Abstract
Introduction
Gastrointestinal involvement is a common complain observed in 40–60% of systemic lupus erythematosus (SLE) patients. We performed a systematic review of clinically severe and potential life-threatening gastrointestinal manifestations and discuss clinical presentation, pathogenesis and treatment.
Methods
We performed a literature search in English literature using PubMed and Embase from 2000 to December 2020. The following MeSH terms: systemic lupus erythematosus, protein-losing enteropathy, ascites, pancreatitis, vasculitis, intestinal vasculitis, enteritis and diarrhea published in the English literature.
Results
We identified 141 studies (case reports, case series and cohort studies). The most frequent presenting symptoms are acute abdominal pain, nausea, and vomiting. Many of the manifestations were associated with disease activity. Histological features are rarely available, but both vasculitis and thrombosis have been described. There is no treatment guideline. The majority of patients were treated with corticosteroids and the most common immunososupressant were azathioprine, cyclophosphamide and mycophenolate.
Conclusion
Vasculitis and thrombosis may be responsible for severe life-threatening manifestations such as pancreatitis, protein loosing gastroenteritis, acalculous cholecistyitis and enteritis.
Keywords: Gastrointestinal manifestations, Pancreatitis, Enteritis, Ascites, Acalculous cholecistytis, Protein loosing enteritis, Vasculitis and Diarrhea
Highlights
-
•
Severe Gastrointestinal manifestations are rare, however potential life threatening.
-
•
Vasculitis and thrombosis are the most frequent pathological mechanism described.
-
•
Cohort studies with analysis of genetic risk factors and the role of autoantibodies could improve diagnosis and prognosis.
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease with systemic inflammatory manifestations. Skin, kidney, central nervous system involvement, in addition to hematological abnormalities are most frequently observed [1].
Gastrointestinal involvement is a common complain observed in 40–60% of SLE patients [2]. A clinically recognized gastrointestinal manifestations have been described in 8–10% of patients [2,3]. Autopsy studies, on the other hand, report findings of gastrointestinal involvement in 60–70% of patients, suggesting that subclinical or unrecognized involvement is common [3].
Most gastrointestinal manifestations are usually mild [4]. Vasculitis and thrombosis may be responsible for life-threatening manifestations, leading to ischemia, perforation and infarction if not early diagnosed and adequately treated [5].
In this article we performed a systematic review of clinically severe and potential life-threatening gastrointestinal manifestations and discuss clinical presentation, pathogenesis and treatment.
2. Methods
We performed a systematic review of the literature and limited our search to articles published in the English literature from January 2000 to December 2020. The search of relevant references for the exploration of the electronic database in PubMed and Embase using the following MeSH terms: systemic lupus erythematosus, protein-losing enteropathy, ascites, pancreatitis, vasculitis, intestinal vasculitis, enteritis and diarrhea. The articles have been selected when presented this terms in Title/Summary. Bibliographies of articles were reviewed for additional literature not identified through the PubMed and Embase search.
An evaluation of the studies was made using as inclusion criteria: 1. Original articles; 2. Articles in English; 3. Patients with Systemic Lupus Erythematosus 4. Patients who presented gastrointestinal manifestations due to SLE.
Exclusion criteria: 1. Studies before to the year 2000; 2. Patients with manifestations not due to disease activity or other connective tissue disease knowingly responsible for gastrointestinal involvement were excluded from the analyses; 3. Articles using animal models; 4. Studies containing overlapping or insufficient data.
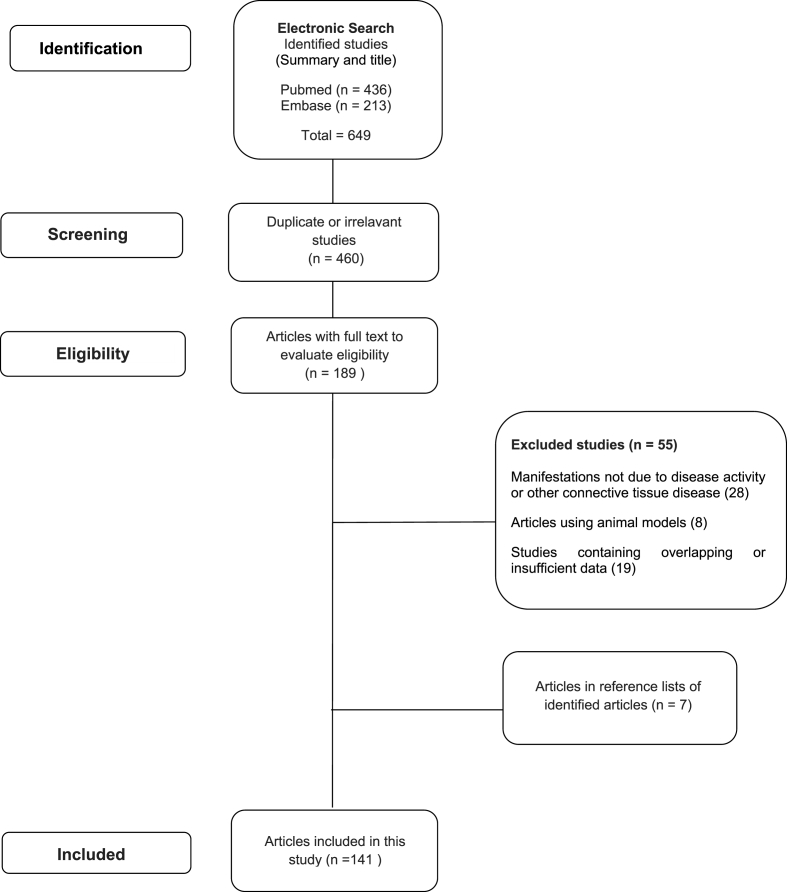
At the end of the analysis, 141 relevant articles were included in this study and the PRISM flowchart is shown in Fig. 1.
Fig. 1.
PRISMA flowchart of this systematic literature review on gastrointestinal involvement in systemic lupus erythematosus.
3. Results
3.1. Pancreatitis
We obtaining a total of 55 articles, including case report or case series and case-control studies of pancreatitis in SLE [4,6–59]. The most frequent presenting symptoms are acute abdominal pain, nausea and vomiting [6,15,[17], [18], [19], [20], [21],[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33],[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50],[52], [53], [54], [55], [56], [57], [58]] (Table 1).
Table 1.
SLE pancreatitis. Summary of case reports and case series (2000–2020).
| Signs and symptoms | Abdominal pain, nausea, vomiting06,15,17-21,23-33,35-50,52-58 |
| Fever15,17,25,38,40,42-44,50,52,55,56,59 | |
| ↓Bowel sounds20,30,43,44,51 | |
| Time at pancreatitis onset | At SLE diagnosis18,19,21,25-33,40,42,44-48,50,55,56,57,59 |
| Concomitant SLE activity06,15,17-20,24-36,38-46,49,50,52,53,57,58 | |
| Associations | Sjogren syndrome16 |
| Macrophage activation syndrome18 | |
| Leukoencephalopathy47 | |
| ↑: D-dimer40 | |
| aPL+33 | |
| anti-La16 | |
| Lower income and less private insurance16 | |
| More disability16 | |
| Urticarial lesions49 | |
| Smoking16 | |
| Organ involvement32,49 | |
| Intraductal papillary mucinous neoplasm of the pancreas51 | |
| Hypertriglyceridemia53 | |
| Laboratory findings | ↑Amylase and lipase06,14-16,18-20,23,26,27,35-37,43,45,46,52,55,57-59 |
| Treatment | Steroids15,17-20,23-32,36-39,41-50,52,55-59 |
| Other immunossupressants: | |
| CFO06,18,20,26,39,46,48,50,52,55,57,58 | |
| IVIG47 | |
| MMF06,18,26 | |
| CYA18,47,56 | |
| Plasma exchange (PE) combined with glucocorticosteroids (GC)54 | |
| Outcome | Resolution15,16,18-20,23,25-27,29-31,38,39,45,47,48,50,52,54-57,59 |
| Death17,18,28,32,35,36,57 | |
| Chronicity16,44 | |
| Complications16,18,24,37,41,43,49,50,53,57,58 |
Fever and reduced bowel sounds were reported in 16 of the cases [15,17,20,25,30,38,40,[42], [43], [44],[50], [51], [52],55,56,59]. Pancreatitis was diagnosed within the first year of SLE in 24 studies [18,19,21,[25], [26], [27], [28], [29], [30], [31], [32], [33],40,42,[44], [45], [46], [47], [48],50,[55], [56], [57],59] and concomitant SLE activity was identified in 34 studies [6,15,[17], [18], [19], [20],[24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36],[38], [39], [40], [41], [42], [43], [44], [45], [46],49,50,52,53,57,58]. Increased amylase and lipase were key laboratory features [6,[14], [15], [16],[18], [19], [20],23,26,27,[35], [36], [37],43,45,46,52,55,[57], [58], [59]].
Imaging studies [computer tomography (CT) or ultrasound (US)] were normal in a few; however enlarged pancreas, uniform enhancement of pancreas, peripancreatic fluid, calcification and cysts of the pancreas has been descryibed [4,[6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29],[31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48],52,55,57,58].
In this review we did include only pancreatitis associated with SLE and excluded reports due to infections or mechanical causes. Most reported considered pancreatitis secondary to disease activity and treatment was based on prednisone [oral or intravenous] [15,[17], [18], [19], [20],[23], [24], [25], [26], [27], [28], [29], [30], [31], [32],[36], [37], [38], [39],[41], [42], [43], [44], [45], [46], [47], [48], [49], [50],52,[55], [56], [57], [58], [59]].
Some authors reported the association of cyclophosphamide, cyclosporine A, immunoglobulin, plasmapheresis or rituximab [6,18,20,26,39,46,48,50,52,55,57,58].
Concomitant antiphospholipid antibodies (aPL) was identified in the minority of the cases [33], however most studies did not report on aPL positivity.
Other associations reported were secondary Sjogren's syndrome, anti-La, lower income, less private insurance, more disability and smoking [16], macrophage activation syndrome [18], increased D-dimer [40] and leukoencephalopathy [47], Resolution of pancreatitis was reported in 24 articles [15,16,[18], [19], [20],23,[25], [26], [27],[29], [30], [31],38,39,45,47,48,50,52,[54], [55], [56], [57],59]. Deaths, complications and chronicity were reported predominantly by case series [[16], [17], [18],24,28,32,[35], [36], [37],41,43,44,49,50,53,57,58] (Table 1).
3.2. Protein-losing enteropathy
We reviewed a total of 51 studies of protein-losing enteropathy [21,55,56,[60], [107]].
