Abstract
Carnitine transporter defect (CTD) is a potentially life-threatening disorder causing acute metabolic decompensation, cardiac arrhythmia, and cardiac and skeletal myopathies. CTD is included in many newborn screening (NBS) programs. The screening parameter free carnitine, however, is influenced by maternal conditions due to placental transfer. This study reviewed the NBS results for CTD as part of a pilot study in Bavaria, Germany, and the long-term follow-up of the identified patients treated in our center between January 1999 and June 2018. Among 1,816,000 Bavarian NBS samples, six newborns were diagnosed with CTD (incidence of 1:302,667; positive predictive value (PPV) of 1.63% from 2008 to 2018). In the 24 newborns presented to our center for confirmatory testing, we detected four newborns and six mothers with CTD, one newborn and three mothers in whom CTD was presumed but not genetically confirmed, and one mother with glutaric aciduria type I. In 11 newborns, no indication for an inborn error of metabolism was found. The newborns and mothers with CTD had no serious cardiac adverse events or relevant muscular symptoms at diagnosis and during treatment for up to 14 years. Three mothers were lost to follow-up. Revealing a lower incidence than expected, our data confirm that NBS for CTD most likely misses newborns with CTD. It rather produces high numbers of false-positives and a low PPV picking up asymptomatic mothers with a diagnosis of uncertain clinical significance. Our data add to the growing evidence that argues against an implementation of CTD in NBS programs.
Keywords: Carnitine transporter defect, Systemic primary carnitine deficiency, Newborn screening, Asymptomatic mothers
Highlights
-
•
Newborn screening (NBS) aims at early detection and treatment of relevant disorders.
-
•
High numbers of false-positives burden the healthy population and health care systems.
-
•
NBS for carnitine transporter defect produces high numbers of false-positives.
-
•
NBS for carnitine transporter defect picks up asymptomatic mothers.
-
•
Selective screening at clinical suspicion more suitable than population screening.
1. Introduction
Carnitine transporter defect (CTD, OMIM 212140), also referred to as systemic primary carnitine deficiency, is a rare autosomal-recessive disease caused by a dysfunction of the OCTN2 carnitine transporter in the plasma membrane [20]. The dysfunction leads to urinary carnitine loss, low serum carnitine concentrations, and decreased intracellular carnitine supply. As carnitine is involved in the transport of long chain fatty acids across the inner mitochondrial membrane, the energy production from beta-oxidation is impaired affecting heart, skeletal muscle and brain function during metabolic stress. Patients with CTD may suffer from acute metabolic decompensation with hypoketotic hypoglycemia and hepatic encephalopathy early in life or from cardiac arrhythmias, cardiac and skeletal myopathies later in life [16]. Treatment with l-carnitine can prevent metabolic and myopathic manifestations of the disease [17].
As CTD is a potentially life-threatening but readily treatable disorder, CTD has been included in several newborn screening (NBS) programs using low concentrations of free carnitine (C0) as screening parameter, detected by tandem mass spectrometry (MS/MS) from dried blood spots (DBS) [8,11,34,36]. However, identifying newborns with CTD by NBS remains challenging as C0 levels in newborns shortly after birth are strongly influenced by maternal C0 levels due to placental transfer [3,35]. As a consequence, newborns with CTD might be missed while mothers with CTD or secondary carnitine deficiencies are detected. Accordingly, previous NBS studies (a) identified lower numbers of newborns with CTD than expected, (b) were associated with high numbers of false-positives, and (c) detected mothers with CTD or inborn errors of metabolism such as glutaric aciduria type I, medium-chain acyl-CoA dehydrogenase deficiency and cobalamin C deficiency rather than newborns with CTD [8,11,34]. The women with CTD identified by their offspring's NBS had mainly no or only minor symptoms and showed that this disorder includes a large group of adults with unremarkable clinical phenotype [4,12,26,32]. Data on the natural course of the disease in these individuals are scarce and the benefit of treatment in asymptomatic patients remains a matter of debate [4,17,34]. The low sensitivity, high false-positive rate and the identification of many asymptomatic adult cases recently led to the discontinuation of CTD screening in New Zealand [34].
Since the introduction of MS/MS based NBS in 1999, the detection of CTD has been part of a pilot project in Bavaria. We report the results of this NBS pilot study and the clinical long-term follow-up of the identified patients with CTD treated in our center during a study period of 19 ½ years.
2. Patients and methods
2.1. Patients
Data on newborns with positive NBS results regarding reduced concentrations of C0 between 1st of January 1999 and 30th of June 2018 were collected. For newborns with confirmed CTD treated at our center, data on treatment and clinical follow-up were assessed. In addition, medical history, clinical follow-up, biochemical, and genetic data of mothers with CTD identified by positive NBS of their offspring, were compiled.
2.2. Newborn screening
NBS samples were to be taken between 36 and 72 h of life on filter paper. The concentration of C0 was determined using MS/MS as previously described [6,18]. An abnormal result was defined as C0 < 9.0 μmol/l. Newborns with concentrations below this cut-off were recalled for the analysis of C0 in a second DBS. If the C0 concentration persisted below 9.0 μmol/l, confirmatory testing was initiated.
