Abstract
Thiamine is essential for the activity of several enzymes associated with energy metabolism in humans. Chronic alcohol use is associated with deficiency of thiamine along with other vitamins through several mechanisms. Several neuropsychiatric syndromes have been associated with thiamine deficiency in the context of alcohol use disorder including Wernicke–Korsakoff syndrome, alcoholic cerebellar syndrome, alcoholic peripheral neuropathy, and possibly, Marchiafava–Bignami syndrome. High-dose thiamine replacement is suggested for these neuropsychiatric syndromes.
Keywords: Alcohol use disorder, alcoholic cerebellar syndrome, alcoholic peripheral neuropathy, Marchiafava–Bignami syndrome, thiamine, Wernicke–Korsakoff syndrome
INTRODUCTION
Thiamine is a water-soluble vitamin (B1) that plays a key role in the activity of several enzymes associated with energy metabolism. Thiamine pyrophosphate (or diphosphate) is the active form that acts as a cofactor for enzymes. The daily dietary requirement of thiamine in adults is 1–2 mg and is dependent on carbohydrate intake.[1,2] The requirement increases if basal metabolic rate is higher, for example, during alcohol withdrawal state. Dietary sources include pork (being the major source), meat, legume, vegetables, and enriched foods. The body can store between 30 and 50 mg of thiamine and is likely to get depleted within 4–6 weeks if the diet is deficient.[2] In those with alcohol-related liver damage, the ability to store thiamine is gradually reduced.[1,2] Lower thiamine levels are found in 30%–80% of chronic alcohol users.[3] Thiamine deficiency occurs due to poor intake of vitamin-rich foods, impaired intestinal absorption, decreased storage capacity of liver, damage to the renal epithelial cells due to alcohol, leading to increased loss from the kidneys, and excessive loss associated with medical conditions.[2,3] Furthermore, alcohol decreases the absorption of colonic bacterial thiamine, reduces the enzymatic activity of thiamine pyrophosphokinase, and thereby, reducing the amount of available thiamine pyrophosphate.[4] Since facilitated diffusion of thiamine into cells is dependent on a concentration gradient, reduced thiamine pyrophosphokinase activity further reduces thiamine uptake into cells.[4] Impaired utilization of thiamine is seen in certain conditions (e.g., hypomagnesemia) which are common in alcohol use disorder.[2,3,4] This narrative review discusses the neuropsychiatric syndromes associated with thiamine deficiency in the context of alcohol use disorder, and the treatment regimens advocated for these conditions. A PubMed search supplemented with manual search was used to identify neuropsychiatric syndromes related to thiamine deficiency in alcohol use disorder patients.
NEUROPSYCHIATRIC SYNDROMES ASSOCIATED WITH THIAMINE DEFICIENCY
Wernicke–Korsakoff syndrome
Wernicke encephalopathy is associated with chronic alcohol use, and if not identified and treated early, could lead to permanent brain damage characterized by an amnestic syndrome known as Korsakoff syndrome. Inappropriate treatment of Wernicke encephalopathy with lower doses of thiamine can lead to high mortality rates (~20%) and Korsakoff syndrome in ~ 80% of patients (ranges from 56% to 84%).[5,6] The classic triad of Wernicke includes oculomotor abnormalities, cerebellar dysfunction, and confusion. Wernicke lesions are found in 12.5% of brain samples of patients with alcohol dependence.[7] However, only 20%–30% of them had a clinical diagnosis of Wernicke encephalopathy antemortem. It has been found that many patients develop Wernicke–Korsakoff syndrome (WKS) following repeated subclinical episodes of thiamine deficiency.[7] In an autopsy report of 97 chronic alcohol users, only16% had all the three “classical signs,” 29% had two signs, 37% presented with one sign, and 19% had none.[8] Mental status changes are the most prevalent sign (seen in 82% of the cases), followed by eye signs (in 29%) and ataxia (23%).[8] WKS should be suspected in persons with a history of alcohol use and presenting with signs of ophthalmoplegia, ataxia, acute confusion, memory disturbance, unexplained hypotension, hypothermia, coma, or unconsciousness.[9] Operational criteria for the diagnosis of Wernicke encephalopathy have been proposed by Caine et al.[10] that requires two out of four features, i.e., (a) dietary deficiency (signs such as cheilitis, glossitis, and bleeding gums), (b) oculomotor abnormalities (nystagmus, opthalmoplegia, and diplopia), (c) cerebellar dysfunction (gait ataxia, nystagmus), and (d) either altered mental state (confusion) or mild memory impairment.
