Abstract
Purpose of Review
Genetic, epidemiological, and translational data indicate that Lipoprotein (a) [Lp(a)] is likely in the causal pathway for atherosclerotic cardiovascular diseases as well as calcification of the aortic valves.
Recent Findings
Lp(a) is structurally similar to low-density lipoprotein, but in addition to apolipoprotein B-100, it has a glycoprotein apolipoprotein(a) [apo(a)], which is attached to the apolipoprotein B-100. Several distinctive properties of Lp(a) can be attributed to the presence of apo(a). This review discusses the current state of literature on pathophysiological and clinical aspects of Lp(a). After five decades of research, the understanding of Lp(a) structure, biochemistry, and pathophysiology of its cardiovascular manifestations still remains less than fully understood.
Summary
Universally, Lp(a) elevation may be the most predominant monogenetic lipid disorder with approximate prevalence of Lp(a)>50 mg/dL among estimated >1.4 billion people. This makes a compelling rationale for diagnosing and managing Lp(a)-mediated risk. In addition to discussing various cardiovascular phenotypes of Lp(a) and associated morbidity, we also outline current and emerging therapies aimed at identifying a definitive treatment for elevated Lp(a) levels.
Keywords: Cardiovascular diseases, Coronary heart disease, Lipoprotein(a), Lp(a), Apolipoprotein(a)
Introduction
Lipoprotein (a) [Lp(a)] was thrust into limelight in 2009 when significant evidence from Mendelian randomization studies [1] and large cohort studies showed its association with atherosclerotic cardiovascular disease (ASCVD) [2–5]. Although Lp(a) was first identified in the 1960s, it is only till recently that its structure and function has been fully understood [4, 5]. There has indeed been an increased understanding of the biochemistry and pathophysiological aspects of Lp(a) and its association with ASCVD risk. However, this enigmatic lipoprotein’s complexity continues to unfold. In this article, we aim to summarize the current state of literature regarding the association of Lp(a) with cardiovascular diseases (CVD), highlight Lp(a) reducing therapies and discuss future directions of therapies in development.
Structure of the lipoprotein(a)
Lp(a) is a lipoprotein very similar to low density lipoprotein (LDL) with its lipid concentration as well as the presence of the protein apoB-100. In addition, each Lp(a) particle has an additional glycoprotein, apo(a), which is attached to the apoB-100 by a single disulfide bond [6–8]. The apo(a) chain contains five cysteine-rich domains known as “kringles.” The apo(a) kringle IV–like sequences are grouped into 10 types depending on the amino acid sequence (KIV1 to KIV10). KIV1 to KIV10 are present in one copy except KIV2, which are highly variable in number and determine the size of Lp(a) isoforms [9]. Among most of the population, the concentration of Lp(a) is inversely correlated to the apo(a) isoform size [10].
Lp(a) has prothrombotic, proinflammatory and proatherogenic properties influencing ASCVD development and progression. Apo(a) is structurally very similar to plasminogen and therefore, Lp(a) can hinder and impair the plasminogen activation, plasmin generation, and fibrinolysis [11, 12]. Lp(a) can also bind to macrophages via a high-affinity receptor that promotes foam cell formation and the deposition of cholesterol in atherosclerotic plaques [13]. It is mainly excreted by the liver with secondary role of the kidneys [14, 15].
Lipoprotein (a) and cardiovascular diseases
Coronary artery disease and stroke
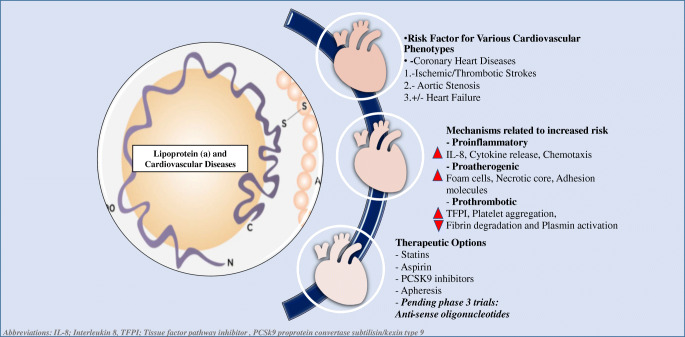
Elevated Lp(a) is one of the leading inherited dyslipidemias in patients with premature ASCVD including coronary artery disease (CAD) or ischemic stroke [16, 17]. Lp(a) particles can be readily oxidized, degraded, and/or aggregated [18]. Lp(a) may also be more easily taken up by macrophage scavenger receptors [19] and may have preferential sequestration into the arterial walls compared with LDL particles (Fig. 1).
Fig. 1.
Lipoprotein (a): a risk factor for cardiovascular diseases, mechanisms and treatment options
Numerous studies [2, 20–25] have demonstrated the association of Lp(a) with incident ASCVD. Kamstrup et al., assessed the causal role of Lp(a) with incidence of CAD in their Mendelian randomization approach from the Copenhagen City Heart Study (CCHS; a prospective general population study with 16 years of follow-up), the Copenhagen General Population Study (CGPS; a cross-sectional general population study in more than 29,000 individuals), and the Copenhagen Ischemic Heart Disease Study (a case–control study in 2461 individuals). They concluded that genetically elevated Lp(a) levels were associated with an increased risk of myocardial infarction (MI) [1]. The hazards ratio (HR) for MI was 1.22 (95% CI 1.09–1.37) per doubling of Lp(a) levels using instrumental variable analyses [1]. In another study, two LPA variants were described to have a strong association with increased risk of coronary heart disease (CHD) events [3]. Clarke et al. showed that the odds ratio for CHD were 1.70 (95% CI 1.49–1.95) for the common variant of the LPA gene (rs10455872) and 1.92 (95% CI 1.48–1.49) for a second, independent variant (rs3798220). These genetic studies have significantly strengthened the evidence for causality of Lp(a) levels and CAD [26].
