Abstract
Sensing noxiously high temperatures is crucial for living organisms to avoid heat-induced injury. The TRPV1 channel has long been known as a sensor for noxious heat. However, the mechanism of how this channel is activated by heat remains elusive. Here we found that a series of polyols including sucrose, sorbitol, and hyaluronan significantly elevate the heat activation threshold temperature of TRPV1. The modulatory effects of these polyols were only observed when they were perfused extracellularly. Interestingly, mutation of residues E601 and E649 in the outer pore region of TRPV1 largely abolished the effects of these polyols. We further observed that intraplantar injection of polyols into the hind paws of rats reduced their heat-induced pain response. Our observations not only suggest that the extracellular regions of TRPV1 are critical for the modulation of heat activation by polyols, but also indicate a potential role of polyols in reducing heat-induced pain sensation.
Keywords: TRPV1, polyols, heat activation, thermal activation threshold, outer pore region
Abbreviation: HA, hyaluronate
Temperature is critical for surviving and thriving of all living beings. In mammals, ambient temperature is sensed through primary afferent sensory neurons of the dorsal root and trigeminal ganglia (1). In these neurons, temperature-sensitive transient receptor potential (TRP) channels such as TRPV1, TRPA1, TRPM3, and TRPM8 are highly expressed. These TRP channels are directly activated at specific temperatures in the range from noxious heat to painful cold (2, 3, 4, 5, 6, 7), so that they convert thermal information into chemical and electrical signals in the sensory nervous system (8). In doing so, these channels constitute the key components of the protective mechanism against noxious temperature in animals to avoid tissue damage and recognize hazardous stimuli (9, 10, 11).
Among these TRP channels, TRPV1 (transient receptor potential vanilloid 1) is a prototypical molecular sensor for detecting noxious heat (10). TRPV1 is activated by temperature above 40 °C with a high temperature sensitivity in channel open probability (Q10 being 20~30) (12, 13). At present, although the high-resolution three-dimensional structures of TRPV1 have been determined by cryo-EM (14, 15), how TRPV1 obtains the high temperature sensitivity is still unclear. Structurally, like all TRP channels, TRPV1 is a tetramer and each subunit contains six transmembrane domains (S1–S6), a hydrophobic pore loop linking transmembrane S5 and S6, and large cytoplasmic N and C terminals (15). At the protein level, different regions of the N-terminal domain as well as the C-terminal domain, the pore turret, and the pore/extracellular loop following the pore have all been suggested to be critical for heat activation (16, 17, 18, 19, 20, 21, 22). To reconcile and understand these experimental observations regarding heat activation of TRPV1, a previous study proposed a theoretical framework for temperature activation of TRP channels: changes in heat capacity of TRP channel proteins caused by buried/exposure of specific residues mediates temperature activation, where how water molecules interact with protein residues is crucial (23). Initial experimental evidence for such a hypothesis was found in voltage-gated potassium channels (24). Our previous study on cold activation of TRPM8 channel also confirmed the validity of this hypothesis, where we found that tuning the hydrophobicity of residues undergoing buried/exposure conformational changes during cold activation can specifically alter the cold sensitivity of TRPM8 channel (25). However, whether such a theoretical framework of temperature activation is applicable to heat activation of TRPV1 remains untested.
Polyols are compounds with different numbers of hydroxyl groups, such as D-sorbitol, sucrose, and sodium hyaluronate (HA) (Fig. 1A). Owing to the ability to stabilize proteins as osmolytes, polyols have a crucial role in protecting organisms against ambient stress such as temperature, where the multiple hydroxyl groups in a polyol molecule may compete with water molecules with the protein residues. Disruption of water–protein interactions by polyols further leads to changes in protein stability (26). High concentrations of polyols impact protein stability in vitro. For example, glycerin and sucrose increase protein stability (26, 27). Previous studies suggest that the stability increases with increasing the molecular volume and concentration of the polyols, and stability is a linear function of osmotic concentration (28, 29, 30, 31). The effect of polyols on proteins may be explained by indirect interaction, where the preferential exclusion mechanism leads to indirect interactions by which polyol molecules are expelled from the protein surface, thereby forming a thin hydration shell around the surface of the protein and ultimately altering the ordering of water in polyol solution. The ordering extent of water increases with enhancement of polyols molecular volume (30). Therefore, we reason that, for the TRPV1 channel, adding polyols in solution may also alter its water–protein interactions so that the heat activation is modulated accordingly.
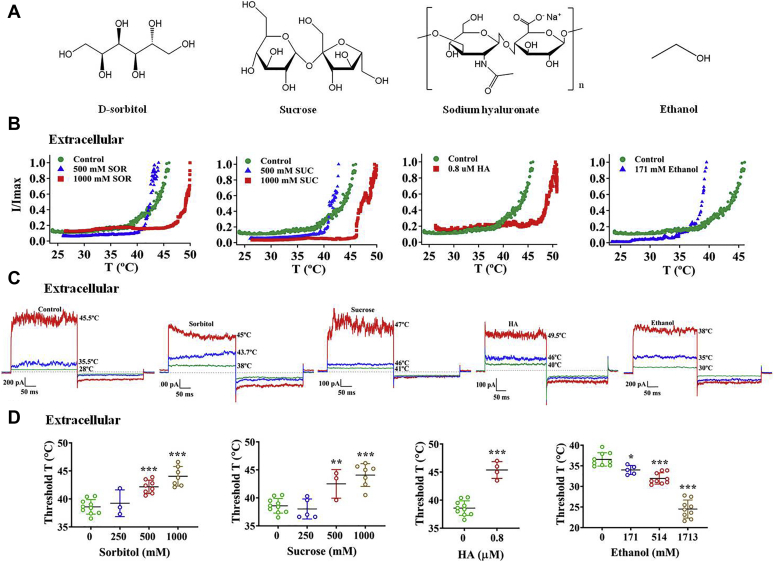
Figure 1.