As it is very difficult to clinically distinguish Wernicke encephalopathy from other associated conditions such as delirium tremens, hepatic encephalopathy, or head injury, it is prudent to have a lower threshold to diagnose this if any of the clinical signs is seen. Magnetic resonance imaging (MRI) brain scan during Wernicke encephalopathy shows mammillary body atrophy and enlarged third ventricle, lesions in the medial portions of thalami and mid brain and can be used to aid diagnosis.[11,12] However, most clinical situations warrant treatment without waiting for neuroimaging report. The treatment suggestions in the guidelines vary widely. Furthermore, hardly any evidence-based recommendations exist on a more general use of thiamine as a preventative intervention in individuals with alcohol use disorder.[13] There are very few studies that have evaluated the dose and duration of thiamine for WKS, but higher doses may result in a greater response.[6,14] With thiamine administration rapid improvement is seen in eye movement abnormalities (improve within days or weeks) and ataxia (may take months to recover), but the effects on memory, in particular, are unclear.[4,14] Severe memory impairment is the core feature of Korsakoff syndrome. Initial stages of the disease can present with confabulation, executive dysfunction, flattened affect, apathy, and poor insight.[15] Both the episodic and semantic memory are affected, whereas, procedural memory remains intact.[15]
Thomson et al.[6] suggested the following should be treated with thiamine as they are at high risk for developing WKS: (1) all patients with any evidence of chronic alcohol misuse and any of the following: acute confusion, decreased conscious level, ataxia, ophthalmoplegia, memory disturbance, and hypothermia with hypotension; (2) patients with delirium tremens may often also have Wernicke encephalopathy, therefore, all of these patients should be presumed to have Wernicke encephalopathy and treated, preferably as inpatients; and (3) all hypoglycemic patients (who are treated with intravenous glucose) with evidence of chronic alcohol ingestion must be given intravenous thiamine immediately because of the risk of acutely precipitating Wernicke encephalopathy.
Alcoholic cerebellar syndrome
Chronic alcohol use is associated with the degeneration of anterior superior vermis, leading to a clinical syndrome characterized by the subacute or chronic onset of gait ataxia and incoordination in legs, with relative sparing of upper limbs, speech, and oculomotor movements.[16] In severe cases, truncal ataxia, mild dysarthria, and incoordination of the upper limb is also found along with gait ataxia. Thiamine deficiency is considered to be the etiological factor,[17,18] although direct toxic effects of alcohol may also contribute to this syndrome. One-third of patients with chronic use of alcohol have evidence of alcoholic cerebellar degeneration; however, population-based studies estimate prevalence to be 14.6%.[19] The effect of alcohol on the cerebellum is graded with the most severe deficits occurring in alcohol users with the longest duration and highest severity of use. The diagnosis of cerebellar degeneration is largely clinical; MRI can be used to evaluate for vermian atrophy but is unnecessary.[20] Anterior portions of vermis are affected early, with involvement of posterior vermis and adjacent lateral hemispheres occurring late in the course could be used to differentiate alcoholic cerebellar degeneration from other conditions that cause more diffuse involvement.[21] The severity of cerebellar syndrome is more in the presence of WKS, thus could be related to thiamine deficiency.[22,23] Therefore, this has been considered as a cerebellar presentation of WKS and should be treated in a similar way.[16] There are anecdotal evidence to suggest improvement in cerebellar syndrome with high-dose thiamine.[24]
Alcoholic peripheral neuropathy
Peripheral neuropathy is common in alcohol use disorder and is seen in 44% of the users.[25] It has been associated predominantly with thiamine deficiency; however, deficiency of other B vitamins (pyridoxine and cobalamin) and direct toxic effect of alcohol is also implicated.[26] Clinically, onset of symptoms is gradual with the involvement of both sensory and motor fibers and occasionally autonomic fibers. Neuropathy can affect both small and large peripheral nerve fibers, leading to different clinical manifestations. Thiamine deficiency-related neuropathy affects larger fiber types, which results in motor deficits and sensory ataxia. On examination, large fiber involvement is manifested by distal limb muscle weakness and loss of proprioception and vibratory sensation. Together, these can contribute to the gait unsteadiness seen in chronic alcohol users by creating a superimposed steppage gait and reduced proprioceptive input back to the movement control loops in the central nervous system. The most common presentations include painful sensations in both lower limbs, sometimes with burning sensation or numbness, which are early symptoms. Typically, there is a loss of vibration sensation in distal lower limbs. Later symptoms include loss of proprioception, gait disturbance, and loss of reflexes. Most advanced findings include weakness and muscle atrophy.