2.3. Confirmatory testing
For confirmatory testing, the concentration of C0 and acylcarnitines in serum and the renal reabsorption of C0 were analyzed in both the newborn and its mother before the initiation of treatment with l-carnitine. If the renal reabsorption of C0 was abnormal, a sequence analysis of the SLC22A5 gene was initiated. If no or only one mutation was detected by sequence analysis, a skin biopsy for the assessment of carnitine transport in cultured fibroblasts was recommended [31]. Patients were diagnosed with CTD based on two pathogenic mutations in the SLC22A5 gene or a markedly reduced carnitine uptake in cultured fibroblasts.
2.4. Clinical management
Newborns and adults diagnosed with CTD were treated with l-carnitine and received an emergency plan. The dosage of l-carnitine was adjusted according to the patient's weight and serum C0 concentration. Infants with CTD were seen in the outpatient clinic on a regular basis and measurements of C0, creatine kinase, and liver transaminases in serum were performed every 6 to 12 months with a re-analysis of serum C0 concentrations after dose adjustments. Electrocardiograms (ECG) and echocardiograms were initiated annually.
Adults with CTD were followed either in our metabolic center or in the adult service. Outpatient visits including blood analysis and cardiac assessment were recommended on an annual basis.
2.5. Statistical analysis
Statistical analyses were not performed due to the small sample sizes. The figure was created using the IBM SPSS Statistics program (version 26).
2.6. Statement of ethics
The study was conducted in accordance with the World Medical Association Declaration of Helsinki. The local ethics committee approved the pilot study within the Bavarian newborn screening program (No 09074). Informed consent for the participation of their newborn was obtained by the parents. For the review of medical records informed consent was waived due to retrospective and anonymized data assessment (No 18–646).
3. Results
3.1. Incidence, recall rate, and positive predictive value
Between 1st of January 1999 and 30th of June 2018, 1,816,000 samples from Bavarian newborns were analyzed. Among them, six newborns were diagnosed with CTD indicating an incidence of 1:302,667 in newborns.
The numbers of recalled newborns have not been registered until 1st of January 2008. Among 890,000 samples screened after this date, 184 NBS samples showed a reduced concentration of C0 and a second DBS was requested (recall rate 0.02%). In 30 (0.003%) of these newborns, the concentration of C0 persisted below the cut-off and confirmatory testing was initiated. Three of these newborns were confirmed to suffer from CTD resulting in a positive predictive value (PPV) of 1.63%.
Of note, two of the six newborns with CTD were diagnosed and treated in another clinic, and thus were taken into account only when calculating the incidence. No information on confirmatory testing or clinical follow-up of these patients were available to us.
No symptomatically diagnosed patient with CTD missed by our NBS pilot study (false-negative) came to our attention.
3.2. Patient cohort
24 newborns were presented to our metabolic center for confirmatory testing. Four newborns (N1 – N4) and six mothers (M1, M3 – M7) were diagnosed with CTD. In one consanguineous family, both the newborn (N4) and the mother (M1) were diagnosed with CTD. In two mothers (M8 and M9), the biochemical parameters and mild muscular symptoms were consistent with CTD, but diagnosis could not be confirmed by genetic testing, and skin biopsy was denied.
One mother showed secondary carnitine deficiency due to glutaric aciduria type I. The diagnosis was confirmed by the characteristic profile of acylcarnitines in serum, the excretion of 3-hydroxy-glutaric acid in urine, and the detection of two mutations in the GCDH gene (c.1156C > T/p.R386X, c.1262C > T/p.A421V).
One newborn (N5) and its mother (M12) had mildly reduced carnitine uptake in fibroblasts, but genetic analysis remained inconclusive. In the remaining 11 cases, no inborn error of metabolism was found in the newborn or the mother.
In addition, one newborn born in another federal state of Germany was presented to our metabolic center with a reduced concentration of C0 in NBS but inconclusive work-up. The newborn was healthy, but its mother (M2) and the sister of the mother (M10) were diagnosed with CTD. Furthermore, a sister of the mother M3 (M11) was diagnosed with CTD by family screening. All three women were included in the clinical data description.
3.3. Biochemical and genetic findings
Results of NBS and confirmatory testing of the four newborns with CTD (N1-N4) are listed in Table 1. In N2-N4, a reduced concentration of C0 was confirmed in serum and the renal reabsorption was decreased. In N1, treatment with l-carnitine was started by the local pediatrician before the patient was referred for confirmatory testing. Thus, initial C0 in serum and renal reabsorption could not be assessed. In all newborns, the diagnosis was confirmed by two mutations in the SLC22A5 gene or a markedly reduced carnitine uptake in cultured fibroblasts. The nonsense mutation c.351G > A (p.W117X) (N2) has not been described in the literature so far.
Table 1.
Clinical and biochemical data of newborns with CTD at diagnosis and long-term follow-up.