Lp(a) concentrations vary by ethnicity [27]. African American individuals have been reported to have higher Lp(a) levels than Caucasians. However, both races remain equally at risk of incident CVD events (CHD or ischemic stroke) conferred by Lp(a) concentration [28].
Heart failure
Combined evidence from the CCHS and CGPS cohorts evaluated the association between elevated Lp(a) levels and heart failure (HF) in a total of 98,097 Danish individuals followed from 1976 to 2013 [29]. The results of this study showed that Lp(a) levels were associated with incident HF risk [HRsHF 1.10 (95% CI 0.97–1.25) for the 34th–66th percentiles of Lp(a) (8–19 mg/dL), 1.24 (95% CI 1.08–1.42) for the 67th–90th percentiles of Lp(a) (20–67 mg/dL), 1.57 (95% CI 1.32–1.87) for the 91st–99th percentiles of Lp(a) (68–153 mg/dL), and 1.79 (95% CI 1.18–2.73) for levels >99th percentile (>153 mg/dL) versus less than the 34th percentile (Lp(a) levels <8 mg/dL) (p < 0.001)]. The equivalent population-attributable risk was determined to be 9%. Furthermore, genetic analyses showed a relative risk of 1.18 (95% CI 1.04–1.34) per 10-fold higher Lp(a) levels for HF [29]. Mediation analyses showed that 47% of the increased risk for HF secondary to elevated Lp(a) levels was mediated through MI and 21% through aortic stenosis, with 37% of the remaining risk unexplained. The authors postulated that high levels of Lp(a) may lead to increased arterial stiffness, including noncompliance of the aorta resulting in HF not explained by development of MI or aortic stenosis [29].
However, in an analysis from Atherosclerosis Risk in Communities (ARIC) study, Lp(a) levels were not independently associated with HF hospitalization [30]. When comparing quintile 5 vs. quintile 1 of Lp(a) levels; there was no significant association with HF hospitalization (HR 1.07, 95%CI [0.91–1.27]). Therefore, whether Lp(a) independently increases the risk of HF or if HF incidence may be a downstream effect of CHD development associated with elevated Lp(a) remains, at best, inconclusive.
Aortic stenosis
High plasma Lp(a) concentrations have been shown to correlate strongly with aortic stenosis [31–34]. In the CCHS and CGPS cohort (combined n = 77,860), an incremental risk associated with aortic stenosis was noted across percentile increases in Lp(a) levels [35].
Thanassoulis et al. showed that LPA single nucleotide polymorphism (SNP) rs10455872, which carries a high risk for MI and CHD, reached significance in a genome-wide analysis and was the strongest genetic causal risk factor for the development of aortic stenosis and calcifications of the aortic valve across multiple ethnic groups [36]. Other studies have since confirmed the association between Lp(a) levels and aortic valve calcifications, aortic stenosis, and progression of aortic stenosis across different racial groups [35, 37, 38].
Recently, Tsimikas et al., have postulated that the high burden of thrombosis and increased risk of stroke conferred by the coronavirus disease 2019 (COVID19) [39] may have linkages to elevated Lp(a) levels [40]. COVID19 is associated with thrombotic events and has also been shown to induce a cytokine storm with high levels of IL-6. Given IL-6’s ability to strongly upregulate Lp(a) levels, and the high homology of Lp(a) to plasminogen, it is speculated that baseline elevated Lp(a) or acutely increased Lp(a) may be responsible for some effects of the COVID19 related thrombotic events [40]. This hypothesis is yet to be validated in large epidemiologic datasets.
Quantification of lipoprotein(a)
Primarily due to the differences in KIV2, the measurement of Lp(a) has been a debated topic. In addition to the aforementioned variations in KIV2, variable glycosylation occurs in the core of the KIV motifs as well as the sequences linking the kringles, which makes measurement challenging [41].
Some of these obstacles include assignment of uniform target value to the assay calibrators, assessment of Lp(a) mass (typically mg/dL) versus particle number (nmol/L), and an absence of implemented guidelines for validation of methodological approaches [42]. The measurement of Lp(a) mass units (mg/dL) expressed the entire lipoprotein’s mass including apo(a), apolipoprotein B-100, cholesterol, phospholipids, cholesteryl esters as well as the triglycerides [43]. Therefore, the heterogeneous size of the apo(a) and the KIV2 units in the Lp(a) can result in challenging standardization using a single calibrant material for measurement. Serum levels of Lp(a) in those with higher numbers of KIV2 repeats may be overestimated and those with smaller numbers could be underestimated until a universal calibrant has the same range of isoforms as the testing samples of Lp(a). A conversion factor of 2.85 for small isoforms and 1.85 for large isoforms, with a mean of 2.4 nmol/L for every 1 mg/dL, was recommended in the past [44] although, this has significant shortfalls. Marcovina et al. have shown that the conversion factor calibrations, if not appropriately followed conversion may alter the correct Lp(a) values [42].
A reagent assay by Denka Seiken can provide precise analytical method for Lp(a) measurement. The assay reports Lp(a) concentrations in nmol/L and can be referenced to the World Health Organization/International Federation of Clinical Chemistry and Laboratory Medicine reference materials [42].
Practical and clinical considerations of lipoprotein(a)
Who should the Lp(a) levels be measured in?
Without intervention during an individual’s lifetime, Lp(a) levels have been noted to remain relatively stable due to their genetic predetermination [45, 46]. Repeat measurement is reserved where a secondary cause of elevated Lp(a) (including chronic kidney disease/nephrotic syndrome, chronic liver disease, hypothyroidism, diabetes mellitus, postmenopausal status and drugs) is suspected or the response to therapeutic interventions needs to be determined. Several leading lipid and cardiovascular societies have put forward screening guidelines for Lp(a) (Table 1).
