Abstract
Recent advances in quantitative proteomics show that WD40 proteins play a pivotal role in numerous cellular networks. Yet, they have been fairly unexplored and their physical associations with other proteins are ambiguous. A quantitative understanding of these interactions has wide-ranging significance. WD40 repeat protein 5 (WDR5) interacts with all members of human SET1/MLL methyltransferases, which regulate methylation of the histone 3 lysine 4 (H3K4). Here, using real-time binding measurements in a high-throughput setting, we identified the kinetic fingerprint of transient associations between WDR5 and 14-residue WDR5 interaction (Win) motif peptides of each SET1 protein (SET1Win). Our results reveal that the high-affinity WDR5-SET1Win interactions feature slow association kinetics. This finding is likely due to the requirement of SET1Win to insert into the narrow WDR5 cavity, also named the Win binding site. Furthermore, our explorations indicate fairly slow dissociation kinetics. This conclusion is in accordance with the primary role of WDR5 in maintaining the functional integrity of a large multisubunit complex, which regulates the histone methylation. Because the Win binding site is considered a key therapeutic target, the immediate outcomes of this study could form the basis for accelerated developments in medical biotechnology.
Keywords: Mixed Lineage Leukemia (MLL), SET1 proteins, Protein-peptide Interactions, Biolayer Interferometry, Fluorescence polarization, Surface Plasmon Resonance, Cancer
Introduction
WD40 repeat protein 5 (WDR5) is a conserved chromatin-associated protein that is involved in a number of transient protein-protein interactions [1]. However, WDR5 is notoriously known for its regulatory role in multisubunit epigenetic complexes, such as Suppressor of Variegation, Enhancer of Zeste, and Trithorax 1 (SET1) lysine methyltransferases (KMT) of histones [2-8]. There are six SET1 family members in humans: MLL1, MLL2, MLL3, MLL4, SETd1A, and SETd1B. Each member forms a large multisubunit complex with functions that appear to have diverged in target gene localization and product specificity. However, features common among the complexes are a C-terminal catalytic SET domain that is regulated by interaction with a conserved subcomplex consisting of WDR5, retinoblastoma binding protein-5 (RbBP5), absent-small-homeotic-2-like protein (Ash2L), and dumpy-30 (DPY-30) (WRAD2) [7, 9-20]. WDR5 functions to bridge the interaction between the SET domain and other WRAD2 subunits by the recognition of an evolutionarily conserved WDR5-interaction (Win) motif found in all SET1 family members [21-25]. Formation of this core complex is required for optimal methyltransferase activity [9, 11, 26]. Therefore, small molecules targeting the Win motif-WDR5 protein-protein interaction show promise as anticancer therapeutic candidates.
Interestingly, recent studies have revealed that WDR5 is involved in numerous interactions with other proteins [27-29], including the transcription factor MYC oncoprotein [30-35], 3-phosphoinositide-dependent protein kinase 1 (PDPK1) [36], and interacting partners involved in phosphatidylinositol 3-kinase (PI3K) signaling [36]. Moreover, WDR5 is implicated in nongenomic activities, such as regulatory mechanisms of cellular shape, polarity, and migration [37, 38]. Therefore, WDR5 is a multitasking protein with diverse roles in cellular processes [36, 39, 40]. Its highly conserved sequence across multiple organisms suggests the fundamental significance of its multiple roles [40].
An essential structural archetype of WDR5 is its internal cavity that hosts a high-affinity binding pocket for an evolutionarily conserved Arg-containing peptide segment of the six SET1 proteins [21-23]. Interestingly, this binding cavity of WDR5, here named the Win binding site, is the same [21, 23] as that previously suggested to bind histone H3 [41-46]. Yet, the WDR5-SET1 interaction is required for the stability and functional operation of the C-terminal catalytic SET domain [11, 21, 22]. Moreover, it has only recently been identified that the Win binding site is implicated in transient protein-protein interactions with dozens of proteins, including those involved in PI3K signaling [36]. Given that WDR5 is overexpressed under various oncogenic conditions [47-49], the Win binding site has become a key therapeutic target for different cancers [24, 25, 50-59]. Therefore, a better mechanistic and quantitative understanding of the interactions of the Win binding site with other Win motif partners has fundamental and clinical significance [60-64].
Several research groups have previously examined interactions of WDR5 protein with Win-motif SET1 (SET1Win) peptides using a variety of approaches, such as analytical ultracentrifugation (AUC) [22], isothermal titration calorimetry (ITC) [21, 24, 65], surface plasmon resonance (SPR) [23], and X-ray crystallography [21, 23, 24, 65]. These explorations have confirmed the high affinity of the Win binding site for different Win motif interaction partners [24, 65]. In this study, we determined the kinetic fingerprint and affinities of these interactions using high-throughput optical and fluorescence approaches, which included biolayer interferometry (BLI), SPR, and steady-state fluorescence polarization (state-state FP).
WDR5 is a 334-residue protein that has a 7-bladed, WD-40 repeat-based β propeller structure surrounding a central cavity (Figure 1A) [29, 66-68]. Each blade contains four anti-parallel β strands. A segment of the central cavity serves as the high-affinity Win binding site for the SET1Win peptides (Figure 1B; Supplementary Figures S1-S2) [21, 23, 24, 65]. Here, we performed a systematic kinetic analysis of the interactions of WDR5 with six 14-residue SET1Win peptides, which include an evolutionarily conserved Arg residue at P0 (Table 1). Moreover, this Arg residue has been shown to be critical to WDR5-SET1Win interactions (Supplementary Table S1, Figure S3) [22, 26, 69, 70]. In addition, SET1Win sequences contain a highly conserved 6-residue Win motif peptide, at positions P−3 through P2, along with 8 residues on their flanking sides, at positions P−7 through P−4 and P3 through P6. These flanking residues diverge among SET1 family members, accounting for differences in the binding affinity of WDR5-SET1Win interactions [24, 65]. The highly conserved 6-residue Win motif peptide has to insert into the WDR5 binding cavity to facilitate these highly specific WDR5-SET1Win interactions [24, 65]. Therefore, the choice of a 14-residue length of SET1Win was based on the requirement of the minimal 6-residue Win motif sequence, along with four residues on each flanking side. This also facilitated the interpretation of our results in light of a prior crystallographic study [24], which was conducted with an identical SET1Win length.
Figure 1: Structure of the binary WDR5-MLL1Win complex.
(A) The insertion of MLL1Win into the WDR5 cavity is shown from the top. (B) The same interaction is shown from the side. These graphic representations were made using the pdb code 4ESG.63
Table 1. Alignment of the amino acid sequence of SET1Win motifs.
| Peptide | P−7 | P−6 | P−5 | P−4 | P−3 | P−2 | P−1 | P0 | P1 | P2 | P3 | P4 | P5 | P6 | Charge |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MLL1Win: MLL13758-3771 | L | N | P | H | G | S | A | R | A | E | V | H | L | S* | 0 |
| MLL2Win: MLL25333-5346 | I | N | P | T | G | C | A | R | S | E | P | K | I | L | +1 |
| MLL3Win: MLL34703-4716 | V | N | P | T | G | C | A | R | S | E | P | K | M | S | +1 |
| MLL4Win: MLL42504-2517 | L | N | P | H | G | A | A | R | A | E | V | Y | L | S* | 0 |
| SETd1AWin: SETd1A1488-1501 | E | H | Q | T | G | S | A | R | S | E | G | Y | Y | P | −1 |
| SETd1BWin: SETd1B1698-1711 | E | H | V | T | G | C | A | R | S | E | G | F | Y | T | −1 |
This is a R3771S substituted MLL1Win peptide.
This is a R2517S substituted MLL4Win peptide.
Here, we employed multiple techniques to probe the effect of the surface immobilization on the kinetic fingerprint of WDR5-SET1Win interactions as well as to establish the efficacy of each technique for the measurement of the kinetic rate constants and binding affinities. We provide a critical analysis of each approach with respect to the kinetics of the Win binding site. BLI and SPR require surface immobilization, while steady-state FP does not. On the other hand, steady-state FP is free from this limitation, but is unable to provide real-time kinetic measurements. Furthermore, the use of multiple approaches allowed us to provide quantitative and qualitative validations of our conclusions and to obtain more generalizable outcomes that were not restrained by any one approach. Finally, we also show that distinctions in the kinetic rate constants of these interactions are correlated with unique sequences on the SET1Win peptides' flanking sides.
Materials and Methods
Protein Expression and Purification
Human WDR5 (UniProtKB - P61964; WDR5_HUMAN) was expressed and purified, as follows. pET3aTr vectors containing the 6×His-TEV-WDR5 sequence were transformed into Rosetta™ 2 BL21(DE3)pLysS (Novagen, Cat #71403) competent E. coli cells. Rosetta™ 2 BL21(DE3)pLysS (Novagen, Cat #71403) competent E. coli cells were grown overnight on Luria-Bertani (LB) agar carbenecillin/chloramphenicol selection plates at 37°C. 50 mL LB broth starter cultures (one per 10 L bioreactor growth) containing 50 μg/mL each of carbenecillin and chloramphenicol were inoculated with 5 colonies and grown for 3-5 hours until turbid at 37°C. 10 L Luria broth bioreactors (Eppendorf BioFlo, Enfield, CT) containing 50 μg/mL each of carbenecillin and chloramphenicol, in addition to Antifoam 204 (Sigma-Aldrich, St. Louis, MO), were then inoculated at 37°C and 800 rpm with the entire turbid starter culture. When the culture attained OD600 = 0.8, agitation was decreased to 600 rpm and the temperature was rapidly dropped to 18°C. Induction of target proteins was initiated with 100 μM Isopropyl-β-d-1-thiogalactopyranoside (IPTG; Goldbio, St. Louis, MO) at 18°C. After 12-24 hours post-induction at 18°C, cells were pelleted by centrifugation at 4,465×g using a J6-MI centrifuge (Beckman Coulter, Brea, CA) for 30 minutes at 4°C. The pellet was resuspended in 50 mM Tris-HCl (pH 7.4), 300 mM NaCl, 10 mM βME, and 20 mM imidazole. The resuspended cells were frozen on dry ice and stored at −80°C until purification.