The most frequent symptoms observed were lower leg or generalized edema, ascites and diarrhea (Table 2). Laboratory abnormalities observed were low serum albumin, normal or low protein loss in nearly all patients [[60], [61], [62], [63], [64],66,67,[69], [70], [71],74,[80], [81], [82], [83],86,[88], [89], [90],93,[96], [97], [98],100,106]. Increased cholesterol level was also frequently observed [61,66,[70], [71], [72],74,81,88] (Table 2).
Table 2.
SLE protein loosing enteropathy. Summary of case reports, case series, case control studies (2000–2020).
| Diagnosis | Tc-99 m albumin scintigraphy60-63,67,69,70,71,73,74,77,80,86-90,92,94-100,102,103,105,106 |
| ↑ alpha 1-antitrypsin clearance in the stool61-63,72,93,95,98,107 | |
| Period of PLE onset | At SLE diagnosis21,62,65,71-74,76,79-83,85-87,90,98,106 |
| SLE flare79,84,102,103 | |
| Symptoms | Edema60-64,66-69,71-74,76-83,85-88,90,91,93,98,104,106,107 |
| Ascites60,63,64,66-69,71-74,77,80,81,83,88,93,98,100,107 | |
| Abdominal pain21,60,65,71,85,90 | |
| Nausea and vomiting21,60 | |
| Diarrhea21,60,63-67,70,76,79,81,83,85,87-89,90,92,96,97,101,106,107 | |
| Laboratory findings | Hypoalbuminemia60-64,66-71,74,80-83,86,88-90,93,96-98,100,106,107 |
| Hyperlipoproteinemia61,66,70-72,74,81,88,106 | |
| ↑CA12581,86 | |
| Association | Raynauds phenomenon21 |
| Sjogren syndrome87 | |
| Endoscopic gastric, duodenal or jejunal biopsies or CT images | Chronic inflammation of lamina propria63,79,86-88,90 |
| Thickening bowel wall61,63,70,85,87,90 | |
| Ulcers87,89 | |
| Treatment | Corticosteroid21,60-107 |
| Other immunosupressants | |
| Azathioprine60,65,73,79-81,85-87,96,107 | |
| CFO63,70,72,88,93,96,107 | |
| MMF65,87,92,96,103,107 | |
| MTX64,65,84,96 | |
| IVIg64 | |
| Anti-TNFα63,64 | |
| Ciclosporine A82 | |
| Rituximab93,107 |
Diagnosis was made by Technetium (Tc-99 m) albumin scintigraphy and/or increased α1 antitrypsin clearance in the stool [[60], [61], [62], [63],67,[69], [70], [71], [72], [73], [74],77,80,[86], [87], [88], [89], [90],[92], [93], [94], [95], [96], [97], [98], [99], [100],102,103,[105], [106], [107]]. Additional imaging findings on CT scans revealed thickening of bowel wall [61,63,70,85,87,90].
Biopsy performed in a few patients revealed unspecific inflammation and the absence of vasculitis in the lamina propria [63,79,[86], [87], [88],90].
Protein losing enteropathy was diagnosed at SLE onset [21,62,65,[71], [72], [73], [74],76,[79], [80], [81], [82], [83],[85], [86], [87],90,98,106] or concomitant with SLE flare [79,84,102,103]. Patients with established SLE that presented with new onset gastrointestinal symptoms had the diagnosis of protein losing enteropathy made earlier than patients without SLE diagnosis [84].
Some larger studies observed an association with Raynaud's phenomenon [21], CA-125 [81,86] and Sjogren's Syndrome [87] (Table 2).
In a retrospective case-control study the odds of an SLE patient with anti-SSA positivity developing protein losing enteropathy was 3 times higher than an SLE patient who were anti-SSA-negative [96].
In addition, the best combination of findings predictive of protein losing enteropathy were the simultaneous detection of serum albumin (<22 g/l) and 24-h urine protein levels (<0.8 g/24 h), with a sensitivity of 0.818 and specificity of 0.989 [96]. With adequate treatment, serum albumin is the first parameter to improve, followed by serum complement C3 levels [96].
Most patients reported were treated with corticosteroids [21,60–107]. Additional immunosuppressant used were azathioprine [60,65,73,[79], [80], [81],[85], [86], [87],96,107], followed by cyclophosphamide [63,70,72,88,93,96,107], mycophenolate [65,87,92,96,103,107], IVIg [64], anti-tumor necrosis factor (TNF) [63,64], methotrexate [64,65,84,96] rituximab [93,107] and cyclosporine A [82] (Table 2).
Additional treatment with octreotide [71,75] or ingestion of medium chain triglyceride (MCT) diet [75,85] may also be beneficial in this setting. Prognosis is generally good with full recovery; mortality is infrequently reported [21,60–104].
3.3. Acalculous cholecystitis
We obtained a total of 8 reports/studies addressing acalculous cholecystitis [19,[108], [109], [110], [111], [112], [113], [114]]. The patients usually present right upper quadrant pain and vomiting in the setting of active SLE features [19,[108], [109], [110], [111], [112], [113], [114]].
Diagnosis was made by abdominal ultrasonography [19,[108], [109], [110], [111], [112], [113], [114]], computed tomography scans [19,112], histopathologic examination [108,111] and laparotomy [19]. In some patients cholecystectomy was performed [19,[108], [109], [110], [111]].
Histopathology varies from medium-size vasculitis to thrombotic microangiopathy associated with aPL [19,[108], [109], [110], [111], [112]]. Acalculous cholecystitis was diagnosed at SLE onset [19,110,111] or during the course of active disease [[108], [109],[112], [113], [114]]
Most patients were treated with IV or oral corticosteroids [19,[108], [109], [110], [111], [112], [113], [114]]. Additional immunosuppressants used were cyclophosphamide [19,108,110,111], azathioprine [19,110,111] and cyclosporine [19]. Three studies reported treatment only with corticosteroids, without additional immunosuppressant [[112], [113], [114]].
3.4. Vasculitis
Vasculitis was described affecting the colon and/or the small bowel [19,40,46,[115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139]].
Most patient present with abdominal pain [19,40,[116], [117], [118], [119], [120],[122], [123], [124], [125],129,[131], [132], [133],138,139], nausea/vomiting [118,122,124,126,127,129,139] and abdominal distention [117,120,130].
Fever [122], reduced bowel sounds [119,120,128,129] and acute surgical abdomen [19,117,128,134] may also be the presenting feature (Table 3).
Table 3.
SLE vasculitis. Summary of case reports and case series (2000–2020).
| Signs and symptoms | Abdominal pain19,40,116-120,122-125,129,131-133,138,139 |
| Acute abdomen19,117,128,134 | |
| Nausea, vomiting118,122,124,126,127,129,139 | |
| Abdominal distention117,120,130 | |
| Fever122 | |
| ↓Bowel sounds119,120,128,129 | |
| Associations | Concomitant SLE activity19,117,120-124,127,131-134,138,139 |
| Imaging findings | Intestinal wall thickening19,115,116,118-120,122-124,126-128,130 Mesenteric vessel engorgement115,116,124,134 |
| Biopsy | Inflammation115,118,122,125 |
| Treatment | ↑Steroids or IV steroids19,40,46,115-139 |
| Other immunossupressants: CFO19,116,117,119,120,132,134,138,139 | |
| IVIG118 | |
| MMF117,134 | |
| Rituximab130 | |
| Outcome | Resolution19,119,123-126,128-132,139 |
| Death122,138 | |
| Complications19,117,118,122 |
Concomitant SLE disease activity has frequently been described [19,117,[120], [121], [122], [123], [124],127,[131], [132], [133], [134],138,139].
Imagining studies described intestinal wall thickening [19,115,116,[118], [119], [120],[122], [123], [124],[126], [127], [128],130] and mesenteric vessel engorgement [115,116,124,134]. Some studies reported biopsy results with overt inflammation [115,118,122,125].
Oral or IV steroid was used in all patients [19,40,46,[115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139]]. Other immunosuppressants used were cyclophosphamide [19,116,117,119,120,132,134,138,139], IVIg [118], mycophenolate [117,134] and rituximab [130].
Although it is a potential severe manifestation, most patients had resolution of symptoms with adequate treatment [19,119,[123], [124], [125], [126],[128], [129], [130], [131], [132],139]. Intestinal perforation [19,117,118,122] and death [122,138] were rarely reported (Table 3).
3.5. Diarrhea
We found four case reports [[140], [141], [142], [143]] and two retrospective studies [144,145].
The most common associated symptoms described were abdominal pain [[140], [141], [142], [143], [144], [145]] and vomiting [[140], [141], [142],144,145]. Other signs and symptoms described were ascites [140,[142], [143], [144]], nausea [140,142,144,145], fever [142,143], colitis [142,143], mild hepatic dysfunction [143], pleuritis [141,143], abdominal sensitivity [145] and serositis [143] (Table 4).
Table 4.
SLE Diarrhea. Summary of case reports and case series (2000–2020).
| Signs and Symptoms | Colitis142,143 |
| Diarrhea140-145 | |
| Mild hepatic dysfunction143 | |
| Abdominal pain140-145 | |
| Enterocolitis140 | |
| Enteropathy144 | |
| Fever142,143 | |
| Nausea140,142,144,145 | |
| Pleurite141,143 | |
| Abdominal sensitivity145 | |
| Serosite143 | |
| Vomiting140-142,144,145 | |
| Ascite140,142-144 | |
| Image exams | CT scan140,142,143,145 |
| Ultrasonography141 | |
| 99 mTc-HAS144 | |
| Radiography144,145 | |
| Gastroendoscopy144 | |
| Colonoscopy144 | |
| Main Laboratory findings | Positive antinuclear antibody (+)140,143,144 Proteinuria140-145 |
| ↓ Serum level of C3140,143,145 | |
| ↓ Serum level of C4140,143,144 | |
| anti-DNA (+)141-145 | |
| Serum albumin144,145 | |
| anti-SSA antibody and anti-SSB antibody143,144 | |
| Medications | Corticosteroids140,141,143-145 |
| Cyclophosphamide140,142,144,145 | |
| Azathioprine140,141,142 | |
| Cyclosporine144 | |
| Hydroxychloroquine140 | |
| Mycophenolate Mofetil140,142 | |
| Rituximab140,142 | |
| Intravenous fluids145 | |
| Clinical progression | Clinical improvement140-145 |
| Death144,145 | |
| Type of Study | Case report140-143 |
| Retrospective study144,145 |
In some studies, patients with diarrhea had active disease [140,141,[143], [144], [145]].