Table 1.
Societal recommendations for lipoprotein (a) population screening
| Society | Currently applicable screening guidelines |
|---|---|
|
2018 ACC/AHA Cholesterol Guidelines [47••] |
– No specific screening recommendation however; elevated Lp(a) (≥50 mg/dL or 125 nmol/L) is noted as a “risk enhancing” factor if measured – Presence of risk enhancing factors favor statin therapy use in those 40–75-year-old adults, without diabetes mellitus, with 10-year ASCVD risk of >5–19.9% |
| 2019 NLA Scientific Statement [48] |
– Lp(a) screening reasonable or “may be” reasonable among: Personal or first-degree family history of premature ASCVD Recurrent or progressive ASCVD despite optimal lipid therapy Family history of elevated Lp(a) Primary severe hypercholesterolemia or suspected familial hypercholesterolemia Intermediate risk patients ( >5–19.9% ACC/AHA 10-year ASCVD risk) Very high risk of ASCVD (to define PCSK9 inhibitor benefit) Statin resistance Progressive aortic stenosis |
|
2019 ESC/EAS Dyslipidemia Guidelines [49••] |
– Measure Lp(a) at least once in each adult’s lifetime to identify those with very high inherited Lp(a) levels >180 mg/dL (>430 nmol/L) – Consider Lp(a) measurement in selected patients with: Family history of premature coronary artery disease or elevated Lp(a) For reclassification of risk in those are at borderline between moderate to high risk of CVD |
|
HEART UK Consensus Statement [43] |
– Personal of family history of premature ASCVD –First degree relative with elevated Lp(a) levels (>200 nmol/L) – Calcified aortic valve stenosis – Borderline increased 10-year risk of ASCVD events* for risk reclassification |
| 2016 Canadian Cardiovascular Society Guidelines [50] |
– Individuals within intermediate Framingham Risk category (10–19%) – Family history of premature ASCVD |
*Lp(a) best re-classifies cardiovascular disease risk in people at intermediate risk calculated by the ACC/AHA 10-year ASCVD risk <15%; >15% risk recommended to be on statin therapy regardless of Lp(a) levels
Abbreviations: ACC/AHA; American College of Cardiology/American Heart Association, ESC; European Society of Cardiology EAS; European Atherosclerosis Society.
The 2018 American Heart Association (AHA)/American College of Cardiology (ACC) and the HEART UK guidelines do not support universal Lp(a) testing as yet [43, 47]. This is in contrast to the 2019 European Atherosclerosis Society and European Society of Cardiology guidelines which endorse the measurement of Lp(a) to be considered at least once in each adult person’s lifetime to identify those with very high inherited Lp(a) levels >180 mg/dL (>430 nmol/L) [49••]. The National Lipid Association (NLA) and HEART UK guidelines cautiously suggest that it may be reasonable to measure Lp(a) amongst those individuals with a personal history of or first-degree relatives with premature ASCVD and amongst those with severe hypercholesterolemia (LDL-C ≥190 mg/dL) [48]. The Canadian Cardiovascular Guidelines of 2016 suggest to measure the Lp(a) in individuals with intermediate Framingham risk category (10–19%) or those with a family history of premature ASCVD [50]. Universal thresholds for what Lp(a) levels identify higher risk are not uniform across guidelines, however in most cases, Lp(a) levels >125 nmol/L (>50 mg/dL) have been deemed as strongly associated with incidence of ASCVD risk (Table 2).
Table 2.
Recommendations for lipoprotein (a) risk thresholds from different societies
| Societal position | Lipoprotein (a) risk threshold |
|---|---|
| 2018 American Heart Association/ American College of Cardiology Multisociety Guidelines [47••] | >50 mg/dL (>125 nmol/L) |
| 2019 National Lipid Association Scientific Statement [48] | >50 mg/dL or >100 nmol/L (based on >80th populations percentile in Caucasians) |
| 2019 European Society of Cardiology and European Atherosclerosis Society [49••] | Lp(a) > 180 mg/dL (>430 nmol/L) is defined as risk threshold equivalent to that for heterozygous FH |
| 2019 HEART UK Consensus Statement [43] | Risk thresholds: 32–90 nmol/L minor; 90–200 nmol/L moderate; 200–400 nmol/L high; >400 nmol/L very high |
| 2016 Canadian Cardiovascular Society Guidelines [50] | >30 mg/dL |
Abbreviations: ESC; European Society of Cardiology EAS; European Atherosclerosis Society, FH; Familial Hypercholesterolemia
Given the strong genetic predisposition, cascade screening of Lp(a) levels amongst family members of patients with elevated Lp(a) levels and those with familial hypercholesterolemia may be considered on a case by case basis with patient-clinical discussion.
Risk stratification evidence
The risk prediction of cardiovascular diseases by addition of Lp(a) has yielded conflicting results. In a study of over 28,000 women from the Women’s Health Initiative, Women’s Health Study and the JUPITER trial (Justification for Use of Statins in Prevention) by Cook et al., Lp(a) was associated with ASCVD only among those with high baseline total cholesterol >220 mg/dL [51]. Further, the risk prediction and reclassification indices had nominal improvement with the addition of Lp(a) to the models including traditional ASCVD risk factors [52].
Reclassification of patients by measuring Lp(a) was addressed by at least two other studies with 15- and 6-year prospective follow-ups [53, 54]. Lp(a) addition reclassified between 15 and 40% of patients to either high or low risk for ASCVD events.
Barriers and strategies for clinicians
From a clinical standpoint, elevated Lp(a) has been the least sought-after dyslipidemia to treat to date [55]. The reasons for this include lack of understanding of the lipoprotein, measurement challenges and a lack of targeted therapies. The electronic health record billing codes for elevated Lp(a) [E78.41] and family history of elevated Lp(a) [Z83.430] were just recently approved in the USA [55]. Most of these barriers persist widely across the world even today.
