Highlights
-
•
Dravet syndrome (DS) patient in super-refractory status epilepticus (SRSE) for 5 weeks.
-
•
Add-on fenfluramine (FFA) successfully treated SRSE in DS.
-
•
Most effective dose of FFA was 0.7 mg/kg/day.
-
•
Long-term FFA provided >90% reduction in seizures and prevented status epilepticus.
-
•
FFA maintenance also resulted in improvement of neurocognitive function.
Keywords: Dravet syndrome, Fenfluramine, Neurocognitive response, Super-refractory status epilepticus
Abstract
A 20-year-old woman with Dravet syndrome and multiple prior episodes of status epilepticus presented to our hospital in November 2018 in super-refractory status epilepticus. After 5 weeks of unsuccessful continuous treatment with anesthetics, including pentobarbital and ketamine, we sought and were granted an emergency approval by the United States Food and Drug Administration to administer fenfluramine, an investigational new drug, to this patient. One week of treatment with fenfluramine at 0.4 mg/kg/day was ineffective. The dose of fenfluramine was titrated to 0.7 mg/kg/day, and after 1 week, electrographic seizures ceased. One week later, the patient was seizure-free and off all anesthetic agents. Add-on treatment with fenfluramine was continued with no further episodes of status epilepticus and >90% reduction in tonic-clonic seizures. This case report illustrates the potential for fenfluramine to prevent reoccurrence of status epilepticus and to manage super-refractory status epilepticus in patients with Dravet syndrome.
1. Introduction
Super-refractory status epilepticus (SRSE) refers to status epilepticus (SE) that fails to be controlled within 24 hours of third-line anti-seizure therapy (typically, continuous infusion of pentobarbital, propofol, or midazolam) or that reoccurs upon withdrawal of a third-line medication [1], [2]. Control of SRSE remains a major challenge, and mortality is reported to be approximately 33% [1], [3], [4].
Dravet syndrome (DS) is a rare, severe, treatment-resistant epileptic encephalopathy presenting in the first year of life that is characterized by frequent disabling seizures of multiple types [5]. Patients with DS experience neurodevelopmental delay; motor, behavioral, and cognitive impairment [5], [6]; and an elevated mortality rate, primarily due to sudden unexpected death in epilepsy (SUDEP) and SE [7].
Fenfluramine was approved in the United States and the European Union in 2020 for treatment of seizures associated with DS in patients 2 years or older [8] based on the mean 54%–62% reductions in seizure frequency compared to placebo (P < 0.0001) observed in two phase 3, double-blind, placebo-controlled clinical trials of fenfluramine in patients 2 to 18 years old [9], [10]. The experience with fenfluramine in adult patients with DS in the phase 3 development program is limited to 10 patients who turned 18 years of age prior to receiving their first dose of fenfluramine in either a double-blind study (n = 3) or during treatment in the open-label extension study (n = 7) [11]. This adult cohort demonstrated a median 73% reduction in convulsive seizure frequency compared with baseline.
Here we report the case of a 20-year-old woman with DS who presented to our hospital and rapidly developed SRSE. She failed treatment with multiple anesthetic and anti-seizure medications for several weeks but responded positively with an excellent neurological outcome when fenfluramine was added to her therapy.
2. Case presentation
The patient experienced her first afebrile seizure at the age of 3 months, but no treatment was initiated due to a normal electroencephalogram (EEG) and absence of risk factors for epilepsy. Seizures, including both tonic and tonic-clonic seizures, reoccurred at age 6 months and could not be controlled with phenobarbital. Carbamazepine, lamotrigine, and phenytoin exacerbated seizures. Felbamate and topiramate failed to control seizures. At age 5 years, patient was implanted with vagal nerve stimulation (VNS) and started on ketogenic diet and, later, cannabidiol oil. Development delays in language, motor, and bulbar functions were pervasive by that time. At age 9 years stiripentol was tried but resulted in excessive drooling without significant improvement in seizure control. At 10 years of age, genetic testing revealed a frameshift mutation in SCN1A (two base-pair [AT] insertion at 3725–3726), confirming the diagnosis of DS.