Cell pellets were lysed with a Qsonica Sonicator Q700 (FisherBrand, Pittsburg,_PA) on ice. 5 L of thawed, resuspended pellets were resuspended in 160 mL of 50 mM Tris-HCl (pH 7.4), 300 mM NaCl, 3 mM DTT, and 30 mM imidazole (lysis/Ni-NTA Buffer A/dialysis buffer) containing an additional 200 μL of phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich) and two Pierce™ Protease Inhibitor Mini Tablets (EDTA-free; Thermo Fisher Scientific, Waltham, MA). The cell suspensions were stirred at 4°C for 15 minutes until homogeneous and then sonicated for 10 minutes at 90% power (2 seconds on, 4 seconds off). Lysate was then centrifuged for 1 hour at 4,465×g and 4°C, and the supernatant was stored at 2-8°C for purification. A Ni-NTA purification process was followed. A Kontes 25 x 200 mm column with 30-40 mL Ni-NTA resin (Qiagen, Hilden, Germany) was equilibrated with at least 10 resin-bed volumes (RBVs) of 0.22 μm filtered de-ionized (DI) H2O to remove ethanol, and then at least 5 RBVs of Ni-NTA Buffer A (above). After equilibration, lysate was added to the column and washed with 5-10 RBVs of Ni-NTA Buffer A. Sample was eluted using at least 5 RBVs of Ni-NTA Buffer B (50 mM Tris-HCl (pH 7.4), 300 mM NaCl, 3 mM DTT, and 500 mM imidazole). 5 mL fractions were collected, analyzed via SDS-PAGE, and pooled. Then, sample was dialyzed, as follows. WDR5-containing fractions were pooled into 12-14 kDa MWCO dialysis tubing (Repligen, Waltham, MA), then 2.5 mg of GST-6H-TEV protease (per 5 L of culture; expressed and purified in-house) was added to cleave the His-tag, and the solution was dialyzed against 4 L of dialysis buffer (above) overnight for 12-18 hours at 2-8°C. Dialyzed protein was analyzed via SDS-PAGE to ensure complete cleavage. A negative Ni-NTA was then conducted. The resulting cleaved WDR5 solution was passed through a Bio-Rad Ni-NTA IMAC cartridge (Bio-Rad, Hercules, CA) using a Bio-Rad NGC chromatography system (Bio-Rad) to remove the cleaved His-tag and GST-6H-TEV protease. The flowthrough was collected, analyzed via SDS-PAGE and UV-Vis, and concentrated for size-exclusion chromatography (SEC) purification. As a final polish, the WDR5 proteins were purified via SEC using a HiLoad® 26/600 Superdex® 200 pg column (GE Healthcare, Chicago, IL) into 20 mM Tris-HCl (pH 7.5), 300 mM NaCl, 1 μM ZnCl2, 1 mM TCEP. Fractions of interest were analyzed via SDS-PAGE and UV-Vis, pooled, concentrated where necessary, aliquoted to 1 mL, flash-frozen in liquid nitrogen, and stored at −80°C.
Peptide synthesis, labeling, purification, and analysis for BLI measurements
All peptides for biolayer interferometry measurements were synthesized by GenScript (Piscataway, NJ). The peptides werepurified to ≥ 95% purity. Amino acid analysis, purity confirmation, and solubility testing were provided by GenScript. All peptides were biotinylated at the N terminus. Their C terminus was amidated.
Peptide synthesis, labeling, purification, and analysis for SPR and FP measurements
For Fmoc-SPPS, peptides were synthesized at theoretical 100 μmol scale using the standard double coupling workflow pre-programmed on the Biotage Syro I peptide synthesizer (Biotage, Charlotte, NC). 278 mg of Rink Amide AM Resin LL (100-200 mesh, 0.36 mmol/g functionalization; Novabiochem (EMD Millipore)) was loaded into each 10 mL reactor vial, corresponding to the 100 μmol synthesis scale. Each resin aliquot was swelled with DMF for 30 minutes, followed by an initial resin deprotection step using 40% piperidine in DMF. Each double coupling cycle was comprised of two independent, 45-minute coupling sub-steps that differed in the choice of activation reagents: 1) DIC/Oxyma (1:2 ratio with respect to Fmoc-[AA]), followed by 2) HBTU/DIPEA (0.95:2 ratio with respect to Fmoc-[AA]). For each coupling sub-step, four molar equivalents of each respective 0.5 M Fmoc-[AA] stock was added to each vial. Each coupling cycle terminated with a double Fmoc deprotection step using 40% piperidine in DMF. Coupling reactions proceeded with interspersed vortexing of the vials and under an air atmosphere at ambient temperature. Following the conclusion of peptide synthesis, resins were washed with three successive aliquots of DCM, followed by three minutes under vacuum to dry.
Each peptidyl-resin aliquot was swelled with DMF for 30 minutes and drained. DMF (1,200 μL) was added to each aliquot, followed by 300 μL of DIPEA in NMP (6 molar equivalents, 600 μmol), then followed by 600 μL of Lissamine Rhodamine B sulfonyl chloride in DMF (3 molar equivalents, 300 μmol). The mixture was shielded from light and allowed to react with intermittent vortexing overnight at ambient temperature. Following conjugation, the resin bed was drained, washed successively with DMF until no further change in the color of the flowthrough was observed (faint pink), washed successively with DCM, and then held under vacuum for three minutes to dry.
Cleavage cocktail containing 90% TFA, 5% TIS, 2.5% DODT, and 2.5% H2O was freshly prepared. 4 mL of cleavage cocktail was added to each peptidyl-resin aliquot, sealed, and placed on a rocking platform to react for 4 hours at ambient temperature. After incubation, the contents of each reactor vial were plunged into separate 50 mL conical tubes. The resin was then treated with an additional 2 mL of cleavage cocktail and allowed to react on the rocking platform for 30 minutes at ambient temperature. Following the second incubation, the contents of each reactor vial were pooled into their respective 50 mL conical tubes and the resin containing reactor vials were discarded. Cleavage aliquots were triturated by the fast addition of ~45 mL cold (−80°C) diethyl ether. Precipitate was compacted by centrifugation at 1,000×g for 10 minutes at 0°C using an Allegra X-22R centrifuge (Beckman Coulter, Brea, CA). The supernatant was discarded, and the peptide pellets were washed with a second ~30 mL aliquot of cold diethyl ether, centrifuged, and decanted as before. The resulting peptide pellets were flash frozen in liquid nitrogen and lyophilized using a FreeZone 2.5L lyophilizer (Labcono, Kansas City, MO) overnight to remove residual solvents. The crude lyophilized peptide was stored at −80°C until purification.
Crude peptide aliquots were purified using reversed-phase chromatography through two stages: 1) Flash chromatography using a Biotage Isolera One (Biotage AB, Uppsala, Sweden), 2) Semi-preparative HPLC using a Waters 2695 separations module equipped with a Waters 2996 photodiode array detector (PDA). 1) Biotage Isolera One purifications: Each crude peptide aliquot was solubilized in 2.5 mL total of either DMSO or DMF (DMF only if the peptide contained oxidizable Cys or Met residues) and loaded onto a Biotage Sfär C18 Samplet for 25 g Column (Biotage, Uppsala, Sweden) with the aid of a vacuum. Each samplet was placed in a Biotage Sfär Bio C18 D Duo (300 Å, 20 μm) 25 g column (Biotage). Peptides were eluted using a 15-column volume (CV) gradient of 9-90% MeCN in H2O containing 0.1% TFA at a flowrate of 30 mL/min. The collection threshold was 75 mAU for λ = 200-400 nm, with monitoring at λ = 215 nm and 355 nm (rhodamine). Rhodamine labeled fractions were pooled and MeCN was removed using a rotary evaporator for 30 minutes at 25°C. Samples were flash-frozen in liquid nitrogen and lyophilized using a FreeZone 2.5L lyophilizer (Labcono) for three days. The semipure lyophilized peptide was stored at −80°C until further purification. 2) Semi-preparative HPLC: Peptide samples were resuspended in 50-80 μL aliquots of DMSO or 1:2 DMSO/H2O. Aliquots were injected onto a Waters XBridge Peptide BEH C18 OBD Prep Column (5 μm, 300 Å, 10 mm x 150 mm). A gradient of 10-50% MeCN containing 0.1% (v/v) TFA in H2O containing 0.1% (v/v) TFA was applied over 40 minutes (Δ1%/min.) at a flow rate of 4.73 mL/min. at ambient temperature using a Waters 2695 separations module. Sample detection occurred at 215 nm, 280 nm, 355 nm (rhodamine), and 560 nm (rhodamine) using a Waters 2996 photodiode array detector (PDA). Sample purity was determined by baseline integration using Waters Empower 3 software (>90% purity in all cases). Rhodamine containing fractions were analyzed by MALDI-TOF mass spectroscopy for the identity and purity confirmatory tests. Target fractions were flash-frozen in liquid nitrogen and lyophilized using a FreeZone 2.5L lyophilizer (Labcono) for three days. The resulting purified, lyophilized peptides were reconstituted as concentrated stocks in ultrapure water, aliquoted, flash-frozen in liquid nitrogen, and stored at −80°C until use.