| Subject number |
Newborn screening (dried blood spots) and confirmatory testing |
Clinical data at diagnosis |
Long-term follow-up |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C0 in first DBSa (μmol/l) |
C0 in second DBS (μmol/l) |
C0 in serum (μmol/l) |
RRa of C0 (%) |
Carnitine uptake in fibroblasts (pmol/min x mg) |
Genotype SLC22A5 cDNA (protein) |
Gender |
CTD-related symptoms (metabolic decompensation, muscular/cardiac symptoms) |
current age (years) |
Mean l-carnitine treatment (mg/kg/d) |
Mean C0 in serum during treatment (μmol/l) |
Hospital admissions and/or CTD-related symptoms |
|
| Ref.a | [>9] | [>9] | [8.9-93.3] | [>90] | [1.06 ± 0.30] | [8.9–93.3] | ||||||
| Newborns with CTD | ||||||||||||
| N1 | 6 | 6 | NAa | NA | 0.09 | c.761G > A/− (p.R254Q/−) | Male | None | 18 | 65 | 28 | ECG: RBBBa, Sokolow-Lyon criterion for LVHa (both transient) |
| N2 | 6 | 2.5 | 4.9 | 61 | 0 | c.351G > A/deletion exon 2 (p.W117X/deletion exon 2) | Female | None | 15 | 75 | 15 | None |
| N3 | 5 | 1 | 0.8 | 0 | 0 | c.458_459delTG/c.1403C > G (p.V153Afs*41/p.T468R) | Male | None | 14 | 150 | 14 | 14 prophylactic hospital admissions |
| N4 | 4 | 4 | 7.6 | 95b | NA | c.641C > T/c.641C > T (p.A214V/p.A214V) | Male | None | 4 | 85 | 54 | None |
| Newborn with mild functional CTD | ||||||||||||
| N5 | 3.5 | 4.5 | 17.5 | 91 | 0.26 | – | Female | None | 18 | 50 | 25 | None |
Ref., reference; DBS, dried blood spot; RR, renal reabsorption; NA, not available; ECG, electrocardiogram; RBBB, right bundle branch block; LVH, left ventricular hypertrophy.
FTR was assessed in a different laboratory with a reference value of >98%. Novel mutations in bold and italic.
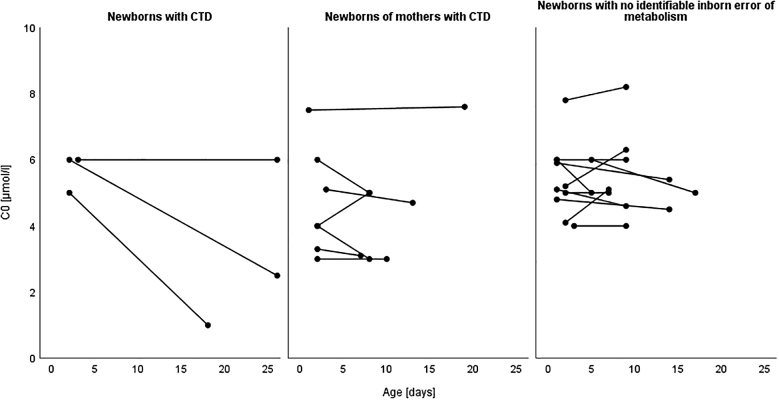
The mean concentration of C0 decreased from the first (5.7 ± 0.6 μmol/l, range 5–6) to the second DBS (3.2 ± 2.6 μmol/l, range 1–6) due to a decrease in N2 and N3, while the concentration of C0 stayed unchanged in N1 (Fig. 1). N4 was not included because its mother (M1) was also affected with CTD.
Fig. 1.
Concentrations of free carnitine (C0) in first and second dried blood spots (DBS). The progression of the C0 concentrations from the first to the second DBS is illustrated for newborns with carnitine transporter defect (CTD), newborns of mothers with CTD and newborns with no identifiable inborn error of metabolism. The newborn N4 was not included because both N4 and his mother M1 were affected by CTD. The newborn of M2 was not included as the newborn screening (NBS) was performed in a different screening laboratory.
As reduced concentrations of C0 in NBS can be a consequence of maternal conditions, the mothers of the newborns were also tested. In seven mothers (M1 – M7) the diagnosis of CTD was established by two mutations in the SLC22A5 gene or markedly reduced carnitine uptake in cultured fibroblasts (Table 2). Two additional women were diagnosed with CTD by family screening (M10 and M11). In two mothers (M8 and M9) only one mutation in the SLC22A5 gene was found despite reduced concentration of C0 in serum and decreased renal reabsorption. A skin biopsy to determine the carnitine uptake in fibroblasts was recommended, but denied by both women.
Table 2.
Medical history, biochemical data and long-term follow-up of mothers and women identified by family screening with CTD.