[20] Progression is very gradual over months and involvement of upper limbs may occur late in the course. Diagnosis begins with laboratory evaluation to exclude other causes of distal, sensorimotor neuropathy including hemoglobin A1c, liver function tests, and complete blood count to evaluate for red blood cell macrocytosis. Cerebrospinal fluid studies may show increased protein levels but should otherwise be normal in cases of alcohol neuropathy and are not recommended in routine evaluation. Electromyography and nerve conduction studies can be used to distinguish whether the neuropathy is axonal or demyelinating and whether it is motor, sensory, or mixed type. Alcoholic neuropathy shows reduced distal, sensory amplitudes, and to a lesser extent, reduced motor amplitudes on nerve conduction studies.[20] Abstinence and vitamin supplementation including thiamine are the treatments advocated for this condition.[25] In mild-to-moderate cases, near-complete improvement can be achieved.[20] Randomized controlled trials have showed a significant improvement in alcoholic polyneuropathy with thiamine treatment.[27,28]
Marchiafava–Bignami syndrome
This is a rare but fatal condition seen in chronic alcohol users that is characterized by progressive demyelination and necrosis of the corpus callosum. The association of this syndrome with thiamine deficiency is not very clear, and direct toxic effects of alcohol are also suggested.[29] The clinical syndrome is variable and presentation can be acute, subacute, or chronic. In acute forms, it is predominantly characterized by the altered mental state such as delirium, stupor, or coma.[30] Other clinical features in neuroimaging confirmed Marchiafava–Bignami syndrome (MBS) cases include impaired gait, dysarthria, mutism, signs of split-brain syndrome, pyramidal tract signs, primitive reflexes, rigidity, incontinence, gaze palsy, diplopia, and sensory symptoms.[30] Neuropsychiatric manifestations are common and include psychotic symptoms, depression, apathy, aggressive behavior, and sometimes dementia.[29] MRI scan shows lesions of the corpus callosum, particularly splenium. Treatment for this condition is mostly supportive and use of nutritional supplements and steroids. However, there are several reports of improvement of this syndrome with thiamine at variable doses including reports of beneficial effects with high-dose strategy.[29,30,31] Early initiation of thiamine, preferably within 2 weeks of the onset of symptoms is associated with a better outcome. Therefore, high-dose thiamine should be administered to all suspected cases of MBS.
LABORATORY DIAGNOSIS OF THIAMINE DEFICIENCY
Estimation of thiamine and thiamine pyrophosphate levels may confirm the diagnosis of deficiency. Levels of thiamine in the blood are not reliable indicators of thiamine status. Low erythrocyte transketolase activity is also helpful.[32,33] Transketolase concentrations of <120 nmol/L have also been used to indicate deficiency, while concentrations of 120–150 nmol/L suggest marginal thiamine status.[1] However, these tests are not routinely performed as it is time consuming, expensive, and may not be readily available.[34] The ETKA assay is a functional test rather than a direct measurement of thiamin status and therefore may be influenced by factors other than thiamine deficiency such as diabetes mellitus and polyneuritis.[1] Hence, treatment should be initiated in the absence of laboratory confirmation of thiamine deficiency. Furthermore, treatment should not be delayed if tests are ordered, but the results are awaited. Electroencephalographic abnormalities in thiamine deficiency states range from diffuse mild-to-moderate slow waves and are not a good diagnostic option, as the prevalence of abnormalities among patients is inconsistent.[35]
Surrogate markers, which reflect chronic alcohol use and nutritional deficiency other than thiamine, may be helpful in identifying at-risk patients. This includes gamma glutamate transferase, aspartate aminotransferase: alanine transaminase ratio >2:1, and increased mean corpuscular volume.[36] They are useful when a reliable history of alcohol use is not readily available, specifically in emergency departments when treatment needs to be started immediately to avoid long-term consequences.