Imagining studies included CT scan [140,142,143,145], the main findings included: gastrointestinal thickening such as diffuse hypodense submucosal thickening of the stomach [140], extensive small bowel thickening from the antrum of the stomach through to the distal ileum [142] and wall thickening and trilayered enhancement of small bowel loops [143]. In addition, there were also abdominal ultrasonography [141], 99 mTc-HAS [144], radiography [144,145], gastroendoscopy [144] and colonoscopy [144] (Table 4).
Treatment described mainly included corticosteroids [140,141,[143], [144], [145]], cyclophosphamide [140,142,144,145], azathioprine, rituximab and mycophenolate mofetil [140,142] (Table 4).
Although clinical improvement was the most frequent reported outcome [[140], [141], [142], [143], [144], [145]], there were also some cases of patient deaths [144,145].
3.6. Ascites
We identified a total of 33 studies reporting ascites in SLE [[2], [3], [4], [5], [6], [7],[9], [10], [11], [12], [13], [14],[16], [17], [18],[20], [21], [22], [23],[25], [26], [27],30,32,33,36,40,42,48,63,69,72,146].
Ascites is characterized by a non-normal fluid accumulation located in the peritoneal cavity, which can be acute or chronic in SLE, and may cause various symptoms such as abdominal distension [2,3,12,[16], [17], [18],[20], [21], [22],25,26,30] abdominal discomfort [14,30] or abdominal pain [2,3,12,14,20,22,25,36,63,146].
Marked ascites has also been attributed to chronic lupus peritonitis, characterized by the insidious onset of massive, painless ascites, and found to be unrelated to disease activity [3]. In SLE patients, ascites and/or effusions are more frequently a complication of nephrotic syndrome rather than SLE peritonitis [5].
The most common exams performed were computed tomography [3,6,9,12,18,[20], [21], [22], [23],25,27,32,36,40,42,72,146] followed by ultrasound [3,7,16,17,21,25,30] radiography [9,13,16,17,21,25,30] and colonoscopy [9,13,14,17,22,25,30].
Association with disease activity has been reported in most studies [3,6,[12], [13], [14],17,18,20,21,32,36,40,48,63].
The most recurrent laboratory findings were positive anti-nuclear antibody [2,5,6,[11], [12], [13], [14],17,18,20,21,23,[25], [26], [27],36,69], low level of C3 [5,6,[12], [13], [14],[16], [17], [18],27,30,36] low level of C4 [5,6,12,13,[16], [17], [18],21,36] and increase of CA-125 [12,13,17,18,23,48,146].
Most SLE patients received corticosteroids [2,3,5,7,9,10,[12], [13], [14],[16], [17], [18],[20], [21], [22], [23],[25], [26], [27],30,42,146], hydroxychloroquine [[13], [14], [15], [16], [17], [18],20,21,26], azathioprine [10,14,[20], [21], [22],30], cyclophosphamide [2,[5], [6], [7],17,20,26], mycophenolate mofetil [7,14,16,20,23] and rituximab [7,14,16] (Table 5).
Table 5.
Ascites. Summary of case reports, case series, case control studies (2000–2020).
| Symptoms | Acute abdomen20 |
| Diarrhea03,14,17,20,25,30,72,146 | |
| Abdominal distension02,03,12,16-18,20-22,25,26,30 | |
| Abdominal discomfort14,30 | |
| Abdominal pain02,03,12,14,20,22,25,36,63,146 | |
| Splenomegaly02,11 | |
| Fever02,06,07,11,12,16,20,25,26,72 | |
| Hepatomegaly02 | |
| Hypertension02,03,07 | |
| Nausea or vomiting02,03,20,30,36,72 | |
| Weight loss06,20,22,23,25 | |
| Lack of appetite12,23,27 | |
| Lymphadenopathy11 | |
| Hepatic insufficiency11 | |
| Laboratory findings | Anemia03,05,07,09,12,20,25,26 |
| Positive anti-nuclear antibody02,05,06,11-14,17,18,20,21,23,25-27,36,69 | |
| Anti-dsDNA02,03,05,10,11,12,16,20-23,26,30,36 | |
| Anti-Sm03,05,06,20,22 | |
| Low level of C305,06,12-14,16-18,27,30,36 | |
| Low level of C405,06,12,13,16-18,21,36 | |
| Increase of CA-12512,13,17,18,23,48,146 | |
| Hyperglobulinaemia12 | |
| Hypoalbuminemia02,12,14,23 | |
| Hyperalbuminemia05,21 | |
| Thrombocytopenia02,03,36 | |
| Exams | Endoscopy14,22 |
| Ultrasound03,07,16,17,21,25,30 | |
| Magnetic resonance imaging (MRI)03,06,09,12,13 | |
| Colonoscopy09,13,14,17,22,25,30 | |
| Computed Tomography (CT)03,06,09,12,18,20-23,25,27,32,36,40,42,72,146 | |
| Radiography09,13,16,17,21,25,30 | |
| Changes found by imaging exams | Abdominal edema03,13,30 |
| Intestinal edema13,20,30 | |
| Pleural effusion03,07,11-14,16,18,21,22,27,42,69 | |
| Serosite10,12,13,16,22,25,42,69 | |
| Lupus enteritis03,20 | |
| Peritonitis09,10,12,16,42 | |
| Main Treatment | Corticosteroids02,03,05,07,09,10,12-14,16-18,20-23,25-27,30,42,146 |
| Azathioprine10,14,20-22,30 | |
| Cyclophosphamide02,05-07,17,20,26 | |
| Mycophenolate mofetil07,14,16,20,23 | |
| Hydroxychloroquine13,14,16-18,20,21,26 | |
| Rituximab07,14,16 | |
| Disease progression | Death09,11,26,27,63 |
| Clinical improvement03-07,10,12-14,16,18,20-23,25,26,33,146 |
Although clinical improvement was the most frequent outcome reported [-7,3,10,[12], [13], [14],16,18,[20], [23],25,26,33,146], there were some cases of patient deaths reported [9,11,26,27,63].
4. Discussion
The association of SLE with gastrointestinal autoimmune diseases is common, however mostly mild. Although severe gastrointestinal manifestations are uncommon, they are potentially life-threatening and have to be diagnosed timely to allow adequate treatment [5].
Acute pancreatitis has described to occur in 0.8% of patients screened for pancreatitis and 8% of patients with abdominal pain [7,14,16]. Common clinical symptoms as shown findings include nausea, vomiting, fever, absent bowel sounds and abdominal distension. In most studies pancreatitis may be associated with a history of active SLE, suggesting vasculitis as possible etiology [18,19,21,[25], [26], [27], [28], [29], [30], [31], [32], [33],40,[42], [43], [44], [45], [46], [47], [48]].
This hypothesis is further corroborated with the improvement or resolution with corticosteroid or other immunosuppressant use. In a few cases associations with aPL have been made and thrombosis as etiology cannot be excluded, however it is important to remember that we included only studies with pancreatitis due to SLE and other causes such as mechanical, infections (e.g CMV) and drug related (corticosteroid, azathioprine) have to be always excluded [7,16].
Outcome of pancreatitis is difficult to determine due to many case reports that may have publication bias. Most studies confirm that it is a life-threatening manifestation. In a literature review, corticosteroids were shown to decrease mortality [14].
Cohort studies show a higher frequency of chronic pancreatitis and complications. The overall mortality of pancreatitis in SLE is around 30% associated with other features of active SLE (macrophage activation syndrome, central nervous system manifestations), hypocalcemia, and pancreatic complications [18].
Comparing pancreatitis in childhood onset to adult onset SLE, the first group presents a higher prevalence, a higher disease activity score, more complications and greater mortality rate [17]. We previously reported a case series of acute pancreatitis (AP) and macrophage activation syndrome (MAS) in childhood (cSLE) patients [52].
Protein-losing gastroenteropathy is a clinical syndrome suspected in the presence of hypoalbuminaemia without significant proteinuria, severe liver disease, malabsorption, or poor oral intake [5,[60], [61], [62], [63], [64],[66], [67], [68], [69], [70], [71],[74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86],[88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99],103]. Protein-loss through the intestinal tract is usually diagnosed in an advanced stage with varying degree of edema and less commonly with initially symptoms of diarrhea or abdominal pain [88]. Hypoalbuminemia is the characteristic laboratorial finding, however serum cholesterol may be elevated, and lymphatic leakage may result in hypoglobulinemia, lymphopenia and steatorrhea [88].
Diagnosis can be made by dosage of fecal alpha-1 antitrypsin, a serum protein that is not digested in the gastrointestinal tract and is excreted mostly intact in stool but radioisotopic tests are usually more specific and sensitive [100]. Although α1-antitrypsin clearance is a reliable and easy test, it cannot detect the location of the leakage and results can be influenced by diarrhea or gastrointestinal hemorrhage [101]. False positives of 99 mTc HSA may result from active gastrointestinal bleeding and in vivo breakdown of 99 mTc HSA yielding free pertechnetate from radiolabeling [99,103].
Several mechanisms may underlie the pathogenesis of protein loosing enteropathy in SLE, including intravascular activation and conversion of complement, non-necrotizing vasculitis, acquired lymphangiectasia and increased microvascular/endothelial permeability [64,84].
Since most of the studies are case report, and no systematic approach for the diagnosis protein loosing enteropathy has been made, it is difficult to determine predominant causes in the literature. When performed, biopsy usually showed non-specific low grade inflammation [63,79,[86], [87], [88],90]. Enterocytes regulate their own permeability and secrete cytokines [64]. The lamina propria contains nerves and T and B-lymphocytes, plasma cells, macrophages, mast cells, eosinophils, and neutrophils [64]. Antigenic stimulus results in expansion of resident immune cell populations and the release of chemotactic molecules or cytokines in reaction and attracts circulating immunocytes. Thus, in the presence of an altered immune system activity, such as in SLE flare, this mechanism may lead to increased permeability in the intestinal mucosa [21,62,64,65,[71], [72], [73], [74],76,[79], [80], [81], [82], [83], [84], [85], [86], [87],90,98,102,103].
Literature review has shown response to corticosteroid and other immunosupressants. Beneficial role of octreotide and microcomplement consumption test (MCT) has also been described [71,75,85]. Octreotide is potentially able to reduce microvascular intestinal blood flow, therefore decreasing local lymph formation and lymphatic flow. In addition, octreotide binds to somatostatin receptor SST2RA and exerts an immunomodulatory action [75]. MCT may be beneficially in protein-losing enteropathy because they are better absorbed than long chain fatty acids and they are transported by portal blood to the liver bypassing the lymphatic system [75].
There are only few reports of acalculous cholecystitis in SLE, making estimate prevalence difficult. It is a rare manifestation and usually associated with active SLE features. Histopathology varies from medium-size vasculitis to thrombotic microangiopathy associated with aPL in some articles, as written in the results. Corticosteroid and immunosuppressants have been used, but may not avoid cholecystectomy in all.
