Highlights
-
•
Studies have shown that AR-V7 may be correlated with the poor prognosis of castration resistant prostate cancer (CRPC), however, clinicopathological characteristics of AR-V7 have not been fully elucidated.
-
•
We enrolled 24 studies with 2307 eligible patients for a systemic review and meta-analysis.
-
•
AR-V7 positivity was associated with higher Gleason score, bone or any site metastasis, presence of pain and worse ECOG performance score in CRPC.
-
•
Therefore, AR-V7 positivity may be a particular type of prostate cancer subtype in CRPC.
Keywords: Androgen receptor splicing variant 7, Clinicopathological characteristics, Castration resistance prostate cancer, TNM stage, Meta – analysis
Abbreviations: AR-V7, androgen receptor splicing variant 7; CRPC, castration resistance prostate cancer; mCRPC, metastatic castration-resistant prostate cancer; AR, androgen receptor; PRISMA, preferred reporting items for systematic reviews and meta-analyses; T, tumor; N, node; M, metastasis; ECGO, Eastern Cooperative Oncology Group; HSPC, Hormone sensitive prostate cancer; PSA, Prostate specific antigen; OR, odds radio
Abstract
Background
Studies have shown that AR-V7 may be correlated with the poor prognosis of castration resistant prostate cancer (CRPC), however, clinicopathological characteristics of AR-V7 have not been fully elucidated.
Objective
This study aimed at evaluating the clinicopathological features of AR-V7 in CRPC patients.
Materials and methods
To evaluate the clinicopathological features of AR-V7 in CRPC patients. A search of PubMed, Embase, and Web of Science was performed using the keywords prostate cancer, prostate tumor, prostate neoplasm, prostate carcinoma, AR-V7, AR3, androgen receptor splicing variant-7, or androgen receptor-3. Twenty-four trials published by February 2020 were included in this study.
Results
The proportion of Gleason score ≥ 8 was found to be significantly higher in AR-V7-positive CRPC (69.5%) than negative (54.9%) (OR 1.68, 95% CI 1.25–2.25, p < 0.001), while the rates of T3/T4 stage (OR 1.16, 95% CI 0.60–2.24, p = 0.65) and N1 stage (OR 0.99, 95% CI 0.65–1.51, p = 0.96) were not statistically correlated with AR-V7 status. The AR-V7-positive patients exhibited a significantly higher proportion of any site metastasis (61.3% versus 35.0%; OR 2.19, 95% CI 1.57–3.05, p < 0.001) and bone metastasis (81.7% versus 69.0%; OR 1.97, 95% CI 1.44–2.69, p < 0.001), and a trend close to significance was expected in visceral metastasis (28.8% versus 22.1%; OR 1.29, 95% CI 0.96–1.74, p = 0.09). Incidences of pain in AR-V7-positive CRPC (54.6%) were significantly higher than in negative CRPC (28.1%; OR 4.23, 95% CI 2.52–7.10, p < 0.001), line with worse ECOG performance status (56.7% versus 35.0%, OR 2.18, 95% CI 1.51–3.16, P < 0.001). Limitations of the study include differences in sample sizes and designs, AR-V7 detection assays, as well as disease characteristics of the included studies.
Conclusions
AR-V7 positivity is associated with a higher Gleason score, bone or any site metastasis, pain and worse ECOG performance scores in CRPC. However, it is not correlated with tumor stage or lymph node metastasis. More studies are needed to confirm these findings.
Introduction
According to a recent study published in 2019, prostate cancer is the most common cancer among American men [1]. Unfortunately, most cases of prostate cancer eventually progress to metastatic castration-resistant prostate cancer (mCRPC) [2,3]. There is a need to identify predictive biomarkers for worse prognosis and to develop precise therapeutic options. The androgen receptor (AR) signaling pathway is the primary therapeutic target of prostate cancer. The AR axis is the major driver for tumor progression [4,5]. Blocking AR [6,7] or inhibiting ligand production [8,9] can suppress AR signaling and prolong the survival of men with mCRPC. The appearance of Androgen receptor variants (AR-Vs), spliced isoforms of the AR and encoded truncated AR proteins lack the C-terminal ligand-binding domain but retain the trans-activating N-terminal domain, which may lead to AR signal based therapeutic resistance [10,11].
The AR variant 7 (AR-V7, also known as AR3), one of the most abundant AR-Vs in CRPC, are associated with prostate cancer aggressiveness, castration-resistant prostate cancer (CRPC) development [4,12] and primary resistance to Enzalutamide and Abiraterone therapy in men with CRPC [13], [14], [15]. Despite being poor at binding the ligand, AR-V7 is constitutively active in a ligand-independent manner and is capable of driving CRPC growth [12,16]. Therefore, AR-V7 may inform therapeutic decisions in CRPC patients and serve as a predictive biomarker [17,18].
Although the prognostic value of AR-V7 in CRPC has been reported, clinicopathological characteristics of AR-V7 expression have not been clearly elucidated [13,14,[19], [20], [21]]. Some studies have reported that AR-V7 positivity is associated with clinicopathological characteristics, in contrast with findings of other studies [13,[21], [22], [23]]. To the best of our knowledge, there is no systematic review of this topic. This study aimed at determining the correlation between AR-V7 expression and clinicopathological features, including Gleason score, tumor stage, node stage, metastasis status, pain and ECOG performance status score in CRPC. 24 studies were enrolled to evaluate the clinicopathological significance of AR-V7 expression in CRPC patients.
Materials and methods
Search strategy
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [24]. Studies published between February 2009 and before February 2020 were retrieved. The published studies were retrieved from Embase, PubMed, and the Web of Science. The key search terms were: prostate cancer OR prostate neoplasm OR prostate tumor OR prostate carcinoma; AR-V7 OR AR3 OR androgen receptor splicing variant 7 OR androgen receptor 3. References cited in the selected articles were also referred to identify applicable studies. Two or three reviewers independently evaluated each study to determine whether they met the predefined inclusion criteria. Differences and disagreements in the studies were solved by discussion.
Selection criteria
Titles and abstracts of the studies were primarily screened, and full papers were further vetted to confirm eligibility. For eligibility, the following criteria were used: i. Studies on prostate cancer and AR-V7; ii. Published results included AR-V7 positive and patients’ clinicopathological characteristics in castrated refractory prostate cancer (CRPC) including Gleason score, tumor (T) stage, node (N) stage, metastasis (M) status, pain and/or Eastern Cooperative Oncology Group (ECOG) performance status score; iii. The studies were to have been clinical trials, including prospective series or retrospective cohort studies or comparative series or case-series studies. The exclusion criteria were: i. Studies that reported only on the AR-V7-positive proportion in hormone sensitive prostate cancer (HSPC) or other prostate neoplasms; ii. Studies that did not report on any clinicopathological features; iii. In vitro or animal studies; iv. Studies reported in other languages other than English, unless there were translations; v. Case letters, reports, editorials, comments, and review papers. When more than one report of the same trial was available, the most recent report with longer follow-ups and a larger patient population was included.
