonist protocol or GnRH antagonist protocol for pituitary downregulation. The details for both protocols were previously described [15,16]. They received either (human menopausal gonadotropin) HMG or recombinant follicular stimulating hormone (rFSH) for ovarian stimulation following the menstrual period with dosage adjustment according to the response.
Transvaginal ultrasonography along with measurement of blood estradiol was used to assess the ovarian response. When 3 leading follicles reached 17–18 mm, we administered 10,000 IU of Human chorionic gonadotropin (HCG), and oocytes retrieval was done 35–36 h later. Fertilization was carried out in vitro by ICSI. In the cleavage stage (Day 3), at most three embryos were transferred with ultrasound guidance. Luteal phase support was achieved with vaginal micronized progesterone. Correspondingly, clinical pregnancies were confirmed by positive urine HCG tests and transvaginal ultrasonographic evidence of a gestational sac.
2.3. Collection of clinical information
Clinical information that was collected included age, place of residence, assessment for infertility type (primary or secondary), duration of infertility [17], history of undergoing assisted reproduction, and IVF indications. In addition, the data included tests recorded before ovarian stimulation in IVF/ICSI cycle such as Basal Follicular Stimulating Hormone (Basal FSH), Day 2 Estradiol (E2) and Anti Müllerian Hormone (AMH).
During the IVF treatment, data recorded included stimulation protocol, stimulation drug, sperm collection procedure, endometrium thickness, number of oocytes retrieved, M − II oocytes, fertilization rate, number of transferred embryos, and pregnancy rate.
2.4. Determination of antibodies and hormone levels
All tests were done in the same hospital lab. Lab Tests were primarily measured on the instrument Cobas 6000 (Roche Diagnostics). The instrument is a closed system, random access auto-analyzer. Performance characteristics of measurement are previously explained [18]. Particularly, two lots of calibrators were used in each instrument, four lots of reagents for Cobas were used during the procedures. Method calibrations were done on the instrument roughly once every month for the change of lots. Two levels of 3rd party commercial control materials were run for every working day.
Lab tests cutoff values were as follows: TPO antibodies ≥35 IU/ml, TG antibodies ≥40 IU/ml, TSH (0.45–4.5) uIU/ml, Basal FSH ≥ 12.5 mIU/ml, Day 2 Estradiol (E2) ≥ 50 pg/ml and AMH ≥ 1.5 ng/ml.
2.5. Data analysis
Only women with normal TSH levels were included in the study (0.45–4.5 μIU/ml). The primary outcome measure was defined as the Clinical Pregnancy Rate. Thyroid autoimmunity status was considered positive in the presence of anti-TPO and/or anti-TG higher than the upper limit of the reference range.
Subgroup analysis was done for a stricter TSH threshold of <2.5 [19,20], for prognosis related to age (Blue for less than 40 years and Red for 40 years and higher), low and normal AMH levels as an indicator for ovarian reserve, in addition to normal and poor ovarian reserve according to FSH (considering Day 2 Estradiol levels). The pregnancy rate was calculated as per completed embryo transfer.
Statistical analysis was done using the Statistical Program for Social Sciences (Version 25; SPSS Inc., Chicago, IL, USA). Mann–Whitney U test, Kruskal–Wallis H test, chi-squared test, and Fisher's exact test were used as appropriate. The cutoff value of p < 0.05 was considered statistically significant.
3. Results
A total of 584 patients who underwent 954 IVF/ICSI cycles were included in the study.
The prevalence of positive TAI was 148 patients (25.3%) on their first visit to our center. The mean age was 34.25 ± 5.601 years (mean ± SD) for TAI positive patients compared with 33.8 ± 6.143 years for the TAI Negative. Sixty four percent (n = 374) of patients were residents of Damascus Metropolitan area or Rural Damascus governorate, 25.0% (n = 146) lived in all other governorates of Syria and 11.0% (n = 64) lived abroad (mostly in Iraq). There was no significant difference in TAI positivity between places of residence. 32.9% of our patients were older than 40 years of age at the start of the cycle. No statistical difference was observed in TAI positivity between both groups of age. Demographic data are summarized in Table 1.
Table 1.