THIAMINE REPLACEMENT THERAPY
Oral versus parenteral thiamine
Intestinal absorption of thiamine depends on active transport through thiamine transporter 1 and 2, which follow saturation kinetics.[1] Therefore, the rate and amount of absorption of thiamine in healthy individuals is limited. In healthy volunteers, a 10 mg dose results in maximal absorption of thiamine, and any doses higher than this do not increase thiamine levels. Therefore, the maximum amount of thiamine absorbed from 10 mg or higher dose is between 4.3 and 5.6 mg.[37] However, it has been suggested that, although thiamine transport occurs through the energy-requiring, sodium-dependent active process at physiologic concentrations, at higher supraphysiologic concentrations thiamine uptake is mostly a passive process.[38] Smithline et al. have demonstrated that it is possible to achieve higher serum thiamine levels with oral doses up to 1500 mg.[39]
In chronic alcohol users, intestinal absorption is impaired; hence, absorption rates are expected to be much lower. It is approximately 30% of that seen in healthy individuals, i.e., 1.5 mg of thiamine is absorbed from 10 mg oral thiamine.[3] In those consuming alcohol and have poor nutrition, not more than 0.8 mg of thiamine is absorbed.[2,3,6] The daily thiamine requirement is 1–1.6 mg/day, which may be more in alcohol-dependent patients at risk for Wernicke encephalopathy.[1] It is highly likely that oral supplementation with thiamine will be inadequate in alcohol-dependent individuals who continue to drink. Therefore, parenteral thiamine is preferred for supplementation in deficiency states associated with chronic alcohol use. Therapy involving parenteral thiamine is considered safe except for occasional circumstances of allergic reactions involving pruritus and local irritation.
There is a small, but definite risk of anaphylaxis with parenteral thiamine, specifically with intravenous administration (1/250,000 intravenous injections).[40] Diluting thiamine in 50–100 mg normal saline for infusion may reduce the risk. However, parenteral thiamine should always be administered under observation with the necessary facilities for resuscitation.
A further important issue involves the timing of administration of thiamine relative to the course of alcohol abuse or dependence. Administration of thiamine treatment to patients experiencing alcohol withdrawal may also be influenced by other factors such as magnesium depletion, N-methyl-D-aspartate (NMDA) receptor upregulation, or liver impairment, all of which may alter thiamine metabolism and utilization.[6,14]
Thiamine or other preparations (e.g., benfotiamine)
The thiamine transporters limit the rate of absorption of orally administered thiamine. Allithiamines (e.g., benfotiamine) are the lipid-soluble thiamine derivatives that are absorbed better, result in higher thiamine levels, and are retained longer in the body.[41] The thiamine levels with orally administered benfotiamine are much higher than oral thiamine and almost equals to intravenous thiamine given at the same dosage.[42]
Benfotiamine has other beneficial effects including inhibition of production of advanced glycation end products, thus protecting against diabetic vascular complications.[41] It also modulates nuclear transcription factor κB (NK-κB), vascular endothelial growth factor receptor 2, glycogen synthase kinase 3 β, etc., that play a role in cell repair and survival.[41] Benfotiamine has been found to be effective for the treatment of alcoholic peripheral neuropathy.[27]
Dosing of thiamine
As the prevalence of thiamine deficiency is very common in chronic alcohol users, the requirement of thiamine increases in active drinkers and it is difficult to rapidly determine thiamine levels using laboratory tests, it is prudent that all patients irrespective of nutritional status should be administered parenteral thiamine. The dose should be 100 mg thiamine daily for 3–5 days during inpatient treatment. Commonly, multivitamin injections are added to intravenous infusions. Patients at risk for thiamine deficiency should receive 250 mg of thiamine daily intramuscularly for 3–5 days, followed by oral thiamine 100 mg daily.[6]
Thiamine plasma levels reduce to 20% of peak value after approximately 2 h of parenteral administration, thus reducing the effective “window period” for passive diffusion to the central nervous system.[6] Therefore, in thiamine deficient individuals with features of Wernicke encephalopathy should receive thiamine thrice daily.