Treatment of elevated Lp(a)
Primary prevention patients
Aggressive management of all risk factors amongst patients with elevated Lp(a) with or without established ASCVD and/or aortic stenosis has shown outcomes benefit. In addition to a heart healthy lifestyle [56]; the following are some favorable therapeutic interventions.
Statins
Statins have an established role in primary and secondary prevention of ASCVD events. Statins only marginally affect Lp(a) plasma levels with reports of either no effect on or an increase of Lp(a) levels after statin treatment [57]. The mechanisms by which statins raise apo(a) and Lp(a) require further investigation. In the Scandinavian Simvastatin Survival Study (4S) subgroup analysis performed among 4402 high-risk men with CHD, the numbers of deaths were significantly lower (192 deaths) vs the top (240 deaths) half of the Lp(a) distribution in the simvastatin and placebo groups combined (P < .05). Individuals aged 40–75 years with 10y ASCVD risk of 7.5% to ≤20% with a Lp(a) ≥100 nmol/L may derive benefit from being on moderate- to high-intensity statin therapy [47••].
Aspirin
In the Women’s Health Initiative, carriers of rs3798220, a minor variant of LPA, had elevated Lp(a) levels and an increased CVD risk (HR 2.11, 95% CI 1.39–2.52) versus noncarriers. The carriers appeared to benefit more from the use of aspirin therapy than noncarriers over 9.9y follow up [58]. The benefit of aspirin therapy in individuals with elevated Lp(a) levels could likely be due to its antiplatelet effect given the prothrombotic properties of Lp(a). Another study showed marginal lowering of Lp(a) with aspirin use in a subset of Japanese patients; a benefit hypothesized to be secondary to the preferential action of aspirin in reducing LPA gene transcription [59, 60].
Secondary prevention patients
The use of statin therapy and low dose aspirin has shown CVD outcomes benefit in individuals with established ASCVD and should be continued. In addition, those with elevated Lp(a) and established ASCVD may be considered for the following therapies.
PCSK9i
Proprotein convertase subtilisin/kexin 9 inhibitors ((PCSK9i) have shown to significantly reduce plasma Lp(a) concentration. Whether the PCSK9i induced reduction in Lp(a) translates into ASCVD event reduction was tested amongst 25,096 patients in the FOURIER trial (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk), a randomized trial of evolocumab versus placebo in patients with ASCVD [61]. Evolocumab significantly reduced Lp(a) levels, and patients with higher baseline Lp(a) levels experienced greater absolute reductions in Lp(a) and tended to derive greater cardiovascular benefit from PCSK9 inhibition. Evolocumab reduced the risk of CHD death, myocardial infarction, or urgent revascularization by 23% (HR 0.77 [95% CI, 0.67–0.88]) in patients with a baseline Lp(a)>37 nmol/L (median). The absolute risk reductions, and number needed to treat over 3 years were 2.49% and 40 versus 0.95% and 105, respectively. The PCSK9i derived reduction in Lp(a) and associated benefit in reducing ASCVD events was further strengthened by the results of ODYSSEY Outcomes trial. Alirocumab reduced Lp(a) by 5.0 mg/dL (interquartile range [IQR]: 0–13.5 mg/dL), corrected LDL-C by 51.1 mg/dL (IQR: 33.7-67.2 mg/dLl), and reduced the risk of MACE (HR: 0.85 [95% CI, 0.78 to 0.93) [62]. Relative risk reduction was similar across Lp(a) quartiles. In contrast, absolute risk reduction for MACE was greater at higher baseline Lp(a) levels exceeding 2% in the upper two quartiles.
In a study of pooled data from 10 phase 3 ODYSSEY trials, Ray and colleagues did not find a significant association in the overall cohort between reductions in Lp(a), and incidence of ASCVD events (once changes in LDL-C were controlled in the overall population) [63]. However, they did show a significant association between reductions in Lp(a) and ASCVD events in the subgroup of patients with baseline Lp(a) ≥50 mg/dL (p = 0.02).
Mipomersen
Mipomersen is an antisense oligonucleotide (ASO) which binds the messenger RNA (mRNA) of ApoB-100, which in turn triggers the selective degradation of mRNA molecule for ApoB-100. Mipomersen reduces the atherogenic lipids and lipoprotein production (including Lp(a) and LDL) by binding to a specific mRNA preventing the translation of ApoB protein and, consequently, reducing the production of LDL and Lp(a) [Lp(a) (mg/dL) reduction in mipomersen vs placebo group (% median change of −26.4(−42.8, 5.4) vs. −0.0 (10.7, 15.3), p<0.001)] [64]. The efficacy and safety of long-term mipomersen treatment are currently under evaluation and therefore, it is not part of the usual Lp(a) reduction strategy in clinical practice.
Apheresis
Lipoprotein apheresis (LA) is currently the only available therapy that reduces Lp(a) levels by 50% [65, 66]. This process encompasses the selective or nonselective removal of plasma constituents including lipoproteins in an extracorporeal fashion [67]. Patients with stable CHD as well as elevated Lp(a) levels (>50 mg/dL; mean 103 mg/dL) were shown to have regression of coronary atherosclerosis (by quantitative coronary angiography) after weekly Lp(a)-specific apheresis for at least 18 months [68]. Lp(a) level was reduced by a mean of 73%, to a mean level of 29 mg/dL.