TAI prevalence according to patients' demographics and cycles characteristics.
| TAI Positive | Total (% of cycles) | p Value | |||
|---|---|---|---|---|---|
| Patients' No.* | 148 | 25.3% | 584 | ||
| Age* | 34.25 | ±5.601 | 34.19 | ±6.007 | NS |
| Place of residence*, n (%) | |||||
| Damascus and Rif Dimashq | 91 | 24.3% | 374 | 64.0% | NS |
| All other Syrian governorates | 40 | 27.4% | 146 | 25.0% | |
| Outside Syria | 17 | 26.6% | 64 | 11.0% | |
| IVF Cycles No. | 250 | 26.2% | 954 | ||
| Age group | NS | ||||
| Blue < 40 | 156 | 26.2% | 595 | 62.4% | |
| Red > 40 | 94 | 26.2% | 359 | 37.6% | |
| Infertility Type | NS | ||||
| Primary | 218 | 25.9% | 841 | 88.2% | |
| Secondary | 32 | 28.3% | 113 | 11.8% | |
| Infertility Duration (years) | NS | ||||
| 0 - 4 | 78 | 27.9% | 280 | 29.4% | |
| 5 - 9 | 94 | 25.7% | 366 | 38.4% | |
| 10 - 14 | 49 | 28.8% | 170 | 17.8% | |
| > 15 | 29 | 21.0% | 138 | 14.5% | |
| IVF Indication | p < 0.05 | ||||
| Male Factor | 90 | 23.2% | 388 | 40.7% | |
| Female Factor | 107 | 26.4% | 405 | 42.5% | |
| Combined | 31 | 40.3% | 77 | 8.1% | |
| Unexplained | 4 | 18.2% | 22 | 2.3% | |
| PGD | 18 | 29.0% | 62 | 6.5% | |
| IVF History | NS | ||||
| < 2 | 157 | 30.3% | 518 | 54.3% | |
| 2 - 3 | 65 | 21.0% | 309 | 32.4% | |
| 4 - 5 | 22 | 24.4% | 90 | 9.4% | |
| ≥ 6 | 6 | 16.2% | 37 | 3.9% | |
NS: non-significant.
Regarding IVF/ICSI procedures, 250 patients (26.2%) tested positive (per cycle) for TAI prior to IVF/ICSI cycle initiation. The sample was divided according to IVF/ICSI indication into 405 cycles (42.5%) for female factor infertility, 40.7% (n = 388) for male factors, 8.1% (n = 77) for combined male and female infertility causes, and 2.3% (n = 22) referred for unexplained infertility (Table 1).
Patients’ infertility duration at cycle initiation was between 0 and 4 years for 290 cycles (29.4%), 5–9 years for 366 (38.4%), 10–14 years for 170 (17.8%) and >15 years for 138 cycles (14.5%). In addition, 54.3% (n = 518) had a history of having only one or no prior IVF/ICSI cycles, 32.4% (n = 309) underwent 2 to 3 cycles, and 13.3% (n = 127) underwent 4 or more cycles (Table 1).
TAI positivity was significantly more prevalent in women undergoing IVF/ICSI for combined factors compared with other indications (40.3%, p < 0.05), no statistical difference in TAI status was found when comparing cycles concerning age group prognosis, infertility type, infertility duration, and history of IVF/ICSI procedures (Table 1).
Most of the cycles were carried out using long protocol IVF/ICSI method 84.5% (n = 799). There was no significant difference in TAI status regarding stimulation protocol or stimulation drug.
The response to ovarian stimulation in terms of endometrium thickness at the day of oocyte trigger, the numbers of oocytes retrieved, M − II oocytes, fertilization rate, and the number of transferred embryos were not statistically different according to TAI status (Table 2). In addition, similar results were found when subgrouping the data to TSH <2.5 only (Table 2).
Table 2.
Initial IVF outcomes according to TAI positivity in Low and High Normal TSH subgroups.
| TSH <2.5 |
TSH <4.5 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAI Positive | Total (%) | p value | TAI Positive | Total (% of cycles) | p value | |||||
| Endometrium (n = 909) | 9.77 | ±5.60 | 9.67 | ±5.57 | NS | 9.47 | ±4.68 | 9.70 | ±6.05 | NS |
| Oocytes Retrieved (n = 930) | NS | NS | ||||||||
| 0 - 4 | 42 | 24.3% | 173 | 26.9% | 60 | 26.4% | 227 | 24.4% | ||
| 5 - 9 | 56 | 27.1% | 207 | 32.2% | 79 | 27.8% | 284 | 30.5% | ||
| ≥ 10 | 64 | 24.3% | 263 | 40.9% | 107 | 25.5% | 419 | 45.1% | ||
| MII (n = 907) | NS | NS | ||||||||
| 0–25% | 6 | 37.5% | 16 | 2.5% | 12 | 41.4% | 29 | 3.2% | ||
| 25%–50% | 15 | 22.1% | 68 | 10.8% | 23 | 22.1% | 104 | 11.5% | ||
| 50%–75% | 88 | 28.8% | 306 | 48.7% | 133 | 28.5% | 466 | 51.4% | ||
| 75%–100% | 47 | 20.0% | 235 | 37.4% | 70 | 22.7% | 308 | 34.0% | ||
| Fertilization Rate (n = 865) | NS | NS | ||||||||
| 0–25% | 30 | 23.6% | 127 | 21.2% | 51 | 27.6% | 185 | 21.4% | ||
| 25%–50% | 57 | 25.3% | 225 | 37.6% | 88 | 25.0% | 352 | 40.7% | ||
| 50%–75% | 46 | 27.5% | 167 | 27.9% | 69 | 29.5% | 234 | 27.1% | ||
| 75%–100% | 15 | 18.8% | 80 | 13.4% | 17 | 18.1% | 94 | 10.9% | ||
| Transferred embryos (n = 866) | NS | NS | ||||||||
| 0 | 9 | 32.1% | 28 | 4.7% | 12 | 34.3% | 35 | 4.0% | ||
| 1 | 22 | 27.5% | 80 | 13.3% | 37 | 32.7% | 113 | 13.0% | ||
| 2 | 20 | 17.7% | 113 | 18.8% | 29 | 19.0% | 153 | 17.7% | ||
| 3 | 19 | 19.8% | 96 | 16.0% | 31 | 22.5% | 138 | 15.9% | ||
| ≥ 4 | 78 | 27.6% | 283 | 47.2% | 116 | 27.2% | 427 | 49.3% | ||
NS: non-significant.