For MALDI-TOF mass spectroscopy of the purified peptides, 1 μL of peptide stock in H2O (1-100 μM) was mixed with 9 μL spotting matrix (10 mg/mL α-cyano-4-hydroxycinnamic acid (CHCA) in 50:50 MeCN/0.1% (v/v) TFA in H2O). 2 μL of each peptide spotting solution was spotted onto a Bruker MTP 384 Target Plate, which was calibrated using Anaspec Peptide Mass Standard Kit, and allowed to dry at room temperature for at least 30 minutes prior to analysis. Samples were analyzed on a Bruker Autoflex iii Mass Spectrometer as an average of 1200 shots using 35% laser power, an m/z range from 840-6000 Da with suppression <400 Da in linear mode, 3.6x detector gain, 2.00 sample rate, and medium gating strength.
Biolayer interferometry (BLI)
Octet RED384 (FortéBio, Fremont, CA) was used for the BLI studies [71-73]. Streptavidin (SA) sensors were presoaked in buffer for ~30 minutes. The buffer solution contained 150 mM NaCl, 20 mM Tris-HCl, 1 mM TCEP, 1 mg/mL bovine serumalbumin (BSA), pH 7.5. 5 nM tagged peptide was then loaded onto sensors for 15 minutes. Sensors were then dipped in buffer again for 5 minutes to wash off unbound peptides from the surface. A 3-fold serial dilution of WDR5 was conducted ranging from 0.1 μM to 9 μM for the association process and then placed into the buffer solution for the dissociation process. The association and dissociation processes were ~200 and ~600 seconds long, respectively. For all WDR5 concentrations, unloaded sensors were run concurrently as controls and were used to subtract the baseline and the drift in the sensorgrams to extract the binding curves. The BLI experiments were performed at 24°C. All reagents were prepared in the above- mentioned buffer and were loaded into 96-well flat bottom black plates for the sensorgram recordings. The binding curves were fitted using the Octet Data Analysis software (FortéBio). The curves of the association process, which were recorded for various analyte concentrations, [C], were fitted using the following equation [74]:
| (1) |
Here, Y0 and Y∞ are the BLI response signals at time zero and infinity, respectively, of the association process. t denotes the cumulative time of the association reaction. kobs is the apparent first-order reaction rate constant of the association process. The curves of the dissociation process were fitted using the following equation:
| (2) |
Here, Y0 and Y∞ are the BLI response signals at time zero and infinity, respectively, of the dissociation process. koff indicates the dissociation rate constant. Finally, the association rate constant, kon, was determined using the slope of the linear curve [75, 76]:
| (3) |
Global fitting, which was conducted using several analyte concentrations, provided the corresponding kon and koff values. The dissociation constant, KD, were indirectly determined using the kon and koff values. Three distinct BLI measurements were conducted for all inspected interactions.
Surface plasmon resonance (SPR)
All SPR experiments [77-79] were conducted on a Cytiva Biacore 8K (Cytiva Life Sciences, Marlborough, MA). All buffers and dilutions were freshly made in-house using ultrapure water obtained from an IQ 7000 Milli-Q system (Millipore-Sigma, Burlington, MA). WDR5 protein was immobilized onto the active flow cell of each channel of a Cytiva Series S Sensor Chip CM5 (Cytiva Life Sciences) according to the following parameters and protocol. A CM5 chip was inserted into the instrument and equilibrated for 1 h at 25°C in PBS-P+ running buffer (PBS-P+ Buffer 10×, Cytiva Life Sciences). The chip surface was activated using an injection of 1:1 N- hydroxysuccinimide (NHS)/1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) (Cytiva Amine Coupling Kit, Cytiva Life Sciences) for 420 seconds at 10 μL/min across both active and reference flow cells. This activation process was followed by a wash of the microfluidics with 1 M ethanolamine-HCl (pH 8). Following activation, wild-type WDR5 (1.75-2.50 μg/mL; analyte dependent) in 50 mM sodium phosphate (pH 6.5), 50 mM NaCl was then injected across the active flow cell for 150 seconds at 10 μL/min. Following ligand immobilization, both active and passive flow cells were chemically deactivated with an injection of 1 M ethanolamine-HCl (pH 8) for 420 seconds at 10 μL/min. SET1Win peptide dilutions were freshly prepared from HPLC-purified peptide stocks in ultrapure water; the peptides were identical to those used in the steady-state FP experiments and contained an N-terminal sulforhodamine B and a C-terminal amide. Titrations of each labeled SET1Win peptide analyte were conducted to span an approximate range of 0.1-10 × KD (approximately 1 nM to 7 μM; 40 μM for MLL1). Each SET1Win peptide was analyzed in a separate channel. Multicycle kinetic analyses were conducted at a flow cell temperature of 25°C and a sample compartment temperature of 20°C in a running buffer composed of 20 mM Tris- HCl (pH 7.5), 150 mM NaCl, 1 mM TCEP, 0.05% Tween 20. Each analysis cycle consisted of the following steps: 1) SET1Win peptide analyte injection: 120-second association, 360-second dissociation, 30 μL/min.; 2) regeneration injection with 100% ethylene glycol for 15 seconds at 10 μL/min. (high viscosity setting); 3) wash of the microfluidics system with running buffer; 4) regeneration injection with 1 μM ZnCl2 for 30 second at 10 μL/min. Prior to curve fitting, all data generated from the active flow cell of each channel are double referenced to both the appropriate buffer blanks (first/last) and the reference flow cell. For MLL2Win, MLL3Win, MLL4Win, SETd1AWin, and SETd1BWin, the affinity constants, KD, were calculated indirectly using KD = koff/kon. For MLL1Win, a plot of relative response versus the MLL1Win analyte concentration was constructed and data were fitted using a four-parameter logistic regression to obtain the KD. Therefore, an affinity analysis (relative response vs. concentration dose- response curve) was used to calculate the KD, in this instance due to kinetic rate constants falling outside of the instrument detection range. All interactions were independently determined in triplicate (e.g., separate ligand immobilizations). Experimental data and fits were plotted using GraphPad Prism 8 (GraphPad Software).
Steady-state fluorescence polarization (steady-state FP) measurements
All steady-state fluorescence polarization (FP) measurements wererecorded using a SpectraMax i3x plate reader (Molecular Devices, San Jose, CA).[80, 81] HPLC-purified peptides, which contained an N-terminal sulforhodamine B and a C-terminal amide, were reconstituted as concentrated stocks in ultrapure water and used in all subsequent experiments. All steady-state FP assays were conducted in a buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM TCEP, 0.005% Tween 20 and plated in black untreated 96-well polystyrene microplates (Corning Inc., Corning, NY). Steady-state FP assays were conducted in triplicate, 24-point serial dilution for each of the six SET1Win peptides against WDR5. 200 μL of WDR5 stock solutions, ranged from 21.6 to 131 μM, were added to each well on the first column of one of two adjacent black 96-well dilution plates, and 100 μL of the assay buffer was added to wells A2-H24 over the two plates. WDR5 variants were then diluted down the two plates by transferring 100 μL from each well to the next, for a total of 24, 2-fold dilutions which range from low μM to low pM (variant specific). Following dilutions, 100 μL of the appropriate 20 nM labeled SET1Win peptide, which was dissolved in the assay buffer, was added to each well on each set of plates at a final concentration of 10 nM. The steady-state FP anisotropy was measured on the plates after a 1 h incubation at room temperature in the dark. The resulting dose-response data were averaged and fitted using a four-parameter logistic regression to obtain the binding affinity (KD) for each interaction. Data were plotted and analyzed using GraphPad Prism 8 (GraphPad Software, San Diego, CA).
Molecular graphics
All cartoons showing molecular graphics were prepared using thePyMOL Molecular Graphics System (Version 2.4.0 Schrödinger, LLC).
Results and Discussion
The kinetic fingerprint of WDR5 – SET1Win interactions
We first explored the association (kon) and dissociation (koff) rate constants of WDR5-SET1Win interactions using BLI measurements [71, 72]. This technology probes ligand-receptor interactions that lead to accumulations of bimolecular complexes at the surface of the BLI sensor. This process is facilitated by immobilizing one interacting partner (ligand) onto the surface of the BLI sensor and supplying the analyte partner (receptor) from solution. In this way, the association and dissociation phases of the ligand-receptor complex are optically measured in real time using alterations in the interference pattern between reflected light waves at the surface of the BLI sensor. The N-terminus of each SET1Win was biotinylated and their C-terminus was amidated (Table 1). A 9-residue Gly/Ser-rich peptide spacer was inserted between the biotinylated site and the SET1Win sequence. This 3 nm-long spacer ensures that there is a satisfactory distance between the BLI sensor and SET1Win for WDR5 to interact without steric hinderance from the sensor surface.
The association binding curves were acquired through a 3-fold serial dilution of WDR5 (Figure 2). After the BLI response reached a saturation level, individual dissociation binding curves were recorded when sensors were placed in a WDR5-free buffer. The association and dissociation BLI phases underwent a time-dependent single-exponential increase and decrease, respectively. The binding curves were fitted using the Octet Data Analysis software (Materials and Methods; eqns. (1)-(3)). The equilibrium dissociation constants using BLI, KD-BLI, were indirectly determined using kon and koff.
Figure 2: Label-free optical BLI sensorgrams of WDR5-SET1Win interactions.
5 nM biotin-tagged SET1Win peptides were loaded onto streptavidin (SA) sensors for 15 minutes. Titration series of WDR5 were injected as analytes and the corresponding association and dissociation curves are shown for the six SET1Win peptides.