High-dose parenteral thiamine administered thrice daily has been advocated in patients at risk for Wernicke encephalopathy.[43] The Royal College of Physicians guideline recommends that patients with suspected Wernicke encephalopathy should receive 500 mg thiamine diluted in 50–100 ml of normal saline infusion over 30 min three times daily for 2–3 days and sometimes for longer periods.[13] If there are persistent symptoms such as confusion, cerebellar symptoms, or memory impairment, this regimen can be continued until the symptoms improve. If symptoms improve, oral thiamine 100 mg thrice daily can be continued for prolonged periods.[6,40] A similar treatment regimen is advocated for alcoholic cerebellar degeneration as well. Doses more than 500 mg intramuscular or intravenous three times a day for 3–5 days, followed by 250 mg once daily for a further 3–5 days is also recommended by some guidelines (e.g., British Association for Psychopharmacology).[44]
Other effects of thiamine
There are some data to suggest that thiamine deficiency can modulate alcohol consumption and may result in pathological drinking. Benfotiamine 600 mg/day as compared to placebo for 6 months was well tolerated and found to decrease psychiatric distress in males and reduce alcohol consumption in females with severe alcohol dependence.[45,46]
OTHER FACTORS DURING THIAMINE THERAPY
Correction of hypomagnesemia
Magnesium is a cofactor for many thiamine-dependent enzymes in carbohydrate metabolism. Patients may fail to respond to thiamine supplementation in the presence of hypomagnesemia.[47] Magnesium deficiency is common in chronic alcohol users and is seen in 30% of individuals.[48,49] It can occur because of increased renal excretion of magnesium, poor intake, decreased absorption because of Vitamin D deficiency, the formation of undissociated magnesium soaps with free fatty acids.[48,49]
The usual adult dose is 35–50 mmol of magnesium sulfate added to 1 L isotonic (saline) given over 12–24 h.[6] The dose has to be titrated against plasma magnesium levels. It is recommended to reduce the dose in renal failure. Contraindications include patients with documented hypersensitivity and those with heart block, Addison's disease, myocardial damage, severe hepatitis, or hypophosphatemia. Do not administer intravenous magnesium unless hypomagnesemia is confirmed.[6]
Other B-complex vitamins
Most patients with deficiency of thiamine will also have reduced levels of other B vitamins including niacin, pyridoxine, and cobalamin that require replenishment. For patients admitted to the intensive care unit with symptoms that may mimic or mask Wernicke encephalopathy, based on the published literature, routine supplementation during the 1st day of admission includes 200–500 mg intravenous thiamine every 8 h, 64 mg/kg magnesium sulfate (≈4–5 g for most adult patients), and 400–1000 μg intravenous folate.[50] If alcoholic ketoacidosis is suspected, dextrose-containing fluids are recommended over normal saline.[50]
PRECAUTIONS TO BE TAKEN WHEN ADMINISTERING PARENTERAL THIAMINE
It is recommended to monitor for anaphylaxis and has appropriate facilities for resuscitation and for treating anaphylaxis readily available including adrenaline and corticosteroids. Anaphylaxis has been reported at the rate of approximately 4/1 million pairs of ampoules of Pabrinex (a pair of high potency vitamins available in the UK containing 500 mg of thiamine (1:250,000 I/V administrations).[40] Intramuscular thiamine is reported to have a lower incidence of anaphylactic reactions than intravenous administration.[40] The reaction has been attributed to nonspecific histamine release.[51] Administer intravenous thiamine slowly, preferably by slow infusion in 100 ml normal saline over 15–30 min.
CONCLUSIONS
Risk factors for thiamine deficiency should be assessed in chronic alcohol users. A high index of suspicion and a lower threshold to diagnose thiamine deficiency states including Wernicke encephalopathy is needed. Several other presentations such as cerebellar syndrome, MBS, polyneuropathy, and delirium tremens could be related to thiamine deficiency and should be treated with protocols similar to Wernicke encephalopathy. High-dose thiamine is recommended for the treatment of suspected Wernicke encephalopathy and related conditions [Figure 1]. However, evidence in terms of randomized controlled trials is lacking, and the recommendations are based on small studies and anecdotal reports. Nevertheless, as all these conditions respond to thiamine supplementation, it is possible that these have overlapping pathophysiology and are better considered as Wernicke encephalopathy spectrum disorders.
Figure 1.