Regarding adjuvant drugs taken during IVF/ICSI procedure, prednisolone did not affect pregnancy rate in both TAI positive and TAI negative groups (p > 0.05 for both subgroups).
TAI status according to lab tests before IVF/ICSI procedure: TAI status did not statistically differ when comparing different ovarian reserves observed according to basal FSH and AMH. Similar results were found for the TSH <2.5 and the blue age subgroup (Table 3).
Table 3.
TAI positivity in Low and High Normal TSH subgroups according to pre-cycle tests.
| TSH <2.5 |
TSH <4.5 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAI Positive | Total (% of cycles) | p Value | TAI Positive | Total (% of cycles) | p Value | |||||
| Basal FSH and E2 (n = 719) | NS | NS | ||||||||
| Normal | 78 | 24.7% | 316 | 64.4% | NS | 122 | 26.6% | 459 | 63.8% | NS |
| Poor Ovarian Reserve | 9 | 16.4% | 55 | 11.2% | 20 | 27.0% | 74 | 10.3% | ||
| Negative Feedback | 32 | 26.7% | 120 | 24.4% | 41 | 22.0% | 186 | 25.9% | ||
| AMH (n = 580) | NS | NS | ||||||||
| Low | 51 | 23.8% | 214 | 54.2% | 78 | 25.7% | 304 | 52.4% | ||
| Normal | 51 | 28.2% | 181 | 45.8% | 85 | 30.8% | 276 | 47.6% | ||
NS: non-significant.
3.1. Main outcomes
The pregnancy rate did not differ statistically between TAI positive and TAI negative groups. Also, no statistical difference was found regarding pregnancy rate when including only male factor infertility and PGD cases together as an IVF/ICSI indication (Table 4).
Table 4.
Clinical pregnancy rate among TAI positive and negative cycles in different subgroups.
| Pregnant | Total (% of cycles) | Cancelled | p Value | ||||
|---|---|---|---|---|---|---|---|
| All Cycles | NS | ||||||
| TAI Negative | 239 | 38.2% | 625 | 74.4% | 72 | 10.3% | |
| TAI Positive | 97 | 45.1% | 215 | 25.6% | 32 | 13.0% | |
| TSH > 2.5 | NS | ||||||
| TAI Negative | 170 | 39.0% | 436 | 75.7% | 55 | 11.2% | |
| TAI Positive | 67 | 47.9% | 140 | 24.3% | 22 | 13.6% | |
| TSH: 2.5–4.5 | NS | ||||||
| TAI Negative | 69 | 36.5% | 189 | 71.6% | 17 | 8.3% | |
| TAI Positive | 30 | 40.0% | 75 | 28.4% | 10 | 11.8% | |
| Male Factor and PGD | NS | ||||||
| TAI Negative | 111 | 37.4% | 297 | 66.9% | 40 | 11.9% | |
| TAI Positive | 45 | 45.9% | 98 | 22.1% | 9 | 8.4% | |
| Primary Infertility | p < 0.05 | ||||||
| TAI Negative | 206 | 37.3% | 552 | 66.3% | 64 | 10.4% | |
| TAI Positive | 89 | 47.1% | 189 | 22.7% | 27 | 12.5% | |
| IVF History < 2 | NS | ||||||
| TAI Negative | 147 | 43.6% | 337 | 65.4% | 22 | 6.1% | |
| TAI Positive | 67 | 45.9% | 146 | 28.3% | 10 | 6.4% | |
| Blue Age | NS | ||||||
| TAI Negative | 174 | 42.6% | 408 | 74.0% | 27 | 6.2% | |
| TAI Positive | 67 | 46.9% | 143 | 26.0% | 11 | 7.1% | |
| Red Age | NS | ||||||
| TAI Negative | 65 | 30.0% | 217 | 75.1% | 45 | 17.2% | |
| TAI Positive | 30 | 41.7% | 72 | 24.9% | 21 | 22.6% | |
| Normal AMH | NS | ||||||
| TAI Negative | 77 | 43.8% | 176 | 70.4% | 15 | 7.9% | |
| TAI Positive | 30 | 40.5% | 74 | 29.6% | 11 | 12.9% | |
| Low AMH | NS | ||||||
| TAI Negative | 49 | 26.9% | 182 | 74.6% | 43 | 19.1% | |
| TAI Positive | 23 | 37.1% | 62 | 25.4% | 16 | 20.5% | |
| Normal Ovarian Reserve | NS | ||||||
| TAI Negative | 110 | 36.3% | 303 | 74.3% | 29 | 8.7% | |
| TAI Positive | 42 | 40.0% | 105 | 25.7% | 15 | 12.5% | |
| Poor Ovarian Reserve | p < 0.05 | ||||||
| TAI Negative | 10 | 24.4% | 41 | 78.8% | 13 | 24.1% | |
| TAI Positive | 7 | 63.6% | 11 | 21.2% | 9 | 45.0% | |
NS: non-significant.