Data collection and study quality
i. The extracted patient features included age, Gleason score, tumor stage, node stage, metastasis status, presence of pain, ECOG performance status score, baseline prostate specific antigen (PSA) and alkaline phosphatase levels. ii. Descriptions of the study designs, countries in which the trials were performed, detection assays of AR-V7, numbers of patients enrolled, treatment administered, and the median follow-up time. iii. The relationship between AR-V7 status and patients’ clinicopathological features. iv. The number and percentage of patients with different AR-V7 status in various groups of clinicopathological characteristics. The above characteristics were manually extracted from each paper by WZZ and checked by either of LQC, YJH, or SHX. The authors of publications found in our search were contacted to provide further data where necessary, and to check that data extraction was correct.
Statistical methods
After data were extracted, comparisons were performed using Review Manager Software (RevMan v.5.3; The Nordic Cochrane Center, Copenhagen, Denmark). Proportions of patients with different AR-V7 status in various groups of clinicopathological characteristics were evaluated. For analysis, patients were grouped based on their AR-V7 status after which their CRPC Gleason scores, tumor stages, node stages, metastasis status, presence of pain and ECOG performance status score, were compared with OR (95% CI) as the summary measure. Statistical heterogeneities among studies were evaluated using the chi-square test and the I2 statistic; a higher I2 value indicates higher between study heterogeneity. For significant heterogeneous studies (I2 ≥ 50%), a random effects model was adopted. Publication bias was evaluated and small-study effects were assessed by a funnel plot (supplement Figs. 1–4B). Odds ratio (OR) estimates were weighted and merged using the Mantel–Hansel random effects model. All statistical tests were two-sided and p ≤ 0.05 was defined as statistically significant. No correction was made for multiple comparisons.
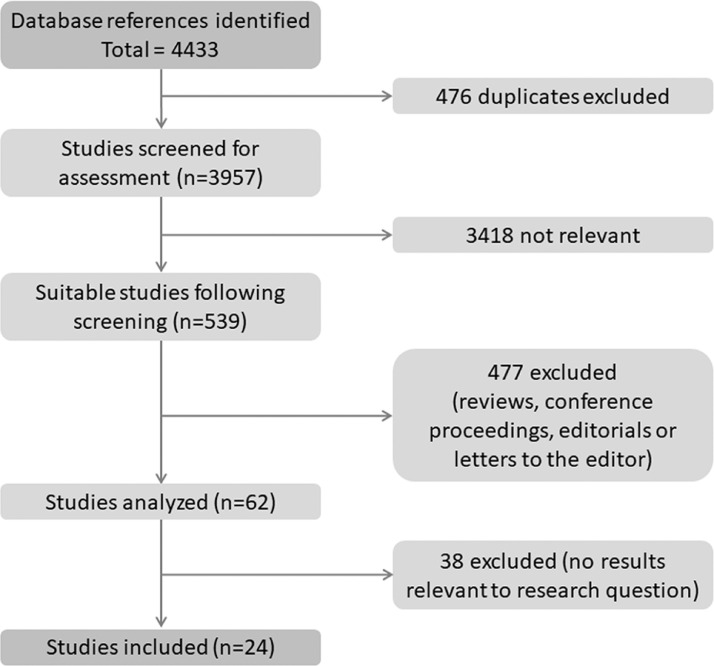
Fig. 1.
Schematic presentation of the study selection process.
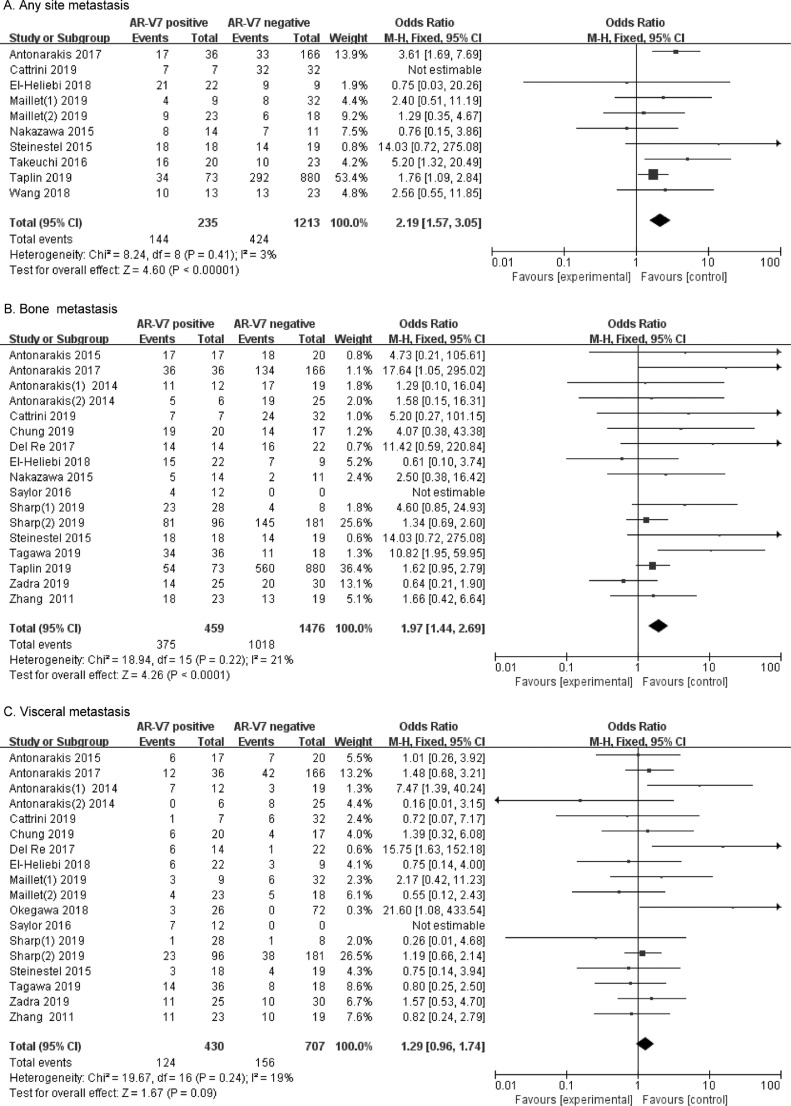
Fig. 4.
Forest plots of CRPC metastasis in AR-V7-positive and negative groups. (A) Rates of any site metastasis in different AR-V7 status CRPC. (B) Rates of bone metastasis in different AR-V7 status CRPC. (C) Rates of visceral metastasis in different AR-V7 status CRPC. Bars indicate 95% CIs. AR-V7 = androgen receptor splicing variant 7; CI = confidence interval; OR = odds ratio.
Results
Characteristics and qualities of the included studies
The enrollment process for this study was done as shown in Fig. 1. Results of the search were updated in February 2020, and 4409 of the 4433 full text published papers were excluded. The excluded studies were: 476 repeated studies; 3418 studies that were irrelevant to the research question; 477 studies such as conference abstracts, reviews, letters, and editorials that were unable pass the quality assessment test; and 38 studies that reported irrelevant results. There were no additional studies that were included from the reference lists. A total of 24 studies were selected in the present meta-analysis.