LA therapy also showed a decrease in overall recurrent ASCVD events amongst patients with elevated Lp(a) (>60 mg/dL) [69]. Among those on LA, there was a reported 64 and 63% reduction in LDL-C and Lp(a) levels from baseline, respectively. Over a period of ~48 months follow up, a 94% reduction in major adverse CVD events was noted for those on LA. The effect of LA in these studies may have been confounded by the removal of other atherogenic lipoproteins, as LA eliminates any apoB-containing lipoprotein, including LDL as well as triglyceride-rich lipoproteins and their remnants. Furthermore, fibrinogen (a procoagulant) and other proinflammatory molecules may also be removed by LA [67]. Currently, an ongoing randomized multi-center study (MultiSELECt trial) is evaluating the cardiovascular effects of apheresis (vs maximal medical therapy) in secondary prevention patients with elevated Lp(a) levels [70].
Apo-anti-sense nucleotide
Recently, the phase II trial studies evaluating the use of AKCEAAPO(a)-LRx, an ASO targeting LPA mRNA (which encodes the main Lp(a) constituent, apolipoprotein(a)) conjugated with N-acetylgalactosamine to direct the therapy specifically to hepatocytes [71], were concluded with promising results [72]. Among 268 patients with high levels of Lp(a) (≥60 mg/dL) and preexisting ASCVD randomly assigned to AKCEA-APO(a)-LRx or placebo; AKCEA-APO(a)-LRx showed significant reductions in Lp(a) levels versus placebo. Approximately 98% of patients achieved Lp(a) ≤50 mg/dL (mean reduction 80%) at the highest cumulative dose regimen (equivalent to 80 mg monthly) [72]. In a recent study, AKCEA-APO(a)-LRx and PCSK9i were given to individuals (14 and 18, respectively) with ASCVD and elevated Lp(a) levels. AKCEA-APO(a)-LRx led to a more significant Lp(a)-lowering (mean % change of −47 vs −16% by PCSK9i) and also reduced the pro-inflammatory markers. [73] The pivotal phase III study (HORIZON trial; ClinicalTrials.govIdentifier: NCT04023552) will evaluate the AKCEA-APO(a)-LRx associated reduction of Lp(a) and CVD outcomes.
Conclusions
The past decade has brought significant improvements in our understanding of Lp(a) structure, its causal association with various cardiovascular phenotypes, challenges in optimal measurement techniques, and the development of efficacious therapies. With enhanced awareness of Lp(a) as a risk factor for cardiovascular diseases, the scientific community has indeed made strides towards improved understanding of Lp(a) with potential disease modifying therapies within sight. Future paradigms including implementation of early referral to specialists, increased educational efforts and further research will be essential to gain momentum in the quest towards championing the care of patients with elevated Lp(a).
Declarations
Conflict of Interest
Anum Saeed and Sina Kianoush have no disclosures or conflicts of interests.
Salim S. Virani has received research support from the Department of Veterans Affairs, World Heart Federation, and Tahir and Jooma Family. He has received honorarium from the American College of Cardiology as Associate Editor for Innovations for acc.org.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Coronary Heart Disease
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anum Saeed, Email: saeeda2@upmc.edu.
Sina Kinoush, Email: Sina.Kianoush@bcm.edu.
Salim S. Virani, Email: virani@bcm.edu
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
- 1.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. Jama. 2009;301(22):2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 2.Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. Jama. 2009;302(4):412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de Faire U, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 4.McLean JW, Tomlinson JE, Kuang WJ, Eaton DL, Chen EY, Fless GM, et al. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987;330(6144):132–137. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- 5.Lawn RM. Lipoprotein(a) in heart disease. Sci Am. 1992;266(6):54–60. doi: 10.1038/scientificamerican0692-54. [DOI] [PubMed] [Google Scholar]
- 6.Koschinsky ML, Cote GP, Gabel B, van der Hoek YY. Identification of the cysteine residue in apolipoprotein(a) that mediates extracellular coupling with apolipoprotein B-100. J Biol Chem. 1993;268(26):19819–19825. doi: 10.1016/S0021-9258(19)36587-1. [DOI] [PubMed] [Google Scholar]
- 7.Albers JJ, Kennedy H, Marcovina SM. Evidence that Lp[a] contains one molecule of apo[a] and one molecule of apoB: evaluation of amino acid analysis data. J Lipid Res. 1996;37(1):192–196. doi: 10.1016/S0022-2275(20)37647-1. [DOI] [PubMed] [Google Scholar]
- 8.Lamina C, Kronenberg F. The mysterious lipoprotein(a) is still good for a surprise. Lancet Diabetes Endocrinol. 2013;1(3):170–172. doi: 10.1016/S2213-8587(13)70085-8. [DOI] [PubMed] [Google Scholar]
- 9.van der Hoek YY, Wittekoek ME, Beisiegel U, Kastelein JJ, Koschinsky ML. The apolipoprotein(a) kringle IV repeats which differ from the major repeat kringle are present in variably-sized isoforms. Hum Mol Genet. 1993;2(4):361–366. doi: 10.1093/hmg/2.4.361. [DOI] [PubMed] [Google Scholar]
- 10.Utermann G, Hoppichler F, Dieplinger H, Seed M, Thompson G, Boerwinkle E. Defects in the low density lipoprotein receptor gene affect lipoprotein (a) levels: multiplicative interaction of two gene loci associated with premature atherosclerosis. Proc Natl Acad Sci U S A. 1989;86(11):4171–4174. doi: 10.1073/pnas.86.11.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loscalzo J, Weinfeld M, Fless GM, Scanu AM. Lipoprotein(a), fibrin binding, and plasminogen activation. Arteriosclerosis (Dallas, Tex) 1990;10(2):240–245. doi: 10.1161/01.atv.10.2.240. [DOI] [PubMed] [Google Scholar]