In addition, there was not any significant correlation between TAI status and pregnancy rate with respect to cases with a history of cycles of less than two. On the other hand, TAI significantly affected the pregnancy rate in only primary infertility cases (p < 0.05) (Table 4).
Furthermore, the pregnancy rate was also not significantly affected by TAI status when comparing TAI positive and TAI negative cases in the subgroups of TSH <2.5, TSH 2.5–4.5, blue and red Age, normal and low AMH and ovarian reserve regarding FSH. Meanwhile, it only affected the pregnancy rate in the poor ovarian reserve subgroup (p < 0.05) (Table 4).
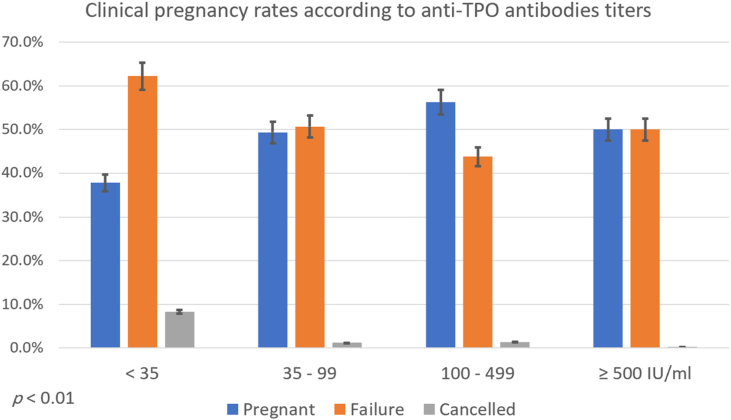
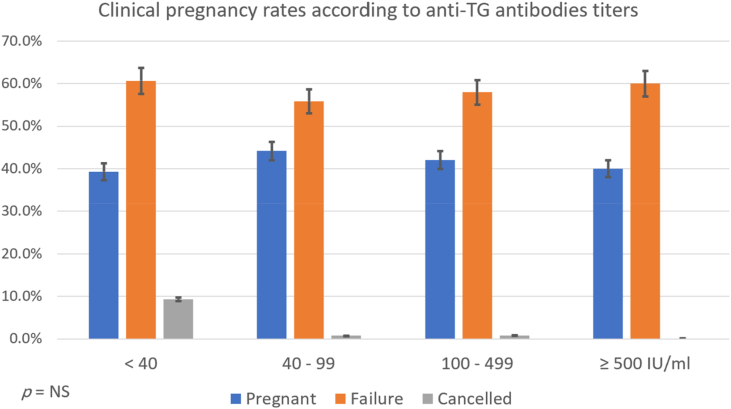
Finally, clinical pregnancy rate differed significantly between different Anti-TPO antibodies titers subgroups (p < 0.05), but not with anti-TG antibodies titers subgroups (Fig. 1 and Fig. 2).
Fig. 1.
Clinical pregnancy rates according to anti-TPO antibodies titers.
Fig. 2.
Clinical pregnancy rates according to anti-TG antibodies titers.
4. Discussion
About 18–20% of couples of reproductive age have fertility problems. Infertility is defined as not being able to conceive after a year of unprotected intercourse [21,22].