A number of terms have been used to describe vasculitis in the gastrointestinal tract including lupus mesenteric vasculitis, mesenteric arteritis, lupus enteritis, lupus arteritis, lupus vasculitis, gastrointestinal vasculitis, intra-abdominal vasculitis and acute gastrointestinal syndrome [5]. Although the underlying lesion in most cases of gastrointestinal (GI) vasculitis in SLE is a small vessel arteritis or venulitis, vasculitis in biopsy studies is not found in all cases [135].
Lupus vasculitis generally presents with abdominal pain, and other signs of abdominal obstruction (distention, nausea and vomiting and reduced bowel sounds) in the setting of SLE activity [19,117,[120], [121], [122], [123], [124],127,[131], [132], [133], [134],138].
Marked ascites has been attributed to chronic lupus peritonitis, characterized by the insidious onset of massive, painless ascites and unrelated to disease activity [72].
Most studies are case reports and case series, making adequate prevalence of this manifestation difficult. Large studies report an overall prevalence of enteritis in SLE patients ranging from 0.2 to 9.7% and from 29% to 65% in patients who had acute abdominal pain [121,133,135,136]. Imaging findings and biopsy help adequate diagnosis in most patients [115,[118], [119], [120],[122], [123], [124],126,128,130,134].
Although the above reviewed gastrointestinal manifestations are rare they have a potential for severe complications and even death. A high index of suspicions is necessary to differentiate disease activity from infection or other secondary causes. Corticosteroids (oral or intravenous) is still the first line treatment. Responses to cyclophosphamide, cyclosporine A, azathioprine, mycophenolate, methotrexate, IVIg, rituximab and anti-TNFα have been described, however no randomized control trial exist up to date to determine the most adequate immunosuppressant medication.
Supportive foundations
Supported by Coordination for the Improvement of Higher Education Personnel, Brazil (CAPES 001); National Counsel of Technological and Scientific Development, Brazil (CNPq 306723/2019-0, 401477/2016-9).
Data sharing statement
Not applicable
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Simone Appenzeller reports financial support was provided by National Council for Scientific and Technological Development. Renan Bazuco Frittoli reports financial support was provided by Coordination of Higher Education Personnel Improvement (001). Jessica Fernandes Vivaldo reports financial support was provided by Coordination of Higher Education Personnel Improvement (CAPES 001).
References
- 1.Ruiz-Irastorza G., Khamashta M.A., Castellino G., Hughes G.R. Systemic lupus erythematosus. Lancet. 2001;357:1027–1032. doi: 10.1016/S0140-6736(00)04239-2. PMID:11293608. [DOI] [PubMed] [Google Scholar]
- 2.Takeno M., Ishigatsubo Y. Intestinal manifestations in systemic lupus erythematosus. Intern. Med. 2006;45:41–42. doi: 10.2169/internalmedicine.45.0136. PMID: 16484736. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman B.I., Katz W.A. The gastrointestinal manifestations of systemic lupus erythematosus: a review of the literature. Semin. Arthritis Rheum. 1980;9:237–247. doi: 10.1016/0049-0172(80)90016-5. PMID: 6996096. [DOI] [PubMed] [Google Scholar]
- 4.Ebert E.C., Hagspiel K.D. Gastrointestinal and hepatic manifestations of systemic lupus erythematosus. J. Clin. Gastroenterol. 2011;45:436–441. doi: 10.1097/MCG.0b013e31820f81b8. PMID: 21422947. [DOI] [PubMed] [Google Scholar]
- 5.Tian X.P., Zhang X. Gastrointestinal involvement in systemic lupus erythematosus: insight into pathogenesis, diagnosis and treatment. World J. Gastroenterol. 2010;16:2971–2977. doi: 10.3748/wjg.v16.i24.2971. PMID: 20572299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrin L., Giurgea I., Baudet-Bonneville V., Deschênes G., Bensman A., Ulinski T. Acute pancreatitis in paediatric systemic lupus erythematosus. Acta Paediatr. 2006;95:121–124. doi: 10.1080/08035250500325090. PMID: 16373310. [DOI] [PubMed] [Google Scholar]
- 7.Izzedine H., Caramella C., Ratziu V., Deray G. Chronic calcifying pancreatitis and systemic lupus erythematous. Pancreas. 2005;31:289–290. doi: 10.1097/01.mpa.0000175162.34704.6b. PMID: 16163063. [DOI] [PubMed] [Google Scholar]
- 8.Ozenc A., Altun H., Hamaloglu E., Ozdemir A. A case of acute pancreatitis in a patient with systemic lupus erythematosus. Acta Chir. Belg. 2005;105:319–321. doi: 10.1080/00015458.2005.11679726. [PMID: 16018530] [DOI] [PubMed] [Google Scholar]
- 9.Nishiyama S., Miyawaki S., Asakawa T. A case of systemic lupus erythematosus with severe acute pancreatitis. Mod. Rheumatol. 2002;12:174–177. doi: 10.3109/s101650200029. PMID: 24383907. [DOI] [PubMed] [Google Scholar]
- 10.Al-Mayouf S.M., Majeed M., Al-Mehaidib A., Alsuhaibani H. Pancreatic pseudocyst in paediatric systemic lupus erythematosus. Clin. Rheumatol. 2002;21:264–266. doi: 10.1007/s10067-002-8294-3. PMID: 12111636. [DOI] [PubMed] [Google Scholar]
- 11.Langlet P., Karmali R., Deprez C., Brandelet B., Kleynen P., Dratwa M., de Koster E., Denis P., Deltenre M. Severe acute pancreatitis associated with peliosis hepatis in a patient with systemic lupus erythematosus. Acta Gastroenterol. 2001;64:298–300. [PMID: 11680052] [PubMed] [Google Scholar]
- 12.Penalva J.C., Martínez J., Pascual E., Palanca V.M., Lluis F., Peiró F., Pérez H., Pérez-Mateo M. Chronic pancreatitis associated with systemic lupus erythematosus in a young girl. Pancreas. 2003;27:275–277. doi: 10.1097/00006676-200310000-00016. PMID: 14508137. [DOI] [PubMed] [Google Scholar]
- 13.Rojas-Serrano J., Cardiel M.H. Lupus patients in an emergency unit. Causes of consultation, hospitalization and outcome. A cohort study. Lupus. 2000;9:601–606. doi: 10.1191/096120300678828785. PMID: 11035435. [DOI] [PubMed] [Google Scholar]
- 14.Nesher G., Breuer G.S., Temprano K., Moore T.L., Dahan D., Baer A., Alberton J., Izbicki G., Hersch M. Lupus-associated pancreatitis. Semin. Arthritis Rheum. 2006;35:260–267. doi: 10.1016/j.semarthrit.2005.08.003. PMID: 16461071 DOI: [DOI] [PubMed] [Google Scholar]
- 15.Fasano S., Coscia M.A., Valentini G. Subclinical pancreatitis as onset of systemic lupus erythematosus. JCR. J. Clin. Rheumatol. 2019;1 doi: 10.1097/rhu.0000000000001133. [DOI] [PubMed] [Google Scholar]
- 16.Makol A., Petri M. Pancreatitis in systemic lupus erythematosus: frequency and associated factors—a review of the Hopkins lupus cohort. J. Rheumatol. 2010;37:341–345. doi: 10.3899/jrheum.090829. PMID: 20032096. [DOI] [PubMed] [Google Scholar]
- 17.Wang C.H., Yao T.C., Huang Y.L., Ou L.S., Yeh K.W., Huang J.L. Acute pancreatitis in pediatric and adult-onset systemic lupus erythematosus: a comparison and review of the literature. Lupus. 2011;20:443–452. doi: 10.1177/0961203310387179. PMID: 21335396. [DOI] [PubMed] [Google Scholar]
- 18.Campos L.M., Omori C.H., Lotito A.P., Jesus A.A., Porta G., Silva C.A. Acute pancreatitis in juvenile systemic lupus erythematosus: a manifestation of macrophage activation syndrome? Lupus. 2010;19:1654–1658. doi: 10.1177/0961203310378863. PMID: 20837568. [DOI] [PubMed] [Google Scholar]
- 19.Richer O., Ulinski T., Lemelle I., Ranchin B., Loirat C., Piette J.C., Pillet P., Quartier P., Salomon R., Bader-Meunier B., French Pediatric-Onset SLE Study Group Abdominal manifestations in childhood-onset systemic lupus erythematosus. Ann. Rheum. Dis. 2007;66:174–178. doi: 10.1136/ard.2005.050070. PMID: 16818463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malaviya A.N., Sharma A., Agarwal D., Kapoor S., Garg S., Singh S., Rawat R. Acute abdomen in SLE. Int J Rheum Dis. 2011;14:98–104. doi: 10.1111/j.1756-185X.2010.01581.x. PMID: 21303489. [DOI] [PubMed] [Google Scholar]
- 21.Xu D., Yang H., Lai C.C., Li P., Zhang X., Yang X.O., Zhang F.C., Qian J.M. Clinical analysis of systemic lupus erythematosus with gastrointestinal manifestations. Lupus. 2010;19:866–869. doi: 10.1177/0961203310365883. PMID: 20410154. [DOI] [PubMed] [Google Scholar]
- 22.Phongkitkarun S., Boonnumsirikij M., Jatchavala J., Tong-u-thaisri P. Abdominal manifestation and complications in systemic lupus erythematosus: emphasis on CT findings. J. Med. Assoc. Thai. 2007;90:2112–2120. PMID: 18041431] [PubMed] [Google Scholar]
- 23.Rodriguez E.A., Sussman D.A., Rodriguez V.R. Systemic lupus erythematosus pancreatitis: an uncommon presentation of a common disease. Am J Case Rep. 2014;15:501–503. doi: 10.12659/AJCR.891281. PMID: 25399483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh M., Wani S., Murtaza M., Joglekar S., Kasubhai M. Systemic lupus erythematosus presenting with acute fatal pancreatitis as an initial manifestation. Am. J. Gastroenterol. 2001;96:2280–2281. doi: 10.1111/j.1572-0241.2001.03992.x. PMID: 11467682. [DOI] [PubMed] [Google Scholar]