Polyols elevate the thermal activation threshold of the wildtype TRPV1 channel only from the extracellular side.A, comparison of chemical structures of D-sorbitol, sucrose, sodium hyaluronate (HA), and ethanol. B, all extracellular polyols, D-sorbitol (SOR), sucrose (SUC), and HA, apparently shift the temperature–current curves of the TRPV1 channel to higher temperature. On the contrary, ethanol with only one hydroxyl group shifted it to lower temperature. C, representative macroscopic current traces recorded from TRPV1 channels after applying different polyols from the extracellular side. D, polyols increase the thermal activation threshold of wildtype TRPV1 in a concentration-dependent manner, but ethanol decreases it (n = 3–10). The data are represented as the mean ± SD. Statistical analyses were performed by one-way ANOVA with Dunnett’s multiple comparisons post hoc test or Student’s t test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 versus control group.
In this study, we investigated how three typical polyols and ethanol modulate TRPV1 heat activation. We first conducted patch clamp recordings with HEK293 cells expressing the wildtype or mutant TRPV1 channel while applying polyols extracellularly or intracellularly. We found that extracellular polyols increase the TRPV1 heat activation threshold temperature, and residues in the outer pore region critical for proton activation are also essential for polyol modulation of TRPV1 heat activation. Based on our in vitro findings, we further carried out behavioral tests with rats and observed that polyols protect rats against noxious heat as polyols can reduce rat’s heat sensitivity monitored by increasing thermal paw withdrawal latency. This work tried to elucidate the molecular mechanism of structural stability of the TRPV1 in polyol solutions, providing a new idea for the development of novel TRPV1 antagonists from the perspective of biophysics.
Results
Polyols reduce the potency of heat by increasing threshold for TRPV1 channel activation only from the extracellular side
Since the temperature sensitivity of TRPV1 is modulated by the transmembrane voltage (32), the biphasic pulses (−80 and +80 mV) were used when recording the thermal activation current. We first conducted inside-out recording while raising the temperature of the recording chamber and found that the thermal activation threshold of wildtype human TRPV1 was 38.6 ± 1.3 °C (n = 10, Fig. 1, B–D). Then, we applied various concentrations of different polyols from the extracellular side (polyols in the pipette solution) and observed the significantly delayed steep increase of current amplitude in response to temperature jumps compared with the control group (Fig. 1B). Furthermore, we analyzed these data and found that three polyols significantly raised the threshold temperature of TRPV1 as well as ΔT (shift in threshold temperature compared with control) in a concentration-dependent manner (Fig. 1D). On the contrary, ethanol containing only one hydroxyl group in its chemical structure is known to activate the TRPV1 channel (33). In either inside-out or outside-out recordings where ethanol was perfused to the extracellular or intracellular side of the TRPV1 channel, it left-shifted the thermal activation threshold compared with the control group likely due to its high membrane permeability (last panels of Fig. 1, B–D, and Fig. S1, A and B). When the two key residues for proton activation of the TRPV1 channel were mutated (E601Q-E649Q) (34), ethanol no longer decreased the heat activation threshold (Fig. S1, C and D). It is well known that adding polyols increases the osmolarity of the solution (Fig. S2A). To test whether the increase in heat activation threshold of TRPV1 by polyol was caused by changes in osmolarity, we performed the control experiments. We observed that, although 500 mM sorbitol increased the osmolarity of bath solution to 784 mOsm/kg and elevated the heat activation threshold of TRPV1, adding urea to bath solution to the same 784 mOsm/kg did not elevate the heat activation threshold (Fig. S2, B–D). Therefore, we believe that it was not the increase in osmolarity that caused changes in heat activation of TRPV1.
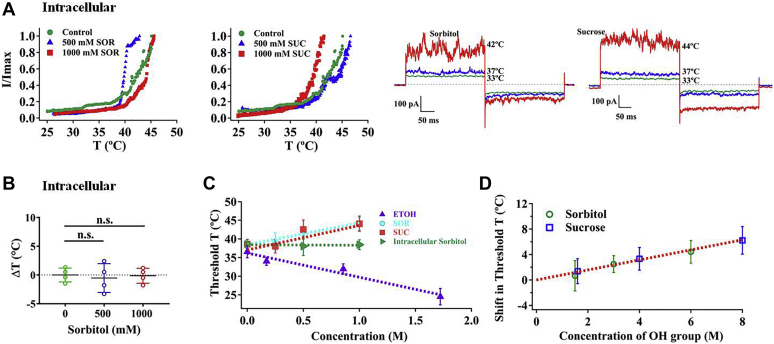
To examine whether polyols applied to the intracellular side alter TRPV1 heat activation as when they were applied extracellularly, we also carried out inside-out patch recording with various concentrations of polyols in the bath. Interestingly, unlike extracellular polyols, intracellular polyols did not affect the TRPV1 heat activation (Fig. 2, A and B). Therefore, these findings indicate that polyols are likely to interact with the extracellular region of TRPV1 to stabilize this membrane-embedded channel protein. To quantify for protein-stabilizing effects of polyols, we plotted the thermal activation threshold of TRPV1 against various concentrations of different polyols as well as ethanol and conducted regression analysis. The linear regression line has a positive slope with correlation coefficient (R2) of 0.94 for sorbitol and 0.96 for sucrose, respectively (Fig. 2C, dashed lines in cyan and red, respectively). Different from polyols, ethanol exhibits a negative slope with a correlation coefficient of 0.96 (Fig. 2C, dashed line in purple). These results indicate that a polyol of larger concentration has a stronger protein-stabilizing effect.
Figure 2.
Intracellular polyols did not affect the TRPV1 heat activation.A, representative macroscopic current traces recorded from TRPV1 channels after applying different polyols from the intracellular side. B, intracellularly perfused polyols did not change thermal activation threshold of wildtype TRPV1 at both concentrations of sorbitol (n = 4). C and D, the relationship between the thermal activation threshold of wildtype TRPV1 and the concentration of polyols or hydroxyl groups (n = 3–10). The data are represented as the mean ± SD. Statistical analyses were performed by one-way ANOVA with Dunnett’s multiple comparisons post hoc test. n.s., not significant.