Thyroid hormones interfere with numerous aspects of reproduction. Normal ovarian function and pregnancy outcomes are adversely affected by hypothyroidism and hyperthyroidism [23,24]. Since the early 1990s, many studies about the influence of thyroid autoantibodies on recurrent miscarriages and infertility in euthyroid women have been published [23,24]. They concluded that recurrent abortions and failure to conceive are associated with increased positivity of thyroid autoantibodies [9,25,26]. Additionally, recent systematic reviews emphasized this association in the presence of TAI and the increased number of miscarriages among subfertile women who achieved pregnancy through IVF [27].
This study is an assessment of a large cohort of patients in our region, most baseline characteristics of both groups were highly similar and thorough subanalysis was done, which in turn helped in excluding confounding factors affecting the outcome of interest.
Studies found an increased prevalence of TAI in women referred to fertility clinics compared with the normal population [9,28], previous study by Aljarad et al. in the Syrian population showed the same result with a prevalence of 22.1% [10]. In our study, we found that the prevalence of TAI positivity in our sample was 25.3% in patients undergoing IVF/ICSI at our center. Chai et al. reported that it was 19.7% among women who underwent their first ART in Hong Kong [5] and Sakar et al. reported TAI positivity of 19.52% in Turkey [6]. Such differences may be arising due to heterogeneity of women samples studied, differences due to small sample sizes, differences due to the assays used to detect TAI, and finally differences arising from ethnicity as well as geographical locations, and the role of the highly variable iodine nutritional intake in the populations investigated [8].
Whether to screen or not for thyroid function and TAI regarding fertility care remains debatable [20] and cost-effectiveness of tests remains a crucial factor for low-income countries.
Despite the difference in the prevalence of TAI in our study population, the pregnancy rate did not statistically differ between TAI positive and TAI negative groups. A previous meta-analysis showed the same result [29]. In addition, similar to a previous study [5], a similar result was found in cases with a history of cycles of less than two.
Subgroup analysis of the outcome of clinical pregnancy rates when using a stricter threshold of TSH of 2.5 mIU/L also showed no difference to using an upper limit of 4.5 mIU/L (Table 4), this was also similar to the analysis done in the Unuane et al. paper on cumulative delivery rates [20].
Moreover, to exclude factors induced by female infertility factor as an indication for IVF/ICSI, we conducted the analysis including only male factor and PGD together as an IVF/ICSI indication. The result was also similar with no significant pregnancy outcome difference between TAI positive and negative groups, similar to a previous study published by Tan et al., 2018 [30]. On the other hand, we found that TAI affected pregnancy rates when including only primary infertility cases (p < 0.05). The absence of a significant difference in secondary infertility cases may be due to an additional onset of a secondary disease as the primary etiology. In addition, immune causes may be more prevalent in primary infertility cases [31].
In the presented study, the presence of TAI did not affect the fertilization rate (Table 2) similar to a previous study by Kutteh et al. [32], but contrary to Zhong et al. [13].
Clinical pregnancy rate did not differ statistically between blue and red age subgroups regardless of TAI status. Whereas a study on cumulative delivery rates by Unuane et al. [20]. Showed the same result for different age categories. Although 32.9% of our studied women were more than 40 years of age compared to approximately only 15% in the Unuane et al. [20]. paper.
Pregnant women using prednisolone supplementation during IVF/ICSI procedure were found to have a significantly higher TAI (69.8%, n = 67, p < 0.05). Although in our study this supplementation did not affect pregnancy rates in both TAI groups, a previous study's findings were contrary to ours regarding prednisolone supplementation. There was also a strong association between the presence of thyroid autoantibodies and poor IVF outcomes [33]. More studies showed pregnancy rates were significantly improved in women undergoing IVF with the conjugated use of glucocorticoids [[34], [35], [36]]. Though these findings are applicable to the everyday use of glucocorticoids, the same might not be true for women with autoantibodies [[35], [36], [37]].
4.1. Study limitations
We could not include clinical, subclinical hypothyroidism, or central hypothyroidism patients as our data did not include T3, Free T3, T4 or Free T4 Laboratory tests, which were already studied previously. Further studies are needed to include other immunological antibodies such as anti-thyroid stimulating hormone receptors to evaluate their effect on assisted reproductive techniques. Additionally, we could not document long follow up for our studied population in order to detect the effect of TAI positivity status on live birth rate and fetus wellbeing. The benefits of thyroxine supplementation should also be studied in our region-specific population.
Statistical analysis in our large cohort retrospective study controlled most variables possible for the determination of the main outcome. Other small case-control prospective studies resulted in the same outcome [6,38], while others found contrary results [33]. Thus, further large multi-center well-controlled prospective studies are needed to deeply evaluate this outcome.