| Subject number |
Medical history |
Laboratory work-up |
Long-term follow-up |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C0 in newborn's first DBSa (μmol/l) |
C0 in newborn's second DBS (μmol/l) |
Age at diagnosis (years) |
CTD-related history (metabolic decompensations, muscular/cardiac symptoms) |
C0 in serum (μmol/l) |
RRa of C0 (%) |
Carnitine uptake in fibroblasts (pmol/min x mg) |
Genotype SLC22A5 cDNA (protein) |
Duration of follow-up (years) |
Mean l-carnitine treatment (mg/kg/d) |
Mean C0 in serum during treatment (μmol/l) |
|
| Ref.a | [>9] | [>9] | [8.9–93.3] | [>90] | [1.06 ± 0.30] | [8.9–93.3] | |||||
| Mothers with CTD | |||||||||||
| M1b | 4 | 4 | 28 | None | 6 | 93c | NAa | c.641C > T/c.641C > T (p.A214V/p.A214V) | 4 | – | – |
| M2 | NA | NA | 31 | None | 2.1 | 60 | 0.02 | c.136C > T/c.769-786del18 (p.P46S/p.Δ257–262) | 14 | 80 | 11 |
| M3 | 4 | 3 | 36 | None | 3.1 | 82.1 | NA | c.136C > T/c.1531G > C (p.P46S/p.E511Q) | 4 | 70 | 13 |
| M4 | 3 | 3 | 34 | None | 2.3 | 87.8 | NA | c.136C > T/c.98G > T (p.P46S/p.G33V) | 10 | 80 | 18 |
| M5 | 5.1 | 4.7 | 32 | Noned | 5.2 | 87.5 | NA | c.136C > T/c.1195C > T (p.P46S/p.R399W) | Lost to follow-up | – | – |
| M6 | 3.3 | 3.1 | 23 | Noned | <2.0 | 99.5e | NA | c.136C > T/c.506G > A (p.P46S/p.R169Q) | Lost to follow-up | – | – |
| M7 | 4 | 5 | 25 | Noned | 8 | NA | 0 | c.394-16 T > A/− (IVS1-16 T > A/−) | 1 | 30 | 14 |
| M8 | 6 | 5 | 38 | Muscle pain | 4.6 | 87.3 | NA | c.1385G > T/− (p.G462V/−) | 8 | 40 | 20 |
| M9 | 7.5 | 7.6 | 29 | Muscle pain, cramps | 5.5 | 90.1 | NA | c.136C > T/− (p.P46S/−) | Lost to follow-up | – | – |
| Family screening | |||||||||||
| M10b | 32 | None | 0.5 | NA | NA | c.136C > T/c.769-786del18 (p.P46S/p.del257–262) | 13 | 60 | 12 | ||
| M11b | 37 | None | 2.5 | 60.4 | NA | c.136C > T/c.1531G > C (p.P46S/p.E511Q) | 6 | 80 | 11 | ||
| Mother with mild functional CTD | |||||||||||
| M12 | 3.5 | 4.5 | 30 | Supraventricular extrasystolesf | 10.5 | 74 | 0.34 | – | 18 | 45 | 30 |
Novel mutations in bold and italic.
Ref., reference; DBS, dried blood spot; RR, renal reabsorption; NA, not available.
M1 is the mother of N4, both diagnosed with CTD. M10 and M11 are the sisters of M2 and M3, respectively.
RR was assessed in a different laboratory with a reference value of >98%.
Electrocardiogram and echocardiography were not performed.
Result not informative due to very low carnitine levels.
The 1st child died of sudden infant death syndrome with 15 months of life.
The genotypes of mothers with CTD are listed in Table 2. All women showed at least one missense mutation or a mutation previously described in an asymptomatic mother with the most common being c.136C > T (p.P46S) identified in eight of 11 women. Three novel mutations were identified, two missense mutations (c.98G > T/p.G33V and c.1531G > C/p.E511Q) and an in-frame deletion of 18 base pairs (c.769-786del18/p.Δ257–262).
There was no difference in the mean concentrations of C0 in newborns of maternal CTD between the first (4.7 ± 1.6 μmol/l, range 3–7.5) and the second DBS (4.5 ± 1.7 μmol/l, range 3–7.6) (Fig. 1).
One newborn (N5) and its mother (M12) showed C0 concentrations in serum in the lower normal range (17.5 and 10.5 μmol/l, respectively; reference value 8.9–93.3 μmol/l), a mildly decreased renal reabsorption (91% and 74%, respectively; reference value >90%) and mildly reduced carnitine uptake in fibroblasts (25% and 36% of control, respectively). Sequence analysis of the SLC22A5 gene revealed no mutation. Both individuals are indicated as “mild functional CTD” (Table 1 and Table 2).
In 11 newborns, no indication for CTD or another inborn error of metabolism was found in the newborn or the mother. Confirmatory testing showed C0 concentrations in serum within the normal range in all newborns and mothers. In eight of these cases, renal reabsorption of C0 was available and gave unremarkable results. No further follow-up was required.
The concentrations of C0 of these newborns in the first DBS (5.4 ± 1.1 μmol/l, range 4–7.8) were reduced to values comparable to the newborns with CTD (5.7 ± 0.6 μmol/l, range 5–6) and newborns of mothers with CTD (4.7 ± 1.6 μmol/l, range 3–7.5). Furthermore, the mean concentration of C0 in the second DBS (5.4 ± 1.1 μmol/l, range 4–8.2) stayed unchanged (Fig. 1).
3.4. Treatment, clinical findings, and long-term follow-up
All newborns with CTD were asymptomatic at the time of diagnosis. They showed no evidence of metabolic decompensation and no ECG or echocardiogram abnormalities. Serum concentrations of C0 were determined on a regular basis and l-carnitine supplementation was required at a daily dose between 65 and 150 mg/kg, divided into 2 to 4 doses. N3 required the highest daily dose and a frequency of at least 4 doses per day in order to maintain serum C0 concentrations in the low normal range (>10 μmol/l). He suffered considerably from fishy body odor and was admitted to hospital on 14 occasions due to vomiting or reluctance to eat during viral infections. In the remaining children, no prophylactic admission to hospital was necessary during intercurrent illnesses. No metabolic decompensations occurred, no muscular symptoms were reported. Routine ECG and echocardiograms were unremarkable in all children except for N1, who showed a right bundle branch block and signs of ventricular hypertrophy (Sokolow-Lyon criterion) in the ECG in childhood, both normalizing during puberty. All patients showed normal neurocognitive development, motor skills, and endurance at the age of 4, 14, 15, and 18 years, respectively. Clinical information on the patients is listed in Table 1.
