Abstract
The relationship between the lower airway microbiota in humans and respiratory illness has gained attention recently. However, the relationship between nontuberculous mycobacterial lung disease (NTM-LD) and the lower airway microbiota is not fully understood yet. We conducted a study to characterize the lower airway microbiota in Mycobacterium avium complex lung disease (MAC-LD), a representative subclass of the NTM-LD. The subject sample included 25 patients clinically suspected of having mild MAC disease whose condition could not be diagnosed using sputum culture. Upon testing MAC antibodies (anti-glycopeptidolipid (GPL)-core IgA antibodies), mycobacterial culture of bronchoalveolar lavage fluid (BALF), and performing BALF 16S rRNA gene sequencing, we divided the subjects into two groups of patients: those in whom MAC was detected in BALF mycobacterial culture (MAC-LD group) and in whom MAC was not detected in BALF mycobacterial culture (non-MAC-LD group), which was then comparatively examined. BALF mycobacterial culture showed that 9 out of 25 patients were positive for NTM; the detected Mycobacterium was MAC in all. No patients were positive for acid-fast bacteria other than MAC. Eighteen patients were positive for MAC antibodies (anti-glycopeptidolipid (GPL)-core IgA antibodies), including nine patients positive for mycobacterial culture. On BALF 16S rRNA gene sequencing, six patients were positive for the genus Mycobacterium and were culture-positive. Among the 16 patients in the non-MAC-LD group, the genus Pseudomonas was detected by 16S rRNA gene sequencing in 7 patients, 4 among whom were positive for MAC antibodies (anti-GPL-core IgA antibodies). Conversely, the genus Pseudomonas was not detected among the nine patients in the MAC-LD group. Other than the genus Pseudomonas, there was no clear difference in the composition of and no significant difference in the diversity of the bacterial flora between the MAC-LD and non-MAC-LD groups. However, we found that the genus Pseudomonas and MAC tended to exist exclusively.
Keywords: Nontuberculous mycobacteria, Pseudomonas aeruginosa, Bronchoalveolar lavage fluid, Microbiota, Microbiome
Nontuberculous Mycobacteria, Pseudomonas aeruginosa, Bronchoalveolar Lavage Fluid, Microbiota, Microbiome
1. Introduction
Nontuberculous mycobacteria (NTM) are indigenous bacteria belonging to the genus Mycobacterium found in water and soil, and although they are not usually transmitted from person to person, some hosts contract infection in the lungs and develop nontuberculous mycobacterial lung disease (NTM-LD) [1]. There has been an increasing trend of NTM-LD worldwide, and over 150 different species of NTM have been identified. However, NTM-LD is primarily caused by a limited few of these species, such as Mycobacterium avium, M. intracellulare, M. kansasii, and M. abscessus[2].
For the diagnostic criteria of NTM-LD, the criteria listed in Table 1 are generally used worldwide and consist of clinical criteria and microbiologic criteria [3]. NTM-LD is diagnosed when all the clinical criteria and microbiologic criteria are met. Among NTM-LD, patients in whom M. avium and M. intracellulare are detected are diagnosed as having Mycobacterium avium complex lung disease (MAC-LD).
Table 1.
Diagnostic criteria of NTM-LD.
| Clinical criteria |
|---|
|
| Microbiologic criteria |
|
AFB: acid-fast bacillus, HRCT: high-resolution computed tomography, NTM: nontuberculous mycobacteria.
In some patients suspected of having NTM-LD, there are cases where no NTM is detected in the mycobacterial culture despite the patients meeting the clinical criteria [4]. Tanaka et al. examined the mycobacterial culture of sputum and bronchial washing in 26 patients suspected of NTM-LD with bronchodilatation and clusters of small nodules surrounding the bronchi and reported that the sputum samples from 6 patients were positive for NTM, and the bronchial washing samples from 13 patients were NTM-positive [4]. While the sensitivity of detection with bronchial washing is considered higher than that with sputum, this does not mean that NTM are always detected in bronchial washing.