Patient characteristics
A total of twenty-four studies involving 2307 patients were included in the analysis of clinicopathological features of AR-V7-positivity CRPC (supplementary Table 1). Target samples and detection assays for AR-V7 are presented in Table 1. Sixteen studies involving 1699 patients were included in the Gleason score meta-analysis; six studies involving 169 patients were included in T stage analysis; ten trials involving 587 patients were included in N stage analysis while eighteen trials involving 1935 patients were included in the metastasis analysis. A total of 418 patients from five studies were used in the analysis of the presence of pain while seventeen trials involving 2047 patients were included in the ECOG performance status meta-analysis.
Table 1.
Characteristics of studies included in the clinicopathological features of different AR-V7 status CRPC.
| Study | Year | Country | Study design | AR-V7 detection assay | Patients characteristics |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Patients (n) | Age (range) |
Gleason score (%) |
Tumor stage at diagnosis (%) | Baseline PSA (ng/ml) median | Baseline alkaline phosphatase (U/L) | Follow-up time(month) Median[] |
|||||
| Antonarakis [14] | 2017 | USA | prospective | CTC mRNA | ABT or ENZ | 53 CTC- | 70 | ≥8 (68.0%) | – | 13.7 | 80 | 28.7 |
| 113CTC+/ AR-V7- | 71 | ≥8 (60.0%) | – | 31.4 | 96 | 29.5 | ||||||
| 36 CTC+/ AR-V7+ | 70 | ≥8 (83.0%) | – | 92.0 | 120 | 11.2 | ||||||
| Del Re [44] | 2017 | Italy | prospective | Exosomal RNA | ENZ or ABT | 36 | 66 (51–81) |
≤7 (44%) ≥8 (53%) |
T1/T2 (8.0%) T3/T4 (36.0%) |
26.3 (0.63–4581) |
180 (49–917) |
9 (2.0–31.0) |
| Takeuchi T [45]. | 2016 | Japan | cohort study | Whole blood mRNA |
ENZ or ABT | 43 | 73 (59–88) |
≤7 (20.9%) ≥8 (72.1%) |
130 (5.3–9529) | |||
| Lee [46] | 2016 | Korea | retrospective | IHC | ADT | 3 | 70 (56–70) |
≥8 (100%) | T3/T4 (100%) | 8.78 (8.6–173.7) |
12 (11–33) |
|
| Sharp [38] | 2019 | USA | prospective | IHC | ENZ or ABT | 28 AR-V7+ | 69.0 (SD=7.5) | M1a (14%) M1b (82%) M1c (4%) |
87.5 (35.5–272.5) | 90.0 (63.5–176.8) | 25.2(20.9–46.2) | |
| 8 AR-V7- | 63.0 (SD=4.8) | M1a (3%) M1b (50%) M1c (4%) |
154.0 (8.9–238.3) | 90.0 (80.3–170.5) | 74.3(36.9-NA) | |||||||
| Wang [47] | 2018 | China | retrospective | CTC mRNA | ENZ or ABT | 36 | 56.2 (SD=8.6) | M0 (36%) M1 (64%) |
||||
| Tagawa [20] | 2019 | USA | prospective | CTC mRNA | Docetaxel or Cabazitaxel | 54 | 71 (53–84) | ≤6 (13.7%) 7 (25.5%) ≥8 (60.8%) |
N1 (51.9%) M1b (90.7%) M1c (40.7%) |
92.1 (2.4–1558) |
217.8 (SD=260.35) | |
| Antonarakis [13] | 2014 | USA | prospective | CTC mRNA | ABT | 31 | 69 (48–79) |
≤7 (26.7%) ≥8 (73.3%) |
T1/T2 (26.7%) T3/T4 (61.3%) |
37.8 (2.2–2045.0) |
118 (59–1348) |
4.6 (0.9–8.2) |
| ENZ | 31 | 70 (56–84) |
≤7 (40%) ≥8 (60%) |
T1/T2 (54.8%) T3/T4 (45.2%) |
44.3 (4.3–3204.2) |
108 (58–872) |
5.4 (1.4–9.9) |
|||||
| Steinestel [34] | 2015 | Germany | prospective | CTC mRNA | ENZ or ABT | 24 | 75 (53–87) |
≤7 (41.3%) ≥8 (58.7%) |
– | 96.5 (0.1–4282) |
– | – |
| Nakazawa [22] | 2015 | USA | prospective | CTC mRNA | NHT or chemotherapy | 14 | 65 (50–82) |
≤7 (92.9%) ≥8 (0%) |
58.7 (2.2–895) | 127 (52–838) |
11 (6–18) |
|
| Antonarakis [31] | 2015 | USA | prospective | CTC mRNA | Docetaxel or cabazitaxel | 37 | 67 (46–82) |
≤7 (17%) ≥8 (83%) |
T1/T2 (38.0%) T3/T4 (62.0%) |
126 (0.1–2270) |
161 (53–1243) |
7.7 (0.7–19.0) |
| Onstenk [32] | 2015 | Netherlands | prospective | CTC mRNA | Cabazitaxel | 29 | 70 (SD±7) |
– | – | 321 IQR (76–649) | 163 (106–375) |
7 (2–27) |
| Zhang [48] | 2011 | USA | retrospective | IHC | ADT | 42 | 63 (42–93) |
413.2 (0.15–7402) |
19.5 (1–92) |
|||
| Saylor [49] | 2016 | USA | retrospective | RNA ISH | ABT or ENZ | 12 | ||||||
| Zadra [23] | 2019 | USA | retrospective | Immune-fluorescence | ABT or ENZ | 55 | 55 | |||||
| Belderbos [43] | 2019 | Netherlands | prospective | CTC mRNA | ENZ, ABT or Cabazitaxel | 94 | 69 IQR (65–75) | 186 IQR (67–356) | ||||
| Cattrini [50] | 2019 | Italy | prospective | CTC mRNA | ENZ,ABT or Docetaxel | 39 | 72 (56–84) |
M1b (79.5%) M1c (17.9%) |
35.2 (0.33–4688) | |||