- 25.Duncan H.V., Achara G. A rare initial manifestation of systemic lupus erythematosus--acute pancreatitis: case report and review of the literature. J. Am. Board Fam. Pract. 2003;16:334–338. doi: 10.3122/jabfm.16.4.334. PMID: 12949035. [DOI] [PubMed] [Google Scholar]
- 26.Kim J.S., Ptaszny M.E., Hajmomenian H.R., Wallace W.D. Proceedings of UCLA healthcare. 2011;15 http://www.med.ucla.edu/modules/xfsection/article.php?articleid=518 [Google Scholar]
- 27.Wang F., Wang N.S., Zhao B.H., Tang L.Q. Acute pancreatitis as an initial symptom of systemic lupus erythematosus: a case report and review of the literature. World J. Gastroenterol. 2005;11:4766–4768. doi: 10.3748/wjg.v11.i30.4766. PMID: 16094728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pascual-Ramos V., Duarte-Rojo A., Villa A.R., Hernández-Cruz B., Alarcón-Segovia D., Alcocer-Varela J., Robles-Díaz G. Systemic lupus erythematosus as a cause and prognostic factor of acute pancreatitis. J. Rheumatol. 2004;31:707–712. [PMID: 15088295] [PubMed] [Google Scholar]
- 29.Masoodi I. The simultaneous incidence of acute pancreatitis and autoimmune hemolytic anemia: a rare duo in a patient with SLE. Ger. Med. Sci. 2014;12:12. doi: 10.3205/000197. PMID: 25276114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia Y., Ortiz A., Mccallum R., Salameh H., Serrato P. Acute pancreatitis as the initial presentation of systematic lupus erythematosus. Case Rep Gastrointest Med. 2014;2014:571493. doi: 10.1155/2014/571493. PMID: 25197582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Limwattana S., Dissaneewate P., Kritsaneepaiboon S., Dendumrongsup T., Vachvanichsanong P. Systemic lupus erythematosus-related pancreatitis in children. Clin. Rheumatol. 2013;32:913–918. doi: 10.1007/s10067-013-2242-2. PMID: 23673437. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y., Ye Y., Liang L., Wu T., Zhan Z., Yang X., Xu H. Systemic-lupus-erythematosus-related acute pancreatitis: a cohort from South China. Clin. Dev. Immunol. 2012;2012:568564. doi: 10.1155/2012/568564. PMID: 22761631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goel R., Danda D., Mathew J., Chacko A. Pancreatitis in systemic lupus erythematosus - case series from a tertiary care center in South India. Open Rheumatol. J. 2012;6:21–23. doi: 10.2174/1874312901206010021. PMID: 22529883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medeiros M.M., Fernandes G.H., Pinto N.S., Silveira V.A. Clinical and subclinical pancreatitis in a cohort of patients diagnosed with systemic lupus erythematosus. Clin. Exp. Rheumatol. 2011;29:776–782. [PMID: 21962107] [PubMed] [Google Scholar]
- 35.Lariño Noia J., Macías García F., Seijo Ríos S., Iglesias García J., Domínguez Muñoz J.E. Pancreatitis and systemic lupus erythematosus. Rev. Esp. Enferm. Dig. 2009;101:571–579. doi: 10.4321/s1130-01082009000800009. PMID: 19785498. [DOI] [PubMed] [Google Scholar]
- 36.Cairoli E., Pérez G., Briva A., Cancela M., Alonso J. Fatal acute pancreatitis complicated by pancreatic pseudocysts in a patient with systemic lupus erythematosus. Rheumatol. Int. 2010;30:675–678. doi: 10.1007/s00296-009-0964-x. PMID: 19466420. [DOI] [PubMed] [Google Scholar]
- 37.Myung D.S., Kim T.J., Lee S.J., Park S.C., Kim J.S., Kim J.C., Yoon W., Lee S.S., Park Y.W. Lupus-associated pancreatitis complicated by pancreatic pseudocyst and central nervous system vasculitis. Lupus. 2009;18:74–77. doi: 10.1177/0961203308093462. PMID: 19074172. [DOI] [PubMed] [Google Scholar]
- 38.Fan H.C., Cheng S.N., Hua Y.M., Chu C.H., Juan C.J., Lee M.Y., Hung C.H. Systemic lupus erythematosus-related acute pancreatitis: a case report. J. Microbiol. Immunol. Infect. 2003;36:212–214. PMID: 14582568] [PubMed] [Google Scholar]
- 39.Gonzalez-Echavarri C., Pernas B., Ugarte A., Ruiz-Irastorza G. Severe multiorganic flare of systemic lupus erythematosus successfully treated with rituximab and cyclophosphamide avoiding high doses of prednisone. Lupus. 2014;23:323–326. doi: 10.1177/0961203314520842. PMID: 24531426. [DOI] [PubMed] [Google Scholar]
- 40.Yuan S., Lian F., Chen D., Li H., Qiu Q., Zhan Z., Ye Y., Xu H., Liang L., Yang X. Clinical features and associated factors of abdominal pain in systemic lupus erythematosus. J. Rheumatol. 2013;40:2015–2022. doi: 10.3899/jrheum.130492. PMID: 24187097. [DOI] [PubMed] [Google Scholar]
- 41.Koga T., Miyashita T., Koga M., Izumi Y., Onizuka S., Fujioka H., Fujiwara S., Nakamichi C., Nakashima K., Migita K. A case of lupus-associated pancreatitis with ruptured pseudoaneurysms. Mod. Rheumatol. 2011;21:428–431. doi: 10.1007/s10165-011-0415-x. PMID: 21308389. [DOI] [PubMed] [Google Scholar]
- 42.Ko H.S., Park K.S., Shin J.C. Refractory fever with pancytopenia in postpartum and SLE-induced pancreatitis. Acta Obstet. Gynecol. Scand. 2010;89:1616–1617. doi: 10.3109/00016349.2010.520301. PMID: 21080902. [DOI] [PubMed] [Google Scholar]
- 43.Hoorn E.J., Flink H.J., Kuipers E.J., Poley J.W., Mensink P.B., Dolhain R.J. Complicated systemic lupus erythematosus pancreatitis: pseudocyst, pseudoaneurysm, but real bleeding. Lupus. 2011;20:305–307. doi: 10.1177/0961203310383071. PMID: 20956462. [DOI] [PubMed] [Google Scholar]
- 44.Gutierrez S.C., Pasqua A.V., Casas H., Cremaschi M.B., Valenzuela M.L., Cubilla A.A., Garcia A. Chronic pancreatitis and systemic lupus erythematosus: an uncommon association. Case Rep Gastroenterol. 2008;2:6–10. doi: 10.1159/000112861. PMID: 21490830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi S., Yoshida M., Kitahara T., Abe Y., Tsuchida A., Nojima Y. Autoimmune pancreatitis as the initial presentation of systemic lupus erythematosus. Lupus. 2007;16:133–136. doi: 10.1177/0961203306073137. PMID: 17402370. [DOI] [PubMed] [Google Scholar]
- 46.Vergara-Fernandez O., Zeron-Medina J., Mendez-Probst C., Salgado-Nesme N., Borja-Cacho D., Sanchez-Guerrero J., Medina-Franco H. Acute abdominal pain in patients with systemic lupus erythematosus. J. Gastrointest. Surg. 2009;13:1351–1357. doi: 10.1007/s11605-009-0897-4. PMID: 19415401. [DOI] [PubMed] [Google Scholar]
- 47.Swol-Ben J., Bruns C.J., Müller-Ladner U., Hofstädter F., Link J., Hechenrieder C., Jauch K.W. Leukoencephalopathy and chronic pancreatitis as concomitant manifestations of systemic lupus erythematosus related to anticardiolipin antibodies. Rheumatol. Int. 2004;24:177–181. doi: 10.1007/s00296-003-0366-4. PMID: 12937945. [DOI] [PubMed] [Google Scholar]
- 48.Singh R., Saunders B., Scopelitis E. Pancreatitis leading to thrombotic thrombocytopenic purpura in systemic lupus erythematosus: a case report and review of literature. Lupus. 2003;12:136–139. doi: 10.1191/0961203303lu258cr. PMID: 12630759. [DOI] [PubMed] [Google Scholar]
- 49.Takao M., Hamada T., Kaji T., Ikeda-Mizuno K., Takehara-Yasuhara C., Ichimura K., Yanai H., Yshino T., Iwatsuki K. Hypocomplementemic urticarial vasculitis arising in a patient with immunoglobulin G4-related disease. Int. J. Dermatol. 2016 doi: 10.1111/ijd.12868. [DOI] [PubMed] [Google Scholar]
- 50.Marques V.L., Gormezano N.W., Bonfá E., Aikawa N.E., Terreri M.T., Pereira R.M., Magalhães C.S., Guariento A., Appenzeller S., Ferriani V.P., Barbosa C.M., Ramos V.C., Lotufo S., Silva C.A. Pancreatitis subtypes survey in 852 childhood-onset systemic lupus erythematosus patients. J. Pediatr. Gastroenterol. Nutr. 2016 Feb;62(2):328–334. doi: 10.1097/MPG.0000000000000990. [DOI] [PubMed] [Google Scholar]
- 51.Roch A.M., Rosati C.M., Cioffi J.L., Ceppa E.P., DeWitt J.M., Al-Haddad M.A., House M.G., Zyromski N.J., Nakeeb A., Schmidt C.M. Intraductal papillary mucinous neoplasm of the pancreas, one manifestation of a more systemic disease? Am. J. Surg. 2016 Mar;211(3):512–518. doi: 10.1016/j.amjsurg.2015.12.009. Epub 2015 Dec 31. [DOI] [PubMed] [Google Scholar]
- 52.Gormezano N.W., Otsuzi C.I., Barros D.L., da Silva M.A., Pereira R.M., Campos L.M., Borba E.F., Bonfá E., Silva C.A. Macrophage activation syndrome: a severe and frequent manifestation of acute pancreatitis in 362 childhood-onset compared to 1830 adult-onset systemic lupus erythematosus patients. Semin. Arthritis Rheum. 2016 Jun;45(6):706–710. doi: 10.1016/j.semarthrit.2015.10.015. Epub 2015 Nov 5. [DOI] [PubMed] [Google Scholar]
- 53.Wang Q., Shen M., Leng X., Zeng X., Zhang F., Qian J. Prevalence, severity, and clinical features of acute and chronic pancreatitis in patients with systemic lupus erythematosus. Rheumatol. Int. 2016 Oct;36(10):1413–1419. doi: 10.1007/s00296-016-3526-z. Epub 2016 Jul 5. [DOI] [PubMed] [Google Scholar]