The patient first came under our care at age 17 years (May 2015) when she was hospitalized for SE. At that time her ASM regimen included phenobarbital, valproic acid and clobazam in addition to a vagus nerve stimulator (VNS) and a ketogenic diet. Multiple anesthetic agents, including propofol, midazolam, pentobarbital, and ketamine, were required for treatment of SE. VNS battery was changed, and she was placed on levetiracetam. After 3½ weeks, the patient returned to her neurocognitive baseline level: alert and interactive but with minimal language, requiring a wheelchair for ambulation and assistance with all activities of daily living. She remained on the following regimen for three years: levetiracetam (1000 mg q AM, 1500 q PM), valproate (1000 mg bid), clobazam (10 mg bid), and levocarnitine (600 mg bid), in addition to VNS and a ketogenic diet. Seizure burden was high: 10 brief tonic seizures nightly, typically in clusters, as well prolonged, nocturnal tonic-clonic seizures typically once per week. No observable seizures occurred while awake. Her neurocognitive function remained unchanged.
In November 2018, following a 2-week hospitalization at another center for parainfluenza respiratory infection, she presented to our ED due to a dramatic increase in seizure frequency. Tonic seizures consisting of eye-opening, head deviation and bilateral upper extremity elevation occurred every 2 to 6 minutes, suggesting tonic SE. Initial treatment consisted of intravenous lorazepam (4 mg), followed by intubation, transfer to the neuro intensive care unit for continuous video-EEG monitoring, intravenous propofol and midazolam. Subsequent treatment is illustrated in Fig. 1.
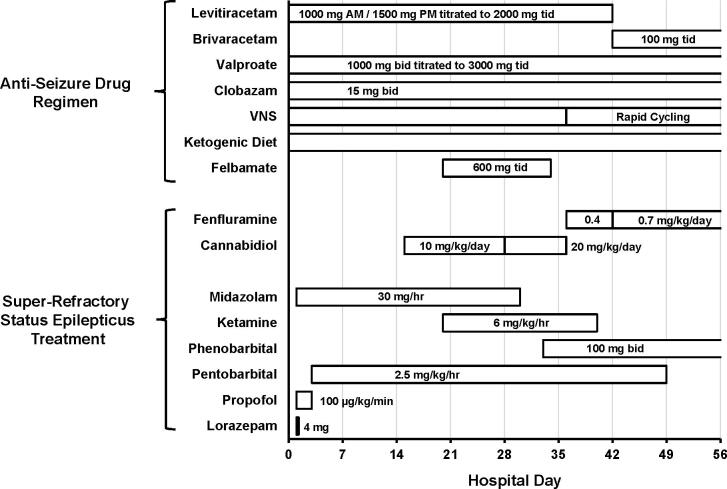
Fig. 1.
Schematic representation of in-hospital treatment of patient with tonic SE. Clobazam, brivaracetam, cannabidiol, and fenfluramine were dosed via the G-tube. All other drugs were administered intravenously. Phenobarbital was added in an effort to wean off pentobarbital.
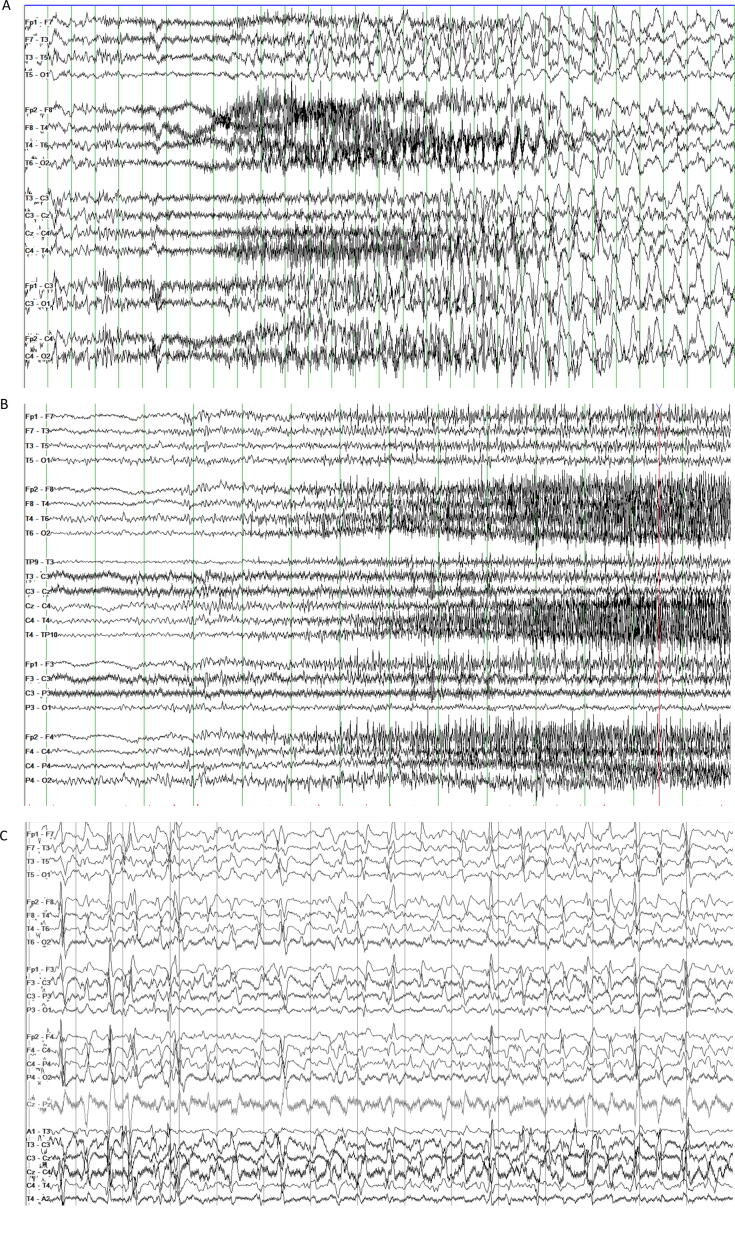
Video-EEG monitoring confirmed frequent seizures involving a buildup of fast activity evolving into generalized 5 Hz ictal rhythm, then 1 Hz spike-wave discharge, in addition to generalized spike-wave and polyspike-wave discharges and independent left and right temporal epileptiform discharges (Fig. 2A). Over the first 3 days of treatment, there was marked resolution of electrographic seizures, but sporadic epileptiform transients persisted. After propofol infusion syndrome developed on Day 3, propofol was replaced with