All mothers and adult family members with CTD were asymptomatic at the time of diagnosis with no history of metabolic decompensations or cardiac abnormalities. No muscular symptoms were reported with the exception of M8 and M9, who described muscle pain and leg cramps, respectively (Table 2). ECG and echocardiogram showed no signs of arrhythmia or cardiac hypertrophy when available. Eight women with CTD were followed up at our outpatient clinic for a duration between 1 and 14 years (Table 2). They required l-carnitine supplementation between 30 and 80 mg/kg per day in 1 to 3 doses adjusted to the individual's serum C0 level. Three women (M2, M10, M11) reported side effects of l-carnitine treatment i.e. unpleasant body odor and gastrointestinal symptoms. No metabolic decompensations occurred. Follow-up ECGs and echocardiograms revealed no cardiac abnormalities. M8 reported subjective improvement of muscular symptoms with l-carnitine treatment.
Three women were not followed-up in our outpatient clinic. To our knowledge, they decided against a supplementation with l-carnitine but remained asymptomatic.
In N5 and its mother M12, supplementation with l-carnitine was recommended as serum C0 concentrations decreased significantly below the normal range, whenever treatment was withdrawn. In addition, family history revealed that the first child of the family died from sudden infant death syndrome at the age of 15 months. Medical history of N5 was uneventful. M12 suffered from supraventricular extrasystoles and required a catheter ablation. The unclear diagnosis and the side-effects of l-carnitine treatment were reported to be very burdensome.
4. Discussion
The primary aim of NBS is the early detection and treatment of clinically important disorders in order to minimize morbidity and mortality in early childhood [33]. CTD is a potentially life-threatening but readily treatable disorder and low levels of C0 are detectable by the widely used screening technique MS/MS. Hence, CTD appeared to be a reasonable candidate disorder for the detection by NBS and was included in many NBS programs, among them our pilot study within the Bavarian NBS program [8,11,14,24,34,36]. Previous results of NBS programs for CTD, however, have challenged the assumption of CTD being a suitable candidate. For instance, high false-positive rates and low PPV between 1.9 and 4.7% have been described [8,11,13,34]. This finding is confirmed by a PPV of 1.63% found in our cohort between 2008 and 2018. During that time, only three newborns with CTD were confirmed out of 184 initial NBS specimens flagged positive. Within the entire study period of 19 ½ years, six newborns with CTD were identified suggesting an incidence of 1:302,667. This corresponds to the previously estimated incidence of 1:382,247 using NBS data from Bavaria and Baden-Wuerttemberg [9] and low incidences were also reported from NBS programs in USA (1:142,236), Australia (1:120,000), and New Zealand (1:290,000) [30,33,34]. However, data suggest that these incidences might be too low indicating that a considerable number of individuals with CTD are missed by NBS [8,29,34]. Given the frequency of pathogenic SLC22A5 gene variants in the normal population, the predicted incidence would be 1:59,465 [7], and may be even higher considering the recently described frequent variant within the 5′ untranslated region (UTR) of the gene [5]. Of note, in Japan, an incidence as high as 1:40,000 was reported by population screening in adults as opposed to 1:199,000 by NBS [10,27].
Both low specificity and low sensitivity can be explained by the strong influence of maternal concentrations of C0 on the newborn concentrations of C0. Carnitine is transferred via the placenta to the fetus during pregnancy and the concentrations of C0 during the neonatal period reflect those of the mother rather than the newborn. As a consequence, unaffected newborns of mothers with CTD can have low concentrations of C0 shortly after birth and affected newborns of healthy, heterozygous mothers may still display sufficient concentrations of C0 to escape detection by NBS [8,29]. In addition, maternal concentrations of C0 decrease physiologically during pregnancy and thus add to the number of false-positives [15,35]. Accordingly, NBS programs detect considerable numbers of mothers with CTD or other conditions leading to secondary carnitine deficiency [8,11,13,34,36]. In our cohort, we identified nine mothers with CTD and one mother with glutaric aciduria type 1, as opposed to only four newborns with CTD. In line with previous reports [4,12,13,26,32,36], the nine mothers and two adult family members diagnosed with CTD in our cohort were asymptomatic at the time of diagnosis with the exception of M8 and M9 who reported minor muscular symptoms. No woman carried null mutations on both SLC22A5 alleles. All women showed at least one missense mutation or a mutation previously described in an asymptomatic mother identified by NBS of the child. The frequent mutation c.136C > T (p.P46S) was found in eight women (36% of alleles). This mutation has mainly been encountered in asymptomatic women so far [1,12,26] and retains a considerable residual activity [1,5,25]. It has therefore been suggested that this and other similar missense mutations might be protective against early clinical manifestations [25]. In four individuals a definitive diagnosis could not be established. In M8 and M9, only one mutation in the SLC22A5 gene could be identified. Both women reported mild muscular symptoms that improved with l-carnitine supplementation. In N5 and M12, mild impairment of carnitine uptake in fibroblasts was detected, but no underlying genetic variation could be identified. This finding is in line with reports describing mutation analysis in CTD to be inconclusive in about 16% of alleles [7]. Of note, the recently described prevalent 5’UTR variant (c.-149G > A) was not analyzed in our cohort [5]. Genetic testing in our patients was performed before this variant came to attention and comprised Sanger sequencing of the coding exons and flanking regions, a technique that is unlikely to cover the relevant gene region [5]. One might speculate that this variant may well be the underlying cause, particularly in N5 and M12, and re-testing needs to be considered.