- 54.Yuan S., Ye Y., Chen D.D., Qiu C.Q., Zhan Z., F L., Li H., Liang L., Xu H., Yang X. Lupus mesenteric vasculitis: clinical features and associated factors for the recurrence and prognosis of disease. Semin. Arthritis Rheum. 2014;43:759–766. doi: 10.1016/j.semarthrit.2013.11.005. PMID: 24332116 DOI: [DOI] [PubMed] [Google Scholar]
- 55.Lin Q., Zhang M., Tang H., Shen Y., Zhu Y., Xu Q., Li X. Acute pancreatitis and macrophage activation syndrome in pediatric systemic lupus erythematosus: case-based review. Rheumatol. Int. 2019 doi: 10.1007/s00296-019-04388-4. [DOI] [PubMed] [Google Scholar]
- 56.Li Y., Wei M. Acute pancreatitis in childhood-onset systemic lupus erythematosus: case report. (2019) Arch. Argent. Pediatr. 2019;117(3) doi: 10.5546/aap.2019.eng.e279. [DOI] [PubMed] [Google Scholar]
- 57.Dwivedi P., Kumar R.R., Dhooria A., Adarsh M.B., Malhotra S., Kakkar N., Dhir V. Corticosteroid-associated lupus pancreatitis: a case series and systematic review of the literature. Lupus. 2019 doi: 10.1177/0961203319844004. [DOI] [PubMed] [Google Scholar]
- 58.Gayam V., Mandal A.K., Khalid M., Kaler J., Thapa S., Garlapati P., Shrestha B. A rare case of systemic lupus erythematosus with gastric ulcer and acute pancreatitis: a case report and literature review. Gastroenterol. Res. 2018;11(4):321–325. doi: 10.14740/gr1048w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basturk A., Artan R., Yilmaz A., Senol U. A paediatric systemic lupus erythematosus patient presenting with acute pancreatitis: a rare case. Gastroenterol. Rev. 2016;4:296–298. doi: 10.5114/pg.2016.61469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carneiro F.O., Sampaio L.R., Brandão L.A., Braga L.L., Rocha F.A. Protein-losing enteropathy as initial manifestation of systemic lupus erythematosus. Lupus. 2012;21:445–448. doi: 10.1177/0961203311425523. PMID: 21997967. [DOI] [PubMed] [Google Scholar]
- 61.Aoki T., Noma N., Takajo I., Yamaga J., Otsuka M., Yuchi H., Ishikawa N., Inatsu H., Sakata J., Asada Y., Eto T. Protein losing gastropathy associated with autoimmune disease: successful treatment withprednisolone. J. Gastroenterol. 2002;37:204–209. doi: 10.1007/s005350200022. PMID: 11931534. [DOI] [PubMed] [Google Scholar]
- 62.Awazawa R., Yamamoto Y., Mine Y., Nakamura I., Kishimoto K., Kinjyo F., Hagiwara K., Fujita J., Uezato H., Takahashi K. Systemic lupus erythematosus complicated with protein-losing enteropathy: a case report andreview of the published works. J. Dermatol. 2012;39:454–461. doi: 10.1111/j.1346-8138.2011.01404.x. PMID: 22035257. [DOI] [PubMed] [Google Scholar]
- 63.Al-Mogairen S.M. Lupus protein-losing enteropathy (LUPLE): a systematic review. Rheumatol. Int. 2011;31:995–1001. doi: 10.1007/s00296-011-1827-9. PMID: 21344315. [DOI] [PubMed] [Google Scholar]
- 64.Yazici Y., Erkan D., Levine D.M., Parker T.S., Lockshin M.D. Protein-losing enteropathy in systemic lupus erythematosus: report of a severe, persistent case and review of pathophysiology. Lupus. 2002;11:119–123. doi: 10.1191/0961203302lu152cr. PMID: 11958575. [DOI] [PubMed] [Google Scholar]
- 65.Gornisiewicz M., Rodriguez M., Smith J.K., Saag K., Alarcón G.S. Protein-losing enteropathy in a young African-American woman with abdominal pain, diarrhea and hydronephrosis. Lupus. 2001;10:835–840. doi: 10.1191/096120301701548472. PMID: 11787872. [DOI] [PubMed] [Google Scholar]
- 66.Nakajima A., Ohnishi S., Mimura T., Kubo K., Suzuki A., Yazaki Y., Matsuhashi N. Protein-losing enteropathy associated with hypocomplementemia and anti-nuclear antibodies. J. Gastroenterol. 2000;35:627–630. doi: 10.1007/s005350070063. PMID: 10955602. [DOI] [PubMed] [Google Scholar]
- 67.Miyata M., Yoshida M., Saka M., Kasukawa R. Protein-losing gastroenteropathy in system lupus erythematosus: diagnosis with 99mTc--human serum albumin scintigraphy. Arthritis Rheum. 2000;43:1900. [PMID: 10943884] [PubMed] [Google Scholar]
- 68.Law S.T., Ma K.M., Li K.K. Protein-losing enteropathy associated with or without systemic autoimmune disease: what are the differences? Eur. J. Gastroenterol. Hepatol. 2012;24:294–302. doi: 10.1097/MEG.0b013e32834f3ea0. PMID: 22157233. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y.F., Tseng K.C., Chiu J.S., Chuang M.H., Chung M.I., Lai N.S. Outcome of surgical resection for protein-losing enteropathy in systemic lupus erythematosus. Clin. Rheumatol. 2008;27:1325–1328. doi: 10.1007/s10067-008-0929-6. PMID: 18500433. [DOI] [PubMed] [Google Scholar]
- 70.Kim Y.G., Lee C.K., Byeon J.S., Myung S.J., Oh J.S., Nah S.S., Moon H.B., Yoo B. Serum cholesterol in idiopathic and lupus-related protein-losing enteropathy. Lupus. 2008;17:575–579. doi: 10.1177/0961203307087407. PMID: 18539712. [DOI] [PubMed] [Google Scholar]
- 71.Hung J., Wood C.A., Woronik V., Vieira J.M., Jr., Barros R.T. Protein-losing gastroenteropathy in a patient with systemic lupus erythematosus and antiphospholipid antibody syndrome simulating nephrotic syndrome. Nephrol. Dial. Transplant. 2006;21:202–208. doi: 10.1093/ndt/gfl043. PMID: 16490742. [DOI] [PubMed] [Google Scholar]
- 72.Lee C.K., Han J.M., Lee K.N., Lee E.Y., Shin J.H., Cho Y.S., Koh Y., Yoo B., Moon H.B. Concurrent occurrence of chylothorax, chylous ascites, and protein-losing enteropathy insystemic lupus erythematosus. J. Rheumatol. 2002;29:1330–1333. [PMID: 12064855] [PubMed] [Google Scholar]
- 73.Mok C.C., Ying K.Y., Mak A., To C.H. Szeto ML Outcome of protein-losing gastroenteropathy in systemic lupus erythematosus treated with prednisolone and azathioprine. Rheumatology. 2006;45:425–429. doi: 10.1093/rheumatology/kei164. PMID: 16234272. [DOI] [PubMed] [Google Scholar]
- 74.Wu C.C., Lin S.H., Chu P., Lai J.H., Chang D.M., Lin Y.F. An unrecognized cause of oedema in a patient with lupus nephritis: protein losing enteropathy. Nephrol. Dial. Transplant. 2004;19:2149–2150. doi: 10.1093/ndt/gfh226. PMID: 15252179. [DOI] [PubMed] [Google Scholar]
- 75.Ossandon A., Bombardieri M., Coari G., Graziani G., Valesini G. Protein losing enteropathy in systemic lupus erythematosus: role of diet and octreotide. Lupus. 2002;11:465–466. doi: 10.1191/0961203302lu233xx. PMID: 12195791. [DOI] [PubMed] [Google Scholar]
- 76.Li H., Zhang X., Chen J. Successful treatment of steroid-refractory systemic lupus erythematosus-associated protein-losing enteropathy using combination therapy with tacrolimus and steroid. Lupus. 2011;20:1109–1111. doi: 10.1177/0961203311406766. PMID: 21768173. [DOI] [PubMed] [Google Scholar]
- 77.Chng H.H., Tan B.E., Teh C.L., Lian T.Y. Major gastrointestinal manifestations in lupus patients in Asia: lupus enteritis, intestinal pseudo-obstruction, and protein-losing gastroenteropathy. Lupus. 2010;19:1404–1413. doi: 10.1177/0961203310374337. PMID: 20947549. [DOI] [PubMed] [Google Scholar]
- 78.Tsao Y.T., Wu C.C., Hsu Y.J., Chu P. Acute right ventricular failure in lupus-associated protein-losing enteropathy. Am. J. Emerg. Med. 2010;845:1–3. doi: 10.1016/j.ajem.2009.12.009. PMID: 20837273. [DOI] [PubMed] [Google Scholar]
- 79.Law S.T., Ma K.M., Li K.K. The clinical characteristics of lupus related protein-losing enteropathy in Hong Kong Chinese population: 10 years of experience from a regional hospital. Lupus. 2012;21:840–847. doi: 10.1177/0961203312438113. PMID: 22343095. [DOI] [PubMed] [Google Scholar]
- 80.Aguiar F.M., Menescal Z.L., Costa D.M., Correia J.W., Paiva J.G., Correia J.M. Protein-losing enteropathy in systemic lupus erythematosus: case report. Rev. Bras. Reumatol. 2012;52:960–964. doi: 10.1590/S0482-50042012000600013. PMID: 23223705. [DOI] [PubMed] [Google Scholar]
- 81.Ranawaka N., Atukorala I., Fernandopulle N., Nawarathna M. An unusual cause of generalized oedema in systemic lupus erythematosus. Rheumatology. 2012;51:2298–2300. doi: 10.1093/rheumatology/kes157. PMID: 22753772 DOI: [DOI] [PubMed] [Google Scholar]
- 82.Chang Y.S., Lai C.C., Chen W.S., Wang S.H., Chou C.T., Tsai C.Y. Protein-losing enteropathy and premature ovarian failure in a young woman with systemic lupus erythematosus. Lupus. 2012;21:1237–1239. doi: 10.1177/0961203312449492. PMID: 22627066. [DOI] [PubMed] [Google Scholar]
- 83.Ratnayake E.C., Riyaaz A.A., Wijesiriwardena B.C. Sytemic lupus erythematosus presenting with protein losing enteropathy in a resource limited centre: a case report. Int. Arch. Med. 2012;5:1. doi: 10.1186/1755-7682-5-1. PMID: 22281038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Law S.T., Ma K.M., Li K.K. Clinical characteristics of concurrent and sequentially presented lupus-related protein-losing enteropathy: what are their differences? Rheumatol. Int. 2013;33:85–92. doi: 10.1007/s00296-011-2356-2. PMID: 22218644. [DOI] [PubMed] [Google Scholar]