pentobarbital. Break-through tonic seizures persisted despite another week of aggressive treatment (Fig. 2B). Over the next 4 weeks, multiple attempts to wean the patient off anesthetic agents failed despite aggressive management of ASMs. Cannabidiol (10 mg/kg/d; Epidiolex®, Greenwich Biosciences, Inc., Carlsbad, CA, USA) was added on Day 15, and ketamine (2 mg/kg bolus followed by 2 mg/kg/hr titrated to final dose of 6 mg/kg/hr) was started on Day 20 of hospitalization. VNS was adjusted to rapid cycling (7 seconds on/14 seconds off), and felbamate (600 mg tid) was added. Despite these interventions, high doses of ketamine and pentobarbital were required for seizure control. An EEG after 1 month of treatment is shown in Fig. 2C.
Fig. 2.
Panel A. EEG of tonic seizure on day of presentation showing brief 1–2 second burst of generalized 6–7 Hz activity, followed by several seconds of diffuse attenuation with superimposed fast activity and myogenic artifact that evolves into 2 Hz generalized rhythmic activity. Panel B. Breakthrough tonic seizures after 1 week of treatment for SRSE: gradual buildup of low voltage fast activity over 15–20 seconds, often followed by generalized slow wave and diffuse attenuation. Panel C. Interictal EEG one month into hospitalization showing generalized epileptiform discharges. In addition to multifocal epileptiform discharges, most frequent over the left temporal region. All EEGs displayed at 7 uV/mm, HFF 70 Hz, LFF 1 Hz.
Because of the grave prognosis for prolonged refractory tonic SE and failure to improve with currently available medications, the study authors contacted Zogenix, Inc., the manufacturer of fenfluramine, and the US Food and Drug Administration (FDA) to request emergency treatment investigational new drug (IND) approval for administration of fenfluramine. The emergency IND was approved by the FDA, and on hospital Day 36, following approval by our hospital’s institutional review board and informed consent from the patient’s parent, cannabidiol was discontinued and fenfluramine was administered via G-tube at a dose of 0.4 mg/kg/day (18.4 mg/day) in equal divided doses about 12 hours apart. The starting dose was approximately at the midpoint of doses used in the phase 3 clinical trials. During the first week of treatment with fenfluramine, no significant changes in the patient’s clinical condition were observed; in fact, seizures became more frequent. The EEG remained abnormal and was characterized by frequent electrographic seizures, occasional multifocal sharp waves, and diffuse background slowing.