The clinical benefit of treating asymptomatic women identified by low concentrations of C0 in their children's NBS remains a matter of debate [4,17,34]. The women identified and treated at our center experienced no serious cardiac adverse events or relevant muscular symptoms even when compliance with l-carnitine supplementation was low or absent. Some dropped out of clinical follow-up seeing no further need. On the other hand, several women experienced a high psychological burden due to the unclear clinical risk of their condition, especially when the diagnosis remained inconclusive. Furthermore, some women were affected by the side-effects of l-carnitine supplementation such as unpleasant body odor and gastrointestinal symptoms. In general, NBS aims at the early detection of relevant disease in infants in order to prevent serious adverse events. Occasionally, the target disease was detected in the mother rather than the newborn [19]. In NBS for CTD, however, maternal CTD has been shown to be detected in high numbers. This could be regarded as an additional benefit when clinically relevant phenotypes are identified. Picking up considerable numbers of mothers carrying missense mutations associated with significant residual OCTN2 activity and thus largely benign conditions, might not be justified considering the potentially unnecessary interventions and therapies, that cause psychological, physical, and social distress as well as financial burdens to the health care systems [2].
The newborns diagnosed with CTD displayed one null mutation (N3), two null mutations (N2), or a distinctively decreased carnitine transporter activity (N1) consistent with findings reported from symptomatic patients [25]. N4, born to consanguineous parents, showed the mutation c.641C > T (p.A214V) in a homozygous state. This mutation has been identified in asymptomatic mothers showing significantly higher residual activities as compared to symptomatic patients [25]. This patient might have escaped detection by NBS, had not his mother (N1) also been affected and thus had revealed low concentrations of C0 to be detected [29]. Of note, the missense mutation c.136C > T (p.P46S) was not identified in a newborn with CTD, supporting the hypothesis that mild CTD might be missed by NBS [29]. The children followed at our center showed a normal development without hypoglycemia or metabolic decompensation during intercurrent infections in childhood or relevant cardiac abnormalities. Within 19 ½ years, no clinically ascertained patient, missed by NBS, came to our attention. This observation further highlights the question whether mild CTD phenotypes within the Bavarian population are clinically relevant.
In order to improve the performance of NBS for CTD, blood sampling at a later age has been suggested [8,15,22,29]. In line with these considerations, we found a trend to lower C0 concentrations in the group of newborn CTD in the second DBS. However, these suggestions contradict the requirements of a possibly early screening in order to identify disorders with a short pre-symptomatic window such as methylmalonic aciduria in due time [28]. On the Faroe Islands, the collection of a second, post‑neonatal screening sample for CTD beyond the age of two months has been introduced [29]. Re-screening all newborns seems justified in a population with an incidence of CTD as high as 1:300 and a prevalent founder mutation (c.95A > G; p.N32S) identified in 86% of cases, which has been shown to be strongly associated with a severe phenotype and an increased risk of sudden cardiac death in homozygous individuals [21,23]. It might not be feasible in Germany/Bavaria given the low incidence and a mutational spectrum including numerous variants with an unknown clinical relevance in many detected individuals. A second tier strategy applying genetic testing could serve as an alternative.
5. Conclusions
Our data confirm that NBS for CTD produces high numbers of false-positives and shows a low PPV, while it most likely misses newborns with CTD. It rather picks up asymptomatic mothers confronting them with an unsettling diagnosis of uncertain clinical significance. Our data add to the growing evidence that argues against an implementation of CTD in NBS programs applying the current screening strategy relying on the determination of C0 within the first days of life. In the context of the high frequency of benign phenotypes, a targeted diagnostic work-up in patients with suggestive clinical features of CTD, such as hypoketotic hypoglycemia, infantile-onset cardiomyopathy and cardiac arrhythmias, might be preferable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Katharina A. Schiergens: Conceptualization, Methodology, Investigation, Writing - original draft. Katharina J. Weiss: Conceptualization, Methodology, Investigation, Writing - original draft. Wulf Röschinger: Investigation, Writing - review & editing. Amelie S. Lotz-Havla: Investigation. Joachim Schmitt: Investigation. Robert Dalla Pozza: Investigation. Sarah Ulrich: Investigation. Birgit Odenwald: Investigation. Joachim Kreuder: Investigation. Esther M. Maier: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing, Supervision.
Acknowledgment
We are grateful to our patients and their families. We appreciate the efforts of all colleagues who collected data for this study. This article is part of a thesis by Joachim Schmitt to fulfill the requirements for a medical degree at the Ludwig-Maximilians-University of Munich.