It is noteworthy that sorbitol has two fewer hydroxyl groups than sucrose. In order to investigate how the numbers of hydroxyl groups of a polyol affects the TRPV1 heat activation, we plotted ΔT against concentration of hydroxyl groups and carried out regression analysis. The linear regression line has a positive slope of 1.25 ± 0.09 °C/mol with a good correlation coefficient of 0.98 (Fig. 2D). More importantly, both sorbitol and sucrose exhibited the same hydroxyl group concentration dependence of ΔT, indicating that there is a common physical basis underlying the modulatory effects of polyols (Fig. 2D). Collectively, these findings demonstrate that the numbers of hydroxyl groups of a polyol play a pivotal role in protecting TRPV1 protein against thermal stimulus.
Turret of TRPV1 modulates the effect of polyols on heat activation
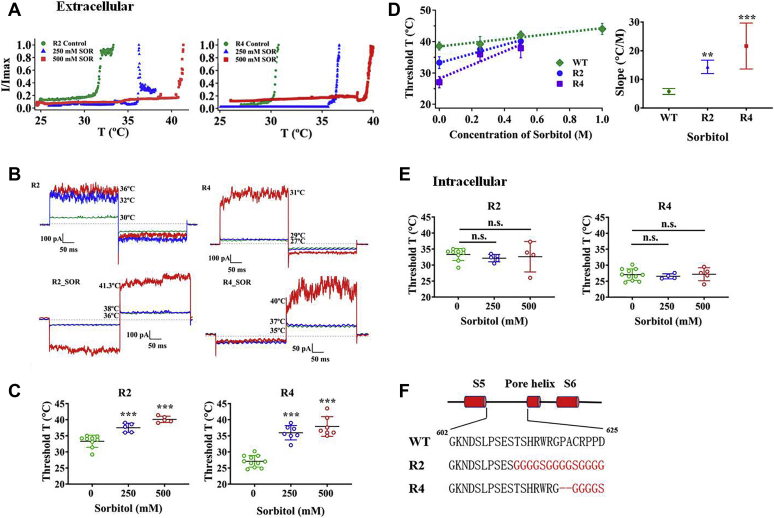
The discernable increase in the threshold temperature of polyols prompted us to explore the structural basis for this change. Because we have observed that polyols delay TRPV1 heat activation only from the extracellular side but not intracellular side, we reasoned that the channel structure(s) mediating the effect of reducing heat sensitivity should be exposed to the extracellular aqueous environment. Moreover, previous studies have demonstrated that the outer pore region is essential for TRPV1 heat activation (21, 35, 36). Therefore, our research for sites related to heat-evoked TRPV1 activation focused on the pore turret region. To test our hypothesis, we first generated two different turret replacement mutants, namely, R2 and R4, based on our previous study (Fig. 3F) (17) and observed that they have similar responsiveness to capsaicin compared with wildtype (Fig. S3). Then, we recorded the thermal threshold activation of two mutants in bath solution and found that their values are in line with our previous observations, showing lower threshold temperature than wildtype with 33.3 ± 1.9 °C (n = 9, p < 0.001) for R2 and 27.1 ± 1.8 °C for R4 (n = 11, p < 0.001) (Fig. 3, A–C). After applying sorbitol to these two mutants from the extracellular side, we found that sorbitol still could significantly increase the thermal threshold activations of two mutants compared with their control groups at both concentrations (Fig. 3, A–D). In contrast, intracellular polyol still had little effect on the thermal threshold of activation of two mutants, resembling the response of wildtype TRPV1 to polyols with the same conditions (Fig. 3E).
Figure 3.
Pore turret of TRPV1 modulates the effects of polyols on heat activation.A, polyols increase the thermal activation threshold of R2 and R4 turret mutants. B, representative macroscopic current traces recorded from R2 and R4 after applying sorbitol from the extracellular side. C, comparisons of thermal activation threshold of different mutant channels with various concentrations of sorbitol (n = 5–11). D, the sorbitol concentration dependence of shifts in heat activation threshold of two mutants exhibits larger slopes than that of the wildtype channel. E, polyols did not increase the thermal activation threshold of the mutant channels from the intracellular side (n = 4–11). The data are represented as the mean ± SD. Statistical analyses were performed by one-way ANOVA with Dunnett’s multiple comparisons post hoc test. ∗∗p < 0.01; ∗∗∗p < 0.001 versus corresponding control group; n.s., not significant. F, diagram illustrating the design of two TRPV1 mutants.
However, when we plotted thermal activation thresholds of the wildtype and two mutant TRPV1 channels against two concentrations of sorbitol and conducted regression analysis, we found that the sorbitol concentration dependence of shifts in heat activation threshold of two mutants exhibits larger slopes (14.40 ± 2.33 °C/mol for R2 and 21.66 ± 8.03 °C/mol for R4) than wildtype TRPV1 (5.80 ± 1.03 °C/mol) (Fig. 3D and Table 1). These observations show that the magnitude of polyol’s effects on TRPV1 heat activation is modulated by the turret of TRPV1.
Table 1.
The intercepts (a) and slopes (b and c, b for ethanol and polyols original concentration; c for those of hydroxyl group concentration) from ethanol/polyols concentration-thermal activation threshold curves in wildtype and mutant TRPV1 channels
| Threshold temperature for heat activation | a (°C) | b (°C/mol) Polyol | c (°C/mol) -OH |
|---|---|---|---|
| WT-ETOH | 36.22 ± 0.91 | −6.49 ± 0.95 | −6.49 ± 0.95 |
| WT-SUC | 37.05 ± 0.51 | 6.57 ± 0.90 | 0.82 ± 0.11 |
| WT-SOR | 38.46 ± 0.59 | 5.80 ± 1.03 | 0.98 ± 0.17 |
| R2-SOR | 33.24 ± 0.75 | 14.40 ± 2.33∗∗ | 2.40 ± 0.39∗∗ |
| R4-SOR | 28.23 ± 2.60 | 21.66 ± 8.03∗∗∗ | 3.61 ± 1.34∗∗∗ |
| E601Q-E649Q double mutant-SOR | 45.23 ± 0.70 | −1.31 ± 1.22∗∗∗ | −0.22 ± 0.20∗∗ |
| WT-pH6.0-SOR | 34.39 ± 0.87 | 20.34 ± 2.69∗∗∗ | 3.39 ± 0.45∗∗∗ |
ETOH, ethanol; SOR, D-sorbitol; SUC, sucrose.