The fenfluramine dose was increased to 0.7 mg/kg/day in divided doses (16.1 mg bid) on hospital Day 42. In addition to the increased dose of fenfluramine, levetiracetam was replaced with brivaracetam (100 mg tid) for improved CNS penetration. One week after the increase in fenfluramine dose, clinical and electrographic seizures began to resolve, and by Day 55, the patient was seizure-free and was off all anesthetic agents. The EEG recording on Day 57 revealed resolution of interictal epileptiform discharges and electrographic seizures. Diffuse background slowing remained evident.
Fenfluramine was continued at 0.7 mg/kg/day. During 2 months of in-patient rehabilitation no tonic-clonic seizures occurred, and the frequency of tonic-clonic seizures during sleep was substantially reduced. After discharge, the patient has demonstrated an enduring improvement in seizure control, neurocognitive function, and quality of life. At present, she has no awake seizures, only 2 or 3 brief tonic seizures nightly, and as of February 2021, has experienced a single tonic-clonic seizure while continuing treatment with fenfluramine (0.7 mg/kg/day). She has not experienced another episode of SE and has not required further rescue benzodiazepines for prolonged seizures or seizure clusters (compared with 2 times per week prior to this tonic SE episode). The patient’s neurocognitive status has significantly improved over baseline level. She is more alert and interactive, and her vocabulary and communication skills have significantly improved. Additional anti-seizure medications at the time of manuscript preparation included valproate (2000 mg bid), brivaracetam (100 mg bid), phenobarbital (97.2 mg bid), clobazam (20 mg bid), and levocarnitine (660 mg bid). Other ongoing therapies included rapid cycle VNS therapy and ketogenic diet.
The patient has had regular transthoracic echocardiographic examinations during treatment with fenfluramine, and as of March 2021, no clinically significant abnormalities have been observed.
3. Discussion
SE is common in DS, occurring in about 30% of patients. In studies of mortality in DS, SE is listed as the cause of death in about one-third of cases [7], [12]. In addition to death, serious neurological sequalae are associated with SE. For example, in a study of 15 DS patients with acute encephalopathy precipitated by SE, 4 patients died, and all remaining patients demonstrated moderate (n = 1) or severe (n = 10) neurological dysfunction, including severe cognitive and/or motor impairment in most cases [13]. As is true with other epilepsies, SE may present as refractory SE or as SRSE in patients with DS. A case series of seven patients aged 1.3–23.4 years who presented in refractory SE triggered by fever has been recently reported [14]. All patients were treated with benzodiazepines; four patients died during or shortly following SE, and the remaining three patients died while in a deep coma after 13–60 days. In one of the patients, the condition was likely SRSE based on the 25-day duration of SE before death.
One previously reported case described the use of fenfluramine to treat nonconvulsive SE [15]. In that case, an 8-year-old boy was admitted for nonconvulsive SE characterized by reduced responsiveness. He was also experiencing 10 to 15 generalized tonic-clonic seizures per day. Fenfluramine was available in his region through an early access program; it was added to the patient’s treatment regimen and was titrated to 26 mg/day (the maximum approved daily dose) over 4 days. The patient became more responsive after 4 days, and seizures ceased. Fenfluramine treatment continued, and after 1 month, seizures remained controlled, with frequency reduced from weekly to monthly.
We believe the present case is the first reported case of fenfluramine used for treatment of tonic SRSE. Although the initial dose of fenfluramine, 0.4 mg/kg/day, was ineffective at treating SRSE, once fenfluramine was titrated to 0.7 mg/kg/day, the response was rapid and dramatic. The profound and sustained response to fenfluramine seen in this patient is similar to that observed in double-blind clinical trials and their open-label extension study in patients with DS [9], [10], [16]. Although brivaracetam replaced levetiracetam at the same time that fenfluramine was added, it seems unlikely that this contributed to the patient’s response, based on the fact that its antiseizure mechanism of action is similar to that of levetiracetam [17]. Despite the similar mechanisms of action, brivaracetam enters the CNS more rapidly than levetiracetam and has a 15- to 30-fold greater affinity for the synaptic vesicle protein 2A, the presumed site of action of the two drugs [18]. Thus the potential confounding effect of this switch on the response of the patient cannot be ruled out. It is important to note that concomitant administration of fenfluramine with valproate and clobazam has no effect on the pharmacokinetics of either valproate or clobazam, and therefore, increases in blood concentration of these anti-seizure medications cannot be invoked as an explanation of the patient’s response [19]. In addition to attaining profound seizure control, the patient has made significant neurobehavioral gains, including improved alertness and acquisition of new language and speech skills.