Association rate constants
The BLI-determined kon values were in the order of 104 M−1s−1, clearly indicating slow association kinetics for a protein-peptide system (Table 2). It is likely that this outcome resulted from two distinct physical restrictions: (i) tethering SET1Win onto the surface of the BLI sensor, thus reducing its local mobility, and (ii) partitioning of SET1Win into the WDR5 cavity. The latter physical process was illustrated in early crystallographic studies [21], which revealed that an MLL1Win peptide penetrates into the WDR5 cavity to undergo a bimolecular association process. Here, we were unable to acquire an accurate mean value of kon for the WDR5-MLL1Win interaction due to its relatively fast koff constant, whose binding time constant was shorter than the time-resolution limit of this approach (~1 s). Furthermore, kon measured by BLI for various SET1Win peptides were within the same order of magnitude, suggesting a similar insertion mechanism of the 6-residue Win motif peptide into the Win binding site (Table 1; Supplementary Figures S1-S2) [24, 65].
Table 2. Kinetic rate constants of association, kon, and dissociation, koff, and equilibrium dissociation constants, KD-BLI, of WDR5 with SET1Win peptides using BLI.
Numbers represent mean ± s.d. determined from three independent experiments.
| Peptide sequence |
kon (M−1s−1) × 10−4 |
koff (s−1) × 103 |
KD-BLI (nM) |
|---|---|---|---|
| Biotinyl-(GGS)3MLL1Win-NH2 | ND* | ND** | ND*** |
| Biotinyl-(GGS)3MLL2Win-NH2 | 4.4 ± 0.4 | 7.7 ± 0.2 | 170 ± 20 |
| Biotinyl-(GGS)3MLL3Win-NH2 | 5.4 ± 0.6 | 5.3 ± 0.1 | 100 ± 5 |
| Biotinyl-(GGS)3MLL4Win-NH2 | 2.3 ± 0.2 | 39 ± 2 | 1,700 ± 200 |
| Biotinyl-(GGS)3 SETd1AWin-NH2 | 8.2 ± 0.8 | 51 ± 6 | 620 ± 20 |
| Biotinyl-(GGS)3SETd1BWin-NH2 | 7.1 ± 0.5 | 17 ± 1 | 250 ± 30 |
kon was not quantitatively determined. Although WDR5-MLL1Win interactions were detectable using a BLI measurement (Fig. 2), no accurate quantitative determination was made due to the limited time resolution of this approach. In this case, we assume that the kon was in the same order of magnitude with the kon of the other SET1Win peptides (~104 M−1s−1).
koff was not quantitatively determined due to a fast dissociation rate constant. The upper-limit value for the detection of koff is 1 s−1 according to instrument specifications.
KD-BLI was not quantitatively determined due to the limited time resolution of the approach. In this case, KD-BLI determined by BLI for WDR5-MLL1Win interactions should be greater than ~105 nM. This value results from dividing the upper-limit value of detection of koff (ND**) by the value of the kon approximation (ND*).
Dissociation rate constants
In contrast, koff values spanned between two and three orders of magnitude (Table 2), highlighting significant distinctions in the WDR5-SET1Win interactions. This assumes that the koff of the WDR5-MLL1 interactions is faster than 1 s−1, showing that MLL1Win has the weakest interaction with WDR5 among all SET1Win peptides. On the other hand, the strongest WDR5-SET1Win interactions were monitored with MLL2Win and MLL3Win, which had average koff values of (7.7 ± 0.2) × 10−3 s−1 and (5.3 ± 0.1) × 10−3 s−1, respectively. This finding provides unusually long binding times of ~130 s and ~190 s, respectively.
It was previously demonstrated that WDR5-SET1Win interactions have a common archetype: a highly conserved Arg residue at P0 (Table 1) [22]. This Arg residue is the pivotal player of WDR5-SET1Win interactions, contributing to most of the binding affinity through a complex network of contacts with neighboring residues (Supplementary Table S1, Figure S3) [22, 24]. To test this hypothesis, we conducted BLI measurements using a control MLL3Win peptide, whose native form exhibited the longest binding time with WDR5. The key Arg residue at P0 was replaced by an Ala residue, resulting in the R4710A MLL3Win mutant. No interaction was detected with this R4710A MLL3Win mutant (Supplementary Figure S4), confirming the critical role of Arg at P0 for the strength of WDR5-SET1Win interactions. In addition, this finding validates the efficacy of our BLI measurements for examining the kinetic landscape of the Win binding site of WDR5.
Does the tethering restriction of SET1Win onto the surface of the BLI sensor impact the kinetic rate constants?
Association rate constants
We postulated above that the slow association kinetics of WDR5-SET1Win interactions were caused at least in part by the immobilization of SET1Win onto the surface of the BLI sensor. To assess this hypothesis, we next employed SPR [77-79] as an orthogonal, label-free approach for examining these interactions of SET1Win peptides with WDR5. SPR is an optoelectronic technique that monitors accumulation of bimolecular ligand-receptor complexes onto the surface of the SPR sensor by changes in the refractive index. In this case, SET1Win analyte is supplied by using a flow-driven fluidic device. WDR5 was immobilized onto the surface of the SPR chips as the "receptor" (Materials and Methods). In this way, we recorded the association and dissociation phases in real time when SET1Win was not immobilized onto a surface (Figure 3). Biacore™ Software was used to analyze and fit the SPR sensorgrams using a 1:1 binding interaction model to provide the kon and koff rate constants (Supplementary Figure S5). In accord with our expectation, kon values obtained by SPR were significantly increased by almost an order of magnitude to quantities greater than 105 M−1s−1 (Table 3; Supplementary Figure S6, Figure S7A). Again, kon measured by SPR for various SET1Win peptides were within the same order of magnitude, potentially indicating an identical insertion mechanism of the 6-residue Win motif peptide into the Win binding site (Table 1; Supplementary Figures S1-S2) [24, 65]. In addition, this insertion mechanism prevails no matter whether SET1Win is in either physically restricted (e.g., BLI) or unrestricted (e.g., SPR) conditions.
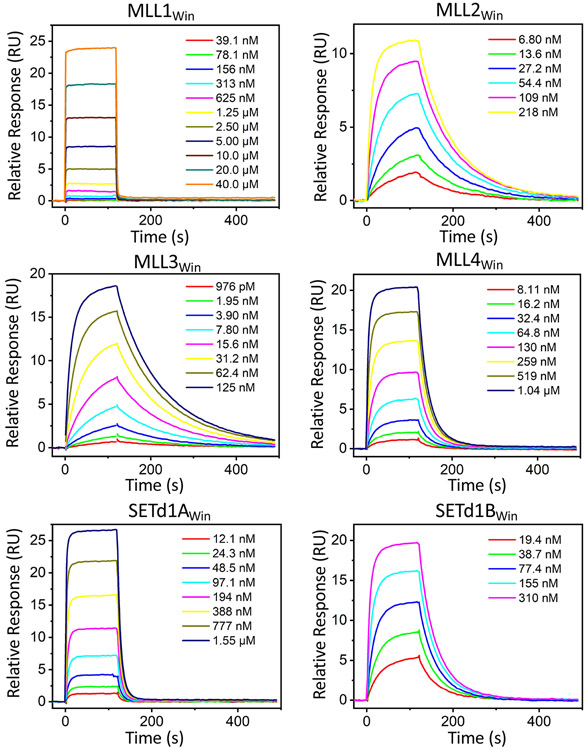
Figure 3: Label-free optoelectronic SPR sensorgrams of the WDR5-SET1Win interactions.
Titration series of the respective SET1Win peptides were injected as analytes and the corresponding association and dissociation curves are shown for the six SET1Win peptides.
Table 3. Kinetic and affinity determinations of WDR5-SET1Win interactions using SPR.
Values represent mean ± s.d. acquired from at least three independent experimental determinations (separate receptor immobilizations). For MLL2Win, n = 4 independent experimental determinations were used.
| Peptide sequence |
kon (M−1s−1) × 10−5 |
koff (s−1) × 103 |
KD-SPR (nM) |
|---|---|---|---|
| Sulforhodamine B-(GGS)3MLL1Win-NH2 | ND* | ND** | 16,000 ± 3,000*** |
| Sulforhodamine B-(GGS)3MLL2Win-NH2 | 3.7 ± 0.3 | 12 ± 1 | 33 ± 2 |
| Sulforhodamine B-(GGS)3MLL3Win-NH2 | 4.9 ± 0.4 | 9 ± 1 | 19 ± 1 |
| Sulforhodamine B-(GGS)3MLL4Win-NH2 | 2.1 ± 0.3 | 41 ± 3 | 190 ± 20 |
| Sulforhodamine B-(GGS)3 SETd1AWin-NH2 | 3.1 ± 0.2 | 110 ± 10 | 350 ± 10 |
| Sulforhodamine B-(GGS)3SET1dBWin-NH2 | 3.4 ± 0.3 | 24 ± 1 | 69 ± 6 |
kon was not quantitatively determined. Although the WDR5-MLL1Win interactions were detectable using an SPR measurement (Fig. 3), no accurate quantitative determination was made due to the limited time resolution of the approach. In this case, we assume that the kon was in the same order of magnitude with the kon of other SET1Win peptides (~105 M−1s−1).
koff was not quantitatively determined due to a fast dissociation rate constant. The upper-limit value for the detection of koff is 0.5 s−1 according to instrument specifications. The Biacore 8K+ cannot measure rate constants of dissociation, koff, faster than 0.5 s−1.
Here, KD-SPR was determined using a steady-state SPR measurement (Supplementary Figure S6).