| Taplin [51] | 2019 | USA | prospective | CTC mRNA | Galeterone or ENZ | 953 | 72 (62–77) |
≤7 (43%) ≥8 (57%) |
M0 (58%) M1 (42%) |
15.5 IQR (8.98–31.70) |
50.04 IQR (25.56–88.08) |
|
| Sharp [52] | 2019 | UK/USA | prospective | CTC mRNA /IHC |
ENZ, ABT or Taxane | 95 CTC- | 71.0 IQR (66.8–75.6) | M1b (74.7%) M1c (17.9%) |
110.0 IQR (29–300.5) |
83.0 IQR (66.0–163.0) | ||
| 86 CTC+ ARV7- |
69.6 IQR (64.9–72.3) | M1b (86.1%) M1c (24.4%) |
147.0 IQR (51.0–345) |
111.5 IQR (76.3–200.5) |
||||||||
| 96 CTC+ ARV+ |
70.4 IQR (65.3–74.6) | M1b (84.4%) M1c (24.0%) |
244.5 IQR (109.3–746.8) |
180.0 IQR (93.8–346.0) |
||||||||
| Maillet [53] | 2019 | France | prospective | CTC mRNA | ENZ or ABT | 41 | 73 | ≥8 (56%) | M1 (29%) | 35 | 10.5 95%CI (8.7–13.7) | |
| Okegawa [54] | 2018 | Japan | retrospective | CTC mRNA | ENZ or ABT | 49 CTC− | 69 | ≥8 (81.6%) | 75.7 | 317 | ||
| 23 CTC+ AR-V7− |
71 | ≥8 (91.3%) | 71.5 | 323 | 20.7 (3.0–37.0) |
|||||||
| 26 CTC+ AR-V7+ |
72 | ≥8 (96.2%) | 79.1 | 378 | ||||||||
| Chung [19] | 2019 | USA | prospective | CTC mRNA | ENZ or ABT | 37 | 72 (67–79) |
≤7 (43.2%) 8 (8.1%) ≥9 (46%) |
N1 (64.9%) M1b (89.2%) M1c (27%) |
20.9 IQR (11.6–96.8) | 102.0 IQR (80.5–170.5) | 11.43 IQR (4.73–21.3) |
| Sieuwerts [55] | 2019 | Netherlands | prospective | CTC mRNA | Cabazitaxel | 52 | 69 (SD=7) |
209 IQR (72–510) | 174 IQR (98–339) | |||
| El-Heliebi [21] | 2018 | Austria Germany Netherlands Sweden |
prospective | CTC FISH | ENZ, ABT or Taxane | 31 | 70.5 (42–83) |
≤7 (38.7%) ≥8 (51.6%) |
T1/T2 (22.6%) T3/T4 (45.2%) |
48.81 (0.8–4623) |
||
IQR=inter quartile range; SD= standard deviation; AR-V7 =androgen receptor splice variant 7; CTC = circulating tumor cell; PSA = prostate specific antigen; ADT=androgen deprivation therapy;NHT = novel hormonal therapy; LHRH= luteinizing hormone releasing hormone;PCa = prostate cancer; CRPC= castration resistance prostate cancer; ABT= Abiraterone; ENZ= Enzalutamide; IHC= Immunohistochemistry.
Gleason score
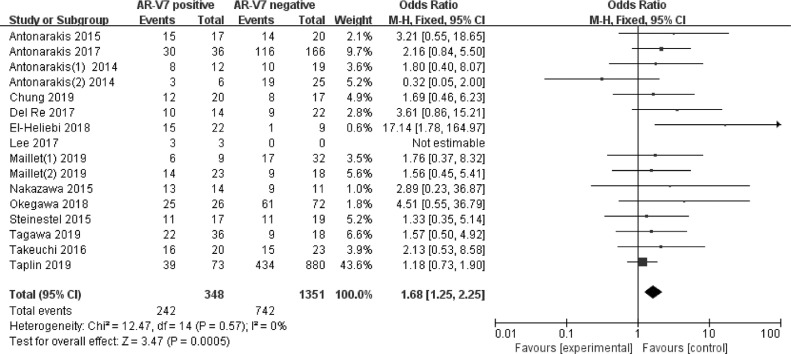
Gleason scores ≥8 between AR-V7-positive and negative CRPC groups were compared in sixteen studies involving 1699 patients. As shown in Fig. 2, 242 (69.5%) of the 348 AR-V7-positive men had Gleason scores ≥8, whereas 742 (54.9%) of 1351 AR-V7-negative men had Gleason scores ≥8. Gleason score was significantly higher in AR-V7-positive than in AR-V7-negative CRPC (OR 1.68, 95% CI 1.25–2.25, p < 0.001). Significant heterogeneity was not found among the studies (I2 = 0.0%, p = 0.57) and fixed-effects model was adopted (Supplementary Fig. 1).
Fig. 2.
Forest plots of the proportion of Gleason scores ≥8 between the group of AR-V7-positive and negative CRPC from sixteen studies. Bars indicate 95% CIs. AR-V7 = androgen receptor splicing variant 7; CI = confidence interval; OR = odds ratio.
T stage
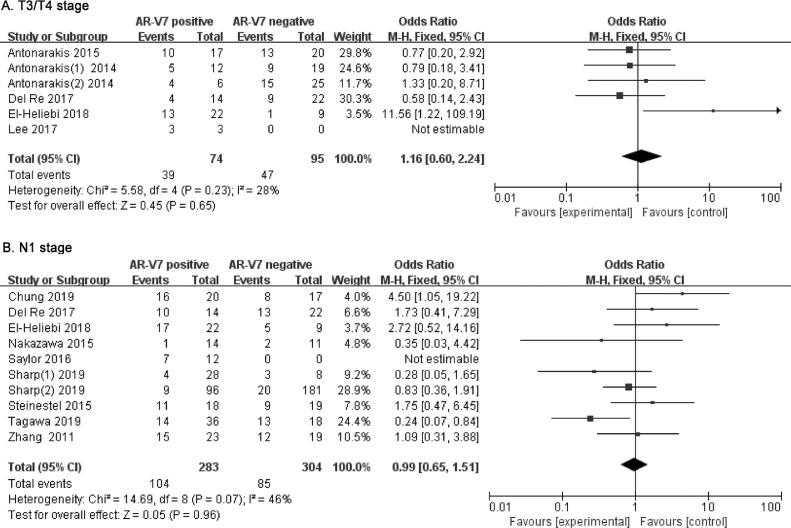
The rate of higher tumor stage (T3/T4) in different AR-V7 status CRPC was analyzed in six studies involving 169 patients. Fig. 3A shows that 39 (52.7%) of 74 AR-V7-positive men had higher T stages compared to 49.5% (47 of 95) of AR-V7-negative CRPC. There was no significant difference in T stage in CRPC, grouped by different AR-V7 status (OR 1.16, 95% CI 0.60–2.24, p = 0.65). There was no evidence for heterogeneity between the studies (I2=28%, p = 0.23) and the fixed-effects model was applied (Supplementary Fig. 2A).
Fig. 3.
Forest plots of CRPC T stage and N stage for AR-V7-positive and negative groups. (A) Ratio of T3/T4 stage in different AR-V7 status CRPC. (B) Ratio of N1 stage in different AR-V7 status CRPC. Bars indicate 95% CIs. AR-V7 = androgen receptor splicing variant 7; CI = confidence interval; OR = odds ratio.
N stage
Lymph node metastatic rate was evaluated in ten studies involving 587 CRPC patients, grouped by AR-V7-positivity or negativity. As shown in Fig. 3B, 104 (36.7%) of the 283 AR-V7-positive men had lymph node invasion compared to 28.0% (85 of 304) of AR-V7-negative CRPC men. There were no significant differences in N stages in CRPC of different AR-V7 status (OR 0.99, 95% CI 0.65–1.51, p = 0.96). Significant heterogeneity was not found (I2 = 46%, p = 0.07) and fixed-effects model was used (Supplementary Fig. 2B).