Fenfluramine was previously approved globally as an appetite suppressant to aid in weight loss in overweight and obese adults but was withdrawn following reports of cardiac valve disease in treated patients [20]. Based on this history, all patients with DS who have been treated with fenfluramine have been followed with regular echocardiographic examinations. In the most recent report, 330 patients with DS in the phase 3 program had been treated for a median 631 days (range, 7 to 1086 days) without emergence of cardiac valve disease [21].
During treatment, the patient’s daily dose of fenfluramine 32.2 mg/day exceeded the maximum daily dose for treatment of DS as approved by the United States Food and Drug Administration and the European Medicines Agency (26 mg/day) [8]. It is important to note that treatment with fenfluramine described in this report occurred before fenfluramine was approved in the United States or in Europe, and its use had been approved on an emergency investigational new drug protocol. Further research is necessary to determine the appropriate maximum dosing for treatment of SRSE in patients with DS.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors received professional medical writing and editing assistance provided by Edward Weselcouch, PhD, of PharmaWrite (Princeton, NJ, USA), and paid for by Zogenix, Inc. (Emeryville, CA, USA). Zogenix reviewed the final manuscript to confirm the accuracy of statements about fenfluramine and its use in company-sponsored clinical studies.
Author contributions
Dr. Millett and Suzanne Pach have provided care and treatment for this patient, and because of the dramatic response of the patient to fenfluramine, proposed publishing this case report. Both investigators contributed to drafting and revising of the manuscript and have approved the final copy.
Ethics statement
The work described in this manuscript was conducted in accord with the Declaration of Helsinki for experiments involving humans. The protocol governing the experimental treatment of this patient was approved by the US Food and Drug Administration and was reviewed and approved by the Hoag Hospital Institutional Review Board prior to initiation of treatment. In addition, the parent of the patient provided written signed informed consent prior to initiation of treatment for the patient. The authors have obtained written approval from the parent of the patient for publication of this case report (data on file).
Contributor Information
David Millett, Email: david.millett@hoag.org.
Suzanne Pach, Email: suzanne.pach@hoag.org.
References
- 1.Vasquez A., Farias-Moeller R., Tatum W. Pediatric refractory and super-refractory status epilepticus. Seizure. 2019;68:62–71. doi: 10.1016/j.seizure.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Rai S., Drislane F.W. Treatment of refractory and super-refractory status epilepticus. Neurotherapeutics. 2018;15(3):697–712. doi: 10.1007/s13311-018-0640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantanen A.M., Reinikainen M., Parviainen I., Ruokonen E., Ala-Peijari M., Bäcklund T. Incidence and mortality of super-refractory status epilepticus in adults. Epilepsy Behav. 2015;49:131–134. doi: 10.1016/j.yebeh.2015.04.065. [DOI] [PubMed] [Google Scholar]
- 4.Delaj L., Novy J., Ryvlin P., Marchi N.A., Rossetti A.O. Refractory and super-refractory status epilepticus in adults: a 9-year cohort study. Acta Neurol Scand. 2017;135(1):92–99. doi: 10.1111/ane.12605. [DOI] [PubMed] [Google Scholar]
- 5.Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(Suppl 2):3–9. doi: 10.1111/j.1528-1167.2011.02994.x. [DOI] [PubMed] [Google Scholar]
- 6.Lagae L., Brambilla I., Mingorance A., Gibson E., Battersby A. Quality of life and comorbidities associated with Dravet syndrome severity: a multinational cohort survey. Dev Med Child Neurol. 2018;60(1):63–72. doi: 10.1111/dmcn.13591. [DOI] [PubMed] [Google Scholar]
- 7.Cooper M.S., McIntosh A., Crompton D.E., McMahon J.M., Schneider A., Farrell K. Mortality in Dravet syndrome. Epilepsy Res. 2016;128:43–47. doi: 10.1016/j.eplepsyres.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Zogenix, Inc. Fintepla® (fenfluramine) oral solution prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212102s000lbl.pdf. Published June 2020. Accessed January 14, 2021.