The data are represented as the mean ± SD, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, versus the corresponding control group.
Outer pore residues are critical for the effects of polyols on heat activation
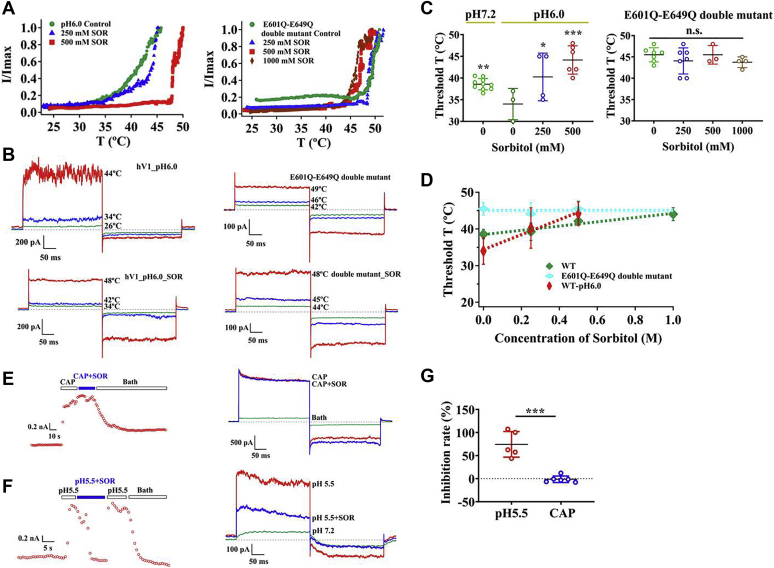
Extracellular protons potentiate heat sensitivity of TRPV1. E601 and E649 (amino acid numbers are for mouse TRPV1) within the pore region are two crucial pH sensors for acidification of extracellular environment (34). Therefore, we hypothesized that these two sites may also be critical for the effects of polyols on heat activation. We first conducted patch clamping with extracellular acidic solution (pH 6.0) while raising the temperature of the recording chamber. As expected, extracellular proton lowered the heat activation threshold as compared with the neutral pH (Fig. 4C). We further observed that, when sorbitol was perfused with acidic solution, it can still reverse the synergistic effect of protons on wildtype TRPV1 heat activation in a concentration-dependent manner (Fig. 4, A–C, left panels; Fig. 4D).
Figure 4.
Residues in the outer pore region of TRPV1 play a pivotal role in threshold temperature modulating effects of polyols.A, polyols reverse the synergistic effect of protons on wildtype TRPV1 heat activation but did not increase the threshold temperature of the E601Q-E649Q double mutant. B, representative macroscopic current traces recorded from wildtype TRPV1 at pH 6.0 and E601Q-E649Q double mutant after applying sorbitol from the extracellular side. C and D, comparisons of thermal activation threshold of wildtype TRPV1 at pH 6.0 as well as that of the E601Q-E649Q double mutant after perfusing various concentrations of sorbitol (n = 3–10). E, representative macroscopic current traces recorded from TRPV1 channels after applying 3 μM capsaicin or 3 μM capsaicin with 500 mM sorbitol from the extracellular side at room temperature. F, representative macroscopic current traces recorded from TRPV1 channels after applying acid (pH 5.5) or acid (pH 5.5) with 500 mM sorbitol from the extracellular side at room temperature. G, sorbitol, 500 mM, inhibits the activation of TRPV1 by proton at room temperature but not by 3 μM capsaicin (n = 5–6). The data are represented as the mean ± SD. Statistical analyses were performed by one-way ANOVA with Dunnett’s multiple comparisons post hoc test or Student’s t test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 versus corresponding control group; n.s., not significant.
The E601Q-E649Q double mutant virtually eliminates proton potentiation of the TRPV1 channel (34), so we then repeated the above-mentioned experiment with this mutant and found that sorbitol can no longer elevate the heat activation threshold even at a high concentration of 1000 mM (Fig. 4, A–C, right panels; Fig. 4D). Taken together, our observations suggest that E601 and E649 in the outer pore region of TRPV1 are important for modulation of heat activation threshold by polyols.
Effects of polyols on capsaicin and proton activation of TRPV1
Besides being activated by noxious heat, the TRPV1 channel is also activated by capsaicin and extracellular protons (10). Previous studies suggested that the pungency of oral capsaicin was reduced by polyol-containing solutions (37). However, we observed that, when the TRPV1 channel was activated by capsaicin (3 μM), adding sorbitol (500 mM) did not reduce channel activation (Fig. 4, E and G). In contrast, we found that polyols could significantly inhibit the TRPV1 activation by extracellular protons (Fig. 4, F and G). These observations are not surprising, because capsaicin binds to the transmembrane domains of TRPV1 (14, 38, 39), whereas the residues critical for proton activation locate in the outer pore domain (34, 40).