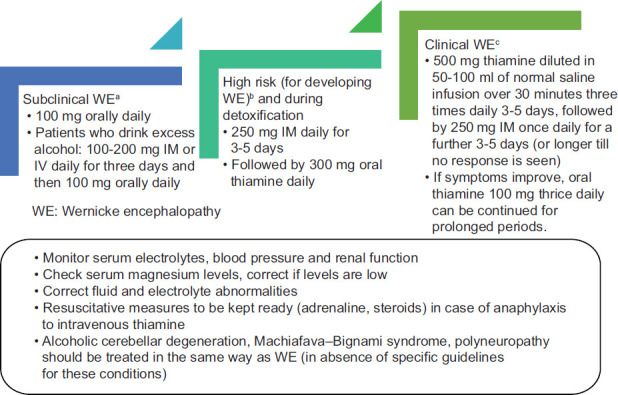
Thiamine recommendations for patients with alcohol use disorder. aHistory of alcohol use, but no clinical features of WE; bNo clinical features of WE, but with risk factors such as complicated withdrawal (delirium, seizures); cClinical features of WE (ataxia, opthalmoplegia, global confusion)
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Frank LL. Thiamin in clinical practice. JPEN J Parenter Enteral Nutr. 2015;39:503–20. doi: 10.1177/0148607114565245. [DOI] [PubMed] [Google Scholar]
- 2.Thomson AD, Marshall EJ. The natural history and pathophysiology of Wernicke's Encephalopathy and Korsakoff's Psychosis. Alcohol Alcohol. 2006;41:151–8. doi: 10.1093/alcalc/agh249. [DOI] [PubMed] [Google Scholar]
- 3.Thomson AD, Guerrini I, Marshall EJ. Wernicke's encephalopathy: Role of thiamine. Pract Gastroenterol. 2009;33:21–30. [Google Scholar]
- 4.Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: Under-recognized and under-treated. Psychosomatics. 2012;53:507–16. doi: 10.1016/j.psym.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Wood B, Currie J, Breen K. Wernicke's encephalopathy in a metropolitan hospital. A prospective study of incidence, characteristics and outcome. Med J Aust. 1986;144:12–6. doi: 10.5694/j.1326-5377.1986.tb113623.x. [DOI] [PubMed] [Google Scholar]
- 6.Thomson AD, Cook CC, Touquet R, Henry JA Royal College of Physicians London. The Royal College of Physicians report on alcohol: Guidelines for managing Wernicke's encephalopathy in the accident and Emergency Department. Alcohol Alcohol. 2002;37:513–21. doi: 10.1093/alcalc/37.6.513. [DOI] [PubMed] [Google Scholar]
- 7.Harper C. Thiamine (vitamin B1) deficiency and associated brain damage is still common throughout the world and prevention is simple and safe! Eur J Neurol. 2006;13:1078–82. doi: 10.1111/j.1468-1331.2006.01530.x. [DOI] [PubMed] [Google Scholar]
- 8.Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: A retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49:341–5. doi: 10.1136/jnnp.49.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook CC. Prevention and treatment of Wernicke-Korsakoff syndrome. Alcohol Alcohol. 2000;35:19–20. doi: 10.1093/alcalc/35.supplement_1.19. [DOI] [PubMed] [Google Scholar]
- 10.Caine D, Halliday GM, Kril JJ, Harper CG. Operational criteria for the classification of chronic alcoholics: Identification of Wernicke's encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62:51–60. doi: 10.1136/jnnp.62.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan EV, Pfefferbaum A. Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol. 2009;44:155–65. doi: 10.1093/alcalc/agn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung YC, Chanraud S, Sullivan EV. Neuroimaging of Wernicke's encephalopathy and Korsakoff's syndrome. Neuropsychol Rev. 2012;22:170–80. doi: 10.1007/s11065-012-9203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pruckner N, Baumgartner J, Hinterbuchinger B, Glahn A, Vyssoki S, Vyssoki B. Thiamine substitution in alcohol use disorder: A narrative review of medical guidelines. Eur Addict Res. 2019;25:103–10. doi: 10.1159/000499039. [DOI] [PubMed] [Google Scholar]
- 14.Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff Syndrome in people who abuse alcohol? Cochrane Database Syst Rev. 2013;7:CD004033. doi: 10.1002/14651858.CD004033.pub3. doi: 10.1002/14651858.CD004033.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arts NJ, Walvoort SJ, Kessels RP. Korsakoff's syndrome: A critical review. Neuropsychiatr Dis Treat. 2017;13:2875–90. doi: 10.2147/NDT.S130078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laureno R. Nutritional cerebellar degeneration, with comments on its relationship to Wernicke disease and alcoholism. Handb Clin Neurol. 2012;103:175–87. doi: 10.1016/B978-0-444-51892-7.00010-3. [DOI] [PubMed] [Google Scholar]