Dissociation rate constants
Interestingly, with the exception of SETd1A, the koff values acquired by the SPR measurement of WDR5-SET1Win interactions closely resembled those measured by BLI (Table 3; Supplementary Figure S7B). Moreover, this finding provides indirect evidence that WDR5 did not undergo denaturation upon its immobilization onto the surface of the SPR sensor. Tethering SET1Win onto the surface of the BLI sensor via its C-terminus was considered inconvenient, because prior crystallographic information indicated the interaction of the C-terminus residues (Table 1) with the WDR5 surface [24]. However, does the SET1Win tethering onto the surface of the BLI sensor via its N-terminus impose an additional physical restriction on the binding mechanism? Since the koff measured by BLI and SPR are almost identical, we conclude that the SET1Win tethering onto the surface of the BLI sensor via its N-terminus did not produce any additional restrain on the detachment mechanism of SET1Win from the high-affinity Win binding site.
Comparisons of binding affinities of WDR5-SET1Win interactions in restricted and unrestricted conditions
Next, we asked how immobilization-free (i.e., unrestricted) conditions influence the binding affinities. It is expected that the equilibrium dissociation constant slightly decreases if both WDR5 and SET1Win move freely in solution. Hence, we determined equilibrium dissociation constant values using steady-state FP measurements, KD-FP (Materials and Methods) [80-82]. This fluorescence technique monitors changes in the polarity of the emitted light due to modifications in the rotational diffusion coefficient of a fluorescently labeled ligand. For example, the formation of the ligand-receptor complex is accompanied by a decline in the rotational mobility of the ligand, increasing the polarity of the emitted light. Here, Sulforhodamine B, an optically stable and bright fluorophore [83], was chemically attached to the N terminus of SET1Win via a 9-residue Gly/Ser-rich flexible spacer, whereas their C terminus was amidated. WDR5 concentration-dependent, steady-state FP binding curves were collected using a plate reader in a high-throughput setting (Fig. 4). In this way, we determined the immobilization-free KD-FP (Supplementary Table S2). In accord with our anticipation, KD-FP values were slightly lower than those acquired by SPR, KD-SPR, meaning that WDR5-SET1Win interactions appeared somewhat stronger when probed in unrestricted conditions.
Figure 4: Steady-state FP curves of the WDR5-SET1Win interactions.
The N terminus of the SET1Win peptides was tagged with sulforhodamine B, whereas the C terminus was amidated. The final concentration of labeled SET1Win peptides in each well was 10 nM. Three independent experiments were conducted to obtain the WDR5 dose response.
The KD-FP values are also in agreement with results of prior ITC measurements (e.g., in immobilization-free conditions) at either lower [65] or higher salt concentrations [24]. It is worth mentioning that we preferred replacing Arg by Ser at the C terminus of MLL1Win and MLL4Win to avoid potential rebinding events of the native Arg at P6 to the WDR5 cavity (Table 1). Regardless, we employed similar peptide sequences and identical buffer conditions to obtain meaningful comparisons among the three approaches. Then, we have discovered the following relationship for each WDR5-SET1Win interaction: KD-BLI > KD-SPR > KD-FP (Fig. 5; Supplementary Table S2). In addition, numerical values of KD-FP replicated similar trends noted with different SET1Win peptides using both BLI and SPR.
Figure 5: Quantitative comparisons of dissociation equilibrium constants of WDR5-SET1Win interactions using BLI, SPR, and steady-state FP measurements.
"*" is the upper-limit value for the detection of KD-BLI. This value results from dividing the upper-limit value of the detection of koff by the value of the kon approximation (Table 2).
Tentative interpretations
The kinetics and affinity measurements of the WDR5-SET1Win interactions were conducted with no significant variation among independently recorded BLI sensorgrams. The precision of this technique allowed us to quantitatively compare kinetics that were within the same order of magnitude. The SET1Win motif is located about 60 residues N-terminal to the SET domain, both of which are at the C-terminal end of the large SET1 proteins. These proteins range between ~1,700 – 5,500 amino acids in length. The rotational and diffusional rates of SET1 proteins relative to WDR5 are slow enough that these proteins can be considered as stationary compared to WDR5. Therefore, a more physiologically-relevant approach that recapitulates the binding of the full-length SET1 is that in which the 14-residue SET1Win peptide is attached onto a surface, whereas WDR5 is free insolution. In this way, the actual kon measured by BLI would be conceivably closer to that value in physiological conditions. In addition, it should be mentioned that SET1Win is physically restrained at both ends when it is part of the SET1 subunit, suggesting an even lower kon value. However, SET1Win may have stabilized conformations that promote binding within the context of the large SET1 subunit. In other words, the unrestricted SET1Win may undergo significantly greater degrees of freedom to adopt numerous non-productive conformations. Furthermore, binding of SET1 proteins to WDR5 may be coupled with additional interaction pockets that can amplify the binding strength either by increasing kon or by decreasing koff or both. For example, prior sedimentation velocity experiments have shown that a 225-residue MLL1Win-containing polypeptide exhibits a binding affinity of ~120 nM to WDR5 [22].
kon values measured by SPR are yet at least an order of magnitude lower than those predicted for a protein-peptide interaction system (107 - 108 M−1s−1) [84-88]. Here, WDR5-SET1Win interactions require a precise insertion of the 6-residue, Arg-containing SET1Win peptide into the WDR5 cavity. Therefore, we interpret that kon values are limited by the entropic penalty determined by the SET1Win partitioning into the Win binding site. The high-affinity Win binding site features a conical geometry with a maximum internal diameter of ~1.5 nm, as measured from side chain to side chain. For example, MLL1Win partitions ~1.0 nm into the Win binding site (Fig. 1) [24]. In support to this interpretation, previous single-molecule studies have shown that kon values of the interactions of peptides, up to 25 residues in length, with a narrow 2 nm-wide nanopore are in the order of 105 M−1s−1 [89, 90]. This is the same order of magnitude with kon values that we determined by SPR.
However, the kon is a composite parameter, which includes contributions of the diffusion-limited rate factor, kon0, and electrostatic free energy of the WDR5-SET1Win complex, ΔG*e1. Zhou and coworkers have demonstrated that the rate constant of association of two proteins can be accurately computed using the following expression: kon = kon0 exp(−ΔG*e1/kBT), where kB and T are Boltzmann's constant and the absolute temperature, respectively [91, 92]. For this equation, they employed kinetic-rate theory of rigid-body docking and Poisson-Boltzmann formalism [93-95]. Using the same approach [93] and crystallographic information of the WDR5-SET1Win complex [24], we found kon values of several SET1Win peptides (Supplementary Table S3). For example, computed kon values for MLL2Win and MLL3Win were 2.9 × 105 M−1s−1 and 1.2 × 105 M−1s−1, respectively. These values compare well with our corresponding experimental data determined by SPR, which were (3.7 ± 0.3) × 105 M−1s−1 and (4.9 ± 0.4) × 105 M−1s−1, respectively. Yet, computed kon values for MLL1Win and MLL4Win were in the order of 104 M−1s−1. The TransComp server: Web Server for Predicting Protein Association Rate Constants [91, 93] was not able to produce computed kon values for SETd1AWin and SETd1BWin, likely because these WDR5-SET1Win complex formations do not undergo a single-step association mechanism. This outcome suggests that the WDR5-SET1Win interactions of SETd1AWin and SETd1BWin exhibit some subtle structural distinctions with respect to MLL1Win, MLL2Win, MLL3Win, and MLL4Win, which is in accord with prior crystallographic data [24, 65].
Concluding remarks, practical implications, and future prospects
In summary, we present a detailed kinetic fingerprint of the multitasking high-affinity Win binding site of WDR5, a protein with major regulatory implications in the methylation of H3K4 and in multiple physical associations with other proteins. This study reveals slow kinetics of association and dissociation of the SET1Win peptides with WDR5. It is known that WDR5 bridges the interaction between the SET domain of large SET1 subunits and other WRAD2 constituents. A long-binding time of WDR5-SET1Win interaction, meaning slow dissociation kinetics of the high-affinity Win binding site, is a pivotal mechanism by which WDR5 assists the functional integrity of the multisubunit WRAD2 complex. Furthermore, slow dissociation rates detected in this study point out a fundamental consideration for future therapeutics aimed at targeting interactions of the Win binding site with other proteins. The stability of these multisubunit complexes would decrease the opportunities for inhibitors to interfere in WDR5-SET1/MLL interactions. Consequently, this stresses the need for both fast association rates and slow dissociation rates when designing potential inhibitors to modulate WDR5 function. Newly designed small-molecule drugs would need to bind strongly to the WDR5 cavity and stave off other binding partners. Slow dissociation rate constants also reiterate the efficacy of SET1Win peptidomimetics as a fundamental platform for such drugs. Modifications of these sequences that enhance the association rates, while maintaining the disassociation rates, could be very effective at inhibiting Win binding site interactions. A good place to start would be the alteration of either the net charge or charge distribution of these peptides for amplifying the rate constants of association. Any approach employed in this work can be used in the high-throughput screening of libraries of small-molecule compounds against WDR5-SET1Win interactions. However, WDR5 is a more relevant target for drug discovery when immobilized onto the SPR sensor, where the free inhibitor in solution would better mimic in vivo function. This experimental design enables determinations of kinetic rate constants, contrasting the steady-state FP measurements. Our results also indicate that WDR5 did not undergo denaturation upon its immobilization onto the SPR sensor. In the future, it would be desirable to extend these kinetic studies to full-length WRAD2 subunits, because of the suitability of these high-throughput approaches for examining long-lived protein-protein interactions. For instance, it would be interesting to conduct SPR measurements, in which a full-length SET1 subunit is immobilized on the chip surface and use WDR5 as analyte in solution.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to our colleagues in the Movileanu and Cosgrove laboratories and at Ichor Therapeutics Laboratories for their comments on the manuscript and stimulating discussions as well as for their assistance during the early stage of this project.