For patients clinically suspected of having MAC-LD, MAC antibodies (anti-GPL-core IgA antibodies) are measured in some instances as diagnostic aid, although they are not used in the diagnostic criteria of NTM-LD. MAC antibody tests measure serum antibodies against GPL-core, which is a part of the glycolipid antigen GPL constituting the cell wall of NTM and is the antigen common to NTM other than M. tuberculosis and M. kansasii [5]. GPL has been known to be the antigen determining the MAC serotype. According to the classification by Schaefer et al., there are 28 MAC serotypes that have been reported to differ in terms of regional distribution and pathogenicity [6]. Furthermore, even when the test result for MAC antibodies (anti-GPL-core IgA antibodies) is positive and NTM suspected lesions are found in the lung, MAC is not always identified in the sputum mycobacterial culture and bronchoalveolar lavage fluid (BALF) mycobacterial culture tests. The sensitivity and specificity of MAC antibodies (anti-GPL-core IgA antibodies) vary depending on the report. According to a multicenter study in Japan, it has been reported that when the cutoff value for the diagnosis of MAC-LD is defined as 0.7 U/mL, the sensitivity is 84.3% and specificity is 100% [7]. According to a study in the United States of America, when the cutoff value for MAC antibody (anti-GPL-core IgA antibody) positivity was 0.3 U/mL, the sensitivity was 70.1% and specificity was 93.9% [8]. In contrast, when the cutoff value was 0.7 U/mL, the sensitivity was 51.7% and the sensitivity was 93.9% [8]. Shu et al. [9] have reported that when the cutoff value for MAC antibody (anti-GPL-core IgA antibody) is 0.73 U/mL, the sensitivity and specificity are 60% and 91%, respectively, and have pointed that in a state of immunosuppression, the sensitivity might decrease. In terms of lesion morphology also, antibody titers have been reported to be higher in nodular-bronchiectasis (NB) type than in fibro-cavitary (FC) type [7]. Furthermore, it has been reported that MAC antibody (anti-GPL-core IgA antibody) titers may reflect disease activity [10].
The lower airway of humans is not aseptic; rather, there is bacterial flora, which is believed to correlate with one's health status. In recent years, 16S rRNA gene sequencing has been applied as a method of identifying microorganisms. Although the conventional bacterial culture method is useful for identification, it cannot be used for estimating the proportion of each bacterium in a sample because some bacteria can grow rapidly and others cannot under culture test conditions. The 16S rRNA gene sequencing technique enables identification of microorganisms present in a sample and measurement of the proportion of each microorganism; further, it helps to understand the microbiota found in various sites of the body and in association with different illnesses. The 16S rRNA gene sequencing technique has been used to characterize lower airway microbiota in various respiratory diseases. Denner et al. have reported that in the bacterial flora of the asthma patient group, the genera Lactobacillus, Pseudomonas, and Rickettsia are common, whereas in the non-asthmatic patient group, the genera Prevotella, Veillonella, and Streptococcus are common [11]. It has also been reported that in the patient group with chronic obstructive pulmonary disease (COPD) and asthma, among the phyla of bacteria, the phylum Proteobacteria was more commonly detected, and among the genera of bacteria, the genus Haemophilus was more commonly detected, compared with the patient group with FEV1% ≥ 95% and without COPD or asthma [12]. Sulaiman et al. compared BALF 16S rRNA gene sequencing in 8 patients with NTM-LD and 12 non-NTM-LD patients with bronchiectasis and reported that the sensitivity of NTM detection with this technique was lower than that with mycobacterial culture, and that with regard to the bacterial flora, the genus Mycobacterium and the family Oxalobacteraceae were more common in the NTM-LD group, and the genus Porphyromonas was more common in the non-NTM-LD group [13]. However, there are few reports of the lower airway microbiota in NTM-LD and many elements remain unclear. We compared BALF 16S rRNA sequencing and BALF mycobacterial culture in the sensitivity of acid-fast bacteria detection. Moreover, among NTM-LD, we focused on MAC-LD, and in patients who satisfied the clinical criteria of NTM-LD, we classified those who were positive and negative for MAC on BALF mycobacterial culture as the MAC-LD group and the non-MAC-LD group, respectively; further, we compared the difference in the diversity and microbial composition of lower airway microbiota between the two groups using BALF 16S rRNA sequencing.