5. Conclusion
The prevalence of TAI in Syrian women having IVF/ICSI is 25.3% which is higher than the normal population. Although several arguments were in favor of the correlation between IVF/ICSI outcomes and Thyroid autoimmune diseases, we concluded that the presence of TAI positivity did not adversely affect the Clinical Pregnancy Rate (CPR), which is the most relevant concern for women undergoing IVF treatment and their physicians. Screening of Thyroid Autoimmunity constitutes a financial burden on patients more than its benefit which is consistent with many previous studies.
Ethics approval and consent to participate
Ethical approval was obtained from the Ethical and Research Committee in both Faculty of Medicine, Damascus University and Syrian Private University with the approval of Orient Hospital Board of Directors.
Consent for publication
Not applicable.
Competing interests
None.
Data availability
The study data are available from the corresponding author upon reasonable request.
Funding
None.
Authors' contributions
All authors contributed to the study concept and design. MA clinically approached the patients. NA, AH, and NN retrospectively checked all patients’ files for the completeness and accuracy of the computerized data before initiating the study. NA and FA conducted the statistical analysis. NA, AH and NN drafted the initial manuscript. MA, HA and NI revised the statistical analysis and the manuscript for scientific accuracy. All authors revised the final version of the manuscript and approved it for publication.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Registration of research studies
-
1.
Name of the registry: Impact of Thyroid Autoimmunity in Euthyroid Women on the Outcomes of In Vitro Fertilization
-
2.
Unique Identifying number or registration ID: researchregistry6786
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.researchregistry.com/register-now#home/registrationdetails/608d54193da41d001dd343fd/
Guarantor
Ahed Hamed, Nawras Alhalabi.
Acknowledgement
We would like to express our sincere thanks and appreciation to the dean of Faculty of Medicine of Syrian Private University; Prof Nizar AlDhaher, Head of Alkhatib Lab; Dr Adnan Alkhatib; Dr Mohammad Marwan Alhalabi, Dr Mohammad Aljarad, in addition to the Faculty of Medicine of Syrian Private University Undergraduate Research Group and We Research Team for their endless encouragement and scientific motivation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102473.
Contributor Information
Ahed Hamad, Email: ahedhamed278@gmail.com.
Nawras Alhalabi, Email: nawras@me.com, http://www.nawrasalhalabi.com.
Nazht Nmr, Email: NazhtNmr@outlook.com.
Fatima Abbas, Email: Fatima.abbas174@gmail.com.
Hisham Al-Hammami, Email: profhishamalhammami@outlook.com.
Nazir Ibrahim, Email: profnaziribraim@gmail.com.
Marwan Alhalabi, Email: Profalhalabi@icloud.com.
List of Abbreviations
- AMH
Anti Müllerian Hormone
- Anti-TG
Anti Thyroglobulin antibodies
- Anti-TPO
Anti Thyroid Peroxidase antibodies
- ART
Assisted Reproduction Techniques
- Basal FSH
Basal Follicular Stimulating Hormone
- CPR
Clinical Pregnancy Rate
- E2
Day 2 Estradiol
- ECL
Electrochemiluminescence
- ET
Embryo transfer
- FRs
Fertilization Rates
- GnRH
Gonadotropin-Releasing Hormone
- HCG
Human chorionic gonadotropin
- HMG
Human Menopausal Gonadotropin
- IVF/ICSI
Vitro Fertilization/Intra Cytoplasmic Sperm Injection
- IVF
In Vitro Fertilization
- IVF-ET
in Vitro fertilization-embryo transfer
- PGD
Pre-implantation Genetic Diagnosis
- rFSH
Recombinant Follicular Stimulating Hormone
- SD
Standard Deviation
- T3
Triiodothyronine
- T4
Thyroxine
- TAI
Thyroid Autoimmunity
- TESA
Testicular/Epididymal Sperm Aspiration
- TG
Thyroglobulin
- TPA
Tripropylamine
- TPO
Thyroid peroxidase
- TSH
Thyroid Stimulating Hormone
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bergström S. Reproductive failure as a health priority in the Third World: a review. East Afr. Med. J. 1992;69(4):174–180. [PubMed] [Google Scholar]
- 2.Steptoe P.C., Edwards R.G. Birth after reimplantation of a human embryo. Lancet. 1978;2(8085):366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 3.Alexander E.K., Pearce E.N., Brent G.A., Brown R.S., Chen H., Dosiou C. Guidelines of the American thyroid association for the Diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315–389. doi: 10.1089/thy.2016.0457. 2017. PubMed PMID: 28056690. [DOI] [PubMed] [Google Scholar]