M stage
The rates of metastases were assessed in ten studies involving 1448 CRPC patients, grouped by different AR-V7 status. As shown in Fig. 4A, 144 (61.3%) of 235 AR-V7-positive men had metastases compared to 424 (35.0%) of 1213 AR-V7-negative CRPC patients. The rates of metastases in AR-V7-positive CRPC were significantly higher than those in AR-V7-negative (OR 2.19, 95% CI 1.57–3.05, p < 0.001). There being no heterogeneity between the studies (I2=3%, p = 0.41), fixed-effects model was applied to evaluate OR and 95% CI (Supplementary Fig. 3A).
We further compared the rates of bone metastases between the AR-V7-positive and negative CRPC groups in seventeen studies involving 1935 patients. Fig. 4B shows that 375 (81.7%) of 459 AR-V7-positive men had bone metastases compared to 1018 (69.0%) of 1476 AR-V7-negative men. Significantly higher rates of bone metastases were found in AR-V7-positive CRPC men (OR 1.97, 95% CI 1.44–2.69, p < 0.001). Significant heterogeneity was not found (I2=21%, p = 0.22) (Supplementary Fig. 3B), and a fixed effects model was performed to calculate the OR and 95% CI.
Visceral metastatic ratio was also evaluated in eighteen studies involving 1137 CRPC patients, grouped by different AR-V7 status. Fig. 4C shows that 124 (28.8%) of 430 AR-V7-positive men had visceral metastases compared to 156 (22.1%) of 707 AR-V7-negative men. There was no significant difference in visceral metastatic ratios for different AR-V7 status (OR 1.29, 95% CI 0.96–1.74, p = 0.09). No significant study heterogeneity was detected (I2 = 19%, p = 0.24) and fixed-effects model was adopted (Supplementary Fig. 3C).
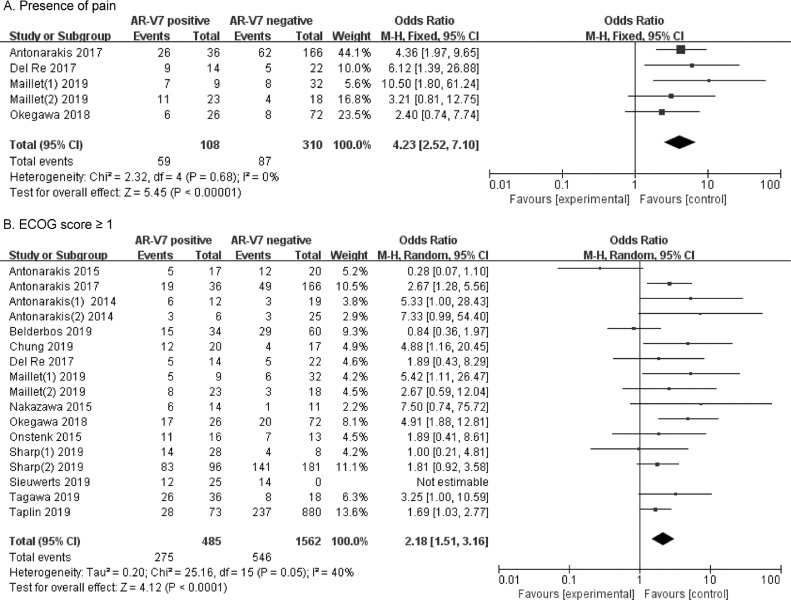
Presence of pain
The rates of pain occurrence were compared in five studies involving 418 CRPC patients, grouped by different AR-V7 status. As shown in Fig. 5A, 59 (54.6%) of 108 AR-V7-positive men suffered pain compared to 87 (28.1%) of 310 AR-V7-negative CRPC men. Significantly higher rates of pain were determined in AR-V7-positive CRPC (OR 4.23, 95% CI 2.52–7.10, p < 0.001). There being no heterogeneity between the studies (I2=0%, p = 0.68), fixed-effects model was used to calculate OR and 95% CI (Supplementary Fig. 4A).
Fig. 5.
Forest plots of performance status for AR-V7-positive and negative CRPC groups. (A) Rates of pain sufferance in different AR-V7 status CRPC. (B) Rates of ECOG score ≥ 1 in different AR-V7 status CRPC. Bars indicate 95% CIs. AR-V7 = androgen receptor splicing variant 7; CI = confidence interval; OR = odds ratio.
ECOG performance status score
A total of 17 studies involving 2047 CRPC patients were included in the comparisons of the relations between high ECOG scores (ECOG score ≥ 1) and AR-V7 status. Fig. 5B shows that 275 (56.7%) of 485 AR-V7-positive men had ECOG scores ≥ 1, compared to 546 (35.0%) of 1562 AR-V7-negative men. AR-V7-positive CRPC patients had significantly higher ECOG scores than AR-V7-negative patients (OR 2.18, 95% CI 1.51–3.16, p < 0.001). A random effects model was used to calculate OR and 95% CI because significant heterogeneity was found (I2=40%, p = 0.05) (Supplementary Fig. 4B).
Discussion
This study aimed at establishing whether AR-V7 positivity was associated with worse clinicopathological features. Biomarkers of clinical utility are important in optimizing treatment for patients. AR-V7 is the most common AR splice variant in advanced stage patients [25], [26], [27], [28], [29]. Studies have reported that AR-V7 is a novel AR splice variant that is capable of initiating and promoting CRPC progression [6,7,9]. Therefore, AR-V7 positive CRPC should be considered as a novel subtype of CRPC with specific clinicopathological characteristics and resistance to AR signal targeted therapy.
AR-V7 positivity is associated with unfavorable baseline characteristics and may reflect a larger neoplasm burden [20,30,][56]. We found that AR-V7 status is associated with clinicopathological characteristics for CRPC patients. However, an insignificant correlation between AR-V7-positive and higher Gleason scores has been reported [14], with some studies reporting that AR-V7-negative patients have higher Gleason scores [13]. We found that AR-V7 positive patients had significantly higher Gleason scores, inconsistent with previous findings [13].
AR-V7-positive men have been reported to exhibit higher T stages and lower N stages [20,21]. We found that the correlations between AR-V7 with T stage and lymph node metastasis were not significant.
Bone or any site metastasis, presence of pain and ECOG performance scores were compared in CRPC, respectively, with different AR-V7 status. A limited number of studies have reported on the clinicopathological features of AR-V7, which can be used to inform clinical decision and potential therapeutic targets of CRPC. As a specific treatment biomarker in men with mCRPC, expression of the AR-V7 protein in circulating tumor cells has been correlated with superior survival after taxane therapy in clinical practice [18]. Therefore, AR-V7 targeted therapy strategies are necessary and AR-V7 levels should be continuously monitored during treatment as they are correlated with faster disease progression in CRPC [31], [32], [33], [34]. Given the prognostic value and specific clinicopathological characteristics of AR-V7 in CRPC, AR-V7-positive CRPC should be taken as a novel subtype of prostate cancer that requires a more aggressive, personalized and AR-V7 targeted therapeutic strategy.