2. Materials and methods
2.1. Patients and study design
Among patients who consulted the outpatient services of the department of respiratory medicine at the Toho University Medical Center Sakura Hospital between November 2018 and March 2020, we retrospectively examined patients neither with fever nor with respiratory failure who met the clinical criteria of NTM-LD (Table 1) but were not proven to have NTM by sputum culture examination and were required to undergo bronchoalveolar lavage (BAL) for definite diagnosis. The clinical background of the 25 patients is presented in Table 2. BAL was performed by wedging a bronchofiberscope into the bronchi with the largest number of lesions and infusing 50 ml of physiological saline three times successively. The collected BALF samples were subjected to bacterial and mycobacterial culture tests immediately after bronchoscopy. The residual BALF samples (20 ml) were stored at −80 °C until they were used for 16S rRNA gene sequencing. Mycobacterial cultures were all performed using the manual Mycobacterial Growth Indicator Tube (Nippon Becton Dickinson Co., Ltd.). After testing positive and negative for MAC in BALF mycobacterial culture, patients were classified into the MAC-LD group and the non-MAC-LD group, respectively. This was followed by comparing the bacterial composition ratios by 16S rRNA gene sequencing and the diversity using principal coordinate analysis (PCoA). This study was approved by the Ethics Committee of Toho University Sakura Medical Center on December 28, 2020 (Ethics Committee of Toho University Sakura Medical Center). The ethical approval number is S20061. This was a retrospective, observational study, which was conducted using the opt-out method of Toho University Sakura Medical Center for participant consent.
Table 2.
Clinical background of the patients.
| All | MAC-LD | non-MAC-LD | Normal value | |
|---|---|---|---|---|
| N | 25 | 9 | 16 | |
| Age mean ± SD | 68.6 ± 10.1 | 68.2 ± 9.7 | 68.3 ± 10.8 | |
| Sex (m/f) | 5/20 | 1/8 | 4/12 | |
| BMI (kg/m2) mean ± SD | 20.0 ± 2.4 | 19.1 ± 2.2 | 20.5 ± 2.4 | |
| TP (g/dl) mean ± SD | 7.7 ± 0.5 | 7.8 ± 0.5 | 7.7 ± 0.5 | 6.7–8.3 |
| Alb (g/dl) mean ± SD | 4.1 ± 0.4 | 4.2 ± 0.3 | 4.0 ± 0.4 | 3.8–5.2 |
| AST (IU/l) mean ± SD | 20.0 ± 4.6 | 20.6 ± 5.2 | 19.3 ± 4.1 | 10–40 |
| ALT (IU/l) mean ± SD | 16.9 ± 9.6 | 16.8 ± 4.0 | 16.8 ± 12.2 | 5–45 |
| LDH (IU/l) mean ± SD | 188 ± 32.5 | 204 ± 30 | 182 ± 31 | 120–240 |
| ALP (IU/l) mean ± SD | 267 ± 96 | 270 ± 96 | 265 ± 102 | 100–325 |
| CRP (g/dl) mean ± SD | 0.5 ± 0.8 | 0.5 ± 0.8 | 0.7 ± 1.0 | ≦0.30 |
| WBC (/μl) mean ± SD | 7123 ± 2501 | 7578 ± 1693 | 6842 ± 2977 | 3300–9000 |
| MAC antibody (U/ml) mean ± SD | 2.7 ± 3.2 | 5.1 ± 3.9 | 1.5 ± 1.6 | <0.7 |
| CT type (NB/FC) | 22/3 | 6/3 | 16/0 |
Data are presented as mean ± SD, unless otherwise indicated.
MAC-LD: Mycobacterium avium complex lung disease, BMI: body mass index, TP: total protein, Alb: albumin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, CRP: C-reactive protein, WBC: white blood cell count, MAC antibody: Mycobacterium avium complex antibody, CT: computed tomography, SD: standard deviation, NB: Nodular-bronchiectasis type, FC: Fibro-cavitary type.
2.2. Chest radiographic and computed tomography findings
In all patients, chest computed tomography (CT) scans (1–5 mm) were used for image diagnosis of NTM-LD. Image diagnosis was performed by at least one radiologist and at least one physician of the department of respiratory medicine, who evaluated the images to determine whether the clinical criteria for NTM-LD were satisfied. The CT was classified into FC type and NB type. Of the 25 patients, 22 patients presented NB type, and 3 patients presented FC type. The details of the CT type are presented in Table 2.
2.3. Anti-glycopeptidolipid(GPL)-core IgA antibody
MAC antibodies (anti-GPL-core IgA antibodies) were measured using an anti-GPL-core IgA antibody measuring kit from TAUNS Co., Ltd. With this kit, when the cutoff value is set at 0.7 U/mL, the sensitivity to MAC-LD is said to be 84% [7]. In the present study, results exceeding the cutoff value of 0.7 U/mL were judged to be positive.