- 4.Casey B.M., Dashe J.S., Wells C.E., McIntire D.D., Byrd W., Leveno K.J. Subclinical hypothyroidism and pregnancy outcomes. Obstet. Gynecol. 2005;105(2):239–245. doi: 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- 5.Chai J., Yeung W.Y.T., Lee C.Y.V., Li H.W.R., Ho P.C., Ng H.Y.E. Live birth rates following in vitro fertilization in women with thyroid autoimmunity and/or subclinical hypothyroidism. Clin. Endocrinol. 2014;80(1):122–127. doi: 10.1111/cen.12220. [DOI] [PubMed] [Google Scholar]
- 6.Sakar M., Unal A., Atay A., Zebitay A., Verit F., Demir S. Is there an effect of thyroid autoimmunity on the outcomes of assisted reproduction? J. Obstet. Gynaecol. 2016;36(2):213–217. doi: 10.3109/01443615.2015.1049253. [DOI] [PubMed] [Google Scholar]
- 7.Poppe K., Velkeniers B., Glinoer D. Thyroid disease and female reproduction. Clin. Endocrinol. 2007;66(3):309–321. doi: 10.1111/j.1365-2265.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 8.Poppe K., Glinoer D., Tournaye H., Devroey P., Schiettecatte J., Haentjens P. Thyroid autoimmunity and female infertility. Verh. - K. Acad. Geneeskd. Belg. 2006;68(5–6):357–377. [PubMed] [Google Scholar]
- 9.Kaprara A., Krassas G.E. Thyroid autoimmunity and miscarriage. Hormones (Basel) 2008;7(4):294–302. doi: 10.14310/horm.2002.1210. [DOI] [PubMed] [Google Scholar]
- 10.Aljarad M., Alhalabi N., Hamad A., Nmr N., Abbas F., Alkhatib A. Prevalence of thyroid autoimmune antibodies in women seeking fertility care in Damascus. Syria. 2019;11(8) doi: 10.7759/cureus.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krassas G., Poppe K., Glinoer D. Thyroid function and human reproductive health. Endocr. Rev. 2010;31(5):702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 12.Vissenberg R., Manders V., Mastenbroek S., Fliers E., Afink G., Ris-Stalpers C. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum. Reprod. Update. 2015;21(3):378–387. doi: 10.1093/humupd/dmv004. [DOI] [PubMed] [Google Scholar]
- 13.Zhong Y-p, Ying Y., Wu H-t, Zhou C-q, Xu Y-w, Wang Q. Relationship between antithyroid antibody and pregnancy outcome following in vitro fertilization and embryo transfer. Int. J. Med. Sci. 2012;9(2):121. doi: 10.7150/ijms.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agha R., Abdall-Razak A., Crossley E., Dowlut N., Iosifidis C., Mathew G., for the STROCSS Group The STROCSS 2019 guideline: strengthening the reporting of cohort studies in Surgery. Int. J. Surg. 2019;72:156–165. doi: 10.1016/j.ijsu.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Yu Ng E.H., Yeung W.S.B., Yee Lan Lau E., So W.W.K., Ho P.C.J.H.R. High serum oestradiol concentrations in fresh IVF cycles do not impair implantation and pregnancy rates in subsequent frozen–thawed embryo transfer cycles. 2000;15(2):250–255. doi: 10.1093/humrep/15.2.250. [DOI] [PubMed] [Google Scholar]
- 16.Blockeel C., Engels S., De Vos M., Haentjens P., Polyzos N.P., Stoop D. Oestradiol valerate pretreatment in GnRH-antagonist cycles: a randomized controlled trial. 2012;24(3):272–280. doi: 10.1016/j.rbmo.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Sexual and Reproductive Health. WHO; 2018. https://www.who.int/reproductivehealth/topics/infertility/definitions/en/ [cited 2018 10 December]. Available from. [Google Scholar]
- 18.Sarkar R. TSH comparison between chemiluminescence (Architect) and electrochemiluminescence (Cobas) immunoassays: an Indian population perspective. Indian J. Clin. Biochem. 2014;29(2):189–195. doi: 10.1007/s12291-013-0339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C., Shan Z., Mao J., Wang W., Xie X., Zhou W. Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J. Clin. Endocrinol. Metab. 2014;99(1):73–79. doi: 10.1210/jc.2013-1674. [DOI] [PubMed] [Google Scholar]
- 20.Unuane D., Velkeniers B., Deridder S., Bravenboer B., Tournaye H., De Brucker M.J.A.C.B. Impact of thyroid autoimmunity on cumulative delivery rates in IVF/ICSI patients. 2016;71(S2):6. doi: 10.1016/j.fertnstert.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Hoxsey R., Rinehart J.S. Infertility and subsequent pregnancy. Clin. Perinatol. 1997;24(2):321–342. [PubMed] [Google Scholar]