This systematic review has several advantages. First, this is the first meta-analysis of clinicopathological characteristics of AR-V7 in CRPC patients. Second, our study offers a scientific basis for individualized estimations of clinicopathological features of CRPC patients and identifies more aggressive CRPC patients. In this way, it informs precision medicine and individualized treatment for CRPC patients. In the near future, the challenge will be to correctly classify patients according to AR-V7 status within a suitable time for clinical treatment [35]. Additional clinical studies with larger sample sizes are need to validate our findings.
Since the discovery of AR-V7 in 2009 [27,36] to the discovery of clinical role of AR-V7 in 2013 [13], it can be used to predict CRPC resistance to enzalutamide and abiraterone. Recently, various studies on AR-V7 have been published. As a clinical biomarker, AR-V7 can be used to predict the prognosis of prostate cancer treatment. Therefore, AR-V7 positive prostate cancer can be used as a special prostate cancer subtype with unique clinical outcomes and treatment options. We performed a meta-analysis of studies published to date, and preliminarily found that AR-V7 positive prostate cancer is associated with Gleason scores, bone or any site metastasis, pain presence and ECOG performance status. This study informs precision medicine and individualized treatment of prostate cancer. The AR-V7 research in prostate cancer is at an early stage. Therapeutic options for patients are diverse, detection methods for AR-V7 are different, clinical samples for detecting AR-V7 are different, and the threshold for determining AR-V7 positivity has not yet been established. These outcomes have caused great differences in research designs of AR-V7 studies, leading to knowledge gaps between researchers and readers.
For researchers, the above-mentioned prerequisites should be fully considered when designing AR-V7 studies, and a scientifically designed plan as well as detailed description should be made, so that readers can better understand their research and establish a unified detection criteria for AR-V7 in future. Methods, interpretation of AR-V7 positivity and application standards provide scientific and traceable evidence.
Within the next 5 years, based on different detection methods of AR-V7, AR-V7 positivity and clinical application efficacies will be evaluated in multi-center prospective large samples. Findings from such studies with enhance AR-V7 positive prostate cancer awareness. Moreover, AR-V7, combined with other biomarkers (AR-V9, PCA3, PARP, etc.) can be used to accurately predict the prognosis of prostate cancer and promote individualized treatment.
This systematic review has some limitations. The sample size (ranging from 3 to 953 participants) is rather small, which limits its statistical power. Smaller sample sizes are less reliable and tend to have publication bias. Therefore, large-cohort clinical trials are needed to elucidate on the clinic-pathological features of CRPC patients. Second, designs of the various studies were not unified. Many studies were associated with an uncertain selection bias as they enrolled patients from a single center. Others studies were single or multicenter clinical trials, where enrolled patients might have been selected by different criteria. Third, only studies published in English were included in our meta-analysis. Fourth, included patients may be the same between prospective and retrospective studies. For example, Antonarakis et al. published 3 articles between 2014 and 2017 [13,14,31]. Although some patients in their studies were the same, the studies were performed at different times. The findings are valuable, but these repeated cases may lead to statistical biases. Fifth, cut-off values for positive and negative AR-V7 expression differed among various studies while the different detection assays and antibodies used in the included studies might impact on the sensitivity and specificity of AR-V7 positivity [37], [38], [39], [40]. Different assays, including qRT-PCR, immunohistochemistry (IHC), fluorescence in-situ hybridization and RNA in-situ hybridization (RISH) were used to measure AR-V7 expression levels. These detection methods have different advantages and disadvantages [41,42]. Therefore, positivity rates may vary across studies because of different cutoff values. Sensitivity and specificity of tissue-based detections are not optimal because of non-specific detections of nuclear AR-V7 pre-mRNA by RISH and non-specific binding reactions of the AR-V7 antibody. In addition, these studies used different clinical specimens, leading to bias. Expression levels of AR-V7 in blood cells may not truly reflect the expression levels of AR-V7 in tumor tissues or CTCs. Therefore, consensus on analytical methods and cut-off values are needed [43]. Large multicenter studies are capable of providing more precise and credible results. Moreover, clinicopathological features of CRPC patients, including T stage, N stage and M stage, clinical definition of PSA, alkaline phosphatase responses, and Gleason scores varied among different studies, which might be responsible for heterogeneities. We admitted that AR-V7 has not been extensively and sufficiently studied in CRPC patients, which may have led to controversial conclusions.
To overcome these drawbacks, first, we performed a comprehensive, systematic, and repeatable search strategy for the most relevant studies in multiple online databases, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Selection bias could not be eliminated, but was minimized by our strict screening for inclusion eligibility. Second, study designs, methods of AR-V7 detection, types of therapy, Gleason scores, tumor stages, node stages, metastasis status, presence of pain and ECOG performance status scores in CRPC, and follow-up period were tabulated for further analysis. Third, fixed or random effect models were used based on different heterogeneity for more authentic and credible results. Additionally, publication bias was evaluated and small-study effects were assessed by funnel plot (Supplementary Figs. 1–4B).
Conclusions
AR-V7 expression is significantly associated with Gleason scores, bone or any site metastasis, pain presence and ECOG performance status, but not statistically correlated with tumor stage or lymph node metastasis. Specific clinicopathological features of AR-V7-positive CRPC were associated with higher Gleason scores, more metastasis and pain presence, and worse ECOG performance status. Given the prognostic value of AR-V7 in resistance to Abiraterone and Enzalutamide treatment for CRPC patients, our results may guide clinicians in identifying patients with more aggressive cancers, and selecting the most active anti-tumor therapy. However, heterogeneities in sample sizes and study designs, assays for AR-V7 detection assessment, and cut-off value definition for positive versus negative expression were evident within the included studies. Cross-institutional large-cohort prospective studies are needed to confirm these findings. Moreover, the clinical utility of AR-V7 as a biomarker in CRPC should also be evaluated.
Funding support
There was no funding source for this review. All authors had full access to all the data and the corresponding author had final responsibility for the decision to submit for publication.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81972374 and 81772744).
Ethics statement
The studies involving human participants were reviewed and approved by First Affiliated Hospital of Zhejiang University.
Consent for publication
All authors have authorized the publication of this manuscript.
CRediT authorship contribution statement
Qinchen Li: Formal analysis, Writing - original draft. Zhize Wang: Project administration, Formal analysis, Writing - review & editing. Jiahe Yi: Data curation. Haixiang Shen: Data curtion. Zitong Yang: Investigation, Investigation. Libin Yan: Investigation. Liping Xie: Writing – review & editing.
Declaration of Competing Interest
Authors declare no conflicts of interest in this study.
Acknowledgment
We appreciate the technical support by laboratory staff of the Urology Laboratory of the First Affiliated Hospital of Zhejiang University. Thanks for the help and support of the Zhejiang University Cancer Center.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101145.
Contributor Information
Qinchen Li, Email: liqinchen@zju.edu.cn.
Zhize Wang, Email: wangzhize@zju.edu.cn.
Jiahe Yi, Email: 22018150@zju.edu.cn.
Haixiang Shen, Email: 21618129@zju.edu.cn.
Zitong Yang, Email: 21918408@zju.edu.cn.
Libin Yan, Email: chosenyan@126.com.
Liping Xie, Email: xielp@zju.edu.cn.