2.4. 16S rRNA gene sequencing
The 16S rRNA gene sequencing technique enables identification of microorganisms that are difficult to identify by culture and enables measurement of the proportion of each microorganism in a specimen. We implemented 16S rRNA gene sequencing for 20 mL of residual specimen of BALF and examined the characteristics of the bacterial flora. The residual BALF specimen was stored at −80 °C until it was used for 16S rRNA gene sequencing. The 16S rRNA gene sequencing and data analysis were performed using the following procedure.
To begin with, residual BALF specimens were freeze-dried using a VD-250R Freeze Dryer (TAITECH Co., Ltd.). The freeze-dried specimens were ground for 2 min at 1,500 rpm using a Multi-Beads Shocker (YASUI KIKAI Co., Ltd.). Lysis Solution F (NIPPON GENE Co., Ltd.) was added to the ground specimen and left to stand for 10 min at 65 °C. Thereafter, centrifugal separation was performed for 1 min at 12,000 × g, and the supernatant was collected. Using an MPure Bacterial DNA Extraction Kit (MP Biomedicals, Inc) and MPure-12 system, DNA was extracted from the collected specimen. Density measurement of the extracted DNA solution was performed using Synergy LX (Bio Tek Co., LTD.) and QuantiFluor dsDNA System (Promega Corporation), and a library was created from the extracted DNA solution using the two-step tailed polymerase chain reaction method. Density measurement of the library created was performed using Synergy H1 (Bio Tek Co., LTD.) and QuantiFluor dsDNA System (Promega Corporation), and the quality of the library created was verified using a dsDNA 915 Reagent Kit (Advanced Analytical Technologies, Inc.) and fragment analyzer. After adjusting the library, sequencing was performed at 2 × 300 bp using a MiSeq Kit v3 (Illumina, Inc.) and MiSeq system. Next, we began to read the lead sequence obtained using the FASTX Barcode Splitter (Hannon lab) of the FASTX Toolkit (ver. 0.0.14), and only the lead sequence that was completely consistent with the primer sequence used was extracted. From these, after removing 50 bp, chimeric sequence, and noise sequence at the 3’ end in the primer sequence using the DADA2 plugin of the Qiime2 (Illumina, Inc., ver. 2020.2), a typical sequence was created. Last, a phylogenic estimation of the bacteria was performed using the typical sequence obtained using the feature-classifier plugin and the EzBioCloud 16S database.
In this study, the V4 region of 16S rRNA gene was amplified for sequencing. The raw sequence data were in FASTQ format. The reported nucleotide sequence data are available in the DNA Data Bank of Japan (DDBJ) Sequenced Read Archive under the accession number DRA011799.
2.5. Statistical analysis
Data without explanatory notes were expressed as mean ± standard deviation. PCoA was performed using the diversity plugin of QIIME2.0 (ver. 2020.2). Bacteria composition ratios were compared using Fisher's exact test using the SPSS 21.0 software (IBM Co., Arming, NY, USA).
3. Results
Of the 25 patients, 9 patients had positive results on BALF mycobacterial culture with the presence of MAC. For MAC antibodies (anti-GPL-core IgA antibodies), 18 patients were positive, including 9 patients with positive BALF mycobacterial culture results. Furthermore, MAC-LD was diagnosed upon identification of MAC from BALF mycobacterial culture in 9 of the 25 patients, and the genus Mycobacterium was identified by 16S rRNA gene sequencing in 6 of the 25 patients. The detection rate of the acid-fast bacteria was 24% for BALF 16S rRNA analysis and 36% for BALF mycobacterial culture. The acid-fast bacterial detection sensitivity of BALF 16S rRNA analysis was lower than that of BALF mycobacterial culture. In the present study, NTM could not be detected by BALF mycobacterial culture unless the test for MAC antibodies (anti-GPL-core IgA antibodies) was positive, and the genus Mycobacterium could not be detected by 16S rRNA gene sequencing unless NTM was detected by BALF mycobacterial culture (Figure 1).
Figure 1.
Patient classification. MAC antibody (+): Patients with Mycobacterium avium complex antibody positive, MAC antibody (−): Patients with Mycobacterium avium complex antibody negative, BALF mycobacterial culture MAC (+): Patients with bronchoalveolar lavage fluid mycobacterial culture-positive for Mycobacterium avium complex, BALF mycobacterial culture MAC (−): Patients with bronchoalveolar lavage fluid mycobacterial culture negative for Mycobacterium avium complex, 16S rRNA gene sequencing Mycobacterium (+): Patients with 16S rRNA gene sequencing positive for the genus Mycobacterium, 16S rRNA gene sequencing Mycobacterium (−): Patients with 16S rRNA gene sequencing negative for the genus Mycobacterium.