- 17.Maschke M, Weber J, Bonnet U, Dimitrova A, Bohrenkämper J, Sturm S, et al. Vermal atrophy of alcoholics correlate with serum thiamine levels but not with dentate iron concentrations as estimated by MRI. J Neurol. 2005;252:704–11. doi: 10.1007/s00415-005-0722-2. [DOI] [PubMed] [Google Scholar]
- 18.Mulholland PJ, Self RL, Stepanyan TD, Little HJ, Littleton JM, Prendergast MA. Thiamine deficiency in the pathogenesis of chronic ethanol-associated cerebellar damage in vitro. Neuroscience. 2005;135:1129–39. doi: 10.1016/j.neuroscience.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 19.Del Brutto OH, Mera RM, Sullivan LJ, Zambrano M, King NR. Population-based study of alcoholic cerebellar degeneration: The Atahualpa Project. J Neurol Sci. 2016;367:356–60. doi: 10.1016/j.jns.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 20.Hammoud N, Jimenez-Shahed J. Chronic neurologic effects of alcohol. Clin Liver Dis. 2019;23:141–55. doi: 10.1016/j.cld.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Heo SH, Chang DI. Early-stage alcoholic cerebellar degeneration: Diagnostic imaging clues. J Korean Med Sci. 2015;30:1539. doi: 10.3346/jkms.2015.30.11.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips SC, Harper CG, Kril JJ. The contribution of Wernicke's encephalopathy to alcohol-related cerebellar damage. Drug Alcohol Rev. 1990;9:53–60. doi: 10.1080/09595239000185071. [DOI] [PubMed] [Google Scholar]
- 23.Baker KG, Harding AJ, Halliday GM, Kril JJ, Harper CG. Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke's encephalopathy. Neuroscience. 1999;91:429–38. doi: 10.1016/s0306-4522(98)90664-9. [DOI] [PubMed] [Google Scholar]
- 24.Graham JR, Woodhouse D, Read FH. Massive thiamine dosage in an alcoholic with cerebellar cortical degeneration. Lancet. 1971;2:107. doi: 10.1016/s0140-6736(71)92089-7. [DOI] [PubMed] [Google Scholar]
- 25.Julian T, Glascow N, Syeed R, Zis P. Alcohol-related peripheral neuropathy: A systematic review and meta-analysis. J Neurol. 2018;22:1–3. doi: 10.1007/s00415-018-9123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chopra K, Tiwari V. Alcoholic neuropathy: Possible mechanisms and future treatment possibilities. Br J Clin Pharmacol. 2012;73:348–62. doi: 10.1111/j.1365-2125.2011.04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woelk H, Lehrl S, Bitsch R, Köpcke W. Benfotiamine in treatment of alcoholic polyneuropathy: An 8-week randomized controlled study (BAP I Study) Alcohol Alcohol. 1998;33:631–8. doi: 10.1093/alcalc/33.6.631. [DOI] [PubMed] [Google Scholar]
- 28.Peters TJ, Kotowicz J, Nyka W, Kozubski W, Kuznetsov V, Vanderbist F, et al. Treatment of alcoholic polyneuropathy with vitamin B complex: A randomised controlled trial. Alcohol Alcohol. 2006;41:636–42. doi: 10.1093/alcalc/agl058. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes LM, Bezerra FR, Monteiro MC, Silva ML, de Oliveira FR, Lima RR, et al. Thiamine deficiency, oxidative metabolic pathways and ethanol-induced neurotoxicity: How poor nutrition contributes to the alcoholic syndrome, as Marchiafava-Bignami disease. Eur J Clin Nutr. 2017;71:580–6. doi: 10.1038/ejcn.2016.267. [DOI] [PubMed] [Google Scholar]
- 30.Hillbom M, Saloheimo P, Fujioka S, Wszolek ZK, Juvela S, Leone MA. Diagnosis and management of Marchiafava-Bignami disease: A review of CT/MRI confirmed cases. J Neurol Neurosurg Psychiatry. 2014;85:168–73. doi: 10.1136/jnnp-2013-305979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemlekar SS, Mehta RY, Dave KR, Shah ND. Marchiafava: Bignami disease treated with parenteral thiamine. Indian J Psychol Med. 2016;38:147–9. doi: 10.4103/0253-7176.178810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brin M. Erythrocyte transketolase in early thiamine deficiency. Ann N Y Acad Sci. 1962;98:528–41. doi: 10.1111/j.1749-6632.1962.tb30574.x. [DOI] [PubMed] [Google Scholar]
- 33.Dreyfus PM. Clinical application of blood transketolase determinations. N Engl J Med. 1962;267:596–8. doi: 10.1056/NEJM196209202671204. [DOI] [PubMed] [Google Scholar]