- 12.Palabrica TM, Liu AC, Aronovitz MJ, Furie B, Lawn RM, Furie BC. Antifibrinolytic activity of apolipoprotein(a) in vivo: human apolipoprotein(a) transgenic mice are resistant to tissue plasminogen activator-mediated thrombolysis. Nat Med. 1995;1(3):256–259. doi: 10.1038/nm0395-256. [DOI] [PubMed] [Google Scholar]
- 13.Zioncheck TF, Powell LM, Rice GC, Eaton DL, Lawn RM. Interaction of recombinant apolipoprotein(a) and lipoprotein(a) with macrophages. J Clin Invest. 1991;87(3):767–771. doi: 10.1172/JCI115079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cain WJ, Millar JS, Himebauch AS, Tietge UJ, Maugeais C, Usher D, et al. Lipoprotein [a] is cleared from the plasma primarily by the liver in a process mediated by apolipoprotein [a] J Lipid Res. 2005;46(12):2681–2691. doi: 10.1194/jlr.M500249-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Hoover-Plow J, Huang M. Lipoprotein(a) metabolism: potential sites for therapeutic targets. Metab Clin Exp. 2013;62(4):479–491. doi: 10.1016/j.metabol.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genest JJ, Martin-Munley SS, McNamara JR, Ordovas JM, Jenner J, Myers RH, et al. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation. 1992;85:2025–2033. doi: 10.1161/01.CIR.85.6.2025. [DOI] [PubMed] [Google Scholar]
- 17.Boffa MB, Marcovina SM, Koschinsky ML. Lipoprotein(a) as an Emerging Risk Factor for Atherothrombosis. In: Davidson MH, Toth PP, Maki KC, Gotto AM, editors. Therapeutic Lipidology. Totowa: Humana Press; 2007. pp. 241–266. [Google Scholar]
- 18.Hoff HF, O'Neil J, Yashiro A. Partial characterization of lipoproteins containing apo[a] in human atherosclerotic lesions. J Lipid Res. 1993;34(5):789–798. doi: 10.1016/S0022-2275(20)39699-1. [DOI] [PubMed] [Google Scholar]
- 19.Haberland ME, Fless GM, Scanu AM, Fogelman AM. Malondialdehyde modification of lipoprotein(a) produces avid uptake by human monocyte-macrophages. J Biol Chem. 1992;267(6):4143–4151. doi: 10.1016/S0021-9258(19)50640-8. [DOI] [PubMed] [Google Scholar]
- 20.Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117(2):176–184. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 21.Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol. 2013;61(11):1146–1156. doi: 10.1016/j.jacc.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Saeed A, Virani SS. Lipoprotein(a) and cardiovascular disease: current state and future directions for an enigmatic lipoprotein. Front Biosci (Landmark edition) 2018;23:1099–1112. doi: 10.2741/4635. [DOI] [PubMed] [Google Scholar]
- 23.Saeed A, Sun W, Agarwala A, Virani SS, Nambi V, Coresh J, Selvin E, Boerwinkle E, Jones PH, Ballantyne CM, Hoogeveen RC. Lipoprotein(a) levels and risk of cardiovascular disease events in individuals with diabetes mellitus or prediabetes: The Atherosclerosis Risk in Communities study. Atherosclerosis. 2019;282:52–56. doi: 10.1016/j.atherosclerosis.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erqou S, Thompson A, Di Angelantonio E, Saleheen D, Kaptoge S, Marcovina S, et al. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J Am Coll Cardiol. 2010;55(19):2160–2167. doi: 10.1016/j.jacc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 25.Craig WY, Neveux LM, Palomaki GE, Cleveland MM, Haddow JE. Lipoprotein(a) as a risk factor for ischemic heart disease: metaanalysis of prospective studies. Clin Chem. 1998;44(11):2301–2306. doi: 10.1093/clinchem/44.11.2301. [DOI] [PubMed] [Google Scholar]
- 26.Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res. 2016;57(11):1953–1975. doi: 10.1194/jlr.R071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldeyer C, Makarova N, Zeller T, Schnabel RB, Brunner FJ, Jorgensen T, et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J. 2017;38:2490–2498. doi: 10.1093/eurheartj/ehx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virani SS, Brautbar A, Davis BC, Nambi V, Hoogeveen RC, Sharrett AR, Coresh J, Mosley TH, Morrisett JD, Catellier DJ, Folsom AR, Boerwinkle E, Ballantyne CM. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125(2):241–249. doi: 10.1161/CIRCULATIONAHA.111.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamstrup PR, Nordestgaard BG. Elevated lipoprotein(a) levels, LPA risk genotypes, and increased risk of heart failure in the general population. JACC Heart Fail. 2016;4(1):78–87. doi: 10.1016/j.jchf.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Agarwala A, Pokharel Y, Saeed A, Sun W, Virani SS, Nambi V, et al. The association of lipoprotein(a) with incident heart failure hospitalization: Atherosclerosis Risk in Communities Study. Atherosclerosis. [DOI] [PMC free article] [PubMed]
- 31.Bozbas H, Yildirir A, Atar I, Pirat B, Eroglu S, Aydinalp A, Ozin B, Muderrisoglu H. Effects of serum levels of novel atherosclerotic risk factors on aortic valve calcification. J Heart Valve Disease. 2007;16(4):387–393. [PubMed] [Google Scholar]
- 32.Glader CA, Birgander LS, Soderberg S, Ildgruben HP, Saikku P, Waldenstrom A, et al. Lipoprotein(a), Chlamydia pneumoniae, leptin and tissue plasminogen activator as risk markers for valvular aortic stenosis. Eur Heart J. 2003;24(2):198–208. doi: 10.1016/S0195-668X(02)00385-8. [DOI] [PubMed] [Google Scholar]