In the MAC-LD group and non-MAC-LD group, we compared diversity using PCoA by 16S rRNA gene sequencing and observed no significant difference between the two groups in terms of diversity (Figure 2).
Figure 2.
Weighted UniFrac-based Principal Coordinates Analysis (PCoA). MAC-LD: Mycobacterium avium complex lung disease, PC: Principal Component.
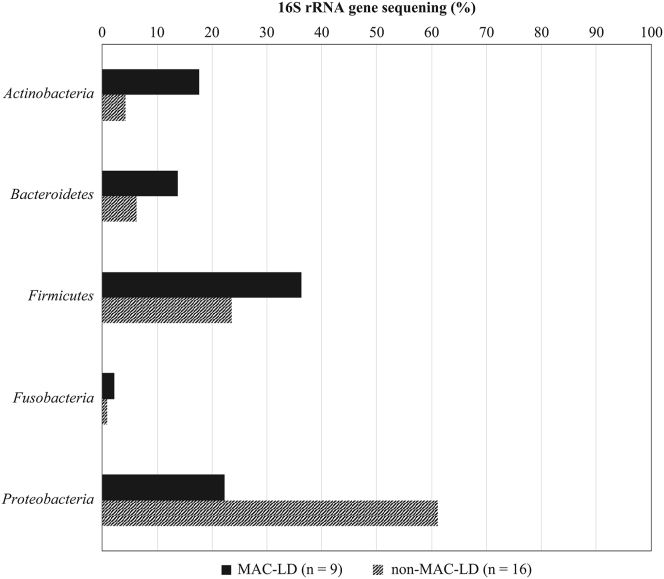
The bacteria composition rate on 16S rRNA gene sequencing was compared between the MAC-LD group and non-MAC-LD group. Figure 3 presents the bacteria composition rates between the two groups for different phyla of bacteria, and Figure 4 presents the bacteria composition rates for different genera of bacteria. With regard to the mean ratio on 16S rRNA gene sequencing of each phylum of bacteria shown in Figure 3, the phyla Actinobacteria, Bacteroidetes, and Firmicutes were most common in the MAC-LD group, whereas the phylum Proteobacteria tended to be more common in the non-MAC-LD group. The mean ratios of bacteria of various genera detected by 16S rRNA gene sequencing shown in Figure 4 indicated that the genera Mycobacterium, Streptococcus, and Prevotella were more common in the MAC-LD group, whereas the genera Pseudomonas and Haemophilus were more common in the non-MAC-LD group. With 16S rRNA gene sequencing, the genus Mycobacterium was detected in 6 of the 9 patients in the MAC-LD group, whereas the genus Mycobacterium was detected in none of the 16 patients in the non-MAC-LD group. With 16S rRNA gene sequencing, the genus Pseudomonas was not detected in 9 patients of the MAC-LD group, whereas the genus Pseudomonas was detected in 7 of the 16 patients in the non-MAC-LD group. On 16S rRNA analysis, it was difficult to detect the genus Pseudomonas in the MAC-LD group, and the genus Pseudomonas tended to be easily detected in the non-MAC-LD group (Fisher's exact test, p = 0.0267). Among the nine patients who tested negative for MAC and the genus Mycobacterium with BALF mycobacterial culture and 16S rRNA gene sequencing but tested positive for MAC antibodies (anti-GPL-core IgA antibodies), the genus Pseudomonas was detected by 16S rRNA gene sequencing in four patients.
Figure 3.
Bacterial composition ratio on 16S rRNA gene sequencing (phylum). MAC-LD: Mycobacterium avium complex lung disease.
Figure 4.
Bacterial composition ratio on 16S rRNA gene sequencing (genus). MAC-LD: Mycobacterium avium complex lung disease.