References
- 1.Amat di San Filippo C., Ardon O., Longo N. Glycosylation of the OCTN2 carnitine transporter: study of natural mutations identified in patients with primary carnitine deficiency. Biochim. Biophys. Acta. 2011;1812:312–320. doi: 10.1016/j.bbadis.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andermann A., Blancquaert I., Beauchamp S., Costea I. Guiding policy decisions for genetic screening: developing a systematic and transparent approach. Public Health Genomics. 2011;14:9–16. doi: 10.1159/000272898. [DOI] [PubMed] [Google Scholar]
- 3.Christodoulou J., Teo S.H., Hammond J., Sim K.G., Hsu B.Y., Stanley C.A., Watson B., Lau K.C., Wilcken B. First prenatal diagnosis of the carnitine transporter defect. Am. J. Med. Genet. 1996;66:21–24. doi: 10.1002/(SICI)1096-8628(19961202)66:1<21::AID-AJMG5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 4.El-Hattab A.W., Li F.Y., Shen J., Powell B.R., Bawle E.V., Adams D.J., Wahl E., Kobori J.A., Graham B. Maternal systemic primary carnitine deficiency uncovered by newborn screening: clinical, biochemical, and molecular aspects. Genet Med. 2010;12:19–24. doi: 10.1097/GIM.0b013e3181c5e6f7. [DOI] [PubMed] [Google Scholar]
- 5.Ferdinandusse S., Te Brinke H., Ruiter J.P.N., Haasjes J., Oostheim W., van Lenthe H., Ebberink M.S., Wanders R.J.A. A mutation creating an upstream translation initiation codon in SLC22A5 5’UTR is a frequent cause of primary carnitine deficiency. Hum. Mutat. 2019;40:1899–1904. doi: 10.1002/humu.23839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fingerhut R., Roschinger W., Muntau A.C., Dame T., Kreischer J., Arnecke R., Superti-Furga A., Troxler H., Liebl B. Hepatic carnitine palmitoyltransferase I deficiency: acylcarnitine profiles in blood spots are highly specific. Clin. Chem. 2001;47:1763–1768. [PubMed] [Google Scholar]
- 7.Frigeni M., Balakrishnan B., Yin X., Calderon F.R.O., Mao R., Pasquali M., Longo N. Functional and molecular studies in primary carnitine deficiency. Hum. Mutat. 2017;38:1684–1699. doi: 10.1002/humu.23315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallant N.M., Leydiker K., Wilnai Y., Lee C., Lorey F., Feuchtbaum L., Tang H., Carter J., Enns G.M. Biochemical characteristics of newborns with carnitine transporter defect identified by newborn screening in California. Mol. Genet. Metab. 2017;122:76–84. doi: 10.1016/j.ymgme.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann G.F., von Kries R., Klose D., Lindner M., Schulze A., Muntau A.C., Roschinger W., Liebl B., Mayatepek E. Frequencies of inherited organic acidurias and disorders of mitochondrial fatty acid transport and oxidation in Germany. Eur. J. Pediatr. 2004;163:76–80. doi: 10.1007/s00431-003-1246-3. [DOI] [PubMed] [Google Scholar]
- 10.Koizumi A., Nozaki J., Ohura T., Kayo T., Wada Y., Nezu J., Ohashi R., Tamai I., Shoji Y. Genetic epidemiology of the carnitine transporter OCTN2 gene in a Japanese population and phenotypic characterization in Japanese pedigrees with primary systemic carnitine deficiency. Hum. Mol. Genet. 1999;8:2247–2254. doi: 10.1093/hmg/8.12.2247. [DOI] [PubMed] [Google Scholar]
- 11.Lee N.C., Tang N.L., Chien Y.H., Chen C.A., Lin S.J., Chiu P.C., Huang A.C., Hwu W.L. Diagnoses of newborns and mothers with carnitine uptake defects through newborn screening. Mol. Genet. Metab. 2010;100:46–50. doi: 10.1016/j.ymgme.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Li F.Y., El-Hattab A.W., Bawle E.V., Boles R.G., Schmitt E.S., Scaglia F., Wong L.J. Molecular spectrum of SLC22A5 (OCTN2) gene mutations detected in 143 subjects evaluated for systemic carnitine deficiency. Hum. Mutat. 2010;31:E1632–E1651. doi: 10.1002/humu.21311. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y., Xu H., Zhou D., Hu Z., Zhang C., Hu L., Zhang Y., Zhu L., Lu B. Screening 3.4 million newborns for primary carnitine deficiency in Zhejiang Province, China. Clin. Chim. Acta. 2020;507:199–204. doi: 10.1016/j.cca.2020.04.039. [DOI] [PubMed] [Google Scholar]
- 14.Loeber J.G., Platis D., Zetterström R.H., Almashanu S., Boemer F., Bonham J.R., Borde P., Brincat I., Cheillan D. Neonatal screening in Europe revisited: an ISNS perspective on the current state and developments since 2010. International Journal of Neonatal Screening. 2021;7:15. doi: 10.3390/ijns7010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longo N., Amat di San Filippo C., Pasquali M. Disorders of carnitine transport and the carnitine cycle. Am J Med Genet C Semin Med Genet. 2006;142C:77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longo N., Frigeni M., Pasquali M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta. 2016;1863:2422–2435. doi: 10.1016/j.bbamcr.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magoulas P.L., El-Hattab A.W. Systemic primary carnitine deficiency: an overview of clinical manifestations, diagnosis, and management. Orphanet J Rare Dis. 2012;7:68. doi: 10.1186/1750-1172-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maier E.M., Liebl B., Roschinger W., Nennstiel-Ratzel U., Fingerhut R., Olgemoller B., Busch U., Krone N., Kries R. Population spectrum of ACADM genotypes correlated to biochemical phenotypes in newborn screening for medium-chain acyl-CoA dehydrogenase deficiency. Hum. Mutat. 2005;25:443–452. doi: 10.1002/humu.20163. [DOI] [PubMed] [Google Scholar]