FUNDING
This work was supported by the National Cancer Institute of the U.S. National Institutes of Health grant R01 CA140522 (to M.S.C) and by the National Institute of General Medical Sciences of the U.S. National Institutes of Health grant R01 GM129429 (to L.M.).
ABBREVIATIONS
- AUC
analytical ultracentrifugation
- BLI
Biolayer Interferometry
- FP
Fluorescence polarization anisotropy
- H3K4
histone 3 lysine 4
- ITC
isothermal titration calorimetry
- KMT
lysine methyltransferases of histones
- kon
the association rate constant
- koff
the dissociation rate constant
- KD
the equilibrium dissociation constant
- KD-BLI
the equilibrium dissociation constant obtained by biolayer interferometry
- KD-SPR
the equilibrium dissociation constant obtained by surface plasmon resonance
- KD-FP
the equilibrium dissociation constant obtained by steady-state fluorescence polarization
- MLL
Mixed lineage leukemia
- MYC
transcription factor oncoprotein
- PDPK1
3- phosphoinositide-dependent protein kinase 1
- PI3K
phosphatidylinositol 3-kinase
- SET1
Suppressor of Variegation, Enhancer of Zeste, and Trithorax 1 lysine methyltransferases of histones
- SET1Win
14-residue WDR5 interaction (Win) motif peptides of each SET1 protein
- SPR
Surface plasmon resonance
- WDR5
WD40 repeat protein 5
- Win
evolutionarily conserved WDR5-interaction motif found in all SET1 family members
- WRAD2
subcomplex consisting of WDR5, retinoblastoma binding protein-5 (RbBP5), absent-small-homeotic-2-like protein (Ash2L), and dumpy-30 (DPY-30)
Footnotes
ASSOCIATED CONTENT. Supplementary Information. This content includes the following: Complex formation of WDR5 with SET1Win peptides (Figures S1-S2). List of hydrogen bonds at the WDR5-SET1Win protein interface (Table S1, Figure S3). Negative control of BLI measurements using an arginine-replaced MLL3Win derivative (Figure S4). Quantitative kinetic determinations of WDR5-SET1Win interactions using SPR (Figure S5). Determination of the equilibrium dissociation constant of WDR5-MLL1Win interactions using SPR (Figure S6). Quantitative comparisons of kinetic rate constants of WDR5-SET1Win interactions between BLI and SPR measurements (Figure S7). Quantitative comparisons of the binding affinities of WDR5-SET1Win interactions among BLI, SPR, and steady-state FP (Table S2). Computational prediction of association rate constants of WDR5-SET1Win interactions using the basal rate constants and electrostatic free energies of the transient WDR5-SET1Win complex (Table S3). Accession codes. Supporting references.
COMPETING INTERESTS
The authors declare no competing financial interest.
DATA AVAILABILITY
All supporting data, materials, and sequence information are included within the main article and its supplementary file.
REFERENCES
- 1.Trievel RC and Shilatifard A (2009) WDR5, a complexed protein. Nat. Struct. Mol. Biol 16, 678–680 [DOI] [PubMed] [Google Scholar]
- 2.Crawford BD and Hess JL (2006) MLL core components give the green light to histone methylation. ACS Chem. Biol 1, 495–498 [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove MS and Patel A (2010) Mixed lineage leukemia: a structure-function perspective of the MLL1 protein. FEBS J. 277, 1832–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Han J, Zhang Y, Cao F, Liu Z, Li S, Wu J, Hu C, Wang Y, Shuai J, Chen J, Cao L, Li D, Shi P, Tian C, Zhang J, Dou Y, Li G, Chen Y and Lei M (2016) Structural basis for activity regulation of MLL family methyltransferases. Nature. 530, 447–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vedadi M, Blazer L, Eram MS, Barsyte-Lovejoy D, Arrowsmith CH and Hajian T (2017) Targeting human SET1/MLL family of proteins. Protein Sci. 26, 662–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue H, Yao T, Cao M, Zhu G, Li Y, Yuan G, Chen Y, Lei M and Huang J (2019) Structural basis of nucleosome recognition and modification by MLL methyltransferases. Nature. 573, 445–449 [DOI] [PubMed] [Google Scholar]
- 7.Jiang H (2020) The complex activities of the SET1/MLL complex core subunits in development and disease. Biochim. Biophys. Acta. Gene. Regul. Mech 1863, 194560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sha L, Ayoub A, Cho US and Dou Y (2020) Insights on the regulation of the MLL/SET1 family histone methyltransferases. Biochim. Biophys. Acta Gene. Regul. Mech 1863, 194561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, Allis CD and Roeder RG (2006) Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat. Struct. Mol. Biol 13, 713–719 [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Tate CM, You JS and Skalnik DG (2007) Identification and characterization of the human Set1B histone H3-Lys4 methyltransferase complex. J. Biol. Chem 282, 13419–13428 [DOI] [PubMed] [Google Scholar]
- 11.Patel A, Dharmarajan V, Vought VE and Cosgrove MS (2009) On the mechanism of multiple lysine methylation by the human mixed lineage leukemia protein-1 (MLL1) core complex. J. Biol. Chem 284, 24242–24256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odho Z, Southall SM and Wilson JR (2010) Characterization of a novel WDR5-binding site that recruits RbBP5 through a conserved motif to enhance methylation of histone H3 lysine 4 by mixed lineage leukemia protein-1. J. Biol. Chem 285, 32967–32976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avdic V, Zhang P, Lanouette S, Groulx A, Tremblay V, Brunzelle J and Couture JF (2011) Structural and biochemical insights into MLL1 core complex assembly. Structure. 19, 101–108 [DOI] [PubMed] [Google Scholar]
- 14.Patel A, Vought VE, Dharmarajan V and Cosgrove MS (2011) A novel non-SET domain multi-subunit methyltransferase required for sequential nucleosomal histone H3 methylation by the mixed lineage leukemia protein-1 (MLL1) core complex. J. Biol. Chem 286, 3359–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Nuland R, Smits AH, Pallaki P, Jansen PW, Vermeulen M and Timmers HT (2013) Quantitative dissection and stoichiometry determination of the human SET1/MLL histone methyltransferase complexes. Mol. Cell Biol 33, 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P, Bergamin E and Couture JF (2013) The many facets of MLL1 regulation. Biopolymers. 99, 136–14523175388 [Google Scholar]
- 17.Froimchuk E, Jang Y and Ge K (2017) Histone H3 lysine 4 methyltransferase KMT2D. Gene. 627, 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali A and Tyagi S (2017) Diverse roles of WDR5-RbBP5-ASH2L-DPY30 (WRAD) complex in the functions of the SET1 histone methyltransferase family. J. Biosci 42, 155–159 [DOI] [PubMed] [Google Scholar]
- 19.Han J, Li T, Li Y, Li M, Wang X, Peng C, Su C, Li N, Li Y, Xu Y and Chen Y (2019) The internal interaction in RBBP5 regulates assembly and activity of MLL1 methyltransferase complex. Nucleic Acids Res. 47, 10426–10438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaustov L, Lemak A, Wu H, Faini M, Fan L, Fang X, Zeng H, Duan S, Allali-Hassani A, Li F, Wei Y, Vedadi M, Aebersold R, Wang Y, Houliston S and Arrowsmith CH (2019) The MLL1 trimeric catalytic complex is a dynamic conformational ensemble stabilized by multiple weak interactions. Nucleic Acids Res. 47, 9433–9447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel A, Dharmarajan V and Cosgrove MS (2008) Structure of WDR5 bound to mixed lineage leukemia protein-1 peptide. J. Biol. Chem 283, 32158–32161 [DOI] [PubMed] [Google Scholar]
- 22.Patel A, Vought VE, Dharmarajan V and Cosgrove MS (2008) A conserved arginine-containing motif crucial for the assembly and enzymatic activity of the mixed lineage leukemia protein-1 core complex. J. Biol. Chem 283, 32162–32175 [DOI] [PubMed] [Google Scholar]
- 23.Song JJ and Kingston RE (2008) WDR5 interacts with mixed lineage leukemia (MLL) protein via the histone H3-binding pocket. J. Biol. Chem 283, 35258–35264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dharmarajan V, Lee JH, Patel A, Skalnik DG and Cosgrove MS (2012) Structural basis for WDR5 interaction (Win) motif recognition in human SET1 family histone methyltransferases. J. Biol. Chem 287, 27275–27289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alicea-Velázquez NL, Shinsky SA, Loh DM, Lee JH, Skalnik DG and Cosgrove MS (2016) Targeted Disruption of the Interaction between WD-40 Repeat Protein 5 (WDR5) and Mixed Lineage Leukemia (MLL)/SET1 Family Proteins Specifically Inhibits MLL1 and SETd1A Methyltransferase Complexes. J. Biol. Chem 291, 22357–22372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinsky SA, Monteith KE, Viggiano S and Cosgrove MS (2015) Biochemical reconstitution and phylogenetic comparison of human SET1 family core complexes involved in histone methylation. J. Biol. Chem 290, 6361–6375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stirnimann CU, Petsalaki E, Russell RB and Müller CW (2010) WD40 proteins propel cellular networks. Trends Biochem. Sci 35, 565–574 [DOI] [PubMed] [Google Scholar]