4. Discussion
We examined the sensitivity of BALF 16S rRNA gene sequencing in patients who met the clinical criteria of NTM-LD. Among the 25 patients who satisfied the clinical criteria of NTM-LD, 18 were positive for MAC antibodies (anti-GPL-core IgA antibodies). Furthermore, MAC-LD could be diagnosed upon identifying MAC from BALF mycobacterial culture in 9 of the 25 patients, and the genus Mycobacterium was identified by 16S rRNA gene sequencing in 6 of the 25 patients. In 18 patients who were positive for MAC antibodies (anti-GPL-core IgA antibodies), MAC-LD could be diagnosed in 9 patients, indicating that MAC-LD could be diagnosed in 50% of patients who were positive for MAC antibodies (anti-GPL-core IgA antibodies). In the present study, MAC could not be detected by BALF mycobacterial culture unless patients were positive for MAC antibodies (anti-GPL-core IgA antibodies), and the genus Mycobacterium could not be identified by 16S rRNA gene sequencing unless MAC was detected by BALF mycobacterial culture. Therefore, the results of the present study suggest that even if MAC is detected from BALF mycobacterial culture, the genus Mycobacterium is not necessarily detectable on 16S rRNA gene sequencing; however, in patients with the genus Mycobacterium detected on 16S rRNA gene sequencing, MAC can be easily identified from BALF mycobacterial culture. Sulaiman et al. compared BALF 16S rRNA analysis in 8 patients with NTM-LD and 12 non-NTM-LD patients with bronchiectasis and reported that the sensitivity for detecting NTM with 16S rRNA gene sequencing was lower than that with culture [13]. NTM account for a small proportion in the population, and this is inferred as a reason for why the detection sensitivity for a less abundant pathogen with 16S rRNA gene sequencing is lower than that with culture detection [13]. Caverly et al. also performed 16S rRNA gene sequencing and mycobacterial culture using sputum as well as BALF from cystic fibrosis patients with NTM and reported that, in both samples, the sensitivity with 16S rRNA gene sequencing was lower than that with mycobacterial culture [14]. In agreement with these previous studies, our results indicated higher detection sensitivity with BALF mycobacterial culture than with 16S rRNA gene sequencing. In the group of 18 patients who were positive for MAC antibodies (anti-GPL-core IgA antibodies) and met the clinical criteria of NTM-LD, we found that the sensitivity for detecting the genus Mycobacterium by 16S rRNA gene sequencing was lower than that by culture.
Sulaiman et al. evaluated diversity with BAL 16S rRNA gene sequencing in the NTM-LD group and non-NTM-LD group; however, they found no significant difference [13]. With the 25 patients in the present study classified into the MAC-LD group and non-MAC-LD group, we evaluated BAL 16S rRNA gene sequencing using PCoA. Upon conducting PCoA of the MAC-LD group and non-MAC-LD group shown in Figure 2, we observed no significant difference in diversity. These results are similar to those reported by Sulaiman et al.
Moreover, we studied the composition ratios of different phyla and genera of bacteria from BALF 16S rRNA analysis results. Among the different phyla of bacteria, the phyla Actinobacteria, Bacteroidetes, and Firmicutes were more common in the MAC-LD group, whereas the phylum Proteobacteria was more common in the non-MAC-LD group; however, the differences were not significant. The differences were not presumably because a bacterial phylum comprises various genera. Among the different genera, the genera Mycobacterium, Streptococcus, and Prevotella accounted for larger proportions in the MAC-LD group, whereas the genera Pseudomonas and Haemophila were more common in the non-MAC-LD group; however, the differences were not of significance except for the genus Pseudomonas. As we found in the microbial composition ratios from BALF 16S rRNA analysis that the genus Pseudomonas was less likely to be detected in the MAC-LD group, and that the genus Pseudomonas was more likely to be detected in the non-MAC-LD group, we considered the relationship between MAC and Pseudomonas aeruginosa in the lower respiratory tract. In the patient group with non-cystic fibrosis bronchiectasis and NTM identified from respiratory mycobacterial culture, it has been reported that P. aeruginosa is more difficult to identify from sputum culture compared with identification in the patient group with non-cystic fibrosis bronchiectasis and without NTM detected from respiratory mycobacterial culture [15]. In patients with cystic fibrosis, it has been reported that P. aeruginosa is more difficult to identify in the group with NTM identified from mycobacterial culture of the airway, compared with the group without NTM detected [16]. In contrast, Wickremasinghe et al. have reported that P. aeruginosa is more commonly identified in sputum culture in bronchiectasis patients in whom MAC is frequently identified by sputum mycobacterial culture, compared with bronchiectasis patients without concurrent MAC [17]. Fujita et al. have reported that in MAC-LD, P. aeruginosa tends to be more often identified in sputum culture prior to MAC treatment than after MAC treatment [18]. In patients with non-cystic fibrosis, it has been reported that the severe patients with a rapid decrease over time in forced expiratory volume 1 (FEV1) and forced vital capacity (FVC) on respiratory function test often have mixed infection by P. aeruginosa and NTM, whereas the mild patients with slow changes in FEV1 and FVC over time often have single infection by P. aeruginosa or NTM [19]. In summary, these reports suggest that the likelihood of co-infection by NTM and P. aeruginosa in NTM-LD differs in different circumstances, such as mild vs. severe cases and before vs. after treatment; in mild cases, co-infection by NTM and P. aeruginosa is unlikely, whereas in severe cases and post-treatment cases, co-infection by NTM and P. aeruginosa easily occur.