- 85.Cohen M.J., Theodor I., Elazary A.S., Bekerman P., Nahmias A., Rubinov A., Ben-Yehuda A. Severe malnutrition due to systemic lupus erythematosus associated protein losing enteropathy. Nutrition. 2012;28:220–223. doi: 10.1016/j.nut.2011.07.017. PMID: 22208557. [DOI] [PubMed] [Google Scholar]
- 86.Türkçapar N., Ozyüncü N., Cinar K., Ensari A., Küçük O., Idilman R., Duman N., Ozden A. A case of systemic lupus erythematosus presenting with protein-losing enteropathy. Turk. J. Gastroenterol. 2006;17:226–230. [PMID: 16941261] [PubMed] [Google Scholar]
- 87.Ong C.S., Cheah T.E., Jasmin R., Yahya F., Sockalingam S., Ng C.T. Painless as/cites and elevated CA125: initial presentation of lupus-associated protein-losing enteropathy. Lupus. 2013;22:1174–1177. doi: 10.1177/0961203313498792. PMID: 23886639. [DOI] [PubMed] [Google Scholar]
- 88.Werner de Castro G.R., Appenzeller S., Bértolo M.B., Costallat L.T. Protein-losing enteropathy associated with systemic lupus erythematosus: response to cyclophosphamide. Rheumatol. Int. 2005;25:135–138. doi: 10.1007/s00296-004-0483-8. PMID: 15249982. [DOI] [PubMed] [Google Scholar]
- 89.Sano S., Yamagami K., Tanaka A., Nishio M., Nakamura T., Kubo Y., Inoue T., Ueda W., Okawa K., Yoshioka K. A unique case of collagenous colitis presenting as protein-losing enteropathy successfully treated with prednisolone. World J. Gastroenterol. 2008;14:6083–6086. doi: 10.3748/wjg.14.6083. PMID: 18932290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng W.J., Tian X.P., Li L., Jing H.L., Li F., Zeng X.F., Tang F.L. Protein-losing enteropathy in systemic lupus erythematosus: analysis of the clinical features of fifteen patients. J. Clin. Rheumatol. 2007;13 doi: 10.1097/RHU.0b013e31815bf9c6. PMID: 18176138. [DOI] [PubMed] [Google Scholar]
- 91.Murali A., Narasimhan D., Krishnaveni J., Rajendiran G. Protein losing enteropathy in systemic lupus erythematosus. J. Assoc. Phys. India. 2013;61:747–749. [PMID: 24772735] [PubMed] [Google Scholar]
- 92.Rajendiran A., Viswanathan S., Remalayam B., Muthu V., Alexander T. Chronic diarrhea as the presenting complaint of systemic lupus erythematosus in a man. Intern. Med. 2012;51:3131–3134. doi: 10.2169/internalmedicine.51.8518. PMID: 23154718. [DOI] [PubMed] [Google Scholar]
- 93.Sansinanea P., Carrica S.A., Marcos J., García M.A. Protein-losing enteropathy associated with refractory systemic lupus erythematosus with a good response to rituximab. Reumatol. Clínica. 2015;1699–258 doi: 10.1016/j.reuma.2015.01.009. PMID: 25818375. [DOI] [PubMed] [Google Scholar]
- 94.Chen Z., Li M.T., Xu D., Yang H., Li J., Zhao J.L., Zhang H.H., Han S.M., Xu T., Zeng X.F. Protein-losing enteropathy in systemic lupus erythematosus: 12 years' experience from a Chinese academic center. PloS One. 2014;9:e114684. doi: 10.1371/journal.pone.0114684. PMID: 25490025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.El-Shafie K.T., Al-Shirawi A., Al-Maskari B., Samir N. A possible case of systemic lupus erythematosus presenting with generalised oedema. Sultan Qaboos Univ Med J. 2014;14:582–584. PMID: 25364567] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhen C., Meng-Tao L., Dong X., Hong Y., Jing L., Jiu-Liang Z., Heng-Hui Z., Shao-Mei H., Tao X., Xiao-Feng Z. Protein-losing enteropathy in systemic lupus erythematosus: 12 Years' experience from a Chinese academic center. PloS One. 2014;9(9) doi: 10.1371/journal.pone.0114684. PMID: 25490025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soichi S., Keiko Y., Ayako T., Minako N., Tomoyuki N., Yuki K., Takeshi I., Wataru U., Kiyotaka O., Katsunobu Y. A unique case of collagenous colitis presenting as proteinlosing enteropathy successfully treated with prednisolone. World J. Gastroenterol. 2008;14:6083–6086. doi: 10.3748/wjg.14.6083. PMID: 18932290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Florent C., Vidon N., Flourie B. Gastric clearance of alpha-1-antitrypsin under cimetidine perfusion: new test to detect protein-losing gastropathy? J. Dermatol. (Tokyo) 2012;39:454–461. doi: 10.1007/BF01347903. [PMID: 3484447] [DOI] [PubMed] [Google Scholar]
- 99.Hsu Y.J., Lin S.H., Lin Y.F. Pitfalls of technetium-99 mlabeled human serum albumin scintigraphy for protein-losing enteropathy. Kidney Int. 2009;76 doi: 10.1038/ki.2009.271. PMID: 19789543. [DOI] [PubMed] [Google Scholar]
- 100.Chen Y.C., Hwang S.J., Chiu J.S. Chronic edema from protein-losing enteropathy: scintigraphic diagnosis. Kidney Int. 2009;75:1124. doi: 10.1038/ki.2008.337. PMID: 19404299. [DOI] [PubMed] [Google Scholar]
- 101.Matan J.C., Iris Theodor R.N., Anat S.E., Pazit B., Avital N., Alan R., Arie B.Y. Severe mal nutrition due to systemic lupus erythematosus associated protein losing enteropathy. Nutrition. 2012;28:220–223. doi: 10.1016/j.nut.2011.07.017. PMID: 22208557. [DOI] [PubMed] [Google Scholar]
- 102.Mikako Y., Masayuki M., Mitsuru S., Tomomi S., Hiroshi S., JunkoT Hideo S., Ayako S., Masae K., Tomoe N., Reiji K. Protein-losing enteropathy exacerbated with the appearance of symptoms of. systemic lupus erythematosus Internal Medicine. 2001;40:449–453. doi: 10.2169/internalmedicine.40.449. PMID: 11393422. [DOI] [PubMed] [Google Scholar]
- 103.Ashwin R., Stalin V., Bhavith R., Vivekanandan M., Thomas A. Chronic diarrhea as the presenting complaint of systemic lupus erythematosus in a man. Intern. Med. 2012;51:3131–3134. doi: 10.2169/internalmedicine.51.8518. PMID: 23154718. [DOI] [PubMed] [Google Scholar]
- 104.Christian M.H., Hildegard Z., Simon S., Martin W.L., Kathrin W., Gabriele H., Georg H., Manfred G. Early onset systemic lupus erythematosus: differential diagnoses, clinical presentation, and treatment options. Clin. Rheumatol. 2011;30:275–283. doi: 10.1007/s10067-010-1576-2. PMID: 20886250. [DOI] [PubMed] [Google Scholar]
- 105.Ryoko A., Yu-ichi Y., Yoshiko M., 1 Ikumi N. Systemic lupus erythematosus complicated with protein-losing enteropathy: a case report and review of the published works. J. Dermatol. (Tokyo) 2012;39:454–461. doi: 10.1111/j.1346-8138.2011.01404.x. PMID: 22035257. [DOI] [PubMed] [Google Scholar]
- 106.Lim D.H., Kim Y.G., Bae S.H., Ahn S., Hong S., Lee C.K., Yoo B. Factors related to outcomes in lupus-related protein-losing enteropathy. Korean J Intern Med. 2015 Nov;30(6):906–912. doi: 10.3904/kjim.2015.30.6.906. Epub 2015 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sansinanea P., Carrica S.A., Marcos J., García M.A. Protein-losing enteropathy associated with refractory systemic lupus erythematosus with a good response to rituximab. Reumatol. Clínica. 2016 Jan-Feb;12(1):47–49. doi: 10.1016/j.reuma.2015.01.009. Epub 2015 Mar 26. [DOI] [PubMed] [Google Scholar]
- 108.Basiratnia M., Vasei M., Bahador A., Ebrahimi E., Derakhshan A. Acute acalculous cholecystitis in a child with systemic lupus erythematosus. Pediatr. Nephrol. 2006;21:873–876. doi: 10.1007/s00467-006-0021-x. PMID: 16575590. [DOI] [PubMed] [Google Scholar]
- 109.Bando H., Kobayashi S., Matsumoto T., Tamura N., Yamanaka K., Yamaji C., Takasaki C., Takasaki Y., Hashimoto H. Acute acalculous cholecystitis induced by mesenteric inflammatory veno-occlusive disease (MIVOD) in systemic lupus erythematosus. Clin. Rheumatol. 2003;22:447–449. doi: 10.1007/s10067-003-0766-6. PMID: 14677025. [DOI] [PubMed] [Google Scholar]
- 110.Mendonça J.A., Marques-Neto J.F., Prando P., Appenzeller S. Acute acalculous cholecystitis in juvenile systemic lupus erythematosus. Lupus. 2009;18:561–563. doi: 10.1177/0961203308098587. PMID: 19395459. [DOI] [PubMed] [Google Scholar]
- 111.De-Leon-Bojorge B., Zaltzman-Girsevich S., Ortega-Salgado A., Prieto-Patron A., Córdoba-Córdoba R., Yamazaki-Nakashimada M. Thrombotic microangiopathy involving the gallbladder as an unusual manifestation of systemic lupus erythematosus and antiphospholipid syndrome: case report and review of the literature. World J. Gastroenterol. 2006;12:7206–7209. doi: 10.3748/wjg.v12.i44.7206. PMID: 17131489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu W., Chen W., He X., Qu Q., Hong T., Li B. Successful treatment using corticosteroid combined antibiotic for acute acalculous cholecystitis patients with systemic lupus erythematosus. Medicine (Baltim.) 2017 Jul;96(27) doi: 10.1097/MD.0000000000007478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu X., Li B., Zheng C. Hepatobiliary and Pancreatic: acute acalculous cholecystitis in systemic lupus erythematosus, successfully treated with corticosteroid. J. Gastroenterol. Hepatol. 2016 Oct;31(10):1673. doi: 10.1111/jgh.13430. [DOI] [PubMed] [Google Scholar]