Polyols relieve noxious heat-evoked pain in rats
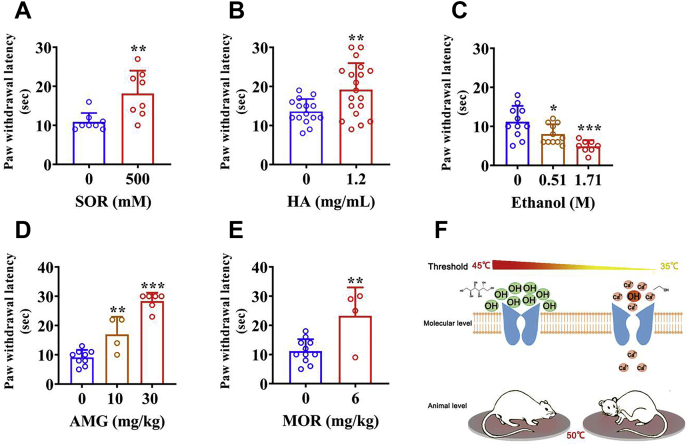
How were the modulatory effects of polyols on the heat activation of the TRPV1 channel that we have observed in cellular electrophysiology experiments manifested at the animal behavioral level? As the heat activation threshold of TRPV1 was increased by polyols, we hypothesized that polyols would decrease TRPV1 activation by noxious heat to relieve pain. To test this hypothesis in vivo, we conducted hot plate test under a constantly high temperature after bilateral intraplantar pretreatment with sorbitol, HA, and ethanol to each group of rats. We set the temperature of the hot plate to be 50 °C so that it readily activates the TRPV1 channel but not the other noxious heat sensor TRPV2 channel with an activation threshold higher than 52 °C (9). In rats pretreated with sorbitol, thermal paw withdrawal latency was significantly higher than in rats receiving saline (saline: 10.9 ± 2.3 s, n = 8; sorbitol: 18.1 ± 5.9 s, n = 8; p < 0.01; Fig. 5A). Similar results were also obtained from HA pretreatment group, as HA has been reported to reduce pain in mice (41) (saline: 13.6 ± 3.2 s, n = 15; HA: 19.2 ± 6.8 s, n = 19; p < 0.01; Fig. 5B). In contrast, each dose group of TRPV1-activating ethanol exhibits significantly shorter thermal paw withdrawal latency than the saline group (saline: 11.2 ± 4.1 s, n = 11; low dose: 8.0 ± 2.5 s, n = 11, p < 0.05; high dose: 4.9 ± 1.6 s, n = 8, p < 0.001; Fig. 5C). In addition, we chose two positive control drugs (AMG9810, which is a competitive TRPV1 antagonist (42), and morphine) and repeated the same tests to prove our animal experimental system is reliable. As expected, both drugs significantly increased the thermal paw withdrawal latency compared with the control group (Fig. 5, D and E). Combined with the results from electrophysiological studies, our findings indicate that the opposite effects of polyols and ethanol on the sensitivity of nociceptor endings to noxious stimuli are mediated, at least in part, by TRPV1 channels (Fig. 5F).
Figure 5.
Polyols significantly relieve pain induced by noxious heat in rats. Effects of intraplantar injection of sorbitol (A), HA (B), and various doses of ethanol (C) on thermal paw withdrawal latency. Effects of two positive control drugs AMG9810 (D) and morphine (E) on thermal paw withdrawal latency. The data are represented as the mean ± SD. Statistical analyses were performed by one-way ANOVA with Dunnett’s multiple comparisons post hoc test or Student’s t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 versus corresponding control group. F, a schematic diagram summarizing distinct effects of polyols and ethanol on thermal activation threshold of the TRPV1 channel.
Discussion
In this study, we first observed that in patch-clamp recordings, polyols such as sucrose, sorbitol, and HA largely elevate the heat activation threshold temperature of TRPV1, whereas the monoalcohol ethanol decreased the threshold. Such effects of polyols on TRPV1 only occur when they are perfused from the extracellular side. In agreement with such a location preference of polyols, we further found that, in the outer pore region of TRPV1, mutating residues E601 and E649 can largely abolish the effects of polyols. Furthermore, as the heat activation threshold is elevated by polyols, paw injection of polyols reduced the heat pain in rats.
The extracellular preference of polyols’ effects on heat activation threshold suggests that key domains for modulation of heat activation in TRPV1 locate extracellularly. Indeed, the outer pore of TRPV1 shows conformational changes specific to heat activation (21), whereas modulators such as cations (36, 43, 44) and peptide toxins (35, 45, 46) binding to the outer pore region have been reported to manipulate the heat activation machineries. Although the intracellular N and C termini have also been suggested to be critical for heat activation (16, 22, 47), we found that, when polyols (Fig. 2, A and B) or Mg2+ ions (36) were perfused to the intracellular side of TRPV1, they exhibit little effects on its heat activation. Moreover, we found in the cold activated TRPM8 channel, residues modulating cold activation properties are all located in the outer pore region (48). Although theoretical analysis suggests that residues serving as temperature sensors in TRP channels can be widely distributed (23), our observation in this study with polyols and previous studies suggest a potential clustering of such residues to the outer pore of TRPV1.
Although our studies reveal that the clustering of residues is important for heat activation in the outer pore of TRPV1, how outer pore residues contribute to heat activation remains unknown. As replacing residues in the turret of TRPV1 with glycine or serine (mutants R2 and R4) did not eliminate the increase in heat activation threshold by polyols (Fig. 3, A–C), we reason that either these turret residues are not critically involved in modulation by polyols or, more likely, polyols exert their effects on TRPV1 through indirect interaction with the channel protein (30). It is likely that the polyol molecules in extracellular solution are excluded from the protein surface, thereby forming a thin water layer on the surface of the protein and finally altering the ordering of water in polyol solution (30). There is a positive correlation between ordering extent of water and polyols molecular volume, so that molecules with large volume and multiple hydroxyl groups such as sorbitol and sucrose exhibit opposite effects on TRPV1 heat activation as compared with the ethanol molecule, which is small in volume with only a single hydroxyl group. In addition, we found that adding polyols (1000 mM sorbitol) decreased the pH of solution (therefore increased the proton concentration) by about 0.17 unit to pH 7.0. The TRPV1 channel is activated by low extracellular pH, so a decrease in pH would allosterically lower the heat activation threshold. However, we observed that, instead of lowering the heat activation threshold, 1000 mM sorbitol significantly increased the heat activation threshold by nearly 5 degrees (Fig. 1D). Therefore, the effect of polyols on heat activation cannot be due to changes in the pH of solution.
Nevertheless, we have identified two key residues (E601 and E649) for the effects of polyols on TRPV1 heat activation. Since these two residues are also critical for the proton activation of the channel (34), we believe that there is an intrinsic link between these two stimuli of the polymodal receptor TRPV1. A previous study shows that these two residues are critical for channel activation by Mg2+ ions (49). Our previous study also demonstrates that E601 and E649 are required for the allosteric coupling of proton and peptide toxin activation of TRPV1 (50). Therefore, these studies strongly suggest that E601 and E649 are critical for TRPV1 activation by extracellular stimuli.
The TRPV1 channel is an established target for analgesic drugs (11). Indeed, the polyol HA has been shown to inhibit TRPV1 to reduce heat and capsaicin nocifensive responses in mice (41). We found that, in agreement with this observation, other polyols like sorbitol also show analgesic effects against heat pain in rats (Fig. 5). HA has been intra-articularly injected to reduce pain in osteoarthritis (41, 51). As polyols generally affect the heat capacitance of thermosensitive ion channels, they may modulate temperature gating properties of other ion channels. Therefore, we speculate that polyols in general have the potential to be translationally developed as analgesics for certain types of pain in future.