We examined the 16S rRNA gene sequencing of BALF in 25 asymptomatic patients who met the clinical criteria of NTM-LD. In the 16S rRNA gene sequencing of BALF, the genus Pseudomonas was not identified in 9 patients of the MAC-LD group; however, the genus Pseudomonas was identified in 7 of the 16 patients of the non-MAC-LD group. On 16S rRNA gene sequencing, the genus Pseudomonas was difficult to detect in the MAC-LD group, but tended to be easy to detect in the non-MAC-LD group (Fisher's exact test, p = 0.0267). In mild patients without fever or respiratory failure in whom the clinical criteria of NTM-LD were met but MAC-LD could not be diagnosed by sputum culture, as in case of the patients in our study, 16S rRNA gene sequencing data also indicate that MAC and the genus Pseudomonas rarely coexist. Furthermore, in four of nine patients who were positive for MAC antibodies (anti-GPL-core IgA antibodies) without MAC identified in BALF mycobacterial culture or 16S rRNA gene sequencing of BALF, the genus Pseudomonas was identified by 16S rRNA gene sequencing. Among patients who were positive for MAC antibodies (anti-GPL-core IgA antibodies) but negative on both BALF mycobacterial culture and BALF 16S rRNA gene sequencing, the genus Pseudomonas was detected in many patients. This suggests the possibility that MAC had previously existed in the lower airway but was substituted with P. aeruginosa in mild patients who met the clinical criteria of NTM-LD, were positive for MAC antibodies (anti-GPL-core IgA antibodies), and were negative on BALF mycobacterial culture and BALF 16S rRNA gene sequencing.
Our study has several limitations. First, we cannot clearly state that the BALF was not contaminated by microorganisms in the environment or upper airway. A previous study investigating changes in the lower airway microbiota in healthy adults has reported that BALF culture is not affected by contaminants from the upper airway [20]. Morris et al. have reported that the lungs have a characteristic bacterial flora; however, the lung microbiota resembled the oropharynx microbiota, and the involvement of contamination from the upper airway was not ruled out [21]. Second, with 16S rRNA gene sequencing that we used in the present study, it is difficult to identify the species of bacteria in the genus Mycobacterium; M. tuberculosis and MAC are both identified as a species of the genus Mycobacterium and are not distinguishable from each other. In this study, MAC happened to be the species of the genus Mycobacterium responsible for all the positive results in the mycobacterial culture and thus the results could be interpreted without difficulty; however, the inability to differentiate between acid-fast bacteria is a major shortcoming in further studies. Third, the study was limited to mild, untreated cases, and we did not compare the subjects with patients without bronchiectasis. Fourth, this study had a small sample size; because of this limitation, we could not propose the best combination of diagnostic methods for detecting NTM-LD in clinically suspected cases. To obtain further findings, studies with a larger subject sample are needed.
5. Conclusions
The detection sensitivity of acid-fast bacteria by 16S rRNA gene sequencing was lower than that by mycobacterial culture. No significant difference between the MAC-LD group and the non-MAC-LD group was observed in the diversity of the lower airway microbiota; however, we found that the genus Pseudomonas and MAC tended to be exclusively present.
Declarations
Author contribution statement
Kotaro Iwasaki: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yasuo Matsuzawa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Hiroki Wakabayashi, Moe Shioya, Sho Hayakawa: Performed the experiments.
Ichiro Tatsuno: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the commercial, public, or not-for-profit sectors.