- 9.Lagae L., Sullivan J., Knupp K., Laux L., Polster T., Nikanorova M. Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a randomised, double-blind, placebo-controlled trial. Lancet. 2019;394:2243–2254. doi: 10.1016/S0140-6736(19)32500-0. [DOI] [PubMed] [Google Scholar]
- 10.Nabbout R., Mistry A., Zuberi S., Villeneuve N., Gil-Nagel A., Sanchez-Carpintero R. Fenfluramine for treatment-resistant seizures in patients with Dravet syndrome receiving stiripentol-inclusive regimens: a randomized clinical trial. JAMA Neurol. 2019;77(3):300–308. doi: 10.1001/jamaneurol.2019.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller I, Devinsky O, Auvin S, Thiele EA, Polster T, Laux L, et al. Efficacy and tolerability with FINTEPLA (fenfluramine) in adult patients with Dravet syndrome: a case series of patients participating in phase 3 studies. Presented at the Virtual Annual Meeting of the American Epilepsy Society; December 4-8, 2020.
- 12.Shmuely S., Sisodiya S.M., Gunning W.B., Sander J.W., Thijs R.D. Mortality in Dravet syndrome: a review. Epilepsy Behav. 2016;64(Pt A):69–74. doi: 10.1016/j.yebeh.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Okumura A., Uematsu M., Imataka G., Tanaka M., Okanishi T., Kubota T. Acute encephalopathy in children with Dravet syndrome. Epilepsia. 2012;53(1):79–86. doi: 10.1111/j.1528-1167.2011.03311.x. [DOI] [PubMed] [Google Scholar]
- 14.De Liso P., Pironi V., Mastrangelo M., Battaglia D., Craiu D., Trivisano M. Fatal status epilepticus in Dravet syndrome. Brain Sci. 2020;10(11):889. doi: 10.3390/brainsci10110889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Specchio N., Pietrafusa N., Ferretti A., Trivisano M., Vigevano F. Successful use of fenfluramine in nonconvulsive status epilepticus of Dravet syndrome. Epilepsia. 2020;61(4):831–833. doi: 10.1111/epi.16474. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan J., Scheffer I.E., Lagae L., Nabbout R., Pringsheim M., Talwar D. Fenfluramine HCl (Fintepla®) provides long-term clinically meaningful reduction in seizure frequency: analysis of an ongoing open-label extension study. Epilepsia. 2020;61(11):2396–2404. doi: 10.1111/epi.16722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanon N.T., Gagné J., Wolf D.C., Aboulamer S., Bosoi C.M., Simard A. Favorable adverse effect profile of brivaracetam vs levetiracetam in a preclinical model. Epilepsy Behav. 2018;79:117–125. doi: 10.1016/j.yebeh.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Steinhoff BJ, Staack AM. Levetiracetam and brivaracetam: a review of evidence from clinical trials and clinical experience. Ther Adv Neurol Disord 2019;12:1756286419873518. [DOI] [PMC free article] [PubMed]
- 19.Boyd B., Smith S., Gammaitoni A., Galer B.S., Farfel G.M. A phase I, randomized, open-label, single-dose, 3-period crossover study to evaluate the drug-drug interaction between ZX008 (fenfluramine HCl oral solution) and a regimen of stiripentol, clobazam, and valproate in healthy subjects. Int J Clin Pharmacol Ther. 2019;57(1):11–19. doi: 10.5414/CP203276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connolly H.M., Crary J.L., McGoon M.D., Hensrud D.D., Edwards B.S., Edwards W.D. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(9):581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal A, Galer B, Wong P, Farfel G, Keane MG, Lai WW. Identification of the intermittent nature of physiologic trace mitral and/or aortic regurgitation: long-term longitudinal echocardiogram study in children with Dravet syndrome treated with fenfluramine. Presented at the European Society of Cardiology Congress 2020 - the Digital Experience; August 29−September 1, 2020.