- 33.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29(3):630–634. doi: 10.1016/S0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 34.Gotoh T, Kuroda T, Yamasawa M, Nishinaga M, Mitsuhashi T, Seino Y, Nagoh N, Kayaba K, Yamada S, Matsuo H, Hosoe M, Itoh Y, Kawai T, Igarashi M, Shimada K. Correlation between lipoprotein(a) and aortic valve sclerosis assessed by echocardiography (the JMS Cardiac Echo and Cohort Study) Am J Cardiol. 1995;76(12):928–932. doi: 10.1016/S0002-9149(99)80263-X. [DOI] [PubMed] [Google Scholar]
- 35.Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63(5):470–477. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 36.Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, Malhotra R, O'Brien KD, Kamstrup PR, Nordestgaard BG, Tybjaerg-Hansen A, Allison MA, Aspelund T, Criqui MH, Heckbert SR, Hwang SJ, Liu Y, Sjogren M, van der Pals J, Kälsch H, Mühleisen TW, Nöthen MM, Cupples LA, Caslake M, di Angelantonio E, Danesh J, Rotter JI, Sigurdsson S, Wong Q, Erbel R, Kathiresan S, Melander O, Gudnason V, O'Donnell CJ, Post WS, CHARGE Extracoronary Calcium Working Group Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368(6):503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, LaRosa JC, Waters DD, DeMicco DA, Simes RJ, Keech AC, Colquhoun D, Hitman GA, Betteridge DJ, Clearfield MB, Downs JR, Colhoun HM, Gotto AM, Jr, Ridker PM, Grundy SM, Kastelein JJP. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64(5):485–494. doi: 10.1016/j.jacc.2014.02.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma GS, Wilkinson MJ, Reeves RR, Yeang C, DeMaria AN, Cotter B, et al. Lipoprotein(a) in patients undergoing transcatheter aortic valve replacement. Angiology. 2019;70(4):332–336. doi: 10.1177/0003319719826461. [DOI] [PubMed] [Google Scholar]
- 39.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ (Clinical research ed) 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moriarty PM, Gorby LK, Stroes ES, Kastelein JP, Davidson M, Tsimikas S. Lipoprotein(a) and its potential association with thrombosis and inflammation in COVID-19: a testable hypothesis. Curr Atheroscler Rep. 2020;22(9):48. doi: 10.1007/s11883-020-00867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scanu AM, Edelstein C. Learning about the structure and biology of human lipoprotein [a] through dissection by enzymes of the elastase family: facts and speculations. J Lipid Res. 1997;38(11):2193–2206. doi: 10.1016/S0022-2275(20)34933-6. [DOI] [PubMed] [Google Scholar]
- 42.Marcovina SM, Albers JJ. Lipoprotein (a) measurements for clinical application. J Lipid Res. 2016;57(4):526–537. doi: 10.1194/jlr.R061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cegla J, Neely RDG, France M, Ferns G, Byrne CD, Halcox J, Datta D, Capps N, Shoulders C, Qureshi N, Rees A, Main L, Cramb R, Viljoen A, Payne J, Soran H, HEART UK Medical, Scientific and Research Committee HEART UK consensus statement on Lipoprotein(a): a call to action. Atherosclerosis. 2019;291:62–70. doi: 10.1016/j.atherosclerosis.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Brown WV, Ballantyne CM, Jones PH, Marcovina S. Management of Lp(a) J Clinical Lipidol. 2010;4(4):240–247. doi: 10.1016/j.jacl.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Ellis KL, Pérez de Isla L, Alonso R, Fuentes F, Watts GF, Mata P. Value of measuring lipoprotein(a) during cascade testing for familial hypercholesterolemia. J Am Coll Cardiol. 2019;73(9):1029–1039. doi: 10.1016/j.jacc.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 46.Enkhmaa B, Anuurad E, Berglund L. Lipoprotein (a): impact by ethnicity and environmental and medical conditions. J Lipid Res. 2016;57(7):1111–1125. doi: 10.1194/jlr.R051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.••.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2018:Cir0000000000000625. The 2018 cholesterol management guidelines by the American Heart Association and American College of Cardiology set the clinical practice guidelines of cholesterol management
- 48.Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, et al. Use of Lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clinical Lipidol. 2019;13(3):374–392. doi: 10.1016/j.jacl.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 49.••.2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140-205. The 2019 cholesterol management guidelines by the European Society of Cardiology/European Atherosclerosis Society set the clinical practice guidelines of cholesterol management in 2019. [DOI] [PubMed]
- 50.Anderson TJ, Grégoire J, Pearson GJ, Barry AR, Couture P, Dawes M, Francis GA, Genest J, Jr, Grover S, Gupta M, Hegele RA, Lau DC, Leiter LA, Lonn E, Mancini GBJ, McPherson R, Ngui D, Poirier P, Sievenpiper JL, Stone JA, Thanassoulis G, Ward R. 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can J Cardiol. 2016;32(11):1263–1282. doi: 10.1016/j.cjca.2016.07.510. [DOI] [PubMed] [Google Scholar]
- 51.Cook NR, Mora S, Ridker PM. Lipoprotein(a) and cardiovascular risk prediction among women. J Am Coll Cardiol. 2018;72(3):287–296. doi: 10.1016/j.jacc.2018.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Sr, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;63(25 Pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willeit P, Kiechl S, Kronenberg F, Witztum JL, Santer P, Mayr M, Xu Q, Mayr A, Willeit J, Tsimikas S. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J Am Coll Cardiol. 2014;64(9):851–860. doi: 10.1016/j.jacc.2014.03.061. [DOI] [PubMed] [Google Scholar]