- 34.Edwards KA, Tu-Maung N, Cheng K, Wang B, Baeumner AJ, Kraft CE. Thiamine assays – Advances, challenges, and caveats. ChemistryOpen. 2017;6:178–91. doi: 10.1002/open.201600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandrakumar A, Bhardwaj A, 't Jong GW. Review of thiamine deficiency disorders: Wernicke encephalopathy and Korsakoff psychosis. J Basic Clin Physiol Pharmacol. 2018;30:153–62. doi: 10.1515/jbcpp-2018-0075. [DOI] [PubMed] [Google Scholar]
- 36.Torruellas C, French SW, Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:11684–99. doi: 10.3748/wjg.v20.i33.11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomson AD, Leevy CM. Observations on the mechanism of thiamine hydrochloride absorption in man. Clin Sci. 1972;43:153–63. doi: 10.1042/cs0430153. [DOI] [PubMed] [Google Scholar]
- 38.Hoyumpa AM, Jr, Strickland R, Sheehan JJ, Yarborough G, Nichols S. Dual system of intestinal thiamine transport in humans. J Lab Clin Med. 1982;99:701–8. [PubMed] [Google Scholar]
- 39.Smithline HA, Donnino M, Greenblatt DJ. Pharmacokinetics of high-dose oral thiamine hydrochloride in healthy subjects. BMC Clin Pharmacol. 2012;12:4. doi: 10.1186/1472-6904-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44:911–5. doi: 10.1111/imj.12522. [DOI] [PubMed] [Google Scholar]
- 41.Raj V, Ojha S, Howarth FC, Belur PD, Subramanya SB. Therapeutic potential of benfotiamine and its molecular targets. Eur Rev Med Pharmacol Sci. 2018;22:3261–73. doi: 10.26355/eurrev_201805_15089. [DOI] [PubMed] [Google Scholar]
- 42.Xie F, Cheng Z, Li S, Liu X, Guo X, Yu P, et al. Pharmacokinetic study of benfotiamine and the bioavailability assessment compared to thiamine hydrochloride. J Clin Pharmacol. 2014;54:688–95. doi: 10.1002/jcph.261. [DOI] [PubMed] [Google Scholar]
- 43.Cook CC, Hallwood PM, Thomson AD. B Vitamin deficiency and neuropsychiatric syndromes in alcohol misuse. Alcohol Alcohol. 1998;33:317–36. doi: 10.1093/oxfordjournals.alcalc.a008400. [DOI] [PubMed] [Google Scholar]
- 44.Lingford-Hughes AR, Welch S, Peters L, Nutt DJ British Association for Psychopharmacology. Expert Reviewers Group. BAP updated guidelines: Evidence-based guidelines for the pharmacological management of substance abuse, harmful use, addiction and comorbidity: Recommendations from BAP. J Psychopharmacol. 2012;26:899–952. doi: 10.1177/0269881112444324. [DOI] [PubMed] [Google Scholar]
- 45.Manzardo AM, He J, Poje A, Penick EC, Campbell J, Butler MG. Double-blind, randomized placebo-controlled clinical trial of benfotiamine for severe alcohol dependence. Drug Alcohol Depend. 2013;133:562–70. doi: 10.1016/j.drugalcdep.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manzardo AM, Pendleton T, Poje A, Penick EC, Butler MG. Change in psychiatric symptomatology after benfotiamine treatment in males is related to lifetime alcoholism severity. Drug Alcohol Depend. 2015;152:257–63. doi: 10.1016/j.drugalcdep.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dingwall KM, Delima JF, Gent D, Batey RG. Hypomagnesaemia and its potential impact on thiamine utilisation in patients with alcohol misuse at the Alice Springs Hospital. Drug Alcohol Rev. 2015;34:323–8. doi: 10.1111/dar.12237. [DOI] [PubMed] [Google Scholar]
- 48.Flink EB. Magnesium deficiency in alcoholism. Alcohol Clin Exp Res. 1986;10:590–4. doi: 10.1111/j.1530-0277.1986.tb05150.x. [DOI] [PubMed] [Google Scholar]
- 49.Grochowski C, Blicharska E, Baj J, Mierzwińska A, Brzozowska K, Forma A, et al. Serum iron, magnesium, copper, and manganese levels in alcoholism: A systematic review. Molecules. 2019;24:E1361. doi: 10.3390/molecules24071361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flannery AH, Adkins DA, Cook AM. Unpeeling the evidence for the banana bag: Evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016;44:1545–52. doi: 10.1097/CCM.0000000000001659. [DOI] [PubMed] [Google Scholar]
- 51.Lagunoff D, Martin TW, Read G. Agents that release histamine from mast cells. Annu Rev Pharmacol Toxicol. 1983;23:331–51. doi: 10.1146/annurev.pa.23.040183.001555. [DOI] [PubMed] [Google Scholar]