Appendix. Supplementary materials
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Ylitalo E.B., Thysell E., Thellenberg-Karlsson C., Lundholm M., Widmark A., Bergh A. Marked response to cabazitaxel in prostate cancer xenografts expressing androgen receptor variant 7 and reversion of acquired resistance by anti-androgens. Prostate. 2020;80(2):214–224. doi: 10.1002/pros.23935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paller C.J., Antonarakis E.S. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin. Adv. Hematol. Oncol. 2013;11(1):14–23. [PMC free article] [PubMed] [Google Scholar]
- 4.Erb H.H.H., Sparwasser P., Diehl T., Hemmerlein-Thomas M., Tsaur I., Jungel E. AR-V7 protein expression in circulating tumour cells is not predictive of treatment response in mCRPC. Urol. Int. 2020:1–10. doi: 10.1159/000504416. [DOI] [PubMed] [Google Scholar]
- 5.Galletti G., Leach B.I., Lam L., Tagawa S.T. Mechanisms of resistance to systemic therapy in metastatic castration-resistant prostate cancer. Cancer Treat. Rev. 2017;57:16–27. doi: 10.1016/j.ctrv.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Beer T.M., Armstrong A.J., Rathkopf D.E., Loriot Y., Sternberg C.N., Higano C.S. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scher H.I., Fizazi K., Saad F., Taplin M.E., Sternberg C.N., Miller K. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 8.Ryan C.J., Smith M.R., de Bono J.S., Molina A., Logothetis C.J., de Souza P. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bono J.S., Logothetis C.J., Molina A., Fizazi K., North S., Chu L. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu R., Lu C., Mostaghel E.A., Yegnasubramanian S., Gurel M., Tannahill C. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72(14):3457–3462. doi: 10.1158/0008-5472.can-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan S.C., Li Y., Dehm S.M. Androgen receptor splice variants activate androgen receptor target genes and support aberrant prostate cancer cell growth independent of canonical androgen receptor nuclear localization signal. J. Biol. Chem. 2012;287(23):19736–19749. doi: 10.1074/jbc.M112.352930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jimenez-Vacas J.M., Herrero-Aguayo V., Montero-Hidalgo A.J., Gomez-Gomez E., Fuentes-Fayos A.C., Leon-Gonzalez A.J. Dysregulation of the splicing machinery is directly associated to aggressiveness of prostate cancer. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonarakis E.S., Lu C., Wang H., Luber B., Nakazawa M., Roeser J.C. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014;371(11):1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonarakis E.S., Lu C., Luber B., Wang H., Chen Y., Zhu Y. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J. Clin. Oncol. 2017 doi: 10.1200/jco.2016.70.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scher H.I., Graf R.P., Schreiber N.A., Jayaram A., Winquist E., McLaughlin B. Assessment of the validity of nuclear-localized androgen receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration-resistant prostate cancer. JAMA Oncol. 2018 doi: 10.1001/jamaoncol.2018.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonarakis E.S., Armstrong A.J., Dehm S.M., Luo J. Androgen receptor variant-driven prostate cancer: clinical implications and therapeutic targeting. Prostate Cancer Prostatic Dis. 2016;19(3):231–241. doi: 10.1038/pcan.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H., Wang Z., Tang K., Zhou H., Liu H., Yan L. Prognostic value of androgen receptor splice variant 7 in the treatment of castration-resistant prostate cancer with next generation androgen receptor signal inhibition: a systematic review and meta-analysis. Eur. Urol. Focus. 2018;4(4):529–539. doi: 10.1016/j.euf.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Tendulkar R.D., Agrawal S., Gao T., Efstathiou J.A., Pisansky T.M., Michalski J.M. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J. Clin. Oncol. 2016;34(30):3648–3654. doi: 10.1200/JCO.2016.67.9647. [DOI] [PubMed] [Google Scholar]
- 19.Chung J.S., Wang Y., Henderson J., Singhal U., Qiao Y., Zaslavsky A.B. Circulating tumor cell-based molecular classifier for predicting resistance to abiraterone and enzalutamide in metastatic castration-resistant prostate cancer. Neoplasia. 2019;21(8):802–809. doi: 10.1016/j.neo.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tagawa S.T., Antonarakis E.S., Gjyrezi A., Galletti G., Kim S., Worroll D. Expression of AR-V7 and ARv(567es) in circulating tumor cells correlates with outcomes to taxane therapy in men with metastatic prostate cancer treated in TAXYNERGY. Clin. Cancer Res. 2019;25(6):1880–1888. doi: 10.1158/1078-0432.CCR-18-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Heliebi A., Hille C., Laxman N., Svedlund J., Haudum C., Ercan E. In situ detection and quantification of AR-V7, AR-FL, PSA, and KRAS point mutations in circulating tumor cells. Clin. Chem. 2018;64(3):536–546. doi: 10.1373/clinchem.2017.281295. [DOI] [PubMed] [Google Scholar]
- 22.Nakazawa M., Lu C., Chen Y., Paller C.J., Carducci M.A., Eisenberger M.A. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann. Oncol. 2015;26(9):1859–1865. doi: 10.1093/annonc/mdv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zadra G., Ribeiro C.F., Chetta P., Ho Y., Cacciatore S., Gao X. Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc. Natl. Acad. Sci. U. S. A. 2019;116(2):631–640. doi: 10.1073/pnas.1808834116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Z., Yang X., Sun F., Jiang R., Linn D.E., Chen H. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hörnberg E., Ylitalo E.B., Crnalic S., Antti H., Stattin P., Widmark A. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS ONE. 2011;6(4) doi: 10.1371/journal.pone.0019059. e19059-e19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu R., Dunn T.A., Wei S., Isharwal S., Veltri R.W., Humphreys E. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16–22. doi: 10.1158/0008-5472.can-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson D., Van Allen E.M., Wu Y.M., Schultz N., Lonigro R.J., Mosquera J.M. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun S., Sprenger C.C.T., Vessella R.L., Haugk K., Soriano K., Mostaghel E.A. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J. Clin. Invest. 2010;120(8):2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plymate S.R., Sharp A., de Bono J.S. Nuclear circulating tumor cell androgen receptor variant 7 in castration-resistant prostate cancer: the devil is in the detail. JAMA Oncol. 2018;4(9):1187–1188. doi: 10.1001/jamaoncol.2018.1615. [DOI] [PubMed] [Google Scholar]