- 114.Obreja E.I., Salazar C., Torres D.G. Polyserositis and acute acalculous cholecystitis: an uncommon manifestation of undiagnosed. Systemic Lupus Erythematosus. 2019 Jun 14;11(6):e4899. doi: 10.7759/cureus.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lawrence J., Brandt G.I. Vasculitis associated with systemic lupus erythematosus. Gastrointest. Endosc. 2010;72:618–619. doi: 10.1016/j.gie.2010.02.033. PMID: 20541196. [DOI] [PubMed] [Google Scholar]
- 116.Freda H.P., Ioannis D.D., Garyfalia P., Zenia S., Herakles K., Dimitrios G., Dimitrios T.B. Intestinal ischemia as the first manifestation of vasculitis. Semin. Arthritis Rheum. 2004;34:431–441. doi: 10.1016/j.semarthrit.2003.12.004. PMID: 15305242. [DOI] [PubMed] [Google Scholar]
- 117.Yuan S., Ye Y., Chen D.D., Qiu C.Q., Zhan Z., F L., Li H., Liang L., Xu H., Yang X. Lupus mesenteric vasculitis: clinical features and associated factors for the recurrence and prognosis of disease. Semin. Arthritis Rheum. 2014;43:759–766. doi: 10.1016/j.semarthrit.2013.11.005. PMID: 24332116 DOI: [DOI] [PubMed] [Google Scholar]
- 118.Albuquerque-Nettoa A.F., Erica G., Cavalcanteb E.G., Adriana M., Sallumc E., Aikawab N.E., Tannurid U., Silva C.A.A. Vasculite mesentérica em paciente com lúpus eritematoso sistêmico juvenil. Rev. Bras. Reumatol. 2013;53:219–222. doi: 10.1590/S0482-50042013000200011. PMID: 23856801. [DOI] [Google Scholar]
- 119.Chu Y., Hsu B., Tseng K. Lupus mesenteric vasculitis with GI and genitourinary tract involvement. Clin. Gastroenterol. Hepatol. 2014;12:69–70. doi: 10.1016/j.cgh.2013.12.024. PMID: 24393805 DOI: [DOI] [PubMed] [Google Scholar]
- 120.Mizoguchi F., Nanki T., Miyasak B. Pneumatosis cystoides: intestinalis following lupus enteritis and peritonitis. Inter Med. 2008;47:1267–1271. doi: 10.2169/internalmedicine.47.0748. PMID: 18591854. [DOI] [PubMed] [Google Scholar]
- 121.Kwok S.K., Seo S.H., Ju J.K., Park K.S., Yoon C.H., Kim W.U., Min J.K., Park S.H., Cho C.S., Kim H.Y. Lupus enteritis: clinical characteristics, risk factor for relapse and association with anti-endothelial cell antibody. Lupus. 2007;16:803–809. doi: 10.1177/0961203307082383. PMID: 17895303. [DOI] [PubMed] [Google Scholar]
- 122.Janssens P., Arnaud L., Galicier L., Mathian A., Hi M., Sene D., Haroche J., Veyssier-Belot C., Huynh-Charlier I., Grenier P.A., Piette J., Amoura Z. Lupus enteritis: from clinical findings to therapeutic management. Orphanet J. Rare Dis. 2013;8:67. doi: 10.1186/1750-1172-8-67. PMID: 23642042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gharabaghi1 M.A., Abdollahi P., Kalany M., Sotoudeh M. Systemic lupus erythematosus presenting with eosinophilic enteritis: a case report. J. Med. Case Rep. 2011;5:235. doi: 10.1186/1752-1947-5-235. PMID: 21702974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alsalameh S., Hauggaard A. Mesenteric vasculitis in active systemic lupus erythematosus causing diffuse abdominal pain. Rheumatology. 2013;52:1889. doi: 10.1093/rheumatology/ket281. PMID: 23934312. [DOI] [PubMed] [Google Scholar]
- 125.Diep J.T., Kerr D.L., Sarebahi S., Tismenetsky M. Opportunistic infections mimicking gastrointestinal vasculitis in systemic lupus erythematosus. J. Clin. Rheumatol. 2007;13:213–216. doi: 10.1097/RHU.0b013e318124fdf1. PMID: 17762457. [DOI] [PubMed] [Google Scholar]
- 126.Kornu R., Oliver Q.Z., Reimold A.M. Recognizing concomitant lupus enteritis and lupus cystitis. J. Clin. Rheumatol. 2008;14:226–229. doi: 10.1097/RHU.0b013e318181a8ec. PMID: 18766123. [DOI] [PubMed] [Google Scholar]
- 127.Sran S., Sran M., Patel N., Anand P. Lupus enteritis as an initial presentation of systemic lupus erythematosus. Gastrointestinal Medicine. 2014;ID962735 doi: 10.1155/2014/962735. PMID: 25295199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaneko Y., Hirakata M., Suwa A., Satoh S., Nojima T., Mimori Y.I.T. Systemic lupus erythematosus associated with recurrent lupus enteritis and peritonitis. Clin. Rheumatol. 2004;23:351–354. doi: 10.1007/s10067-004-0882-y. PMID: 15293099. [DOI] [PubMed] [Google Scholar]
- 129.Lin Y., Chen P., Chen H. Mesenteric vasculitis causing ileocecal intussusception as the initial presentation of systemic lupus erythematosus: a case report. Clin. Rheumatol. 2013;32:37–40. doi: 10.1007/s10067-010-1421-7. PMID: 20238134. [DOI] [PubMed] [Google Scholar]
- 130.Fotis L., Baszis K.W., French A.R., Cooper M.A., White A.J. Mesenteric vasculitis in children with systemic lupus erythematosus. Clin. Rheumatol. 2015 Feb 17 doi: 10.1007/s10067-015-2892-3. [Epub ahead of print][PMID: 25687984. [DOI] [PubMed] [Google Scholar]
- 131.Carvalho J.F. Mesenteric vasculitis in a systemic lupus erythematosus patient with a low SLEDAI: an uncommon presentation. Clinics. 2010;65:337–340. doi: 10.1590/S1807-59322010000300016. PMID: 20360927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alcocer-Gouyonnet F., Chan-Nunes C., Hernandez J., Guzman J., Gamboa-Domingues A. Thrombocytopenia and pneumatosis intestinalis as indicators for surgery. Am. Surg. 2000;66:193–195. [PMID: 10695751] [PubMed] [Google Scholar]
- 133.Buck A.C., Serebro L.H., Quinet R.J. Subacute abdominal pain requiring hospitalization in a systemic lupus erythematosus patient: a retrospective analysis and review of the literature. Lupus. 2001;10:491–495. doi: 10.1191/096120301678416051. PMID: 11480847. [DOI] [PubMed] [Google Scholar]
- 134.Tan T.C., Wansaicheong G.K., Thong B.H. Acute onset of systemic lupus erythematosus with extensive gastrointestinal and genitourinary involvement. Lupus. 2012;21:1240–1243. doi: 10.1177/0961203312455111. PMID: 22833436. [DOI] [PubMed] [Google Scholar]
- 135.Lee C.K., Ahn M.S., Lee E.Y., Shin J.H., Cho Y.S., Ha H.K., Yoo B., Moon H.B. Acute abdominal pain in systemic lupus erythematosus: focus on lupus enteritis (gastrointestinal vasculitis) Ann. Rheum. Dis. 2002;61:547–550. doi: 10.1136/ard.61.6.547. PMID: 12006332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lian T.Y., Edwards C.J., Chan S.P., Chng H.H. Reversible acute gastrointestinal syndrome associated with active systemic lupus erythematosus in patients admitted to hospital. Lupus. 2003;12:612–616. doi: 10.1191/0961203303lu433oa. PMID: 12945720. [DOI] [PubMed] [Google Scholar]
- 137.Ju J.H., Min J.K., Jung C.K., Oh S.N., Kwok S.K., Kang K.Y., Park K.S., Ko H.J., Yoon C.H., Park S.H. Lupus mesenteric vasculitis can cause acute abdominal pain in patients with SLE. Nat. Rev. Rheumatol. 2009;5:273–281. doi: 10.1038/nrrheum.2009.53. PMID: 1941012794. [DOI] [PubMed] [Google Scholar]
- 138.Koo B.S., Hong S., Kim Y.J., Kim Y.G., Lee C.K., Yoo B. Lupus enteritis: clinical characteristics and predictive factors for recurrence. Lupus. 2015;24:628–632. doi: 10.1177/0961203314558858. PMID: 25391541. [DOI] [PubMed] [Google Scholar]
- 139.Lee M.G., Hagley K., Decuaelar K. Intestinal ischemia in systemic lupus erythematosus. J. Natl. Med. Assoc. 2008;100:721. doi: 10.1016/s0027-9684(15)31349-3. PMID: 18595576] [DOI] [PubMed] [Google Scholar]
- 140.Lee H.A., Shim H.G., Seo Y.H., Choi S.J., Lee B.J., Lee Y.H. Panenteritis as an initial presentation of systemic lupus erythematosus. Korean J. Gastroenterol. 2016;67:107–111. doi: 10.4166/kjg.2016.67.2.107. [DOI] [PubMed] [Google Scholar]
- 141.Liao W.C., Yuan W.H., Yaun W.H., Su C.W., Hou M.C., Lin H.C. Vomiting and diarrhea in a woman with systemic lupus erythematosus. J. Chin. Med. Assoc. 2015;78:133–135. doi: 10.1016/j.jcma.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 142.Smith L.W., Petri M. Lupus enteritis: an uncommon manifestation of systemic lupus erythematosus. J. Clin. Rheumatol. 2013;19:84–86. doi: 10.1097/RHU.0b013e318284794e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moon S.J., Kwok S.K., Park K.S., Kim W.U., Park S.H., Kim H.Y. Simultaneous presentation of hemophagocytic syndrome and mesenteric vasculitis in a patient with systemic lupus erythematosus. Mod. Rheumatol. 2011;21:330–333. doi: 10.1007/s10165-010-0401-8. [DOI] [PubMed] [Google Scholar]
- 144.Zheng W., Tian X., Li L., Jing H., Li F., Zeng X. Protein-losing enteropathy in systemic lupus erythematosus: analysis of the clinical features of fifteen patients. J Clin Rheumatol. dezembro de. 2007;13(6):313–316. doi: 10.1097/RHU.0b013e31815bf9c6. [DOI] [PubMed] [Google Scholar]
- 145.Lian T.Y., Edwards C.J., Chan S.P., Chng H.H. Reversible acute gastrointestinal syndrome associated with active systemic lupus erythematosus in patients admitted to hospital. Lupus. 2003;12:612–616. doi: 10.1191/0961203303lu433oa. [DOI] [PubMed] [Google Scholar]
- 146.Honda M., Asano T., Takajo D., Takada K., Nakamura M., Sekinaka-Mitsui K., Wakamatsu H., Nonoyama S. Honda M1, asano T2, takajo D2, takada K2, nakamura M2, sekinaka-mitsui K2, wakamatsu H2, nonoyama S2. Indian J. Pediatr. 2019 Nov 11 doi: 10.1007/s12098-019-03092-2. ([Epub ahead of print]) [DOI] [Google Scholar]