- 28.Santosh Kumar HS, Kumar V, Pattar S and Telkar S (2016) Towards the construction of an interactome for Human WD40 protein family. Bioinformation. 12, 54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain BP and Pandey S (2018) WD40 Repeat Proteins: Signalling Scaffold with Diverse Functions. Protein J. 37, 391–406 [DOI] [PubMed] [Google Scholar]
- 30.Ullius A, Luscher-Firzlaff J, Costa IG, Walsemann G, Forst AH, Gusmao EG, Kapelle K, Kleine H, Kremmer E, Vervoorts J and Luscher B (2014) The interaction of MYC with the trithorax protein ASH2L promotes gene transcription by regulating H3K27 modification. Nucleic Acids Res. 42, 6901–6920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas LR, Foshage AM, Weissmiller AM and Tansey WP (2015) The MYC-WDR5 Nexus and Cancer. Cancer Res. 75, 4012–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas LR, Wang Q, Grieb BC, Phan J, Foshage AM, Sun Q, Olejniczak ET, Clark T, Dey S, Lorey S, Alicie B, Howard GC, Cawthon B, Ess KC, Eischen CM, Zhao Z, Fesik SW and Tansey WP (2015) Interaction with WDR5 promotes target gene recognition and tumorigenesis by MYC. Mol. Cell. Biochem 58, 440–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aho ER, Weissmiller AM, Fesik SW and Tansey WP (2019) Targeting WDR5: A WINning Anti-Cancer Strategy? Epigenet. Insights 12, 2516865719865282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas LR, Adams CM, Wang J, Weissmiller AM, Creighton J, Lorey SL, Liu Q, Fesik SW, Eischen CM and Tansey WP (2019) Interaction of the oncoprotein transcription factor MYC with its chromatin cofactor WDR5 is essential for tumor maintenance. Proceedings of the National Academy of Sciences of the United States of America. 116, 25260–25268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas LR, Adams CM, Fesik SW, Eischen CM and Tansey WP (2020) Targeting MYC through WDR5. Mol. Cell. Oncol 7, 1709388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guarnaccia AD, Rose KL, Wang J, Zhao B, Popay TM, Wang CE, Guerrazzi K, Hill S, Woodley CM, Hansen TJ, Lorey SL, Shaw JG, Payne WG, Weissmiller AM, Olejniczak ET, Fesik SW, Liu Q and Tansey WP (2021) Impact of WIN site inhibitor on the WDR5 interactome. Cell Rep. 34, 108636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, Chu J, Patel S, Schaffer DV and Li S (2013) Biophysical regulation of epigenetic state and cell reprogramming. Nat. Mater 12, 1154–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang P, Dreger M, Madrazo E, Williams CJ, Samaniego R, Hodson NW, Monroy F, Baena E, Sánchez-Mateos P, Hurlstone A and Redondo-Muñoz J (2018) WDR5 modulates cell motility and morphology and controls nuclear changes induced by a 3D environment. Proceedings of the National Academy of Sciences of the United States of America. 115, 8581–8586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guarnaccia AD and Tansey WP (2018) Moonlighting with WDR5: A Cellular Multitasker. J. Clin. Med 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bryan AF, Wang J, Howard GC, Guarnaccia AD, Woodley CM, Aho ER, Rellinger EJ, Matlock BK, Flaherty DK, Lorey SL, Chung DH, Fesik SW, Liu Q, Weissmiller AM and Tansey WP (2020) WDR5 is a conserved regulator of protein synthesis gene expression. Nucleic Acids Res. 48, 2924–2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Couture JF, Collazo E and Trievel RC (2006) Molecular recognition of histone H3 by the WD40 protein WDR5. Nat. Struct. Mol. Biol 13, 698–703 [DOI] [PubMed] [Google Scholar]
- 42.Han Z, Guo L, Wang H, Shen Y, Deng XW and Chai J (2006) Structural basis for the specific recognition of methylated histone H3 lysine 4 by the WD-40 protein WDR5. Mol. Cell 22, 137–144 [DOI] [PubMed] [Google Scholar]
- 43.Schuetz A, Allali-Hassani A, Martín F, Loppnau P, Vedadi M, Bochkarev A, Plotnikov AN, Arrowsmith CH and Min J (2006) Structural basis for molecular recognition and presentation of histone H3 by WDR5. EMBO J. 25, 4245–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruthenburg AJ, Wang W, Graybosch DM, Li H, Allis CD, Patel DJ and Verdine GL (2006) Histone H3 recognition and presentation by the WDR5 module of the MLL1 complex. Nat. Struct. Mol. Biol 13, 704–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avdic V, Zhang P, Lanouette S, Voronova A, Skerjanc I and Couture JF (2011) Fine-tuning the stimulation of MLL1 methyltransferase activity by a histone H3-based peptide mimetic. FASEB J. 25, 960–967 [DOI] [PubMed] [Google Scholar]
- 46.Iberg AN, Espejo A, Cheng D, Kim D, Michaud-Levesque J, Richard S and Bedford MT (2008) Arginine methylation of the histone H3 tail impedes effector binding. J. Biol. Chem 283, 3006–3010 [DOI] [PubMed] [Google Scholar]
- 47.Ge Z, Song EJ, Kawasawa YI, Li J, Dovat S and Song C (2016) WDR5 high expression and its effect on tumorigenesis in leukemia. Oncotarget. 7, 37740–37754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun W, Guo F and Liu M (2018) Up-regulated WDR5 promotes gastric cancer formation by induced cyclin D1 expression. J. Cell. Biochem 119, 3304–3316 [DOI] [PubMed] [Google Scholar]
- 49.Wang F, Zhang J, Ke X, Peng W, Zhao G, Peng S, Xu J, Xu B and Cui H (2020) WDR5-Myc axis promotes the progression of glioblastoma and neuroblastoma by transcriptional activating CARM1. Biochem. Biophys. Res. Commun 523, 699–706 [DOI] [PubMed] [Google Scholar]
- 50.Karatas H, Townsend EC, Bernard D, Dou Y and Wang S (2010) Analysis of the binding of mixed lineage leukemia 1 (MLL1) and histone 3 peptides to WD repeat domain 5 (WDR5) for the design of inhibitors of the MLL1-WDR5 interaction. J. Med. Chem 53, 5179–5185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karatas H, Townsend EC, Cao F, Chen Y, Bernard D, Liu L, Lei M, Dou Y and Wang S (2013) High-affinity, small-molecule peptidomimetic inhibitors of MLL1/WDR5 protein-protein interaction. J. Am. Chem. Soc 135, 669–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Senisterra G, Wu H, Allali-Hassani A, Wasney GA, Barsyte-Lovejoy D, Dombrovski L, Dong A, Nguyen KT, Smil D, Bolshan Y, Hajian T, He H, Seitova A, Chau I, Li F, Poda G, Couture JF, Brown PJ, Al-Awar R, Schapira M, Arrowsmith CH and Vedadi M (2013) Small-molecule inhibition of MLL activity by disruption of its interaction with WDR5. Biochem. J 449, 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou H, Liu L, Huang J, Bernard D, Karatas H, Navarro A, Lei M and Wang S (2013) Structure-based design of high-affinity macrocyclic peptidomimetics to block the menin-mixed lineage leukemia 1 (MLL1) protein-protein interaction. J. Med. Chem 56, 1113–1123 [DOI] [PubMed] [Google Scholar]
- 54.Cao F, Townsend EC, Karatas H, Xu J, Li L, Lee S, Liu L, Chen Y, Ouillette P, Zhu J, Hess JL, Atadja P, Lei M, Qin ZS, Malek S, Wang S and Dou Y (2014) Targeting MLL1 H3K4 methyltransferase activity in mixed-lineage leukemia. Mol. Cell 53, 247–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karatas H, Li Y, Liu L, Ji J, Lee S, Chen Y, Yang J, Huang L, Bernard D, Xu J, Townsend EC, Cao F, Ran X, Li X, Wen B, Sun D, Stuckey JA, Lei M, Dou Y and Wang S (2017) Discovery of a Highly Potent, Cell-Permeable Macrocyclic Peptidomimetic (MM-589) Targeting the WD Repeat Domain 5 Protein (WDR5)-Mixed Lineage Leukemia (MLL) Protein-Protein Interaction. J. Med. Chem 60, 4818–4839 [DOI] [PubMed] [Google Scholar]
- 56.Wang F, Jeon KO, Salovich JM, Macdonald JD, Alvarado J, Gogliotti RD, Phan J, Olejniczak ET, Sun Q, Wang S, Camper D, Yuh JP, Shaw JG, Sai J, Rossanese OW, Tansey WP, Stauffer SR and Fesik SW (2018) Discovery of Potent 2-Aryl-6,7-dihydro-5 H-pyrrolo[1,2- a]imidazoles as WDR5-WIN-Site Inhibitors Using Fragment-Based Methods and Structure-Based Design. J. Med. Chem 61, 5623–5642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aho ER, Wang J, Gogliotti RD, Howard GC, Phan J, Acharya P, Macdonald JD, Cheng K, Lorey SL, Lu B, Wenzel S, Foshage AM, Alvarado J, Wang F, Shaw JG, Zhao B, Weissmiller AM, Thomas LR, Vakoc CR, Hall MD, Hiebert SW, Liu Q, Stauffer SR, Fesik SW and Tansey WP (2019) Displacement of WDR5 from Chromatin by a WIN Site Inhibitor with Picomolar Affinity. Cell Rep. 26, 2916–2928.e2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dennis ML, Morrow BJ, Dolezal O, Cuzzupe AN, Stupple AE, Newman J, Bentley J, Hattarki M, Nuttall SD, Foitzik RC, Street IP, Stupple PA, Monahan BJ and Peat TS (2019) Fragment screening for a protein-protein interaction inhibitor to WDR5. Struct. Dyn 6, 064701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian J, Teuscher KB, Aho ER, Alvarado JR, Mills JJ, Meyers KM, Gogliotti RD, Han C, Macdonald JD, Sai J, Shaw JG, Sensintaffar JL, Zhao B, Rietz TA, Thomas LR, Payne WG, Moore WJ, Stott GM, Kondo J, Inoue M, Coffey RJ, Tansey WP, Stauffer SR, Lee T and Fesik SW (2020) Discovery and Structure-Based Optimization of Potent and Selective WD Repeat Domain 5 (WDR5) Inhibitors Containing a Dihydroisoquinolinone Bicyclic Core. J. Med. Chem 63, 656–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bolshan Y, Getlik M, Kuznetsova E, Wasney GA, Hajian T, Poda G, Nguyen KT, Wu H, Dombrovski L, Dong A, Senisterra G, Schapira M, Arrowsmith CH, Brown PJ, Al-Awar R, Vedadi M and Smil D (2013) Synthesis, Optimization, and Evaluation of Novel Small Molecules as Antagonists of WDR5-MLL Interaction. ACS Med. Chem. Lett 4, 353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Getlik M, Smil D, Zepeda-Velázquez C, Bolshan Y, Poda G, Wu H, Dong A, Kuznetsova E, Marcellus R, Senisterra G, Dombrovski L, Hajian T, Kiyota T, Schapira M, Arrowsmith CH, Brown PJ, Vedadi M and Al-Awar R (2016) Structure-Based Optimization of a Small Molecule Antagonist of the Interaction Between WD Repeat-Containing Protein 5 (WDR5) and Mixed-Lineage Leukemia 1 (MLL1). J. Med. Chem 59, 2478–2496 [DOI] [PubMed] [Google Scholar]