Experimental procedures
HEK293 cells culture and transient transfection
Human embryonic kidney 293 (HEK293) cells were cultured in Dulbecco's modified eagle medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37 °C with 5% CO2. Transient transfection was performed at ~70% cell confluence using Lipofectamine 2000 according to the manufacturer's instruction and was conducted by adding 2 μg plasmid DNA and 2 μl Lipofectamine 2000 in a culture dish. After transfection, cells were incubated overnight (24–36 h), then electrophysiological experiments were recorded.
cDNA constructs
Two mutants were generated in the pore turret region. These two constructs used in this study were based on the human TRPV1. These mutations were designed to perturb the targeted structures and test their potential involvements in activation gating by polyols. For the first mutant, termed R2, the turret segment 602GKNDSLPSESTSHRWRGPACRPPD625 was replaced with GKNDSLPSESGGGGSGGGGSGGGG. For the second mutant, termed R4, it was replaced with GKNDSLPSESTSHRWRGGGGGS. These are among a group of pore turret mutants that we have studied previously, and their functional properties including heat response are described in our previous report (17).
Electrophysiological recordings
Patch-clamp recordings were done using an EPC10 amplifier (HEKA) driven by PatchMaster software (HEKA). The membrane potential was held at 0 mV, and currents were elicited by a protocol consisting of a 300-ms step to +80 mV followed by a 300-ms step to −80 mV at 1-s intervals, and currents were normally measured at +80 mV from inside-out patches. For inside-out measurements, both bath and pipette solutions contained 130 mM NaCl, 0.2 mM EDTA, and 3 mM Hepes (pH 7.2). Patch pipettes were pulled from borosilicate glass capillaries on a P-97 pipette puller (Sutter Instrument) to a resistance of 4 to 8 MΩ. Patch-clamp recordings were conducted at room temperature at 24 °C.
Temperature control
The bath solution was heated by using a Warner temperature controller (Model TC-324C). The patch pipette was placed about 1 mm from the monitor thermistor of Models CC-28 (Warner Instruments) to ensure accurate monitoring of local bath solution temperature. The thermistor’s temperature readout was fed into an analog input of the patch amplifier and recorded simultaneously with current. The speed of temperature change was set at a moderate rate about 0.3 °C/s to ensure the current was recorded at steady state. With this method, we achieved rapid and reliable temperature changes between 24 and 50 °C.
Animals
Wistar female rats (180–200 g, Jinan Pengyue Experimental Animal Breeding Co Ltd) were housed under a 12-h light–dark cycle and allowed access to food and water ad libitum. The ambient temperature of the holding and testing rooms was ~22 °C. All procedures involving animals were carried out in compliance with the National Institutes of Health and institutional guidelines for the humane care of animals and were approved by the Animal Care Committee of Qingdao University (Qingdao, China). All efforts were made to minimize both animal numbers and distress within the experiments.
Reagents and drug administration
AMG9810 and sodium hyaluronate (high molecular weight = 1500 kDa) were purchased from MCE, D-sorbitol was purchased from Sigma. Drugs were dissolved in saline to form a final administering solution (D-sorbitol 0.5 M, HA 0.8 μM, ethanol 0.51 and 1.71 M, and morphine 6 mg/kg) except AMG9810 (10 and 30 mg/kg) that was dissolved in vehicle (10% dimethyl sulfoxide + 10% Tween-80 + 80% saline). D-sorbitol, HA, and ethanol were administered by intraplantar injections using a 29G × 1/2", 3/10-ml insulin syringe; AMG9810 and morphine were injected intraperitoneally. According to preliminary experiments, we carried out drug treatments at several time points for each drug before hot plate test. Five minutes for the ethanol, 1 h for the D-sorbitol, 8 h for the HA, and 30 min for the AMG9810 and morphine group, respectively. The experimenter was blinded to the identity of the injectates in the various behavioral experiments.
Hot plate test
Before the experiment, all rats were placed in the hot plate device one by one for 2 min at room temperature, so that the rats could adapt to the surrounding environment in advance. Then we did a screening experiment, rats were tested before drug treatment, they were placed individually on a hot plate analgesia tester (BME-480, Chinese Academy of Medical Sciences Institute of Biomedical Engineering) maintained at a constant temperature of 50 ± 0.1 °C. The paw withdrawal latency was recorded as the time taken to exhibit distinct pain behavior by hind paw licking. Rats showing a reaction time greater than 20 s were excluded from the subsequent test. To obtain control data, the saline or vehicle was injected. Immediately after the termination of a trial, rats were removed from the hot plate surface and returned to the home cage. A cut-off time of 30 s was established to prevent tissue injury. After the latency of each rat was recorded, the hot plate surface was wiped with 75% alcohol to remove dirt and odor left by the previous rat, so as not to affect the results of the next rat.
Data analysis
To determine a more precise heat activation threshold temperature of TRPV1, we recruited a new method that is different from some previous studies. In detail, our new method starts with smoothing the current trace (in Igor, under “analysis”, choose “smooth…”). The purpose of the first step is to ensure the next step (differentiation) works well. This step can be repeated multiple times to achieve a better result. Next we used the differentiation function to obtain the slope at each data point, and then we found a point where the slope is clearly higher than the zero line. This is where the current took off upon heating, which is the heat activation threshold temperature (Fig. S4). Data display was performed using Igor Pro 6.0. Data are shown as mean ± SD, comparison of experimental data between the two groups was conducted statistically with unpaired Student's t test, and the comparison of experimental data between multiple groups was conducted statistically with one-way ANOVA. Differences were regarded as statistically significant with ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001, n.s., not significant.
Animal experiment data are presented as mean ± SD, comparison of experimental data between the two groups was conducted statistically with unpaired Student's t test, and the comparison of experimental data between multiple groups was conducted statistically with one-way ANOVA. Statistical significance is indicated as follows: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Data availability
All data are contained within the article.