- 19.McGoey R.R., Marble M. Positive newborn screen in a normal infant of a mother with asymptomatic very long-chain acyl-CoA dehydrogenase deficiency. J. Pediatr. 2011;158:1031–1032. doi: 10.1016/j.jpeds.2011.01.063. [DOI] [PubMed] [Google Scholar]
- 20.Nezu J., Tamai I., Oku A., Ohashi R., Yabuuchi H., Hashimoto N., Nikaido H., Sai Y., Koizumi A. Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter. Nat. Genet. 1999;21:91–94. doi: 10.1038/5030. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen J., Duno M., Lund A.M., Steuerwald U., Hansen S.H., Joensen H.D., Kober L., Nielsen O.W. Increased risk of sudden death in untreated primary carnitine deficiency. J. Inherit. Metab. Dis. 2020;43:290–296. doi: 10.1002/jimd.12158. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen J., Hougaard D.M., Sandhu N., Fjaellegaard K., Petersen P.R., Steuerwald U., Lund A.M. Primary carnitine deficiency: is Foetal development affected and can newborn screening be improved? JIMD Rep. 2017;36:35–40. doi: 10.1007/8904_2016_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen J., Lund A.M., Risom L., Wibrand F., Gislason H., Nielsen O.W., Kober L., Duno M. Residual OCTN2 transporter activity, carnitine levels and symptoms correlate in patients with primary carnitine deficiency. Mol Genet Metab Rep. 2014;1:241–248. doi: 10.1016/j.ymgmr.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Röschinger W., Sonnenschein S., Schuhmann E., Nennstiel-Ratzel U., Roscher A., Olgemöller B. Neue Zielerkrankungen im Neugeborenenscreening. Monatsschrift Kinderheilkunde. 2015;163:142–149. [Google Scholar]
- 25.Rose E.C., di San Filippo C.A., Ndukwe Erlingsson U.C., Ardon O., Pasquali M., Longo N. Genotype-phenotype correlation in primary carnitine deficiency. Hum. Mutat. 2012;33:118–123. doi: 10.1002/humu.21607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schimmenti L.A., Crombez E.A., Schwahn B.C., Heese B.A., Wood T.C., Schroer R.J., Bentler K., Cederbaum S., Sarafoglou K. Expanded newborn screening identifies maternal primary carnitine deficiency. Mol. Genet. Metab. 2007;90:441–445. doi: 10.1016/j.ymgme.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Shibata N., Hasegawa Y., Yamada K., Kobayashi H., Purevsuren J., Yang Y., Dung V.C., Khanh N.N., Verma I.C. Diversity in the incidence and spectrum of organic acidemias, fatty acid oxidation disorders, and amino acid disorders in Asian countries: selective screening vs. expanded newborn screening. Mol Genet Metab Rep. 2018;16:5–10. doi: 10.1016/j.ymgmr.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sontag M.K., Miller J.I., McKasson S., Sheller R., Edelman S., Yusuf C., Singh S., Sarkar D., Bocchini J. Newborn screening timeliness quality improvement initiative: impact of national recommendations and data repository. PLoS One. 2020;15 doi: 10.1371/journal.pone.0231050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steuerwald U., Lund A.M., Rasmussen J., Janzen N., Hougaard D.M., Longo N. Neonatal screening for primary carnitine deficiency: lessons learned from the Faroe Islands. Int J Neonatal Screen. 2017;3:1–10. [Google Scholar]
- 30.Therrell B.L., Jr., Lloyd-Puryear M.A., Camp K.M., Mann M.Y. Inborn errors of metabolism identified via newborn screening: ten-year incidence data and costs of nutritional interventions for research agenda planning. Mol. Genet. Metab. 2014;113:14–26. doi: 10.1016/j.ymgme.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaz F.M., Scholte H.R., Ruiter J., Hussaarts-Odijk L.M., Pereira R.R., Schweitzer S., de Klerk J.B., Waterham H.R., Wanders R.J. Identification of two novel mutations in OCTN2 of three patients with systemic carnitine deficiency. Hum. Genet. 1999;105:157–161. doi: 10.1007/s004399900105. [DOI] [PubMed] [Google Scholar]
- 32.Vijay S., Patterson A., Olpin S., Henderson M.J., Clark S., Day C., Savill G., Walter J.H. Carnitine transporter defect: diagnosis in asymptomatic adult women following analysis of acylcarnitines in their newborn infants. J. Inherit. Metab. Dis. 2006;29:627–630. doi: 10.1007/s10545-006-0376-y. [DOI] [PubMed] [Google Scholar]
- 33.Wilcken B., Wiley V., Hammond J., Carpenter K. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N. Engl. J. Med. 2003;348:2304–2312. doi: 10.1056/NEJMoa025225. [DOI] [PubMed] [Google Scholar]
- 34.Wilson C., Knoll D., de Hora M., Kyle C., Glamuzina E., Webster D. The decision to discontinue screening for carnitine uptake disorder in New Zealand. J. Inherit. Metab. Dis. 2019;42:86–92. doi: 10.1002/jimd.12030. [DOI] [PubMed] [Google Scholar]
- 35.Winter S.C., Linn L.S., Helton E. Plasma carnitine concentrations in pregnancy, cord blood, and neonates and children. Clin. Chim. Acta. 1995;243:87–93. doi: 10.1016/0009-8981(95)06148-7. [DOI] [PubMed] [Google Scholar]
- 36.Zhou W., Li H., Huang T., Zhang Y., Wang C., Gu M. Biochemical, molecular, and clinical characterization of patients with primary carnitine deficiency via large-scale newborn screening in Xuzhou area. Front. Pediatr. 2019;7:50. doi: 10.3389/fped.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]