Data availability statement
The data that has been used is confidential.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Prevots D.R., Shaw P.A., Strickland D. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am. J. Respir. Crit. Care Med. 2010;182(7):970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson M.M., Odell J.A. Nontuberculous mycobacterial pulmonary infections. J. Thorac. Dis. 2014;6(3):210–220. doi: 10.3978/j.issn.2072-1439.2013.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffith D.E., Aksamit T., Brown-Elliott B.A. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka E., Amitani R., Niimi A. Yield of computed tomography and bronchoscopy for the diagnosis of Mycobacterium avium complex pulmonary disease. Am. J. Respir. Crit. Care Med. 1997;155(6):2041–2046. doi: 10.1164/ajrccm.155.6.9196113. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi K. Serodiagnosis of Mycobacterium avium complex disease in humans: translational research from basic mycobacteriology to clinical medicine. Jpn. J. Infect. Dis. 2014;67(5):329–332. doi: 10.7883/yoken.67.329. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer W.B. Serologic identification and classification of the atypical mycobacteria by their agglutination. Am. Rev. Respir. Dis. 1965;92(6):85–93. doi: 10.1164/arrd.1965.92.6P2.85. [DOI] [PubMed] [Google Scholar]
- 7.Kitada S., Kobayashi K., Ichiyama S. Serodiagnosis of Mycobacterium avium-complex pulmonary disease using an enzyme immunoassay kit. Am. J. Respir. Crit. Care Med. 2008;177(7):793–797. doi: 10.1164/rccm.200705-771OC. [DOI] [PubMed] [Google Scholar]
- 8.Kitada S., Levin A., Hiserote M. Serodiagnosis of Mycobacterium avium complex pulmonary disease in the USA. Eur. Respir. J. 2013;42(2):454–460. doi: 10.1183/09031936.00098212. [DOI] [PubMed] [Google Scholar]
- 9.Shu C.-C., Ato M., Wang J.-T. Sero-diagnosis of Mycobacterium avium complex lung disease using serum immunoglobulin A antibody against glycopeptidolipid antigen in Taiwan. PloS One. 2013;8(11) doi: 10.1371/journal.pone.0080473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitada S., Yoshimura K., Miki K. Validation of a commercial serodiagnostic kit for diagnosing pulmonary Mycobacterium avium complex disease. Int. J. Tubercul. Lung Dis. 2015;19(1):97–103. doi: 10.5588/ijtld.14.0564. [DOI] [PubMed] [Google Scholar]
- 11.Denner D.R., Sangwan N., Becker J.B. Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J. Allergy Clin. Immunol. 2016;137(5):1398–1405. doi: 10.1016/j.jaci.2015.10.017. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilty M., Burke C., Pedro H. Disordered microbial communities in asthmatic airways. PloS One. 2010;5(1) doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sulaiman I., Wu B.G., Li Y. Evaluation of the airway microbiome in nontuberculous mycobacteria disease. Eur. Respir. J. 2018;52(4):1800810. doi: 10.1183/13993003.00810-2018. [DOI] [PubMed] [Google Scholar]
- 14.Caverly L.J., Carmody L.A., Haig S.J. Culture-independent identification of nontuberculous Mycobacteria in cystic fibrosis respiratory samples. PloS One. 2016;11(4) doi: 10.1371/journal.pone.0153876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maiz L., Girοn R., Olveira C. Prevalence and factors associated with nontuberculous mycobacteria in non-cystic fibrosis bronchiectasis: a multicenter observational study. BMC Infect. Dis. 2016;16(1):437. doi: 10.1186/s12879-016-1774-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binder A.M., Adjemian J., Olivier K.N. Epidemiology of nontuberculous mycobacterial infections and associated chronic macrolide use among persons with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2013;188(7):807–812. doi: 10.1164/rccm.201307-1200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wickremasinghe M., Ozerovitch L.J., Davies G. Non-tuberculous mycobacteria in patients with bronchiectasis. Thorax. 2005;60(12):1045–1051. doi: 10.1136/thx.2005.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita K., Ito Y., Hirai T. Prevalence and risk factors for chronic co-infection in pulmonary Mycobacterium avium complex disease. BMJ Open Respir. Res. 2014;1(1) doi: 10.1136/bmjresp-2014-000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heish M.H., Lin C.Y., Wang C.Y. Impact of concomitant nontuberculous mycobacteria and Pseudomonas aeruginosa isolates in non-cystic fibrosis bronchiectasis. Infect. Drug Resist. 2018;11:1137–1143. doi: 10.2147/IDR.S169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickson R.P., Erb-Downward J.R., Freeman C.M. Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann. Am. Thorac Soc. 2015;12(6):821–830. doi: 10.1513/AnnalsATS.201501-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris A., Beck J.M., Schloss P.D. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am. J. Respir. Crit. Care Med. 2013;187(10):1067–1075. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.