Supporting information
This article contains supporting information (34).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We are grateful to our lab members for assistance and discussion. Initial test of polyols on heat activation of the TRPV1 channel was conducted by F.Y. in Dr Jie Zheng’s lab at University of California, Davis.
Author contributions
Y. T., S. R., and F. Y. data curation; Y. T. and F. Y. supervision; Y. T. and F. Y. funding acquisition; Y. T. and Y. N. writing - original draft; Y. N. and Y. L. formal analysis; Y. N., Y. L., and L. L., investigation; S. R. resources; F. Y. conceptualization; F. Y. writing - review and editing.
Funding and additional information
This study was supported by National Natural Science Foundation of China (31971040 and 31800990 to F. Y.), Zhejiang Provincial Natural Science Foundation of China (LR20C050002 to F. Y.), and Natural Science Foundation of Shandong Province (ZR2020MH161 to Y. T.).
Edited by Mike Shipston
Contributor Information
Yuhua Tian, Email: yhtian05250@qdu.edu.cn.
Fan Yang, Email: fanyanga@zju.edu.cn.
Supporting information
References
- 1.Caterina M.J. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R64–R76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- 2.Clapham D.E. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 3.Feng Q. Temperature sensing by thermal TRP channels: Thermodynamic basis and molecular insights. Curr. Top. Membr. 2014;74:19–50. doi: 10.1016/B978-0-12-800181-3.00002-6. [DOI] [PubMed] [Google Scholar]
- 4.Patapoutian A., Peier A.M., Story G.M., Viswanath V. ThermoTRP channels and beyond: Mechanisms of temperature sensation. Nat. Rev. Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- 5.Vay L., Gu C., McNaughton P.A. The thermo-TRP ion channel family: Properties and therapeutic implications. Br. J. Pharmacol. 2012;165:787–801. doi: 10.1111/j.1476-5381.2011.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vriens J., Owsianik G., Hofmann T., Philipp S.E., Stab J., Chen X., Benoit M., Xue F., Janssens A., Kerselaers S., Oberwinkler J., Vennekens R., Gudermann T., Nilius B., Voets T. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron. 2011;70:482–494. doi: 10.1016/j.neuron.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 7.Vriens J., Nilius B., Voets T. Peripheral thermosensation in mammals. Nat. Rev. Neurosci. 2014;15:573–589. doi: 10.1038/nrn3784. [DOI] [PubMed] [Google Scholar]
- 8.Dhaka A., Viswanath V., Patapoutian A. Trp ion channels and temperature sensation. Annu. Rev. Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 9.Caterina M.J., Rosen T.A., Tominaga M., Brake A.J., Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 10.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 11.Julius D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 12.Vyklický L., Vlachová V., Vitásková Z., Dittert I., Kabát M., Orkand R.K. Temperature coefficient of membrane currents induced by noxious heat in sensory neurones in the rat. J. Physiol. 1999;517(Pt 1):181–192. doi: 10.1111/j.1469-7793.1999.0181z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welch J.M., Simon S.A., Reinhart P.H. The activation mechanism of rat vanilloid receptor 1 by capsaicin involves the pore domain and differs from the activation by either acid or heat. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13889–13894. doi: 10.1073/pnas.230146497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao E., Liao M., Cheng Y., Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao M., Cao E., Julius D., Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brauchi S., Orta G., Salazar M., Rosenmann E., Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J. Neurosci. 2006;26:4835–4840. doi: 10.1523/JNEUROSCI.5080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui Y., Yang F., Cao X., Yarov-Yarovoy V., Wang K., Zheng J. Selective disruption of high sensitivity heat activation but not capsaicin activation of TRPV1 channels by pore turret mutations. J. Gen. Physiol. 2012;139:273–283. doi: 10.1085/jgp.201110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du G., Tian Y., Yao Z., Vu S., Zheng J., Chai L., Wang K., Yang S. A specialized pore turret in the mammalian cation channel TRPV1 is responsible for distinct and species-specific heat activation thresholds. J. Biol. Chem. 2020;295:9641–9649. doi: 10.1074/jbc.RA120.013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grandl J., Kim S.E., Uzzell V., Bursulaya B., Petrus M., Bandell M., Patapoutian A. Temperature-induced opening of TRPV1 ion channel is stabilized by the pore domain. Nat. Neurosci. 2010;13:708–714. doi: 10.1038/nn.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voets T., Droogmans G., Wissenbach U., Janssens A., Flockerzi V., Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 21.Yang F., Cui Y., Wang K., Zheng J. Thermosensitive TRP channel pore turret is part of the temperature activation pathway. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7083–7088. doi: 10.1073/pnas.1000357107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao J., Liu B., Qin F. Modular thermal sensors in temperature-gated transient receptor potential (TRP) channels. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11109–11114. doi: 10.1073/pnas.1105196108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clapham D.E., Miller C. A thermodynamic framework for understanding temperature sensing by transient receptor potential (TRP) channels. Proc. Natl. Acad. Sci. U. S. A. 2011;108:19492–19497. doi: 10.1073/pnas.1117485108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowdhury S., Jarecki B.W., Chanda B. A molecular framework for temperature-dependent gating of ion channels. Cell. 2014;158:1148–1158. doi: 10.1016/j.cell.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu L., Han Y., Chen X., Aierken A., Wen H., Zheng W., Wang H., Lu X., Zhao Z., Ma C., Liang P., Yang W., Yang S., Yang F. Molecular mechanisms underlying menthol binding and activation of TRPM8 ion channel. Nat. Commun. 2020;11:3790. doi: 10.1038/s41467-020-17582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis-Searles P.R., Saunders A.J., Erie D.A., Winzor D.J., Pielak G.J. Interpreting the effects of small uncharged solutes on protein-folding equilibria. Annu. Rev. Biophys. Biomol. Struct. 2001;30:271–306. doi: 10.1146/annurev.biophys.30.1.271. [DOI] [PubMed] [Google Scholar]