- 22.Healy D.L., Trounson A.O., Andersen A.N. Female infertility: causes and treatment. Lancet. 1994;343(8912):1539–1544. doi: 10.1016/s0140-6736(94)92941-6. [DOI] [PubMed] [Google Scholar]
- 23.Choudhury S.R., Knapp L.A. Human reproductive failure I: immunological factors. Hum. Reprod. Update. 2001;7(2):113–134. doi: 10.1093/humupd/7.2.113. [DOI] [PubMed] [Google Scholar]
- 24.Choudhury S.R., Knapp L.A. Human reproductive failure II: immunogenetic and interacting factors. Hum. Reprod. Update. 2001;7(2):135–160. doi: 10.1093/humupd/7.2.135. [DOI] [PubMed] [Google Scholar]
- 25.Stagnaro-Green A., Glinoer D. Thyroid autoimmunity and the risk of miscarriage. Best Pract. Res. Clin. Endocrinol. Metabol. 2004;18(2):167–181. doi: 10.1016/j.beem.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Bussen S., Steck T., Dietl J. Increased prevalence of thyroid antibodies in euthyroid women with a history of recurrent in-vitro fertilization failure. Hum. Reprod. 2000;15(3):545–548. doi: 10.1093/humrep/15.3.545. [DOI] [PubMed] [Google Scholar]
- 27.Toulis K.A., Goulis D.G., Venetis C.A., Kolibianakis E.M., Negro R., Tarlatzis B.C. Risk of spontaneous miscarriage in euthyroid women with thyroid autoimmunity undergoing IVF: a meta-analysis. 2010;162(4):643–652. doi: 10.1530/EJE-09-0850. [DOI] [PubMed] [Google Scholar]
- 28.Krassas G.E., Perros P., Kaprara A. Thyroid autoimmunity, infertility and miscarriage. Expet Rev. Endocrinol. Metabol. 2008;3(2):127–136. doi: 10.1586/17446651.3.2.127. [DOI] [PubMed] [Google Scholar]
- 29.Poppe K., Autin C., Veltri F., Kleynen P., Grabczan L., Rozenberg S. Thyroid autoimmunity and intracytoplasmic sperm injection outcome: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2018;103(5):1755–1766. doi: 10.1210/jc.2017-02633. [DOI] [PubMed] [Google Scholar]
- 30.Tan S., Dieterle S., Pechlavanis S., Janssen O.E., Fuhrer D. Thyroid autoantibodies per se do not impair intracytoplasmic sperm injection outcome in euthyroid healthy women. Eur. J. Endocrinol. 2014;170(4):495–500. doi: 10.1530/EJE-13-0790. [DOI] [PubMed] [Google Scholar]
- 31.Kazemijaliseh H., Tehrani F.R., Behboudi-Gandevani S., Hosseinpanah F., Khalili D., Azizi F. The prevalence and causes of primary infertility in Iran: a population-based study. Global J. Health Sci. 2015;7(6):226. doi: 10.5539/gjhs.v7n6p226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kutteh W.H., Yetman D.L., Carr A.C., Beck L.A., Scott R.T.J.F., Jr. Increased prevalence of antithyroid antibodies identified in women with recurrent pregnancy loss but not in women undergoing assisted reproduction. sterility. 1999;71(5):843–848. doi: 10.1016/s0015-0282(99)00091-6. [DOI] [PubMed] [Google Scholar]
- 33.Litwicka K., Arrivi C., Varricchio M.T., Mencacci C., Greco E. In women with thyroid autoimmunity, does low‐dose prednisolone administration, compared with no adjuvant therapy, improve in vitro fertilization clinical results? J. Obstet. Gynaecol. Res. 2015;41(5):722–728. doi: 10.1111/jog.12615. [DOI] [PubMed] [Google Scholar]
- 34.Moradan S., Ghorbani R. Dexamethasone in unexplained infertility. Saudi Med. J. 2009:1034–1036. [PubMed] [Google Scholar]
- 35.Boomsma C.M., Keay S.D., Macklon N.S. Peri‐implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database Syst. Rev. 2012;(6) doi: 10.1002/14651858.CD005996.pub2. [DOI] [PubMed] [Google Scholar]
- 36.Michael A.E., Papageorghiou A.T. Potential significance of physiological and pharmacological glucocorticoids in early pregnancy. Hum. Reprod. Update. 2008;14(5):497–517. doi: 10.1093/humupd/dmn021. [DOI] [PubMed] [Google Scholar]
- 37.Taniguchi F. Results of prednisolone given to improve the outcome of in vitro fertilization-embryo transfer in women with antinuclear antibodies. J. Reprod. Med. 2005;50(6):383–388. [PubMed] [Google Scholar]
- 38.Muller A., Verhoeff A., Mantel M., Berghout A. Thyroid autoimmunity and abortion: a prospective study in women undergoing in vitro fertilization. Fertil. Steril. 1999;71(1):30–34. doi: 10.1016/s0015-0282(98)00394-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study data are available from the corresponding author upon reasonable request.