- 54.van Wijk DF, Sjouke B, Figueroa A, Emami H, van der Valk FM, MacNabb MH, et al. Nonpharmacological lipoprotein apheresis reduces arterial inflammation in familial hypercholesterolemia. J Am Coll Cardiol. 2014;64(14):1418–1426. doi: 10.1016/j.jacc.2014.01.088. [DOI] [PubMed] [Google Scholar]
- 55.Tsimikas S, Stroes ESG. The dedicated "Lp(a) clinic": a concept whose time has arrived? Atherosclerosis. 2020;300:1–9. doi: 10.1016/j.atherosclerosis.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy J, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsimikas S, Gordts P, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020;41(24):2275–2284. doi: 10.1093/eurheartj/ehz310. [DOI] [PubMed] [Google Scholar]
- 58.Chasman DI, Shiffman D, Zee RY, Louie JZ, Luke MM, Rowland CM, et al. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis. 2009;203(2):371–376. doi: 10.1016/j.atherosclerosis.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akaike M, Azuma H, Kagawa A, Matsumoto K, Hayashi I, Tamura K, Nishiuchi T, Iuchi T, Takamori N, Aihara KI, Yoshida T, Kanagawa Y, Matsumoto T. Effect of aspirin treatment on serum concentrations of lipoprotein(a) in patients with atherosclerotic diseases. Clin Chem. 2002;48(9):1454–1459. doi: 10.1093/clinchem/48.9.1454. [DOI] [PubMed] [Google Scholar]
- 60.Kagawa A, Azuma H, Akaike M, Kanagawa Y, Matsumoto T. Aspirin reduces apolipoprotein(a) (apo(a)) production in human hepatocytes by suppression of apo(a) gene transcription. J Biol Chem. 1999;274(48):34111–34115. doi: 10.1074/jbc.274.48.34111. [DOI] [PubMed] [Google Scholar]
- 61.O'Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139(12):1483–1492. doi: 10.1161/CIRCULATIONAHA.118.037184. [DOI] [PubMed] [Google Scholar]
- 62.Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, Fras Z, Goodman SG, Halvorsen S, Hanotin C, Harrington RA, Jukema JW, Loizeau V, Moriarty PM, Moryusef A, Pordy R, Roe MT, Sinnaeve P, Tsimikas S, Vogel R, White HD, Zahger D, Zeiher AM, Steg PG, Schwartz GG, ODYSSEY OUTCOMES Committees and Investigators Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75(2):133–144. doi: 10.1016/j.jacc.2019.10.057. [DOI] [PubMed] [Google Scholar]
- 63.Ray KK, Vallejo-Vaz AJ, Ginsberg HN, Davidson MH, Louie MJ, Bujas-Bobanovic M, Minini P, Eckel RH, Cannon CP. Lipoprotein(a) reductions from PCSK9 inhibition and major adverse cardiovascular events: pooled analysis of alirocumab phase 3 trials. Atherosclerosis. 2019;288:194–202. doi: 10.1016/j.atherosclerosis.2019.06.896. [DOI] [PubMed] [Google Scholar]
- 64.Santos RD, Raal FJ, Catapano AL, Witztum JL, Steinhagen-Thiessen E, Tsimikas S. Mipomersen, an antisense oligonucleotide to apolipoprotein B-100, reduces lipoprotein(a) in various populations with hypercholesterolemia: results of 4 phase III trials. Arterioscler Thromb Vasc Biol. 2015;35(3):689–699. doi: 10.1161/ATVBAHA.114.304549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bambauer R, Bambauer C, Lehmann B, Latza R, Schiel R. LDL-apheresis: technical and clinical aspects. TheScientificWorldJournal. 2012;2012:314283. doi: 10.1100/2012/314283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan TZ, Hsu LY, Arai AE, Rhodes S, Pottle A, Wage R, Banya W, Gatehouse PD, Giri S, Collins P, Pennell DJ, Barbir M. Apheresis as novel treatment for refractory angina with raised lipoprotein(a): a randomized controlled cross-over trial. Eur Heart J. 2017;38:1561–1569. doi: 10.1093/eurheartj/ehx178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waldmann E, Parhofer KG. Lipoprotein apheresis to treat elevated lipoprotein (a) J Lipid Res. 2016;57(10):1751–1757. doi: 10.1194/jlr.R056549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Safarova MS, Ezhov MV, Afanasieva OI, Matchin YG, Atanesyan RV, Adamova IY, Utkina EA, Konovalov GA, Pokrovsky SN. Effect of specific lipoprotein(a) apheresis on coronary atherosclerosis regression assessed by quantitative coronary angiography. Atheroscler Suppl. 2013;14(1):93–99. doi: 10.1016/j.atherosclerosissup.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 69.Moriarty PM, Gray JV, Gorby LK. Lipoprotein apheresis for lipoprotein(a) and cardiovascular disease. J Clinical Lipidol. 2019;13(6):894–900. doi: 10.1016/j.jacl.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Hohenstein B, Julius U, Lansberg P, Jaeger B, Mellwig KP, Weiss N, Graehlert X, Roeder I, Ramlow W. Rationale and design of MultiSELECt: A European Multicenter Study on the Effect of Lipoprotein(a) Elimination by lipoprotein apheresis on Cardiovascular outcomes. Atheroscler Suppl. 2017;30:180–186. doi: 10.1016/j.atherosclerosissup.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 71.Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet (London, England) 2015;386(10002):1472–1483. doi: 10.1016/S0140-6736(15)61252-1. [DOI] [PubMed] [Google Scholar]
- 72.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif J-C, Baum SJ, Steinhagen-Thiessen E, Shapiro MD, Stroes ES, Moriarty PM, Nordestgaard BG, Xia S, Guerriero J, Viney NJ, O’Dea L, Witztum JL. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382(3):244–255. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 73.Stiekema LCA, Prange KHM, Hoogeveen RM, Verweij SL, Kroon J, Schnitzler JG, Dzobo KE, Cupido AJ, Tsimikas S, Stroes ESG, de Winther MPJ, Bahjat M. Potent lipoprotein(a) lowering following apolipoprotein(a) antisense treatment reduces the pro-inflammatory activation of circulating monocytes in patients with elevated lipoprotein(a) Eur Heart J. 2020;41(24):2262–2271. doi: 10.1093/eurheartj/ehaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]