- 62.Schapira M and Arrowsmith CH (2016) Methyltransferase inhibitors for modulation of the epigenome and beyond. Curr. Opin. Chem .Biol 33, 81–87 [DOI] [PubMed] [Google Scholar]
- 63.Schapira M, Tyers M, Torrent M and Arrowsmith CH (2017) WD40 repeat domain proteins: a novel target class? Nat. Rev. Drug Discov 16, 773–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta A, Xu J, Lee S, Tsai ST, Zhou B, Kurosawa K, Werner MS, Koide A, Ruthenburg AJ, Dou Y and Koide S (2018) Facile target validation in an animal model with intracellularly expressed monobodies. Nat. Chem. Biol 14, 895–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang P, Lee H, Brunzelle JS and Couture JF (2012) The plasticity of WDR5 peptide-binding cleft enables the binding of the SET1 family of histone methyltransferases. Nucleic Acids Res. 40, 4237–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu XH, Chen RC, Gao Y and Wu YD (2010) The effect of Asp-His-Ser/Thr-Trp tetrad on the thermostability of WD40-repeat proteins. Biochemistry. 49, 10237–10245 [DOI] [PubMed] [Google Scholar]
- 67.Xu C and Min J (2011) Structure and function of WD40 domain proteins. Protein & cell. 2, 202–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Denesyuk A, Denessiouk K and Johnson MS (2018) Top surface blade residues and the central channel water molecules are conserved in every repeat of the integrin-like β-propeller structures. J. Struct. Biol 201, 155–161 [DOI] [PubMed] [Google Scholar]
- 69.Shinsky SA, Hu M, Vought VE, Ng SB, Bamshad MJ, Shendure J and Cosgrove MS (2014) A non-active-site SET domain surface crucial for the interaction of MLL1 and the RbBP5/Ash2L heterodimer within MLL family core complexes. J. Mol. Biol 426, 2283–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shinsky SA and Cosgrove MS (2015) Unique Role of the WD-40 Repeat Protein 5 (WDR5) Subunit within the Mixed Lineage Leukemia 3 (MLL3) Histone Methyltransferase Complex. J. Biol. Chem 290, 25819–25833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weeramange CJ, Fairlamb MS, Singh D, Fenton AW and Swint-Kruse L (2020) The strengths and limitations of using biolayer interferometry to monitor equilibrium titrations of biomolecules. Protein Sci. 29, 1018–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Concepcion J, Witte K, Wartchow C, Choo S, Yao D, Persson H, Wei J, Li P, Heidecker B, Ma W, Varma R, Zhao LS, Perillat D, Carricato G, Recknor M, Du K, Ho H, Ellis T, Gamez J, Howes M, Phi-Wilson J, Lockard S, Zuk R and Tan H (2009) Label-free detection of biomolecular interactions using BioLayer interferometry for kinetic characterization. Comb. Chem. High Throughput Screen 12, 791–800 [DOI] [PubMed] [Google Scholar]
- 73.Shah NB and Duncan TM (2014) Bio-layer interferometry for measuring kinetics of protein-protein interactions and allosteric ligand effects. J. Vis. Exp, e51383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Movileanu L, Cheley S, Howorka S, Braha O and Bayley H (2001) Location of a Constriction in the Lumen of a Transmembrane Pore by Targeted Covalent Attachment of Polymer Molecules. J. Gen. Physiol 117, 239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolfe AJ, Gugel JF, Chen M and Movileanu L (2018) Kinetics of Membrane Protein-Detergent Interactions Depend on Protein Electrostatics. J. Phys. Chem. B 122, 9471–9481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jarmoskaite I, AlSadhan I, Vaidyanathan PP and Herschlag D (2020) How to measure and evaluate binding affinities. eLife. 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boozer C, Kim G, Cong S, Guan H and Londergan T (2006) Looking towards label-free biomolecular interaction analysis in a high-throughput format: a review of new surface plasmon resonance technologies. Curr. Opin. Biotechnol 17, 400–405 [DOI] [PubMed] [Google Scholar]
- 78.Masson JF (2017) Surface Plasmon Resonance Clinical Biosensors for Medical Diagnostics. ACS Sens. 2, 16–30 [DOI] [PubMed] [Google Scholar]
- 79.Drescher DG, Selvakumar D and Drescher MJ (2018) Analysis of Protein Interactions by Surface Plasmon Resonance. Adv. Protein Chem. Struct. Biol 110, 1–30 [DOI] [PubMed] [Google Scholar]
- 80.Wolfe AJ, Si W, Zhang Z, Blanden AR, Hsueh YC, Gugel JF, Pham B, Chen M, Loh SN, Rozovsky S, Aksimentiev A and Movileanu L (2017) Quantification of membrane protein-detergent complex interactions. J. Phys. Chem. B 121, 10228–10241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolfe AJ, Hsueh YC, Blanden AR, Mohammad MM, Pham B, Thakur AK, Loh SN, Chen M and Movileanu L (2017) Interrogating Detergent Desolvation of Nanopore-Forming Proteins by Fluorescence Polarization Spectroscopy. Analytical chemistry. 89, 8013–8020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rossi AM and Taylor CW (2011) Analysis of protein-ligand interactions by fluorescence polarization. Nat. Protoc 6, 365–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolfe AJ, Gugel JF, Chen M and Movileanu L (2018) Detergent Desorption of Membrane Proteins Exhibits Two Kinetic Phases. J. Phys. Chem. Lett . 9, 1913–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tolkatchev D, Xu P and Ni F (2003) Probing the kinetic landscape of transient peptide-protein interactions by use of peptide (15)n NMR relaxation dispersion spectroscopy: binding of an antithrombin peptide to human prothrombin. J. Am. Chem. Soc 125, 12432–12442 [DOI] [PubMed] [Google Scholar]
- 85.Gianni S, Engström A, Larsson M, Calosci N, Malatesta F, Eklund L, Ngang CC, Travaglini-Allocatelli C and Jemth P (2005) The kinetics of PDZ domain-ligand interactions and implications for the binding mechanism. J. Biol. Chem 280, 34805–34812 [DOI] [PubMed] [Google Scholar]
- 86.Eildal JN, Hultqvist G, Balle T, Stuhr-Hansen N, Padrah S, Gianni S, Strömgaard K and Jemth P (2013) Probing the role of backbone hydrogen bonds in protein-peptide interactions by amide-to-ester mutations. J. Am. Chem. Soc 135, 12998–13007 [DOI] [PubMed] [Google Scholar]
- 87.Paul F, Wehmeyer C, Abualrous ET, Wu H, Crabtree MD, Schöneberg J, Clarke J, Freund C, Weikl TR and Noé F (2017) Protein-peptide association kinetics beyond the seconds timescale from atomistic simulations. Nat. Commun 8, 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stadmiller SS, Aguilar JS, Parnham S and Pielak GJ (2020) Protein-Peptide Binding Energetics under Crowded Conditions. J. Phys. Chem. B 124, 9297–9309 [DOI] [PubMed] [Google Scholar]
- 89.Mohammad MM and Movileanu L (2008) Excursion of a Single Polypeptide into a Protein Pore: Simple Physics, but Complicated Biology. Eur. Biophys. J 37, 913–925 [DOI] [PubMed] [Google Scholar]
- 90.Bikwemu R, Wolfe AJ, Xing X and Movileanu L (2010) Facilitated Translocation of Polypeptides Through a Single Nanopore. J. Phys.: Condens. Matter 22, 454117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qin S, Pang X and Zhou HX (2011) Automated prediction of protein association rate constants. Structure. 19, 1744–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alsallaq R and Zhou HX (2008) Electrostatic rate enhancement and transient complex of protein-protein association. Proteins. 71, 320–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qin S and Zhou HX (2013) PI(2)PE: A Suite of Web Servers for Predictions Ranging From Protein Structure to Binding Kinetics. Biophys. Rev 5, 41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou HX (2010) Rate theories for biologists. Q. Rev. Biophys 43, 219–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pang X and Zhou HX (2017) Rate Constants and Mechanisms of Protein-Ligand Binding. Annu. Rev. Biophys 46, 105–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supporting data, materials, and sequence information are included within the main article and its supplementary file.