- 31.Antonarakis E.S., Lu C., Luber B., Wang H., Chen Y., Nakazawa M. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1(5):582–591. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onstenk W., Sieuwerts A.M., Kraan J., Van M., Nieuweboer A.J.M., Mathijssen R.H.J. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of ar-v7 in circulating tumor cells. Eur. Urol. 2015;68(6):939–945. doi: 10.1016/j.eururo.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Scher H.I., Lu D., Schreiber N.A., Louw J., Graf R.P., Vargas H.A. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2016;2(11):1441–1449. doi: 10.1001/jamaoncol.2016.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinestel J., Luedeke M., Arndt A., Schnoeller T.J., Lennerz J.K., Wurm C. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget. 2015;10(41):4213–4223. doi: 10.18632/oncotarget.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sieuwerts A.M., Mostert B., van der Vlugt-Daane M., Kraan J., Beaufort C.M., Van M. An in-depth evaluation of the validity and logistics surrounding the testing of AR-V7 mRNA expression in circulating tumor cells. J. Mol. Diagn. 2018;20(3):316–325. doi: 10.1016/j.jmoldx.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Guo Z., Yang X., Sun F., Jiang R., Linn D.E., Chen H. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. DOI: Analytical Validation and Clinical Qualification of a New Immunohistochemical Assay for Androgen Receptor Splice Variant-7 Protein Expression in Metastatic Castration-resistant Prostate Cancer10.1158/0008-5472.can-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Y., Sharp A., Anderson C.M., Silberstein J.L., Taylor M., Lu C. Novel junction-specific and quantifiable in situ detection of AR-V7 and its clinical correlates in metastatic castration-resistant prostate cancer. Eur. Urol. 2018;73(5):727–735. doi: 10.1016/j.eururo.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharp A., Coleman I., Yuan W., Sprenger C., Dolling D., Rodrigues D.N. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J. Clin. Invest. 2019;129(1):192–208. doi: 10.1172/JCI122819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu F., Xie W., Nakabayashi M., Zhang H., Jeong S.H., Wang X. Association of AR-V7 and prostate-specific antigen rna levels in blood with efficacy of abiraterone acetate and enzalutamide treatment in men with prostate cancer. Clin. Cancer Res. 2017;23(3):726–734. doi: 10.1158/1078-0432.CCR-16-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Todenhofer T., Azad A., Stewart C., Gao J., Eigl B.J., Gleave M.E. AR-V7 transcripts in whole blood RNA of patients with metastatic castration resistant prostate cancer correlate with response to abiraterone acetate. J. Urol. 2017;197(1):135–142. doi: 10.1016/j.juro.2016.06.094. [DOI] [PubMed] [Google Scholar]
- 41.Luo J. Development of AR-V7 as a putative treatment selection marker for metastatic castration-resistant prostate cancer. Asian J. Androl. 2016;18(4):580–585. doi: 10.4103/1008-682X.178490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bastos D.A., Antonarakis E.S. CTC-derived AR-V7 detection as a prognostic and predictive biomarker in advanced prostate cancer. Expert Rev. Mol. Diagn. 2018;18(2):155–163. doi: 10.1080/14737159.2018.1427068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belderbos B.P.S., Sieuwerts A.M., Hoop E.O., Mostert B., Kraan J., Hamberg P. Associations between AR-V7 status in circulating tumour cells, circulating tumour cell count and survival in men with metastatic castration-resistant prostate cancer. Eur. J. Cancer. 2019;121:48–54. doi: 10.1016/j.ejca.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Del Re M., Biasco E., Crucitta S., Derosa L., Rofi E., Orlandini C. The detection of androgen receptor splice variant 7 in plasma-derived exosomal RNA strongly predicts resistance to hormonal therapy in metastatic prostate cancer patients. Eur. Urol. 2017;71(4):680–687. doi: 10.1016/j.eururo.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi T., Okuno Y., Hattori-Kato M., Zaitsu M., Mikami K. Detection of AR-V7 mRNA in whole blood may not predict the effectiveness of novel endocrine drugs for castration-resistant prostate cancer. Res. Rep. Urol. 2016;8:21–25. doi: 10.2147/rru.s98877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee C.H., Ku J.Y., Ha J.M., Bae S.S., Lee J.Z., Kim C.S. Transcript levels of androgen receptor variant 7 and ubiquitin-conjugating enzyme 2C in hormone sensitive prostate cancer and castration-resistant prostate cancer. Prostate. 2017;77(1):60–71. doi: 10.1002/pros.23248. [DOI] [PubMed] [Google Scholar]
- 47.Wang S., Yang S., Nan C., Wang Y., He Y., Mu H. Expression of androgen receptor variant 7 (AR-V7) in circulated tumor cells and correlation with drug resistance of prostate cancer cells. Med. Sci. Monit. 2018;24:7051–7056. doi: 10.12659/msm.909669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X., Morrissey C., Sun S., Ketchandji M., Nelson P.S., True L.D. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS ONE. 2011;6(11):e27970. doi: 10.1371/journal.pone.0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saylor P.J., Lee R.J., Arora K.S., Deshpande V., Hu R., Olivier K. Branched chain RNA in situ hybridization for androgen receptor splice variant AR-V7 as a prognostic biomarker for metastatic castration-sensitive prostate cancer. Clin. Cancer Res. 2016 doi: 10.1158/1078-0432.ccr-16-0237. [DOI] [PubMed] [Google Scholar]
- 50.Cattrini C., Rubagotti A., Zinoli L., Cerbone L., Zanardi E., Capaia M. Role of circulating tumor cells (CTC), androgen receptor full length (AR-FL) and androgen receptor splice variant 7 (AR-V7) in a prospective cohort of castration-resistant metastatic prostate cancer patients. Cancers. 2019;11(9) doi: 10.3390/cancers11091365. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taplin M.E., Antonarakis E.S., Ferrante K.J., Horgan K., Blumenstein B., Saad F. Androgen receptor modulation optimized for response-splice variant: a phase 3, randomized trial of galeterone versus enzalutamide in androgen receptor splice variant-7-expressing metastatic castration-resistant prostate cancer. Eur. Urol. 2019 doi: 10.1016/j.eururo.2019.08.034. [DOI] [PubMed] [Google Scholar]
- 52.Sharp A., Welti J.C., Lambros M.B.K., Dolling D., Rodrigues D.N., Pope L. Clinical utility of circulating tumour cell androgen receptor splice variant-7 status in metastatic castration-resistant prostate cancer. Eur. Urol. 2019 doi: 10.1016/j.eururo.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Maillet D., Allioli N., Peron J., Plesa A., Decaussin-Petrucci M., Tartas S. Improved androgen receptor splice variant 7 detection using a highly sensitive assay to predict resistance to abiraterone or enzalutamide in metastatic prostate cancer patients. Eur. Urol. Oncol. 2019 doi: 10.1016/j.euo.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Okegawa T., Ninomiya N., Masuda K., Nakamura Y., Tambo M., Nutahara K. AR-V7 in circulating tumor cells cluster as a predictive biomarker of abiraterone acetate and enzalutamide treatment in castration-resistant prostate cancer patients. Prostate. 2018;78(8):576–582. doi: 10.1002/pros.23501. [DOI] [PubMed] [Google Scholar]
- 55.Sieuwerts A.M., Onstenk W., Kraan J., Beaufort C.M., Van M., De Laere B. AR splice variants in circulating tumor cells of patients with castration-resistant prostate cancer: relation with outcome to cabazitaxel. Mol. Oncol. 2019;13(8):1795–1807. doi: 10.1002/1878-0261.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scher H.I., Anuradha J., Eric W., Brigit M. Assessment of the validity of nuclear-localized androgen receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration-resistant prostate cancer. JAMA Oncol. 2018 Sep;14(9):1179–1186. doi: 10.1001/jamaoncol.2018.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.