- 27.Liao Y.H., Brown M.B., Nazir T., Quader A., Martin G.P. Effects of sucrose and trehalose on the preservation of the native structure of spray-dried lysozyme. Pharm. Res. 2002;19:1847–1853. doi: 10.1023/a:1021445608807. [DOI] [PubMed] [Google Scholar]
- 28.Back J.F., Oakenfull D., Smith M.B. Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry. 1979;18:5191–5196. doi: 10.1021/bi00590a025. [DOI] [PubMed] [Google Scholar]
- 29.Lee S.L., Debenedetti P.G., Errington J.R. A computational study of hydration, solution structure, and dynamics in dilute carbohydrate solutions. J. Chem. Phys. 2005;122:204511. doi: 10.1063/1.1917745. [DOI] [PubMed] [Google Scholar]
- 30.Liu F.F., Ji L., Zhang L., Dong X.Y., Sun Y. Molecular basis for polyol-induced protein stability revealed by molecular dynamics simulations. J. Chem. Phys. 2010;132:225103. doi: 10.1063/1.3453713. [DOI] [PubMed] [Google Scholar]
- 31.O'Connor T.F., Debenedetti P.G., Carbeck J.D. Simultaneous determination of structural and thermodynamic effects of carbohydrate solutes on the thermal stability of ribonuclease A. J. Am. Chem. Soc. 2004;126:11794–11795. doi: 10.1021/ja0481777. [DOI] [PubMed] [Google Scholar]
- 32.Yang F., Xu L., Lee B.H., Xiao X., Yarov-Yarovoy V., Zheng J. An unorthodox mechanism underlying voltage sensitivity of TRPV1 ion channel. Adv. Sci. 2020;7:2000575. doi: 10.1002/advs.202000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trevisani M., Smart D., Gunthorpe M.J., Tognetto M., Barbieri M., Campi B., Amadesi S., Gray J., Jerman J.C., Brough S.J., Owen D., Smith G.D., Randall A.D., Harrison S., Bianchi A. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat. Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- 34.Jordt S.E., Tominaga M., Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang S., Yang F., Wei N., Hong J., Li B., Luo L., Rong M., Yarov-Yarovoy V., Zheng J., Wang K., Lai R. A pain-inducing centipede toxin targets the heat activation machinery of nociceptor TRPV1. Nat. Commun. 2015;6:8297. doi: 10.1038/ncomms9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang F., Ma L., Cao X., Wang K., Zheng J. Divalent cations activate TRPV1 through promoting conformational change of the extracellular region. J. Gen. Physiol. 2014;143:91–103. doi: 10.1085/jgp.201311024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nolden A.A., Lenart G., Hayes J.E. Putting out the fire - efficacy of common beverages in reducing oral burn from capsaicin. Physiol. Behav. 2019;208:112557. doi: 10.1016/j.physbeh.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang F., Xiao X., Lee B.H., Vu S., Yang W., Yarov-Yarovoy V., Zheng J. The conformational wave in capsaicin activation of transient receptor potential vanilloid 1 ion channel. Nat. Commun. 2018;9:2879. doi: 10.1038/s41467-018-05339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang F., Xiao X., Cheng W., Yang W., Yu P., Song Z., Yarov-Yarovoy V., Zheng J. Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat. Chem. Biol. 2015;11:518–524. doi: 10.1038/nchembio.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aneiros E., Cao L., Papakosta M., Stevens E.B., Phillips S., Grimm C. The biophysical and molecular basis of TRPV1 proton gating. EMBO J. 2011;30:994–1002. doi: 10.1038/emboj.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caires R., Luis E., Taberner F.J., Fernandez-Ballester G., Ferrer-Montiel A., Balazs E.A., Gomis A., Belmonte C., de la Pena E. Hyaluronan modulates TRPV1 channel opening, reducing peripheral nociceptor activity and pain. Nat. Commun. 2015;6:8095. doi: 10.1038/ncomms9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gavva N.R., Bannon A.W., Surapaneni S., Hovland D.N., Jr., Lehto S.G., Gore A., Juan T., Deng H., Han B., Klionsky L., Kuang R., Le A., Tamir R., Wang J., Youngblood B. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J. Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao X., Ma L., Yang F., Wang K., Zheng J. Divalent cations potentiate TRPV1 channel by lowering the heat activation threshold. J. Gen. Physiol. 2014;143:75–90. doi: 10.1085/jgp.201311025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jara-Oseguera A., Bae C., Swartz K.J. An external sodium ion binding site controls allosteric gating in TRPV1 channels. Elife. 2016;5 doi: 10.7554/eLife.13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu A., Aierken A., Yao Z., Vu S., Tian Y., Zheng J., Yang S., Yang F. A centipede toxin causes rapid desensitization of nociceptor TRPV1 ion channel. Toxicon. 2020;178:41–49. doi: 10.1016/j.toxicon.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bae C., Anselmi C., Kalia J., Jara-Oseguera A., Schwieters C.D., Krepkiy D., Won Lee C., Kim E.H., Kim J.I., Faraldo-Gomez J.D., Swartz K.J. Structural insights into the mechanism of activation of the TRPV1 channel by a membrane-bound tarantula toxin. Elife. 2016;5 doi: 10.7554/eLife.11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vlachova V., Teisinger J., Susankova K., Lyfenko A., Ettrich R., Vyklicky L. Functional role of C-terminal cytoplasmic tail of rat vanilloid receptor 1. J. Neurosci. 2003;23:1340–1350. doi: 10.1523/JNEUROSCI.23-04-01340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang S., Lu X., Wang Y., Xu L., Chen X., Yang F., Lai R. A paradigm of thermal adaptation in penguins and elephants by tuning cold activation in TRPM8. Proc. Natl. Acad. Sci. U. S. A. 2020;117:201922714. doi: 10.1073/pnas.1922714117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahern G.P., Brooks I.M., Miyares R.L., Wang X.B. Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. J. Neurosci. 2005;25:5109–5116. doi: 10.1523/JNEUROSCI.0237-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang S., Yang F., Zhang B., Lee B.H., Li B., Luo L., Zheng J., Lai R. A bimodal activation mechanism underlies scorpion toxin-induced pain. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1700810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pozo M.A., Balazs E.A., Belmonte C. Reduction of sensory responses to passive movements of inflamed knee joints by hylan, a hyaluronan derivative. Exp. Brain Res. 1997;116:3–9. doi: 10.1007/pl00005742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the article.